- 1Center of Reproductive Medicine, Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
- 2Key Laboratory of Reproductive Dysfunction Disease and Fertility Remodeling of Liaoning Province, Shenyang, China
The duration of ovarian stimulation which is largely dependent on the ovarian response to hormonal stimulation may influence in vitro fertilization (IVF) outcomes. Menstrual cycle length is potentially a good indicator of ovarian reserve and can predict ovarian response. Ovarian stimulation and the follicular phase of the menstrual cycle are both processes of follicular development. There is no published research to predict the duration of ovarian stimulation based on the length of the menstrual cycle. Our retrospective cohort study included 6110 women with regular menstrual cycles who underwent their first IVF treatment between January 2015 and October 2020. Cycles were classified according to quartiles of the ratio of ovarian stimulation duration to original follicular phase length (OS/FP). Multivariate generalized linear models were applied to assess the association between OS/FP and IVF outcomes. The odds ratio (OR) or relative risk (RR) was estimated for each quartile with the lowest quartile as the comparison group. OS/FP of 0.67 to 0.77 had more retrieved and mature oocytes (adjusted RR 1.11, 95% confidence interval [CI] 1.07–1.15, p for trend = 0.001; adjusted RR 1.14, 95% CI 1.09–1.19, p for trend = 0.001). OS/FP of 0.67 to 0.77 showed the highest rate of fertilization (adjusted OR 1.11, 95% CI 1.05–1.17, p for trend = 0.001). OS/FP > 0.77 had the lowest rate of high-quality blastocyst formation (adjusted OR 0.81, 95% CI 0.71–0.93, p for trend = 0.01). No apparent association was noted between OS/FP and clinical pregnancy, live birth, or early miscarriage rate. In conclusion, OS/FP has a significant effect on the number of oocytes, fertilization rate, and high-quality blastocyst formation rate. MCL could be used to predict the duration of ovarian stimulation with an OS/FP of 0.67 to 0.77, which provides a new indicator for the individualized clinical optimization of the trigger time.
Introduction
In recent years, the prevalence of infertility has gradually increased, and assisted reproductive technology (ART) has emerged as the main method to solve intractable infertility. The menstrual cycle is a crucial clinical reference for female reproductive health; it repeats approximately 500 times over the reproductive lifespan, which lasts about 35 to 40 years (menarche to menopause) (1). The median menstrual cycle length (MCL) is 28 days, and most cycles are between 21 and 35 days in length. The menstrual cycle can be separated into two stages: the follicular or proliferative phase and the luteal or secretory phase. The follicular phase accounts for 84% of the variation in MCL (2), whereas the luteal phase is relatively constant at approximately 14 days (3). Epidemiological data demonstrate that menstrual cycles vary in length and regularity and are affected by age, ethnicity, body mass index (BMI), and behavioral, occupational, and environmental factors (4, 5).
Controlled ovarian stimulation (COS) is a key component of successful ART treatment which aims to achieve the synchronized development of multiple follicles in a menstrual cycle (6). Compared with traditional COS methods, personalized ovarian stimulation has a more positive effect on in vitro fertilization (IVF) outcomes (7, 8). Serum anti-Mullerian hormone (AMH) and antral follicle count (AFC) are the most reliable contemporary indicators currently employed to assess ovarian reserve before ovarian stimulation in clinical practice and are highly sensitive and specific in detecting the quantitative aspects of ovarian reserve, including ovarian responsiveness (9, 10). Moreover, MCL is potentially a good indicator of ovarian reserve; a short MCL of 21–27 days, compared with one of 28–31 days, is associated with lower AMH and AFC, reduced fecundability in natural cycles, and poor IVF outcomes (11). MCL correlates with both the quantitative and qualitative aspects of ovarian reserve and may be employed as an earlier, more subtle sign of ovarian aging relative to physiological age (12). In addition, studies have shown that MCL can predict low and high ovarian responses in ART (13–15).
Clinicians may struggle with the actual trigger time, such as the presence of large lead follicles with numerous small follicles at the same time. Several studies have investigated the effects of ovarian stimulation duration and gonadotropin dose on IVF outcomes, but the results are inconsistent (16–25). The process of ovarian stimulation is similar to the follicular phase of the menstrual cycle, and the duration of ovarian stimulation is largely dependent on the ovarian response to hormonal stimulation. We hypothesized that the MCL may be used to predict the duration of ovarian stimulation and that a more favorable IVF outcome would be achieved when the MCL and the duration of ovarian stimulation are within a certain quantitative relationship.
Our study aimed to investigate the effects of the relationship between the duration of ovarian stimulation and the duration of the previous menstrual cycle on IVF/ICSI outcomes and provide a novel index for controlling the duration of ovarian stimulation and optimising the clinical determination of trigger time.
Materials and Methods
Inclusion and Exclusion Criteria
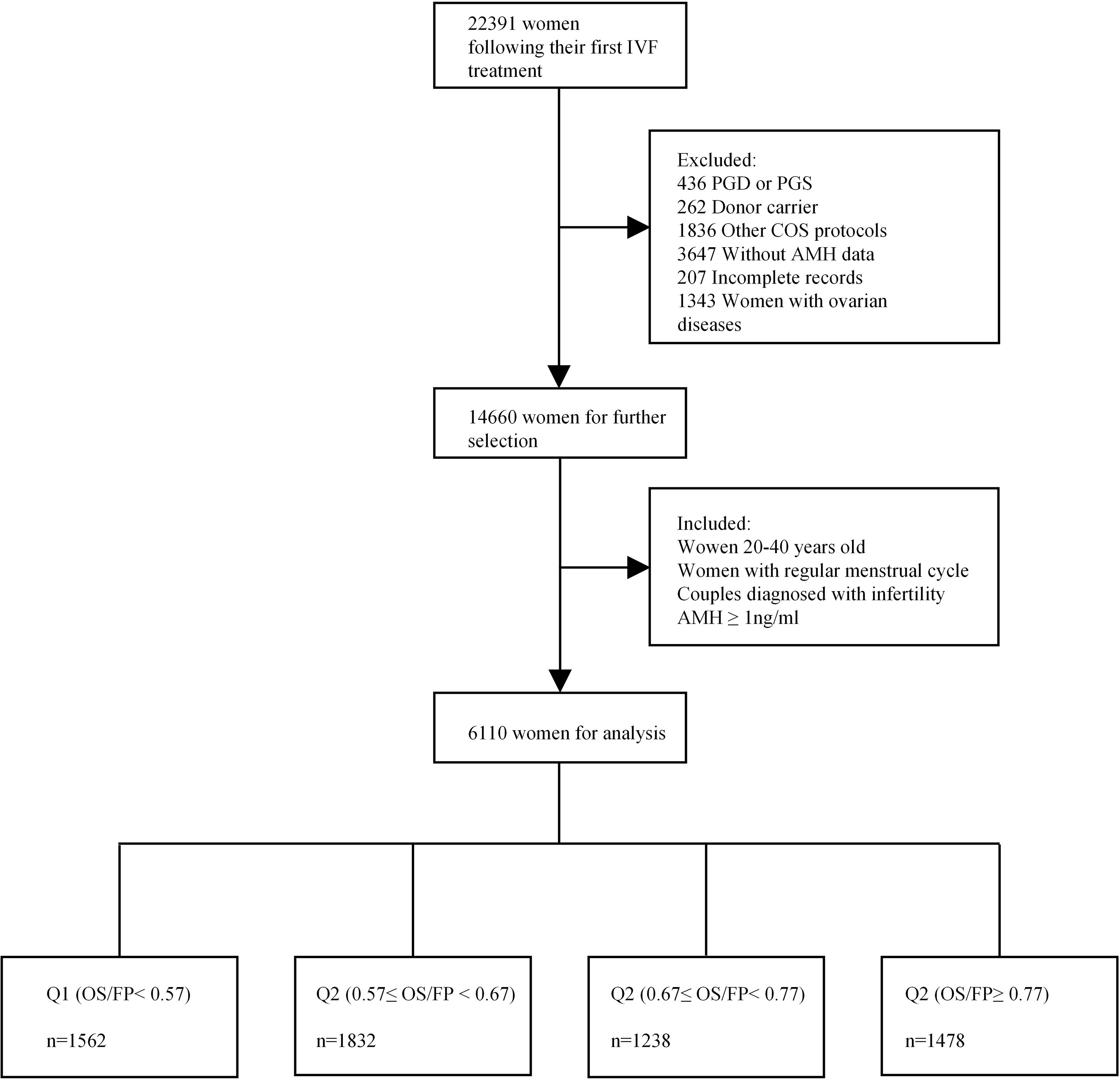
A total of 6110 women with regular menstrual cycles who underwent their first fresh IVF/ICSI cycle at the reproductive centre of Shengjing Hospital in Shenyang, China, between January 2015 and October 2020 were recruited in this study. The infertility diagnosis was assigned according to previously described definitions of the Society for Assisted Reproductive Technology (26). As shown in Figure 1, the inclusion criteria were as follows (1): women between the ages of 20 and 40 years (2); MCL between 21 and 35 days (3); couples diagnosed with infertility (failure to conceive with unprotected intercourse for 12 months or longer) (4); IVF/ICSI indications including unexplained infertility, tubal factors, or a male factor (including oligospermia, asthenospermia, or obstructive azoospermia); and (5) minimum AMH threshold of 1 ng/ml (We excluded women with low ovarian reserve with a cut-off of AMH <1 ng/mL for diminished ovarian reserve (DOR) which was consistent with studies reporting age-specific normal values (27, 28). We did not use DOR diagnosis by the Society for Assisted Reproductive Technology (SART) in our study as it has been shown that DOR is overdiagnosed in the SART reporting system (29).). Those with cycles involving donor oocytes, preimplantation genetic diagnosis, preimplantation genetic screening, or incomplete records were excluded. Women who were diagnosed with polycystic ovary syndrome, ovarian endometriosis, or other ovarian diseases were also excluded. Information on demographics, medical history, and clinical data were extracted from electronic medical systems. Our study was approved by the Ethics Committee of the Shengjing Hospital of China Medical University (2021PS001F), and the requirement of obtaining informed consent was waived owing to the retrospective nature of the study.
Ovarian Stimulation and the IVF/ICSI Procedure
Prior to the IVF cycle, women who underwent ovarian reserve testing were allocated to one of three ovarian stimulation types as clinically indicated, which included the long-term, short-term, or antagonist protocol. The women’s serum oestradiol (E2), luteinizing hormone, follicle sizes, and follicle counts were monitored during ovarian stimulation. Subcutaneous recombinant follicle stimulating hormone (Gonal-f NGPEN, Merck Serono, Germany) or human menopausal gonadotropin (MENOPUR, Ferring, China) was administered with a combined daily dose of 150-300 IU. Ovulation was triggered with hCG (Crinone, Merck Serono, Germany) when three or more follicles reached ≥ 18 mm in diameter. Thirty-six to thirty-eight hours after the hCG trigger, a transvaginal ultrasound-guided oocyte aspiration was performed. The oocytes were then retrieved and fertilized using IVF/ICSI procedures based on the semen quality. One blastocyst or two cleavage embryos were transferred on the third or fifth day after fertilization. Progesterone was administered for luteal support after oocyte retrieval.
Outcome and Embryo Quality Assessment
The numbers of retrieved mature oocytes (metaphase II, MII), oocytes with two pronuclei, and good-quality embryos were evaluated by embryologists. The fertilization rate, defined as the number of oocytes with two pronuclei divided by the number of oocytes inseminated, was determined 17–20 hours after insemination. On day 3, the Peter scoring system (30, 31) was used to assess the quality of the embryos based on the size, shape, and fragmentation of the blastomeres. Embryos with 6–10 cells, even size, regular shape, and < 20% fragmentation were considered good-quality embryos. At the blastocyst stage, the embryos were evaluated using the Gardner system (1): blastocysts were rated as grades 1–6 according to the extent of blastocyst expansion and hatching, and (2) further A–C scores were assigned to grade 3–6 blastocysts based on the number and cohesiveness of the inner cell mass and trophectoderm. A high-quality blastocyst was defined as having a grade ≥ 3BB on day 5 or ≥ 4BB on day 6. The proportion of blastocysts and high-quality blastocysts formed was evaluated.
We defined biochemical pregnancy as a serum β-hCG level > 30 mIU/mL on day 14 after embryo transfer. Clinical pregnancy was defined as the presence of an ultrasound-confirmed intrauterine pregnancy 35 days after embryo transfer. A miscarriage was the spontaneous loss of pregnancy before 12 weeks of gestation. A live birth was referred to a newborn delivered on or after 24 weeks of gestation. The primary outcomes were clinical pregnancy and live birth. Retrieved oocytes, fertilization rate, and embryo quality were considered intermediate outcomes.
Data Classification and Statistical Analyses
The MCL was determined by calculating the average length of the menstrual cycle for the three months prior to the consultation without any hormonal stimulation. Follicular phase length was calculated by subtracting 14 days from the MCL. Patients were classified by quartiles of the ratio of the duration of ovarian stimulation to the follicular phase length (OS/FP) (Supplementary Table 1).
The demographic and clinical characteristics of the participants were reported using mean ± SD or percentages. Associations between categorical variables were analyzed using chi-squared tests or Fisher’s exact tests when one or more cell counts were ≤ 5. Comparisons between various variables and quartiles of the OS/FP were performed using the Kruskal–Wallis test, and the Bonferroni correction was used to account for the increase in type I error. Multivariate generalized linear models were applied to assess the association between OS/FP and IVF outcomes. A Poisson distribution with log link function was used to test the association between the numbers of total and mature oocytes, and a binomial distribution with logit link function was used for fertilization rate, quality of each embryo, and pregnancy outcomes. Overall linear trends were tested across quartiles using the classification number as a continuous variable. The odds ratio (OR) of fertilization, embryo quality, and pregnancy outcomes were estimated for each quartile with the lowest quartile as the comparison group. The relative risk (RR) for the numbers of retrieved oocytes and mature oocytes were estimated for each quartile with the lowest quartile as the comparison group. An RR or > 1 denotes an increase in the prevalence risk and odds of the target events as the ratio between the duration of ovarian stimulation and the follicular phase length increases by one quartile. Moreover, nomograms of fertilization and high-quality blastocyst were developed based on corresponding independent significant factors. Internal validation was performed using the bootstrap method. C-index and calibration curves were used to assess predictive and discriminatory capacity.
Covariates including age (years), BMI (kg/m2), smoking status, fertility status (primary infertility or secondary infertility), etiology of infertility (male factor, female factor, mixed factor, or unexplained infertility), AMH (ng/mL), ovarian stimulation type (long GnRH-agonist, short GnRH-agonist, or GnRH-antagonist), initial gonadotropin dose (IU) and insemination methods (IVF or ICSI) (32) were collected. The variables included in the final model were statistically significant variables (p < 0.15) of the outcomes or those with critical biological significance, such as age and BMI (33). We further assessed the effects of OS/FP on IVF outcomes across female age (≤ 30, 30–35, > 35) and ovarian stimulation types (long GnRH-agonist or GnRH-antagonist) using subgroup analysis. Statistical Package for Social Sciences software (SPSS, version 22.0; Chicago, IL, USA) was used for all statistical calculations. Nomograms were formulated using the package “rms” in R software, version 3.6.3. (http://www.r-project.org/). All reported p values were based on two-sided tests and compared with a significance level of 5%.
Results
In this study, 6110 women who underwent IVF/ICSI treatment for the first time were included. Their mean age and BMI were 31.5 ± 3.9 years and 22.7 ± 3.3 kg/m2, respectively, and 180 (2.9%) women were obese (BMI ≥ 30 kg/m2) (Supplementary Table 2). A total of 2747 (45.0%) women had a history of pregnancy; 436 (7.1%) had a history of live birth; and 2019 (33.0%) had a history of miscarriage. The number of women with a university degree or higher was 2391 (39.1%). The majority of women had never smoked, and only 102 (1.6%) smoked before pregnancy. Secondary infertility was noted in 2424 (39.7%) women. Moreover, the following diagnoses were identified: female factor infertility, 3119 (51.0%); male factor infertility, 1276 (20.9%); mixed male and female factor infertility, 1506 (24.6%); and unexplained infertility, 209 (3.4%). IVF and ICSI were used for fertilization by 3831 (62.7%) and 2279 (37.3%) couples, respectively. A total of 2812 (46.0%) couples had embryos cultured to blastocyst stage. In this cycle, 2648 (43.3%) women had fresh embryos transferred, of which 1258 (47.5%) had clinical pregnancies and 942 (35.6%) had live births.
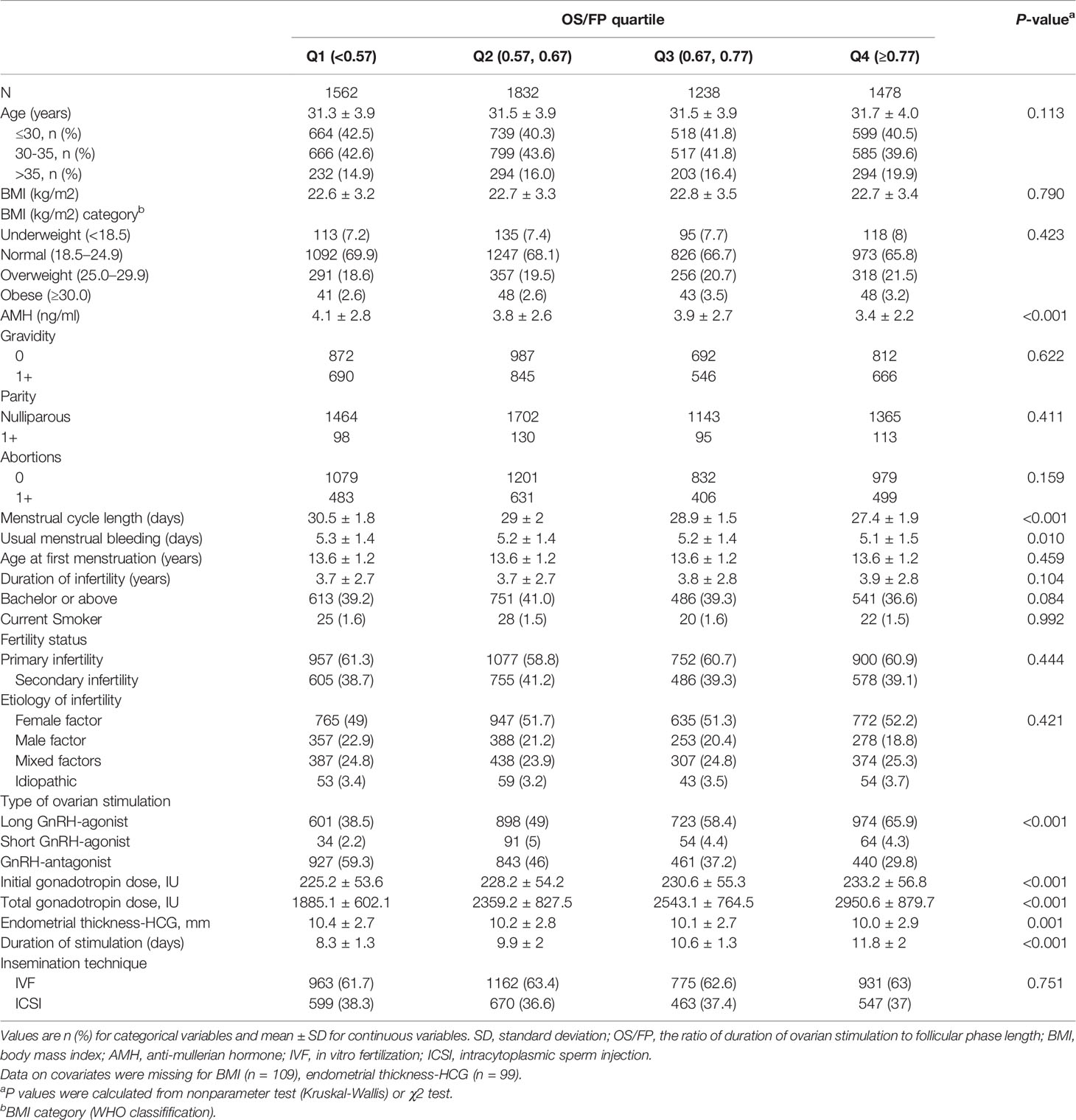
The clinical and reproductive characteristics of the women are shown in Table 1 according to the quartiles of OS/FP. OS/FP < 0.57 had higher AMH levels, a longer MCL and menstrual bleeding duration than OS/FP ≥ 0.77. No differences were noted among the four groups in terms of age, BMI, fertility history, education, smoking, age at menarche, etiology for infertility, and fertilization method. In terms of ovarian stimulation, more women with OS/FP < 0.57 and OS/FP of 0.57 to 0.67 used the GnRH-antagonist protocol for ovulation. The largest proportion of women with OS/FP of 0.67 to 0.77 and OS/FP ≥ 0.77 used the long GnRH-agonist protocol for ovulation. OS/FP ≥ 0.77 had a higher initial gonadotropin dose, a higher total gonadotropin dose, and a higher duration of ovarian stimulation than OS/FP < 0.57. No significant differences were noted in good-quality embryos, blastocyst formation, clinical pregnancy, live birth rate, miscarriage, or ectopic pregnancy rate among the four groups. OS/FP of 0.67 to 0.77 had the highest number of retrieved oocytes (12.6 ± 7.1) and cleaved embryos (8.3 ± 5.0), OS/FP > 0.77 had the highest fertilization rate (70.4 ± 22.4) and OS/FP of 0.57 to 0.67 had the highest rate of high-quality blastocyst formation (36.6 ± 27.2) (Table 2).
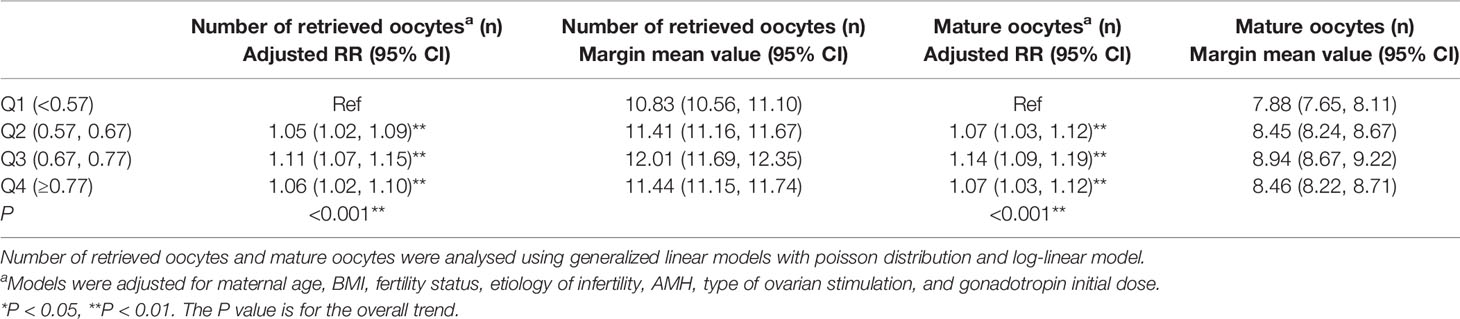
The effects of OS/FP on the number of retrieved and mature oocytes in the corrected multivariate model are described in Table 3. OS/FP ≥ 0.57 had more retrieved and mature oocytes than OS/FP < 0.57, and OS/FP of 0.67 to 0.77 achieved the most (adjusted RR = 1.11, 95% confidence interval [CI]: 1.07–1.15, p for trend = 0.001). In a stratified analysis of age and ovarian stimulation type, OS/FP had a significant effect on the number of retrieved and mature oocytes in women of different ages and ovarian stimulation types (p for trend < 0.001) (Supplementary Tables 3, 4).
Embryos from 3217 couples continued to be cultured, and a total of 2812 couples had embryos forming blastocysts. The relationship between OS/FP and fertilization rate as well as embryo development is shown in Table 4. Fertilization rate were higher in OS/FP of 0.67 to 0.77 than in OS/FP < 0.57 (adjusted OR=1.11, 95% CI: 1.06–1.17, p for trend=0.001). In the age-stratified analysis, the effect of OS/FP on fertilization rate was predominantly present in women of 30 to 35 and >35 years of age (adjusted OR=1.11, 95% CI: 1.01–1.20, p for trend=0.022; adjusted OR=1.27, 95% CI: 1.10–1.48, p for trend=0.001) (Supplementary Table 5). Moreover, the role of OS/FP on fertilization rate was mainly found in the long GnRH-agonist protocol (Supplementary Table 6). Good-quality embryo rate were lower in OS/FP > 0.77 than in OS/FP < 0.57 (adjusted OR=0.90, 95% CI: 0.82–0.98, p for trend=0.013) which only present in women of 30 to 35 years of age (Supplementary Table 5). No significant differences were found in blastocyst formation rate among the four groups. High-quality blastocyst formation rate were lower in OS/FP ≥ 0.77 than in OS/FP < 0.57 (adjusted OR=0.84 95% CI: 0.73–0.96, p for trend=0.01) (Table 4). The effects of OS/FP on high-quality blastocyst formation rate were mainly present in women between 30 to 35 years of age (Supplementary Table 5). The predictive accuracy and discriminatory capacity of nomograms for fertilization and high-quality blastocyst were low (Supplementary Figures 1–4). The corresponding C-indexes were 0.571 and 0.574, respectively.
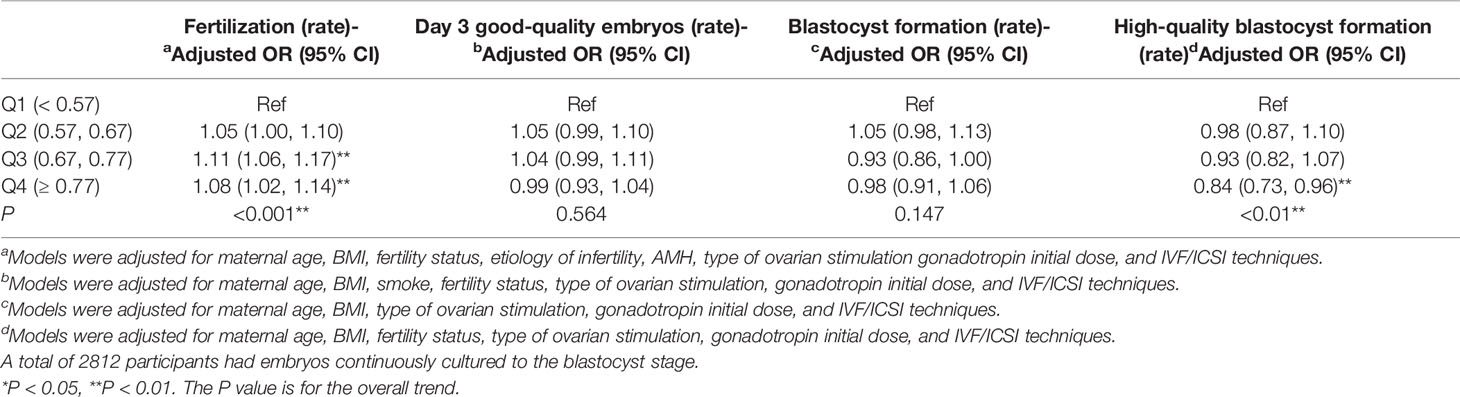
Table 4 Adjusted fertilization, day 3 good-quality embryos, blastocyst formation and high-quality blastocyst (95% CI) by quartile of OS/FP (N = 6110). .
A total of 2753 women had transferred embryos in this fresh cycle. The relationship between OS/FP and pregnancy outcomes is demonstrated in Table 5. No significant differences were found in clinical pregnancy rate, live birth rate, and early miscarriage rate among the four groups. OS/FP was not associated with pregnancy outcomes in the stratified analyses of age and ovarian stimulation type (Supplementary Tables 7, 8).
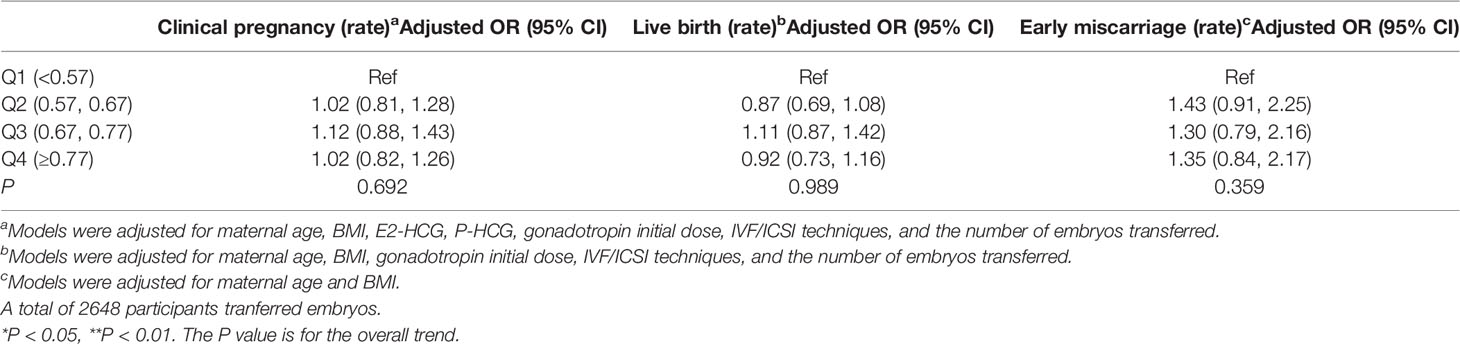
Table 5 Clinical pregnancy, live birth and early miscarriage (95% CI) by quartile of OS/FP (N = 2683).
Discussion
In this study, OS/FP was observed to have an effect on IVF outcomes in women with regular menstrual cycles undergoing IVF for the first time. In particular, women with OS/FP of 0.67 to 0.77 were able to obtain more oocytes, and had the highest rate of fertilization. With regard to embryos, women with OS/FP higher than 0.77 had the lowest high-quality blastocyst formation rate. OS/FP had no correlation with clinical pregnancy rate, live birth rate or miscarriage rate in the first fresh cycles.
Interestingly, the effects of OS/FP on the rate of good-quality embryos and high-quality blastocyst formation are only present in women of 30 to 35 years of age. A woman’s age has a significant effect on ovarian sensitivity, ovarian responsiveness to exogenous gonadotropin, oocyte quality, embryo-related parameters, and IVF outcomes (34–36). Thus, we speculated that such changes would mask the correspondence between the duration of ovarian stimulation and the follicular phase length in the natural state.
Regular menstrual cycles are often considered to allow spontaneous ovulation; this may sometimes be incorrectly interpreted as an indicator of female fertility. As women age, MCL gradually shortens and variability decreases which is a more sensitive indicator of ovarian aging than physiological age (11). As ovaries age, the resting pool of primordial ovarian follicles gradually diminishes, the cohort of recruited growing follicles declines, and the secretion of inhibin B by granulosa cells decreases, leading to a higher secretion of FSH and an earlier onset of follicular development; thus, ovulation occurs earlier and the follicular phase is shorter.
Predicting ovarian responsiveness to gonadotropin is one of the most important procedures in ART treatment which can optimise the success of treatment. AFC in combination with AMH is generally accepted as a useful method to assess ovarian responsiveness (9, 10). However, in clinical practice, patients with the same AMH levels have exhibited varying ovarian responsiveness. The ovarian response to hormone stimulation varies considerably among women with an AMH level below 1.1 ng/mL (low ovarian responders) and is strongly correlated with MCL (12). Recent evidence demonstrated that long MCLs are related to more antral follicular waves and higher ovarian responses (14, 15). Conversely, short MCLs are associated with poor responses to ovarian stimulation, a marker of ovarian aging (13). Furthermore, MCLs are associated with the number of retrieved oocytes and clinical pregnancy rate in IVF (11), whereas AMH cannot be used as a predictor of oocyte quality or clinical pregnancy outcomes (37). Therefore, MCL is likely to be a better biomarker than AMH for predicting the ovarian response. According to our results, clinicians can estimate the range of ovarian stimulation duration by calculating the original length of the follicular phase to obtain more mature oocytes. In addition, when the duration of ovarian stimulation exceeded 0.77 fold of the original follicular phase length, it may lead to a decrease in embryo quality. The potential underlying mechanism was probably that exposure to high doses of gonadotropins caused chromosomal abnormalities in oocytes and increased the aneuploidy rate of granulosa cells (38, 39).
Previous publications revealed conflicting results when investigating the relationship between the duration of ovarian stimulation and IVF outcomes. Although some studies support the association between prolonged gonadotropin stimulation and poor pregnancy outcome (17, 20, 22), some argue that there is no connection (15, 24), while some have obtained negative correlations (23). The lack of consensus is likely due to a lack of standardized cut-off points for ovulation duration, a small sample size, or failure to correct for confounding factors other than age. The studies which suggest that a longer ovarian stimulation duration is associated with lower live birth rate defined a long ovarian stimulation duration as one that is longer than 13 days, which represents a very small proportion of women, thereby increasing the probability of a positive result.
A major strength of our study is that we demonstrated the possibility of predicting the duration of ovarian stimulation by the MCL. MCL is easy to obtain and does not require any invasive tests, the duration of ovarian stimulation can be individually adjusted according to MCL. Furthermore, In contrast to other studies that used 8 and 12 days as time points for ovarian stimulation, our study limited the optimal choice to a fluctuating range of 1-2 days.
However, this study has several limitations. Only the first fresh cycles were considered in this study. Although we did not observe an effect of OS/FP on clinical pregnancy and live birth rates, previous studies have demonstrated that access to more mature oocytes and high quality embryos improved cumulative pregnancy rates (40, 41). In addition, clinicians may freeze all embryos in fresh cycles because of the moderate or severe ovarian hyper-stimulation syndrome (OHSS), elevated progesterone levels on the day of hCG trigger, low oocyte acquisition, or poor embryo quality (42). Women with extremely high or low OS/FP were subsequently less prone to undergo fresh embryo transfer, and this bias was relatively large, which may lead to the failure to determine the effect of OS/FP on clinical outcomes.
Recent studies have found that the live birth rate in fresh cycles increases with an increasing number of retrieved oocytes, with a plateau occurring when the number of retrieved oocytes reaches 15 or more, and the cumulative live birth rates for both fresh and frozen cycles consistently increase with an increasing number of retrieved oocytes, without a plateau (40, 43–45). The incidence of severe OHSS also increases with an increasing number of retrieved oocytes, especially when the number of retrieved oocytes reaches 18 and above, and the incidence of thromboembolism also increases significantly when the number of retrieved oocytes is more than 15 (46). In this study, women with OS/FP of 0.67 to 0.77 had access to more oocytes, but when the number of follicles (> 14 mm) reached 15 and above, an appropriate reduction in the duration of ovarian stimulation was considered depending on the length of the follicular phase to reduce the possibility of OHSS and thromboembolism. In addition, when patients have a diminished capacity for oocyte and embryo development, clinicians are required to optimize the ovarian response to gonadotropin by accurately estimating the ovarian reserve to customize an appropriate strategy to collect the maximum number of oocytes (47). Therefore, when the number of follicles (> 14 mm) is below or equal to 5, it may be beneficial to optimize the duration of ovarian stimulation to obtain more oocytes depending on the length of the follicular phase.
Overall, we present a new clinically valuable parameter, OS/FP, which can affect the number of retrieved oocytes, fertilization rate, and embryo quality. The trigger time can be easily optimized by referring to the length of the woman’s original follicular phase. Future studies will need to explore the role of OS/FP in ART through further expansion of the sample size and interventional studies to reveal the underlying mechanisms and consequently provide guidance for clinicians to optimize the appropriate trigger timing.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Shengjing Hospital of China Medical University (2021PS001F). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
XiZ: Design, conception, analysis and interpretation of data, drafting of article, visualization. XuZ: Acquisition of data, design, drafting of article. SSW: Analysis and interpretation of data, writing - review & editing, visualization. JCT: Conception, resources, writing - review & editing, supervision, funding acquisition. All authors approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the National Key Research and Development Program (2018YFC1002105), the National Natural Science Foundation of China (82071601, 61873257), the Shengjing Freelance Researcher Plan of Shengjing Hospital of China Medical University and the Major Special Construction Plan for Discipline Construction Project of China Medical University (3110118033).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank all the medical staff in the Center of Reproductive Medicine of Shengjing Hospital of China Medical University for their assistance in data collection. We would also like to thank Prof. Liqiang Zheng for his valuable advice regarding the statistical analysis of the data.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.862500/full#supplementary-material
References
1. Critchley HOD, Babayev E, Bulun SE, Clark S, Garcia-Grau I, Gregersen PK, et al. Menstruation: Science and Society. Am J Obstet Gynecol. (2020) 223:624–64. doi: 10.1016/j.ajog.2020.06.004
2. Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL. Use of Urine Biomarkers to Evaluate Menstrual Function in Healthy Premenopausal Women. Am J Epidemiol (1998) 147:1071–80. doi: 10.1093/oxfordjournals.aje.a009401
3. Crawford NM, Pritchard DA, Herring AH, Steiner AZ. Prospective Evaluation of Luteal Phase Length and Natural Fertility. Fertil Steril (2017) 107:749–55. doi: 10.1016/j.fertnstert.2016.11.022
4. Liu Y, Gold EB, Lasley BL, Johnson WO. Factors Affecting Menstrual Cycle Characteristics. Am J Epidemiol (2004) 160:131–40. doi: 10.1093/aje/kwh188
5. Bull JR, Rowland SP, Scherwitzl EB, Scherwitzl R, Danielsson KG, Harper J. Real-World Menstrual Cycle Characteristics of More Than 600,000 Menstrual Cycles. NPJ Digit Med (2019) 2:83. doi: 10.1038/s41746-019-0152-7
6. Paulson RJ. Introduction: Contemporary Approaches to Alternative Ovarian Stimulation Strategies for In Vitro Fertilization. Fertil Steril (2017) 108:555–7. doi: 10.1016/j.fertnstert.2017.08.023
7. Allegra A, Marino A, Volpes A, Coffaro F, Scaglione P, Gullo S, et al. A Randomized Controlled Trial Investigating the Use of a Predictive Nomogram for the Selection of the FSH Starting Dose in IVF/ICSI Cycles. Reprod BioMed Online (2017) 34:429–38. doi: 10.1016/j.rbmo.2017.01.012
8. Nyboe Andersen A, Nelson SM, Fauser BCJM, García-Velasco JA, Klein BM, Arce JC, et al. Individualized Versus Conventional Ovarian Stimulation for In Vitro Fertilization: A Multicenter, Randomized, Controlled, Assessor-Blinded, Phase 3 Noninferiority Trial. Fertil Steril (2017) 107:387–96.e4. doi: 10.1016/j.fertnstert.2016.10.033
9. Keane K, Cruzat VF, Wagle S, Chaudhary N, Newsholme P, Yovich J. Specific Ranges of Anti-Mullerian Hormone and Antral Follicle Count Correlate to Provide a Prognostic Indicator for IVF Outcome. Reprod Biol (2017) 17:51–9. doi: 10.1016/j.repbio.2016.12.002
10. la Marca A, Sunkara SK. Individualization of Controlled Ovarian Stimulation in IVF Using Ovarian Reserve Markers: From Theory to Practice. Hum Reprod Update (2014) 20:124–40. doi: 10.1093/humupd/dmt037
11. Younis JS, Iskander R, Fauser BCJM, Izhaki I. Does an Association Exist Between Menstrual Cycle Length Within the Normal Range and Ovarian Reserve Biomarkers During the Reproductive Years? A Systematic Review and Meta-Analysis. Hum Reprod Update (2020) 26:904–28. doi: 10.1093/humupd/dmaa013
12. Gizzo S, Andrisani A, Noventa M, Quaranta M, Esposito F, Armanini D, et al. Menstrual Cycle Length: A Surrogate Measure of Reproductive Health Capable of Improving the Accuracy of Biochemical/Sonographical Ovarian Reserve Test in Estimating the Reproductive Chances of Women Referred to ART. Reprod Biol Endocrinol (2015) 13:28. doi: 10.1186/s12958-015-0024-1
13. Oehninger S, Nelson SM, Verweij P, Stegmann BJ. Predictive Factors for Ovarian Response in a Corifollitropin Alfa/GnRH Antagonist Protocol for Controlled Ovarian Stimulation in IVF/ICSI Cycles. Reprod Biol Endocrinol (2015) 13:117. doi: 10.1186/s12958-015-0113-1
14. la Cour Freiesleben N, Gerds TA, Forman JL, Silver JD, Nyboe Andersen A, Popovic-Todorovic B. Risk Charts to Identify Low and Excessive Responders Among First-Cycle IVF/ICSI Standard Patients. Reprod BioMed Online (2011) 22:50–8. doi: 10.1016/j.rbmo.2010.08.010
15. Vassena R, Vidal R, Coll O, Vernaeve V. Menstrual Cycle Length in Reproductive Age Women Is an Indicator of Oocyte Quality and a Candidate Marker of Ovarian Reserve. Eur J Obstet Gynecol Reprod Biol (2014) 177:130–4. doi: 10.1016/j.ejogrb.2014.03.027
16. Martin JR, Mahutte NG, Arici A, Sakkas D. Impact of Duration and Dose of Gonadotrophins on IVF Outcomes. Reprod BioMed Online (2006) 13:645–50. doi: 10.1016/S1472-6483(10)60654-2
17. Pal L, Jindal S, Witt BR, Santoro N. Less is More: Increased Gonadotropin Use for Ovarian Stimulation Adversely Influences Clinical Pregnancy and Live Birth After In Vitro Fertilization. Fertil Steril (2008) 89:1694–701. doi: 10.1016/j.fertnstert.2007.05.055
18. Chuang M, Zapantis A, Taylor M, Jindal SK, Neal-Perry GS, Lieman HJ, et al. Prolonged Gonadotropin Stimulation Is Associated With Decreased ART Success. J Assist Reprod Genet (2010) 27:711–7. doi: 10.1007/s10815-010-9476-6
19. Alport B, Case A, Lim H, Baerwald A. Does the Ovarian Stimulation Phase Length Predict In Vitro Fertilization Outcomes? Int J Fertil Steril (2011) 5(3):134–41.
20. Baker VL, Brown MB, Luke B, Smith GW, Ireland JJ. Gonadotropin Dose is Negatively Correlated With Live Birth Rate: Analysis of More Than 650,000 Assisted Reproductive Technology Cycles. Fertil Steril (2015) 104:1145–52. doi: 10.1016/j.fertnstert.2015.07.1151
21. Pereira N, Friedman C, Hutchinson AP, Lekovich JP, Elias RT, Rosenwaks Z. Increased Odds of Live Birth in Fresh In Vitro Fertilization Cycles With Shorter Ovarian Stimulation. Fertil Steril (2017) 107:104–9. doi: 10.1016/j.fertnstert.2016.09.044
22. Gerber RS, Fazzari M, Kappy M, Cohen A, Galperin S, Lieman H, et al. Differential Impact of Controlled Ovarian Hyperstimulation on Live Birth Rate in Fresh Versus Frozen Embryo Transfer Cycles: A Society for Assisted Reproductive Technology Clinic Outcome System Study. Fertil Steril (2020) 114:1225–31. doi: 10.1016/j.fertnstert.2020.06.021
23. Ryan A, Wang S, Alvero R, Polotsky AJ. Prolonged Gonadotropin Stimulation for Assisted Reproductive Technology Cycles Is Associated With Decreased Pregnancy Rates for All Women Except for Women With Polycystic Ovary Syndrome. J Assist Reprod Genet (2014) 31:837–42. doi: 10.1007/s10815-014-0253-9
24. Bakkensen JB, Christou G, Dimitriadis I, James K, Souter I. The Effect of Follicular Phase Length on Cycle Outcomes and Endometrial Development in Gonadotrophin Ovarian Stimulation/Intrauterine Insemination Cycles. Reprod BioMed Online (2020) 40:362–8. doi: 10.1016/j.rbmo.2019.12.007
25. Purandare N, Emerson G, Kirkham C, Harrity C, Walsh D, Mocanu E. The Duration of Gonadotropin Stimulation Does Not Alter the Clinical Pregnancy Rate in IVF or ICSI Cycles. Ir J Med Sci (2017) 186:653–7. doi: 10.1007/s11845-016-1526-3
27. Tal R, Seifer DB, Tal R, Granger E, Wantman E, Tal O. AMH Highly Correlates With Cumulative Live Birth Rate in Women With Diminished Ovarian Reserve Independent of Age. J Clin Endocrinol Metab (2021) 106:2754–66. doi: 10.1210/clinem/dgab168
28. Seifer DB, Baker VL, Leader B. Age-Specific Serum Anti-Müllerian Hormone Values for 17,120 Women Presenting to Fertility Centers Within the United States. Fertil Steril (2011) 95:747–50. doi: 10.1016/j.fertnstert
29. Devine K, Mumford SL, Wu M, DeCherney AH, Hill MJ, Propst A. Diminished Ovarian Reserve in the United States Assisted Reproductive Technology Population: Diagnostic Trends Among 181,536 Cycles From the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Fertil Steril (2015) 104:612–9. doi: 10.1016/j.fertnstert.2015.05.017
30. Wu S, Wang M, Deng Y, Qiu J, Zhang X, Tan J. Associations of Toxic and Essential Trace Elements in Serum, Follicular Fluid, and Seminal Plasma With In Vitro Fertilization Outcomes. Ecotoxicol Environ Saf (2020) 204:110965. doi: 10.1016/j.ecoenv.2020.110965
31. Wu S, Zhang Y, Wu X, Hao G, Ren H, Qiu J, et al. Association Between Exposure to Ambient Air Pollutants and the Outcomes of In Vitro Fertilization Treatment: A Multicenter Retrospective Study. Environ Int (2021) 153:106544. doi: 10.1016/j.envint.2021.106544
32. Correia KFB, Dodge LE, Farland L, Hacker MR, Ginsburg E, Whitcomb BW, et al. Confounding and Effect Measure Modification in Reproductive Medicine Research. Hum Reprod (2022) 35:1013-8. doi: 10.1093/humrep/deaa051
33. Broughton DE, Moley KH. Obesity and Female Infertility: Potential Mediators of Obesity’s Impact. Fertil Steril (2017) 107:840–7. doi: 10.1016/j.fertnstert.2017.01.017
34. Grøndahl ML, Christiansen SL, Kesmodel US, Agerholm IE, Lemmen JG, Lundstrøm P, et al. Effect of women’s age on embryo morphology, cleavage rate and competence - A multicenter cohort study. PLoS One (2017) 12. doi: 10.1371/journal.pone.0172456
35. Seshadri S, Morris G, Serhal P, Saab W. Assisted Conception in Women of Advanced Maternal Age. Best Pract Res Clin Obstet Gynaecol (2021) 70:10–20. doi: 10.1016/j.bpobgyn.2020.06.012
36. Ahmed TA, Ahmed SM, El-Gammal Z, Shouman S, Ahmed A, Mansour R, et al. Oocyte Aging: The Role of Cellular and Environmental Factors and Impact on Female Fertility. Adv Exp Med Biol (2020) 1247:109–23. doi: 10.1007/5584_2019_456
37. Dewailly D, Laven J. AMH as the Primary Marker for Fertility. Eur J Endocrinol (2019) 181:D45–51. doi: 10.1530/EJE-19-0373
38. Roberts R, Iatropoulou A, Ciantar D, Stark J, Becker DL, Franks S, et al. Follicle-Stimulating Hormone Affects Metaphase I Chromosome Alignment and Increases Aneuploidy in Mouse Oocytes Matured In Vitro. Biol Reprod (2005) 72:107–18. doi: 10.1095/biolreprod
39. Kaleli S, Yanikkaya-Demirel G, Erel CT, Senturk LM, Topc¸uoglu A, Irez T. High Rate of Aneuploidy in Luteinized Granulosa Cells Obtained From Follicular Fluid in Women Who Underwent Controlled Ovarian Hyperstimulation. Fertil Steril (2005) 84:802–4. doi: 10.1016/j.fertnstert.2005
40. Polyzos NP, Drakopoulos P, Parra J, Pellicer A, Santos-Ribeiro S, Tournaye H, et al. Cumulative Live Birth Rates According to the Number of Oocytes Retrieved After the First Ovarian Stimulation for In Vitro Fertilization/Intracytoplasmic Sperm Injection: A Multicenter Multinational Analysis Including ∼15,000 Women. Fertil Steril (2018) 110:661–70.e1. doi: 10.1016/j.fertnstert.2018.04.039
41. Niinimäki M, Veleva Z, Martikainen H. Embryo Quality is the Main Factor Affecting Cumulative Live Birth Rate After Elective Single Embryo Transfer in Fresh Stimulation Cycles. Eur J Obstet Gynecol Reprod Biol (2015) 194:131–5. doi: 10.1016/j.ejogrb.2015.08.031
42. Blockeel C, Campbell A, Coticchio G, Esler J, Garcia-Velasco JA, Santulli P, et al. Should We Still Perform Fresh Embryo Transfers in ART? Hum Reprod (2019) 34:2319–31. doi: 10.1093/humrep/dez233
43. Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H, et al. Conventional Ovarian Stimulation and Single Embryo Transfer for IVF/ICSI. How Many Oocytes do We Need to Maximize Cumulative Live Birth Rates After Utilization of All Fresh and Frozen Embryos? Hum Reprod (2016) 31:370–6. doi: 10.1093/humrep/dev316
44. Ji J, Liu Y, Tong XH, Luo L, Ma J, Chen Z. The Optimum Number of Oocytes in IVF Treatment: An Analysis of 2455 Cycles in China. Hum Reprod (2013) 28:2728–34. doi: 10.1093/humrep/det303
45. Vaughan DA, Leung A, Resetkova N, Ruthazer R, Penzias AS, Sakkas D, et al. How Many Oocytes are Optimal to Achieve Multiple Live Births With One Stimulation Cycle? The One-and-Done Approach. Fertil Steril (2017) 107:397–404.e3. doi: 10.1016/j.fertnstert.2016.10.037
46. Magnusson Å, Källen K, Thurin-Kjellberg A, Bergh C. The Number of Oocytes Retrieved During IVF: A Balance Between Efficacy and Safety. Hum Reprod (2018) 33:58–64. doi: 10.1093/humrep/dex334
Keywords: infertility, fertilization, gonadotropins, ovulation stimulation, menstrual cycle
Citation: Zhao X, Zhang X, Wu S and Tan J (2022) Association Between the Ratio of Ovarian Stimulation Duration to Original Follicular Phase Length and In Vitro Fertilization Outcomes: A Novel Index to Optimise Clinical Trigger Time. Front. Endocrinol. 13:862500. doi: 10.3389/fendo.2022.862500
Received: 26 January 2022; Accepted: 27 May 2022;
Published: 25 July 2022.
Edited by:
Tom Kelsey, University of St. Andrews, United KingdomReviewed by:
Shan Xiao, Shenzhen Zhongshan Urological Hospital, ChinaJohannes Ott, Medical University of Vienna, Austria
Copyright © 2022 Zhao, Zhang, Wu and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jichun Tan, dGpjempoQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Xinyang Zhao1,2†
Xinyang Zhao1,2† Shanshan Wu
Shanshan Wu Jichun Tan
Jichun Tan