
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Endocrinol. , 05 July 2022
Sec. Pediatric Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.865913
 Jiale Liu1†
Jiale Liu1† Minjie Luo2†
Minjie Luo2† Siyuan Lv2
Siyuan Lv2 Shaohua Tao3
Shaohua Tao3 Zhu Wu3
Zhu Wu3 Lihua Yu1
Lihua Yu1 Danna Lin1
Danna Lin1 Lulu Huang1
Lulu Huang1 Li Wu1
Li Wu1 Xu Liao1
Xu Liao1 Juan Zi1
Juan Zi1 Xiaorong Lai1
Xiaorong Lai1 Yuting Yuan1
Yuting Yuan1 Wangming Zhang2*
Wangming Zhang2* Lihua Yang1*
Lihua Yang1*In this study, atypical choroid plexus papilloma was treated with high-dose rapamycin for 17 days preoperatively in an infant. Rapamycin significantly reduced the blood supply to the tumor while reducing the tumor volume, and most of the tumor was resected successfully. However, the infant developed hyperglycemia related to the rapamycin dose, which was effectively controlled by adjusting the dose and applying insulin.
Atypical choroid plexus papilloma (aCPP), a subtype of a choroid plexus tumor (CPT) (1–3), is a rare central nervous system tumor characterized by an abundant blood supply and the risk of massive blood loss.
Rapamycin has been widely applied to prevent acute rejection in organ transplantation and successfully treats complicated vascular anomalies and some brain tumors (4, 5).
Here, we report an 11-month-old infant with aCPP treated with high-dose rapamycin for 17 days preoperatively. Rapamycin is effective in reducing the blood supply to the tumor, controlling tumor volume and reducing risk of surgical hemorrhage. However, the infant developed hyperglycemia related to the rapamycin dose, which was effectively controlled by adjusting the dose and applying insulin.
An 11-month-old boy (weight 10 kg) was admitted to the pediatric intensive care unit (PICU) on May 4, 2021 because of increasing head circumference for 1 week and a cerebral hernia for 1 day. He presented with irritability, dysphoria, and vomiting, an enlarged head circumference of 50 cm, and an approximate 3*3 cm anterior fonticulus with increasing tension. He was somnolent with obvious nuchal rigidity but had normal pupils, isometric muscle tension, and normal spinal reflexes at admission. He had a negative family history of neurologic or metabolic diseases.
Routine blood examination, blood biochemistry assays, functional coagulation assays, endocrine function, and diabetes mellitus–associated laboratory tests were normal, except for a slight increase in cortisol levels at 577 nmol/L (May 4, 2021, 23:30) (reference value 166–507 nmol/L at 8:00; 73.8-291 nmol/L at 16:00), which returned to normal 1 week later and might be related to the infant’s state of increased stress. Computed tomography (CT) and magnetic resonance (MR) images revealed a primary malignant tumor (60.00*84.01*94.72 mm) with a rich blood supply in the right lateral ventricle and metastatic tumors in the left lateral ventricle, the third ventricle, and cerebellopontine angle (CPA) (Figure 2).
To reduce blood supply to the tumor, the patient started taking 2.6 mg/m2/day rapamycin orally (divided into two equal doses) (2, 6, 7) on May 6. The patient had a normal diet (approximately 200 ml of formula milk every 3–4 h), and the 2 h postprandial plasma glucose of the patient ranged from 7.7 mmol/L to 10.8 mmol/L. The trough concentration of plasma rapamycin on May 10 after 4 days of rapamycin treatment was 6.8 ng/ml, below the target trough plasma rapamycin concentration of 10–15 ng/ml (6, 8), so we increased the dose of rapamycin to 5.2 mg/m2/day (divided into two equal doses). The patient became conscious with stable vital signs, and vomiting and neck resistance disappeared on May 13 after 7 days of rapamycin treatment (the 9th day after admission). Therefore, he underwent stereotactic biopsy to confirm tumor pathological diagnosis and Ommaya insertion to alleviate and prevent hydrocephalus (9, 10). The pathological diagnosis of the tumor in the biopsy tissue was aCPP. His fasting plasma glucose level rose to 21 mmol/L for no apparent reason on May 15 and returned to normal after 3 days of insulin injection. The trough concentration of plasma rapamycin on May 17 after 11 days of rapamycin treatment was 7.2 ng/ml, remaining below the target trough plasma rapamycin concentration of 10–15 ng/ml, so we continued to increase the dose of rapamycin to 7.8 mg/m2/day (divided into two equal doses) on May 19. The MR images showed that the blood supply to the tumor had notably decreased on May 21 after 14 days of rapamycin treatment (the 17th day after admission), while the volume of the tumor in the right lateral ventricle decreased to 56.82*80.06*92.51 mm (Figure 2). Thus, the patient successfully underwent a subtotal resection of the tumor (110*100*75 mm) on May 23 after 17 days of rapamycin treatment (the 19th day after admission), and the bleeding volume was less than 50 ml. However, after surgery, his recovery was complicated with pneumonia and intracranial staphylococcal infection, which was controlled with clindamycin (8 mg/kg, q8 h) and linezolid (10 mg/kg q12 h). He was also found to have hyperglycemia, rapamycin was withdrawn, and intravenous insulin therapy was given after excluding hyperglycemia caused by abnormal pancreatic structure and function. After 14 days of insulin treatment, his blood glucose concentration returned to normal, so he continued to take 7.8 mg/m2/day (divided into two equal doses) rapamycin orally on June 10 (the 16th day after rapamycin withdrawal), together with continuous subcutaneous insulin infusion (0.5 IU/h) to prevent hyperglycemia. The patient started receiving CPT-SIOP-2000 protocol chemotherapy (11, 12) on July 2. Because the tumor was well controlled, rapamycin and insulin were withdrawn on August 3 (Figure 1). At present, he is still undergoing chemotherapy and maintains normal plasma glucose and insulin levels, and the residual tumor continues to shrink.
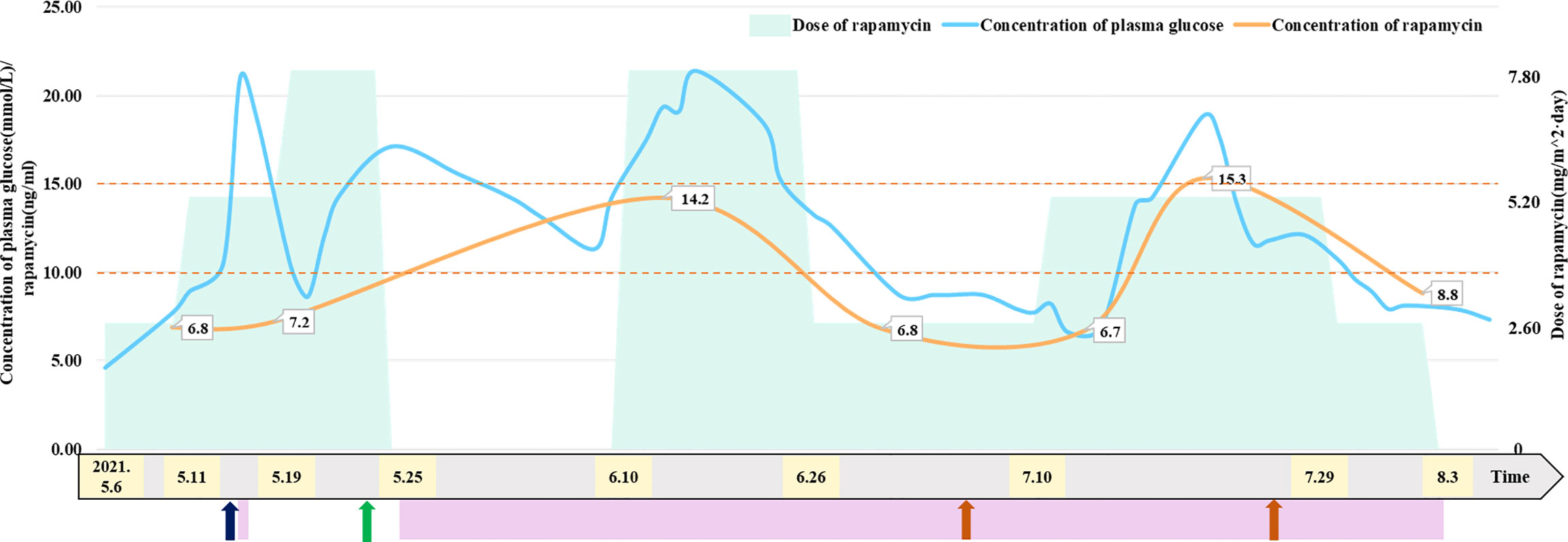
Figure 1 Relationship between dose of rapamycin and concentration of plasma glucose and rapamycin. Patient received insulin treatment to control plasma glucose on May 15 and from May 25 to August 3, 2021. Patient underwent Ommaya insertion and biopsy on May 13, 2021. Patient underwent subtotal resection of tumor on May 23, 2021. Patient received CPT-SIOP-2000 chemotherapy on July 2 and July 26, 2021. The maintaining trough concentration of rapamycin is recommended between 10 to 15 ng/mL. The figure indicated that patient’s concentration of plasma glucose has a positive correlation with the dose of rapamycin.
CPTs with a rich blood supply have a fatal risk of hemorrhaging in the perioperative period and surgery (13–17), and a study found that CPT patients without preoperative vascular embolization lost 182% of their blood volume during surgery (18). In addition, the perioperative mortality of CPT patients can reach 25% (14, 15, 17, 19, 20), and hemorrhage accounts for 12% (20); therefore, it is necessary to reduce the tumor blood supply before surgery. Considering the risk of interventional therapy, the patient’s parents refused patient treatment with preoperative vascular embolization, so we hoped to reduce the blood supply to the tumor and improve the success rate of surgery by rapamycin treatment.
Rapamycin has been applied in brain tumors (4, 5, 21, 22) and complicated vascular anomalies (23, 24) in recent years. To reduce the blood supply to the tumor, the patient, in this case, took rapamycin preoperatively. The MR images showed that the blood supply to the tumor and tumor volume decreased after 17 days of rapamycin treatment, and the subtotal resection of the tumor was successful, with a bleeding volume of less than 50 ml (Figure 2).

Figure 2 Serial intracranial MR images of the patient. Panel 1 Coronal (A) and transverse (B) T2-weighted MRI of the patient showed the tumor size in the right lateral ventricle and (C, D) MRA displayed the tumor with abundant blood supply (high-signal intensity) at admission (May 4, 2021). Panel 2 Coronal (E) and transverse (F) T2-weighted MRI showed a slight decrease in tumor size and (G, H) MRA displayed significant decrease of blood supply (high-signal intensity) to tumor after 14 days of oral rapamycin treatment (May 21, 2021).
The doses and courses of rapamycin vary based on the tumor type, location, and size. The recommended dose of rapamycin based on large-scale randomized scale trials of complicated vascular anomalies is 2.6 mg/m2/day (divided into two equal doses) (2, 6, 7) to achieve the target trough concentration of plasma rapamycin of 10–15 ng/ml (6, 8). However, there is no standard dose for treating solid tumors such as brain tumors. To reduce the blood supply to the tumor and tumor volume, the patient took 2.6 mg/m2/day rapamycin orally (divided into two equal doses) at the initial recommended dose of complicated vascular anomalies, but the trough concentration of plasma rapamycin was under 10–15 ng/ml after 7 days of rapamycin treatment. According to some reports, the recommended dose for children with brain tumors is 3–5 mg/m2/day (25–27), and the maximum dose for recurrent and refractory solid tumors reaches 150 mg/m2/day (once a week) without serious rapamycin-induced adverse effects (28). Furthermore, because of low oral bioavailability (15%–20%) (29) and poor blood–brain barrier penetration (30–32) [the ratio of the cerebrospinal fluid rapamycin concentration to the plasma rapamycin concentration was 0.0057 (33)], the patient with aCPP finally received 7.8 mg/m2/day (divided into two equal doses), and the trough concentration of plasma rapamycin reached 14.8 ng/ml. Of note, the trough concentration of plasma rapamycin reached 10–15 ng/ml when the dose of rapamycin was 2–3 times higher than the recommended dose, which was related to individual differences between patients.
The trough concentration of plasma rapamycin finally reached 10–15 ng/ml by adjusting the rapamycin dose in this case, but the patient developed hyperglycemia. After excluding hyperglycemia caused by pancreatic dysfunction, we suspected that the patient’s hyperglycemia was related to rapamycin treatment and that the concentration of plasma glucose was correlated with the rapamycin dose. Rapamycin-induced hyperglycemia could be controlled by insulin or dosage adjustment and returned to normal after rapamycin withdrawal, in this case, demonstrating that rapamycin-induced hyperglycemia was related to high-dose rapamycin. When the patient developed hyperglycemia, there were no other common adverse effects related to rapamycin treatment, such as oral ulcers, hyperlipidemia, liver dysfunction, and bone marrow suppression (34, 35), because the rapamycin concentration was within 15 ng/ml, and these common adverse effects were usually associated with concentrations of plasma rapamycin.
In conclusion, rapamycin can effectively reduce the blood supply to the tumor and tumor volume but induces reversible hyperglycemia when this aCPP patient received high-dose rapamycin orally to reach the target trough plasma concentration in this case. Therefore, the concentrations of plasma rapamycin and glucose should be monitored during high-dose rapamycin treatment, and dosage adjustment or insulin may be needed if patients develop hyperglycemia.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
JL and ML drafted the manuscript. SL, ST, ZW, LYu, DL, LH, LW XLi JZ, XLa and YY collected materials and prepared figures. WZ and LYa critically revised the final manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
3. Lafay-Cousin L, Keene D, Carret AS, Fryer C, Brossard J, Crooks B, et al. Choroid Plexus Tumors in Children Less Than 36 Months: The Canadian Pediatric Brain Tumor Consortium (CPBTC) Experience. Childs Nerv Syst (2011) 27:259–64. doi: 10.1007/s00381-010-1269-9
4. Adolph JE, Fleischhack G, Gaab C, Mikasch R, Mynarek M, Rutkowski S, et al. Systemic Chemotherapy of Pediatric Recurrent Ependymomas: Results From the German HIT-REZ Studies. J Neurooncol (2021) 155:193–202. doi: 10.1007/s11060-021-03867-8
5. Bruno F, Pellerino A, Bertero L, Soffietti R, Rudà R. Targeted Therapies in Rare Brain Tumours. Int J Mol Sci (2021) 22:7949. doi: 10.3390/ijms22157949
6. Mizuno T, Fukuda T, Emoto C, Mobberley-Schuman PS, Hammill AM, Adams DM, et al. Developmental Pharmacokinetics of Sirolimus: Implications for Precision Dosing in Neonates and Infants With Complicated Vascular Anomalies. Pediatr Blood Cancer (2017) 64:e26458. doi: 10.1002/pbc.26470
7. Mizuno T, Emoto C, Fukuda T, Hammill AM, Adams DM, Vinks AA. Model-Based Precision Dosing of Sirolimus in Pediatric Patients With Vascular Anomalies. Eur J Pharm Sci (2017) 109s:S124–31. doi: 10.1016/j.ejps.2017.05.037
8. Tian R, Liang Y, Zhang W, Wang J, Shan Y, Gao H, et al. Effectiveness of Sirolimus in the Treatment of Complex Lymphatic Malformations: Single Center Report of 56 Cases. J Pediatr Surg (2020) 55:2454–8. doi: 10.1016/j.jpedsurg.2019.12.021
9. Jiang C, Wu X, Lin Z, Wang C, Kang D. External Drainage With an Ommaya Reservoir for Perioperative Hydrocephalus in Children With Posterior Fossa Tumors. Childs Nerv Syst (2013) 29:1293–7. doi: 10.1007/s00381-013-2078-8
10. Peyrl A, Chocholous M, Azizi AA, Czech T, Dorfer C, Mitteregger D, et al. Safety of Ommaya Reservoirs in Children With Brain Tumors: A 20-Year Experience With 5472 Intraventricular Drug Administrations in 98 Patients. J Neurooncol (2014) 120:139–45. doi: 10.1007/s11060-014-1531-1
11. Wrede B, Hasselblatt M, Peters O, Thall PF, Kutluk T, Moghrabi A, et al. Atypical Choroid Plexus Papilloma: Clinical Experience in the CPT-SIOP-2000 Study. J Neurooncol (2009) 95:383–92. doi: 10.1007/s11060-009-9936-y
12. Koh EJ, Wang KC, Phi JH, Lee JY, Choi JW, Park SH, et al. Clinical Outcome of Pediatric Choroid Plexus Tumors: Retrospective Analysis From a Single Institute. Childs Nerv Syst (2014) 30:217–25. doi: 10.1007/s00381-013-2223-4
13. Addo NK, Kamaly-Asl ID, Josan VA, Kelsey AM, Estlin EJ. Preoperative Vincristine for an Inoperable Choroid Plexus Papilloma: A Case Discussion and Review of the Literature. J Neurosurg Pediatr (2011) 8:149–53. doi: 10.3171/2011.5.PEDS1187
14. Due-Tønnessen B, Helseth E, Skullerud K, Lundar T. Choroid Plexus Tumors in Children and Young Adults: Report of 16 Consecutive Cases. Childs Nerv Syst (2001) 17:252–6. doi: 10.1007/PL00013728
15. Guidetti B, Spallone A. The Surgical Treatment of Choroid Plexus Papillomas: The Results of 27 Years Experience. Neurosurg Rev (1981) 4:129–37. doi: 10.1007/BF01743638
16. Pencalet P, Sainte-Rose C, Lellouch-Tubiana A, Kalifa C, Brunelle F, Sgouros S, et al. Papillomas and Carcinomas of the Choroid Plexus in Children. J Neurosurg (1998) 88:521–8. doi: 10.3171/jns.1998.88.3.0521
17. Schijman E, Monges J, Raimondi AJ, Tomita T. Choroid Plexus Papillomas of the III Ventricle in Childhood. Their Diagnosis and Surgical Management. Childs Nerv Syst (1990) 6:331–4. doi: 10.1007/BF00298279
18. Haliasos N, Brew S, Robertson F, Hayward R, Thompson D, Chakraborty A. Preoperative Embolisation of Choroid Plexus Tumours in Children: Part I—does the Reduction of Perioperative Blood Loss Affect the Safety of Subsequent Surgery? Child's Nervous System (2012) 29:65–70. doi: 10.1007/s00381-012-1912-8
19. Fang Y, Westbrook R, Hill C, Boparai RK, Arum O, Spong A, et al. Duration of Rapamycin Treatment has Differential Effects on Metabolism in Mice. Cell Metab (2013) 17:456–62. doi: 10.1016/j.cmet.2013.02.008
20. Hawkins JC 3rd. Treatment of Choroid Plexus Papillomas in Children: A Brief Analysis of Twenty Years' Experience. Neurosurgery (1980) 6:380–4. doi: 10.1227/00006123-198004000-00005
21. Dorrell MI, Kast-Woelbern HR, Botts RT, Bravo SA, Tremblay JR, Giles S, et al. A Novel Method of Screening Combinations of Angiostatics Identifies Bevacizumab and Temsirolimus as Synergistic Inhibitors of Glioma-Induced Angiogenesis. PLos One (2021) 16:e0252233. doi: 10.1371/journal.pone.0252233
22. Huang M, Ke Y, Sun X, Yu L, Yang Z, Zhang Y, et al. Mammalian Target of Rapamycin Signaling is Involved in the Vasculogenic Mimicry of Glioma via Hypoxia-Inducible Factor-1alpha. Oncol Rep (2014) 32:1973–80. doi: 10.3892/or.2014.3454
23. Ren AA, Snellings DA, Su YS, Hong CC, Castro M, Tang AT, et al. PIK3CA and CCM Mutations Fuel Cavernomas Through a Cancer-Like Mechanism. Nature (2021) 594:271–6. doi: 10.1038/s41586-021-03562-8
24. Al-Samkari H, Eng W. A Precision Medicine Approach to Hereditary Hemorrhagic Telangiectasia and Complex Vascular Anomalies. J Thromb Haemost (2022) 20:1077–88. doi: 10.1111/jth.15715
25. Carvalho DM, Richardson PJ, Olaciregui N, Stankunaite R, Lavarino C, Molinari V, et al. Repurposing Vandetanib Plus Everolimus for the Treatment of ACVR1-Mutant Diffuse Intrinsic Pontine Glioma. Cancer Discovery (2022) 12:416–31. doi: 10.1158/2159-8290.CD-20-1201
26. Miklja Z, Yadav VN, Cartaxo RT, Siada R, Thomas CC, Cummings JR, et al. Everolimus Improves the Efficacy of Dasatinib in PDGFRalpha-Driven Glioma. J Clin Invest (2020) 130:5313–25. doi: 10.1172/JCI133310
27. Cacchione A, Lodi M, Carai A, Miele E, Tartaglia M, Megaro G, et al. Upfront Treatment With mTOR Inhibitor Everolimus in Pediatric Low-Grade Gliomas: A Single-Center Experience. Int J Cancer (2020) 148:2522–34. doi: 10.1002/ijc.33438
28. Spunt SL, Grupp SA, Vik TA, Santana VM, Greenblatt DJ, Clancy J, et al. Phase I Study of Temsirolimus in Pediatric Patients With Recurrent/Refractory Solid Tumors. J Clin Oncol (2011) 29:2933–40. doi: 10.1200/JCO.2010.33.4649
29. Kanaujia P, Poovizhi P, Ng WK, Tan RBH. Preparation, Characterization and Prevention of Auto-Oxidation of Amorphous Sirolimus by Encapsulation in Polymeric Films Using Hot Melt Extrusion. Curr Drug Deliv (2019) 16:663–71. doi: 10.2174/1567201816666190416123939
30. Klawitter J, Nashan B, Christians U. Everolimus and Sirolimus in Transplantation-Related But Different. Expert Opin Drug Saf (2015) 14:1055–70. doi: 10.1517/14740338.2015.1040388
31. Abs E, Goorden SM, Schreiber J, Overwater IE, Hoogeveen-Westerveld M, Bruinsma CF, et al. TORC1-Dependent Epilepsy Caused by Acute Biallelic Tsc1 Deletion in Adult Mice. Ann Neurol (2013) 74:569–79. doi: 10.1002/ana.23943
32. Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, et al. Response of a Neuronal Model of Tuberous Sclerosis to Mammalian Target of Rapamycin (mTOR) Inhibitors: Effects on Mtorc1 and Akt Signaling Lead to Improved Survival and Function. J Neurosci (2008) 28:5422–32. doi: 10.1523/JNEUROSCI.0955-08.2008
33. Brandt C, Hillmann P, Noack A, Römermann K, Öhler LA, Rageot D, et al. The Novel, Catalytic Mtorc1/2 Inhibitor PQR620 and the PI3K/mTORC1/2 Inhibitor PQR530 Effectively Cross the Blood-Brain Barrier and Increase Seizure Threshold in a Mouse Model of Chronic Epilepsy. Neuropharmacology (2018) 140:107–20. doi: 10.1016/j.neuropharm.2018.08.002
34. Lacouture M, Sibaud V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am J Clin Dermatol (2018) 19:31–9. doi: 10.1007/s40257-018-0384-3
Keywords: rapamycin, reversible hyperglycemia, insulin, atypical choroid plexus papilloma, infant, case report
Citation: Liu J, Luo M, Lv S, Tao S, Wu Z, Yu L, Lin D, Huang L, Wu L, Liao X, Zi J, Lai X, Yuan Y, Zhang W and Yang L (2022) Case Report: Reversible Hyperglycemia Following Rapamycin Treatment for Atypical Choroid Plexus Papilloma in an Infant. Front. Endocrinol. 13:865913. doi: 10.3389/fendo.2022.865913
Received: 16 February 2022; Accepted: 11 May 2022;
Published: 05 July 2022.
Edited by:
Vandana Jain, All India Institute of Medical Sciences, IndiaReviewed by:
Haotian Zhao, New York Institute of Technology, United StatesCopyright © 2022 Liu, Luo, Lv, Tao, Wu, Yu, Lin, Huang, Wu, Liao, Zi, Lai, Yuan, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wangming Zhang, d3poYW5nQHZpcC4xMjYuY29t; Lihua Yang, ZHJ5YW5nbGlodWFAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.