- 1Department of Endocrinology and Metabolism, West China Hospital of Sichuan University, Chengdu, China
- 2Laboratory of Diabetes and Islet Transplantation Research, Center for Diabetes and Metabolism Research, West China Hospital of Sichuan University, Chengdu, China
Objective: Many gastric artery embolizations (GAE) have been performed in recent years. We try to determine whether GAE caused weight loss by decreasing gastrointestinal hormone through the analysis of weight loss and gastrointestinal hormones changes.
Methods: The PubMed and Medline databases, and the Cochrane Library, were searched using the following keywords. A total of 10 animal trials (n=144), 15 human trials (n=270) were included for analysis. After GAE, we mainly evaluated the changes in body weight loss (BWL) and body mass index (BMI), as well as metabolic indexes, such as blood glucose, lipids, and gastrointestinal hormones levels.
Results: Animal subjects received either chemical or particle embolization, while human subjects only received particle embolization. In animal trials (growing period), the GAE group gained weight significantly slower than the sham-operated group, ghrelin levels decreased. In human trials, GAE brought more weight loss in the early stages, with a trend towards weight recovery after several months that was still lower than baseline levels. Besides weight loss, abnormal metabolic indicators, such as blood glucose and lipids were modified, and the quality of life (QOL) scores of obese patients improved. In addition, weight loss positively correlates with ghrelin.
Conclusion: GAE may help people lose weight and become a new minimally invasive and effective surgery for the treatment of modest obesity. Physiologic changes in gastrointestinal tract of gastrointestinal hormones level may be one reason for weight loss in GAE.
Introduction
Obesity has become a major chronic disease in recent years, with an increasing number of people suffering from it. Obesity-induced insulin resistance is the key factor of the metabolic syndrome, which can lead to metabolic diseases, such as type 2 diabetes (T2D) and hypertension, as well as sleep apnea, cardiovascular, musculoskeletal, reproductive, and psychological disorders (1). More weight loss within a certain range leads to greater benefits and obesity therapy is a critical health initiative.
Weight loss treatment includes lifestyle modification (diet and exercise), drug, and surgery. Lifestyle intervention and drug sometimes fail to reduce weight, especially in severely obese patients (2). Metabolic surgery is a highly effective intervention for metabolic syndrome that can promote substantial weight loss (3). Every year, 685,000 metabolic surgeries are performed worldwide. The three most commonly performed procedures are the Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG), and one-anastomosis gastric bypass (OAGB) (4, 5). The changes in gastrointestinal anatomical structure during weight loss surgery lead to neural and physiological changes that affect hypothalamic signals, intestinal hormones, bile acids, and intestinal microbiota. These changes limit feeding behavior and nutrient absorption to achieve weight loss. RYGB and SG result in the postprandial secretion of glucagon-like peptide-1 (GLP-1), peptide YY (PYY), and oxyntomodulin (OXM), which can increase satiety (6). These hormones regulate glucose homeostasis by altering insulin secretion and sensitivity in the early postoperative period (7). Laparoscopic technology is improving, and the incidence of postoperative complications (including gastric perforation, intestinal obstruction, mucosal tears, and gastrointestinal bleeding) are decreasing. However, still a significant number of patients rely solely on lifestyle intervention and drug due to their fear of surgery, resulting in weight loss failure (8). GAE is a new, minimally invasive surgery in which a physician inserts an angiographic catheter into the groin or wrist, navigates to the aorta, upper abdominal aorta, and left gastric artery through the femoral artery or radial artery. Following that, embolization is performed and the catheter is removed; this is known as transarterial embolization of the gastric fundus (9). Because the gastric fundus is mainly supplied by the left gastric artery (LGA) and a small part by the gastroepiploic artery, embolization has been performed primarily on the left gastric artery in most studies (10). This review describes animal and clinical trials to analyze the therapeutic effects and potential mechanisms of GAE.
Methods
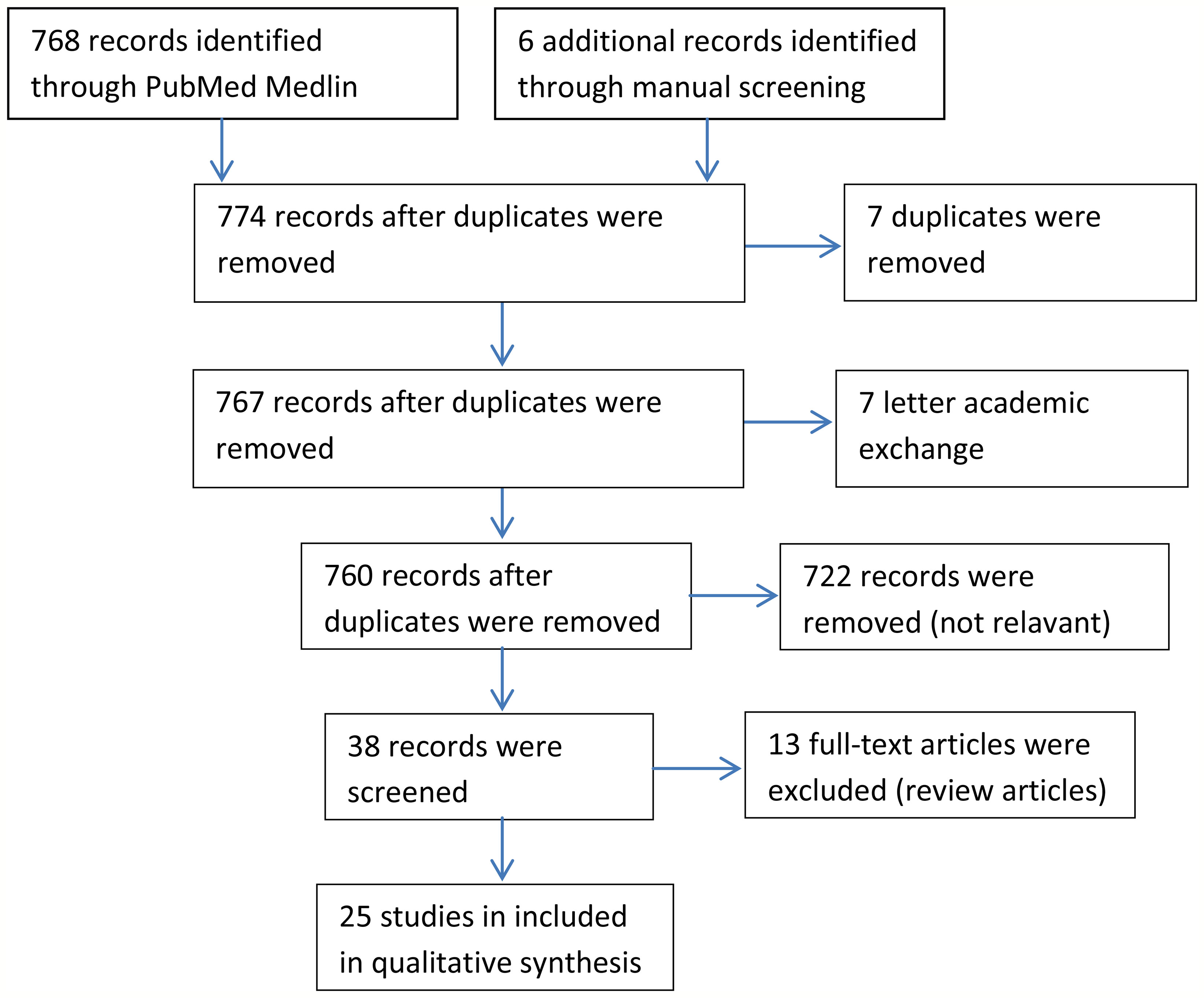
We searched out 768 articles by using gastric artery embolization as a keyword which included 3 meta-analyses (11–13), 7 systematic reviews (14–20), 3 systematic reviews only analysed embolic material choices or procedures (21–23), 15 articles about materials, 722 articles analyzed other indexes just like hepatocellular carcinoma, 7 academic discussions, and 7 duplicates (Figure 1). Finally, we counted 25 articles involving changes in body weight after GAE (10 animal trials [n=144 (24–33), 15 human trials (n=270) (33–48)] different from previous reviews.
Results: The Efficacy of Gastric Artery Embolization
Weight Loss
Animal Experiments
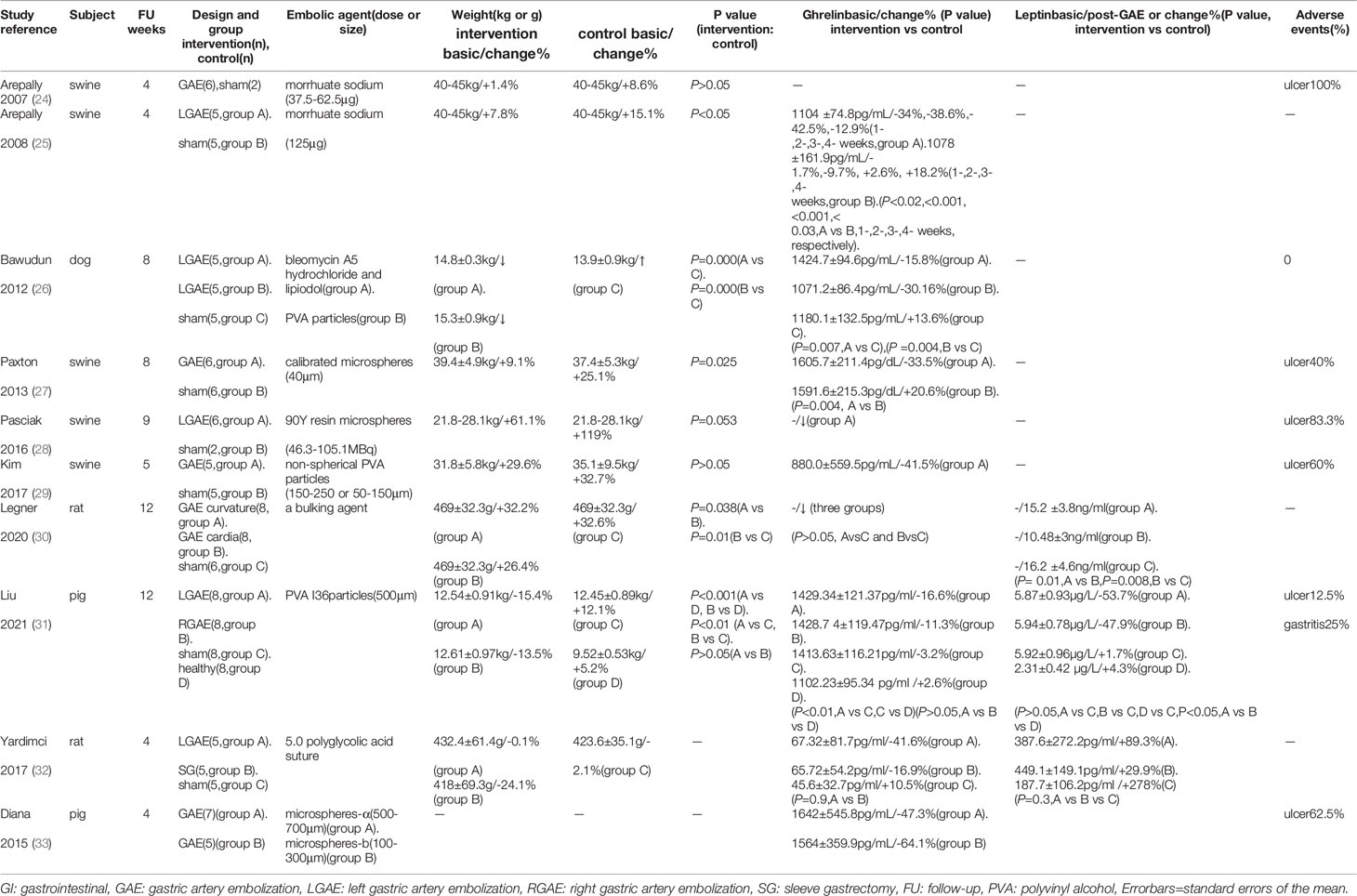
In all animal experiments, control groups were injected with normal saline (Table 1). Arepally et al. (24) initially reported that GAE could slow down the natural weight gain of developing pigs with a low dose of morrhuate sodium. In the following year, they (25) found that injecting a standard dose of morrhuate sodium into the left gastric artery supplying the gastric fundus significantly delayed weight gain in the experimental group compared to the control group. Bawudun et al. (26) used hydrochloride/lipiodol (group A) and polyvinyl alcohol (PVA) particles (group B) in different groups and discovered body weight loss in groups A and B, in contrast to the sham group. Furthermore, subcutaneous fat was significantly reduced in both groups (P<0.5). Later, solid substances were used in the following seven animal experiments (4-12 weeks) (27–33). Although the animals, embolic agents, embolic sites, and follow-up times used in the above studies were not identical, it was shown that GAE either prevented weight gain in developing animals or reduced weight in adult animals. However, GAE cannot achieve equal weight loss as SG.
Human Trials
Originally, two retrospective cohort studies (34, 36) compared fundic artery embolization with other arterial embolization for GI bleeding and showed significantly more body weight loss after GAE (P=0.006, 0.18, at 3-, 13-months, respectively). According to Gunn et al., weight recovery after 13 months was due to the short duration of weight loss, which may be related to revascularization or collateral artery formation after LGAE. In the experimental group, 58% had a history of malignancy and four patients were receiving chemotherapy during the study period, while 50% of the control group had a history of malignancy and six patients were receiving chemotherapy during the study period. Although there was no significant difference in the proportion of patients with a history of malignancy (P=0.29) or receiving chemotherapy (P=0.97) between the two groups, there was still an effect on the results. Then, two similar LGAE studies calculated patients without tumor for GI bleeding (41, 44), also found body weight loss, decreased BMI, excess body weight (EBW, basic 23.3 ± 10.6kg, -24.1%, P=0.003), and body fat index (BFI, basic 128.6 ± 54.6cm2/m2, -3.7%, P=0.03), subcutaneous fat index (SFI, basic 81.7 ± 44.5cm2/m2, -4.1%, P=0.03), and skeletal muscle index (SMI, basic 44.5 ± 7.2cm2/m2, -6.9%, P<0.001), but not visceral fat index (VFI, basic 35.8 ± 17.8cm2/m2, -4.1%, P=0.13) and intramuscular fat index (IMFI, basic 10.2 ± 4.8cm2/m2, -1.6%, P=0.83). Therefore, the latter eleven prospective studies with self-control design only included simple obese patients were conducted. GAE caused greater weight loss in the early stages, with a trend towards weight recovery after several months that was still lower than baseline levels (P<0.05) (Figure 2). Besides the observed weight changes, Levigard et al. (48) showed that both BMI and waist circumference (WC) decreased (basic 108.6 ± 11.16cm, -5.5%, P=0.03). Except WC decreased (basic 114.34 ± 10.40, -10.8%, P=0.122), waist-to-hip ratio(WHtR) decreased (basic 69.99 ± 4.94%, -10.8%, P=0.125), Bai et al. (40) further discovered a significant reduction in abdominal adipose tissue, including the area of subcutaneous adipose tissue (SAT,basic 400.90 ± 79.25cm2, -20.09%, -18.11%, -28.52%, P= 0.006, 0.02, 0.101, at 3-, 6-, 9-months, respectively), and total abdominal adipose tissue (TAAT,basic 695.8 ± 107.43cm2/-9.18%, -11.26%, -24.29%, P=0.169, 0.006, 0.107, at 3-, 6-, 9-months, respectively), using MRI (Table 2).
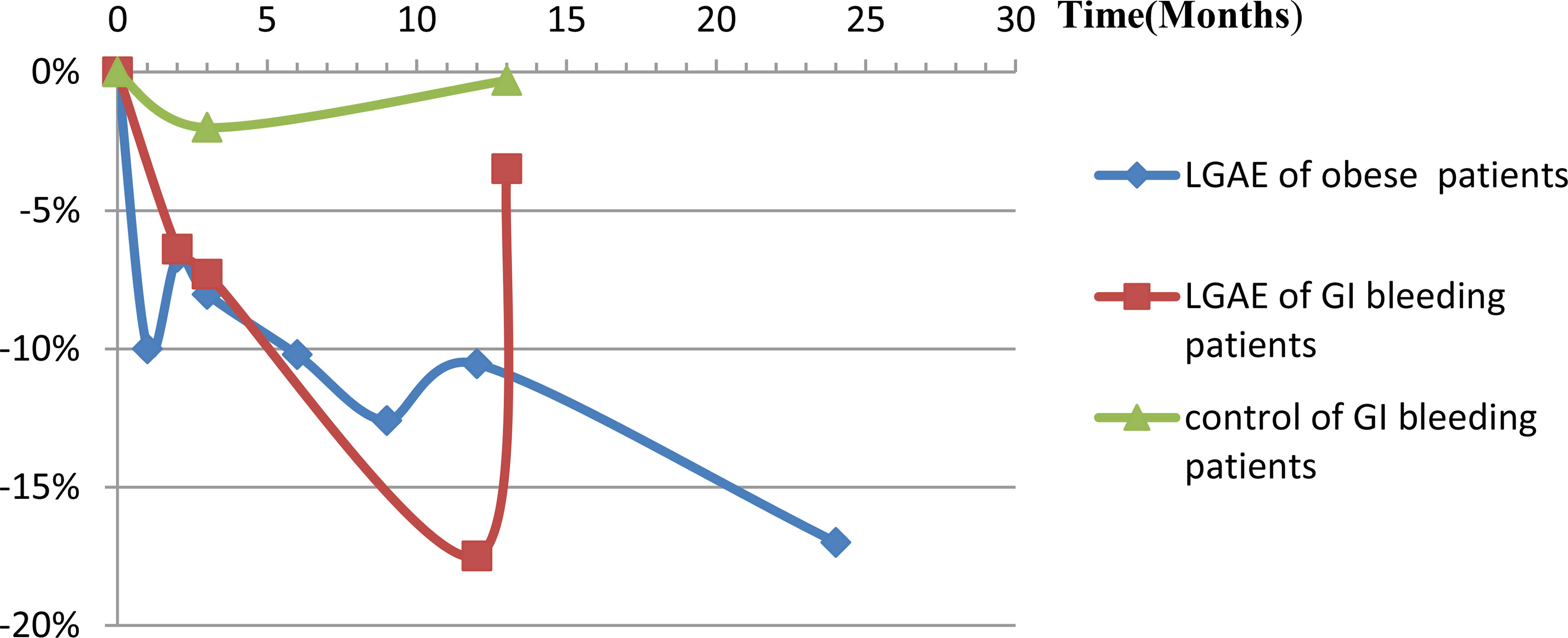
Figure 2 Body weight loss (%) changes post-LGAE in GI bleeding or obese patients. LGAE, left gastric artery embolization; GI, gastrointestinal.
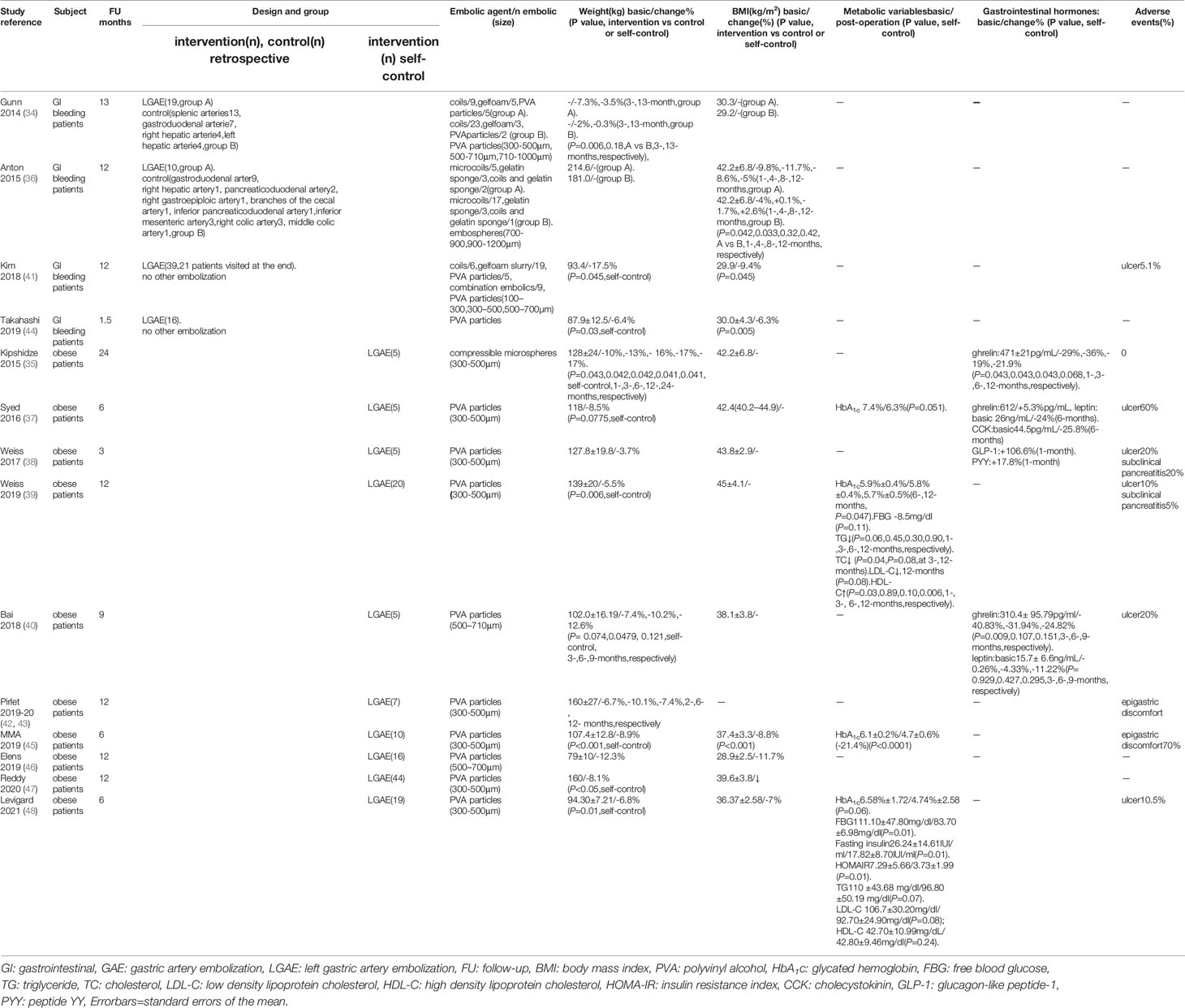
Table 2 Body weight and GI hormones changes post-GAE in GI bleeding patients (retrospective cohort studies and self-control study) and obese patients (self-control studies).
Metabolic Indexes
Only one animal trial (27) found no changes in serum glucose levels (P=0.81). Four human trials examined metabolic indicators (Table 2). Syed et al. (37) reported that one diabetic patient lost weight and HbA1C level dropped from three to six months. Zaitoun et al. (45) showed HbA1c reduced and there was a statistically significant positive correlation between reduced BMI and HbA1c (r=0.91, P=0.0002). Levigard et al. (48) found a decrease in fasting blood glucose (FBG), fasting insulin, insulin resistance index (HOMA-IR) (P=0.01), and HbA1c levels (P=0.06) after six months without medication change (Figure 3). Furthermore, the serum triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C) levels decreased (P>0.05), while high-density lipoprotein cholesterol (HDL-C) levels increased (P>0.05). Weiss et al. (38, 39) discovered that HbA1c and blood glucose decreased at 12 months, while HbA1c change did not correlate with weight change (r2 = 0.24). TG initially decreased but later rebounded to baseline level, total cholesterol (TC) and LDL-C levels decreased at 12 months, with the percentage of weight changes negatively correlating with LDL-C changes after 12 months (r2 = 0.24). HDL decreased one month after embolization but increased at all subsequent time (Figure 4). This suggests that GAE may improve metabolic indexes, but it is not clear whether it works by reducing body weight.
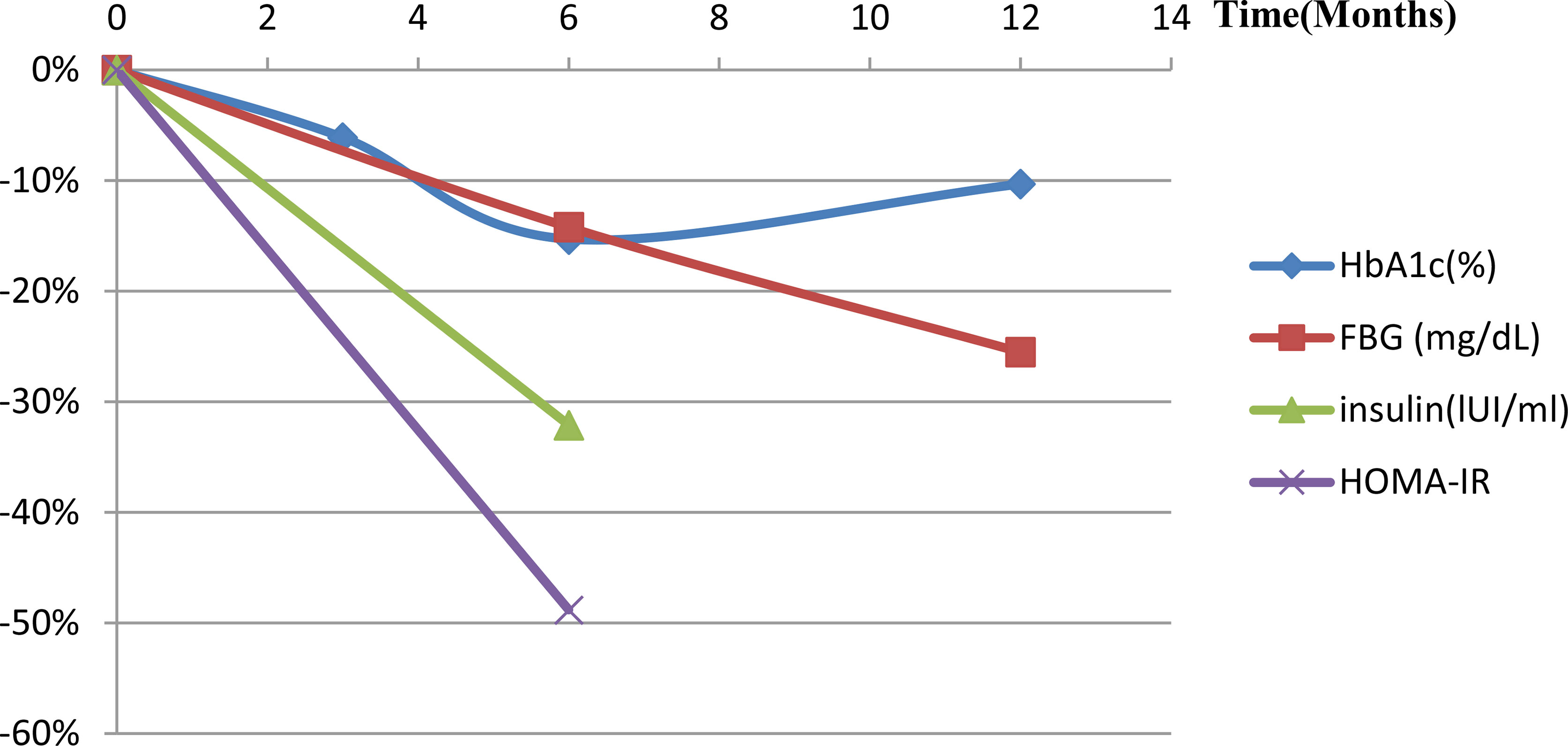
Figure 3 Metabolic index changes post-LGAE in obese patients. LGAE, left gastric artery embolization; HbA1c, glycated hemoglobin; FBG, free blood glucose; HOMA-IR, insulin resistance index.
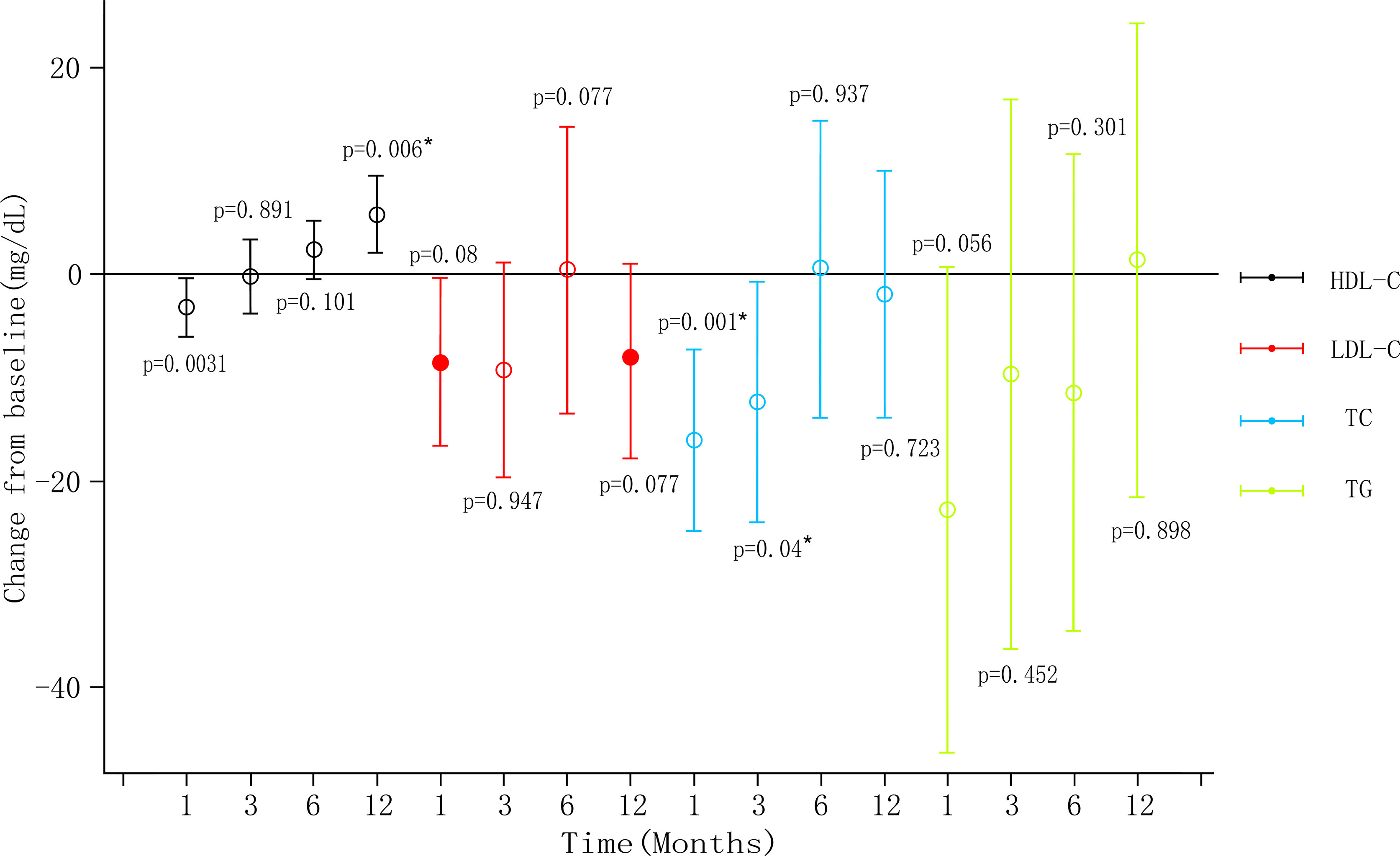
Figure 4 Blood lipid level changes post-LGAE in obese patients. LGAE, left gastric artery embolization; * indicates statistical significance, apllyied from weiss (39).
Gastrointestinal Hormones
Animal Experiments
Serum ghrelin was evaluated in nine animal trials. According to Arepally et al. (24), ghrelin increased in the low-dose group but decreased in the high-dose group. This may be due to incomplete embolization of the gastric fundic mucosa in the low-dose group, which did not effectively inhibit ghrelin. Therefore, a standard-dose test was performed the following year (25), where ghrelin levels decreased in the GAE swine after one week. Following that, several other tests revealed significant ghrelin reductions in the LGAE (33). Bawudun et al. (34) reported that the mean post-procedure relative percentage of ghrelin was decreased, while ghrelin was increased in the control group. Compared to the baseline, the changes in subcutaneous fat in all animals positively correlated with the changes in ghrelin (r=0.632, P=0.011), and the mean percentage changes in body weight of each group also positively correlated with the changes in ghrelin (r=0.740, P=0.000) (Table 1). According to Pasciak et al. (28), ghrelin-immunoreactive cell density was significantly lower in the treated groups (stomach fundus (13.5 ± 6.5) vs sham (34.8 ± 6.6), P<0.05), (body (11.2± 4.2) vs sham (19.8 ± 5.1),P<0.05), (duodenum (5.6 ± 2.2) vs sham (2.4± 0.9),P=0.081), and gastrin immunoreactive cell density in the stomach fundus was higher (76.2 ± 13.2 versus 65.7 ± 14.0, P=0.16). Paxton et al. (27) also discovered that ghrelin-immunoreactive mean cell density was significantly lower (15.3 versus 22.0, P<0.01), while fibrosis was increased in the gastric fundus of treated animals (P=0.07). These findings suggested that GAE reduces the number of gastrin-secreting cells in the gastric sinus with increased fundic fibrosis, leading to a decrease in ghrelin level.
Human Trials
Ghrelin was evaluated in three human trials involving obese patients (35, 37, 40). It was reported that ghrelin decreased most significantly within 1-6 months (P<0.05) but gradually recovered from 6-12 months (P>0.05), which was still lower than the baseline level. In addition, three trials examined other intestinal hormones besides ghrelin. At one and three months after surgery, Weiss et al. (38) reported GLP-1 and PYY levels elevated. Syed et al. (34) found decreased levels of cholecystokinin (CCK) and leptin after LGAE. Bai et al. (40) reported a similar decrease in leptin level. However, they did not perform a correlation analysis between weight loss and changes in gastrointestinal hormones (Table 2).
Quality of Life
Five human trials assessed the quality of life (QOL). Weiss et al. (38) evaluated eating using the appetite and satiety questionnaire, and found that hunger and the 3-day food log decreased by 25.1 ± 27 points after three months. The second trial reported (33) that QOL scores increased from 57 ± 18 to 77 ± 18 (P<0.001) after 12 months. In addition, the mean physical function scores increased from 55 ± 18 to 70 ± 21 (P=0.007), self-esteem scores increased from 50 ± 30 to 72 ± 25 (P=0.011), sexual life scores increased from 61 ± 35 to 88 ± 25 (P=0.003), public distress scores increased from 68 ± 19 to 79 ± 19 (P=0.003), and work scores increased from 73 ± 17 to 88 ± 13 (P=0.007) based on the Short Form 36 (SF-36) questionnaire. Syed et al. (37) found a mean improvement of 9.5 points on the physical component scores and 9.6 points on the mental using the SF-36 questionnaire. Elens et al. (47) reported a satisfaction score of 7.7 ± 1.6 on a scale of 0-10 at the 6-12 months follow-up, with 12.5% of the participants finding the procedure very painful and would not do it again. Levigard et al. (48) found that the QOL score improved from 59.64 ± 5.59 to 69.02 ± 11.97 (P<0.05), while binge eating scores (BES) reduced from 21.50 ± 8.89 to 9.60 ± 4.40 (P=0.01) (Figure 5). These findings demonstrated that postoperative patient quality questionnaire scores increased, but a small number of participants found the experiment process to be too painful.
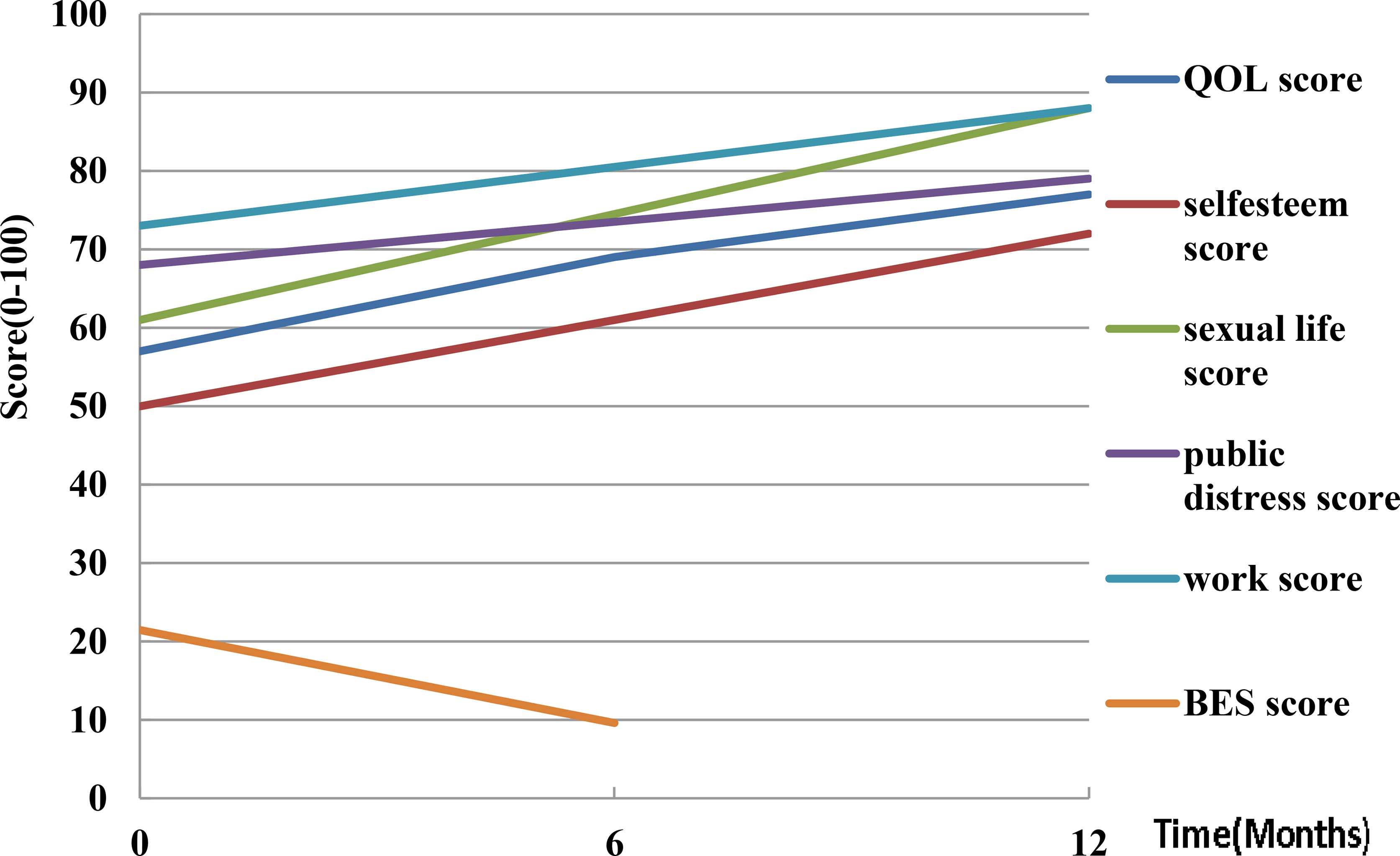
Figure 5 Quality of life changes post-LGAE in obese patients. LGAE, left gastric artery embolization; QOL, quality of life; BES, binge eating scale.
Inflammatory Factors
Chronic inflammatory response is a major cause of glucolipid metabolism disruption in obese patients. Bariatric surgery reduces many inflammatory mediators and cytokines associated with obesity. The levels of inflammatory mediators, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α were decreased after bariatric surgery (49–52). One animal trial (31) showed that serum IL-6 (220.45 ± 7.54pg/mL/-35.1% in LGAE group, 219.23 ± 7.22pg/mL/-33.3% in RGAE group, 219.23 ± 7.22pg/mL/-2% in sham group,131.23 ± 3.43pmol/L/+2.4% in healthy group) and TNF-α (551.23 ± 75.82 pmol/L/-49.3% in LGAE group, 561.11 ± 79.72pmol/L/-47.2% in RGAE group, 558.23 ± 78.82pmol/L/-1.3% in sham group, 224.22 ± 47.81pmol/L/+5.4% in healthy group) levels were lower in the LGAE and RGAE groups than in the sham-operated group, suggesting that the inflammatory syndrome response may be reduced. However, there was a lack of information on human studies.
Discussion: Influencing Factors for Efficacy
Surgical Methods
Although the animals, embolic agents, embolic sites, and follow-up times used in the animal studies were not identical, it was shown that GAE either prevented weight gain in developing animals or reduced weight in adult animals. We re-calculated all human experiments and found GAE caused greater weight loss in the early stages, with a trend towards weight recovery that was still lower than baseline level after 24 months in eleven prospective studies. GAE results in weight loss of no more than 20%, which is far from sufficient for severe obese patients, and perhaps GAE should only be used in patients with mild to moderate obesity, especially those who have failed in lifestyle intervention or pharmacological treatment. We consider that obesity is a chronic disease and may require several successive GAE to achieve remission or conventional metabolic surgery after a first embolization. Pirlet et al. (42) reported that one patient who left gastric angiogram showed recanalization of the distal gastric left artery vascular system, underwent a second embolization procedure 18 months later, suggesting that re-embolization is not recommended. If they receive conventional metabolic surgery after a first embolization, the use of the left gastric artery will reduce the vascularization of the right part of the cardia and could be a handicap for performing a gastric sleeve or a gastric bypass. So, we need to prevent and treat patients recovering from ischemic injury because the rebound of weight and ghrelin levels may be due to re-vascularization of the gastric fundus. In addition, weight regain occurred in classic bariatric surgery after two years, follow up time is insufficient, further studies with larger sample sizes and longer periods are required to obtain stronger evidence. Additionally, Liu et al. (31) comparing left and right gastric artery embolization found that LGAE resulted in greater weight loss but without statistical significance. Legner et al. (30) distinguished embolic site and found the weight gain of the group embolized at the cardia significantly lower than that of the group embolized at the great curvature of the stomach and the sham operation group in rats. Therefore, further comparative studies are needed concerning the specific embolization sites of GAE.
Embolic Materials
Arepally et al. used liquid sclerosants (e. g., sodium morrhuate, bleomycin A5 hydrochloride, and lipiodol emulsion) to achieve distal occlusion of all fundal arteries in developing swine, a procedure termed “gastric artery chemical embolization” (GACE). They found that chemical embolization is not consistently effective in reducing weight. The embolization of this fluid embolization was difficult to pinpoint, which could explain the occurrence of gastric ulceration and necrosis. Solid embolic materials were more likely to promote weight change compared to liquid material. Then, particles were used, including coils, gelatin sponge, and polyvinyl alcohol (PVA) particles ranging from 100-1200µm. Larger PVA particles and microspheres that were biocompatible, as well as a highly compressible embolic agent, have been widely used (21). The size of the embolized particles is a critical factor. Plasma ghrelin levels were similar between GAE pigs and control pigs, regardless of microsphere size. Five ulcers in five pigs embolized by using smaller microspheres (100-300µm), and three ulcers in five pigs embolized by using larger microspheres (300-500µm) (53), it is suggested that large particles may be a more suitable embolic material for GAE. Furthermore, they used x-ray-visible embolic microspheres (XEMs) and an antireflux catheter to improve safety and efficacy of GAE (14, 54–56). Still, comparative studies on the source, size, and method of embolization of embolic materials are required to determine the optimal embolic material.
Gastrointestinal Hormone Changes Post-GAE
The mechanisms underlying bariatric surgery for the treatment of obesity and metabolic syndrome are not fully understood. An increasing number of studies have shown that many GI hormones levels are significantly changed after bariatric surgery, demonstrating that they may be important mediators that influence feeding behavior and regulate glucose level. The main GI hormones associated with energy homeostasis are PYY, GLP-1, CCK, OXM, gastrin, glicentin, and pancreatic precipitin (53). Both RYGB and SG lead to the postprandial secretion of GLP-1, PYY, and OXM, which can increase satiety (14, 54–56), these hormones regulate glucose homeostasis by altering insulin secretion and sensitivity during the early postoperative period (57, 58). Therefore, does GAE also result in weight loss by altering GI hormones? (Figure 6).
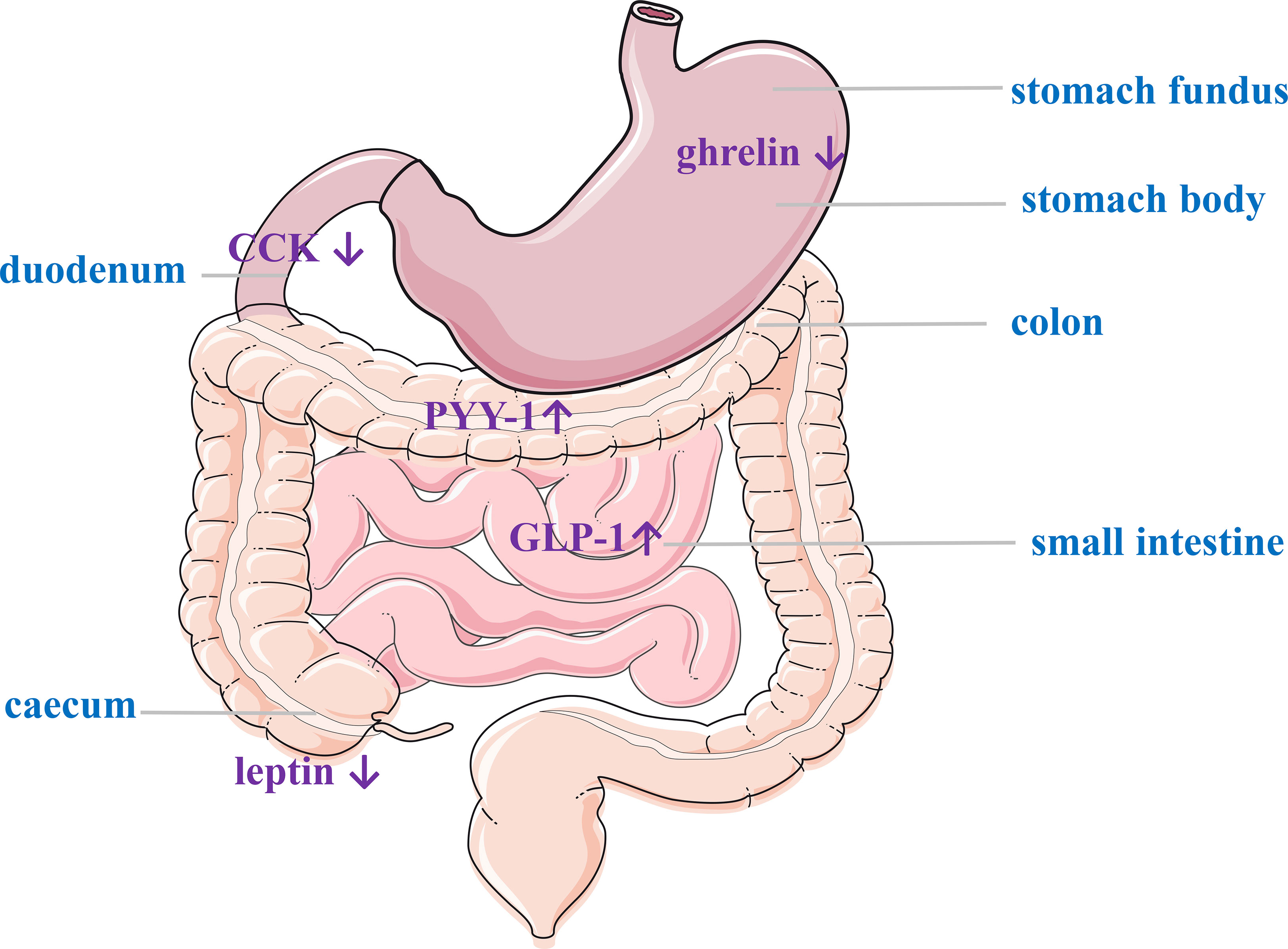
Figure 6 Gastrointestinal hormone changes post-LGAE. LGAE, left gastric artery embolization; CCK, cholecystokinin; GLP-1, glucagon-like peptide-1; PYY, peptide YY.
Ghrelin
Ghrelin is a 28 amino acid peptide secreted primarily from the fundus of the stomach (59) and in small amounts from the duodenum, pancreas, ovaries, adrenal cortex, and brain (60). It may increase appetite and lead to weight gain, and it’s the only gastrointestinal hormone that stimulates appetite. Many studies have reported that ghrelin level decrease after metabolic surgery, receptors in the arcuate nucleus (hypothalamus) bind and integrate signals sent to the satiety center (located in the nucleus solitarius), reducing hunger and appetite (61–63). Ghrelin levels were found to be decreased in vivo in nine animal and four human studies. But there may be some confounding factors in the results. On the one hand, circulating ghrelin consists of two forms: inactive ghrelin (90%) and active acylated ghrelin (10%) (64), none of the published studies have examined the active form (65) because the active form of ghrelin is unstable at a normal temperature (66). On the other hand, the lack of standardization in the measurement of ghrelin level in terms of sample collection schedule, collection method, follow-up period, storage of sample, and radioimmunoassay may affect the precision of the results.
Other Hormones
GLP-1 is produced by L-cells in the distal small intestine and colon in response to food intake and is considered the “ileal brake” on food intake. It provides satiety by activating receptors in the vagus nerve, proximal gastrointestinal tract, pancreas, brainstem, and hypothalamus. There is a wide range of GLP-1 secretion effects, including delayed gastric emptying, increased insulin secretion, and decrease gastric acid and glucagon secretion (67). PYY is mainly secreted by L-cells in the ileum and colon in response to food intake and, to a lesser extent, in the delta-, PP-, and alpha-cells of the pancreatic islets. The biggest change in intestinal hormone secretion after RYGP or SG is the significant increase in peripheral GLP-1and PYY in the days following surgery (68–71). Weiss et al. (38) revealed that there was an increase in GLP-1 and PYY after LGAE, suggesting that LGAE may cause changes in the levels of both of these gastrointestinal hormones associated with weight loss. CCK in the intestinal mucosa has the highest density of I-cells in the duodenum and proximal jejunum (72). The ingestion of protein, amino acids, and digested fat results in the maximal release of CCK (73). CCK promotes satiety by delaying gastric emptying through CCK receptors (stimulating pancreatic enzyme secretion and gallbladder wall contraction). The levels of CCK were elevated after RYGB (57, 74), with a gradual increase in secretion one week after SG (75). The effect of bariatric surgery on CCK homeostasis remains unclear. Similarly, only one trial (37) demonstrated a decrease in CCK after GAE. Leptin is released by subcutaneous white adipose tissue (WAT) (76) that acts as an endocrine signal by reducing appetite, activating pre-opioid melanocortin (POMC)-expressing neurons, and inhibiting hypothalamic agouti-related protein (AgRP) and neuropeptide Y (NPY). In addition, leptin increases the oxidation and absorption of glucose and free fatty acid in skeletal muscles, as well as promotes intrahepatic lipid reduction through fatty acid oxidation (77). The changes in leptin levels after GAE are inconsistent (30–32, 37, 40), and its relationship with weight loss has not been investigated.
Therefore, both animal and human studies have shown a decrease in ghrelin release from gastric fundic cells and an increase in GLP-1 and PYY which associated with reduced hunger and weight loss, and there was a positive correlation between weight loss and ghrelin, suggesting GAE may induce weight loss by lowering ghrelin. Yardimci et al. (32) found that SG can lead to more significant weight loss by altering the anatomy of the gastrointestinal tract compared with GAE although ghrelin decrease similarly in the two procedures. This suggests that GAE is not as effective as conventional metabolic surgery, with the latter causing the most significant weight loss due to alterations in the anatomy of the gastrointestinal tract, with changes in gastrointestinal hormone levels being a secondary factor. One previous meta-analysis (21) although demonstrated a significant reduction in MD of ghrelin levels among two animal studies (27–29) (MD: − 756.56, 95% CI − 1098.78, − 414.33, P < 0.001), but Hedges’ g analysis among three human studies (38, 40, 45) showed that GAE had not significantly affected serum ghrelin level (Hedges’ g statistic: − 0.91, 95% CI − 1.83, 0.01, P = 0.05). We have summarized the reasons for the different statistical results obtained. Firstly, ghrelin increased at 1 month, decreased most at 3 months, recovered slightly at 6 to 9 months, so, the time points for analysis were different. Secondly, we observed a decrease in ghrelin level in 9 animal experiments, with some statistics showing P>0.05 and some P<0.05. We did further correlation analysis, and discovered weight loss positively correlates with ghrelin which indicated that the left gastric artery may be reduce serum ghrelin and induce weight loss after LGAE. But, researchers need to establish a large number of randomized controlled trial (RCT) studies with the same subjects and compare previous metabolic surgery with GAE to complete a correlation analysis between weight loss and hormonal change to confirm whether gastrointestinal hormone change is an important factor in weight loss.
Effects of Feeding Behavior
Decreased appetite is an important factor in weight loss, but whether appetite change is associated with gastrointestinal hormone change or with stomach ischemic injury is not clear. Previous studies didn’t record eating, so the structure and volume of diet require further investigation to accurately assess the effect of GAE surgery on feeding behavior and the efficacy of the latter on weight loss.
Adverse Reactions
One pig that received the highest dose (2000 μg) during GAE died of ruptured gastric ulcers on postoperative day 1. Another pig died of peritonitis due to a surgical technical error that involved gastric fluid leakage from a poorly sealed biopsy site on postoperative day 2. The remaining adverse effects mainly included superficial gastric ulcer (12.5-100%), which was examined by endoscopy in six animal studies, embolization recanalization, and an increased vascular network at the cardia. Furthermore, the main adverse effects in eight human studies were gastric ulcers (10.5-60%) and superficial gastritis (25%), while a few patients had mild subclinical pancreatitis (5-20%) and epigastric discomfort (70%). However, the clinical symptoms of all patients were alleviated after being given proton pump inhibitors (PPI) one week before and one month after surgery (Tables 1, 2). This indicates that the incidence of postoperative GI adverse reactions is mild and can be alleviated by perioperative administration of PPI.
Conclusions
GAE is a new, innovative, image-guided approach for the treatment of obesity. Compared to invasive procedures, it is less harmful and its most common adverse effects are gastric ulcers, mild pancreatitis, and uncomfortable clinical symptoms, which can be alleviated by PPI preparations. It may be useful for mild to moderate obese patients who are struggling with a lifestyle-based weight loss program (78). However, there are still many issues with GAE that need to be addressed. Further RCT studies with large samples are required to compare the effects of different types of GAE to identify the best operation method and the optimal embolic agent, to determine whether GAE reduce serum ghrelin and induce weight loss, to compare the long-term effectiveness, safety, and health economics of GAE to classic bariatric surgery.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYGD 18017).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Haslam DW, James WPT. Obesity. Lancet (2005) 366(9492):1197–209. doi: 10.1016/s0140-6736(05)67483-1
2. Montesi L, El Ghoch M, Brodosi L, Calugi S, Marchesini G, Grave RD, et al. Long-Term Weight Loss Maintenance for Obesity: A Multidisciplinary Approach. Diabetes Metab Syndr Obes (2016) 9:37–46. doi: 10.2147/DMSO.S89836
3. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, et al. Bariatric-Metabolic Surgery Versus Conventional Medical Treatment in Obese Patients With Type 2 Diabetes: 5 Year Follow-Up of an Open-Label, Single-Centre, Randomised Controlled Trial. Lancet (2015) 386(9997):964–73. doi: 10.1016/s0140-6736(15)00075-6
4. Angrisani L, Santonicola A, Iovino P, Vitiello A, Higa K, Himpens J, et al. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes Surg (2018) 28(12):3783–94. doi: 10.1007/s11695-018-3450-2
5. Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J, et al. Bariatric Surgery Worldwide: Baseline Demographic Description and One-Year Outcomes From the Fourth IFSO Global Registry Report 2018. Obes Surg (2019) 29(3):782–95. doi: 10.1007/s11695-018-3593-1
6. Perakakis N, Kokkinos A, Peradze N, Dixon J, Liem R, Ottosson J, et al. Circulating Levels of Gastrointestinal Hormones in Response to the Most Common Types of Bariatric Surgery and Predictive Value for Weight Loss Over One Year: Evidence From Two Independent Trials. Metabolism (2019) 101:153997. doi: 10.1016/j.metabol.2019.153997
7. Bojsen-Møller KN, Dirksen C, Jørgensen NB, Jacobsen SH, Serup AK, Albers PH, et al. Early Enhancements of Hepatic and Later of Peripheral Insulin Sensitivity Combined With Increased Postprandial Insulin Secretion Contribute to Improved Glycemic Control After Roux-En-Y Gastric Bypass. Diabetes (2014) 63(5):1725–37. doi: 10.2337/db13-1307
8. Barondess J, Fromm H, Greenway F, Grundy S, Halsted C. NIH Conference. Gastrointestinal Surgery for Severe Obesity. Consensus Development Conference Panel. Ann Intern Med (1991) 115(12):956–61.
9. Garcia-Reyes K, Fischman A. Anatomical and Technical Considerations for Bariatric Embolization. Tech Vasc Interv Radiol (2020) 23(1):100657. doi: 10.1016/j.tvir.2020.100657
10. Jernigan SR, Osborne JA, Buckner GD. Gastric Artery Embolization: Studying the Effects of Catheter Type and Injection Method on Microsphere Distributions Within a Benchtop Arterial Model. BioMed Eng Online (2020) 19(1):54. doi: 10.1186/s12938-020-00794-z
11. Kordzadeh A, Lorenzi B, Hanif MA, Charalabopoulos A. Left Gastric Artery Embolisation for the Treatment of Obesity: A Systematic Review. Obes Surg (2018) 28(6):1797–802. doi: 10.1007/s11695-018-3211-2
12. Mizandari M, Keshavarz P, Azrumelashvili T, Yazdanpanah F, Lorzadeh E, Hosseinpour H, et al. Left Gastric Artery Embolization for Obesity Treatment: A Systematic Review and Meta-Analysis of Human and Animal Studies. Abdom Radiol (NY) (2021) 46(9):4440–51. doi: 10.1007/s00261-021-03036-5
13. Hafezi-Nejad N, Bailey CR, Gunn AJ, Weiss CR. Weight Loss After Left Gastric Artery Embolization: A Systematic Review and Meta-Analysis. J Vasc Interv Radiol (2019) 30(10):1593–1603.e3. doi: 10.1016/j.jvir.2019.06.020
14. Karra E, Batterham RL. The Role of Gut Hormones in the Regulation of Body Weight and Energy Homeostasis. Mol Cell Endocrinol (2010) 316(2):120–8. doi: 10.1016/j.mce.2009.06.010
15. Hafezi-Nejad N, Bailey CR, Weiss CR. Bariatric Embolization: A Narrative Review of Clinical Data From Human Trials. Tech Vasc Interv Radiol (2020) 23(1):100658. doi: 10.1016/j.tvir.2020.100658
16. Aldawudi I, Katwal PC, Aldawudi SJ, Htun ZM, Khan S. Future of Bariatric Embolization: A Review of Up-To-Date Clinical Trials. Cureus (2020) 12(5):e7958. doi: 10.7759/cureus.7958
17. Shoar S, Saber AA, Aladdin M, Bashah MM, AlKuwari MJ, Rizwan M, et al. Bariatric Manipulation of Gastric Arteries: A Systematic Review on the Potential Concept for Treatment of Obesity. Int J Surg (2016) 36(Pt A):177–82. doi: 10.1016/j.ijsu.2016.10.014
18. Zhong B-Y, Abiola G, Weiss CR. Bariatric Arterial Embolization for Obesity: A Review of Early Clinical Evidence. Cardiovasc Intervent Radiol (2018) 41(11):1639–47. doi: 10.1007/s00270-018-1996-y
19. Weiss CR. Anjaneya S Kathait. Bariatric Embolization: A New and Effective Option for the Obese Patient? Expert Rev Gastroenterol Hepatol (2017) 11(4):293–302. doi: 10.1080/17474124.2017.1294060
20. Fried M, Doležalová K. Overview of Developments in Bariatric Surgery in the Czech Republic and Worldwide and New Trends in Bariatric-Metabolic Surgery. Cas Lek Cesk (2020) 159(3-4):141–3.
21. Fu Y, Kraitchman DL. Rationale and Preclinical Data Supporting Bariatric Arterial Embolization. Tech Vasc Interv Radiol (2020) 23(1):100656. doi: 10.1016/j.tvir.2020.100656
22. Garcia-Reyes K, Fischman A. Anatomical and Technical Considerations for Bariatric Embolization. Tech Vasc Interv Radiol (2020) 23(1):100657. doi: 10.1016/j.tvir.2020.100657
23. Weiss CR, Gunn AJ, Kim CY, Paxton BE, Kraitchman DL, Arepally A. Bariatric Embolization of the Gastric Arteries for the Treatment of Obesity. J Vasc Interv Radiol (2015) 26(5):613–24. doi: 10.1016/j.jvir.2015.01.017
24. Arepally A, Barnett BP, Montgomery E, Patel TH. Catheter-Directed Gastric Artery Chemical Embolization for Modulation of Systemic Ghrelin Levels in a Porcine Model: Initial Experience. Radiology (2007) 244(1):138–43. doi: 10.1148/radiol.2441060790
25. Arepally A, Barnett BP, Patel TH, Howland V, Boston RC, Kraitchman DL, et al. Catheter-Directed Gastric Artery Chemical Embolization Suppresses Systemic Ghrelin Levels in Porcine Model. Radiology (2008) 249(1):127–33. doi: 10.1148/radiol.2491071232
26. Bawudun D, Xing Y, Liu W-Y, Huang Y-J, Ren W-X, Ma M, et al. Ghrelin Suppression and Fat Loss After Left Gastric Artery Embolization in Canine Model. Cardiovasc Intervent Radiol (2012) 35(6):1460–6. doi: 10.1007/s00270-012-0362-8
27. Paxton BE, Kim CY, Alley CL, Crow JH, Balmadrid B, Keith CG, et al. Bariatric Embolization for Suppression of the Hunger Hormone Ghrelin in a Porcine Model. Radiology (2013) 266(2):471–9. doi: 10.1148/radiol.12120242
28. Pasciak AS, Bourgeois AC, Paxton BE, Nodit L, PN C, Kraitchman D, et al. Bariatric Radioembolization: A Pilot Study on Technical Feasibility and Safety in a Porcine Model. J Vasc Interv Radiol 27(10):1509–17. doi: 10.1016/j.jvir.2016.05.020
29. Kim JM, Kim M-D, Han K, Muqmiroh L, Kim SU, Kim GM, et al. Bariatric Arterial Embolization With Non-Spherical Polyvinyl Alcohol Particles for Ghrelin Suppression in a Swine Model. Cardiovasc Intervent Radiol (2017) 40(5):744–9. doi: 10.1007/s00270-017-1600-x
30. Legner A, Kong S-H, Liu Y-Y, Shabat G, Halvax P, Saadi A, et al. The GAMMA Concept (Gastrointestinal Activity Manipulation to Modulate Appetite) Preliminary Proofs of the Concept of Local Vibrational Gastric Mechanical Stimulation. Surg Endosc (2020) 34(12):5346–53. doi: 10.1007/s00464-019-07325-5
31. Liu H, Li X, Chen R, Liu D-C, Tong C. Effect of Left or Right Gastric Artery Interventional Embolization on Obesity and Ghrelin/Leptin Expression in Pigs. Am J Transl Res (2021) 13(5):5444–51.
32. Yardimci E, Bozkurt S, Cengiz MB, Malya FU. Comparison of Weight Loss, Ghrelin, and Leptin Hormones After Ligation of Left Gastric Artery and Sleeve Gastrectomy in Nbv A Rat Model. Med Sci Monit (2017) 23:1442–7. doi: 10.12659/msm.901003
33. Diana M, Pop R, Beaujeux R, Dallemagne B, Halvax P, Schlagowski I, et al. Embolization of Arterial Gastric Supply in Obesity (EMBARGO): An Endovascular Approach in the Management of Morbid Obesity Proof of the Concept in the Porcine Model. Obes Surg (2015) 25(3):550–8. doi: 10.1007/s11695-014-1535-0
34. Gunn AJ, Oklu R. A Preliminary Observation of Weight Loss Following Left Gastric Artery Embolization in Humans. J Obes (2014) 2014:185349. doi: 10.1155/2014/185349
35. Kipshidze N, Archvadze A, Bertog S, Leon MB, Sievert H. Endovascular Bariatrics: First in Humans Study of Gastric Artery Embolization for Weight Loss. JACC Cardiovasc Interv (2015) 8(12):1641–4. doi: 10.1016/j.jcin.2015.07.016
36. Anton K, Rahman T, Bhanushali A, Nadal L, Pierce G, Patel A. Weight Loss Following Left Gastric Artery Embolization in a Human Population Without Malignancy: A Retrospective Review. J Obes Weight Loss Ther (2015) 5(6). doi: 10.4172/2165-7904.1000285
37. Syed MI, Morar K, Shaikh A, Craig P, Khan O, Patel S, et al. Gastric Artery Embolization Trial for the Lessening of Appetite Nonsurgically (GET LEAN): Six-Month Preliminary Data. J Vasc Interv Radiol (2016) 27(10):1502–8. doi: 10.1016/j.jvir.2016.07.010
38. Weiss CR, Akinwande O, Paudel K, Cheskin LJ, Holly B, Hong K, et al. Clinical Safety of Bariatric Arterial Embolization: Preliminary Results of the BEAT Obesity Trial. Radiology (2017) 283(2):598–608. doi: 10.1148/radiol.2016160914
39. Weiss CR, Abiola GO, Fischman AM, Cheskin LJ, Vairavamurthy J, Holly BP, et al. Bariatric Embolization of Arteries for the Treatment of Obesity (BEAT Obesity) Trial: Results at 1 Year. Radiology (2019) 291(3):792–800. doi: 10.1148/radiol.2019182354
40. Bai Z-B, Qin Y-L, Deng G, Zhao G-F, Zhong B-Y, Teng G-J. Bariatric Embolization of the Left Gastric Arteries for the Treatment of Obesity: 9-Month Data in 5 Patients. Obes Surg (2018) 28(4):907–15. doi: 10.1007/s11695-017-2979-9
41. Kim DJ, Raman HS, Salter A, Ramaswamy R, Gunn AJ, Weiss CR, et al. Analysis of Weight Changes After Left Gastric Artery Embolization in a Cancer-Naive Population. Diagn Interv Radiol Mar-Apr (2018) 24(2):94–7. doi: 10.5152/dir.2018.17412
42. Pirlet C, Ruzsa Z, Costerousse O, Nemes B, Merkely B, Poirier P, et al. Transradial Left Gastric Artery Embolization to Treat Severe Obesity: A Pilot Study. Catheter Cardiovasc Interv (2019) 93(3):365–70. doi: 10.1002/ccd.27846
43. Pirlet C, Ruzsa Z, Nemes B, Poirier P, Bertrand OF. Long-Term Outcomes and Weight Loss After Bariatric Embolization of the Left Gastric Artery. J Invasive Cardiol (2020) 32(8):310–4.
44. Takahashi EA, Takahashi N, Reisenauer CJ, Moynagh MR, Misra S. Body Composition Changes After Left Gastric Artery Embolization in Overweight and Obese Individuals. Abdom Radiol (NY) (2019) 44(7):2627–31. doi: 10.1007/s00261-019-02002-6
45. Zaitoun MMA, Abd Alkhalik Basha M, Hassan F, Elsayed SB, Farag AA, Amer M, et al. Left Gastric Artery Embolization in Obese, Prediabetic Patients: A Pilot Study. J Vasc Interv Radiol (2019) 30(6):790–6. doi: 10.1016/j.jvir.2019.02.010
46. Elens S, Roger T, Elens M, Rommens J, Sarafidis A, Capelluto E, et al. Gastric Embolization as Treatment for Overweight Patients; Efficacy and Safety. Cardiovasc Intervent Radiol (2019) 42(4):513–9. doi: 10.1007/s00270-018-2130-x
47. Reddy VY, Neužil P, Musikantow D, Sramkova P, Rosen R, Kipshidze N, et al. Transcatheter Bariatric Embolotherapy for Weight Reduction in Obesity. J Am Coll Cardiol (2020) 76(20):2305–17. doi: 10.1016/j.jacc.2020.09.550
48. Levigard RB, Serrão H, Castro C, Matos P, Mattos F, Madeira E, et al. Bariatric Embolization in the Treatment of Patients With a Body Mass Index Between 30 and 39.9 Kg/M2 (Obesity Class I and II) and Metabolic Syndrome, a Pilot Study. Cardiovasc Intervent Radiol (2021) 44(4):598–606. doi: 10.1007/s00270-021-02776-7
49. Miller GD, Nicklas BJ, Fernandez A. Serial Changes in Inflammatory Biomarkers After Roux-En-Y Gastric Bypass Surgery. Surg Obes Relat Dis (2011) 7(5):618–24. doi: 10.1016/j.soard.2011.03.006
50. Raghavendra Rao S. Inflammatory Markers and Bariatric Surgery: A Meta-Analysis. Inflamm Res (2012) 61(8):789–807. doi: 10.1007/s00011-012-0473-3
51. Saltiel AR, Olefsky JM. Inflammatory Mechanisms Linking Obesity and Metabolic Disease. J Clin Invest (2017) 127(1):1–4. doi: 10.1172/jci92035
52. Viana EC, Araujo-Dasilio KL, Miguel GPS, Bressan J, Lemos EM, Moyses MR, et al. Gastric Bypass and Sleeve Gastrectomy: The Same Impact on IL-6 and TNF-α. Prospective Clinical Trial. Obes Surg (2013) 23(8):1252–61. doi: 10.1007/s11695-013-0894-2
53. Fu Y, Weiss CR, Paudel K, Shin EJ, Kedziorek D, Arepally A, et al. Bariatric Arterial Embolization: Effect of Microsphere Size on the Suppression of Fundal Ghrelin Expression and Weight Change in a Swine Model. Radiology (2018) 289(1):83–9. doi: 10.1148/radiol.2018172874
54. Weiss CR, Fu Y, Beh C, Hu C, Kedziorek D, Shin E-J, et al. Bariatric Arterial Embolization With Calibrated Radiopaque Microspheres and an Antireflux Catheter Suppresses Weight Gain and Appetite-Stimulating Hormones in Swine. J Vasc Interv Radiol (2020) 31(9):1483–91. doi: 10.1016/j.jvir.2020.04.038
55. Syed M, Efridi W, Khan T, Deek F, Kurian M, Shaikh A. A Novel Approach to Catheterizing the Left Gastric Artery for Bariatric Embolization. Radiol Case Rep (2021) 16(11):3574–6. doi: 10.1016/j.radcr.2021.08.051
56. Nielsen MS, Ritz C, Albrechtsen NJW, Holst JJ, Roux CWL, Sjödin A. Oxyntomodulin and Glicentin May Predict the Effect of Bariatric Surgery on Food Preferences and Weight Loss. J Clin Endocrinol Metab (2020) 105(4):dgaa061. doi: 10.1210/clinem/dgaa061
57. Jacobsen SH, Olesen SC, Dirksen C, Jørgensen NB, Bojsen-Møller KN, Kielgast U, et al. Changes in Gastrointestinal Hormone Responses, Insulin Sensitivity, and Beta-Cell Function Within 2 Weeks After Gastric Bypass in Non-Diabetic Subjects. Obes Surg (2012) 22(7):1084–96. doi: 10.1007/s11695-012-0621-4
58. Pérez-Pevida B, Escalada J, Miras AD, Frühbeck G. Mechanisms Underlying Type 2 Diabetes Remission After Metabolic Surgery. Front Endocrinol (Lausanne) (2019) 10:641. doi: 10.3389/fendo.2019.00641
59. Kojima M, Hosoda H, Date Y, Nakazato M, et al. Ghrelin is a Growth-Hormone-Releasing Acylated Peptide From Stomach. Nature (1999) 402(6762):656–60. doi: 10.1038/45230
60. Hameed S, Dhillo WS, Bloom. Gut hormones SR. And Appetite Control. Oral Dis (2009) 15(1):18–26. doi: 10.1111/j.1601-0825.2008.01492.x
61. Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma Ghrelin Levels After Diet-Induced Weight Loss or Gastric Bypass Surgery. N Engl J Med (2002) 346(21):1623–30. doi: 10.1056/NEJMoa012908
62. Anton K, Rahman T, Bhanushali A, Patel AA. Bariatric Left Gastric Artery Embolization for the Treatment of Obesity: A Review of Gut Hormone Involvement in Energy Homeostasis. AJR Am J Roentgenol (2016) 206(1):202–10. doi: 10.2214/ajr.15.14331
63. Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, et al. Ghrelin Causes Hyperphagia and Obesity in Rats. Diabetes (2001) 50(11):2540–7. doi: 10.2337/diabetes.50.11.2540
64. Camilleri M, Papathanasopoulos A, Odunsi ST. Actions and Therapeutic Pathways of Ghrelin for Gastrointestinal Disorders. Nat Rev Gastroenterol Hepatol (2009) 6(6):343–52. doi: 10.1038/nrgastro.2009.72
65. Anderson B, Switzer NJ, Almamar A. The Impact of Laparoscopic Sleeve Gastrectomy on Plasma Ghrelin Levels: A Systematic Review. Obes Surg (2013) 23(9):1476–80. doi: 10.1007/s11695-013-0999-7
66. Gröschl M, Uhr M, Kraus T. Evaluation of the Comparability of Commercial Ghrelin Assays. Clin Chem (2004) 50(2):457–8. doi: 10.1373/clinchem.2003.025429
67. Beckman LM, Beckman TR, Earthman CP. Changes in Gastrointestinal Hormones and Leptin After Roux-En-Y Gastric Bypass Procedure: A Review. J Am Diet Assoc (2010) 110(4):571–84. doi: 10.1016/j.jada.2009.12.023
68. Madsbad S, Dirksen C, Holst JJ. Mechanisms of Changes in Glucose Metabolism and Bodyweight After Bariatric Surgery. Lancet Diabetes Endocrinol (2014) 2(2):152–64. doi: 10.1016/s2213-8587(13)70218-3
69. Guida C, McCulloch LJ, Godazgar M, Stephen SD, Baker C, Basco D, et al. Sitagliptin and Roux-En-Y Gastric Bypass Modulate Insulin Secretion via Regulation of Intra-Islet PYY. Diabetes Obes Metab (2018) 20(3):571–81. doi: 10.1111/dom.13113
70. Guida C, Stephen S, Guitton R. The Role of PYY in Pancreatic Islet Physiology and Surgical Control of Diabetes. Trends Endocrinol Metab (2017) 28(8):626–36. doi: 10.1016/j.tem.2017.04.005
71. Guida C, Stephen SD, Watson M, Dempster N, Larraufie P, Marjot P, et al. PYY Plays a Key Role in the Resolution of Diabetes Following Bariatric Surgery in Humans. EBioMedicine (2019) 40:67–76. doi: 10.1016/j.ebiom.2018.12.040
72. Fakhry J, Wang J, Martins P, Fothergill LJ, Hunne B, Prieur P, et al. Distribution and Characterisation of CCK Containing Enteroendocrine Cells of the Mouse Small and Large Intestine. Cell Tissue Res (2017) 369(2):245–53. doi: 10.1007/s00441-017-2612-1
73. Himeno S, Tarui S, Kanayama S, Kuroshima T, Shinomura Y, Hayashi C, et al. Plasma Cholecystokinin Responses After Ingestion of Liquid Meal and Intraduodenal Infusion of Fat, Amino Acids, or Hydrochloric Acid in Man: Analysis With Region Specific Radioimmunoassay. Am J Gastroenterol (1983) 78(11):703–7.
74. Dirksen C, Jørgensen NB, Bojsen-Møller KN, Kielgast U, Jacobsen SH, Clausen TR, et al. Gut Hormones, Early Dumping and Resting Energy Expenditure in Patients With Good and Poor Weight Loss Response After Roux-En-Y Gastric Bypass. Int J Obes (Lond) (2013) 37(11):1452–9. doi: 10.1038/ijo.2013.15
75. Peterli R, Steinert RE, Woelnerhanssen B, Christoffel-Courtin C, Gass M, et al. Metabolic and Hormonal Changes After Laparoscopic Roux-En-Y Gastric Bypass and Sleeve Gastrectomy: A Randomized, Prospective Trial. Obes Surg (2012) 22(5):740–8. doi: 10.1007/s11695-012-0622-3
76. Scheja L, Heeren J. The Endocrine Function of Adipose Tissues in Health and Cardiometabolic Disease. Nat Rev Endocrinol (2019) 15(9):507–24. doi: 10.1038/s41574-019-0230-6
77. Frithioff-Bøjsøe C, Lund MAV, Lausten-Thomsen U, Hedley PL, Pedersen O, et al. Leptin, Adiponectin, and Their Ratio as Markers of Insulin Resistance and Cardiometabolic Risk in Childhood Obesity. Pediatr Diabetes (2020) 21(2):194–202. doi: 10.1111/pedi.12964
Keywords: gastric artery embolization, obesity, weight loss, gastrointestinal hormone, ghrelin
Citation: Tang Y, Pan X, Peng G and Tong N (2022) Weight Loss and Gastrointestinal Hormone Variation Caused by Gastric Artery Embolization: An Updated Analysis Study. Front. Endocrinol. 13:844724. doi: 10.3389/fendo.2022.844724
Received: 28 December 2021; Accepted: 07 February 2022;
Published: 16 March 2022.
Edited by:
Prasanth K. Chelikani, Texas Tech University, United StatesReviewed by:
Andrew C. Shin, Texas Tech University, United StatesMichel Vix, Hôpitaux Universitaires de Strasbourg, France
Copyright © 2022 Tang, Pan, Peng and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nanwei Tong, dG9uZ253QHNjdS5lZHUuY24=
†These authors have contributed equally to this work
 Yi Tang1,2†
Yi Tang1,2† Nanwei Tong
Nanwei Tong