- 1Department of Health Sciences, University of Florence, Anna Meyer Children’s University Hospital, Florence, Italy
- 2Department of Human Pathology of Adulthood and Childhood, University of Messina, Messina, Italy
Congenital hypothyroidism (CH) is a relatively frequent congenital endocrine disorder, caused by defective production of thyroid hormones (THs) at birth. Because THs are essential for the development of normal neuronal networks, CH is also a common preventable cause of irreversible intellectual disability (ID) in children. Prolonged hypothyroidism, particularly during the THs-dependent processes of brain development in the first years of life, due to delays in diagnosis, inadequate timing and dosing of levothyroxine (l-thyroxine or l-T4), the non-compliance of families, incorrect follow-up and the interference of foods, drugs and medications affecting the absorption of l-T4, may be responsible for more severe ID. In this review we evaluate the main factors influencing levels of THs and the absorption of l-T4 in order to provide a practical guide, based on the existing literature, to allow optimal follow-up for these patients.
1 Introduction
Congenital hypothyroidism (CH) is a relatively frequent congenital endocrine disorder, due to the defective production of thyroid hormones (THs) at birth (1, 2). CH most commonly occurs because of disruption of thyroid gland development or a TH biosynthesis disorder (primary hypothyroidism); it can also be related to a defect in the release of thyroid-stimulating hormone (TSH), a condition which rarely occurs in isolation and is commonly associated with the deficiency of other pituitary hormones (combined pituitary hormone deficiency or CPHD) (secondary or central congenital hypothyroidism or cCH) (1, 2).
CH is also one of the most common preventable causes of irreversible intellectual disability (ID) in children (3) but early diagnosis and treatment can prevent serious consequences (4). THs are involved in the development of normal neuronal networks, and if CH is not treated it can lead to severe, permanent alterations in brain anatomy and function (5). It is important to consider that many complications, such as brain disorders and injuries and nerve growth retardation, may not be clinically recognizable in the first weeks of life leading to delay in the introduction of treatments that can prevent disabling and severe outcomes (4, 6, 7).
Moreover, during pregnancy, absent or inadequate maternal levothyroxine (l-thyroxine or l-T4) treatment correlates to an increased risk of obstetric complications at birth, as well as neuropsychological problems in the unborn child (8), adding to damage associated with neonatal CH.
Screening programs for CH are in place in many countries, allowing for the prompt initiation of treatment during the first months of life, frequently before clinical signs and symptoms appear (1). Generally, the recommended period for collecting blood samples for thyroid screening is between the 3rd and 5th day of life (9). The detection of a suspected thyroid dysfunction should be confirmed with secondary tests, which should be carried out promptly, ideally within the second week of life. However, despite the clear benefits of screening programmes, 70% of infants worldwide are born in areas without routine neonatal screening (10).
In CH, the incidence rate is reported to be about one in 2,000 - 4,000 live births, with a female-to-male ratio of nearly 2:1, although there are significant differences between countries (1–3): for example, in the United States, the incidence is 1 in 3500 to 5000, in Europe 1 in 3000 and in Japan 1 in 5700 (1–3). These differences are due to a number of factors including ethnicity, environmental influences, conditions affecting birth and pregnancy, and the presence or absence of screening programs (1–3). Moreover, sexual dimorphism can affect prenatal thyroid migration (11). The number of low birth weight (LBW) infants, who have a higher risk of CH, has increased in recent years due to improvements in neonatal intensive care (12).
Approximately two thirds of CH cases are secondary to thyroid dysgenesis which can involve both Mendelian and non-Mendelian inheritance: in fact, although CH is typically sporadic, familial cases have been described (1–3). In patients with a genetic form of CH, germline mutations in genes involved in thyroid gland organogenesis (i.e. PAX8, TTF-1/NKX2.1, TTF-2/FOXE1 and TSHR) and responsible for thyroid dysgenesis are rarely found (1–3). Some cases of CH may result from inborn errors in the synthesis of THs (1–3). The clinical expression of mutations in some genes involved in disorders of thyroid function, such as DUOX or DUOX2/DUOXA2, may vary widely between individuals and over time; some patients who present thyroid function impairment in the first weeks of life do not require treatment (13). Initial l-T4 doses and follow-up should be adapted to the needs of each CH patient taking into consideration the formulation used and THs levels.
Practices governing CH diagnosis and treatment have undergone significant changes in recent years: treatment with higher starting doses of l-T4 is now the treatment of choice which has led to improvements in both auxological (14–16) and neurological prognoses (17, 18).
In all cases, there is a significant increase in TSH and/or a significant reduction in FT4. Treatment should be promptly initiated with careful follow-up to determine appropriate dosage (13). Many guidelines (13, 19–21) for treating CH suggest a higher initial l-T4 dose (generally at least 10-15 μg/kg/day) than the 5-10 μg/kg/day previously recommended, in order to normalize thyroid function more quickly (optimally within the first 14 days of treatment) and achieve better development quotient (DQ) scores. It appears that bone maturation at birth may predict psychomotor development in the first 12 months of life (22).
Several studies (13, 23–26) on the neurological outcomes of CH patients confirm that a rapid normalization of thyroid function can reduce the incidence of neurological impairment (19).
However, one study noted, for the first time, that children treated with high doses of l-T4 were more likely to present hyperactivity, aggression and delinquency (27). Other data have also revealed that high serum T4 levels may be associated with reduced attention in children of school age (28) and that there may exist a relationship between permanent attention deficit hyperactivity disorder (ADHD) and overtreatment in early years of life (29). These data show that overtreatment of CH can be dangerous and stress the importance of accurate follow-up to monitor dosage of l-T4.
2 Treatment and Monitoring of Primary CH
2.1 Goals of Treatment for CH
Triiodithyronine (T3) is the biologically active form of TH but it is important to remember that as T3 cannot pass the brain barriers in significant quantities, most T3 in the brain is converted from local deiodination of T4, meaning that in CH patients, it is not necessary to replace T3 to normalise neurologic development (1). Research involving 47 infants treated with different doses of l-T4, found that serum T3 normalized and stayed normal regardless of dosage, indicating that l-T4 alone is an adequate treatment for CH (30).
It is important to start treatment promptly in all infants who test positive in CH screening, after samples for confirmatory tests are taken, the results of which will determine the optimal l-T4 starting dose (31). When the results of venous blood tests are not available on the same day, treatment should be started without waiting for the results, particularly if the patient’s condition is a cause for concern (30, 32, 33),
The treatment goals for CH are outlined by the American Academy of Pediatrics (AAP) (30) and by the European Society for Paediatric Endocrinology (ESPE) (20) and can be summarized in the following main points:
● keeping serum Free T4 (FT4) above the average age-adjusted normal level but within the upper limit, especially in the first 12 months of life.
● keeping serum TSH below 5 mU/L.
Some data suggests that infants with serum T4 levels under 10 μg/dL during the first 12 months of life, associated with serum TSH levels greater than 15 mU/L, have a lower Intelligence Quotient (IQ) than those with serum T4 levels above 10 μg/dL (28). Furthermore, other data show that treatment with higher doses of l-T4 may be connected with higher IQ at 7 and 8 years of life, particularly in verbal working memory and sentence comprehension (27). Frequent and significant alterations in serum T4 and TSH levels during the first 12 months of life are associated with differences in mental development index scores and verbal IQ (27, 33).
Prompt diagnosis and initiation of therapy for CH is essential for ensuring positive outcomes, especially in high-risk infants (34). However, some patients with CH may not achieve normal TSH concentrations despite l-T4 treatment even at higher doses. Poor adherence to treatment is the most frequent reason for poor TSH control. Therefore, in patients who do not respond to treatment it is important to investigate whether l-T4 is being correctly administered and if necessary, review the method of administration. The possibility of impaired l-T4 absorption related to gastrointestinal diseases or the interference of drugs or other substances (see below) should also be considered (35).
Good therapeutic management practices appear to be of particular importance to ensure optimal results in terms of neuromotor and mental development, due to the THs-dependent processes of brain development in the first years of life. Professionals should provide clear explanations and instructions to caregivers to improve adherence to treatment (9, 34, 35). It is critical that families comprehend that treatment is essential for the best patient outcomes.
2.2 Dosage and Timing
Infants with severe forms of CH are at a higher risk for ID; for this reason, the dosage and timing of l-T4 treatment are fundamental for optimising neurocognitive outcomes in the future. Delays in normalizing serum T4 of more than one week can lead to lower IQ (23). Some data suggest that scores in behavioural and cognitive tests were lower for patients whose T4 did not normalize before two weeks than for patients who achieved normal levels in under two weeks (24). Therefore, as stated above, the treatment goal is to bring serum T4 to above below 129 mmol/L (> 10 μg/dL) as quickly as possible. The data available in the literature show that there is an inverse relationship between the initial l-T4 dose, and the time taken to reach target serum T4 levels (24) meaning that the prompt administration of an appropriate starting dose of l-T4 is particularly important.
The starting l-T4 dose recommended both by AAP and the ESPE is from 10 to 15 µg/kg/day (19–21, 30). In infants born at term this translates to an average starting dose of 37.5 - 50 µg per day (20, 21). In the above cited research, infants receiving 50 µg per day (corresponding to 12 - 17 µg/kg/day), versus 37.5 µg (corresponding to 10 - 15 µg/kg/day) performed better in tests measuring behaviour, reading, spelling and maths. The patients on the higher dose achieved IQ scores 11 points higher than those on the lower dose (24). Therefore, in infants whose serum T4 levels are lower than 5 μg/dL, higher l-T4 doses are recommended.
The goal is to normalize T4 and TSH within 2 and 4 weeks, respectively (19, 24). T4 and FT4 concentrations should be in the upper half of the reference range for age, with normalization of TSH (19).
These positive outcomes associated with administering higher doses of l-T4 have been highlighted by studies in Europe, the United States and Canada (24, 36–38). In one study, 83 infants were placed into three different groups, each receiving different initial doses of l-T4 from diagnosis at birth (the first group was treated with 6.0 - 8.0 µg/kg/day, the second with 8.1 - 10.0 µg/kg/day, and the third with 10.1 - 15.0 µg/kg/day) and followed up at four years of age for growth and intellectual development. The infants with severe CH who started on the highest dose achieved the highest intellectual scores (39). Another study involving 61 infants comparing early treatment with delayed treatment and low doses with high doses, found that the outcomes for patients with severe forms of CH were more favourable in those treated early (under 2 weeks) and with higher doses (>9.5 µg/kg/day). Such patients presented normal psychomotor development at 10-30 months of age (23), showing that the time it takes for TSH to normalize is inversely correlated to neurological outcome (19).
Another study which analysed data on the intellectual development of 45 CH children found that differences and variations in serum levels of T4 and TSH during the first year of treatment predicted scores on mental development index (MDI) and verbal IQ tests performed at 2 years and 6 years of age (33). It is clearly imperative to monitor patients so that l-T4 doses can be promptly adjusted and optimal T4 and TSH levels maintained.
2.3 Recommended Follow up During the First Three Years of Life
CH treatment must be carefully monitored with appropriate adjustment of the l-T4 dose, until serum TSH and FT4 concentrations have normalized. Clinical evaluations must be made every few months during the first three years of life together with frequent measurements of serum T4 or FT4 and TSH, in order to avoid prolonged periods of under- or overtreatment (19, 24). Management should focus on maintaining a status of euthyroidism, especially in the first 3 years of life when brain development is most dependent on THs. However, in CPHD patients with associated adrenal insufficiency or in patients in whom this problem cannot be excluded, adrenal function should be investigated, and glucocorticoid therapy should precede l-T4, replacement treatment to avoid inducing an adrenal crisis (19).
The AAP recommends monitoring thyroid function 2 and 4 weeks after initiating l-T4 treatment and every 1 to 2 months during the first 6 months of age. After this, patients should be monitored every 3-4 months between 6 months and three years of age, and then every 6-12 months until the child reaches adult height. Moreover, TH should be evaluated four weeks after any change in dose and follow-up evaluations need to be more frequent if results are abnormal or non-adherence to therapy is suspected (19).
Although in most infants, serum T4 will normalize within seven to fourteen days and serum TSH will normalize within one month of treatment, some patients continue to present altered TSH levels (10-20 mU/L) or serum T4 levels beyond these timeframes (40). This is often caused by under treatment, although it is important to remember that in 10% of CH infants undergoing treatment the FT4 feedback control on TSH secretion does not mature normally (41) due to the resetting of the pituitary-thyroid feedback mechanism in utero (42). In one paper reporting data for 42 CH paediatric patients, a prevalence of pituitary TH resistance of 43% in patients below one year of age was found. However, in childhood and adolescence this problem was found in only 10% of patients (42), suggesting that TH resistance is more common in younger patients and may decrease with age.
2.4 Effects of Inadequate or Non-Compliance
The availability of adequate TH is essential for normal brain development in the first two to three years of life. Low TH levels during this period can lead to irreversible damage, whereas after 3 years of age the effects of hypothyroidism can usually be reversed when the condition is treated. A study by the New England Congenital Hypothyroidism Collaborative highlighted the importance of regular monitoring and dose adjustments to control serum FT4 and T4 levels. Researchers found that eighteen infants included in the study with reduced serum T4 levels, who were taking insufficient doses of l-T4 (< 5 µg/kg/day) and who had an anamnesis showing poor compliance in the first three years of life, had a mean IQ of 87 (43). In comparison a larger group of correctly treated patients, with a history of normal serum T4 levels, achieved a mean IQ score of 105.
Further data suggest that noncompliance after the first three years of life may also negatively affect cognitive achievement. For example, researchers made unannounced home visits on 14-year-old adolescents with CH and found that 44% were not adequately treated, having serum TSH > 15 mU/L and T4 levels < 6.6 µg/dL in the majority (44). Psychometric tests revealed a mean IQ of 106. The importance of complying with TH treatment was explained to patients and their families (the dose was not altered), and 12 to 24 months later, serum TH levels had improved, and the repetition of psychometric tests showed an improvement in mean IQ of 6 points. Although the study did not include a control group, the authors concluded that non-compliance during adolescence is frequent, and that improved compliance can lead to improved cognitive function.
ID caused by CH is preventable but lack of awareness amongst caregivers about the benefits of treatment may be a barrier to clinical improvement in some patients (7). Newborn screening represents a unique opportunity for avoiding damage connected to CH (7).
2.5 Effect of l-T4 Overtreatment
The effects of overtreatment with l-T4 are well known (45–47). A prospective study by Rovet et al. (27) demonstrated for the first time that, despite an improvement in cognitive profiles, an initial high dose of l-T4 can be associated with behavioural problems in childhood. Bongers-Schokking et al. (48) evaluated DQ scores at 11 years, showing that overtreatment can lead to a cognitive impairment of −17.8 points if it is prolonged and −13.4 points when the overtreatment is limited to the first 2 years of life. Another recent study by Bongers-Schokking et al. (29) concluded that episodes of overtreatment during the first 3 months can lead to permanent ADHD, while undertreatment may be related to autism.
3 Treatment and Monitoring of cCH
Many data and recently updated CH consensus guidelines support the treatment of cCH with a once daily administration of l-T4 (13). In severe cCH (serum FT4 levels before treatment below 5 pmol/L), the minimum l-T4 starting dose should be no less than 10 μg/kg/day, in order to promptly bring FT4 within the normal age-adjusted range (49). A lower starting dose of l-T4 (from 5 to 10 μg/kg per day) should be used in milder forms to avoid overtreatment. For primary CH, the long-term biochemical aim must be to bring and maintain serum FT4 within the upper half of the reference interval for the patient’s age (49). Although it remains essential to conduct randomized clinical trials in children to obtain specific results for the paediatric population, data from adult studies support this approach (50, 51).
cCH should not be monitored by measuring serum TSH. Instead, serum FT4 is the most reliable indicator of efficacy of treatment. If TSH levels prior to treatment are low, it is not necessary to take subsequent TSH measurements (13, 49).
Clinical and biochemical follow-up for patients with cCH should be similar to that for patients with primary CH. After diagnosis and the start of l-T4 treatment, a first evaluation should be carried out within 1-2 weeks. If testing indicates a good response, subsequent evaluations should be scheduled every two weeks until serum FT4 normalizes. After this phase, patients should be evaluated every 1-3 months during the first year of life, every 2-4 months during the successive two years, and then every 3-6 months. When it is necessary to take blood samples to measure serum FT4, parents or caregivers must be instructed not to administer the daily dose of l-T4 beforehand. If this is not possible, blood should be taken at least four hours after the last l-T4 dose (13). For cases in which under- or overtreatment is suspected, it may be useful to measure TSH, and free or total T3. Patients with FT4 near the lower limit of the age-adjusted range should be considered at risk for undertreatment, especially if TSH >1.0 mIU/L while those with FT4 near or above the upper limit of the age-adjusted range should be considered at risk for overtreatment, especially if there are typical clinical signs of thyrotoxicosis or high FT3 levels (52).
Naafs et al. found that 86% of 92 Dutch children with cCH detected by newborn screening had both CPHD and ACTH deficiency and that 96% and 74% had GH and gonadotropins deficiency respectively (53). Most of the cases of ACTH deficiency (71%) were diagnosed in the first month of life, but GH deficiency on average was not diagnosed until 1.3 years of age. These findings demonstrate the importance of monitoring all children with cCH over time for the possible emergence of other pituitary defects. Naafs JC et al. (54) also reviewed studies on children with cCH present in literature and found that the full-scale intelligence quotient was below 85 in 27% of patients, and below 70 in 10%. However, the age treatment was begun was not known for most patients (54).
4 Pharmacokinetics and Different Formulations of l-T4
The pharmaceutical preparation of drugs and the mode of administration are important elements in achieving and maintaining euthyroidism in CH and counteracting the effects of external influences on THs values.
Synthetic compounds of thyroxine usually contain the laevo isomer of thyroxine (l-T4), most commonly as a sodium salt (55); however, although l-T4 is frequently prescribed worldwide, the correct daily dosage, mode of administration, and approach to resolving treatment failure remain matters of debate. In primary CH, in order to maintain euthyroidism, thyroxine must be efficiently absorbed and unbiased serum TSH values must be available, which cannot be measured in patients with cCH.
Physiologically, l-T4 is absorbed in the small intestine and, to a lesser extent, in the stomach (56). In patients with short bowel syndrome, the absorption of l-T4 can be compromised (57). Although the pharmacokinetic parameters of l-T4 have not been assessed in children, in adults there are differences between euthyroid and hypothyroid subjects. Hypothyroid subjects achieve Cmax with a delay of 2-3h, associated with a decreased volume of distribution, and a higher bioavailability (than the standard 60–80%) (57–59).
l-T4 permeation through the intestinal epithelium occurs principally in the duodenum and the jejunum, largely within 3 h after ingestion; absorption is most rapid within the first 2 h (57). Approximately 70% of the l-T4 contained in tablets is absorbed and l-T4 ingestion is best in the morning, at least 1 h before breakfast as fasting improves absorption (60).
Concomitant gastrointestinal diseases, such as coeliac disease (CD), Helicobacter pylori infection, lactose intolerance, inflammatory bowel diseases, as well as parasitic infestation (Giardia lamblia) may cause l-T4 malabsorption (56, 57).
Increased gastric pH is also known to influence l-T4 absorption (61); many studies confirm that individuals with impaired gastric acid secretion, as a result of disease or the use of proton pump inhibitors (PPI), often require higher l-T4 doses to reach target TSH levels (62). In comparison, the available data suggest that pantoprazole and esomeprazole do not alter the pharmacokinetic parameters of l-T4 absorption, although the evidence is insufficient for this to be certain (63).
Generally, the half-life of l-T4 in a state of euthyroidism is 6-7 days (3-4 days with hyperthyroidism and 9-10 days with hypothyroidism). The full therapeutic effect of thyroxine is evident 3-4 weeks after treatment is initiated; if treatment is interrupted the therapeutic effect continues to be observed for 1-3 weeks. Because thyroxine has a long half-life, dose changes should not be made any more frequently than every 3-4 weeks (55).
Some studies also suggest that changing the drug’s formulation can significantly reduce problems of l-T4 malabsorption. Liquid l-T4 is absorbed faster than solid pills (64), and has also been found to be less affected by gastric pH and conditions which impair l-T4 absorption (61, 65). Liquid l-T4 seems more effective in reducing TSH levels than tablets of the same dose, independently of whether malabsorption problems are present (65, 66). In patients assuming PPI or other interfering drugs, switching from tablets to a liquid formulation may improve the efficacy of treatment (67, 68). l-T4 in soft gel capsules also dissolves better in increased pH (69) than tablets and appears able to reduce TSH levels both in patients with gastro-intestinal diseases and in patients without apparent malabsorption problems (70, 71).
Serum TSH is not always a reliable marker for pharmacological euthyroidism since values are affected by several drugs and some pathophysiological conditions (72, 73). However, it remains the best available marker as it is not easy to directly measure thyroxine absorption. The daily dose of l-T4 required to obtain the desired TSH value/therapeutic effect does not directly correlate to the ingested dose as absorption of oral l-T4 is between 60–80 %. Often high l-T4 doses are necessary to attain target serum TSH concentrations, which can lead to costly hormonal monitoring. Repeated failure in reaching therapeutic targets is common. Dosage often has to be modified in children as they grow and gain weight, especially in the first year of life, although the need for higher does may also be due to impaired l-T4 absorption.
It is important to consider the time of day that the drug is taken. Tablets should be taken at least 1 h before breakfast (60). In cases of reduced absorption or in patients with CD, lactose intolerance or other chronic inflammatory conditions, liquid l-T4, or soft gel capsules may be better absorbed.
4.1 Different l-T4 Formulations
l-T4 is available in several forms (tablets, soft gel capsules, liquid and, in some countries, powder for preparing an intravenous solution). Unfortunately, tablets are the only form available in many countries.
4.1.1 Tablets
l-T4 tablets are the oldest treatment for CH. l-T4 is absorbed within 20–30 min after ingestion and the absorption process takes about 3 h (59). Taking tablets with food affects the drug’s pharmacokinetics and the efficacy of treatment; tmax is delayed, and the peak value of l-T4 absorption decreases (57, 74), as does drug bioavailability, from 15% to as much as 40% (75, 76). Not refraining from eating for at least 30 min after l-T4 tablet ingestion, may result in significantly higher TSH levels (77). This is important to bear in mind in patients for whom persistent or frequent TSH alterations can be dangerous such as congenital hypothyroid patients, pregnant women, patients with cardiac disease, or oncological patients (78).
For young infants, the tablet should be crushed and mixed with breast milk or water. It should not be mixed with soy formula which interferes with absorption. If the infant requires soy formula, l-T4 should be given midway between feeds and thyroid function must be carefully monitored (79).
Although stable in dry air, l-T4 becomes very unstable in the presence of light, heat and humidity. It is important to make sure that parents and other caregivers store the drug in its original container, away from sunlight and in a cool, dry place (55). Incorrect storage can affect bioavailability, making absorption more difficult which can cause fluctuations in the levels of THs.
4.1.2 Liquid Form
A liquid solution of l-T4 containing ethanol is available in some countries. This form contains l-T4, ethanol, and glycerine and appears to be more stable than tablets. Its main advantage is that the gastric phase of digestion is not necessary (64), meaning that it is absorbed more quickly (64) as has been confirmed by in vitro (80) and in vivo (81–84) studies conducted in hypothyroid adults assuming l-T4 while fasting or with breakfast (81, 84). The faster onset of absorption, compared to tablets, minimizes the risk of drug-food interactions (64).
Studies conducted on paediatric patients affected by CH showed that the liquid formulation is a safe alternative to tablets, ensuring normal growth and neuromotor development (85–87). However, there appears to be greater suppression of serum TSH during the first months of treatment, indicating a higher risk of overtreatment (85–87).
Another concern about the liquid formulation is the potential risk of long-term side effects from the chronic administration of excipient ethanol; however, one millilitre of liquid formulation, equal to 100 μg of l-T4, contains 243 mg of ethanol as excipient, more than four times lower than the safe threshold for alcohol established by the AAP (88). Several studies on treatment during pregnancy or lactation do not demonstrate any side effect on infants (89–91).
A recent multicentric Italian study involving 253 CH patients, with a follow-up period of up to 3 years, confirmed the efficacy of l-T4 at high doses (87) in both liquid and tablet form. The data confirmed findings of previous studies by Cassio et al. and Peroni et al. (85, 86) and are in line with the results of a meta-analysis conducted by Aleksander et al., which showed that a high initial l-T4 dose of between 10 and 15 μg/kg/day was effective and safe in all CH patients and that high FT4 values during infancy did not correlate with IQ impairment at adolescence (18).
In a study by Vigone et al. patients treated with oral l-T4 solution presented, after 15 days and 1 month, but not at 3 months, significantly lower median TSH and higher FT4 values than those treated with tablets, with suppressed TSH levels in about 60% of the CH patients, indicating a higher risk of overtreatment during the first month of therapy (87). This may be due to a higher absorption of liquid l-T4 suggesting that to avoid overtreatment, lower starting doses should be administered with shorter intervals between follow up visits (87). Both tablet and liquid l-T4 were associated with adequate total DQ scores at 1 and 3 years of follow-up (85–87).
Another, prospective randomized control study in children with CH, to assess the efficacy and safety of liquid l-T4 in comparison to tablets, evaluated thirty-nine children, aged 3-12 years old. At six months the group treated with liquid l-T4 had higher TSH levels than the group treated with tablets, while FT4 levels had no statistical difference. Dose adjustments were more frequent in the group treated with liquid. l-T4 (92).
Finally, recently, another liquid formulation containing l-T4, but without ethanol, using a calibrated oral syringe, has been studied, even if only in extremely premature infants (born below 28 weeks’ gestation), without apparent effect on brain size (93).
4.1.3 Soft Gel Capsule
In this form, l-T4 is dissolved in water and glycerine, and conserved in a gelatine matrix, to protect the hormone from degradation. It is free from lactose, gluten, alcohol, sugar, and dyes (94). The literature does not report studies in children with CH. One study, conducted in 60 euthyroid adults, investigated the effect of meals on the absorption of l-T4 in this form. Patients who had been taking oral liquid l-T4 with breakfast, switched to the soft gel capsule, without changing the mean dose. TSH, FT4, and FT3 levels were measured at the beginning of treatment with the soft gel capsule and after 6 months (95). There were no significant differences in TSH, whereas FT4 and FT3 levels were significantly lower in the subjects treated with the soft gel capsule (95). Another study found that, unlike l-T4 tablets, the absorption of soft gel capsules is not affected by the concurrent consumption of coffee (96), suggesting that capsules may be of benefit for patients with CD, lactose intolerance and other chronic inflammatory conditions. A paper, evaluating these new formulations of oral l-T4 for adults with central hypothyroidism, reported that liquid or soft gel l-T4 was more effective at bringing serum FT4 levels into the upper-normal range (97).
5 Effects of Foods, Drugs/Diseases, Supplementation, and Nutrition Route on THs Levels
Many physiological, nutritional, pharmacological or pathological factors can impair the intestinal absorption of oral l-T4 (57, 98, 99). Patients, parents and carers often underestimate the risks of eating concomitantly or too soon after taking l-T4 which can negatively affect absorption and therefore the efficacy of the therapy (100).
This is particularly worrying for paediatric CH patients, especially in the first three years of life given the thyroid-dependence of the central nervous system during this phase. Parents need to be made aware of the importance of correctly administrating l-T4 to avoid interference from food and should be reminded to inform their child’s paediatrician and endocrinologist about any drugs or medicaments the patient has taken or is taking.
Many drugs or medications can alter TH levels by affecting the hypothalamic and pituitary regulation of thyroid hormone production, the synthesis and secretion from the thyroid gland directly, the metabolism of THs (deiodination, sulfation and glucuronidation) or by altering affinity for levels of thyroxine binding globulin (100).
A number of drugs, such as proton-pump inhibitors, sucralfate and aluminium-containing antacids, affect the absorption of l-T4 by changing gastric pH and reducing the solubility of tablets. Others, such as iron and calcium salts, phosphate binders, bile acid sequestrants and other resins, bind with l-T4 to form insoluble complexes. The mechanism of interference for some drugs, such as raloxifene, is unknown (99). Certain herbal remedies also impair the ability to absorb l-T4 (101) and nutritional supplements can also interfere, including infant formulas containing soy protein and iron, calcium and fibre supplements (100).
It is important to inform parents and caregivers about the possible interference of drugs and supplements and the potential damage they can cause in patients who are dependent on exogenous l-T4 (100). The need to increase the dose of l-T4 does not always signal gastrointestinal malabsorption but may indicate interference from food or medications (98). For this reason it is essential to take a patient’s diet and medication use into consideration.
In a study of 925 adult hypothyroid patients aim at identifying factors with an effect on l-T4 therapy, McMillan et al. found that over 50% assumed dietary supplements with a potential interaction with l-T4, such as calcium (over 47% of cases) and iron (almost 12% of cases), while 68% reported frequent intake of foods rich in fibre, iodine, or soy (102). A study by Michel et al. (103) also found that many patients had insufficient awareness of the importance of l-T4 administration schedules.
The problem is also of concern in paediatric patients, as dietary supplements are often given to infants and children (104). Some data suggests that more than 20-30% of children take dietary supplements regularly, most of which have not been recommended by a health care provider (104).
5.1 l-T4 – Food Interaction
It is clear that interference from food can negatively impact the efficacy, and in some cases the safety, of treatment with l-T4. This is also the case for liquid and soft gel capsule formulations (101). Most data, especially sporadic case reports, focus on adults and there is a need to augment our knowledge about the effects of food on l-T4 in children. In the presence of problems of l-T4 absorption, switching from tablets to liquid l-T4 or soft gel capsules can in part solve the problem, and observing appropriate intervals between taking l-T4 and eating can also reduce the possibility of interaction (105).
5.1.1 Infant Formula, Cow’s Milk, and Breast Milk
In newborns and infants with CH, there is also the possibility of interference from infant formula. There are few data in the literature but infant formula milk contains fat, proteins and lactose that can cause l-T4 to remain in the intestinal lumen which prevents it being absorbed (57). If patients are taking l-T4 with foods, they may need larger doses to maintain euthyroidism (55). One study by Chon et al. demonstrated that in adults, cow’s milk which is frequently ingested with breakfast can interfere with absorption (106). 1000 µg l-T4 in tablet form alone or together with 355 mL of 2% cow’s milk was administered to healthy adults. Peak serum TT4 concentrations and the area under the curve were reduced by nearly 8% in patients taking l-T4 with cow’s milk (106). It is possible that breast milk, which contains only 20-30% of the calcium in cow’s milk, is also decreases l-T4 bioavailability (55). However, no data are reported in the literature for children.
5.1.2 Soy Based Formula
Some data, deriving mostly from old paediatric case reports, seems to show that soy infant formulas, as well as soy-containing baby foods, are related to altered thyroid function in early childhood (107–109).
Soy formula is often given to infants as an alternative to milk protein formula, especially in cases of intolerance to other formulas.
In 1959, Van Wyk et al. reported an infant fed with soy-based formula who presented cretinism and goitre at 10 months of age (108), which mostly resolved after the discontinuation of the soy-based diet. In another report on 78 patients under the age of one year, Pinchera et al. described the interaction between soya and l-T4 in a 3-week-old child fed on soy-based baby formula because of suspected lactose intolerance. Despite high doses of l-T4 (15 µg/kg per day), the infant’s TSH values remained elevated (248 mIU/L). Only after substituting the soy-based formula with another formula, did TSH decrease (110).
Since the 1960s soy formulas have been supplemented with iodine and it wasn’t until 1995 that other cases of soy-induced goitres in infants were reported (111, 112). Conrad et al. studied the influence of diet on thyroid function in paediatric CH patients, to investigate whether soy formula-based feeding can prolong the presence of increased TSH. The study included eight children on a soy formula diet and 70 on a non-soy diet. The children fed the soy formula had a more prolonged increase in TSH levels than children on non-soy formulas (113). The authors emphasized the need to frequently control levels of TSH in infants fed with soy formulas so that l-T4 dosage can be adjusted accordingly.
In vitro studies indicate that phytoestrogens may affect the synthesis of T3 and T4 by inhibiting thyroid peroxidise (114). However, in a meta-analysis of 14 studies, Messina and Redmond found no significant adverse clinical effects on thyroid function in healthy adults but the l-T4 dose might need to be increased in hypothyroid patients. For infants below the age of three years with CH (115), maximum attention appears necessary, given the thyroid-dependence of the central nervous system.
5.1.3 Fruit Juices
Some data suggest that fruit juices - particularly grapefruit, orange, and apple - also interfere with l-T4 treatment by blocking the transporters carrying l-T4 from the small intestine into the blood (116, 117). Lilja et al. (118) described an adult hypothyroid female, previously treated with l-T4 with success, who presented a sudden new increase in TSH (more than 60 mU/L) with a reduced FT4 concentration (6.4 pmol/L) after consuming grapefruit juice. Her hormone levels returned to within the normal range only after the woman reduced her consumption of this juice. These results confirm the data of a randomized study of 10 healthy volunteers who drank grapefruit juice 1 h before taking l-T4,and presented a reduction of 9% in l-T4 absorption (118). Meyer et al. observed that THs upregulate expression of the transporter OATP2B1 hypothesising that hypothyroidism influences the interaction of juice and l -T4 (119). Recently Tesic et al. reported a 31-year-old female patient with persistent hypothyroidism despite treatment with high doses of l-T4 (alone or in combination with liothyronine) who had been taking l-T4 tablets with juice or mint tea. The patient was advised to take l-T4 with water, and her TSH levels normalized and her fT4 levels increased in a few days (120). Although the data are scarce and, at times, conflicting, it seems prudent not to take l-T4 with juice, but it does not seem necessary to eliminate fruit juice consumption completely.
Deiana et al. (121) reported a 37-year-old patient treated with l-T4 and with euthyroidism after thyroidectomy, who presented an unexpected TSH increase after eating large quantities of papaya (5-6 fruit a day). She was advised to stop eating the fruit completely and after 45 days her serum TSH concentration returned to within the normal range. Papain reduces gastric acid secretion which lowers l-T4 absorption; other components of the fruit, as well as fibre terpenoids, saponins, alkaloids, and flavonoids, may also reduce l-T4 absorption (121).
5.2 l-T4 – Drugs/Diseases Interaction
Many conditions or drugs may influence the gastric environment and affect the absorption of l-T4. Tablets of l-T4 need to be dissolved in an acid environment. Subjects with achlorhydria, reduced gastric acidity or who are taking PPIs do not have the ideal gastric environment to dissolve l-T4. PPIs are increasingly prescribed to children (122) and are effective treatments for many gastric diseases, duodenal ulcers, non-steroidal anti-inflammatory-induced ulcer-related prophylaxis, Helicobacter pylori in combination with other medications, gastro-esophageal reflux disease and eosinophilic esophagitis. They are also prescribed for functional dyspepsia, chronic cough, and infantile reflux (122).
In adult goitrous patients taking l-T4, omeprazole assumption led to significant increases in serum TSH; this effect was reversed by increasing the dose of l-T4 or by discontinuing the use of omeprazole (123). Similar results have been reported for lansoprazole, but not pantoprazole or esomeprazole taken for one week (57). Antiacids also appear to interfere with thyroxine absorption by influencing gastric pH (57).
The recently developed liquid l-T4, dissolved in an alcoholic solution, does not need an acid environment as it can be directly absorbed through the intestinal mucosa (124).
Besides drugs, pre-existing malabsorption diseases or disorders that impair gastric acidity also affect the bioavailability of l-T4 (125). There are some reports of elevated serum TSH levels in patients with coeliac disease and inflammatory bowel diseases taking doses of thyroxine that were previously sufficient to normalise serum levels (125).
Some studies report similar findings for patients with Helicobacter pylori infection and atrophic gastritis – both of also which impair gastric acidity (125).
Several reports regarding cases of l-T4 malabsorption in patients with various manifestations of CD have been reported (125, 126). For example, in a study conducted on seventy-nine children with permanent CH, 6 patients (4 girls, 2 boys) were positive for CD antibodies, showing a higher-than-normal prevalence of this disease in children with permanent CH. The available data show that a gluten-free diet results in the reduction of l-T4 dose requirements (127). Because the symptoms of some forms of CD are subtle, several authors suggest screening with CD markers in patients with hypothyroidism who need higher than normally expected doses of l-T4.
Franzese et al. report an infant with CH who developed, during l-T4 replacement therapy, a cow’s milk protein intolerance and subsequently CD. Both milk protein intolerance and CD affect the intestinal absorption of l-T4, making the management of CH difficult. After starting a diet with a hydrolyzed milk protein and maintaining the dose of l-T4 at 12 µg/kg dose, serum T4 improved and TSH decreased (128). A case of l-T4 malabsorption with lactose intolerance was reported in a 55-year-old woman with primary hypothyroidism whose TSH levels were persistently high (128).
After being diagnosed with oligo-symptomatic lactose intolerance, the patient was given a lactose-free formulation of l-T4 (150 mg daily) and began following a lactose-restricted diet; after 3 months the problem had resolved (129).
The presence of a pylori infection and atrophic gastritis of the body of the stomach, causing bacterial production of urease that neutralises gastric pH, may determine a decreased TSH suppression (123).
An infant with confirmed CH taking l-T4 experienced a possible drug interaction with simeticone (130) which is used to treat infant colic, a condition frequently seen by paediatricians. In this case, despite adequate l-T4 dosage, TSH was high, suggesting undertreatment. This case highlights the importance of regularly reviewing a patient’s clinical history and considering the possibility of drug interactions in unusual circumstances, especially where patients need high doses despite good compliance and proper administration (131). Clinicians should alert the parents of CH children starting l-T4 about the use of medicines containing simeticone (130).
Small intestinal bacterial overgrowth (SIBO) is a heterogeneous condition with nonspecific symptoms characterized by the presence or increase of atypical bacteria in the small intestine that unbalances intestinal microbiota. SIBO is characterized by symptoms such as flatulence, distension and abdominal pain. These symptoms might be indistinguishable from those presented by patients with functional gastrointestinal disorders. In most cases, SIBO is associated with motility or inflammatory disorders (132). The abnormally high levels of bacteria in the small intestine can cause malabsorption and suboptimal responses to narrow therapeutic index medication which are absorbed in the small intestine, such as l-T4 (133). In one study on patients with SIBO, l-T4 tablets and a compounded oral suspension were not absorbed efficiently, resulting in below optimal control of serum TSH. When patients switched to l-T4 sodium oral solution, TSH levels fell and symptoms resolved (133).
Malabsorption of l-T4 has also been described in patients infected with Giardia lamblia. Giardiasis is a common intestinal infection, but it has only rarely been reported as a cause of impaired l-T4 absorption (124). Giardiasis is probably under-diagnosed; in developing countries with poor sanitation its prevalence is about 20% while in industrialised countries prevalence ranges from 3% to 7% (124). Intestinal giardiasis can cause maldigestion, malabsorption and diarrhoea, leading to anaemia, weight loss and growth retardation (124). In cases of giardiasis infection in patients taking l-T4, switching from tablets to oral solutions revert decreased l-T4 absorption (124).
Short bowel syndrome (SBS), a major cause of intestinal failure in children, is diagnosed when the length of the small intestine is 25% shorter than expected for the child’s age (131). In paediatrics, the most frequent causes of SBS are resections secondary to necrotizing enterocolitis, gastroschisis, intestinal atresia and intestinal volvulus (134). SBS can cause a number of metabolic changes in the body, which can affect growth, intestinal adaptation and lead to metabolic bone disease as well as an impaired functioning of the hypothalamic-pituitary-thyroid (HPT) axis (135). Passos et al. reported six consecutive cases of children with SBS with associated hypothyroidism during the period of intestinal rehabilitation (135).
5.3 l-T4 – Dietary Fibre
A fibre-rich diet or dietary fibre supplements may also significantly reduce the bioavailability of l-T4 due to a non-specific link to fibre in the intestinal lumen causing malabsorption of the hormone (125). Products containing insoluble dietary fibre increase bowel motility, potentially altering the intestinal absorption of l-T4 (78). Although not all authors agree (135), in cases of dietary modifications which increase fibre intake, it may be necessary to monitor TSH levels and increase the dose of l-T4 (136).
5.4 l-T4 – Essential and Trace Elements
The possibility of interactions between levothyroxine and essential and trace elements has been investigated. Di- and trivalent elements, especially calcium and iron, appear to decrease l-T4 bioavailability (137). The mechanisms involved are not entirely clear but may connected to unspecific adsorption and the formation of insoluble complexes in the intestine (138). Parents should thus be informed about the possible adverse effects of calcium and iron supplementation.
5.4.1 Calcium
CH may occur in association with congenital parathyroid hormone (PTH) insufficiency which leads to hypocalcaemia and low secretions of PTH (138). The combination of CH and PTH insufficiency occurs in patients with 22q11 microdeletion/DiGeorge syndrome, or due to PTH resistance in pseudohypoparathyroidism (139, 140). Metabolic bone disease is very frequent in preterm infants, occurring in 20-30% of very LBW infants (VLBW, <1500 g) and in 50-60% of extremely LBW infants (ELBW, <1000 g), who are at an increased risk for CH (141). The introduction of calcium-based supplements to improve PTH insufficiency could interfere with the absorption of l-T4.
In adults, calcium carbonate may reduce l-T4 absorption, resulting in a significative reduction of FT4, higher TSH levels in 20% of patients. These TH alterations were normalized after calcium carbonate discontinuation (142). There are several instances in the literature of interaction between calcium carbonate and l-T4 (143), and in a large observational study on patients assuming l-T4 tablets or iron supplements, in which the authors observed a significant increase of serum TSH in 4.4% of patients in the first group and 7.5% in the second group, concluding that supplementation with calcium and iron may cause a reduction in the absorption of l-T4 (62).
Other calcium preparations also potentially interact with l-T4. Diskin et al. (144) looked at TSH levels in more than 60 patients assuming l-T4 with different phosphate binders (mean dose 95–98 µg/day). The reported that only calcium carbonate, but not calcium acetate, resulted in significantly higher TSH levels. Zamfirescu et al. (145) studied the effect of calcium formulations (acetate, citrate, and carbonate) delivering a dose of 500 mg of elemental calcium on the absorption of l-T4 tablets at a dose of 1000 µg in eight healthy adults. For all the calcium preparations, taking the calcium supplement at the same time as the l-T4 tablets reduced absorption of l-T4 by 20 to 25%. The researchers stressed the importance of taking the examined calcium formulations and l-T4 at different times.
Morini et al. (146) studied a cohort of 50 postmenopausal women with hypothyroidism taking co- calcium supplements containing elemental calcium with a dose of 600-1000 mg for day. When the supplements were taken at the same time as or within 2 hours of l-T4 ingestion, the majority of patients presented significant increases in TSH levels, blood pressure, total cholesterol levels, and fasting glycemia.
Some data suggest that in adults the interaction of l-T4 and calcium can be reduced by replacing l-T4 tablets with liquid l-T4. Benvenga et al. reported data for 12 hypothyroid patients taking calcium carbonate (1000 mg/day) and assuming tablets or liquid l-T4 formulations, concluding that liquid l-T4 may be more resistant to sequestration by calcium (147). Mazokopakis et al. (148) found that only 8.4% of more than 150 patients were taking calcium carbonate at least 4 h before or after l-T4.
5.4.2 Iron
Anaemia, defined as a haemoglobin levels of two standard deviations below the mean for age, is prevalent in infants and children worldwide with microcytic anaemia due to deficient iron intake being the most common type in children (149). Iron deficiency anaemia (IDA) can lead to cognitive problems that can be prevented or ameliorated with iron supplements or increased intake of iron in food (149). The phenolic, carboxylate, and amine functional groups on l-T4 molecules enable them to react with ferrous salts to form insoluble or only partially soluble complexes, which reduces the absorption of l-T4. Iron also plays an important part in TH synthesis and amino acid metabolism (150). Physiologically, IDA is able to impair THs metabolism, decrease T4 and T3 levels, reduce the peripheral conversion of T4 to T3 and T3 metabolism and decrease hepatic T4-5’-deiodinase leading to an increase in circulating TSH activity (151).
Anaemia is often present in infants affected by CH with severity depending on the degree of neonatal hypothyroidism which, if present during development, can lead to persistent health problems also after thyroid replacement therapy is started (152). “Uncomplicated” anaemia secondary to hypothyroidism responds to thyroid replacement therapy alone (153). Anaemia in hypothyroidism must be thoroughly evaluated because treatment must address its cause.
Gökdeniz et al. (154) showed that more than 15% of children with IDA presented concomitantly subclinical hypothyroidism, and Metwalley et al. (155) observed that primary school children with IDA were likely to develop SH and intellectual dysfunction. The presence of anaemia could influence the outcome of l-T4 therapy. Campbell et al. reported a lower efficacy of l-T4 treatment in adults when the drug was administered together with ferrous sulphate, with an increase in TSH levels in 79% of patients (156). A reduced absorption of l-T4 due to the co-administration of ferrous sulphate has been reported in a number of patients (157). Shakir et al. (158) suggest that interaction can occur even if patients maintain an interval of 4–6 h between taking l-T4 and supplements or medicines containing iron. Leger et al. report a 60-year-old female, previously treated with success with l-T4, who developed hypothyroidism after starting to take ferrous fumarate daily (TSH 243 mU/L, T4 <0.52 pmol/L). The woman’s thyroid function normalised 2 months after she stopped taking the iron supplement (159). Finally, Atruktsang et al. (160) recorded the time taken to achieve euthyroidism in over 600 subjects taking l-T4 as well as the number of dose adjustments that were necessary; among the patients whose dose need to be adjusted three or more times, a significant number used ferrous supplements.
Some data seem to suggest that, as with calcium, the use of an oral liquid form of l-T4 can help reduce problems of absorption correlated to iron supplementation. For example, in a small population of hypothyroid patients, Benvenga et al. (147) investigated l-T4 interaction with iron, observing a significant decrease in TSH levels in those who switched from tablets to oral liquid l-T4.
5.4.3 Aluminium
Many antiacids contain aluminium. A number of case studies in adults report l-T4 malabsorption caused by the concomitant use of aluminium hydroxide (161). Liel et al. investigated five hypothyroid subjects treated with l-T4 who took gel tablets containing aluminium hydroxide for 2-4 weeks, observing a significant increase in serum TSH during the period of aluminium hydroxide ingestion (138).
5.4.4 Iodine
Iodine is essential for THs which play an important role in foetal development and early infancy. Iodine deficiency in children may lead to reduced physical and intellectual capacity (162). In iodine-deficient areas, CH caused by iodine deficiency has a significant incidence (34). Preterm infants are at particular risk, also because parenteral nutrition and preterm infant formulas, including those used in hospital settings, do not always provide adequate iodine (163). Excess iodine intake may also cause a physiological decrease in THs synthesis, which is usually transient, due to the Wolff-Chaikoff effect (164). The thyroid’s ability to recover from the Wolff-Chaikoff effect is not fully mature until 36 to 40 weeks of gestation, meaning that preterm infants have a greater risk of prolonged hypothyroidism. Sources of excess iodine include iodine-containing antiseptics, radiographical contrast agents, and excess maternal intake of iodine (from diet or supplements) transmitted to infants in breastmilk (34).
The main dietary sources of iodine are iodized salt, seafood, and dairy products (165). Seaweeds are rich in iodine but the iodine content of seaweeds and dairy products varies considerably (166). Sea salt, Himalayan salt, and salt used in food processing is not always rich in iodine (148). Foods such as soybeans, cruciferous vegetables, and sweet potatoes contain substances capable of interfering with the thyroid’s uptake of iodine but in healthy individuals with adequate iodine intake, consumption of these foods does not lead to thyroid dysfunction (115). Vegans should use iodized salt and/or consume sea vegetables to minimise their risk of developing iodine deficiency (165).
The aetiology of hypothyroidism is multifactorial, but the most frequent causes are excess iodine intake and/or critical illness. Exposure to iodinated Contrast Media (iCM) is an increasingly common source of excess iodine in vulnerable patients (166). The thyroid gland has two auto-release mechanisms to manage high intra-thyroid iodine doses, namely the Via the Wolff–Chaikoff effect and the escape phenomenon (167). However, in newborns and infants these mechanisms are not mature making them prone to iCM toxicity (168). Infants with iCM toxicity typically have low FT3 plasma concentrations with low or normal FT4 and low or normal TSH, consistent with a non-thyroidal disease syndrome.
5.4.5 Selenium
The enzymes, iodothyronine deiodinases, glutathione peroxidases, and thioredoxin reductases, involved in TH biosynthesis and metabolism, regulation of the redox state, and protecting the thyroid from oxidative damage are selenoproteins. Thus, low selenium levels may lead to hypothyroidism: see below in the section related to parenteral nutrition (169).
5.5 l-T4 – Vitamin Interaction
It has been shown that increased gastric pH can influence the absorption of l-T4. Jubiz et al. studied the effects of vitamin C, capable of lowering gastric pH, on l-T4 absorption (170) They conducted a study in 31 hypothyroid subjects with gastritis under l-T4 therapy, taking a median dose of 100 µg in tablet form, with 120 mL of water with or without 500 mg vitamin C in solution. While on vitamin C, TSH levels fell in all patients, and FT3 and FT4 levels increased significantly. Antúnez et al. (171) observed similar results in 28 patients with elevated TSH levels, despite being on a l-T4 dose higher than 1.70 µg/kg. Patients were asked to take l-T4 tablets with 1 g of vitamin C (effervescent tablets, dissolved in 200 mL of water) for 6–8 weeks. Significant decreases in serum TSH levels (from 9.01 ± 5.51 mU/L to 2.27 ± 1.61 mU/L) were achieved.
Biotin is a vitamin commonly used in multivitamin preparations and used to treat progressive multiple sclerosis, several inherited metabolic diseases and acquired dermatologic diseases. It can interfere with many endocrine laboratory assays (including TSH and FT4) (172), which can lead to erroneous or delayed diagnosis of CH. There are cases in the literature of children taking high doses of biotin as therapy for metabolic disease being wrongly diagnosed with hyperthyroidism (172).
The correct assessment of thyroid function may be influenced by biotin therapy (173). High levels of biotin–streptavidin can compete with biotinylated components leading to inaccurate and misleading results characterised by increased FT3, FT4, levels and a reduction in TSH levels, which may lead to misplaced suspicion of Graves’ disease (173). This could lead to erroneous therapeutic strategies with serious consequences in the first three years of life given the thyroid dependency of the central nervous system in this period.
Wijeratne et al. conclude that interference peaks at approximately 2 h after biotin ingestion and can persist for up to 24 h (174). In a newborn with trisomy 21, CH and partial biotinidase deficiency, because of interference with the TSH assay from concurrent biotin administration routine screening detected “normal” TSH levels leading to a delayed diagnosis of CH (172).
5.6 l-T4 – Nutrition Route
In paediatrics, the most basic and important method of nutritional intervention is enteral nutrition (EN) (175). EN is introduced for patients who cannot meet their energy and nutritional needs through normal feeding. EN is often required for children with growth retardation, inadequate weight gain, or weight deficit, and is employed in the treatment of diseases such as Crohn’s, and food allergies or intolerance (175). In neonates, EN is required in situations such as premature or necrotizing enterocolitis (175).
Reis et al. (176) carried a multicentre study in Brazil to evaluate possible drug-EN interactions. The authors found l-T4-EN interaction to be one of the most frequent and clinically significant interactions. In an earlier study, Dickerson et al. (177) evaluated 13 hypothyroid patients in hospital: all participants received EN; their l-T4 doses were kept the same as they were before the patients were hospitalised for 20 ± 5 days. Eight of the thirteen patients developed subclinical or overt hypothyroidism, indicating that it is vital to monitor hypothyroid patients who receive continuous EN and who are being treated with l-T4. Manessi et al. (178) discovered that l-T4, may be adsorbed by enteral feeding tubes and hypothesised that this mechanism was related to a reduction in treatment efficacy. However, Wohlt et al. (179) concluded that the amount of l-T4 absorbed by feeding tubes is likely to be clinically irrelevant, and that impaired l-T4 absorption may be caused by the concomitant ingestion of food. Pirola et al. (180) studied 20 euthyroid patients, a day after surgical invention, to compare the efficacy of different formulations of l-T4 administered while patients were attached to an enteral feeding tube. EN was stopped for 30 min before and after powdered l-T4 tablets were administered, whereas liquid l-T4 was administered via the feeding tube without interrupting EN. There were no significant differences in the results obtained by the two methods, leading the authors to conclude that EN does not impact the absorption of liquid l-T4.
Parenteral Nutrition (PN) is used when normal and enteral feeding are impossible (181). Some reports show that long-term PN is a risk factor for low iodine and/or selenium levels, causing hypothyroidism, which in some cases is severe, stressing the importance of dosing of these elements and evaluating THs (182, 183).
6 Effects on Thyrotropin Releasing Hormone (TRH) and TSH Levels
Drugs and medications frequently influence thyroid function altering the secretion of TRH or TSH (184), even if only a small subgroup (glucocorticoids or GCs, dopamine agonists or DAs, etc) affects the hypothalamus or pituitary (100). Tables 1 and 2 report the drugs and medications which affect thyroid function in children with intact an hypothalamus-pituitary-thyroid axis (Table 1) and in those dependent on exogenous l-T4 (Table 2), also describing the mechanisms of action.
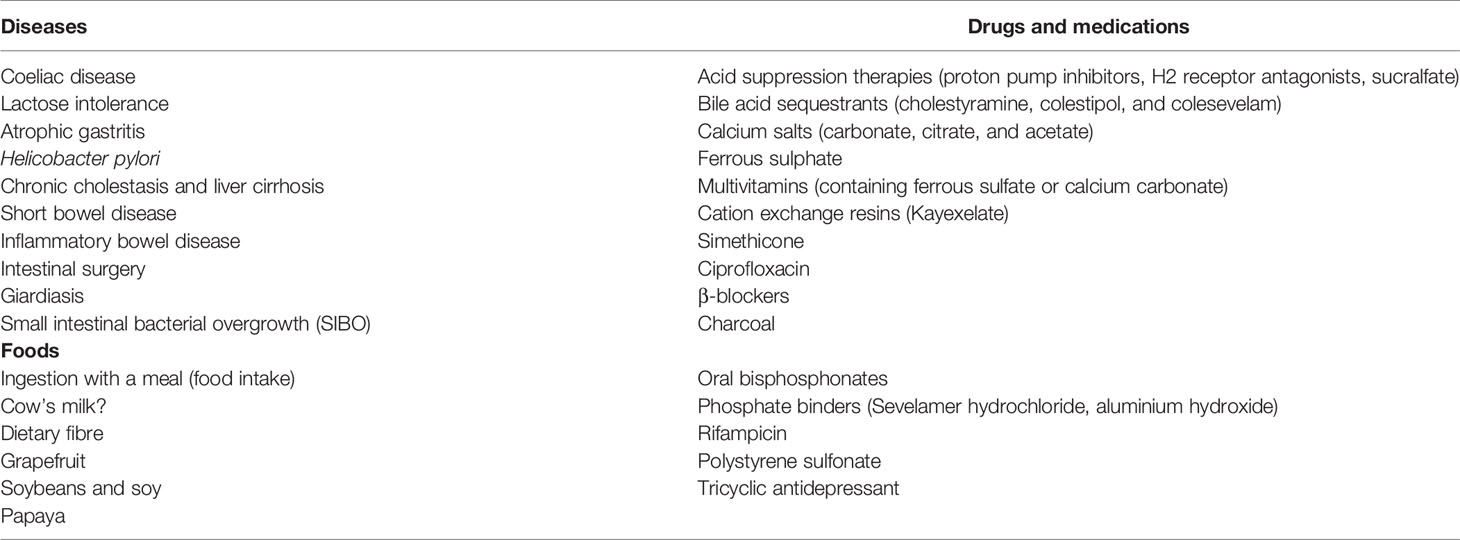
Table 1 Medical conditions, drugs and medications potentially affecting l-T4 absorption in children.
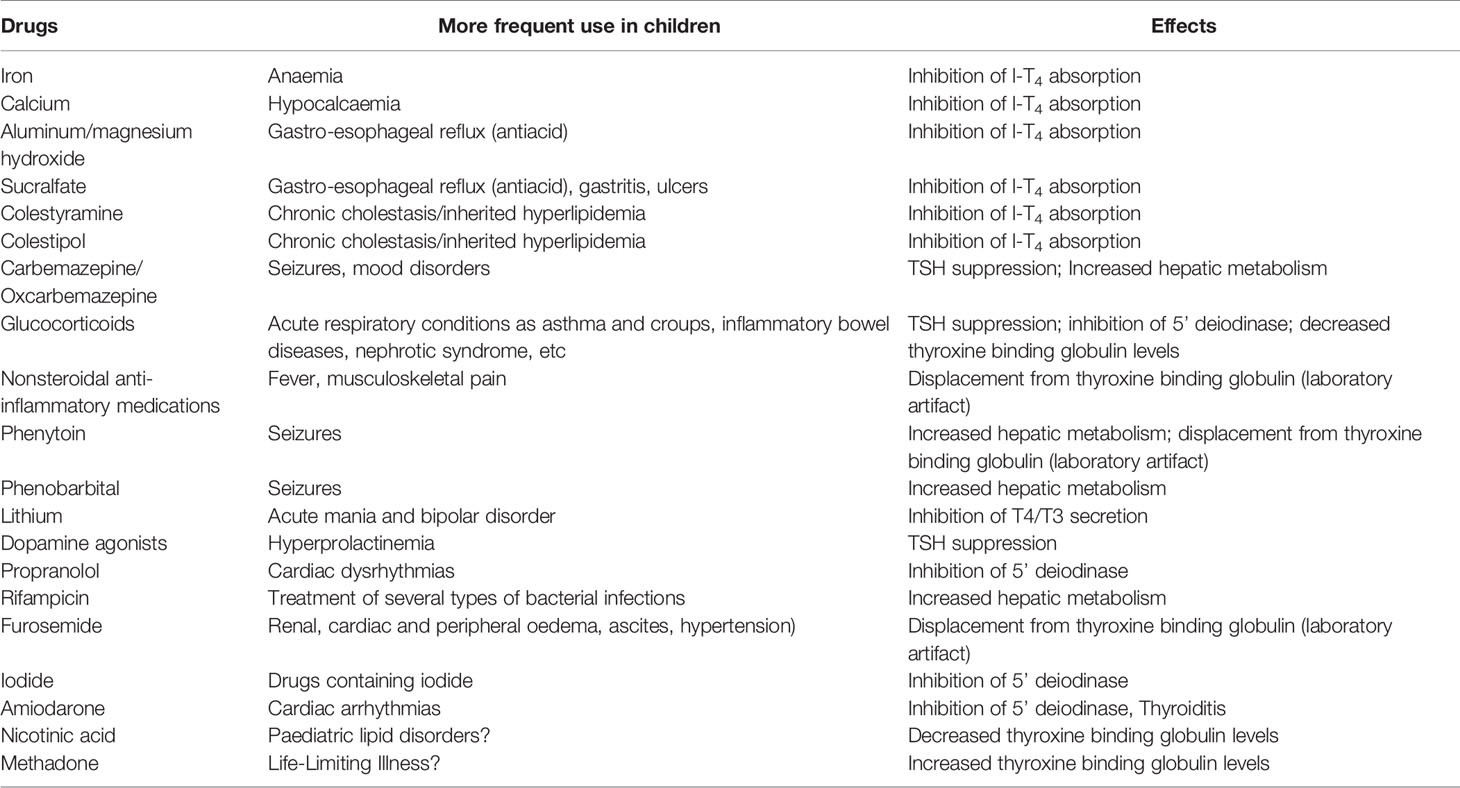
Table 2 Main drugs potentially affecting thyroid function or thyroid hormone levels in patients with congenital hypothyroidism.
Generally, the widely used GCs are not able to induce clinical central hypothyroidism even when used for long periods at high doses. DAs do not typically lead to clinically evident central hypothyroidism, but in patients with nonthyroidal illness they may cause an additional TSH suppression, which could potentially result in iatrogenic central hypothyroidism. Rexinoids, in contrast, frequently induce clinically relevant central hypothyroidism in the majority of subjects, who will require l-T4 treatment and follow up to control serum FT4. This new type of drug is likely to be used on increasing numbers of patients (advanced cancer, metabolic disorders, dermatologic disorders), and it is therefore important to consider the risks of possible side-effects so that they can be managed with treatment.
l-T4 is also the first line treatment for secondary hypothyroidism, which in developing countries is frequently caused by CH, Hashimoto thyroiditis, thyroidectomy, or iodine deficiency (185). The interference of food on l-T4 absorption, as previously mentioned, is known to affect and the efficacy, and in some cases, safety of therapy but there is a lack of awareness among patients and health care workers about this potential problem.
6.1 Glucocorticoids
Glucocorticoids are often used in paediatrics for their immunosuppressant and anti-inflammatory properties, individually or in combination with other drugs both short and long term (186, 187). It is well known that GCs affect serum TSH levels in animals and humans, reducing TSH secretion by directing effecting TRH a hypothalamic level (188). Physiologically, it appears that levels of hydrocortisone have a key role in the diurnal variation of serum TSH, with lower levels in the morning and higher levels at night (189, 190). Some data have shown that high dose GCs may suppress serum TSH in normal subjects and also in hypothyroid patients (191). Nevertheless, long-term high doses of GCs or the cortisol excess typical of Cushing’s syndrome do not seem to result in clinical central hypothyroidism necessitating l-T4 treatment (192). Dexamethasone doses as low as 0.5 mg or prednisone 30 mg can reduce TSH levels significantly (192).
This suppressive effect of GCs on TSH secretion appears to be controlled by the hypothalamus through the inhibition of TRH (193). In humans, GCs receptors are present in the TRH neurons of the paraventricular nucleus and high dose GCs can reduce TRH mRNA levels in the hypothalamus, acting on the TRH gene, probably the primary mechanism for lowering TSH secretion from the hypophysis (194).
The effect of GCs on TSH should be taken into consideration when checking thyroid function in CH patients, in order not to mistake an overtreated condition with a physiological response to GC treatment.
6.2 Dopamine
Dopamine is widely used in critical illnesses to increase blood pressure, cardiac output, urine output, and peripheral perfusion in neonates, infants, and older children with shock and cardiac failure (195).
Dopamine and dopamine agonists can suppress serum TSH and affect the activation of dopamine D2 receptors, reducing TSH pulse amplitude without significantly altering TSH pulse frequency (196, 197). Interestingly, in rats, dopamine stimulates hypothalamic TRH release, but because its overall effect is to lower serum TSH, the inhibitory effect on the pituitary exceeds the first effect (198).
Data on neonates with nonthyroidal illness (NTI) syndrome treated with dopamine infusions indicate that dopamine and NTI have an additive effect on suppressing the HPT axis, placing these individuals at risk of iatrogenic central hypothyroidism (199, 200). It is not clear whether treatment with l-T4 should be given to patients with NTI who are receiving dopamine infusions but, given the thyroid dependence of the central nervous system, the risks of not treating children with CH under 3 years of age should be carefully considered.
6.3 Antiepileptic Drugs
Several drugs used to treat epilepsy such as carbemazepine (CBZ), oxcarbemazepine and valproic acid (VPA) could increase the metabolism of TH through the hepatic P450 system and could also impede pituitary responsiveness to hormonal feedback and cause central hypothyroidism (201, 202). However, several investigations suggest that the hypothalamic-pituitary axis is unaffected by these drugs and a specific mechanism has not been discovered (203). Effects appear to be different in relation to the medications considered. For example, in a study evaluating the use of VPA, CBZ and levetiracetam (LEV), thyroid dysfunction was frequent in children taking VPA or CBZ as a monotherapy, but absent in those taking LEV. The authors suggest that, in children with a predisposition for thyroid disease, LEV should be considered over VPA and CBZ, if appropriate for seizure and epilepsy type (204).
7 Unresolved Questions and Conclusions
Various factors, both exogenous and endogenous, can influence and modify the kinetics of the absorption of l-T4. Pending other targeted studies in the paediatric population, the timing of food intake in relation to l-T4 administration should always be considered as having the potential to interfere with absorption and parents and other caregivers should be advised to delay the consumption of any food for at least 30, and preferably 60 minutes, after taking l-T4.
Caregivers, and also healthcare personnel dealing with CH patients, must be informed about possible interactions with food, drugs and the possible influence of diseases on thyroid function and l-T4 absorption, in order to minimise the negative effects of poorly conducted treatment on the function and development of the central nervous system, especially in the first three years of life.
It is well established that conditions causing malabsorption or reduced gastric acidity can impair the absorption of l-T4 and the sudden onset of apparently resistant hypothyroidism in patients treated with l-T4 may be the only clinically observable feature of some of these disorders.
Drugs may interact with TH levels in patients with CH through several mechanisms, influencing thyroid status at the hypothalamic, pituitary, or thyroid level, or affecting the binding of THs to protein carriers and the conversion of T4 to T3, as well as the final metabolism and recycling of TH. These drugs may also affect the efficiency of exogenous l-T4 treatment, requiring vigilance to prevent both undertreatment and overtreatment and to interpret laboratory findings correctly to avoid inappropriate therapeutic changes.
Data about the interference of different foods on l-T4 absorption in children are very scarce and therefore attention is needed. Liquid formulations are better able to resist interference but all the same, parents and health professionals must remember not to administer l-T4 therapy too close to food.
8 Main Concerns
1. CH is one of the most common preventable causes of ID.
2. Newborn CH screening allows early diagnosis and treatment, significantly reducing the risk of irreversible ID and neurologic damage.
3. Clinicians should be aware that certain forms of cCH or milder forms of primary CH may be missed by newborn screening, and it is therefore fundamental that healthcare works are able to recognise the clinical signs and symptoms of CH in order to avoid erroneous or delayed diagnoses.
4. Prompt diagnosis and treatment with adequate doses of l-T4 leads to excellent neurodevelopmental outcomes in most patients with CH. However, all patients should be closely followed after starting and while under treatment, to monitor their neurological and psychological development.
5. The failure to adequately control CH with oral l-T4 is a frequent clinical problem.
6. Before increasing the l-T4 dose in a patient with CH previously well-controlled, it is mandatory to assess adhesion to treatment.
7. In order to avoid the risk of suboptimal care and patient management, it is essential that caregivers and healthcare personnel are aware of the potential effects of food, drugs and diseases on thyroid function and l-T4 absorption. Possible interferences with l-T4 treatment, should be re-evaluated at each follow-up visit.
8. l-T4 oral solution may have a better absorptive profile than tablets and switching from tablet to liquid l-T4 should be tried before increasing the dose of l-T4.
9. The risk of overtreatment in the first months of life, and especially in the first 4 weeks, makes it fundamental that patients in this age group receive careful follow-up.
Author Contributions
All authors participated in the writing of the manuscript and read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks to Tammy Ann Corkish BA MA for advice on the use of clear English.
References
1. Rastogi MV, LaFranchi SH. Congenital Hypothyroidism. Orphanet J Rare Dis (2010) 5:17. doi: 10.1186/1750-1172-5-17
2. Nazari J, Jafari K, Chegini M, Maleki A, MirShafiei P, Alimohammadi A, et al. Physical and Mental Growth and Development in Children With Congenital Hypothyroidism: A Case-Control Study. Orphanet J Rare Dis (2021) 16(1):393. doi: 10.1186/s13023-021-02017-7
3. Bowden SA, Goldis M. Congenital Hypothyroidism. 2021. In: StatPearls [Internet]. Treasure Island (FL: StatPearls Publishing (2021).
4. Cherella CE, Wassner AJ. Congenital Hypothyroidism: Insights Into Pathogenesis and Treatment. Int J Pediatr Endocrinol (2017) 2017:11. doi: 10.1186/s13633-017-0051-0
5. Prezioso G, Giannini C, Chiarelli F. Effect of Thyroid Hormones on Neurons and Neurodevelopment. Horm Res Paediatr (2018) 90(2):73–81. doi: 10.1159/000492129
6. Tropeano A, Roszkowska AM, Aversa T, Corica D, Pepe G, Aragona P, et al. Ocular Morphology Development and Function in Children With Congenital Hypothyroidism Diagnosed by Neonatal Screening. Endocrine (2021) 72:932–6. doi: 10.1007/s12020-020-02457-4
7. Bruno R, Aversa T, Catena M, Valenzise M, Lombardo F, De Luca F, et al. Even in the Era of Congenital Hypothyroidism Screening Mild and Subclinical Sensorineural Hearing Loss Remains a Relatively Common Complication of Severe Congenital Hypothyroidism. Hear Res (2015) 327:43–7. doi: 10.1016/j.heares.2015.04.018
8. Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal Effects of Maternal Hypothyroxinemia During Early Pregnancy. Pediatrics (2006) 117(1):161–7. doi: 10.1542/peds.2005-0227
9. Brito LNS, Andrade CLO, Alves CAD. Adhesion to Treatment by Children With Congenital Hypothyroidism: Knowledge of Caregivers in Bahia State, Brazil. Rev Paul Pediatr (2021) 39:e2020074. doi: 10.1590/1984-0462/2021/39/2020074
10. Stagi S, Papacciuoli V, Boiro D, Maggioli C, Ndambao NN, Losi S, et al. Auxological and Endocrinological Features in Internationally Adopted Children. Ital J Pediatr (2020) 46(1):82. doi: 10.1186/s13052-020-00832-5
11. Wasniewska M, Arrigo T, Crisafulli G, Aversa T, Messina MF, Salzano G, et al. In the Italian Population Sexual Dimorphism Affects Pre-Natal Thyroid Migration But Not Biochemical Severity of Gland Ectopia and Pre-Natal Bone Maturation. J Endocrinol Invest (2008) 31(4):341–5. doi: 10.1007/BF03346368
12. Mitchell ML, Hsu HW, Sahai I. Massachusetts Pediatric Endocrine Work Group. The Increased Incidence of Congenital Hypothyroidism: Fact or Fancy? Clin Endocrinol (Oxf) (2011) 75(6):806–10. doi: 10.1111/j.1365-2265.2011.04128.x
13. van Trotsenburg P, Stoupa A, Léger J, Rohrer T, Peters C, Fugazzola L, et al. Congenital Hypothyroidism: A 2020-2021 Consensus Guidelines Update-An ENDO-European Reference Network Initiative Endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid (2021) 31(3):387–419. doi: 10.1089/thy.2020.0333
14. Salerno M, Micillo M, Di Maio S, Capalbo D, Ferri P, Lettiero T, et al. Longitudinal Growth, Sexual Maturation and Final Height in Patients With Congenital Hypothyroidism Detected by Neonatal Screening. Eur J Endocrinol (2001) 145(4):377–83. doi: 10.1530/eje.0.1450377
15. Ng SM, Wong SC, Didi M. Head Circumference and Linear Growth During the First 3 Years in Treated Congenital Hypothyroidism in Relation to Aetiology and Initial Biochemical Severity. Clin Endocrinol (Oxf) (2004) 61(1):155–9. doi: 10.1111/j.1365-2265.2004.02087.x
16. Delvecchio M, Salerno M, Acquafredda A, Zecchino C, Fico F, Manca F, et al. Factors Predicting Final Height in Early Treated Congenital Hypothyroid Patients. Clin Endocrinol (Oxf) (2006) 65(5):693–7. doi: 10.1111/j.1365-2265.2006.02651.x
17. Léger J. Congenital Hypothyroidism: A Clinical Update of Long-Term Outcome in Young Adults. Eur J Endocrinol (2015) 172(2):R67–77. doi: 10.1530/EJE-14-0777
18. Aleksander PE, Brückner-Spieler M, Stoehr AM, Lankes E, Kühnen P, Schnabel D, et al. Mean High-Dose L-Thyroxine Treatment Is Efficient and Safe to Achieve a Normal IQ in Young Adult Patients With Congenital Hypothyroidism. J Clin Endocrinol Metab (2018) 103(4):1459–69. doi: 10.1210/jc.2017-01937
19. American Academy of Pediatrics, Rose SRSection on Endocrinology and Committee on Genetics, American Thyroid AssociationBrown RSPublic Health Committee . Update of Newborn Screening and Therapy for Congenital Hypothyroidism. Pediatrics (2006) 117(6):2290–303. doi: 10.1542/peds.2006-0915
20. Cassio A, Corbetta C, Antonozzi I, Calaciura F, Caruso U, Cesaretti G, et al. The Italian Screening Program for Primary Congenital Hypothyroidism: Actions to Improve Screening, Diagnosis, Follow-Up, and Surveillance. J Endocrinol Invest (2013) 36(3):195–203. doi: 10.3275/8849
21. Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. Congenital Hypothyroidism Consensus Conference Group. European Society for Paediatric Endocrinology Consensus Guidelines on Screening, Diagnosis, and Management of Congenital Hypothyroidism. J Clin Endocrinol Metab (2014) 99(2):363–84. doi: 10.1210/jc.2013-1891
22. Wasniewska M, De Luca F, Cassio A, Oggiaro N, Gianino P, Delvecchio M, et al. In Congenital Hypothyroidism Bone Maturation at Birth May Be a Predictive Factor of Psychomotor Development During the First Year of Life Irrespective of Other Variables Related to Treatment. Eur J Endocrinol (2003) 149(1):1–6. doi: 10.1530/eje.0.1490001
23. Bongers-Schokking JJ, de Muinck Keizer-Schrama SM. Influence of Timing and Dose of Thyroid Hormone Replacement on Mental, Psychomotor, and Behavioral Development in Children With Congenital Hypothyroidism. J Pediatr (2005) 147(6):768–74. doi: 10.1016/j.jpeds.2005.09.031
24. Selva KA, Harper A, Downs A, Blasco PA, Lafranchi SH. Neurodevelopmental Outcomes in Congenital Hypothyroidism: Comparison of Initial T4 Dose and Time to Reach Target T4 and TSH. J Pediatr (2005) 147(6):775–80. doi: 10.1016/j.jpeds.2005.07.024
25. Simoneau-Roy J, Marti S, Deal C, Huot C, Robaey P, Van Vliet G. Cognition and Behavior at School Entry in Children With Congenital Hypothyroidism Treated Early With High-Dose Levothyroxine. J Pediatr (2004) 144(6):747–52. doi: 10.1016/j.jpeds.2004.02.021
26. Heyerdahl S, Oerbeck B. Congenital Hypothyroidism: Developmental Outcome in Relation to Levothyroxine Treatment Variables. Thyroid (2003) 13(11):1029–38. doi: 10.1089/105072503770867200
27. Rovet JF, Ehrlich RM. Long-Term Effects of L-Thyroxine Therapy for Congenital Hypothyroidism. J Pediatr (1995) 126(3):380–6. doi: 10.1016/s0022-3476(95)70452-3
28. Rovet J, Alvarez M. Thyroid Hormone and Attention in Congenital Hypothyroidism. J Pediatr Endocrinol Metab (1996) 9(1):63–6. doi: 10.1515/jpem.1996.9.1.63
29. Bongers-Schokking JJ, Resing WCM, Oostdijk W, de Rijke YB, de Muinck Keizer-Schrama SMPF. Relation Between Early Over- and Undertreatment and Behavioural Problems in Preadolescent Children With Congenital Hypothyroidism. Horm Res Paediatr (2018) 90(4):247–56. doi: 10.1159/000494056
30. Selva KA, Mandel SH, Rien L, Sesser D, Miyahira R, Skeels M, et al. Initial Treatment Dose of L-Thyroxine in Congenital Hypothyroidism. J Pediatr (2002) 141(6):786–92. doi: 10.1067/mpd.2002.128887
31. Touati G, Léger J, Toublanc JE, Farriaux JP, Stuckens C, Ponte C, et al. A Thyroxine Dosage of 8 Micrograms/Kg Per Day Is Appropriate for the Initial Treatment of the Majority of Infants With Congenital Hypothyroidism. Eur J Pediatr (1997) 156(2):94–8. doi: 10.1007/s004310050562
32. Pokrovska T, Jones J, Shaikh MG, Smith S, Donaldson MDC. How Well Does the Capillary Thyroid-Stimulating Hormone Test for Newborn Thyroid Screening Predict the Venous Free Thyroxine Level? Arch Dis Child (2016) 101(6):539–45. doi: 10.1136/archdischild-2015-309529
33. Heyerdahl S. Treatment Variables as Predictors of Intellectual Outcome in Children With Congenital Hypothyroidism. Eur J Pediatr (1996) 155(5):357–61. doi: 10.1007/BF01955261
34. Wassner AJ. Congenital Hypothyroidism. Clin Perinatol (2018) 45(1):1–18. doi: 10.1016/j.clp.2017.10.004
35. Oliveira F, Ferreira E. Entry Into the Treatment of the Congenital Hypothyroidism According to Reports of Their Caregivers. Psicol Reflex Crit (2010) 23:19–28. doi: 10.1590/S0102-79722010000100004
36. Kempers MJ, van der Sluijs Veer L, Nijhuis-van der Sanden MW, Kooistra L, Wiedijk BM, Faber I, et al. Intellectual and Motor Development of Young Adults With Congenital Hypothyroidism Diagnosed by Neonatal Screening. J Clin Endocrinol Metab (2006) 91(2):418–24. doi: 10.1210/jc.2005-1209
37. Kooistra L, Laane C, Vulsma T, Schellekens JM, van der Meere JJ, Kalverboer AF. Motor and Cognitive Development in Children With Congenital Hypothyroidism: A Long-Term Evaluation of the Effects of Neonatal Treatment. J Pediatr (1994) 124(6):903–9. doi: 10.1016/s0022-3476(05)83178-6
38. Rovet JF. Children With Congenital Hypothyroidism and Their Siblings: Do They Really Differ? Pediatrics (2005) 115(1):e52–7. doi: 10.1542/peds.2004-1492
39. Salerno M, Militerni R, Bravaccio C, Micillo M, Capalbo D, Di MS, et al. Effect of Different Starting Doses of Levothyroxine on Growth and Intellectual Outcome at Four Years of Age in Congenital Hypothyroidism. Thyroid (2002) 12(1):45–52. doi: 10.1089/105072502753451968
40. Grant DB. Monitoring TSH Concentrations During Treatment for Congenital Hypothyroidism. Arch Dis Child (1991) 66(6):669–70. doi: 10.1136/adc.66.6.669
41. Abusrewil SS, Tyfield L, Savage DC. Serum Thyroxine and Thyroid Stimulating Hormone Concentrations After Treatment of Congenital Hypothyroidism. Arch Dis Child (1988) 63(11):1368–71. doi: 10.1136/adc.63.11.1368
42. Fisher DA, Schoen EJ, La Franchi S, Mandel SH, Nelson JC, Carlton EI, et al. The Hypothalamic-Pituitary-Thyroid Negative Feedback Control Axis in Children With Treated Congenital Hypothyroidism. J Clin Endocrinol Metab (2000) 85(8):2722–7. doi: 10.1210/jcem.85.8.6718
43. New England Congenital Hypothyroidism Collaborative. Characteristics of Infantile Hypothyroidism Discovered on Neonatal Screening. J Pediatr (1984) 104(4):539–44. doi: 10.1016/s0022-3476(84)80543-0
44. New England Congenital Hypothyroidism Collaborative. Correlation of Cognitive Test Scores and Adequacy of Treatment in Adolescents With Congenital Hypothyroidism. J Pediatr (1994) 124(3):383–7. doi: 10.1016/s0022-3476(94)70359-0
45. Alvarez M, Iglesias Fernández C, Rodríguez Sánchez A, Dulín Lñiguez E, Rodríguez Arnao MD. Episodes of Overtreatment During the First Six Months in Children With Congenital Hypothyroidism and Their Relationships With Sustained Attention and Inhibitory Control at School Age. Horm Res Paediatr (2010) 74(2):114–20. doi: 10.1159/000313370
46. Rovet JF. Long-Term Neuropsychological Sequelae of Early-Treated Congenital Hypothyroidism: Effects in Adolescence. Acta Paediatr Suppl (1999) 88(432):88–95. doi: 10.1111/j.1651-2227.1999.tb01168.x
47. Dubuis JM, Glorieux J, Richer F, Deal CL, Dussault JH, Van Vliet G. Outcome of Severe Congenital Hypothyroidism: Closing the Developmental Gap With Early High Dose Levothyroxine Treatment. J Clin Endocrinol Metab (1996) 81(1):222–7. doi: 10.1210/jcem.81.1.8550756
48. Bongers-Schokking JJ, Resing WC, de Rijke YB, de Ridder MA, de Muinck Keizer-Schrama SM. Cognitive Development in Congenital Hypothyroidism: Is Overtreatment a Greater Threat Than Undertreatment? J Clin Endocrinol Metab (2013) 98(11):4499–506. doi: 10.1210/jc.2013-2175
49. Lauffer P, Zwaveling-Soonawala N, Naafs JC, Boelen A, van Trotsenburg ASP. Diagnosis and Management of Central Congenital Hypothyroidism. Front Endocrinol (Lausanne) (2021) 12:686317. doi: 10.3389/fendo.2021.686317
50. Slawik M, Klawitter B, Meiser E, Schories M, Zwermann O, Borm K, et al. Thyroid Hormone Replacement for Central Hypothyroidism: A Randomized Controlled Trial Comparing Two Doses of Thyroxine (T4) With a Combination of T4 and Triiodothyronine. J Clin Endocrinol Metab (2007) 92(11):4115–22. doi: 10.1210/jc.2007-0297
51. Koulouri O, Auldin MA, Agarwal R, Kieffer V, Robertson C, Falconer Smith J, et al. Diagnosis and Treatment of Hypothyroidism in TSH Deficiency Compared to Primary Thyroid Disease: Pituitary Patients Are at Risk of Under-Replacement With Levothyroxine. Clin Endocrinol (Oxf) (2011) 74(6):744–9. doi: 10.1111/j.1365-2265.2011.03984.x
52. Persani L, Brabant G, Dattani M, Bonomi M, Feldt-Rasmussen U, Fliers E, et al. European Thyroid Association (ETA) Guidelines on the Diagnosis and Management of Central Hypothyroidism. Eur Thyroid J (2018) 7(5):225–37. doi: 10.1159/000491388
53. Naafs JC, Verkerk PH, Fliers E, van Trotsenburg ASP, Zwaveling-Soonawala N. Clinical and Genetic Characteristics of Dutch Children With Central Congenital Hypothyroidism, Early Detected by Neonatal Screening. Eur J Endocrinol (2020) 183(6):627–36. doi: 10.1530/EJE-20-0833
54. Naafs JC, Vendrig LM, Limpens J, van der Lee HJ, Duijnhoven RG, Marchal JP, et al. Cognitive Outcome in Congenital Central Hypothyroidism: A Systematic Review With Meta-Analysis of Individual Patient Data. Eur J Endocrinol (2020) 182(3):351–61. doi: 10.1530/EJE-19-0874
55. Roberts GW. Taking Care of Thyroxine. Aust Prescr (2004) 27:75–6. doi: 10.18773/austprescr.2004.061
56. Colucci P, Yue CS, Ducharme M, Benvenga S. A Review of the Pharmacokinetics of Levothyroxine for the Treatment of Hypothyroidism. Eur Endocrinol (2013) 9(1):40–7. doi: 10.17925/EE.2013.09.01.40
57. Virili C, Antonelli A, Santaguida MG, Benvenga S, Centanni M. Gastrointestinal Malabsorption of Thyroxine. Endocr Rev (2019) 40(1):118–36. doi: 10.1210/er.2018-00168
58. Centanni M. Thyroxine Treatment: Absorption, Malabsorption, and Novel Therapeutic Approaches. Endocrine (2013) 43(1):8–9. doi: 10.1007/s12020-012-9814-9
59. Ward LS. The Difficult Patient: Drug Interaction and the Influence of Concomitant Diseases on the Treatment of Hypothyroidism. Arq Bras Endocrinol Metabol (2010) 54(5):435–42. doi: 10.1590/s0004-27302010000500002
60. Bach-Huynh TG, Nayak B, Loh J, Soldin S, Jonklaas J. Timing of Levothyroxine Administration Affects Serum Thyrotropin Concentration. J Clin Endocrinol Metab (2009) 94(10):3905–12. doi: 10.1210/jc.2009-0860
61. Ianiro G, Mangiola F, Di Rienzo TA, Bibbò S, Franceschi F, Greco AV, et al. Levothyroxine Absorption in Health and Disease, and New Therapeutic Perspectives. Eur Rev Med Pharmacol Sci (2014) 18(4):451–6.
62. Irving SA, Vadiveloo T, Leese GP. Drugs That Interact With Levothyroxine: An Observational Study From the Thyroid Epidemiology, Audit and Research Study (TEARS). Clin Endocrinol (Oxf) (2015) 82(1):136–41. doi: 10.1111/cen.12559
63. Dietrich JW, Gieselbrecht K, Holl RW, Boehm BO. Absorption Kinetics of Levothyroxine Is Not Altered by Proton-Pump Inhibitor Therapy. Horm Metab Res (2006) 38(1):57–9. doi: 10.1055/s-2006-924980
64. Yue CS, Scarsi C, Ducharme MP. Pharmacokinetics and Potential Advantages of a New Oral Solution of Levothyroxine vs. Other Available Dosage Forms. Arzneimittelforschung (2012) 62(12):631–6. doi: 10.1055/s-0032-1329951
65. Fallahi P, Ferrari SM, Antonelli A. Oral L-Thyroxine Liquid Versus Tablet in Patients With Hypothyroidism Without Malabsorption: A Prospective Study. Endocrine (2016) 52(3):597–601. doi: 10.1007/s12020-015-0836-y
66. Virili C, Giovanella L, Fallahi P, Antonelli A, Santaguida MG, Centanni M, et al. Levothyroxine Therapy: Changes of TSH Levels by Switching Patients from Tablet to Liquid Formulation. A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) (2018) 9:10. doi: 10.3389/fendo.2018.00010
67. Vita R, Saraceno G, Trimarchi F, Benvenga S. Switching Levothyroxine From the Tablet to the Oral Solution Formulation Corrects the Impaired Absorption of Levothyroxine Induced by Proton-Pump Inhibitors. J Clin Endocrinol Metab (2014) 99(12):4481–6. doi: 10.1210/jc.2014-2684
68. Vita R, Di Bari F, Benvenga S. Oral Liquid Levothyroxine Solves the Problem of Tablet Levothyroxine Malabsorption Due to Concomitant Intake of Multiple Drugs. Expert Opin Drug Deliv (2017) 14(4):467–72. doi: 10.1080/17425247.2017.1290604
69. Pabla D, Akhlaghi F, Zia H. A Comparative pH-Dissolution Profile Study of Selected Commercial Levothyroxine Products Using Inductively Coupled Plasma Mass Spectrometry. Eur J Pharm Biopharm (2009) 72(1):105–10. doi: 10.1016/j.ejpb.2008.10.008
70. Santaguida MG, Virili C, Del Duca SC, Cellini M, Gatto I, Brusca N, et al. Thyroxine Softgel Capsule in Patients With Gastric-Related T4 Malabsorption. Endocrine (2015) 49(1):51–7. doi: 10.1007/s12020-014-0476-7
71. Trimboli P, Virili C, Centanni M, Giovanella L. Thyroxine Treatment With Softgel Capsule Formulation: Usefulness in Hypothyroid Patients Without Malabsorption. Front Endocrinol (Lausanne) (2018) 9:118. doi: 10.3389/fendo.2018.00118
72. Haugen BR. Drugs That Suppress TSH or Cause Central Hypothyroidism. Best Pract Res Clin Endocrinol Metab (2009) 23(6):793–800. doi: 10.1016/j.beem.2009.08.003
73. Wartofsky L, Dickey RA. The Evidence for a Narrower Thyrotropin Reference Range Is Compelling. J Clin Endocrinol Metab (2005) 90(9):5483–8. doi: 10.1210/jc.2005-0455
74. Benvenga S, Bartolone L, Squadrito S, Lo Giudice F, Trimarchi F. Delayed Intestinal Absorption of Levothyroxine. Thyroid (1995) 5(4):249–53. doi: 10.1089/thy.1995.5.249
75. Wenzel KW, Kirschsieper HE. Aspects of the Absorption of Oral L-Thyroxine in Normal Man. Metabolism (1977) 26(1):1–8. doi: 10.1016/0026-0495(77)90121-4
76. Lamson MJ, Pamplin CL, Rolleri RL, Klein I. Quantitation of a Substantial Reduction in Levothyroxine (T4) Absorption by Food. Thyroid (2004) 14:876. doi: 10.1089/thy.2004.14.873
77. Seechurn S, Sharma S, Oyibo S. Administration of Levothyroxine 45—60 Minutes Before Breakfast Improves Biochemical Availability as Evidenced by Reduced Thyrotropin Levels. Open J Endocr Metab Dis (2012) 2:36–9. doi: 10.4236/ojemd.2012.23005
78. Perez CL, Araki FS, Graf H, de Carvalho GA. Serum Thyrotropin Levels Following Levothyroxine Administration at Breakfast. Thyroid (2013) 23(7):779–84. doi: 10.1089/thy.2012.0435
79. LaFranchi SH, Austin J. How Should We Be Treating Children With Congenital Hypothyroidism? J Pediatr Endocrinol Metab (2007) 20(5):559–78. doi: 10.1515/jpem.2007.20.5.559
80. Bernareggi A, Grata E, Pinorini MT, Conti A. Oral Liquid Formulation of Levothyroxine Is Stable in Breakfast Beverages and May Improve Thyroid Patient Compliance. Pharmaceutics (2013) 5(4):621–33. doi: 10.3390/pharmaceutics5040621
81. Marina M, Ceda GP, Aloe R, Gnocchi C, Ceresini G. Circulating Concentrations of Free Thyroxine After an Oral Intake of Liquid LT4 Taken Either During Fasting Conditions or at Breakfast. Acta Biomed (2016) 87(3):247–52.
82. Morelli S, Reboldi G, Moretti S, Menicali E, Avenia N, Puxeddu E. Timing of Breakfast Does Not Influence Therapeutic Efficacy of Liquid Levothyroxine Formulation. Endocrine (2016) 52(3):571–8. doi: 10.1007/s12020-015-0788-2
83. Cappelli C, Pirola I, Daffini L, Formenti A, Iacobello C, Cristiano A, et al. A Double-Blind Placebo-Controlled Trial of Liquid Thyroxine Ingested at Breakfast: Results of the TICO Study. Thyroid (2016) 26(2):197–202. doi: 10.1089/thy.2015.0422
84. Pirola I, Gandossi E, Brancato D, Marini F, Cristiano A, Delbarba A, et al. TSH Evaluation in Hypothyroid Patients Assuming Liquid Levothyroxine at Breakfast or 30 Min Before Breakfast. J Endocrinol Invest (2018) 41(11):1301–6. doi: 10.1007/s40618-018-0867-3
85. Cassio A, Monti S, Rizzello A, Bettocchi I, Baronio F, D'Addabbo G, et al. Comparison Between Liquid and Tablet Formulations of Levothyroxine in the Initial Treatment of Congenital Hypothyroidism. J Pediatr (2013) 162(6):1264–91269.e1–2. doi: 10.1016/j.jpeds.2012.11.070
86. Peroni E, Vigone MC, Mora S, Bassi LA, Pozzi C, Passoni A, et al. Congenital Hypothyroidism Treatment in Infants: A Comparative Study Between Liquid and Tablet Formulations of Levothyroxine. Horm Res Paediatr (2014) 81(1):50–4. doi: 10.1159/000356047
87. Vigone MC, Ortolano R, Vincenzi G, Pozzi C, Ratti M, Assirelli V, et al. Treatment of Congenital Hypothyroidism: Comparison Between L-Thyroxine Oral Solution and Tablet Formulations Up to 3 Years of Age. Eur J Endocrinol (2021) 186(1):45–52. doi: 10.1530/EJE-20-1444
88. Pruitt AW, Anyan WR, Hill RM, Kauffman RE, Mofenson HC, Rumack BH, et al. American Academy of Pediatrics Committee on Drugs: Ethanol in Liquid Preparations Intended for Children. Pediatrics (1984) 73(3):405–7. doi: 10.1542/peds.73.3.405
89. Flores-Huerta S, Hernández-Montes H, Argote RM, Villalpando S. Effects of Ethanol Consumption During Pregnancy and Lactation on the Outcome and Postnatal Growth of the Offspring. Ann Nutr Metab (1992) 36(3):121–8. doi: 10.1159/000177706
90. Little RE, Northstone K, Golding J, ALSPAC Study Team. Alcohol, Breastfeeding, and Development at 18 Months. Pediatrics (2002) 109(5):E72–2. doi: 10.1542/peds.109.5.e72
91. O'Leary CM, Nassar N, Zubrick SR, Kurinczuk JJ, Stanley F, Bower C. Evidence of a Complex Association Between Dose, Pattern and Timing of Prenatal Alcohol Exposure and Child Behaviour Problems. Addiction (2010) 105(1):74–86. doi: 10.1111/j.1360-0443.2009.02756.x
92. Tzifi F, Iliadi A, Voutetakis A, Platis D, Girginoudis P, Kanaka-Gantenbein C. Non-Inferiority of Liquid Thyroxine in Comparison to Tablets Formulation in the Treatment of Children With Congenital Hypothyroidism. J Pediatr Endocrinol Metab (2021) 35(2):239–47. doi: 10.1515/jpem-2021-0458
93. Ng SM, Turner MA, Gamble C, Didi M, Victor S, Manning D, et al. An Explanatory Randomised Placebo Controlled Trial of Levothyroxine Supplementation for Babies Born <28 Weeks' Gestation: Results of the TIPIT Trial. Trials (2013) 14:211. doi: 10.1186/1745-6215-14-211
94. Vita R, Fallahi P, Antonelli A, Benvenga S. The Administration of L-Thyroxine as Soft Gel Capsule or Liquid Solution. Expert Opin Drug Deliv (2014) 11(7):1103–11. doi: 10.1517/17425247.2014.918101
95. Cappelli C, Pirola I, Gandossi E, Cristiano A, Daffini L, Agosti B, et al. Thyroid Hormone Profile in Patients Ingesting Soft Gel Capsule or Liquid Levothyroxine Formulations With Breakfast. Int J Endocrinol (2016) 2016:9043450. doi: 10.1155/2016/9043450
96. Vita R, Saraceno G, Trimarchi F, Benvenga S. A Novel Formulation of L-Thyroxine (L-T4) Reduces the Problem of L-T4 Malabsorption by Coffee Observed With Traditional Tablet Formulations. Endocrine (2013) 43(1):154–60. doi: 10.1007/s12020-012-9772-2
97. Benvenga S, Capodicasa G, Perelli S. L-Thyroxine in an Oral Liquid or Softgel Formulation Ensures More Normal Serum Levels of Free T4 in Patients With Central Hypothyroidism. Front Endocrinol (Lausanne) (2017) 8:321. doi: 10.3389/fendo.2017.00321
98. Hennessey JV. The Emergence of Levothyroxine as a Treatment for Hypothyroidism. Endocrine (2017) 55(1):6–18. doi: 10.1007/s12020-016-1199-8
99. Centanni M, Benvenga S, Sachmechi I. Diagnosis and Management of Treatment-Refractory Hypothyroidism: An Expert Consensus Report. J Endocrinol Invest (2017) 40(12):1289–301. doi: 10.1007/s40618-017-0706-y
100. Wiesner A, Gajewska D, Paśko P. Levothyroxine Interactions With Food and Dietary Supplements-A Systematic Review. Pharmaceut (Basel) (2021) 14(3):206. doi: 10.3390/ph14030206
101. Skelin M, Lucijanić T, Amidžić Klarić D, Rešić A, Bakula M, Liberati-Čizmek AM, et al. Factors Affecting Gastrointestinal Absorption of Levothyroxine: A Review. Clin Ther (2017) 39(2):378–403. doi: 10.1016/j.clinthera.2017.01.005
102. McMillan M, Rotenberg KS, Vora K, Sterman AB, Thevathasan L, Ryan MF, et al. Comorbidities, Concomitant Medications, and Diet as Factors Affecting Levothyroxine Therapy: Results of the CONTROL Surveillance Project. Drugs R D (2016) 16(1):53–68. doi: 10.1007/s40268-015-0116-6
103. Michel R, Neafsey P. Self Medication Practices Among Patients Taking Levothyroxine. Internet J Adv Nurs Pract (2012) 6:2–9.
104. Agostoni C, Esposito S, Nobili A. Dietary Supplements in Infants and Children: Only Beneficial? J Pediatr Gastroenterol Nutr (2016) 63(2):177–80. doi: 10.1097/MPG.0000000000001180
105. Castellana M, Castellana C, Giovanella L, Trimboli P. Prevalence of Gastrointestinal Disorders Having an Impact on Tablet Levothyroxine Absorption: Should This Formulation Still be Considered as the First-Line Therapy? Endocrine (2020) 67(2):281–90. doi: 10.1007/s12020-019-02185-4
106. Chon DA, Reisman T, Weinreb JE, Hershman JM, Leung AM. Concurrent Milk Ingestion Decreases Absorption of Levothyroxine. Thyroid (2018) 28(4):454–7. doi: 10.1089/thy.2017.0428
107. Bhatia J, Greer F, American Academy of Pediatrics Committee on Nutrition. Use of Soy Protein-Based Formulas in Infant Feeding. Pediatrics (2008) 121(5):1062–8. doi: 10.1542/peds.2008-0564
108. Wyk JJ, Arnold MB, Wynn J, Pepper F. The Effects of a Soybean Product on Thyroid Function in Humans. Pediatrics (1959) 24:752–60. doi: 10.1542/peds.24.5.752
109. Hydovitz JD. Occurrence of Goiter in an Infant on a Soy Diet. N Engl J Med (1960) 262:351–3. doi: 10.1056/NEJM196002182620707
110. Pinchera A, Macgillivray MH, Crawford JD, Freeman AG. Thyroid Refractoriness in an Athyreotic Cretin Fed Soybean Formula. N Engl J Med (1965) 273:83–7. doi: 10.1056/NEJM196507082730205
111. Chorazy PA, Himelhoch S, Hopwood NJ, Greger NG, Postellon DC. Persistent Hypothyroidism in an Infant Receiving a Soy Formula: Case Report and Review of the Literature. Pediatrics (1995) 96(1 Pt 1):148–50. doi: 10.1542/peds.96.1.148
112. Fruzza AG, Demeterco-Berggren C, Jones KL. Unawareness of the Effects of Soy Intake on the Management of Congenital Hypothyroidism. Pediatrics (2012) 130(3):e699–702. doi: 10.1542/peds.2011-3350
113. Conrad SC, Chiu H, Silverman BL. Soy Formula Complicates Management of Congenital Hypothyroidism. Arch Dis Child (2004) 89(1):37–40. doi: 10.1136/adc.2002.009365
114. Divi RL, Doerge DR. Inhibition of Thyroid Peroxidase by Dietary Flavonoids. Chem Res Toxicol (1996) 9(1):16–23. doi: 10.1021/tx950076m
115. Messina M, Redmond G. Effects of Soy Protein and Soybean Isoflavones on Thyroid Function in Healthy Adults and Hypothyroid Patients: A Review of the Relevant Literature. Thyroid (2006) 16(3):249–58. doi: 10.1089/thy.2006.16.249
116. Kinne A, Schülein R, Krause G. Primary and Secondary Thyroid Hormone Transporters. Thyroid Res (2011) 4 Suppl 1(Suppl 1):S7. doi: 10.1186/1756-6614-4-S1-S7
117. Bailey DG. Fruit Juice Inhibition of Uptake Transport: A New Type of Food-Drug Interaction. Br J Clin Pharmacol (2010) 70(5):645–55. doi: 10.1111/j.1365-2125.2010.03722.x
118. Lilja JJ, Laitinen K, Neuvonen PJ. Effects of Grapefruit Juice on the Absorption of Levothyroxine. Br J Clin Pharmacol (2005) 60(3):337–41. doi: 10.1111/j.1365-2125.2005.02433.x
119. Meyer Zu Schwabedissen HE, Ferreira C, Schaefer AM, Oufir M, Seibert I, Hamburger M, et al. Thyroid Hormones Are Transport Substrates and Transcriptional Regulators of Organic Anion Transporting Polypeptide 2b1. Mol Pharmacol (2018) 94(1):700–12. doi: 10.1124/mol.117.111161
120. Tesic D, Stokic E, Medic/Stojanoska M, Mitrovic M, Tomic-Naglic D, Icin T, et al. Tea and Juice as a Causes of Levothyroxine Malabsorption and Intermittent Hypothyreoidism: A Case Report. Endocr Abstr (2018) 3947:3–4. doi: 10.1530/endoabs.56.p996
121. Deiana L, Marini S, Mariotti S. Ingestion of Large Amounts of Papaya Fruit and Impaired Effectiveness of Levothyroxine Therapy. Endocr Pract (2012) 18(1):98–100. doi: 10.4158/EP11233.CO
122. Pasman EA, Ong B, Witmer CP, Nylund CM. Proton Pump Inhibitors in Children: The Good, the Bad, and the Ugly. Curr Allergy Asthma Rep (2020) 20(8):39. doi: 10.1007/s11882-020-00926-4
123. Centanni M, Gargano L, Canettieri G, Viceconti N, Franchi A, Delle Fave G, et al. Thyroxine in Goiter, Helicobacter Pylori Infection, and Chronic Gastritis. N Engl J Med (2006) 354(17):1787–95. doi: 10.1056/NEJMoa043903
124. Tortora A, La Sala D, Vitale M. Switch From Tablet Levothyroxine to Oral Solution Resolved Reduced Absorption by Intestinal Parasitosis. Endocrinol Diabetes Metab Case Rep (2019) 2019:19–0026. doi: 10.1530/EDM-19-0026
125. Liwanpo L, Hershman JM. Conditions and Drugs Interfering With Thyroxine Absorption. Best Pract Res Clin Endocrinol Metab (2009) 23(6):781–92. doi: 10.1016/j.beem.2009.06.006
126. Stagi S, Manoni C, Cecchi C, Chiarelli F, de Martino M. Increased Risk of Coeliac Disease in Patients With Congenital Hypothyroidism. Horm Res Paediatr (2011) 76(3):186–92. doi: 10.1159/000328723
127. McDermott JH, Coss A, Walsh CH. Celiac Disease Presenting as Resistant Hypothyroidism. Thyroid (2005) 15(4):386–8. doi: 10.1089/thy.2005.15.386
128. Franzese A, Limauro R, Ecuba P, Campanile F, De Martino F, Tenore A. Malassorbimento Di L-T4 Determinato Da Intolleranza Alle Proteine Del Latte Vaccino E Malattia Celiaca in Paziente Affetto Da Ipotiroidismo Congenito. Caso Clinico [L-T4 Malabsorption Determined by Intolerance to Cow's Milk Proteins and Celiac Disease in a Patient With Congenital Hypothyroidism. A Clinical Case]. Minerva Pediatr (1993) 45(3):113–6.
129. Muñoz-Torres M, Varsavsky M, Alonso G. Lactose Intolerance Revealed by Severe Resistance to Treatment With Levothyroxine. Thyroid (2006) 16(11):1171–3. doi: 10.1089/thy.2006.16.1171
130. Balapatabendi M, Harris D, Shenoy SD. Drug Interaction of Levothyroxine With Infant Colic Drops. Arch Dis Child (2011) 96(9):888–9. doi: 10.1136/archdischild-2011-300333
131. Salvia G, Guarino A, Terrin G, Cascioli C, Paludetto R, Indrio F, et al. Neonatal Onset Intestinal Failure: An Italian Multicenter Study. J Pediatr (2008) 153(5):674–6676.e1–2. doi: 10.1016/j.jpeds.2008.05.017
132. Martín-Masot R, Molina Arias M, Díaz Martín JJ, Cilleruelo Pascual ML, Gutiérrez Junquera C, Donat E, et al. Management of Small Intestinal Bacterial Overgrowth by Pediatric Gastroenterologists in Spain. Rev Esp Enferm Dig (2021) 113(6):436–41. doi: 10.17235/reed.2020.7582/2020
133. Bohinc Henderson B. Levothyroxine Sodium Oral Solution Normalizes Thyroid Function in a Patient With Hashimoto's Disease, Gastritis, Diabetic Gastroparesis, and Small Intestinal Bacterial Overgrowth (SIBO). Int Med Case Rep J (2021) 14:627–32. doi: 10.2147/IMCRJ.S326481
134. Chiu AC, Sherman SI. Effects of Pharmacological Fiber Supplements on Levothyroxine Absorption. Thyroid (1998) 8(8):667–71. doi: 10.1089/thy.1998.8.667
135. Passos ACV, Barros F, Damiani D, Semer B, Cespedes WCJ, Sannicola B, et al. Hypothyroidism Associated With Short Bowel Syndrome in Children: A Report of Six Cases. Arch Endocrinol Metab (2018) 62(6):655–60. doi: 10.20945/2359-3997000000093
136. Liel Y, Harman-Boehm I, Shany S. Evidence for a Clinically Important Adverse Effect of Fiber-Enriched Diet on the Bioavailability of Levothyroxine in Adult Hypothyroid Patients. J Clin Endocrinol Metab (1996) 81(2):857–9. doi: 10.1210/jcem.81.2.8636317
137. Levy I, Attias S, Ben-Arye E, Goldstein L, Schiff E. Adverse Events Associated With Interactions With Dietary and Herbal Supplements Among Inpatients. Br J Clin Pharmacol (2017) 83(4):836–45. doi: 10.1111/bcp.13158
138. Liel Y, Sperber AD, Shany S. Nonspecific Intestinal Adsorption of Levothyroxine by Aluminum Hydroxide. Am J Med (1994) 97(4):363–5. doi: 10.1016/0002-9343(94)90303-4
139. Mantovani G, Elli FM, Corbetta S. Hypothyroidism Associated With Parathyroid Disorders. Best Pract Res Clin Endocrinol Metab (2017) 31(2):161–73. doi: 10.1016/j.beem.2017.04.004
140. Gaudino R, Garel C, Czernichow P, Léger J. Proportion of Various Types of Thyroid Disorders Among Newborns With Congenital Hypothyroidism and Normally Located Gland: A Regional Cohort Study. Clin Endocrinol (Oxf) (2005) 62(4):444–8. doi: 10.1111/j.1365-2265.2005.02239.x
141. Çakır U, Tayman C. The Effect of Thyroid Functions on Osteopenia of Prematurity in Preterm Infants. J Pediatr Endocrinol Metab (2019) 32(1):65–70. doi: 10.1515/jpem-2018-0429
142. Singh N, Singh PN, Hershman JM. Effect of Calcium Carbonate on the Absorption of Levothyroxine. JAMA (2000) 283(21):2822–5. doi: 10.1001/jama.283.21.2822
143. Mazokopakis EE, Giannakopoulos TG, Starakis IK. Interaction Between Levothyroxine and Calcium Carbonate. Can Fam Phys (2008) 54(1):39.
144. Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. Effect of Phosphate Binders Upon TSH and L-Thyroxine Dose in Patients on Thyroid Replacement. Int Urol Nephrol (2007) 39(2):599–602. doi: 10.1007/s11255-006-9166-6
145. Zamfirescu I, Carlson HE. Absorption of Levothyroxine When Coadministered With Various Calcium Formulations. Thyroid (2011) 21(5):483–6. doi: 10.1089/thy.2010.0296
146. Morini E, Catalano A, Lasco A, Morabito N, Benvenga S. L-Thyroxine Malabsorption Due to Calcium Carbonate Impairs Blood Pressure, Total Cholesterolemia, and Fasting Glycemia. Endocrine (2019) 64(2):284–92. doi: 10.1007/s12020-018-1798-7
147. Benvenga S, Di Bari F, Vita R. Undertreated Hypothyroidism Due to Calcium or Iron Supplementation Corrected by Oral Liquid Levothyroxine. Endocrine (2017) 56(1):138–45. doi: 10.1007/s12020-017-1244-2
148. Mazokopakis EE. Counseling Patients Receiving Levothyroxine (L-T4) and Calcium Carbonate. Mil Med (2006) 171(11):vii, 1094.
149. Wang M. Iron Deficiency and Other Types of Anemia in Infants and Children. Am Fam Phys (2016) 93(4):270–8.
150. Craig WJ, Mangels AR, Fresán U, Marsh K, Miles FL, Saunders AV, et al. The Safe and Effective Use of Plant-Based Diets With Guidelines for Health Professionals. Nutrients (2021) 13(11):4144. doi: 10.3390/nu13114144
151. Soliman AT, De Sanctis V, Yassin M, Wagdy M, Soliman N. Chronic Anemia and Thyroid Function. Acta Biomed (2017) 88(1):119–27. doi: 10.23750/abm.v88i1.6048
152. Franzese A, Salerno M, Argenziano A, Buongiovanni C, Limauro R, Tenore A. Anemia in Infants With Congenital Hypothyroidism Diagnosed by Neonatal Screening. J Endocrinol Invest (1996) 19(9):613–9. doi: 10.1007/BF03349027
153. Chu JY, Monteleone JA, Peden VH, Graviss ER, Vernava AM. Anemia in Children and Adolescents With Hypothyroidism. Clin Pediatr (Phila) (1981) 20(11):696–9. doi: 10.1177/000992288102001102
154. Gökdeniz E, Demir C, Dilek I. The Effects of Iron Deficiency Anemia on the Thyroid Functions. J Clin Exp Invest (2010) 1:156–60. doi: 10.5799/ahinjs.01.2010.03.0033
155. Metwalley KA, Farghaly HS, Hassan AF. Thyroid Status in Egyptian Primary School Children With Iron Deficiency Anemia: Relationship to Intellectual Function. Thyroid Res Pract (2013) 10:91–5. doi: 10.4103/0973-0354.116131
156. Campbell NR, Hasinoff BB, Stalts H, Rao B, Wong NC. Ferrous Sulfate Reduces Thyroxine Efficacy in Patients With Hypothyroidism. Ann Intern Med (1992) 117(12):1010–3. doi: 10.7326/0003-4819-117-12-1010
157. Fiaux E, Kadri K, Levasseur C, Le Guillou C, Chassagne P. Hypothyroïdie Secondaire À Une Interaction Médicamenteuse Entre Lévothyroxine Et Sels De Fer [Hypothyroidism as the Result of Drug Interaction Between Ferrous Sulfate and Levothyroxine]. Rev Med Interne (2010) 31(10):e4–5. doi: 10.1016/j.revmed.2009.09.038
158. Shakir KM, Chute JP, Aprill BS, Lazarus AA. Ferrous Sulfate-Induced Increase in Requirement for Thyroxine in a Patient With Primary Hypothyroidism. South Med J (1997) 90(6):637–9. doi: 10.1097/00007611-199706000-00011
159. Leger CS, Ooi TC. Ferrous Fumarate-Induced Malabsorption of Thyroxine. Endocrinologist (1999) 9:493–55. doi: 10.1097/00019616-199911000-00011
160. Atruktsang TS, Zaborek NA, Imbus JR, Long K, Pitt SC, Sippel RS, et al. Identifying Predictors of Prolonged Levothyroxine Dose Adjustment After Thyroidectomy. J Surg Res (2019) 242:166–71. doi: 10.1016/j.jss.2019.03.049
161. Mersebach H, Rasmussen AK, Kirkegaard L, Feldt-Rasmussen U. Intestinal Adsorption of Levothyroxine by Antacids and Laxatives: Case Stories and In Vitro Experiments. Pharmacol Toxicol (1999) 84(3):107–9. doi: 10.1111/j.1600-0773.1999.tb00883.x
162. Teas J, Pino S, Critchley A, Braverman LE. Variability of Iodine Content in Common Commercially Available Edible Seaweeds. Thyroid (2004) 14(10):836–41. doi: 10.1089/thy.2004.14.836
163. Belfort MB, Pearce EN, Braverman LE, He X, Brown RS. Low Iodine Content in the Diets of Hospitalized Preterm Infants. J Clin Endocrinol Metab (2012) 97(4):E632–6. doi: 10.1210/jc.2011-3369
164. Wolff J, Chaikoff IL. Plasma Inorganic Iodide as a Homeostatic Regulator of Thyroid Function. J Biol Chem (1948) 174(2):555–64. doi: 10.1016/S0021-9258(18)57335-X
165. Groufh-Jacobsen S, Hess SY, Aakre I, Folven Gjengedal EL, Blandhoel Pettersen K, Henjum S. Vegans, Vegetarians and Pescatarians Are at Risk of Iodine Deficiency in Norway. Nutrients (2020) 12(11):3555. doi: 10.3390/nu12113555
166. Kubicki R, Grohmann J, Kunz KG, Stiller B, Schwab KO, van der Werf-Grohmann N. Frequency of Thyroid Dysfunction in Pediatric Patients With Congenital Heart Disease Exposed to Iodinated Contrast Media - A Long-Term Observational Study. J Pediatr Endocrinol Metab (2020) 33(11):1409–15. doi: 10.1515/jpem-2020-0032
167. Koukkou EG, Roupas ND, Markou KB. Effect of Excess Iodine Intake on Thyroid on Human Health. Minerva Med (2017) 108(2):136–46. doi: 10.23736/S0026-4806.17.04923-0
168. Smerdely P, Lim A, Boyages SC, Waite K, Wu D, Roberts V, et al. Topical Iodine-Containing Antiseptics and Neonatal Hypothyroidism in Very-Low-Birthweight Infants. Lancet (1989) 2(8664):661–4. doi: 10.1016/s0140-6736(89)90903-3
169. Köhrle J. Selenium and the Thyroid. Curr Opin Endocrinol Diabetes Obes (2015) 22(5):392–401. doi: 10.1097/MED.0000000000000190
170. Jubiz W, Ramirez M. Effect of Vitamin C on the Absorption of Levothyroxine in Patients With Hypothyroidism and Gastritis. J Clin Endocrinol Metab (2014) 99(6):E1031–4. doi: 10.1210/jc.2013-4360
171. Antúnez P, Licht SD. Vitamin C Improves the Apparent Absorption of Levothyroxine in a Subset of Patients Receiving This Hormone for Primary Hypothyroidism. Rev Argent Endocrinol Metab (2011) 48:16–24.
172. Feldt MM. Delayed Diagnosis of Congenital Hypothyroidism in a Child With Trisomy 21 and Biotinidase Deficiency and Successful Use of Levothyroxine Sodium Oral Solution. Case Rep Endocrinol (2020) 2020:8883969. doi: 10.1155/2020/8883969
173. Nagafuji M, Hitaka D, Iwabuchi A, Miyazono Y, Takada H. A Neonate With a Diagnosis of Pontocerebellar Hypoplasia Type 6 Treated With Biotin and Developed Biotin Interference With Laboratory Thyroid Function Tests. Am J Case Rep (2021) 22:e934417. doi: 10.12659/AJCR.934417
174. Wijeratne NG, Doery JC, Lu ZX. Positive and Negative Interference in Immunoassays Following Biotin Ingestion: A Pharmacokinetic Study. Pathology (2012) 44(7):674–5. doi: 10.1097/PAT.0b013e32835a3c17
175. Yi DY. Enteral Nutrition in Pediatric Patients. Pediatr Gastroenterol Hepatol Nutr (2018) 21(1):12–9. doi: 10.5223/pghn.2018.21.1.12
176. Reis AM, de Carvalho RE, de Faria LM, de Oliveira RC, Zago KS, Cavelagna MF, et al. Prevalencia E Significancia Clinica De Interacoes Farmaco-Nutricao Enteral Em Unidades De Terapia Intensiva [Prevalence and Clinical Significance of Interactions Drug-Enteral Nutrition in Intensive Care Units]. Rev Bras Enferm (2014) 67(1):85–90. doi: 10.5935/0034-7167.20140011
177. Dickerson RN, Maish GO 3rd, Minard G, Brown RO. Clinical Relevancy of the Levothyroxine-Continuous Enteral Nutrition Interaction. Nutr Clin Pract (2010) 25(6):646–52. doi: 10.1177/0884533610385701
178. Manessis A, Lascher S, Bukberg P, Darmody T, Yen V, Sadek S, et al. Quantifying Amount of Adsorption of Levothyroxine by Percutaneous Endoscopic Gastrostomy Tubes. JPEN J Parenter Enteral Nutr (2008) 32(2):197–200. doi: 10.1177/0148607108314770
179. Wohlt PD, Zheng L, Gunderson S, Balzar SA, Johnson BD, Fish JT. Recommendations for the Use of Medications With Continuous Enteral Nutrition. Am J Health Syst Pharm (2009) 66(16):1458–67. doi: 10.2146/ajhp080632
180. Pirola I, Daffini L, Gandossi E, Lombardi D, Formenti A, Castellano M, et al. Comparison Between Liquid and Tablet Levothyroxine Formulations in Patients Treated Through Enteral Feeding Tube. J Endocrinol Invest (2014) 37(6):583–7. doi: 10.1007/s40618-014-0082-9
181. Mantegazza C, Landy N, Zuccotti GV, Köglmeier J. Indications and Complications of Inpatient Parenteral Nutrition Prescribed to Children in a Large Tertiary Referral Hospital. Ital J Pediatr (2018) 44(1):66. doi: 10.1186/s13052-018-0505-x
182. Ikomi C, Cole CR, Vale E, Golekoh M, Khoury JC, Jones NY. Hypothyroidism and Iodine Deficiency in Children on Chronic Parenteral Nutrition. Pediatrics (2018) 141(4):e20173046. doi: 10.1542/peds.2017-3046
183. Uchoa KMCB, Evangelista NMA, Carvalho de Camargo MF, Leite HP. Severe Hypothyroidism in a Child Receiving Long-Term Home Parenteral Nutrition Without Selenium. JPEN J Parenter Enteral Nutr (2020) 44(5):944–7. doi: 10.1002/jpen.1807
184. Surks MI, Sievert R. Drugs and Thyroid Function. N Engl J Med (1995) 333(25):1688–94. doi: 10.1056/NEJM199512213332507
185. Chiovato L, Magri F, Carlé A. Hypothyroidism in Context: Where We've Been and Where We're Going. Adv Ther (2019) 36(Suppl 2):47–58. doi: 10.1007/s12325-019-01080-8
186. Aljebab F, Choonara I, Conroy S. Systematic Review of the Toxicity of Long-Course Oral Corticosteroids in Children. PloS One (2017) 12(1):e0170259. doi: 10.1371/journal.pone.0170259
187. Aljebab F, Choonara I, Conroy S. Systematic Review of the Toxicity of Short-Course Oral Corticosteroids in Children. Arch Dis Child (2016) 101(4):365–70. doi: 10.1136/archdischild-2015-309522
188. Brabant A, Brabant G, Schuermeyer T, Ranft U, Schmidt FW, Hesch RD, et al. The Role of Glucocorticoids in the Regulation of Thyrotropin. Acta Endocrinol (Copenh) (1989) 121(1):95–100. doi: 10.1530/acta.0.1210095
189. Samuels MH, McDaniel PA. Thyrotropin Levels During Hydrocortisone Infusions That Mimic Fasting-Induced Cortisol Elevations: A Clinical Research Center Study. J Clin Endocrinol Metab (1997) 82(11):3700–4. doi: 10.1210/jcem.82.11.4376
190. Samuels MH. Effects of Variations in Physiological Cortisol Levels on Thyrotropin Secretion in Subjects With Adrenal Insufficiency: A Clinical Research Center Study. J Clin Endocrinol Metab (2000) 85(4):1388–93. doi: 10.1210/jcem.85.4.6540
191. Wilber JF, Utiger RD. The Effect of Glucocorticoids on Thyrotropin Secretion. J Clin Invest (1969) 48(11):2096–103. doi: 10.1172/JCI106176
192. John CD, Christian HC, Morris JF, Flower RJ, Solito E, Buckingham JC. Kinase-Dependent Regulation of the Secretion of Thyrotrophin and Luteinizing Hormone by Glucocorticoids and Annexin 1 Peptides. J Neuroendocrinol (2003) 15(10):946–57. doi: 10.1046/j.1365-2826.2003.01081.x
193. Cintra A, Fuxe K, Wikström AC, Visser T, Gustafsson JA. Evidence for Thyrotropin-Releasing Hormone and Glucocorticoid Receptor-Immunoreactive Neurons in Various Preoptic and Hypothalamic Nuclei of the Male Rat. Brain Res (1990) 506(1):139–44. doi: 10.1016/0006-8993(90)91210-8
194. Alkemade A, Unmehopa UA, Wiersinga WM, Swaab DF, Fliers E. Glucocorticoids Decrease Thyrotropin-Releasing Hormone Messenger Ribonucleic Acid Expression in the Paraventricular Nucleus of the Human Hypothalamus. J Clin Endocrinol Metab (2005) 90(1):323–7. doi: 10.1210/jc.2004-1430
195. Bhatt-Mehta V, Nahata MC. Dopamine and Dobutamine in Pediatric Therapy. Pharmacotherapy (1989) 9(5):303–14. doi: 10.1002/j.1875-9114.1989.tb04142.x
196. Morley JE. Neuroendocrine Control of Thyrotropin Secretion. Endocr Rev (1981) 2(4):396–436. doi: 10.1210/edrv-2-4-396
197. Kok P, Roelfsema F, Frölich M, van Pelt J, Meinders AE, Pijl H. Bromocriptine Reduces Augmented Thyrotropin Secretion in Obese Premenopausal Women. J Clin Endocrinol Metab (2009) 94(4):1176–81. doi: 10.1210/jc.2008-2303
198. Lewis BM, Dieguez C, Lewis MD, Scanlon MF. Dopamine Stimulates Release of Thyrotrophin-Releasing Hormone From Perfused Intact Rat Hypothalamus via Hypothalamic D2-Receptors. J Endocrinol (1987) 115(3):419–24. doi: 10.1677/joe.0.1150419
199. Van den Berghe G, de Zegher F, Lauwers P. Dopamine and the Sick Euthyroid Syndrome in Critical Illness. Clin Endocrinol (Oxf) (1994) 41(6):731–7. doi: 10.1111/j.1365-2265.1994.tb02787.x
200. Filippi L, Pezzati M, Cecchi A, Poggi C. Dopamine Infusion: A Possible Cause of Undiagnosed Congenital Hypothyroidism in Preterm Infants. Pediatr Crit Care Med (2006) 7(3):249–51. doi: 10.1097/01.PCC.0000216680.22950.D9
201. Isojärvi JI, Myllylä VV, Pakarinen AJ. Effects of Carbamazepine on Pituitary Responsiveness to Luteinizing Hormone-Releasing Hormone, Thyrotropin-Releasing Hormone, and Metoclopramide in Epileptic Patients. Epilepsia (1989) 30(1):50–6. doi: 10.1111/j.1528-1157.1989.tb05280.x
202. Miller J, Carney P. Central Hypothyroidism With Oxcarbazepine Therapy. Pediatr Neurol (2006) 34(3):242–4. doi: 10.1016/j.pediatrneurol.2005.08.032
203. Hirfanoglu T, Serdaroglu A, Camurdan O, Cansu A, Bideci A, Cinaz P, et al. Thyroid Function and Volume in Epileptic Children Using Carbamazepine, Oxcarbazepine and Valproate. Pediatr Int (2007) 49(6):822–6. doi: 10.1111/j.1442-200X.2007.02456.x
Keywords: congenital hypothyroidism, L-thyroxine (L-T4), central nervous system, children, plastic period, pharmacological interferences
Citation: Stagi S, Municchi G, Ferrari M and Wasniewska MG (2022) An Overview on Different L-Thyroxine (l-T4) Formulations and Factors Potentially Influencing the Treatment of Congenital Hypothyroidism During the First 3 Years of Life. Front. Endocrinol. 13:859487. doi: 10.3389/fendo.2022.859487
Received: 21 January 2022; Accepted: 29 April 2022;
Published: 09 June 2022.
Edited by:
Sally Radovick, The State University of New Jersey, United StatesReviewed by:
Hideyuki Iwayama, Aichi Medical University, JapanTim Cheetham, Newcastle University, United Kingdom
Copyright © 2022 Stagi, Municchi, Ferrari and Wasniewska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefano Stagi, c3RlZmFuby5zdGFnaUB1bmlmaS5pdA==
 Stefano Stagi
Stefano Stagi Giovanna Municchi1
Giovanna Municchi1 Malgorzata Gabriela Wasniewska
Malgorzata Gabriela Wasniewska