
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 29 April 2022
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.861746
This article is part of the Research TopicSubclinical Thyroid Disease: Present Knowledge and Future DirectionView all 10 articles
Background: Despite patients with thyroid dysfunction show obvious abnormal hemostatic indicators in the peripheral blood, the current research on whether and how subclinical hypothyroidism (SCH) influence hemostatic function (the coagulation and fibrinolytic system) still remains controversial.
Objective: We conducted this study to evaluate how SCH influence on the coagulation and fibrinolytic system in human body.
Methods: Prior to March 2022, Web of Science, Embase, PubMed, WanFang, CNKI data and reference lists were searched to identify eligible researches. Two of us independently extracted the data and evaluated study quality. The effect size is represented by standard mean difference (SMD). Both fixed and random-effects models were used where appropriate. Review Manager 5.3 and STATA 16.0 were used to analyze the eligible data.
Results: 1325 patients from twelve observational studies were involved in our research. Our study revealed that SCH changed the heamostatic balance towards hypercoagulable and hypofibrinolytic conditions accompanied by an increase in tissue fibrinogen, plasminogen activator and plasminogen activator inhibitor-1. By contrast, there was no statistically difference in acivated partial thromboplastin time (APTT) and D-Dimer in SCH group compared with that in control subjects.
Conclusions: Our study confirmed that SCH is related with a prothrombotic state, as reflected by changes in both coagulation and fibrinolysis. It is highly recommended for screening cardiovascular risk factors in combination with an adequate evaluation of SCH state.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/#recordDetails] PROSPERO [CRD42021275313]
Subclinical hypothyroidism (SCH) is a condition associated with an elevated thyroid stimulating hormone (TSH) level with normal levels of free thyroid hormone (1). Since advances have been made recently on assays for TSH measurement with better sensitivity and specificity, SCH is becoming more prevalent, resulting in increased attention (2). Despite the fact that most patients with SCH don’t present with classical symptoms and signs of hypothyroidism due to the abnormal thyroid hormone level, the increase in atherosclerosis and cardiovascular disease in SCH patients is similar to that in patients with clinical hypothyroidism (3, 4).
Thromboembolism and cardiovascular disease are linked to various abnormalities of the haemostatic indicators related to coagulation and the fibrinolytic system. However, the published data about the heamostatic abnormalities among SCH patients remain controversial. Accumulating evidence has shown that levels of factor VII (FVII):C, the ratio FVII:C/FVII : Ag, fibrinogen (5) and plasminogen activator inhibitor-1 (PAI-1) (6, 7) are elevated, while von-Willebrand factor (vWF), antithrombin III (AT III) concentration and factor VIII (FVIII) activities (8) are decreased in patients diagnosed with SCH. However, conflicting outcomes exist in other researches (9, 10).
In this study, we aimed to analyze systematically the impact of SCH on the coagulation-fibrinolytic system in the human body, develop well-founded hypotheses, and provide recommendations for future research.
The meta-analyses of observational researches face special challenges due to inherent biases and design differences from different studies. Hence, we conducted and detailed the analysis in accordance with the guidelines of the Meta-analysis of Observational Studies in Epidemiology Group (11).
A publication search was performed for studies in the Web of Science, PubMed, Embase, CNKI and WanFang data up to March 2022 by two independent investigators. Search strategies consisted of the following: the Medical Subject Headings terms “Hypothyroidism” or “Thyroid Disease” or “Thyrotropin”; and the text word terms “subclinical hypothyroidism” or “subclinical thyroid dysfunction” or “thyroid-stimulating hormone”; and the text word terms “haemostasis” or “blood coagulation/clotting” or “blood coagulation/clotting tests” or “blood coagulation/clotting factors” or “blood coagulation/clotting disorders”. In order to avoid omitting any relevant research, we also scanned the references of the retrieved articles for more studies. Language was not restricted in the document retrieval. Unpublished researches were not included within this study. The titles and abstracts of all retrieved articles were scanned. After that, we read the full text of studies which were possibly related for further appropriateness assessments to accomplish the article. Within the meta-analysis, all researches were firstly published in the primary literature with no reproduction in other articles. The whole researches that met the inclusion criteria were retrieved for additional assessment and information extraction.
Principle consideration basis was that the research needed to assess the impact of SCH on the coagulation-fibrinolytic system in human. Take it one step further, a review need to meet the accompanying terms: 1) reported SCH whose TSH was high while free thyroxin within normal range; 2) reported the coagulation-fibrinolytic framework information (including tissue plasminogen activator (t-PA), plasminogen activator inhibitor type 1(PAI-1), fibrinogen, activated partial thromboplastin time (APTT) and D-Dimer) for SCH patients and that was contrasted with data of the control whose thyroid function was normal; and 3) the 95% CIs were given or we could compute the 95% CI with given data.
The following sorts of studies were excluded: 1) Patients who were taking drugs or received treatment which could influence TSH and free thyroxin levels; 2) Participants suffered from clinical hypothyroidism or hyperthyroidism; 3) Case series, Case reports, editorials, reviews, in vitro, and non-human researches; 4) Because tumor may influence TSH and free thyroxin levels, researches on tumor patients were likewise removed; 5) the same study published before; 6) researches without sufficient to figure out the statistic or value.
Headlines and summary of these original studies were scanned to see whether the inclusion criteria were met by two researchers separately. When we could not remove a study just from headlines and summary, we needed to review this article thoroughly. Choices with respect to incorporation were made independently, outcomes were contrasted, and discussion was made for dispute resolution if there was any difference. If different publications came from the same research, we chose the recent article. When there was a need, information from other prior articles was used to replenish it. These data below was collected from every article: features of the research (writer, publication year, region, research design, exclusion criteria, TSH assay), particulars of member features (number of patients enrolled, mean age, sex and TSH level), coagulation and fibrinolysis indexes of the SCH groups and the control [fibrinogen, tissue plasminogen Activator (t-PA), PAI-1, D-Dimer and activated partial thromboplastin time (APTT)].
The Newcastle-Ottawa Quality Assessment Scale (NOS) for evaluating quality of observational researches was utilized as a direct to evaluate research quality of cross-sectional and intervention researches (12, 13). Three categories were hence recognized: high quality (low risk of bias), medium quality (moderate risk of bias), or low quality (high risk of bias). Quality of the included researches was evaluated by two independent analysts and any contrasts were settled by agreement or the supposition of the third analyst, when necessary.
We drew the effect sizes that compared the experimental and non-exposure situations from each study. And then, for all eligible studies, the weighted mean difference (WMD) or Standard mean difference (SMD) and 95% CIs in coagulation and fibrinolysis indexes were calculated. Both fixed- and random-effects models were used where appropriate (14) in the meta-analysis. We examined the heterogeneity across studies through Q test and I2 statistics. If P< 0.1 or I2≥50%, when heterogeneity was thought to be obvious across these study outcomes, we chose the random-effect modeling the combinational analysis. If not, the fixed-effect model was used. We assessed the stability and reliability of our study through sensitivity analysis. The possible publication bias was evaluated through Egger’s and Begg’s test (15, 16). We performed the analysis by Review Manager 5.3 software (Cochrane Collaboration, http://www.cochrane.org) and STATA 16.0 software (Stata: Software for Statistics and Data Science | Stata https://www.stata.com/).
Figure 1 shows a flow diagram from which we can understand the search tactics and study selection process. The initial search tactics identified 1238 articles. Two records were involved when search of reference lists and review articles were performed further. In view of the headlines and abstract, we involved 70 potentially relevant publications in all. Of these, 58 failed to match the inclusion criteria (7 case reports,16 review articles,7 duplicate data,19 articles with insufficient data,3 studies not define TSH cut-off and 6 studies on cancer patients) and a total of 12 studies (5, 6, 8–10, 17–23) with 1325 patients were involved in the final analysis (Figure 1).
Table 1 presents the features of the individuals in the SCH and control groups and the main characteristics of these studies, including region, study design, quality, exclusion criteria, outcome parameters, TSH assay and matched or adjusted factors. Among the final 12 articles, 4 were (19, 20, 22, 23) were reported in Chinese, 8 were in English (5, 6, 8–10, 17, 18, 21). Of these, ten were case–control studies and two prospective cohort studies. Of these involved researches, four medium quality studies were confirmed. The rest of researches were confirmed to be of inferior quality. Table 1 summed up the features of these involved researches. Moreover, Table 2 summed up information of age, sex, the sample size and numerical value of TSH, and Table 3 displayed the coagulation and fibrinolytic parameters.
Here, we included 8 studies (5, 6, 8, 9, 18, 19, 21, 23) (388 SCHs) for the impact of SCH on tPA. Taking the heterogeneity (heterozygosity test, Chi2 = 33.24, P <0.0001, I2 = 79%) into account, 1 study was removed at a time to identify the heterogeneous source. When the research by Y. H. Chen et al. (2009) (19) was detached, the heterogeneity decreased significantly (the I2 reduced from 79% to 44%, P increased from <0.0001 to 0.10). After a careful reading, age difference may be one of the sources of heterogeneity, but may not the only one. Therefore, SMD values was merged by means of the fixed-effect model and the pooled SMD was 0.20 (95%CI, 0.05 to 0.35; P = 0.01; Figure 2), which means that SCH patients showed higher level of tPA compared with control subjects.
A total of 8 studies were included (5, 6, 8, 9, 18, 19, 21, 23) (388 SCHs) for the effect of SCH on PAI-1. There was significant statistical heterogeneity in these studies (P<0.00001, I2 = 88%). Therefore, a random-effect model was used to pool SMD. A significant increase was represented when estimated together in PAI-1 among subjects in SCH group compared with the control (SMD,0.61, 95% CI 0.17 to 1.06; P =0.007, Figure 3).
The effect of SCH on fibrinogen was favorable in 6 studies (5, 8, 9, 20, 22, 23). Overall, the alter within the SMD for fibrinogen was 0.35 (95% CI, 0.20 to 0.50, P<0.00001; Figure 4). Heterogeneity analysis shows a moderate heterogeneity (heterozygosity test, Chi2 = 8.65, P=0.12, I2 = 42%). It indicated that there was significantly higher in fibrinogen in SCH group, compared with euthyroid subjects. Further analysis based on whether TSH higher than 10uIU/mL or not (20, 23) showed higher fibrinogen levels in patients with TSH >10uIU/mL compared to those with TSH ≤ 10uIU/mL (WMD,0.43; 95%CI, 0.24 to 0.62; P<0.0001,I2 = 0%). Due to the limited numbers of article, no heterogeneity was found (heterozygosity test, Chi2 = 0.65, P=0.42, I2 = 0%).
Six studies (5, 8–10, 20, 23) compared APTT levels between SCH patients with controls. Due to the large heterogeneity was found (heterozygosity test, Chi2 = 41.05, P<0.0001, I2 = 88%), random-effect model was used to pooled the data. In our analysis, it was of no statistically difference in APTT (WMD,0.65;95% CI, −1.22 to 2.51; P<0.00001; Figure 5). Further analysis based on difference TSH level (20, 23) did not found the effect of TSH level difference on APTT level (WMD, 2.25; 95%CI, -6.86 to 11.36; P=0.63). Also, a large heterogeneity was found (heterozygosity test, Chi2 = 28.44, P<0.0001, I2 = 96%), D-Dimer. Here, the association between D-Dimer and SCH was analyzed in 6 independent studies (8, 9, 17, 19, 21, 23) (268 SCHs). No statistically difference was found in D-Dimer between SCH group and normal thyroid function group (SMD, 0.28;95% CI, −0.28 to 0.83; P = 0.33) from analysis and the heterogeneity among trials was obvious (heterozygosity test, Chi2 = 41.14, P<0.00001, I2 = 88%; Figure 6).
We made further efforts to conduct a subgroup and sensitivity analysis on account of study heterogeneity. Subgroup analyses were conducted by ethnicity, age, gender, TSH cut-off value and study design. However, the results of PAI-1 in SCH patients were not affected. A sensitivity analysis was performed to assess the influence of each research on the final results. There was no any single study that had an impact on the total pooled effect, sensitivity analysis, which indicated that none of the studies interfered with OR or 95% CI (Figure 7).
Publication bias was examined using funnel plot. There was no notable publication bias among articles included in our meta-analysis (Figure 8). Furthermore, no significant bias were found both by Egger’s and Begg’s test (both P>0.1).
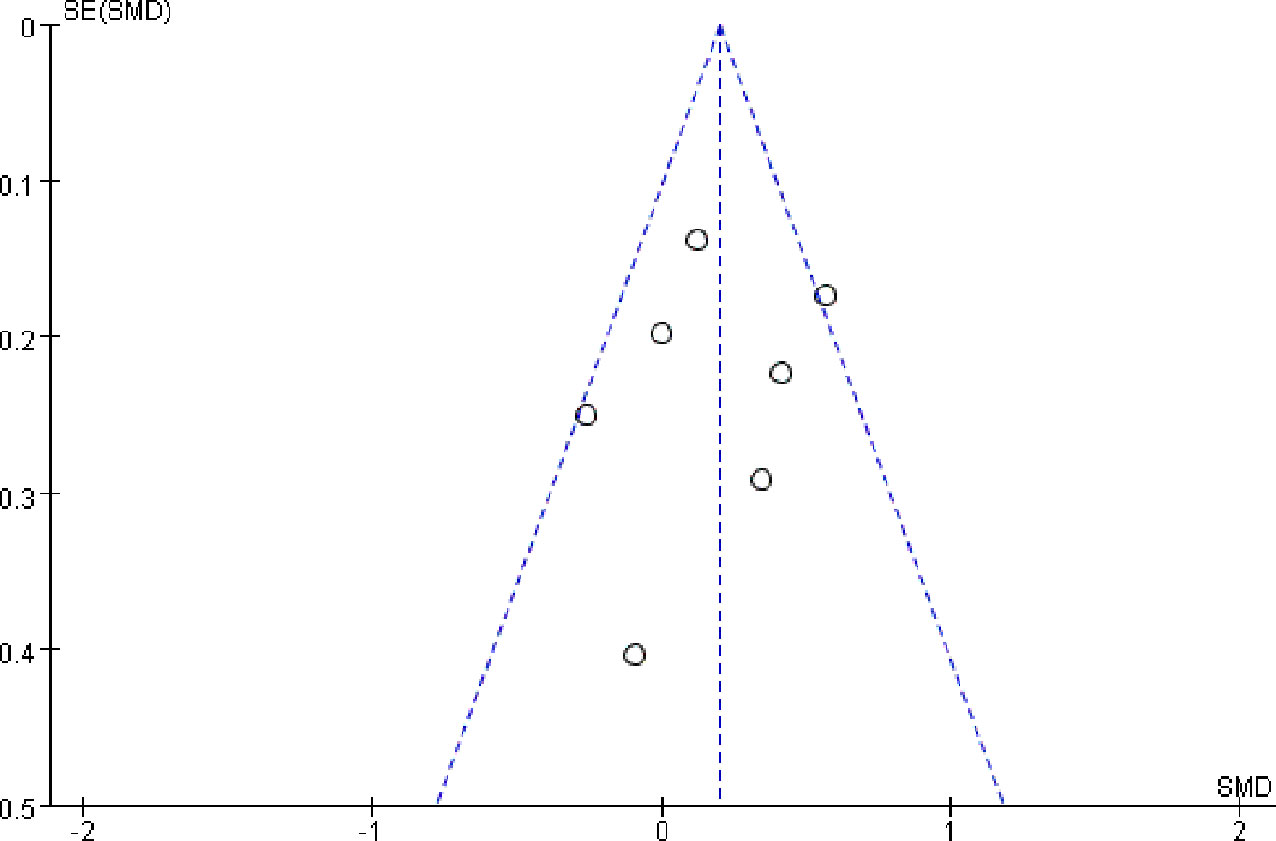
Figure 8 Funnel plot of publication bias-qualitative evaluation of publication bias, performed by Review Manager 5.3.
Subclinical hypothyroidism (SCH) is defined when serum TSH is above the reference range but circulating thyroid hormones are still normal. In Iniodine-sufficient populations, SCH affects up to 16% of the population (24). Notably, researches have indicated that the risk of atherosclerosis and myocardial infarction increased independently in patients with SCH (4). However, it is still controversial that whether SCH influences coagulation and fibrinolysis in the human body. To reply this question, we did this systematic review and discovered that people suffered from SCH had hemostasis and fibrinolysis changes, consistently reflecting a prothrombotic condition.
Our study revealed that patients diagnosed with SCH displayed a prothrombotic tendency. It was showed that both t-PA and PAI-1 were above the normal range in these people, compared with the control, which indicated a hypercoagulable condition with a decline in fibrinolysis because of the pattern of alterations (21, 25). Especially, the equilibriums of t-PA and PAI-1 determine the total fibrinolytic potential of human blood, which has been extensively regarded as a predictive factor of venous as well as arterial thrombosis (26). In addition, increased t-PA levels could give expression to a compensatory reaction to a hypofibrinolysis as a result of an increased inhibitory effect of PAI-1 (26). Moreover, elevated PAI-1 concentrations may increase the tendency to myocardial infarction (MI), which suggests clinical significance of high PAI-1 levels (21, 27).
We analyzed the changes in various other hemostatic parameters and evaluate fibrinolytic balance in the current study. Especially, the increment of fibrinogen in SCH patients comparing to normal populations, which reflected both the human inflammatory state and the tendency of thrombosis and haemorrhage, contributes to atherosclerosis as well as thrombotic complications (28, 29). Data from clinical and epidemiological studies suggested that higher serum fibrinogen level could predict the risk of both primary cardiovascular events and secondary events (29–32).
Existing literatures that show an impaired fibrinolysis and a hypercoagulable in SCH patients agree to our observations (8, 17, 21, 33). This condition may be the precipitating factors of cardiovascular disease, as a mechanism how mild thyroid failure is associated with cardiovascular disease. Just as Chadarevian et al. observed (7), there was an overall decrease of fibrinolytic activity, manifested as lower D-Dimer levels, increased α2-antiplasmin reaction and increased numerical value of tPA and PAI-1, in SCH groups whose TSH level lies in 10 and 50 mIU/l. Meanwhile, Muller et al. (5) reported that subjects with SCH had a significant elevation in factor VII reaction. In a group of individuals with SCH, Canturk et al. (8) suggested elevated fibrinogen, factor VII and PAI-1 levels along with decreased AT III concentrations. It came to light that the condition resulted in more severe atherosclerosis. Furthermore, Lupoli et al. (21) have seen recovery improvements in these parameters after LT4 replacement therapy, such as a significant reduction in PAI-1 and tPA. It was concluded that SCH was a state of hypercoagulable and hypofibrinolytic and this can be reverted by L-T4 treatment. Above all, we propose the following recommendations: for patients with SCH, active LT4 treatment is recommended to reduce the incidence of thrombotic events, especially for patients with a personal history of coronary heart disease, cerebrovascular disease or early family history.
As with any study, there are some limitations in our research. Firstly, by the inclusion of no randomized clinical studies, trials with only data from observational researches, and studies mainly discussed the connection between SCH and the coagulation or fibrinolysis, it is necessary to cautiously interpret the results of our analysis (34). Secondly, the inconsistency of the standard TSH cut-off value and the definition of SCH in the final included studies may add the clinical heterogeneity in our study. Finally, factors including study samples, participants’ characteristics, the method used to test the coagulation and fibrinolysis indexes, and various confounding factors included for the adjustment, may all also added the clinical heterogeneity to our analysis.
In summary, we suggest that SCH is related to a prothrombotic state in our study, which may be caused by alterations in coagulation and fibrinolysis. Therefore, it is important to screen SCH dysfunction for detecting the earliest signs of cardiovascular disease. However, larger and high-quality studies are necessary to evaluate our observations. If future studies clearly revealed the casual association of prothrombotic state with SCH leading to an elevated risk of cardiovascular disease, effective treatments, such as replacement therapy with Levothyroxine (LT4), to revert the abnormalities should be recommended routinely.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
All the authors contributed to the work. WZ and QF defined the research theme. QX and YW designed the methods, analyzed the data, interpreted the results and wrote the manuscript. XS and YZ prepared tables and figures. All authors have read and agreed to the published version of the manuscript.
This study was supported by the Natural Science Foundation under Grant No. ZR2009CQ023 and Medical Science Development Plan of Shandong Province under Grant No. 2009QZ025.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors express sincere thanks to Dr. Junyu Zhao and Dr. Yuying Cui, Shandong Provincial Qianfoshan Hospital, for the technical support.
1. Peeters RP. Subclinical Hypothyroidism. N Engl J Med (2017) 376(26):2556–65. doi: 10.1056/NEJMcp1611144
2. Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH. Carotid Intima-Media Thickness in Patients With Subclinical Hypothyroidism: A Meta-Analysis. Atherosclerosis (2013) 227(1):18–25. doi: 10.1016/j.atherosclerosis.2012.10.070
3. Ahirwar AK, Singh A, Jain A, Kaim K, Bhardwaj S, Patra SK, et al. Association of Prothrombotic Adipokine (Plasminogen Activator Inhibitor-1) With TSH in Metabolic Syndrome: A Case Control Study. Horm Mol Biol Clin Investig (2017) 34(1):1–11. doi: 10.1515/hmbci-2017-0046
4. Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical Hypothyroidism is an Independent Risk Factor for Atherosclerosis and Myocardial Infarction in Elderly Women: The Rotterdam Study. Ann Intern Med (2000) 132(4):270–8. doi: 10.7326/0003-4819-132-4-200002150-00004
5. Müller B, Tsakiris DA, Roth CB, Guglielmetti M, Staub JJ, Marbet GA. Haemostatic Profile in Hypothyroidism as Potential Risk Factor for Vascular or Thrombotic Disease. Eur J Clin Invest (2001) 31(2):131–7. doi: 10.1046/j.1365-2362.2001.00777.x
6. Ozcan MA, Cömlekçi A, Demirkan F, Yüksel F, Sari I, Demir T, et al. Plasma Levels of Free Tissue Factor Pathway Inhibitor in Patients With Various Thyroid Disorders. Thromb Res (2003) 110(4):243–7. doi: 10.1016/s0049-3848(03)00408-0
7. Chadarevian R, Bruckert E, Leenhardt L, Giral P, Ankri A, Turpin G. Components of the Fibrinolytic System are Differently Altered in Moderate and Severe Hypothyroidism. J Clin Endocrinol Metab (2001) 86(2):732–7. doi: 10.1210/jcem.86.2.7221
8. Cantürk Z, Cetinarslan B, Tarkun I, Cantürk NZ, Ozden M, Duman C. Hemostatic System as a Risk Factor for Cardiovascular Disease in Women With Subclinical Hypothyroidism. Thyroid (2003) 13(10):971–7. doi: 10.1089/105072503322511382
9. Erem C. Blood Coagulation, Fibrinolytic Activity and Lipid Profile in Subclinical Thyroid Disease: Subclinical Hyperthyroidism Increases Plasma Factor X Activity. Clin Endocrinol (Oxf) (2006) 64(3):323–9. doi: 10.1111/j.1365-2265.2006.02464.x
10. Gullu S, Sav H, Kamel N. Effects of Levothyroxine Treatment on Biochemical and Hemostasis Parameters in Patients With Hypothyroidism. Eur J Endocrinol (2005) 152(3):355–61. doi: 10.1530/eje.1.01857
11. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis Observational Stud Epidemiol (MOOSE) Group JAMA (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
12. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses (2009). Ottawa (ON: Ottawa Hospital Research Institute. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 2012 Jan).
13. Stuijver DJ, van Zaane B, Romualdi E, Brandjes DP, Gerdes VE, Squizzato A. The Effect of Hyperthyroidism on Procoagulant, Anticoagulant and Fibrinolytic Factors: A Systematic Review and Meta-Analysis. Thromb Haemost (2012) 108(6):1077–88. doi: 10.1160/TH12-07-0496
14. DerSimonian R, Laird N. Meta-Analysis in Clinical Trials. Control Clin Trials (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
15. Begg CB, Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics (1994) 50(4):1088–101. doi: 10.2307/2533446
16. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
17. Guldiken S, Demir M, Turgut B, Altun BU, Arikan E, Kara M. Global Fibrinolytic Capacity in Patients With Subclinical Hypothyroidism. Endocr J (2005) 52(3):363–7. doi: 10.1507/endocrj.52.363
18. Jorde R, Figenschau Y, Hansen JB. Haemostatic Function in Subjects With Mild Subclinical Hypothyroidism. Tromsø Study Thromb Haemost (2006) 95(4):750–1. doi: 10.1160/TH05-10-0655
19. Chen YH. Changes and Significance of Serum Lipid and Prethrombotic State Markers in Elderly Women With Subclinical Hypothyroidism. Modern Pract Med (2009) 21(12):1300–8. doi: CNKI:SUN:NBYX.0.2009-12-015
20. Zhong SC, An JH, Chen JC, Wang AE, Dong FZ, Wang L, et al. Value of Platelet Parameters and 4 Coagulation Parameters Subclinical Hypothyroidism Patients. Lab Med Clin (2014) 11(19):2690–5. doi: 10.3969/j.issn.1672-9455.2014.19.019
21. Lupoli R, Di Minno MN, Tortora A, Scaravilli A, Cacciapuoti M, Barba L, et al. Primary and Secondary Hemostasis in Patients With Subclinical Hypothyroidism: Effect of Levothyroxine Treatment. J Clin Endocrinol Metab (2015) 100(7):2659–65. doi: 10.1210/jc.2015-1726
22. Ren YX, Zhang JQ, Fan CY. Clinical Characteristics and Prognosis of Coronary Heart Disease Patients With Subclinical Hypothyroidism. Chin J Integr Med Cardio-/Cerebrovascular Dis (2018) 16(10):1381–4. doi: 10.12102/j.issn.1672-1349.2018.10.019
23. Gao F, Zhang YN, Zhao NR, Li CL, Yang XX. Clinical Characteristics and Prognosis of Coronary Heart Disease Patients With Subclinical Hypothyroidism. Lab Med (2018) 33(2):110–4. doi: 10.3969/j.issn.1673-8640.2018.02.004
24. Leng O, Razvi S. Treatment of Subclinical Hypothyroidism: Assessing When Treatment is Likely to be Beneficial. Expert Rev Endocrinol Metab (2021) 16(2):73–86. doi: 10.1080/17446651.2020.1738924
25. De Meyer SF, Deckmyn H, Vanhoorelbeke K. Von Willebrand Factor to the Rescue. Blood (2009) 113(21):5049–57. doi: 10.1182/blood-2008-10-165621
26. Di Minno MN, Palmieri V, Lombardi G, Pezzullo S, Cirillo F, Di Somma C, et al. Lack of Change in Insulin Levels as a Biological Marker of PAI-1 Lowering in GH-Deficient Adults on R-HGH Replacement Therapy. Thromb Res (2009) 124(6):711–3. doi: 10.1016/j.thromres.2009.06.018
27. Thögersen AM, Jansson JH, Boman K, Nilsson TK, Weinehall L, Huhtasaari F, et al. High Plasminogen Activator Inhibitor and Tissue Plasminogen Activator Levels in Plasma Precede a First Acute Myocardial Infarction in Both Men and Women: Evidence for the Fibrinolytic System as an Independent Primary Risk Factor. Circulation (1998) 98(21):2241–7. doi: 10.1161/01.cir.98.21.2241
28. Drouet L, Bal Dit Sollier C. Fibrinogène Et Risque D'accident Cardio-Vasculaire [Fibrinogen: Factor and Marker of Cardiovascular Risk]. J Mal Vasc (2002) 27(3):143–56. doi: 10.1161/01.HYP.0000018956.63639.7F
29. Drouet L, Bal dit Sollier C. Le Fibrinogène a-T-Il Une Place Pour Évaluer Le Risque D'accident Cardiovasculaire Ischémique? Is Fibrinogen a Predictor or a Marker of the Risk of Cardiovascular Events?. Therapie (2005) 60(2):125–36. doi: 10.2515/therapie:2005017
30. Tracy RP, Arnold AM, Ettinger W, Fried L, Meilahn E, Savage P. The Relationship of Fibrinogen and Factors VII and VIII to Incident Cardiovascular Disease and Death in the Elderly: Results From the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol (1999) 19(7):1776–83. doi: 10.1161/01.atv.19.7.1776
31. Sato S, Nakamura M, Iida M, Naito Y, Kitamura A, Okamura T, et al. Plasma Fibrinogen and Coronary Heart Disease in Urban Japanese. Am J Epidemiol (2000) 152(5):420–3. doi: 10.1093/aje/152.5.420
32. Smith FB, Lee AJ, Hau CM, Rumley A, Lowe GD, Fowkes FG. Plasma Fibrinogen, Haemostatic Factors and Prediction of Peripheral Arterial Disease in the Edinburgh Artery Study. Blood Coagul Fibrinolysis (2000) 11(1):43–50. doi: 10.1097/00001721-200011010-00005
33. Akinci B, Comlekci A, Ali Ozcan M, Demir T, Yener S, Demirkan F, et al. Elevated Thrombin Activatable Fibrinolysis Inhibitor (TAFI) Antigen Levels in Overt and Subclinical Hypothyroid Patients Were Reduced by Levothyroxine Replacement. Endocr J (2007) 54(1):45–52. doi: 10.1507/endocrj.k06-062
Keywords: coagulation, fibrinolysis, subclinical hypothyroidism, cardiovascular disease, meta-analysis
Citation: Xu Q, Wang Y, Shen X, Zhang Y, Fan Q and Zhang W (2022) The Effect of Subclinical Hypothyroidism on Coagulation and fibrinolysis: A Systematic Review and Meta-Analysis. Front. Endocrinol. 13:861746. doi: 10.3389/fendo.2022.861746
Received: 25 January 2022; Accepted: 24 March 2022;
Published: 29 April 2022.
Edited by:
Daniel Elías-López, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), MexicoReviewed by:
Anne H. Van Der Spek, Cornell University, United StatesCopyright © 2022 Xu, Wang, Shen, Zhang, Fan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhang, emh3MDhhb3l1bkAxNjMuY29t; Qingyun Fan, ZmFucWluZ3l1bnh0enl5QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.