- Department of Thyroid Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Background and Purpose: This study aimed to analyze the diagnostic ability of the combination of stimulated thyroglobulin (sTg) and antithyroglobulin antibody (TgAb) in predicting the efficacy and prognosis of radioactive iodine (131I) therapy (RAIT) in patients with differentiated thyroid carcinomas (DTCs) after total thyroidectomy (TT).
Methods: This retrospective study comprised 409 DTC patients who underwent 131I treatment following TT in the First Affiliated Hospital of Zhengzhou University from January 2019 to August 2020, and they were followed up to November 2021. Patients were divided into the successful ablation and the unsuccessful ablation group based on the classification of the efficacy of RAIT in the 2015 American Thyroid Association guidelines. The clinical characteristics and the efficacy of the initial RAIT were evaluated. The cutoffs of preablation sTg, sTg/thyroid-stimulating hormone (TSH) ratio, and sTg×TgAb product were calculated to predict the efficacy of RAIT. Univariate and multivariate logistic regression analyses were used to identify the independent risk factors for unsuccessful ablation. Kaplan–Meier curves were used to estimate the prognostic value of sTg×TgAb product affecting progression-free survival (PFS).
Results: The cohort consisted of 222 cases in the successful ablation group and 187 cases in the unsuccessful ablation group. Between the two groups, preablation sTg, sTg/TSH ratio, and sTg×TgAb product were significantly higher in the unsuccessful ablation group. The area under the curve (AUC) of the sTg×TgAb product was the highest among the above three factors. The cutoffs for the worse therapeutic effect of the initial RAIT in sTg, sTg/TSH ratio, and sTg×TgAb were >2.99 ng/ml, >0.029 mg/IU, and >34.18, respectively. STg >2.99 ng/ml and sTg×TgAb product >34.18 were independent risk factors for unsuccessful ablation. Patients with sTg×TgAb product >34.18 had shorter PFS than that of patients with sTg×TgAb product ≤34.18. In separate analyses of TgAb-negative and TgAb-positive subgroups, higher sTg×TgAb was both associated with a lower success rate of RAIT and a shorter PFS.
Conclusion: STg×TgAb product predicted the efficacy and prognosis of 131I therapy for both TgAb-negative and TgAb-positive DTC patients before the initial 131I treatment following TT. Thus, it can be used as a clinical reference indicator for the surveillance of DTC patients.
Introduction
Differentiated thyroid carcinoma (DTC) is the most common thyroid malignant tumor. Presently, the standard treatments for DTC include surgery, thyroid-stimulating hormone (TSH) suppression therapy, and selective radioactive iodine-131 treatment (1, 2). Also, selective postoperative radioiodine (131I) treatment might improve prognosis (3). Thyroglobulin (Tg) is a widely used biomarker in the follow-up of DTC patients after thyroidectomy that assists in the diagnosis of persistent disease, distant metastasis, or disease recurrence (4, 5). The stimulated thyroglobulin (sTg) is the Tg value measured after thyroidectomy without or stopped treatment with thyroid hormone (TSH >30 mU/L) (6). Several studies indicated that the sTg before 131I treatment has predictive value for the efficacy of the treatment (7–12). However, sTg can be affected by TSH levels, antithyroglobulin antibody (TgAb) levels, residual thyroid, metastatic lesions, and other factors, and the impact of these factors cannot be excluded.
Antithyroglobulin antibody is an autoantibody against Tg, and the detection rate of DTC patients exceeds 25% (13). TgAb can limit the utility of Tg in the surveillance of DTC patients by interfering with the accuracy of the determination of Tg (14–16) and accelerating its clearance when Tg is complexed with TgAb (17), no matter if the status of TgAb is negative or positive (18, 19). However, the effects of TgAb on sTg have not yet been solved.
This study aimed to use TgAb to correct the predictive value of the sTg to see whether sTg×TgAb product can be a predictive index of the efficacy of radioactive iodine therapy (RAIT) and whether it can predict the prognosis of 131I therapy, irrespective of the positive or negative TgAb status.
Patients and Methods
Patients
In this retrospective study, we reviewed the medical record of DTC patients who underwent total thyroidectomy (TT) and received 131I treatment 4 weeks after surgery between January 2019 and August 2020 at the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China. The inclusion criteria were as follows: (a) cases with histological confirmation of DTC; (b) patients with 4 weeks of levothyroxine (L-T4) withdrawal with a low iodine diet after TT; (c) patients with sTg, TgAb, and TSH outcomes before postoperative 131I treatment; (d) patients who stopped L-T4 for 4 weeks and underwent sTg and TgAb measurement, cervical ultrasound (US), chest computed tomography (CT) scan, whole-body scan (WBS), and single-photon emission computed tomography-computed tomography (SPECT-CT) 6 months after initial RAIT; and (e) patients with positive TgAb status (TgAb >115 kU/L) should have a substantial value. The exclusion criteria were as follows: (a) patients with partial thyroidectomy; (b) patients lost to follow-up; (c) iodine refractory thyroid cancer patients; and (d) cases with undetectable sTg or TSH or TgAb (below or above the detection limit).
Data Collection
The following data were collected: gender; age; histopathological type; tumor size; unilateral or bilateral primary foci; tumor stage; with or without extrathyroidal extension; with or without lymph node (LN) metastasis; risk stratification of recurrence; the initial RAIT dose; sTg, TSH, and TgAb measurements before the initial 131I ablation; results of reexamination 6 months after initial ablation, including clinical and laboratory assessments; and progression-free survival (PFS) and follow-up duration in months. TSH, sTg, and TgAb were measured using an electrochemiluminescence immunoassay (Cobas, Germany), and all of the patients used the same method.
131I Treatment and Follow-Up
Patients were forbidden to take LT4 and strictly followed a low-iodine diet after TT for 4 weeks to achieve the goal TSH of >30 mU/L before RAIT. TSH, sTg, and TgAb measurements, cervical US, and chest CT scan were completed 3 days before RAIT. The treatment dose of RAIT was referred to as the specific disease extent of every patient according to the recommendations of the 2015 American Thyroid Association (ATA) guidelines and Chinese Thyroid Association guidelines on the management of DTC (6, 20). WBS and SPECT-CT were performed 3–5 days after 131I treatment. Patients started to receive TSH suppression therapy when the 131I treatment was completed. After 6 months of the initial 131I treatment, patients were required to stop taking LT4 for 4 weeks and undergo the reexamination similar to the initial RAIT. DTC patients who received the initial RAIT after the TT and the reexamination in our hospital 6 months after the initial RAIT were all followed up. After the reexamination, patients were followed up through regular check-ups, including serum-free triiodothyronine (FT3), free thyroxine (FT4), TSH, Tg, and TgAb measurements every 3 months, and the cervical US every 6 months, until November 2021. The end event was the recurrence or metastasis of DTC.
Grouping Method
According to the 2015 ATA guidelines (6), the clinical therapeutic results of patients who underwent the initial 131I treatment after TT were divided into four categories: excellent response (ER), indeterminate response (IDR), biochemical incomplete response (BIR), and structural incomplete response (SIR). Based on the results of the serological and imaging examinations 6 months after the initial RAIT and the above categories, patients with ER were included in the successful ablation group, while patients with IDR, BIR, or SIR were included in the unsuccessful ablation group. To be more specific, patients were considered to have a successful ablation when subjects met the following four conditions: (a) serum suppressed Tg <0.2 ng/ml or sTg <1 ng/ml; (b) absence of radioactive iodine uptake in thyroid bed on radioactive iodine scanning; (c) TgAb levels stable or declining; and (d) absence of structural or functional evidence of disease. After evaluating the efficacy and prognosis of RAIT, we divided the patients into TgAb-positive group (TgAb >115 kU/L) and TgAb-negative group (TgAb ≤115 kU/L) according to the TgAb levels measured before the initial RAIT to further analyze the predictive value of the sTg×TgAb product in patients with different TgAb status, respectively.
Statistical Analyses
Data were analyzed using the IBM SPSS 25.0 statistical software (IBM Corp, Armonk, NY, USA). Categorical variables were expressed as numbers with percentages, and the differences between groups were evaluated by the Chi-square test. The quantitative data in this study did not conform to a normal distribution and were represented by medians with interquartile ranges; the comparisons between groups were analyzed by the Mann–Whitney U test, and Z-test was used to determine the p-value. The Spearman’s correlation test was used to assess the correlation between sTg and TgAb. The receiver operating characteristic (ROC) curve analysis determined the best cutoff values of preablation sTg, sTg/TSH ratio, and sTg×TgAb product for predicting the unsuccessful ablation of patients and assessed the predictive values of the above variables. The ROC curves of the three predictors were compared using MedCalc version 20.009 software. The ROC curve analysis was also used to evaluate the predictive value of sTg×TgAb product in the TgAb-positive and the TgAb-negative groups, respectively. Univariate and multivariate logistic regression analyses were performed to assess the independent risk factors of successful ablation. Any variable with a p-value <0.1 in the univariate analysis was included in the multivariate regression analysis using forward selection procedures. Kaplan–Meier curves were used to estimate the prognostic value of different factors affecting PFS. The statistical comparisons were carried out using the log-rank test. A p-value <0.05 was considered statistically significant.
Results
Clinical Evaluation of 131I Treatment
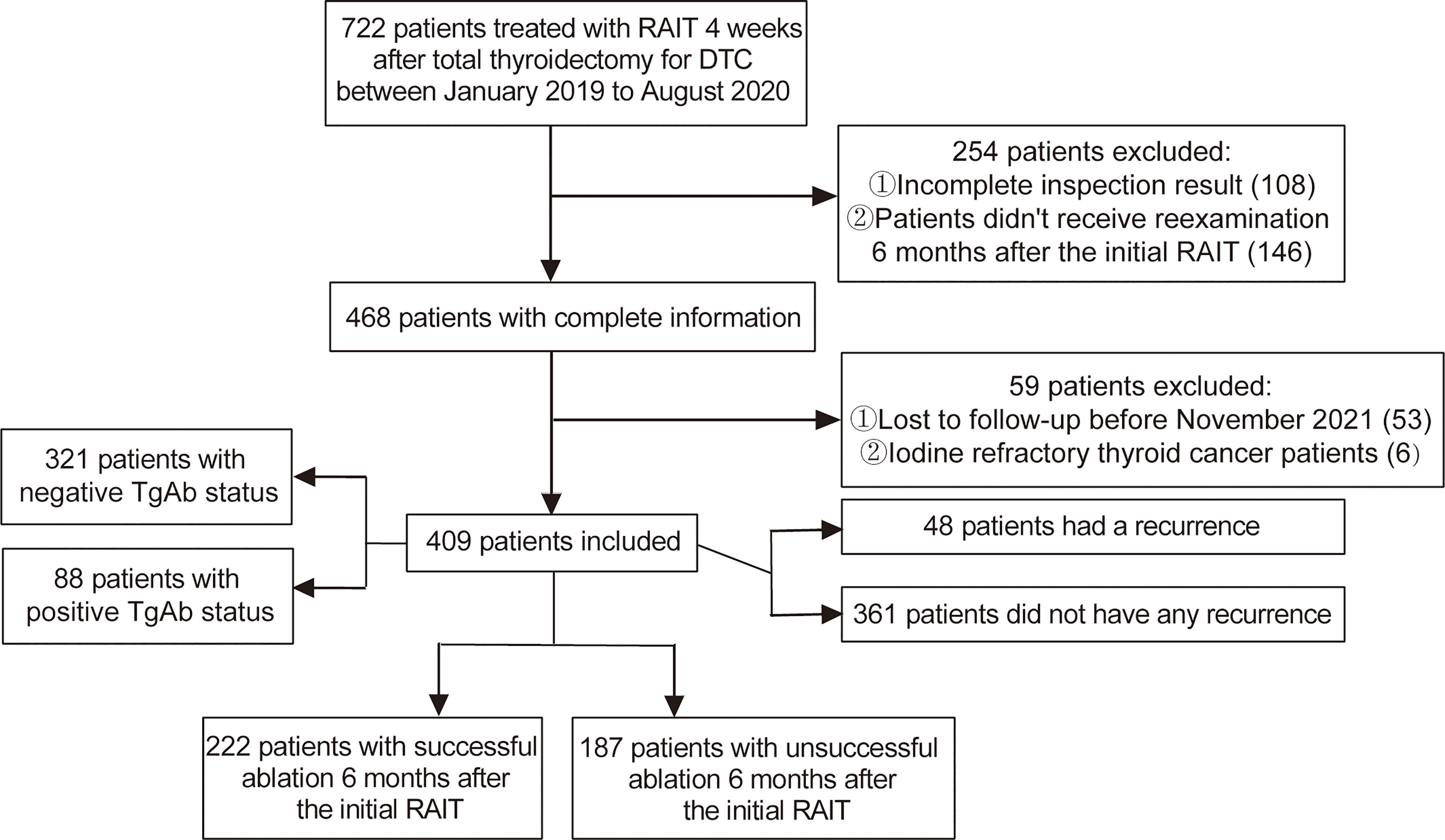
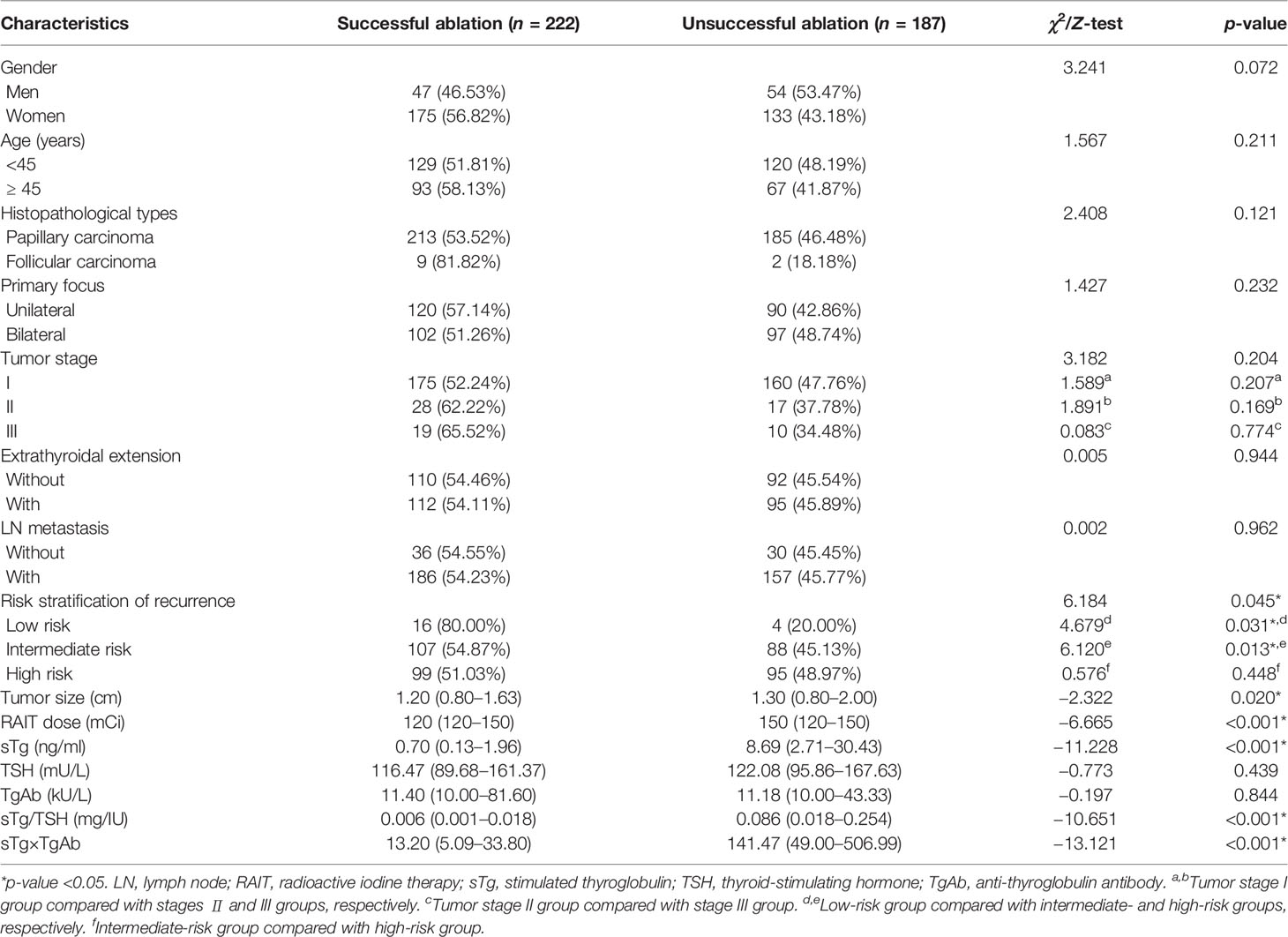
After screening according to the inclusion and exclusion criteria, a total of 409 DTC patients were included in this retrospective study (Figure 1). The cohort comprised 222 (54.28%) cases that had a successful ablation, while 187 (45.72%) cases had an unsuccessful ablation. Among them, 48 (11.74%) cases had a recurrence, including 41 cases with a cervical lymph node metastasis and 7 cases with a distant metastasis. The preablation TgAb-positive group consisted of 88 (21.52%) patients, while TgAb-negative group had 321 (78.48%) patients. The median follow-up period was 23 months (IQR, 18–26 months). Correlation analysis between sTg and TgAb before the first 131I treatment showed that sTg and TgAb were negatively correlated (r = −0.474, p < 0.001). Tumor size, RAIT dose, preablation sTg, sTg/TSH ratio, and sTg×TgAb product of patients with successful ablation were significantly lower than those of patients with unsuccessful ablation (all p < 0.05). The risk stratification of recurrence of the successful ablation group was significantly different from that of the unsuccessful ablation group; intermediate- to high-risk stratification of recurrence was significantly associated with unsuccessful ablation (p < 0.05). Conversely, gender, age, pathological type, unilateral or bilateral primary foci, tumor stage, extrathyroidal extension, LN metastasis, TSH level, and TgAb level did not display any significant differences between the successful and unsuccessful ablation subgroups (all p > 0.05) (Table 1).
ROC Curves to Predict Unsuccessful Ablation
Preablation sTg, sTg/TSH ratio, and sTg×TgAb product were included in the ROC curve analysis (Figure 2), the area under ROC curve (AUC) to predict unsuccessful ablation was 0.822, 0.806, and 0.876, respectively, and the cutoff was 2.99 ng/ml, 0.029 mg/IU, and 34.18, respectively (Table 2). A pairwise comparison analysis of the ROC curves for the three predictors showed that the AUC for sTg×TgAb product was significantly greater than that for the other two factors (all p < 0.05). The AUC of the three factors was arranged in descending order as sTg×TgAb product, sTg, and sTg/TSH ratio, and the differences were statistically significant (all p < 0.05) (Table 3).
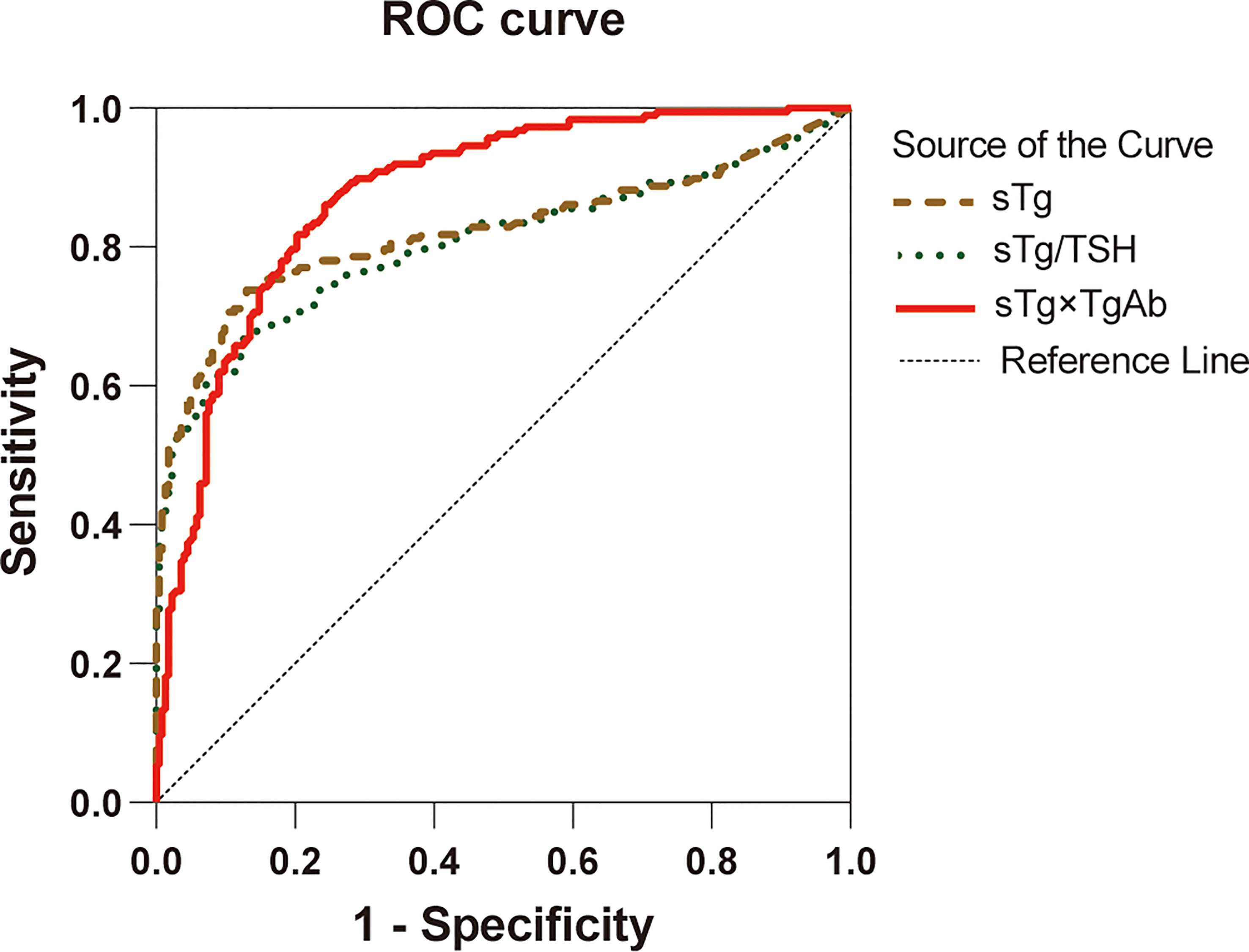
Figure 2 ROC curves for sTg, sTg/TSH ratio, and sTg×TgAb product before initial RAIT to predict unsuccessful ablation.
Univariate and Multivariate Logistic Regression Analyses
Univariate logistic regression analysis found that the following clinical characteristics were not significantly related to the efficacy of 131I treatment: age, unilateral or bilateral primary foci, tumor stage, extrathyroidal extension, LN metastasis, and TSH level (all p > 0.05). On the other hand, gender as male, histopathological types as PTC, higher TgAb level, higher RAIT dose, bigger tumor size, intermediate-risk stratification of recurrence, sTg >2.99 ng/ml, sTg/TSH ratio >0.029 mg/IU, and sTg×TgAb product >34.18 were significantly related to unsuccessful ablation (all p < 0.1), and the above variables were included in the multivariate logistic regression analysis. The results showed that TgAb level, RAIT dose, sTg >2.99 ng/ml, and sTg×TgAb product >34.18 were independent risk factors for unsuccessful ablation (all p < 0.05). The odds ratio (OR) and 95% confidence interval (CI) of the above factors were 1.001 (1.000–1.002), 1.022 (1.007–1.037), 6.120 (2.202–17.008), and 4.200 (1.986–8.884), respectively (Table 4).
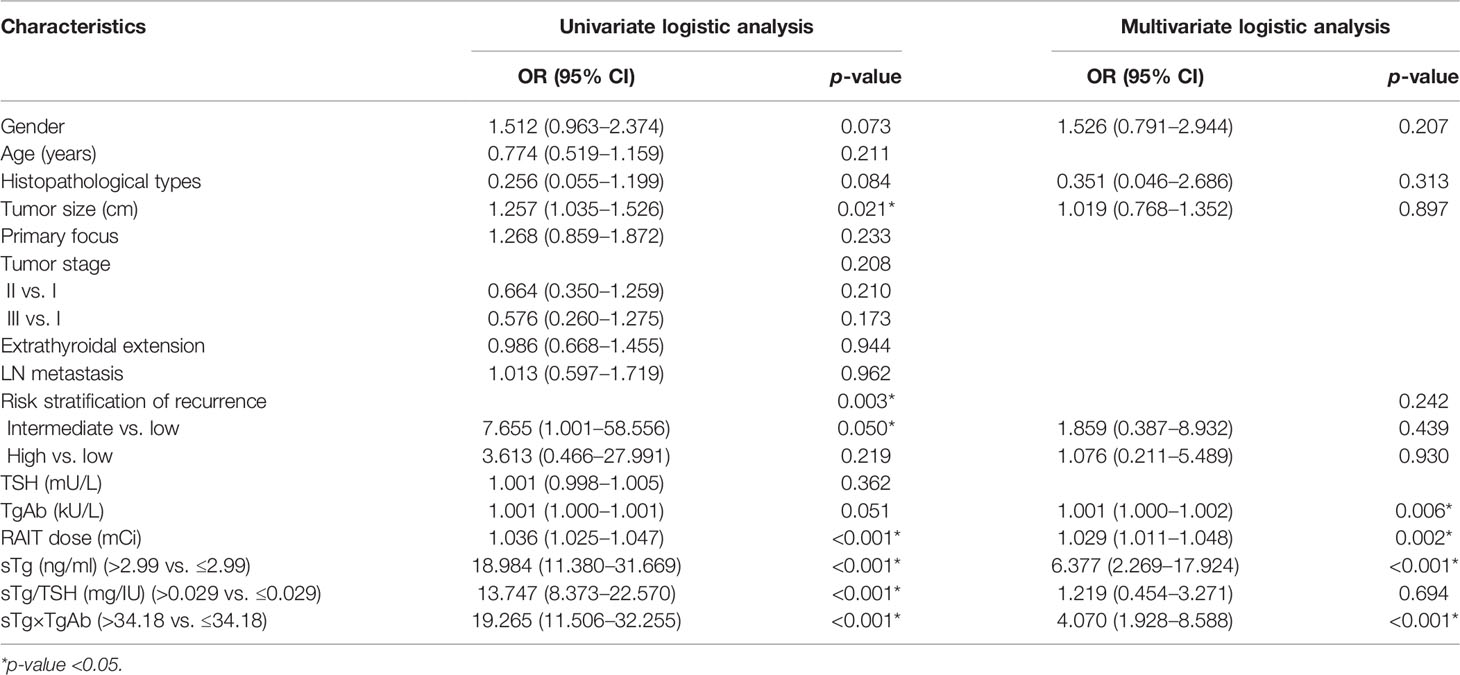
Table 4 Univariate and multivariate logistic regression analyses of tumor characteristics and therapeutic effect.
Kaplan−Meier Analysis of PFS
The median of TgAb levels and RAIT dose and the cutoff values of sTg and sTg×TgAb product were used as grouping standards to draw the Kaplan–Meier curves (Figure 3). Patients with TgAb ≤11.24 kU/L had a mean PFS of 30.96 months, while patients with TgAb >11.24 kU/L had a mean PFS of 31.34 months, without any significant difference (p = 0.740). The mean PFS of patients with RAIT dose ≤150 mCi was 31.91 months, which was significantly longer than that of patients with RAIT dose >150 mCi (mean 22.21 months, p < 0.001). The PFS of patients with sTg ≤2.99 ng/ml was significantly longer than that of patients with sTg >2.99 ng/ml (mean 33.26 vs. 28.06 months, p < 0.001), and that of patients with sTg×TgAb ≤34.18 was significantly longer than of those with sTg×TgAb >34.18 (mean 33.64 vs. 28.92 months, p < 0.001).
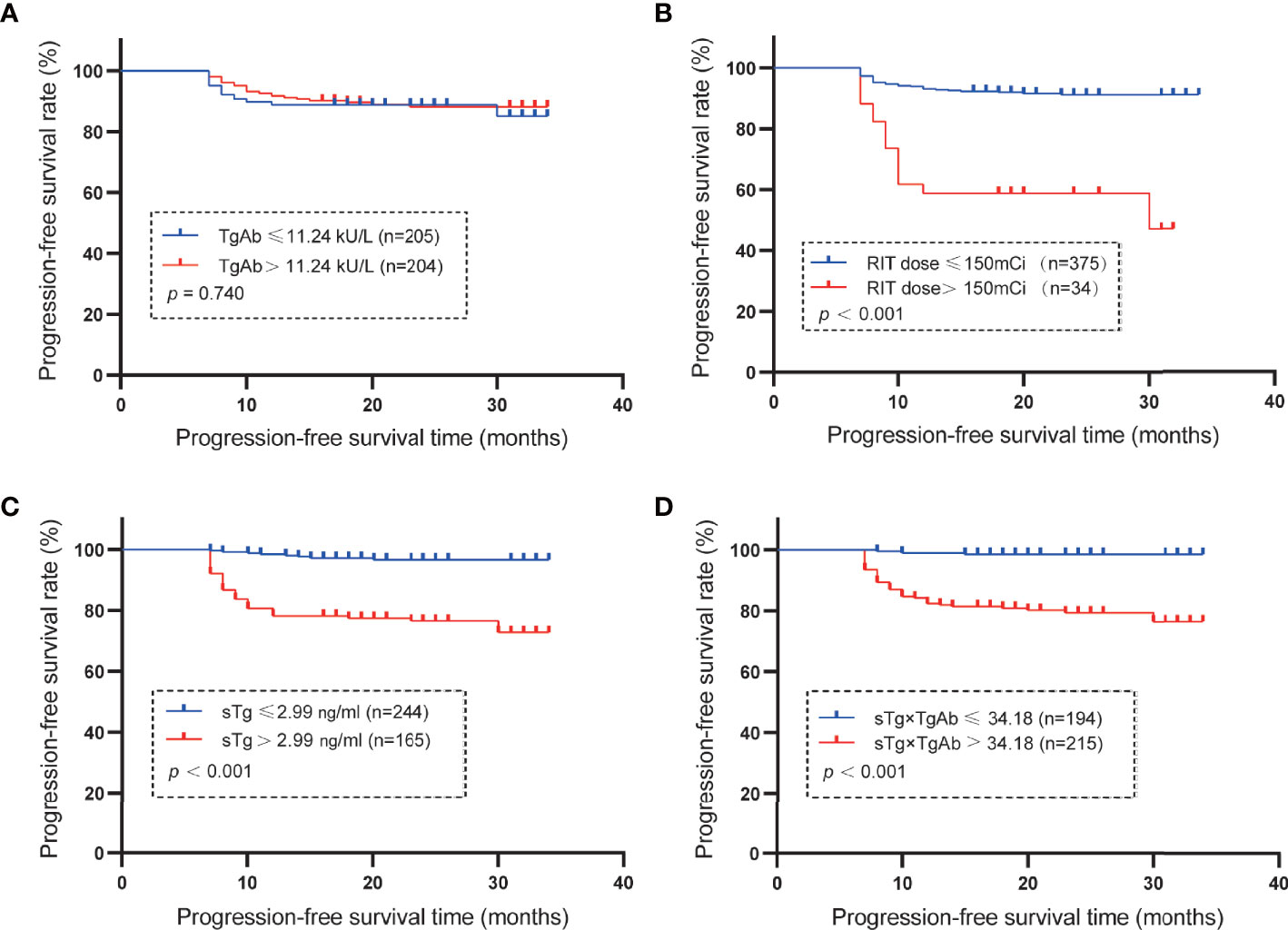
Figure 3 Mean PFS. (A) Mean PFS in the group of patients with TgAb ≤11.24 kU/L or >11.24 kU/L (the median of TgAb). The PFS was 30.96 months with TgAb ≤11.24 kU/L and 31.34 months for TgAb >11.24 kU/L (p = 0.740). (B) Mean PFS of the subset with RAIT dose ≤150 or > 150 mCi (the median of RAIT dose). The PFS was 31.91 months in patients with RAIT dose ≤150 mCi and 22.21 months with RAIT dose >150 mCi (p < 0.001). (C) Mean PFS in the subgroup of patients with sTg ≤2.99 or >2.99 ng/ml. The PFS was 33.26 months in patients with sTg ≤2.99 ng/ml and 28.06 months with sTg >2.99 ng/ml (p < 0.001). (D) Mean PFS in the group of patients with sTg×TgAb ≤34.18 or >34.18. The PFS was 33.64 months with sTg×TgAb ≤34.18 and 28.92 months for sTg×TgAb >34.18 (p < 0.001).
Results of the TgAb-Negative and TgAb-Positive Groups
According to the results of the multivariate logistic regression analysis of the 409 patients, TgAb level, RAIT dose, sTg, and sTg×TgAb product were independent risk factors for unsuccessful ablation. These factors were included in the clinical evaluation of the TgAb-negative and TgAb-positive groups (Table 5). We found that only sTg×TgAb product was significantly lower in patients with successful ablation compared with those with unsuccessful ablation in both groups (p < 0.05). The parameter was included in the ROC curve analysis to predict the unsuccessful ablation of both groups (Figure 4). The cutoffs were 36.80 (AUC: 0.937) and 466.36 (AUC: 0.656), respectively, the sensitivities were 87.6% and 35.7%, and the specificities were 85.2% and 91.3%, respectively. According to the Kaplan–Meier curves of the two groups, the PFS of patients with sTg×TgAb ≤36.80 was significantly longer than that of patients with sTg×TgAb >36.80 (mean 32.74 vs. 27.82 months, p < 0.001) in the TgAb-negative group. In the TgAb-positive group, the PFS of patients with sTg×TgAb ≤466.36 was significantly longer than that of patients with sTg×TgAb >466.36 (mean 33.01 vs. 28.84 months, p = 0.004) (Figure 5).
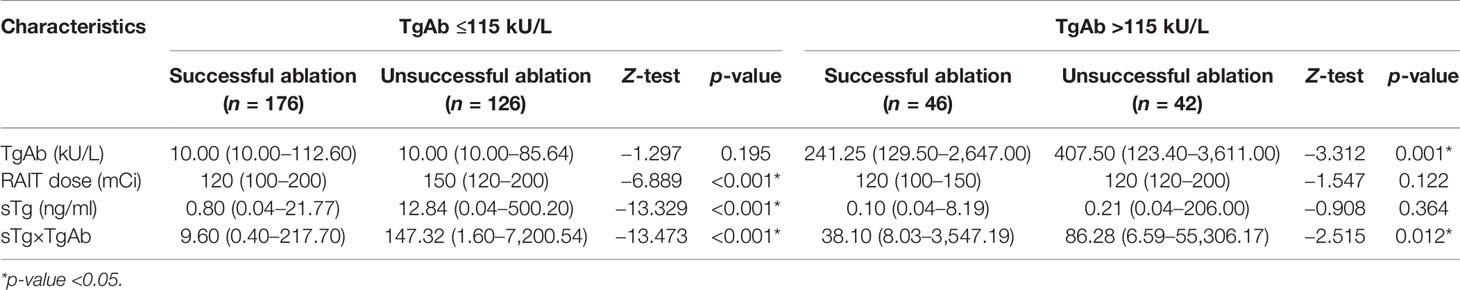
Table 5 Comparison of the three independent risk factors for unsuccessful ablation of the TgAb-negative group (≤115 kU/L) and the TgAb-positive group (>115 kU/L) on the efficacy of the initial 131I treatment.
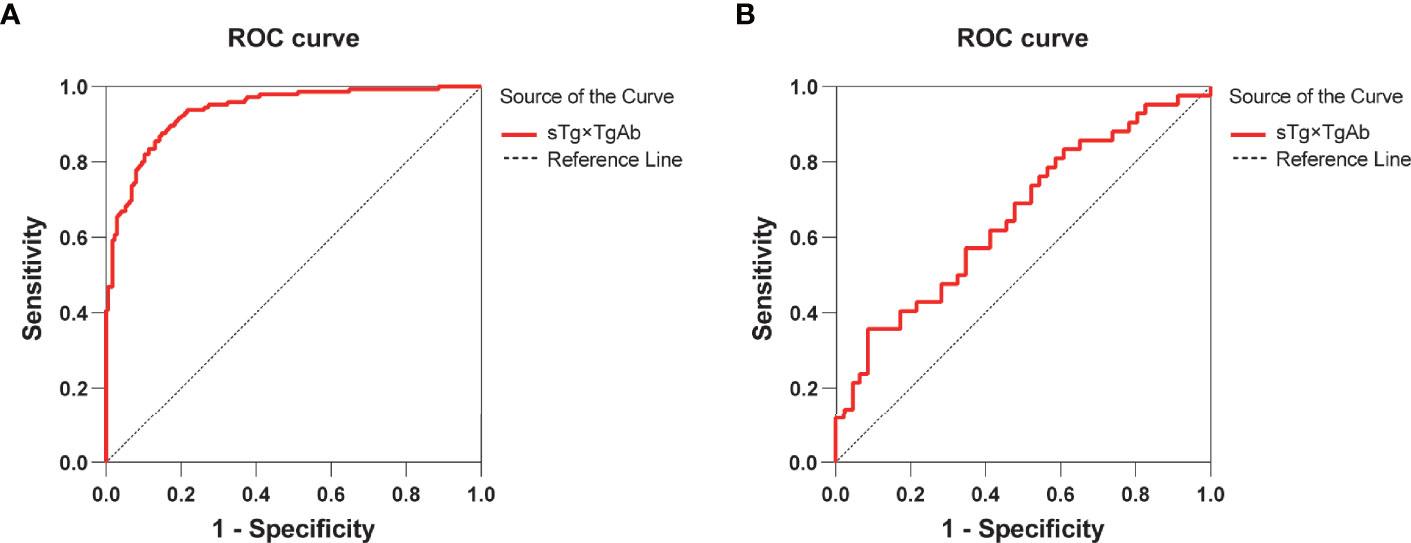
Figure 4 (A) ROC curve for sTg×TgAb product before initial RAIT to predict unsuccessful ablation of the TgAb-negative group. The cutoff for sTg×TgAb product was 36.80 (AUC: 0.937, 95% CI: 0.911–0.962) with 87.6% sensitivity and 85.2% specificity (p < 0.001). (B) ROC curves for sTg×TgAb product before initial RAIT to predict the unsuccessful ablation of the TgAb-positive group. The cutoff for sTg×TgAb product was 466.36 (AUC: 0.656, 95% CI: 0.542–0.770) with 35.7% sensitivity and 91.3% specificity (p = 0.012).
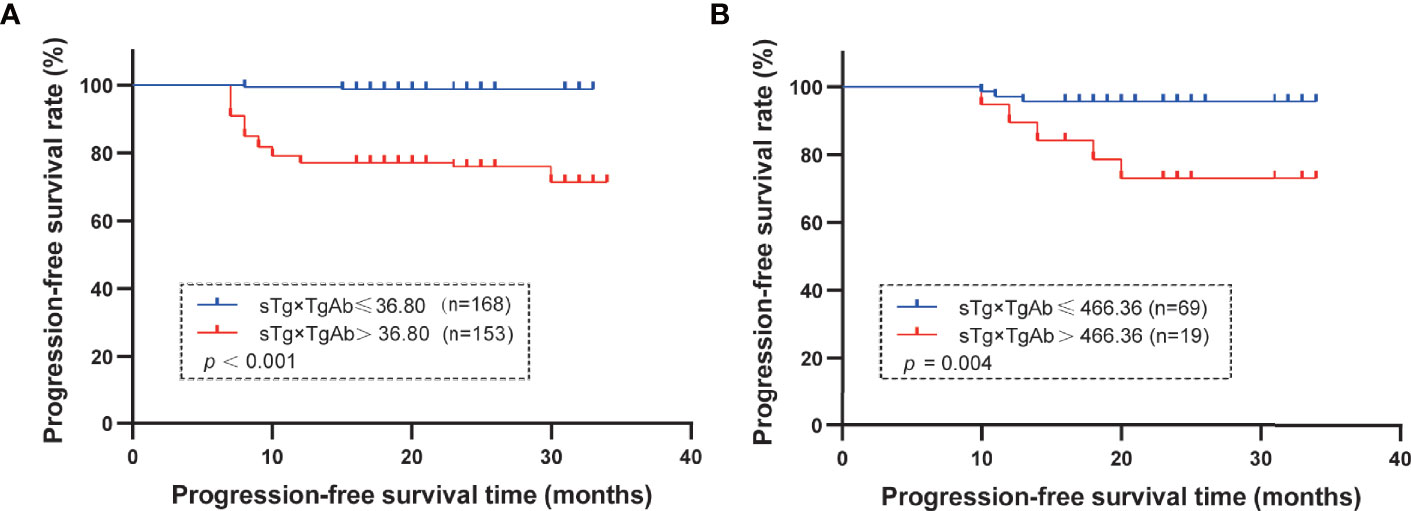
Figure 5 (A) TgAb-negative group: mean PFS in the group of patients with sTg×TgAb ≤36.80 or >36.80. The PFS was 32.74 months with sTg×TgAb ≤36.80 and 27.82 months for sTg×TgAb > 36.80 (p < 0.001). (B) TgAb-positive group: mean PFS by group of patients with sTg×TgAb ≤36.80 or >36.80. The PFS was 33.01 months with sTg×TgAb ≤466.36 and 28.84 months for sTg×TgAb >466.36 (p = 0.004).
Discussion
Under the concept of comprehensive multidisciplinary treatment, selective 131I therapy has become one of the main methods of postoperative adjuvant treatment to improve the prognosis of DTC patients (6, 21). However, the success rate of the initial 131I treatment after DTC surgery is varied (8, 22–25). Therefore, analyzing the factors that affect the efficacy of RAIT and predicting the efficacy and prognosis of RAIT in advance can be valuable in stratifying the risk of patients after DTC surgery and adjusting the treatment plan.
To avoid the interference of TgAb to sTg, the previous studies on the factors affecting the efficacy of RAIT selected TgAb-negative patients as the research subjects (4, 7–12). However, even if TgAb is negative, it affects sTg measurements (18). The present study included TgAb-negative and TgAb-positive patients and studied the influencing factors and the predictors of the efficacy and prognosis of RAIT in the total sample and the two groups. According to the correlation analysis between sTg and TgAb, the present study corrected their mutual influence by sTg×TgAb product and tested its ability to predict the efficacy and prognosis of RAIT.
The clinical characterization demonstrated that preablation sTg and sTg/TSH ratio are significantly related to the efficacy of RAIT, which is consistent with the results of previous studies (13–15, 17). In addition, statistical analysis revealed that sTg×TgAb product, tumor size, risk stratification for recurrence, and RAIT dose are significantly related to the efficacy of RAIT, while the remaining clinical characteristics were similar between the successful and unsuccessful ablation groups. It has been reported that 131I dose is an important factor affecting the therapeutic effect of RAIT ablation, but the results of the studies were different. Kim et al. (26) stated that high doses of iodine treatment are associated with a high success rate, while Trevizam et al. (12) got a result similar to this study that the RAIT dose of the unsuccessful ablation group was significantly higher than that of the successful ablation group. The results of the present study also indicated that higher RAIT dose was an independent risk factor for unsuccessful ablation. The risk of unsuccessful ablation increases by 1.029-fold for every increase of 1 mCi in the RAIT dose. These differences may be attributable to the difference in grouping methods or the research objects.
To confirm the predictive value of sTg×TgAb product, preablation sTg, sTg/TSH ratio, and sTg×TgAb product were included in the ROC curve analysis, and the results showed that these parameters predicted the therapeutic effects of 131I treatment. When preablation sTg was ≤2.99 ng/ml, 79.51% of DTC patients receiving RAIT are probable to succeed in ablation. About 75.98% of patients are likely to succeed in ablation when sTg/TSH ratio was ≤0.029 mg/IU. Comparing our results with those of Wang et al. (8) and Trevizam et al. (12), we found that the cutoff values, sensitivities, and the AUC of sTg and sTg/TSH ratio obtained in this study are the lowest, and the specificities are the highest. These inconsistent results may be related to the different inclusion criteria or grouping methods across studies. Moreover, the results of this study showed that when sTg×TgAb product was ≤34.18, up to 86.60% of DTC patients receiving RAIT is probable to succeed in ablation, which was higher than that of the sTg and sTg/TSH ratio. Subsequently, we compared the ROC curves for these three predictors and found that the AUC for sTg×TgAb product was the highest and significantly greater than that for the sTg and sTg/TSH ratio, indicating that the predictive ability of the sTg×TgAb product is better than the sTg and sTg/TSH ratio. Compared with the sTg and sTg/TSH ratio, the sTg×TgAb product has a higher sensitivity and lower specificity. In clinical application, we can reduce the false-positive rate of the sTg×TgAb product by taking all of these predictors into consideration.
In recent years, TgAb has been gaining increasing attention as a critical indicator of the surveillance of DTC. The ATA guidelines indicated that the increased TgAb levels after total thyroidectomy and RAIT suggested an increased risk of recurrence (6). The results of univariate and multivariate logistic regression analyses showed that the risk of unsuccessful ablation increases by 1.001-fold for every 1 kU/L increase in TgAb level. Combining with the result, the influence of TgAb on the prognosis of DTC was further confirmed. In addition, the results indicated that the risk of unsuccessful ablation in patients with sTg >2.99 ng/ml was 6.377-fold than that of patients with sTg ≤2.99 ng/ml, and the risk of unsuccessful ablation in patients with sTg×TgAb >34.18 is 4.070-fold than that of sTg×TgAb ≤34.18.
After a median follow-up of 23 months, we found that the PFS of patients with sTg >2.99 ng/ml was significantly shorter than that of patients with sTg ≤2.99 ng/ml similar to patients with sTg×TgAb >34.18 compared with patients with sTg×TgAb ≤34.18. This phenomenon further confirmed the predictive value of preablation sTg level and sTg×TgAb product for the prognosis of 131I treatment. According to the ATA guidelines, TgAb is an alternative tumor marker in TgAb-positive DTC patients (6). However, in this study, no significant difference was observed between the mean PFS of patients with TgAb ≤11.24 kU/L and that of patients with TgAb >11.24 kU/L, indicating TgAb cannot be an independent predictor of the prognosis of RAIT in the total population.
The analysis of the TgAb-negative and TgAb-positive groups showed that sTg×TgAb product predicts the efficacy and prognosis of RAIT and whether patients are in TgAb-negative or TgAb-positive status. Nevertheless, sTg was significantly associated with the efficacy of RAIT only in the TgAb-negative group, and TgAb showed a significant association with the efficacy of RAIT only in the TgAb-positive group, which further confirmed that sTg×TgAb product is a universal predictor.
The limitation of this study is the small sample size, especially of the TgAb-positive group. The selection bias cannot be avoided due to the limitation of retrospective analysis and the short follow-up duration. Because the pre-ablation TSH level is difficult to control, we did not select patients with the same TSH level even though it could affect sTg level (2, 27, 28). However, sTg/TSH ratio was included in this study, and its predictive ability was compared with the other predictors.
In summary, this study confirmed that before the first 131I treatment following total thyroidectomy, sTg×TgAb product could predict the efficacy and prognosis of 131I therapy for both TgAb-negative and TgAb-positive DTC patients. It can be used as a clinical reference indicator, and for patients with sTg×TgAb above the cutoff value, the risk can be restratified and the treatment plan improved. In future studies, we will expand the sample size and conduct prolonged surveillance to verify the reliability of this indicator further.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
MP and XL contributed to the concept and design of this study. MP collected data, completed statistical analyses, and drafted the manuscript. MJ and ZL critically revised the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the study and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge Dr. Yansong Lin (Peking Union Medical College Hospital, Beijing, China) for helpful suggestions on this manuscript.
References
1. Cabanillas ME, de Souza JA, Geyer S, Wirth LJ, Menefee ME, Liu SV, et al. Cabozantinib as Salvage Therapy for Patients With Tyrosine Kinase Inhibitor-Refractory Differentiated Thyroid Cancer: Results of a Multicenter Phase Ii International Thyroid Oncology Group Trial. J Clin Oncol (2017) 35(29):3315–21. doi: 10.1200/JCO.2017.73.0226
2. Mazzaferri EL, Kloos RT. Clinical Review 128: Current Approaches to Primary Therapy for Papillary and Follicular Thyroid Cancer. J Clin Endocrinol Metab (2001) 86(4):1447–63. doi: 10.1210/jcem.86.4.7407
3. Verburg FA, de Keizer B, Lips CJ, Zelissen PM, de Klerk JM. Prognostic Significance of Successful Ablation With Radioiodine of Differentiated Thyroid Cancer Patients. Eur J Endocrinol (2005) 152(1):33–7. doi: 10.1530/eje.1.01819
4. Kendler DB, Vaisman F, Corbo R, Martins R, Vaisman M. Preablation Stimulated Thyroglobulin Is a Good Predictor of Successful Ablation in Patients With Differentiated Thyroid Cancer. Clin Nucl Med (2012) 37(6):545–9. doi: 10.1097/RLU.0b013e31824852f8
5. Webb RC, Howard RS, Stojadinovic A, Gaitonde DY, Wallace MK, Ahmed J, et al. The Utility of Serum Thyroglobulin Measurement at the Time of Remnant Ablation for Predicting Disease-Free Status in Patients With Differentiated Thyroid Cancer: A Meta-Analysis Involving 3947 Patients. J Clin Endocrinol Metab (2012) 97(8):2754–63. doi: 10.1210/jc.2012-1533
6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
7. Gonzalez C, Aulinas A, Colom C, Tundidor D, Mendoza L, Corcoy R, et al. Thyroglobulin as Early Prognostic Marker to Predict Remission at 18-24 Months in Differentiated Thyroid Carcinoma. Clin Endocrinol (Oxf) (2014) 80(2):301–6. doi: 10.1111/cen.12282
8. Wang C, Diao H, Ren P, Wang X, Wang Y, Zhao W. Efficacy and Affecting Factors of (131)I Thyroid Remnant Ablation After Surgical Treatment of Differentiated Thyroid Carcinoma. Front Oncol (2018) 8:640. doi: 10.3389/fonc.2018.00640
9. Zheng W, Rui Z, Wang X, Li N, Tan J, Liu W. The Influences of Tsh Stimulation Level, Stimulated Tg Level and Tg/Tsh Ratio on the Therapeutic Effect of (131)I Treatment in Dtc Patients. Front Endocrinol (Lausanne) (2021) 12:601960. doi: 10.3389/fendo.2021.601960
10. De Marchi AFJ, de Macedo ABT, Soares CSP, Bolfi F, Iessi MRG, de Oliveira CC, et al. Thyroglobulin/Thyrotropin Ratio for Predicting Long-Term Response in Differentiated Thyroid Carcinoma: A Retrospective Study. Arch Endocrinol Metab (2021) 65(4):428–35. doi: 10.20945/2359-3997000000387
11. Wang C, Zhang R, Wang R, Meng Z, Zhang G, Dong F, et al. Predictive Value of Thyroglobulin Changes for the Curative Effect of Radioiodine Therapy in Patients With Metastatic Differentiated Thyroid Carcinoma. Front Endocrinol (Lausanne) (2021) 12:667544. doi: 10.3389/fendo.2021.667544
12. Trevizam PG, Tagliarini JV, Castilho EC, de Alencar Marques M, Kiy Y, Mazeto G. Thyroglobulin Levels and Thyroglobulin/Thyrotropin Ratio Could Predict the Success of the Ablative/Therapeutic (131)I in the Differentiated Thyroid Cancers. Endocr Res (2017) 42(1):42–8. doi: 10.3109/07435800.2016.1173056
13. Spencer C, Fatemi S. Thyroglobulin Antibody (Tgab) Methods - Strengths, Pitfalls and Clinical Utility for Monitoring Tgab-Positive Patients With Differentiated Thyroid Cancer. Best Pract Res Clin Endocrinol Metab (2013) 27(5):701–12. doi: 10.1016/j.beem.2013.07.003
14. Spencer CA, Takeuchi M, Kazarosyan M, Wang CC, Guttler RB, Singer PA, et al. Serum Thyroglobulin Autoantibodies: Prevalence, Influence on Serum Thyroglobulin Measurement, and Prognostic Significance in Patients With Differentiated Thyroid Carcinoma. J Clin Endocrinol Metab (1998) 83(4):1121–7. doi: 10.1210/jcem.83.4.4683
15. Chiovato L, Latrofa F, Braverman LE, Pacini F, Capezzone M, Masserini L, et al. Disappearance of Humoral Thyroid Autoimmunity After Complete Removal of Thyroid Antigens. Ann Intern Med (2003) 139(5 Pt 1):346–51. doi: 10.7326/0003-4819-139-5_part_1-200309020-00010
16. Tuttle RM, Leboeuf R. Follow Up Approaches in Thyroid Cancer: A Risk Adapted Paradigm. Endocrinol Metab Clin North Am (2008) 37(2):419–35, ix-x. doi: 10.1016/j.ecl.2008.02.008
17. Weigle WO, High GJ. The Behavior of Autologous Thyroglobulin in the Circulation of Rabbits Immunized With Either Heterologous or Altered Homologous Thyroglobulin. J Immunol (1967) 98(6):1105–14.
18. Spencer C, Petrovic I, Fatemi S. Current Thyroglobulin Autoantibody (Tgab) Assays Often Fail to Detect Interfering Tgab That Can Result in the Reporting of Falsely Low/Undetectable Serum Tg Ima Values for Patients With Differentiated Thyroid Cancer. J Clin Endocrinol Metab (2011) 96(5):1283–91. doi: 10.1210/jc.2010-2762
19. Cubero JM, Rodriguez-Espinosa J, Gelpi C, Estorch M, Corcoy R. Thyroglobulin Autoantibody Levels Below the Cut-Off for Positivity Can Interfere With Thyroglobulin Measurement. Thyroid (2003) 13(7):659–61. doi: 10.1089/105072503322240013
20. Endocrinology Branch of Chinese Medical Association. Guidelines for Diagnosis and Treatment of Thyroid Nodules and Differentiated Thyroid Cancer. Chin J Clin Oncol (2012) 28:779–97. doi: 10.3969/j.issn.1000-8179.2012.17.001
21. Wang R, Zhang Y, Tan J, Zhang G, Zhang R, Zheng W, et al. Analysis of Radioiodine Therapy and Prognostic Factors of Differentiated Thyroid Cancer Patients With Pulmonary Metastasis: An 8-Year Retrospective Study. Med (Baltimore) (2017) 96(19):e6809. doi: 10.1097/MD.0000000000006809
22. Ha S, Oh SW, Kim YK, Koo do H, Jung YH, Yi KH, et al. Clinical Outcome of Remnant Thyroid Ablation With Low Dose Radioiodine in Korean Patients With Low to Intermediate-Risk Thyroid Cancer. J Korean Med Sci (2015) 30(7):876–81. doi: 10.3346/jkms.2015.30.7.876
23. Verburg FA, Lassmann M, Mäder U, Luster M, Reiners C, Hänscheid H. The Absorbed Dose to the Blood Is a Better Predictor of Ablation Success Than the Administered 131i Activity in Thyroid Cancer Patients. Eur J Nucl Med Mol Imaging (2011) 38(4):673–80. doi: 10.1007/s00259-010-1689-5
24. Liu Y, Jin JH, Liu JZ, Li SJ, Wu ZF, Lu KY. Efficacy and Influencing Factors of Radioactive 131i for Elimination of Thyroid Remnants Following Differentiated Thyroid Carcinoma Resection. Chin Remedies Clin (2013) 13:556–8. doi: 10.11655/zgywylc2013.05.003
25. Shen Y, Liu HC, Hu YQ, Tao J, Sun WL, Yu ZY. Clinical Efficacy of 131i Treatment in 92 Cases of Differentiated Thyroid Cancer. Chin J Gen Pract (2011) 9:1517–9. doi: 10.16766/j.cnki.issn.1674-4152.2011.10.082
26. Kim EY, Kim TY, Kim WG, Yim JH, Han JM, Ryu JS, et al. Effects of Different Doses of Radioactive Iodine for Remnant Ablation on Successful Ablation and on Long-Term Recurrences in Patients With Differentiated Thyroid Carcinoma. Nucl Med Commun (2011) 32(10):954–9. doi: 10.1097/MNM.0b013e32834956ec
27. Rosario PW, Xavier AC, Calsolari MR. Value of Postoperative Thyroglobulin and Ultrasonography for the Indication of Ablation and (1)(3)(1)I Activity in Patients With Thyroid Cancer and Low Risk of Recurrence. Thyroid (2011) 21(1):49–53. doi: 10.1089/thy.2010.0145
28. Valadao MM, Rosario PW, Borges MA, Costa GB, Rezende LL, Padrao EL, et al. Positive Predictive Value of Detectable Stimulated Tg During the First Year After Therapy of Thyroid Cancer and the Value of Comparison With Tg-Ablation and Tg Measured After 24 Months. Thyroid (2006) 16(11):1145–9. doi: 10.1089/thy.2006.16.1145
Keywords: differentiated thyroid carcinomas, stimulated thyroglobulin, thyroid-stimulating hormone, antithyroglobulin antibody, iodine 131I therapeutic, predictive value
Citation: Pan M, Li Z, Jia M and Lu X (2022) Combination of Stimulated Thyroglobulin and Antithyroglobulin Antibody Predicts the Efficacy and Prognosis of 131I Therapy in Patients With Differentiated Thyroid Cancer Following Total Thyroidectomy: A Retrospective Study. Front. Endocrinol. 13:857057. doi: 10.3389/fendo.2022.857057
Received: 18 January 2022; Accepted: 25 February 2022;
Published: 06 April 2022.
Edited by:
Laura Sterian Ward, State University of Campinas, BrazilReviewed by:
Rosalia Padovani, Santa Casa of Sao Paulo, BrazilGlaucia M. F. S. Mazeto, São Paulo State University, Brazil
Copyright © 2022 Pan, Li, Jia and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Jia, MzU3NTkyMjEwQHFxLmNvbQ==; Xiubo Lu, ZG9jdG9ybHV4aXVib0AxMjYuY29t
 Mengjiao Pan
Mengjiao Pan Zhuyao Li
Zhuyao Li Xiubo Lu
Xiubo Lu