- 1Assisting Nature, Centre of Assisted Reproduction and Genetics, Thessaloniki, Greece
- 23rd Department of Obstetrics and Gynecology, Aristotle University of Thessaloniki, Thessaloniki, Greece
Oocyte donation programs involve young and healthy women undergoing heavy ovarian stimulation protocols in order to yield good-quality oocytes for their respective recipient couples. These stimulation cycles were for many years beset by a serious and potentially lethal complication known as ovarian hyperstimulation syndrome (OHSS). The use of the short antagonist protocol not only is patient-friendly but also has halved the need for hospitalization due to OHSS sequelae. Moreover, the replacement of beta-human chorionic gonadotropin (b-hCG) with gonadotropin-releasing hormone agonist (GnRH-a) triggering has reduced OHSS occurrence significantly, almost eliminating its moderate to severe presentations. Despite differences in the dosage and type of GnRH-a used across different studies, a comparable number of mature oocytes retrieved, fertilization, blastulation, and pregnancy rates in egg recipients are seen when compared to hCG-triggered cycles. Nowadays, GnRH-a tend to be the triggering agents of choice in oocyte donation cycles, as they are effective and safe and reduce OHSS incidence. However, as GnRH-a triggering does not eliminate OHSS altogether, caution should be practiced in order to avoid unnecessary lengthy and heavy ovarian stimulation that could potentially compromise both the donor’s wellbeing and the treatment’s efficacy.
Introduction
Oocyte donation is a fundamental procedure for many women and in vitro fertilization (IVF) laboratories as the number of couples seeking such treatment is steadily increasing. Based on the latest European IVF-monitoring Consortium research, egg donation treatments represent about 32.4% of all assisted reproductive technology (ART) cycles in European countries (1). These highly complicated programs, in which very young women undergo heavy ovarian stimulation protocols, were for many years beset by a serious and potentially lethal complication known as ovarian hyperstimulation syndrome (OHSS). OHSS is characterized by extravasation of fluids into the third space due to increased vascular permeability mediated by vasoactive factors released by stimulated ovaries when exposed to beta-human chorionic gonadotropin (b-hCG) leading to effusions and vascular dehydration. Clinically, symptoms can be classified from mild to severe, ranging from mild abdominal discomfort and nausea to breathlessness, affected renal/liver function, and thromboembolic events.
In recent years, the innovative gonadotropin-releasing hormone (GnRH) antagonist has made it possible for us to apply new protocols, patient-friendly and safer, in the field of assisted reproduction. Implementing the antagonist protocol alone has significantly decreased the incidence of severe OHSS and the need for hospitalization by 50% (2, 3).
Nevertheless, OHSS was still present in young women with good ovarian response (such as donors). Therefore, the need for an even safer therapeutic approach led the scientific community to opt for oocyte maturation triggering with GnRH agonists (GnRH-a) rather than the use of classical human chorionic gonadotropin (hCG). The use of GnRH-a triggering in these cases has almost eliminated any hyperstimulation complication, changing risky, old procedures of long agonist stimulation protocols into modern, friendly, and safe short antagonist treatments with GnRH-a triggering (4). This modification has really brought about significant changes in IVF cycles and oocyte donation programs in particular (4). The donation programs are an excellent field for utilizing GnRH-a-induced maturation, as its luteolytic effect is not detrimental, since the donor herself will not undergo fresh embryo transfer.
The current mini review aims to review all available literature related to GnRH-a ovulation triggering in oocyte donation cycles in terms of dose administrated, OHSS occurrence, oocyte number, and embryo quality additionally to recipient pregnancy outcomes.
Materials and methods
The present mini review, however, followed the reporting recommendations of the Preferred Reporting Items for Systematic Review and Meta Analyses (PRISMA) statement. Two authors (TC and ET) searched the electronic databases PubMed (MEDLINE) and CENTRAL covering the period from 1990 until December 2021. The reference list of all studies was scanned. Studies had to fulfill the following eligibility criteria: oocyte donation stimulated cycles, GnRH-antagonist protocol, OHSS incidence, type and dose of GnRH-a triggering, oocyte number, and embryo quality. Three authors (RN, GM, and EP) independently screened each of the identified studies based on the predefined eligibility criteria. Any disagreements were resolved by discussion.
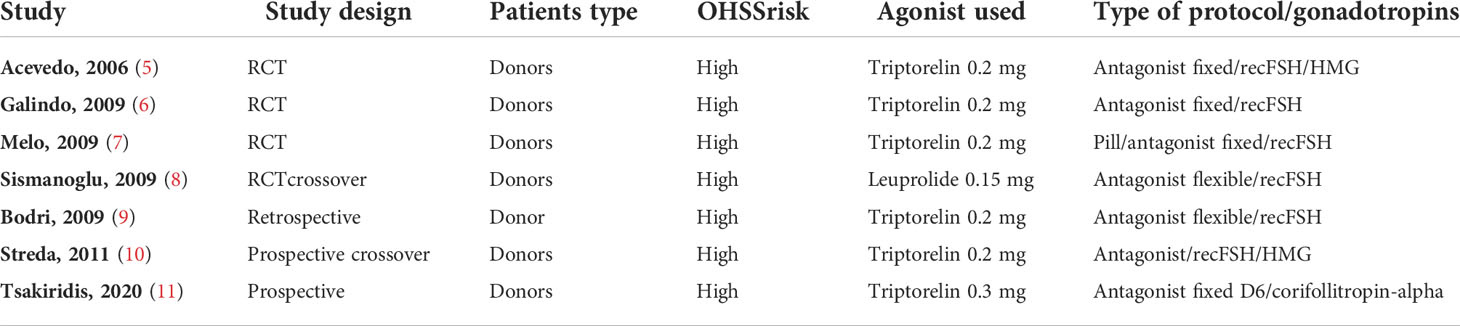
The literature search yielded 110 studies. The titles and abstracts were screened, and the full text of 22 studies was evaluated. Seven studies fulfilled the inclusion criteria for the current review (Table 1).
Pathophysiology of ovarian hyperstimulation syndrome in stimulated cycles and rationale for using agonist triggering instead of human chorionic gonadotropin triggering
GnRH was isolated in the early 1970s by the Nobel laureates Roger Guillemin and the group of Andrew V. Schally. It was one of the earliest well-characterized releasing hormones of the hypothalamic axis. GnRH consists of 10 amino acids. It is produced in the hypothalamus during the physiological menstrual cycle and periodically released to specific gonadotropin receptors located in the anterior pituitary (12).
In humans, in natural cycles, rapidly rising estradiol levels, through a positive feedback, increase GnRH levels by permissive mechanism and therefore gonadotropin secretion [follicle-stimulating hormone (FSH) and luteinizing hormone (LH)] inducing ovulation in women.
ιn IVF-stimulated cycles, supraphysiological estradiol levels cause untimely LH surges necessitating LH suppression in order to avoid premature ovulation. For many years, the long agonist protocol was the gold standard. The GnRH-a was used for LH downregulation, thus, agonist triggering was impossible to be implemented, as the receptors are already desensitized. Therefore, hCG was opted as a well-known substitute for the pre-ovulatory LH surge, presenting a very similar biochemical behavior as the urinary purified or recombinant form (13, 14).
However, OHSS remained the most serious complication of ovarian stimulation in ART (15). The presence of hCG is the basic OHSS pathogenetic factor. Exogenous levels of hCG are responsible for the early-onset OHSS, while the endogenous levels of hCG produced by the implanting fetus are responsible for the late OHSS. The hCG half-life is over 30 h, whereas LH half-life is less than 1.5 h (16, 17). The activation of LH receptors is significantly higher in case of hCG triggering, leading to a higher vascular permeability boosted by the secretion of the vascular endothelial growth factor (VEGF) (18).
The GnRH-a flare-up activity, in the antagonist-downregulated cycle setting, seems to be more closely mimicking the endogenous LH surge seen in natural cycles compared to hCG triggering (18, 19). The flare-up effect is caused by a temporary increase of LH/FSH secretion, but the surge has a shorter and different effect, comparing to hCG induction or natural cycles (19). Three independent studies by Cerrillo et al. (18), Lamb et al. (20), and Zelinski-Wooten et al. (21) reported lower steroid blood levels in case of GnRH-a triggering than those in hCG triggering cycles. It was found that the gene expression of different enzymes that take part in steroidogenesis at the moment of oocyte pickup was lower in cases of GnRH-a triggering (22). Significant reduction of VEGF expression in these patients may be a key factor in early OHSS prevention, such us a lack of hyperstimulated corpora luteal (8, 9, 16, 22).
Is there an optimal dose for agonist triggering?
Nowadays, there are many types of GnRH-a available, and all of them have been thoroughly studied. Bodri et al. (9), Humaidan et al. (23), and Papanikolaou et al. (24) tested a single dose of triptorelin 0.2 mg; other researchers have explored other types and different administration protocols, such as buserelin 0.2 mg in intranasal administration (5), buserelin 0.5 mg (23, 25–27), leuprolide 1 mg (28, 29), and nafarelin (25). The main conclusion of their research was that there is no significant difference between different forms of GnRH-a with regard to effectiveness on ovulation induction. The data presented by Vuong et al. (30), in 2016, confirm the efficacy of triptorelin 0.2 mg vs. 0.3 and 0.4 mg based on the number of metaphase II (MII) oocytes and good transferable embryos.
Triptorelin in doses of 0.2 or 0.3 mg is the most used protocol for ovulation induction in egg donation programs (9, 31).
Gonadotropin-releasing hormone triggering impact on oocyte number and embryo quality during oocyte donation
Embryo yield rates, during IVF, depend on multiple variables. However, the initial goal is to obtain a reasonable number of mature oocytes after final maturation triggering. Therefore, when replacing hCG with GnRH-a started, there was a lot of caution in the scientific community on whether the new triggering agonist regimen could efficiently replace the classical and lengthy use of hCG.
Initially, Humaidan et al. (23), in 2014, repeatedly reported no difference in the number of MII oocytes in both protocols in non-donor IVF cycles. Similarly, other authors have shown an equal number of mature oocytes between the classical hCG and agonist triggering (23).
Later studies that included oocyte donation cycles (32), fertility-preservation oncologic cycles (33, 34), and Pre implantation Genetic Testing for Aneuploidies (PGT-A) cycles (35) mentioned a significant difference and a higher number of MII oocytes and qualified transferable embryos in GnRH-a- vs. hCG-triggered cycles. Reddy et al. (33), in 2014, reported a higher number of MII oocytes (10.5 ± 5.1 vs. 7.7 ± 5.3, p = 0.002) and a higher number of cryopreserved embryos (7.7 ± 4.2 vs. 5.4 ± 3.8, p = 0.002) in oncologic patients triggered by GnRH-a. A large fertility preservation study by Pereira et al. (34), in 2017, confirmed higher effectiveness of GnRH-a triggering, demonstrating an increased number of MII oocytes and embryos frozen (11.8 vs. 9.9 with p = 0.04 and 9.2 vs. 6.4 with p < 0.001, respectively).
Some authors have raised concerns of empty follicle syndrome (EFS) being more frequent after GnRH-a triggering. EFS is a condition in which no oocytes are retrieved despite adequate ovarian stimulation and meticulous follicular aspiration. The incidence of EFS has been reported to range from 1% to 3.5% in literature (36). However, the data from large retrospective analyses, including 2,034 oocyte donation cycles, showed no statistically significant difference in the incidence of EFS between the GnRH triggering group (3.5%) and the hCG triggering group (3.1%) (37). An interesting case report suggests retriggering with hCG after failed GnRH-a triggering as a means of rescuing the cycle while retrieving oocytes that can lead to a normal pregnancy (38).
Regarding embryo yield, Galindo et al. (6), in 2009, studied 257 oocyte donor cycles and showed in both hCG and GnRH-a triggering groups no differences with regard to the number and status of embryos successfully transferred. Interestingly, Erb et al. (35), in 2010, based on his donation cycle retrospective research, concluded that GnRH-a triggering resulted in a higher number of all oocytes, MII oocytes, and embryos (p = 0.06) vs. the hCG triggering group (35).
Gonadotropin-releasing hormone triggering impact on recipient’s pregnancy outcome during oocyte donation
A meta-analysis by Humaidan et al., in 2011, included four studies and concluded that the clinical pregnancy rates and delivery rates were similar between GnRH-a and hCG trigger in donor cycles (39). Three other studies have also reported similar implantation rates and clinical pregnancy rates (5, 7, 8). Moreover, very similar results were presented by Galindo et al. (6), in 2009, with clinical pregnancy, ongoing pregnancy, and life birth rates as primary outcomes in both triggering groups.
Furthermore, retrospective studies by Shapiro et al. (40), in 2007, and Bodri et al. (9), in 2009, in oocyte donor cycles reported very similar results. The effectiveness of GnRH-a triggering with respect to pregnancy rate was confirmed by the research of Castillo et al. (37), in 2012, showing a high pregnancy rate (79/123, 64.2%) per recipient. These results are in accordance with other publications (31, 32).
Are ovarian hyperstimulation syndrome-free oocyte donation programs a reality?
Initial studies, administering GnRH-a triggering in the classical antagonist stimulation cycle setting, demonstrated total clinical OHSS prevention, including both moderate and severe forms of OHSS (34, 35).
In donor cycles, OHSS was not reported after GnRH-a triggering in seven clinical studies. However, in hCG triggering cycles, OHSS occurred between 4% and 17% of cases (5, 6, 8, 34, 41) (Table 2).
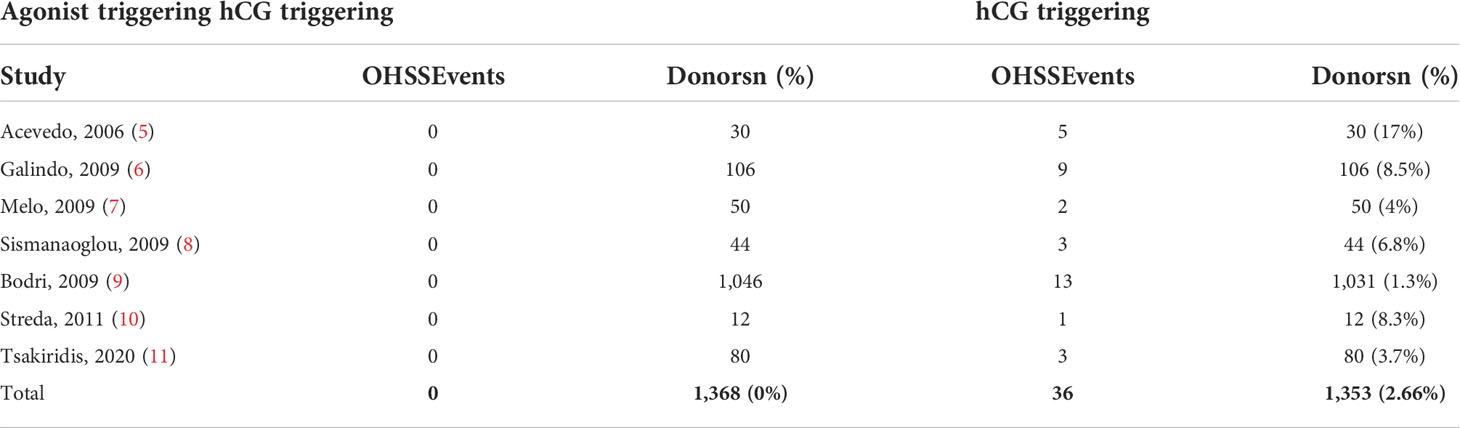
Table 2 OHSS rate in oocyte donation cycles comparing classical hCG triggering vs. GnRH-agonist triggering.
Similarly, recently, Tsakiridis et al. (11) reported three cases of moderate OHSS (3.75%) after hCG triggering without any case of OHSS occurring after GnRH-a triggering (p = 0.25).
Despite an extremely low OHSS incidence after GnRH-a triggering, we cannot forget that the oocyte donors are, a priori, a group at higher risk for OHSS due to a significantly higher number of mature follicles on the day of triggering (42, 43). Based on no donor, but still high-risk patient group analysis, there are similar case reports published by Fatemi et al. (15), in 2014, Santos-Ribeiro et al. (44), in 2015, and Iorio et al. (45), in 2021, in patients who developed severe OHSS even after GnRH-a triggering.
The main limitation in the precise differential diagnosis of OHSS vs. post retrieval bleeding is also the lack of sufficient surgical data, which are available only in severe cases after laparoscopic confirmation of bleeding and the confusion concerning the definition of severe OHSS (41).
That is why we must always be vigilant, as severe OHSS after agonist triggering, although extremely rare, is still possible (46).
Issues that should be taken into consideration
Although agonist triggering became the gold standard of ovulation induction especially in oocyte donors, precautions should still be taken.
1) LH levels on the day of triggering should be seriously considered, as deep downregulation might be present with LH levels <0.1 mIU/ml, and maybe in these cases, a dose of 0.2 triptorelin might not be sufficient to induce adequate gonadotropin release, and in turn, EFS might be encountered.
2) Clinicians should also consider low levels of LH during first days of hormonal stimulation, since some authors linked low follicular LH with poor oocyte collection cohort following GnRH-a triggering. They also noticed that in this group of poor, suboptimal responders, oral contraceptive priming might be associated with low LH starting levels (36).
3) Some reports indicated suboptimal hypophysis response, defined as a serum LH level lower than 12–15 mIU/ml measured 8–12 h after agonist triggering in young low-BMI donors. They postulated a possible connection of iatrogenically induced pituitary downregulation (LH <2 IU/L) caused by previous prolonged oral contraceptive pill (OCP) therapies with low LH levels on the day of GnRH-a triggering (47, 48). The only risk factor, significantly confirmed, was the long-term hormonal contraception [odds ratio (OR) 20.97; 95% confidence interval (CI), 5.29–83.14; p < 0.0001) rather than the short-term OCP use as part of a fertility pretreatment (49).
Conclusions
1) The most popular type of GnRH-a used today is triptorelin in doses of 0.2 or 0.3 mg with confirmed effectiveness in ovulation induction.
2) Most of the authors confirm higher effectiveness of GnRH-a triggering compared to traditional hCG triggering, resulting in a higher number of both mature oocytes and good quality frozen embryos.
3) The implantation rates, clinical pregnancy rates, and life birth rates in recipients, presented in reviewed literature, were very similar in both triggering groups.
4) The main conclusion in terms of safety and the OHSS occurrence, drawn from retrospective studies and clinical trials, is that GnRH-a triggering almost completely eliminates early OHSS during and after stimulation in donation programs.
Further research is needed to increase the accuracy of the data in donation cycles.
Author contributions
RN wrote and edited the manuscript, GM constructed the tables and edited the manuscript, NP edited the manuscript and looked into statistics, ET searched the literature and edited the manuscript, TC searched the literature and edited the manuscript, SK wrote the manuscript, FC reviewed and edited the manuscript, AM reviewed the manuscript and the statistics, EP wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.838236/full#supplementary-material
Abbreviations
ART, assisted reproductive technology; IVF, in vitro fertilization; OHSS, ovarian hyperstimulation syndrome; GnRH, gonadotropin-releasing hormone; GnRH-a, gonadotropin-releasing hormone agonist; hCG, human chorionic gonadotropin; LH, luteinizing hormone; FSH, follicle-stimulating hormone; MII, metaphse II.
References
1. De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2014: Results generated from European registries by ESHRE. the European IVF-monitoring consortium (EIM)‡ for the European society of human reproduction and embryology (ESHRE). Hum Reprod (2018) 33(9):1586–601. doi: 10.1093/humrep/dey242
2. Kolibianakis EM, Schultze-Mosgau A, Schroer A, van Steirteghem A, Devroey P, Diedrich K, et al. A lower ongoing pregnancy rate can be expected when GnRH agonist is used for triggering final oocyte maturation instead of HCG in patients undergoing IVF with GnRH antagonists. Hum Reprod (2005) 20:2887–92. doi: 10.1093/humrep/dei150
3. Kolibianakis EM, Venetis CA, Papanikolaou EG, Diedrich K, Tarlatzis BC, Griesinger G. Oestrogen addition to progesterone for luteal phase support in cycles stimulated with GnRH analogues and gonadotropins for IVF: A systematic review and meta-analysis. Hum Reprod (2008) 23:1346–54. doi: 10.1093/humrep/den115
4. Humaidan P, Alsbjerg B. GnRHa trigger for final oocyte maturation: Is HCG trigger history? Reprod BioMed Online (2014) 29:274–80. doi: 10.1016/j.rbmo.2014.05.008
5. Acevedo B, Gomez-Palomares JL, Ricciarelli E, Hernandez ER. Triggering ovulation with gonadotropin-releasing hormone agonists does not compromise embryo implantation rates. Fertil Steril (2006) 86:1682–7. doi: 10.1016/j.fertnstert.2006.05.049
6. Galindo A, Bodri D, Guillén JJ, Colodrón M, Vernaeve V, Coll O. Triggering with HCG or GnRH agonist in GnRH antagonist treated oocyte donation cycles: A randomised clinical trial. Gynaecological Endocrinol (2009) 25(1):60–6. doi: 10.1080/09513590802404013
7. Melo M, Busso CE, Bellver J, Alama P, Garrido N, Meseguer M, et al. GnRH agonist versus recombinant HCG in an oocyte donation programme: A randomised, prospective controlled, assessor-blind study. Reprod BioMed Online (2009) 19:486–92. doi: 10.1016/j.rbmo.2009.06.001
8. Sismanoglu A, Tekin HI, Erden HF, Ciray NH, Ulug U, Bahceci M. Ovulation triggering with GnRH agonist vs. hCG in the same egg . donor population undergoing donor oocyte cycles with GnRH antagonist: A prospective randomised cross-over trial. J Assist Reprod Genet (2009) 26:251–6. doi: 10.1007/s10815-009-9326-6
9. Bodri D, Guillen JJ, Galindo A, Mataro D, Pujol A, Coll O. Triggering with human chorionic gonadotropin or a gonadotropin-releasing . hormone agonist in gonadotropin-releasing hormone antagonist treated oocyte donor cycles: Findings of a Large retrospective cohort study. Fertil Steril (2009) 91:365–71. doi: 10.1016/j.fertnstert.2007.11.049
10. Streda R, Mardesic T, Sobotka V, Koryntová D, Hybnerová G, Jindra M, et al. Comparison of human chorionic gonadotropin (Pregnyl 10 000 IU I.M.) versus GnRH agonist (Triptorelin 0,2 mg s.C) for final oocytes maturation in the same egg donors–clinical and embryological characteristics. Ceska Gynecol (2011) 76(2):113–8.
11. Tsakiridis I, Najdecki R, Tatsi P, Timotheou E, Kalinderi K, Michos G, et al. Evaluation of the safety and efficacy of corifollitropin Alfa combined with GnRH agonist triggering in oocyte donation cycles. a prospective longitudinal study. JBRA Assisted Reprod (2020) 24(4):436–41. doi: 10.5925/1518-0557.20200033
12. Ehlers K, Halvorson L. Global library of women's medicines. (ISSN:1756-2228) (2013). doi: 10.3843/GLOWM.10285
13. Conn PM, Crowley WF Jr. Gonadotropin-releasing hormone and its analogs. Annu Rev Med (1994) 45:391–405. doi: 10.1146/annurev.med.45.1.391
14. Schwanzel-Fukada M, Pfaff DW. Origin of luteinising hormone-releasing hormone neurones. Nature (1989) 338:161–4. doi: 10.1038/338161a0
15. Fatemi HM, Popovic-Todorovic B, Humaidan P, Kol S, Banker M, Devroey P, et al. Severe ovarian hyper-stimulation syndrome after gonadotropin- releasing hormone (GnRH) agonist trigger and “Freeze-all” approach in GnRH antagonist protocol. Fertil Steril (2014) 101:1008–11. doi: 10.1016/j.fertnstert.2014.01.019
16. Radicioni M, Leuratti C, Cometti B. Randomized pharmacokinetic study of a highly purified human chorionic gonadotropin and of a recombinant human chorionic gonadotropin following single subcutaneous administration in healthy women. Clin Drug Invest (2022) 42:199–206. doi: 10.1007/s40261-022-01118-w
17. Casarini L, Santi D, Brigante G, Simoni M. Two hormones for one receptor: Evolution, biochemistry, actions, and pathophysiology of LH and hCG. Endocrine Rev (2018) 39(5):549–92. doi: 10.1210/er.2018-00065
18. Cerrillo M, Pacheco A, Rodríguez S, Gómez R, Delgado F, Pellicer A, et al. Effect of GnRH agonist and hCG treatment on VEGF, angiopoietin-2, and VE-cadherin: Trying to explain the link to ovarian hyper-stimulation syndrome. Fertil Steril (2011) 95:2517–9. doi: 10.1016/j.fertnstert.2010.12.054
19. Nakano R, Mizuno T, Kotsuji F, Katayama K, Washio M, Tojo S. “Triggering” of ovulation after infusion of synthetic luteinising hormone releasing factor (LRF). Acta Obstet Gynecol Scand (1973) 52:269–72. doi: 10.3109/00016347309158325
20. Lamb JD, Shen S, McCulloch C, Jalalian L, Cedars MI, Rosen MP. Follicle-stimulating hormone administered at the time of human chorionic gonadotropin trigger improves oocyte developmental competence in in vitro fertilisation cycles: A randomised, double-blind, placebo-controlled trial. Fertil Steril (2011) 95:1655–60. doi: 10.1016/j.fertnstert.2011.01.019
21. Zelinski-Wooten M, Hutchison J, Hess D, Wolf D, Stouffer R. Endocrinology: Follicle stimulating hormone alone supports follicle growth and oocyte development in gonadotrophin-releasing hormone antagonist-treated monkeys. Hum Reprod (1995) 10:1658–66. doi: 10.1093/oxfordjournals.humrep.a136151
22. Moedemhe D, Sigue A, Pacpaco E, Olazo A. Stimulation of endogenous surge of luteinising hormone with gonadotropin-releasing hormone analog after ovarian stimulation for in vitro fertilisation. Fertil Steril (1991) 55:328–32. doi: 10.1016/S0015-0282(16)54125-9
23. Humaidan P, Bungum L, Bungum M, Yding Andersen C. Rescue of corpus luteum function with peri-ovulatory HCG supplementation in IVF/ICSI GnRH antagonist cycles in which ovulation was triggered with a GnRH agonist: A pilot study. Reprod BioMed Online (2006) 13:173–8. doi: 10.1016/S1472-6483(10)60612-8
24. Papanikolaou EG, Verpoest W, Fatemi H, Tarlatzis B, Devroey P, Tournaye H. A novel method of luteal supplementation with recombinant luteinising hormone when a gonadotropin-releasing hormone agonist is used instead of human chorionic gonadotropin for ovulation triggering: A randomised prospective proof of concept study. Fertil Steril (2011) 95:1174–7. doi: 10.1016/j.fertnstert.2010.09.023
25. Parneix I, Emperaire JC, Ruffie A, Parneix P. Comparison of different protocols of ovulation induction, by GnRH agonists and chorionic gonadotropin. Gynecol Obstet Fertil (2001) 29:100–5. doi: 10.1016/S1297-9589(00)00064-3
26. Humaidan P, Ejdrup Bredkjaer H, Westergaard LG, Andersen CY. 1,500 IU human chorionic gonadotropin administered at oocyte retrieval rescues the luteal phase when gonadotropin-releasing hormone agonist is used for ovulation induction: A prospective, randomised, controlled study. Fertil Steril (2010) 93:847–54. doi: 10.1016/j.fertnstert.2008.12.042
27. Humaidan P, Polyzos NP, Alsbjerg B, Erb K, Mikkelsen AL, Elbaek HO, et al. GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: Two prospective randomised controlled multi-centre studies in IVF patients. Hum Reprod (2013) 28:2511–21. doi: 10.1093/humrep/det249
28. Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C. The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after Co-treatment with GnRH antagonist in high-risk patients undergoing In vitro fertilisation prevents the risk of ovarian hyper-stimulation syndrome: A prospective randomised controlled study. Fertil Steril (2008) 89:84–91. doi: 10.1016/j.fertnstert.2007.02.002
29. Castillo JC, Dolz M, Moreno J, Gijon L, Ferrer R, Ferrero E, et al. Triggering with GnRH agonist in oocyte-donation cycles: Estradiol monitoring is not necessary during ovarian stimulation. Reprod BioMedicine Online (2012) 24:247–50. doi: 10.1016/j.rbmo.2011.11.006
30. Vuong TNL, Ho MT, Ha TD, Phung HT, Huynh GB, Humaidan P. Gonadotropin-releasing hormone agonist trigger in oocyte donors Co-treated with a gonadotropin-releasing hormone antagonist: A dose-finding study. FertilSteril (2016) 105:356–63. doi: 10.1016/j.fertnstert.2015.10.014
31. Papanikolaou EG, Humaidan P, Polyzos NP, Tarlatzis B. Identification of the high risk for ovarian hyper stimulation syndrome. Semin.Reprod.Med (2010) 28(6):458. doi: 10.1055/s-0030-1265671
32. Thorne J, Loza A, Kaye L, Nulsen J, Benadiva C, Grow D, et al. Euploidy rates between cycles triggered with gonadotropin releasing hormone agonist and human chorionic gonadotropin. Fertil Steril (2019) 112:258–65. doi: 10.1016/j.fertnstert.2019.03.040
33. Reddy J, Turan V, Bedoschi G, Moy F, Oktay K. Triggering final oocyte maturation with gonadotropin-releasing hormone agonist (GnRHa) versus human chorionic gonadotropin (hCG) in breast cancer patients undergoing fertility preservation: An extended experience. J Assist Reprod Genet (2014) 31:927–32. doi: 10.1007/s10815-014-0248-6
34. Pereira N, Kelly AG, Stone LD, Witzke JD, Lekovich JP, Elias RT, et al. Gonadotropin-releasing hormone agonist trigger increases the number of oocytes and embryos available for cryopreservation in cancer patients undergoing ovarian stimulation for fertility preservation. Fertil Steril (2017) 108:532–8. doi: 10.1016/j.fertnstert.2017.06.027
35. Erb TM, Vitek W, Wakim A. Gonadotropin-releasing hormone agonist or human chorionic gonadotropin for final oocyte maturation in an oocyte donor program. Fertil Steril (2010) 93:374–8. doi: 10.1016/j.fertnstert.2008.12.015
36. Popovic-Todorovic B, Santos-Ribeiro S, Drakopoulos P, De Vos M, Racca A, Mackens S, et al. Predicting suboptimal oocyte yield following GnRH agonist trigger by measuring serum LH at the start of ovarian stimulation. Hum Reprod (2019) 34:2027–35. doi: 10.1093/humrep/dez132
37. Castillo JC, Garcia-Velasco J, Humaidan P. Empty follicle syndrome after GnRHa triggering versus hCG triggering in COS. J Assist Reprod Genet (2012) 29:249–53. doi: 10.1007/s10815-011-9704-8
38. Liest S, Christiansen IR, Praetorius L, Bogstad J, Freiesleben NC, Pinborg A, et al. HCG trigger after failed GnRH agonist trigger resulted in two consecutive live births: A case report. Front Reprod Health (2021) 3:764299. doi: 10.3389/frph.2021.764299
39. Humaidan P, Kol S, Papanikolaou E. GnRH agonist for triggering of final oocyte maturation: Time for a change of practice? Hum Reprod Update (2011) 17:510–24. doi: 10.1093/humupd/dmr008
40. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Ross R. Comparison of human chorionic gonadotropin and gonadotropin-releasing hormone agonist for final oocyte maturation in oocyte donor cycles. Fertil Steril (2007) 88:237–9. doi: 10.1016/j.fertnstert.2006.11.069
41. Bodri D, Guillen JJ, Polo A, Trullenque M, Esteve C, Coll O. Complications related to ovarian stimulation and oocyte retrieval in 4052 oocyte donor cycles. Reprod BioMed Online (2008) 17:237–43. doi: 10.1016/S1472-6483(10)60200-3
42. Papanikolaou EG, Pozzobon C, Kolibianakis E, Camus M, Tournaye H, Fatemi H, et al. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin- releasing hormone antagonist in vitro fertilization cycles. FertilSteril (2006) 85(1):112–20. doi: 10.1016/j.fertnstert.2005.07.1292
43. Papanikolaou EG, Humaidan P, Polyzos N, Kalantaridou S, Kol S, Benadiva C, et al. New algorithm for OHSS prevention. Reprod Biol Endocrinol (2011) 9:147. doi: 10.1186/1477-7827-9-147
44. Santos-Ribeiro S, Polyzos N, Stouffs K, De Vos M, Seneca S, Tournaye H, et al. Ovarian hyperstimulation syndrome after gonadotropin-releasing hormone agonist triggering and bfreeze-all: In-depth analysis of genetic predisposition. J Assist Reprod Genet (2015) 32:1063–8. doi: 10.1007/s10815-015-0498-y
45. Iorio GG, Rovetto MY, Conforti A, Carbone L, Vallone R, Cariati F, et al. Severe ovarian hyperstimulation syndrome in a woman with breast cancer under letrozole triggered with GnRH agonist: A case report and review of the literature. Front Reprod Health (2021) 3:704153. doi: 10.3389/frph.2021.704153
46. Ioannidou P, Bosdou J, Lainas G, Lainas T, Grimbizis G, Kolibianakis E. How frequent is severe ovarian hyperstimulation syndrome after GnRH agonist triggering in high-risk women? A systematic review and meta-analysis. RBMO (2021) 42(3):635–50. doi: 10.1016/j.rbmo.2020.11.008
47. Melnick AP, Rosenwaks Z. Oocyte donation: Insights gleaned and future challenges. Fertil Steril (2018) 110(6):988–93. doi: 10.1016/j.fertnstert.2018.09.021
48. Humaidan P, Kol S. Suboptimal response to GnRH agonist trigger: Causes and practical management. Curr Opin Obstet Gynecol (2021) 33(3):213–7. doi: 10.1097/GCO.0000000000000701
Keywords: agonist triggering, oocyte donation programs, OHSS-free programs, optimal dose for agonist triggering, impact on acceptor’s pregnancy outcome
Citation: Najdecki R, Michos G, Peitsidis N, Timotheou E, Chartomatsidou T, Kakanis S, Chouliara F, Mamopoulos A and Papanikolaou E (2022) Agonist triggering in oocyte donation programs—Mini review. Front. Endocrinol. 13:838236. doi: 10.3389/fendo.2022.838236
Received: 17 December 2021; Accepted: 04 July 2022;
Published: 26 August 2022.
Edited by:
Ralf Jockers, Université de Paris, FranceReviewed by:
Luigi Carbone, University of Naples Federico II, ItalyBella Martazanova, National Medical Research Center of Obstetrics, Gynecology and Perinatology Named After Academician V.I. Kulakova, Russia
Copyright © 2022 Najdecki, Michos, Peitsidis, Timotheou, Chartomatsidou, Kakanis, Chouliara, Mamopoulos and Papanikolaou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert Najdecki, cm5hamRlY2tpQGFzc2lzdGluZ25hdHVyZS5ncg==
 Robert Najdecki
Robert Najdecki Georgios Michos1
Georgios Michos1 Evangelia Timotheou
Evangelia Timotheou Tatiana Chartomatsidou
Tatiana Chartomatsidou