- Reproduction Center, The Third Affiliated Hospital of ZhengZhou University, ZhengZhou, China
Background: With the increasing use of frozen embryo transfer (FET), the best endometrial preparation protocol is continuously being discussed. The hormone replacement therapy (HRT) cycle and letrozole-induced ovulation (L-OI) cycle are available protocols for patients with abnormal ovulation. Previous comparisons of the two protocols have focused on pregnancy outcomes, with less attention to perinatal outcomes, and population heterogeneity was large; thus, convincing conclusions about which protocol is more appropriate could not be drawn.
Methods: We performed a retrospective cohort study using propensity score matching (PSM) analysis for a population of patients undergoing FET cycles in the reproductive center of the Third Affiliated Hospital of Zhengzhou University from January 2016 to September 2020. The main outcome measures were clinical pregnancy rate, live birth rate, very preterm delivery (VPTD), preterm delivery (PTD), low birth weight (LBW), macrosomia, small for gestational age (SGA), large for gestational age (LGA), hypertensive disorders of pregnancy (HDP), gestational diabetes mellitus (GDM), premature rupture of membranes (PROM), placenta previa, and congenital abnormality.
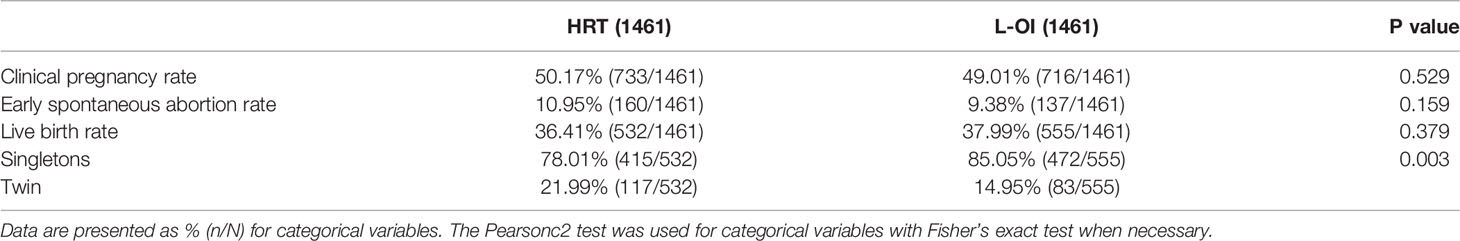
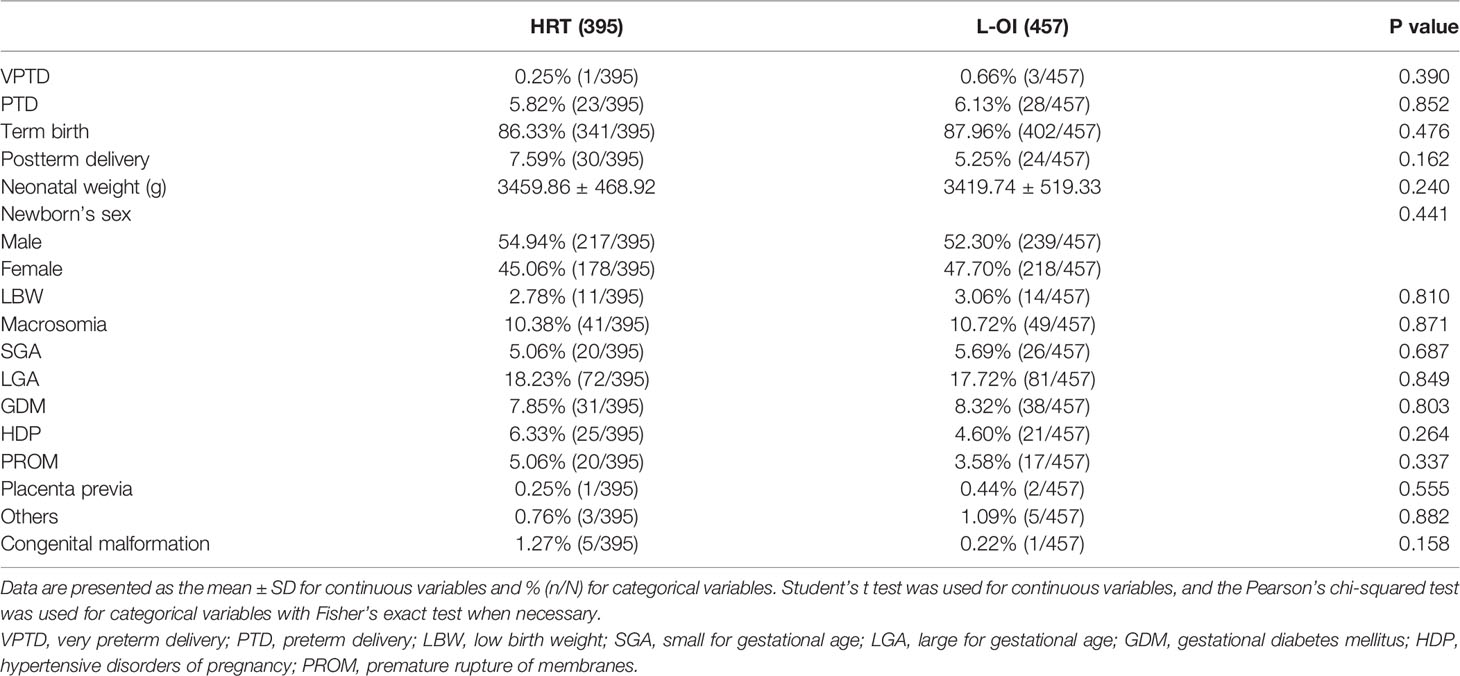
Results: A total of 8010 women were enrolled. Due to the large heterogeneity among the patients, we conducted 1:1 PSM, and 1461 women matched in each group. Compared with the HRT group, the L-OI group had a smaller proportion of thin endometrium (27.38% vs. 41.07%) and thicker endometrium on the day of embryo transfer (9.63 ± 1.82 vs. 8.91 ± 1.38). There were no significant differences in clinical pregnancy rate, early abortion rate or live birth rate between the groups. There was no significant difference in perinatal outcomes of singleton live birth, including VPTD, PTD, postterm delivery, LBW, macrosomia, SGA, LGA, GDM, HDP, placenta previa, and congenital malformation.
Conclusion: For women with abnormal ovulation, the pregnancy and perinatal outcomes of HRT and L-OI protocols are reassuring. It seems that both protocols are safe and effective for endometrial preparation in frozen-thawed embryo transfer in the clinic.
Introduction
Since the first successful live birth following human frozen embryo transfer (FET) reported by Zeilmaker’s team (1), the number of FET cycles has increased steadily worldwide due to improvements in laboratory technology, especially vitrification technology, and an increase in the number of available embryos (2, 3). In addition, the “whole-embryo freezing” strategy, i.e., selective freezing of all embryos before FET, has become a suitable option, especially for patients with a high risk of ovarian hyperstimulation syndrome (OHSS), preimplantation genetic testing (PGT) and double ovarian stimulation (DuoStim), as it reduces complications while simultaneously enhancing the live birth rate (4, 5).
Recently, many studies have shown that the outcome of frozen embryo transfer cycles was not inferior to that of FET cycles (6, 7), and some studies have even suggested that FET was associated with a higher pregnancy rate and lower complication rate (8, 9). Nevertheless, a recent meta-analysis showed that FET was associated with an increased risk of hypertensive disorders of pregnancy (HDP), postterm delivery, macrosomia and large for gestational age (LGA) (10, 11), but with reduced risk of preterm birth (PTD), low birth weight (LBW) and small for gestational age (SGA) (12, 13).
Endometrial preparation protocols optimize the success rate of FET by synchronizing endometrial receptivity and embryonic development stage. Multiple protocols for endometrial preparation for FET have been explored. A natural cycle (NC), an artificial cycle with hormone replacement therapy (HRT), and a cycle with ovulation induction (OI) are the most common protocols. All three protocols are suitable for patients with normal ovulation, and the latter two are also appropriate for patients with ovulatory disorders. Several recent retrospective studies found that NC was the best choice for women with normal ovulation (14, 15); however, there is no unified conclusion on the optimal choice for patients with abnormal ovulation (16, 17).
Clomiphene (CC) and letrozole (LE) are commonly used drugs in the OI cycle. In recent years, LE has been most widely used in OI for patients with polycystic ovary syndrome (PCOS), and it is the first-line OI drug for PCOS patients (18, 19). LE, a third-generation aromatase inhibitor, is commonly used in the clinic because it does not consume estrogen receptors, maintains a normal central feedback system, and promotes normal follicular growth, and it has no negative impact on the endometrium (20, 21) or pregnancy or fetal development (22). Because of its convenience, low cost and time controllability, HRT cycles have been widely applied for patients with abnormal ovulation (2, 23).
Recent studies have demonstrated that by using exogenous estrogen and progesterone to prepare the endometrium and inhibit ovulation, the HRT protocol in FET affected maternal and neonatal outcomes, resulting in the loss of the corpus luteum (CL), which can lead to adverse perinatal outcomes (14, 24). However, at present, there are few studies on the specific population of patients with abnormal ovulation, and heterogeneity in this population is large. Furthermore, as most comparisons between HRT and OI cycles have focused on the clinical pregnancy rate or live birth rate and paid little attention to maternal and neonatal outcomes, convincing conclusions cannot be drawn.
Therefore, this study aimed to explore the relationship between exposure of patients with abnormal ovulation to different endometrial preparation protocols and pregnancy and perinatal outcomes, including pregnancy rate, live birth rate, adverse obstetric complications and neonatal outcomes, to further optimize maternal and infant health after FET in patients with abnormal ovulation.
Materials and Methods
Patients
A total of 8010 women who were undergoing FET cycles from January 2016 to September 2020 at our center were enrolled. We included FET cycles of oligoanovulation (menstrual cycle>37 d), anovulation with letrozole-induced ovulation (L-OI) or HRT after in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI). The following exclusion criteria were applied: 1) maternal age >40 years; 2) adenomyosis, uterine malformations or recurrent miscarriage; and 3) use of donor oocytes and PGT cycles.
Endometrial Preparation Protocols
For HRT cycles, patients were prescribed 2 mg of estradiol valerate (Bayer Co., Germany) to be taken orally three times daily, starting at days 2-4 of menstruation for 7 days. Then, the drug dose was adjusted according to the thickness of the endometrium (up to 9 mg per day). Endometrial transformation was performed when the medication was taken for more than 12 days and endometrial thickness was ≥7 mm; the cycle was cancelled if endometrial thickness was less than 7 mm.
LE (2.5 mg/5 mg) was administered orally for 5 days on the 3rd-5th days of menstruation, and the follicular development speed was monitored by ultrasound. If follicular development was poor, HMG (Lizhu Pharmaceutical Trading Co., China) (37.5-75 IU daily) was added as appropriate to aid in the development of follicles. When the dominant follicle developed to 14 mm, the serum luteinizing hormone (LH) level indicated that ovulation was about to occur, the estradiol (E2) level was more than 150 pg/mL, and the endometrium thickness was more than 7 mm, 10,000 IU urinary hCG was injected (Lizhu Pharmaceutical Trading Co., China). Endometrial transformation was then performed. The cycle was cancelled if follicular dysplasia occurred.
For HRT or L-OI cycles, oral dydrogesterone (2 times daily, 10 mg once) (Abbott Co. USA) and intravaginal administration of 90 mg of a progesterone sustained-release vaginal gel (Merck Co. Germany) were given as luteal phase support until the 12th week of pregnancy. The same dose of estrogen valerate as before transformation was taken until 14 days after embryo transfer. In the case of pregnancy, the drug was continued until clinical pregnancy, which was defined as the presence of an intrauterine gestational sac by ultrasonography at 7–8 weeks of gestation.
Data Collection and Outcome Definition
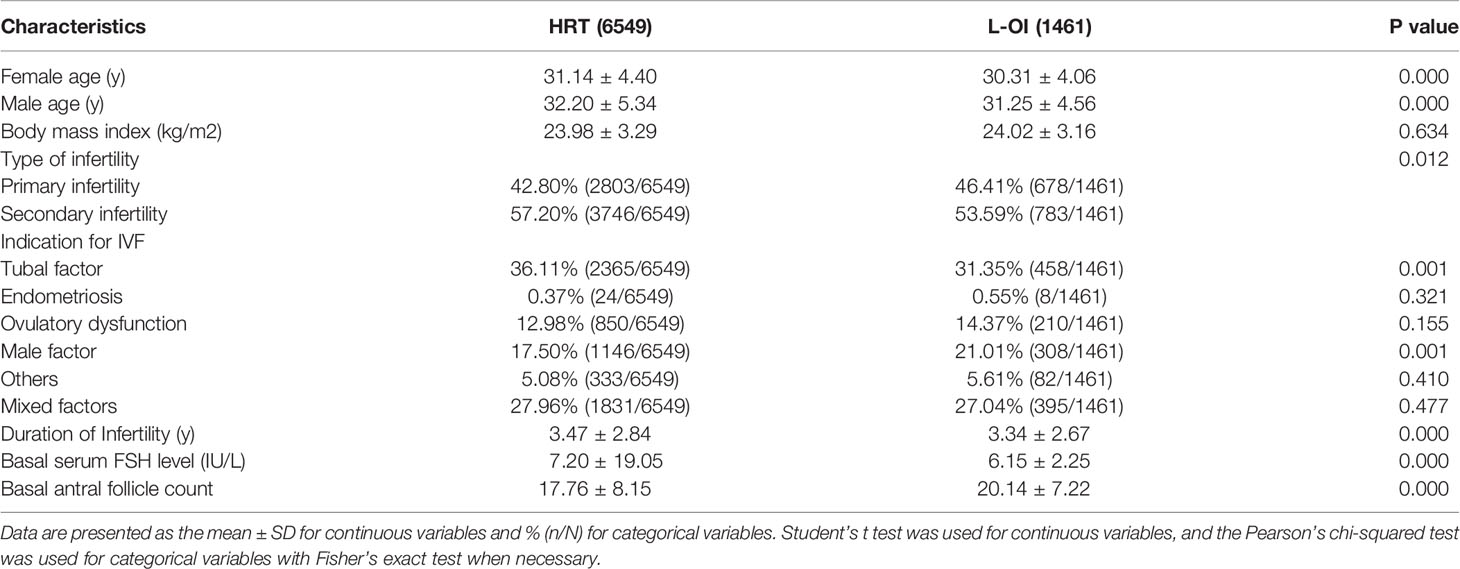
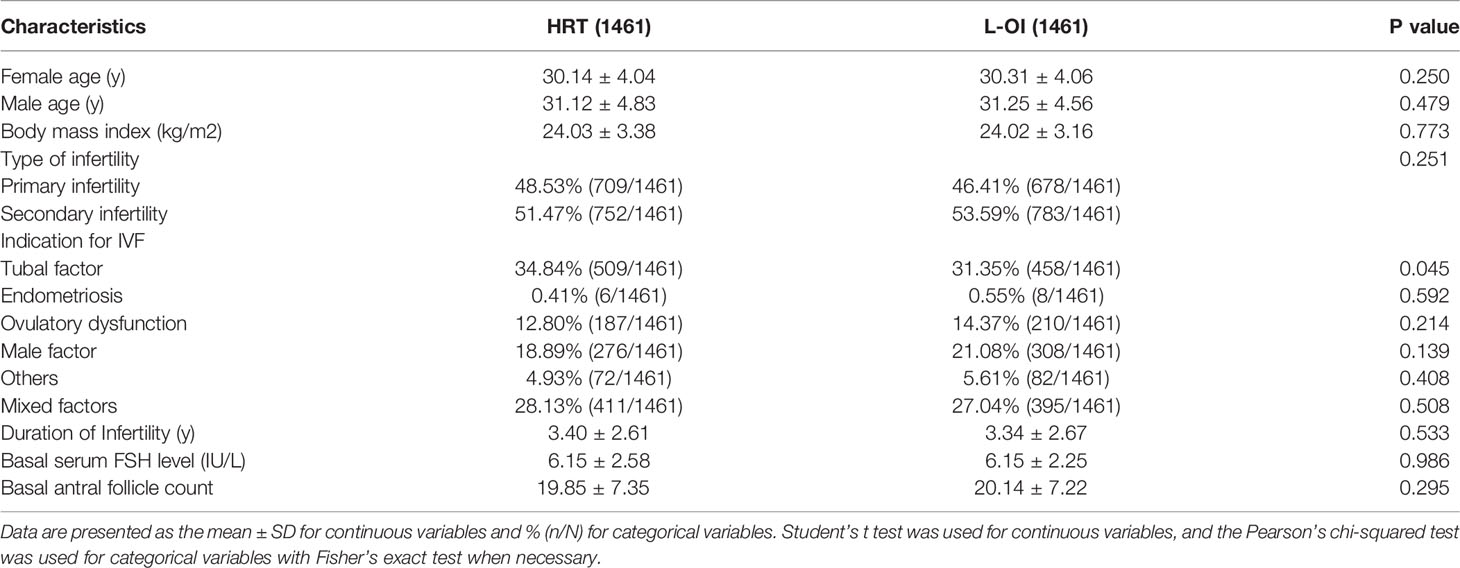
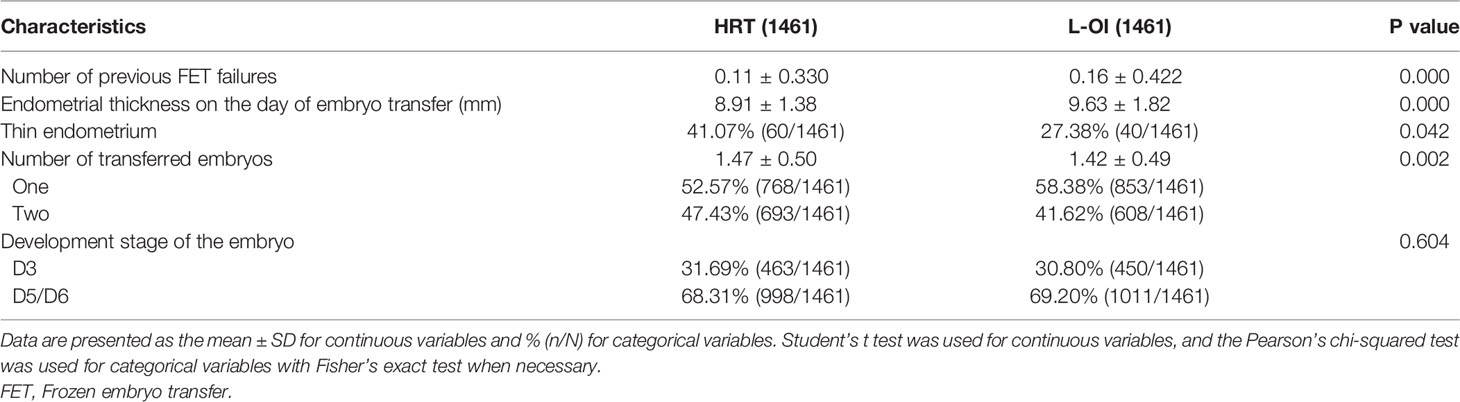
Patient characteristics, such as age, body mass index (BMI), type of infertility, indication for IVF, duration of infertility, basal serum follicle stimulating hormone (FSH), basal antral follicle count (AFC), the number of previous FET failures, endometrial thickness, number of transferred embryos, developmental stage of embryo, pregnancy or live birth, and singleton or twins, were collected through the electronic case system of our center.
For patients with a gestational sac echo and singleton live birth after embryo transfer, pregnancy complications were collected during a telephone follow-up and recorded by a designated nurse in our center. Maternal and neonatal outcomes were recorded and classified according to the information provided by the patients.
Early spontaneous abortion was defined as a clinical pregnancy that failed to reach the 12th gestational week. Live birth was defined as the birth of a live child after 28 weeks of gestation per embryo transfer cycle. Very preterm delivery (VPTD), preterm delivery (PTD), term birth and postterm delivery were defined as a baby born after <32 weeks, <37 weeks of gestation, ≤37 weeks ≤ 41 weeks and >41 weeks of gestation, respectively. The neonatal birth weight of singleton live births was as follows: LBW (<2500 g), SGA (<10th percentile for gestational age) (25), macrosomia (≥4000 g), and LGA (>90th percentile for gestational age) (25).
Statistical Analysis
All statistical management and analyses were performed using SPSS software, version 22.0.
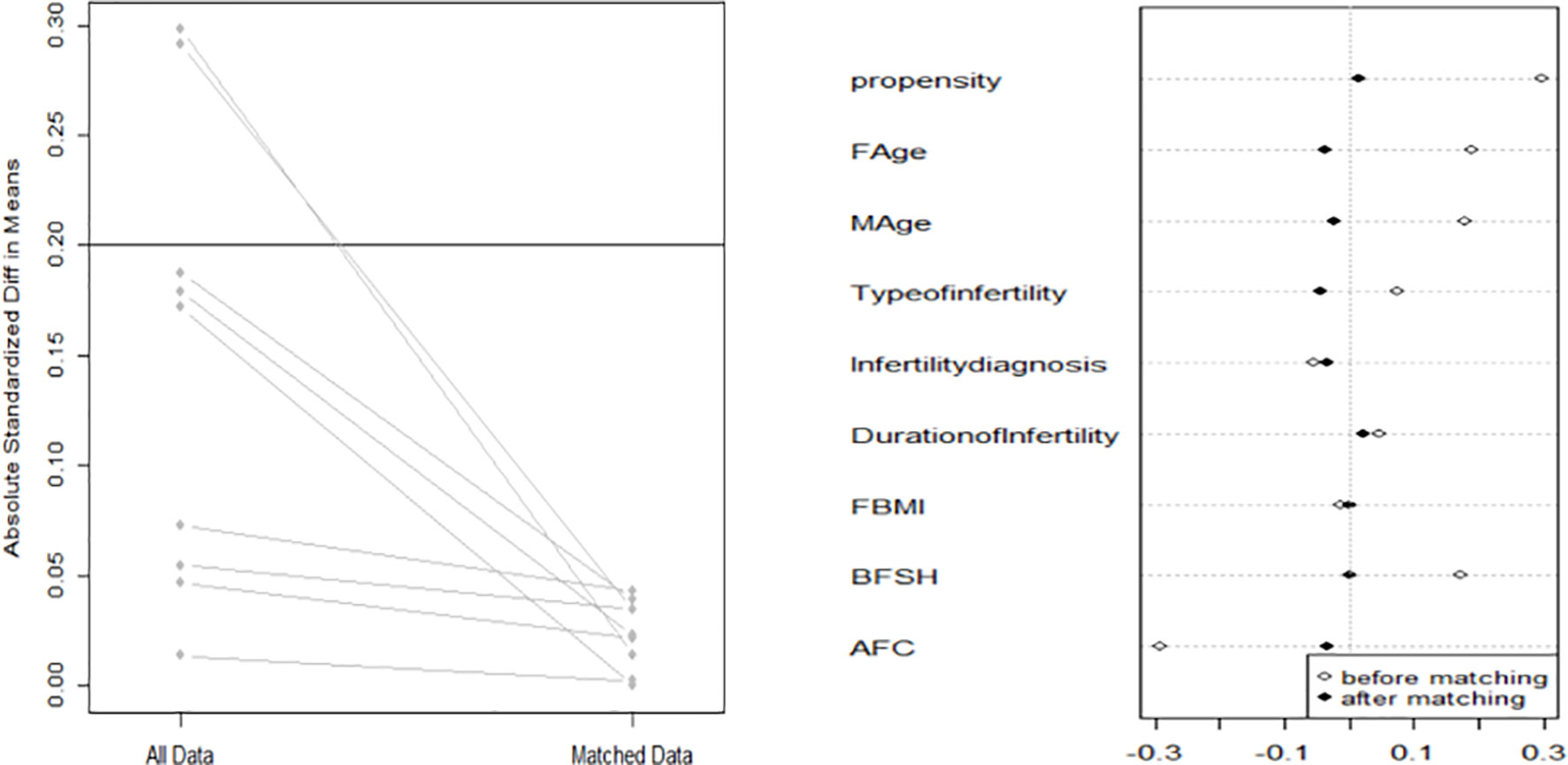
Because there was obvious heterogeneity in basic characteristics, the data were analyzed after 1:1 propensity score matching (PSM).
The one-sample K-S test was used to check for normality. Continuous variables with abnormal distributions are expressed as the mean ± SD, and Student’s t test was used to assess between-group differences. Categorical variables are represented as the number of cases (n) and percentage (%).
Means from chi-square analyses were used to assess differences between the groups. Multiple logistic regression was applied to further analyze different items. Unadjusted odds ratios and adjusted odds ratios with 95% confidence intervals (CIs) were calculated. Statistical significance was set at P<0.05.
Results
Study Population
From January 2016 to September 2020, 8010 FET cycles were evaluated according to the inclusion and exclusion criteria. There were 6549 patients in the HRT group and 1461 patients in the L-OI group. We separately analyzed the patients with a gestational sac echo and singleton live birth after embryo transfer, with 395 patients in the HRT group and 457 in the L-OI group.
Baseline Characteristics
When comparing basic characteristics between the two groups, we found that there were differences in female and male age, type of infertility, indication for IVF, duration of infertility, basal serum FSH, and basal AFC (Table 1). Therefore, based on these differences, we conducted 1:1 PSM, and 1461 women were matched in each group. After matching, there were no significant differences in basic characteristics between the groups (Table 2 and Figure 1).
We found that the number of previous FET failures was higher in the L-OI group than that of the HRT group. In terms of clinical data, the endometrium was thicker and the proportion of thin endometrium lower in the L-OI group (Table 3).
Clinical Outcomes
In terms of clinical outcome, there were no significant differences in clinical pregnancy rate, early abortion rate or live birth rate between the two groups, but the twin rate was higher in the HRT group, which may be because the number of transferred embryos was greater than that in the L-OI group (Table 4).
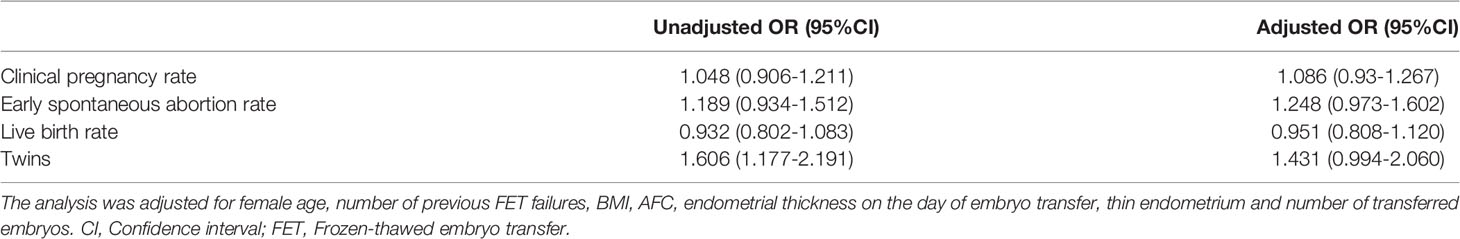
Regarding the main outcome measures, we conducted a multiple logistic regression analysis to adjust for the influence of confounding factors. The included factors were female age, number of previous FET failures, BMI, AFC, endometrial thickness on the day of embryo transfer, thin endometrium and number of transferred embryos. After adjustments for confounding factors, the clinical pregnancy rate, early spontaneous abortion rate, live birth rate and twin rate were not significantly different between the groups (Table 5).
We mainly analyzed maternal and neonatal outcomes and observed no significant differences in perinatal outcomes, including VPTD, PTD, postterm delivery, LBW, macrosomia, SGA, LGA, GDM, HDP, placenta previa, and congenital malformation, between the groups (Table 6). The same conclusion was reached after further multiple logistic regression analysis (Table 7).
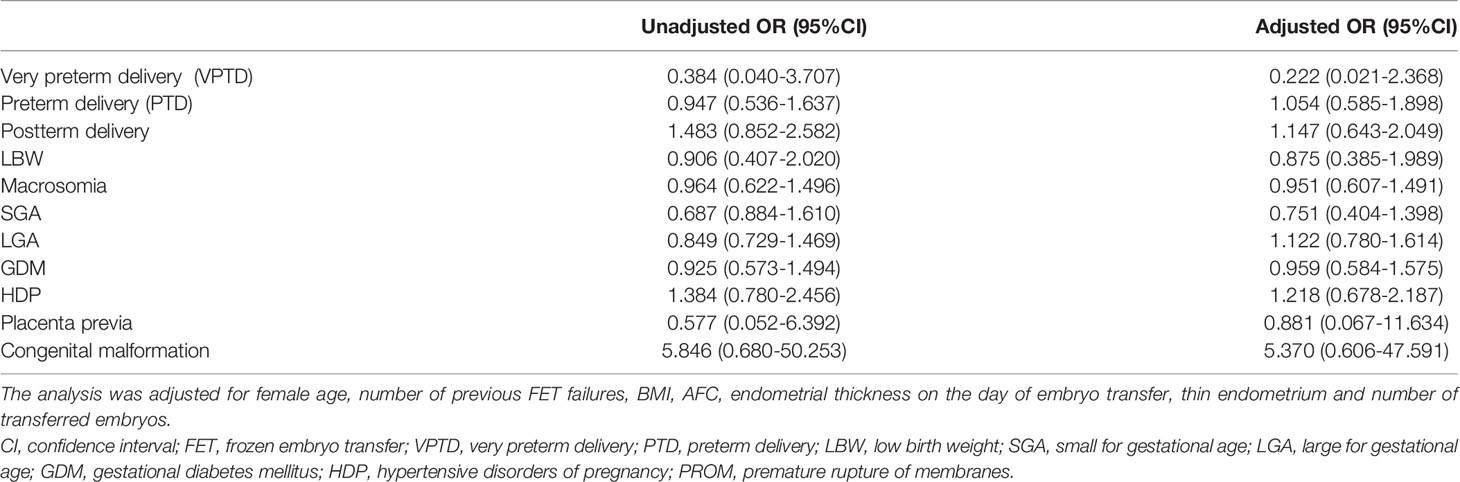
Table 7 Unadjusted and adjusted odds ratios of perinatal and neonatal outcomes of singleton live birth undergoing L-OI versus HRT FET cycles.
Discussion
Our study showed no difference in pregnancy rate, live birth rate or abortion rate between HRT and L-OI cycles for patients with abnormal ovulation. Moreover, there was no difference between the two groups regarding perinatal outcomes.
For patients with abnormal ovulation, both HRT and L-OI are common endometrial preparation protocols in the clinic. The safety and OI effect of LE have also been generally recognized (26). Previous studies have suggested that L-OI cycles resulted in a higher live birth rate than HRT cycles; nevertheless, these studies did not examine maternal and neonatal outcomes (16, 27). Interestingly, some studies reached the same conclusions as in our study. A prospective study including 116 PCOS patients reported similar clinical pregnancy rates for HRT and L-OI protocols (28). Another randomized controlled study including 100 patients found that the L-OI protocol did not improve pregnancy outcomes compared with HRT (17). However, these studies did not focus on maternal and infant outcomes.
Despite no difference in pregnancy outcomes between the two groups, endometrial thickness on the day of embryo transfer in the L-OI group was greater than that in the HRT group in our study, which was consistent with a previous study from our center (29). The reason may be that the proportion of patients with thin endometrium was relatively high in the HRT group. In addition, LE has no negative effects on endometrial or cervical mucus. LE can enable full endometrial pinopode expression and increase integrin αvβ3 expression in the endometrium during implantation (18, 30). LE further decreases intraovarian and serum estrogen levels by blocking conversion of androgens to estrogens in ovarian granulosa cells (20). Subsequently, low estrogen levels reduce ubiquitination of estrogen receptors. This process leads to faster endometrial proliferation and increased blood levels in the uterus and endometrium, with positive effects on pregnancy outcomes (31, 32). Thus, L-OI can be used to prepare endometrial tissue for FET for patients with thin endometrial tissue. However, a previous study in our center reported that L-OI cycles were associated with a higher live birth rate than HRT cycles. Although the live birth rate was increased in our study by using the L-OI protocol, there was no significant differences between the groups possibly due to a large difference in the number of cases included in the L-OI (502) and HRT (2280) groups. There was also heterogeneity in basic characteristics; a previous study adopted regression analysis for correction (29), which was different from the 1:1 PSM in our study.
Moreover, our study found no significant difference in maternal or infant health between the two groups. A large retrospective cohort study in Japan in 2017 that included 110,772 FET cycles, which were divided into an LE-induced ovulation group, NC group and HRT group according to the endometrial preparation protocol used, found that neonatal outcomes of the different treatment schemes were basically similar, consistent with our results. A previous study in our center also reached similar conclusions (29).
There have been few studies on the perinatal complications and infant safety of the two protocols. Studies have shown that newborns were likely to have LBW and macrosomia after HRT cycles (14, 33) while pregnant women had an increased risk of HDP and cesarean section (15, 34). Saito et al.’s study suggested that the HRT cycles were associated with a higher risk of HDP and placental implantation and a lower risk of GDM (35). Another meta-analysis demonstrated that compared with the NC protocol, the OI protocol was associated with an increased incidence of PTD and LBW (36). However, the studies mentioned above compared three protocols, and the study population was not limited.
Recent studies have shown that the HRT protocol lacks CL, which is a crucial hormone for embryo implantation, placenta and pregnancy maintenance. Recent studies emphasized that loss of CL is associated with altered vascular health and insufficient cardiovascular adaptation in early pregnancy, leading to the occurrence of preeclampsia, affecting placental formation and causing placental hyperplasia (37, 38), with impacts on the mother and newborn. CL not only provides estrogen and progesterone but also vasoactive substances, such as relaxin and vascular endothelial growth factor, which may be important for placental formation. These substances are not available in the HRT cycle, which may increase the incidence of obstetric complications (39, 40). In our study, there was no difference between the two groups with regard to singleton delivery. The reason may be due to the different doses and types of luteal support after FET.
In FET cycles, it is necessary to add progesterone to obtain sufficient corpus luteum support to obtain a good pregnancy outcome due to the lack of endogenous progesterone production. In our study, corpus luteum support was provided by a combination of oral and vaginal administration, and the dose was sufficient. In Hu et al.’s study, only oral dydrogesterone (20 mg/d) was applied as luteal support in HRT cycles (14). Previous studies have suggested that dydrogesterone alone was likely not effective as a monotherapy in FET (41) but that the combination of oral and vaginal administration increased the concentration of progesterone in the serum and endometrium and improved the reproductive outcome (42, 43). In the study of Zong et al. dydrogesterone (40 mg/d) and progesterone capsules (Utrogestan, Capsugel) (200 mg/d) were given as luteal-phase support in HRT and OI cycles (33). This was not consistent with our study, in which oral dydrogesterone (60 mg/d) and intravaginal administration of 90 mg of a progesterone sustained-release vaginal gel were given as luteal-phase support. A recent meta-analysis suggested that once-daily Crinone gel or micronized progesterone (200 mg) three times per day is the most suitable luteal support dose (44).
Another reason may be that in some studies, when there were significant differences in basic characteristics and obvious differences in the number of included populations between the groups, logistic regression was used to correct confounding factors instead of ex ante PSM. Although they could correct some confounding factors, the statistical effectiveness did not seem to be more convincing than PSM.
Several limitations associated with this study warrant mentioning. 1) The number of samples was lower after PSM than before, and the study was a retrospective study with some deviation; hence, additional prospective research is needed to verify our results. 2) Patients with diabetes and hypertension were not excluded, but blood pressure and blood glucose were controlled normally before FET, which might have led to some inaccuracy in the results. 3) Because maternal complications and offspring outcomes were obtained by telephone and reported by patients, incomplete and missing data were present. 3) Not all the patients included were undergoing their first FET cycle, though the number of previous transplantation failures between the two groups was compared, some bias in outcome may exist.
In conclusion, for women with abnormal ovulation undergoing FET, both HRT and L-OI protocols are safe and effective in the clinic. Although maternal and infant outcomes appear to be reassuring, they need to be confirmed by additional prospective research with large samples.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
WZ and YG designed the study and selected the population to be included and excluded. WZ and ZL were involved in the data extraction and analysis. JZ and BR reviewed the data. WZ and ZL were involved in drafting this article. ML, JL, and WZ modified the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to the physicians and coordinators who enrolled the patients and collected the data from all of the women who participated in this study. We acknowledge the patients who participated in the study. We also thank American Journal Experts for their professional manuscript editing service.
References
1. Zeilmaker GH, Alberda AT, Gent IV, Rijkmans C, Drogendijk A. Two Pregnancies Following Transfer of Intact Frozen-Thawed Embryos. Fertil Steril (1984) 42:293–6. doi: 10.1016/S0015-0282(16)48029-5
2. De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2014 Results Generated From European Registries by ESHRE The European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod (2018) 33(9):1586–601. doi: 10.1093/humrep/dey242
3. Groenewoud ER, Cohlen BJ, Macklon NS. Programming the Endometrium for Deferred Transfer of Cryopreserved Embryos: Hormone Replacement Versus Modified Natural Cycles. Fertil Steril (2018) 109:768–74. doi: 10.1016/j.fertnstert.2018.02.135
4. Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and Perinatal Outcomes in Singleton Pregnancies Resulting From the Transfer of Frozen Thawed Versus Fresh Embryos Generated Through In Vitro Fertilization Treatment: A Systematic Review and Meta-Analysis. Fertil Steril (2012) 98:368. doi: 10.1016/j.fertnstert.2012.05.019
5. Tiitinen H, Halttunen M, Härkki V, Vuoristo P, Hyden-Granskog C. Elective Single Embryo Transfer: The Value of Cryopreservation. Hum Reprod (2001) 16(6):1140–4. doi: 10.1093/humrep/16.6.1140
6. Evans J, Hannan NJ, Edgell TA, Vollenhoven BJ, Lutjen PJ, Osianlis T, et al. Fresh Versus Frozen Embryo Transfer: Backing Clinical Decisions With Scientific and Clinical Evidence. Hum Reprod Update (2014) 20(6):808–21. doi: 10.1093/humupd/dmu027
7. Groenewoud ER, Cohlen BJ, Al-Oraiby A, Brinkhuis EA, Broekmans FJ, de Bruin JP, et al. A Randomized Controlled, Non-Inferiority Trial of Modified Natural Versus Artificial Cycle for Cryo-Thawed Embryo Transfer. Hum Reprod (2016) 31(7):1483–92. doi: 10.1093/humrep/dew120
8. Belva H, Henriet S, Van den Abbeel C, Camus M, Devroey P, Van der Elst J, et al. Neonatal Outcome of 937 Children Born After Transfer of Cryopreserved Embryos Obtained by ICSI and IVF and Comparison With Outcome Data of Fresh ICSI and IVF Cycles. Hum Reprod (Oxf Engl) (2008) 23(10):2227–38. doi: 10.1093/humrep/den254
9. Palomba S, Homburg R, Santagni S, Sala GL, Orvieto R. Risk of Adverse Pregnancy and Perinatal Outcomes After High Technology Infertility Treatment: A Comprehensive Systematic Review. Reprod Biol Endocrinol (2016) 14:76. doi: 10.1186/s12958-016-0211-8
10. Sha T, Yin X, Cheng W, Massey IY. Pregnancy-Related Complications and Perinatal Outcomes Resulting From Transfer of Cryopreserved Versus Fresh Embryos In Vitro Fertilization: A Meta-Analysis. Fertil Steril: Off J Am Fertil Soc Pac Coast Fertil Soc Can Fertil Androl Soc (2018) 109(2):330–42. doi: 10.1016/j.fertnstert.2017.10.019
11. Sites CK, Wilson D, Barsky M, Bernson D, Bernstein IM, Boulet S, et al. Embryo Cryopreservation and Preeclampsia Risk. Fertil Steril (2017) 108(5):784–90. doi: 10.1016/j.fertnstert.2017.08.035
12. Maheshwari A, Raja E, Bhattacharya S. Obstetric and Perinatal Outcomes After Either Fresh or Thawed Frozen Embryo Transfer: An Analysis of 112,432 Singleton Pregnancies Recorded in the Human Fertilisation and Embryology Authority Anonymized Dataset. Fertil Steril (2016) 106(7):1703–8. doi: 10.1016/j.fertnstert.2016.08.047
13. Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S. Is Frozen Embryo Transfer Better for Mothers and Babies? Can Cumulative Meta-Analysis Provide a Definitive Answer? Hum Reprod (2018) 24(1):35–58. doi: 10.1093/humupd/dmx031
14. Hu KL, Zhang D, Li R. Endometrium Preparation and Perinatal Outcomes in Women Undergoing Single-Blastocyst Transfer in Frozen Cycles. Fertil Steril (2021) 115(6):1487–94. doi: 10.1016/j.fertnstert.2020.12.016
15. Li C, He YC, Xu JJ, Wang Y, Liu H, Duan CC, et al. Perinatal Outcomes of Neonates Born From Different Endometrial Preparation Protocols After Frozen Embryo Transfer: A Retrospective Cohort Study. BMC Pregnancy Childbirth (2021) 21(1):341. doi: 10.1186/s12884-021-03791-9
16. Zhang J, Liu H, Wang Y, Mao X, Kuang Y. Letrozole Use During Frozen Embryo Transfer Cycles in Women With Polycystic Ovary Syndrome. Fertil Steril (2019) 112:371–7. doi: 10.1016/j.fertnstert.2019.04.014
17. Aleyasin A, Aghahosseini M, Safdarian L, Noorzadeh M, Fallahi P. Can Letrozole Plus HMG Protocol Improve Pregnancy Outcomes in Frozen-Thawed Embryo Transfer? An RCT. Int J Reprod Biomed (2017) 15:83–6. doi: 10.29252/ijrm.15.2.83
18. Mitwally M, Casper RF. Use of an Aromatase Inhibitor for Induction of Ovulation in Patients With an Inadequate Response to Clomiphene Citrate. Fertil Steril (2001) 75:305–9. doi: 10.1016/S0015-0282(00)01705-2
19. Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Welt CK. Diagnosis and Treatment of Polycystic Ovary Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2013) 98:4565–92. doi: 10.1210/jc.2013-2350
20. Miller PB, Parnell BA, Bushnell G, Tallman N, Forstein DA, Higdon HL 3rd, et al. Endometrial Receptivity Defects During IVF Cycles With and Without Letrozole. Hum Reprod (2012) 27(3):881–8. doi: 10.1093/humrep/der452
21. Garcia-Velasco JA. The Use of Aromatase Inhibitors in Invitro Fertilization. Fertil Steril (2012) 98(6):1356–8. doi: 10.1016/j.fertnstert.2012.09.042
22. Requena A, Herrero J, Landeras J, Navarro E, Neyro JL, Salvador C, et al. Use of Letrozole in Assisted Reproduction: A Systematic Review and Meta-Analysis. Hum Reprod Update (2008) 14(6):571–82. doi: 10.1093/humupd/dmn033
23. Peeraer K, Couck I, Debrock S, De Neubourg D, De Loecker P, Tomassetti C, et al. Frozen-Thawed Embryo Transfer in a Natural or Mildly Hormonally Stimulated Cycle in Women With Regular Ovulatory Cycles: A RCT. Hum Reprod (2015) 30(11):2552–62. doi: 10.1093/humrep/dev224
24. Llaa B, Als A, Akah A, Tdc C, Szd A, Rbj B, et al. Adverse Obstetric and Perinatal Outcomes in 1,136 Singleton Pregnancies Conceived After Programmed Frozen Embryo Transfer (FET) Compared With Natural Cycle FET - ScienceDirect. Fertil Steril (2021) 115:947–56. doi: 10.1016/j.fertnstert.2020.10.039
25. Dai L, Deng C, Li Y, Zhu J, Zhang Y. Birth Weight Reference Percentiles for Chinese. PloS One (2014) 9:e104779. doi: 10.1371/journal.pone.0104779
26. Healey S, Tan SL, Tulandi T, Biljan MM. Effects of Letrozole on Superovulation With Gonadotropins in Women Undergoing Intrauterine Insemination. Fertil Steril (2003) 80:1325–9. doi: 10.1016/j.fertnstert.2003.03.001
27. Li SJ, Zhang YJ, Chai XS, Nie MF, Zhou YY, Chen JL, et al. Letrozole Ovulation Induction: An Effective Option in Endometrial Preparation for Frozen-Thawed Embryo Transfer. Arch Gynecol Obstet (2014) 289:687–93. doi: 10.1007/s00404-013-3044-0
28. Hosseini-Najarkolaei A, Moini A, Kashani L, Farid Mojtahedi M, Hosseini-Najarkolaee H, Salehi E. The Effect of Letrozole Versus Artificial Hormonal Endometrial Preparation on Pregnancy Outcome After Frozen-Thawed Embryos Transfer Cycles: A Randomized Clinical Trial. Reprod Biol Endocrinol (2020) 18(1):115. doi: 10.1186/s12958-020-00675-z
29. Zhang J, Li Z, Sun L, Guan Y, Du M. Comparison of Pregnancy and Neonatal Outcomes of Single Frozen Blastocyst Transfer Between Letrozole-Induction and HRT Cycles in Patients With Abnormal Ovulation. Front Endocrinol (Lausanne) (2021) 12:664072. doi: 10.3389/fendo.2021.664072
30. Badawy A, Aal IA, Abulatta M. Clomiphene Citrate or Letrozole for Ovulation Induction in Women With Polycystic Ovarian Syndrome: A Prospective Randomized Trial. Fertil Steril (2009) 92:849–52. doi: 10.1016/j.fertnstert.2007.02.062
31. Tatsumi T, Jwa SC, Kuwahara A, Irahara M, Kubota T, Saito H. Pregnancy and Neonatal Outcomes Following Letrozole Use in Frozen-Thawed Single Embryo Transfer Cycles. Hum Reprod (2017) 32(6):1244–8. doi: 10.1093/humrep/dex066
32. Hu YJ, Chen YZ, Zhu YM, Huang HF. Letrozole Stimulation in Endometrial Preparation for Cryopreserved-Thawed Embryo Transfer in Women With Polycystic Ovarian Syndrome: A Pilot Study. Clin Endocrinol (2014) 80:283–9. doi: 10.1111/cen.12280
33. Zong L, Liu P, Zhou L, Wei D, Ding L, Qin Y. Increased Risk of Maternal and Neonatal Complications in Hormone Replacement Therapy Cycles in Frozen Embryo Transfer. Reprod Biol Endocrinol (2020) 18(1):36. doi: 10.1186/s12958-020-00601-3
34. Jing S, Li XF, Zhang S, Gong F, Lu G, Lin G. Increased Pregnancy Complications Following Frozen-Thawed Embryo Transfer During an Artificial Cycle. J Assisted Reprod Genet (2019) 36(5):925–33. doi: 10.1007/s10815-019-01420-1
35. Saito K, Kuwahara A, Ishikawa T, Morisaki N, Miyado M, Miyado K, et al. Endometrial Preparation Methods for Frozen-Thawed Embryo Transfer are Associated With Altered Risks of Hypertensive Disorders of Pregnancy, Placenta Accreta, and Gestational Diabetes Mellitus. Hum Reprod (2019) 34(8):1567–75. doi: 10.1093/humrep/dez079
36. Horcajadas JA, Mínguez P, Dopazo J, Esteban FJ, Domínguez F, Giudice LC, et al. Controlled Ovarian Stimulation Induces a Functional Genomic Delay of the Endometrium With Potential Clinical Implications. J Clin Endocrinol Metab (2008) 93:4500–10. doi: 10.1210/jc.2008-0588
37. von Versen-Höynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, et al. Increased Preeclampsia Risk and Reduced Aortic Compliance With In Vitro Fertilization Cycles in the Absence of a Corpus Luteum. Hypertension (2019) 73(3):640–9. doi: 10.1161/HYPERTENSIONAHA.118.12043
38. von Versen-Hynck F, Narasimhan P, Selamet Tierney E, Martinez N, Conrad KP, Baker VL. Absent or Excessive Corpus Luteum Number Is Associated With Altered Maternal Vascular Health in Early Pregnancy. Hypertension (2019) 73(3):680–90. doi: 10.1161/HYPERTENSIONAHA.118.12046
39. Novak J, Danielson LA, Kerchner LJ, Sherwood OD, Conrad KP. Relaxin is Essential for Renal Vasodilation During Pregnancy in Conscious Rats. J Clin Invest (2001) 107:1469–75. doi: 10.1172/JCI11975
40. Singh B, Reschke L, Segars J, Baker VL. Frozen-Thawed Embryo Transfer: The Potential Importance of the Corpus Luteum in Preventing Obstetrical Complications. Fertil Steril (2020) 113(2):252–7. doi: 10.1016/j.fertnstert.2019.12.007
41. Zarei A, Sohail P, Parsanezhad ME, Alborzi S, Samsami A, Azizi M. Comparison of Four Protocols for Luteal Phase Support in Frozen-Thawed Embryo Transfer Cycles: A Randomized Clinical Trial. Arch Gynecol Obstet (2017) 295:1–8. doi: 10.1007/s00404-016-4217-4
42. Ebrahimi M, Asbagh FA, Darvish S. The Effect of Luteal Phase Support on Pregnancy Rates of the Stimulated Intrauterine Insemination Cycles in Couples With Unexplained Infertility. Int J Fertil Steril (2010) 4:51–6.
43. Miles RA, Paulson RJ, Lobo RA, Press MF, Dahmoush L, Sauer MV. Pharmacokinetics and Endometrial Tissue of Progesterone After Administration by Instramuscular and Vaginal Routes:a Comparative Study. Fertil Steril (1994) 62(3):485–90. doi: 10.1016/s0015-0282(16)56935-0
Keywords: frozen-thawed embryo transfer, pregnancy outcomes, perinatal outcomes, letrozole-induced ovulation protocol, hormone replacement therapy protocol
Citation: Zhang W, Liu Z, Zhang J, Ren B, Liu M, Li J, Zhang W and Guan Y (2022) Comparison of Perinatal Outcomes of Letrozole-Induced Ovulation and Hormone Replacement Therapy Protocols in Patients With Abnormal Ovulation Undergoing Frozen-Thawed Embryo Transfer: A Propensity Score Matching Analysis. Front. Endocrinol. 13:837731. doi: 10.3389/fendo.2022.837731
Received: 17 December 2021; Accepted: 17 February 2022;
Published: 16 March 2022.
Edited by:
Yimin Zhu, Zhejiang University, ChinaReviewed by:
Kok-Min Seow, Shin Kong Wu Ho-Su Memorial Hospital, TaiwanBo Sun, First Affiliated Hospital of Zhengzhou University, China
Copyright © 2022 Zhang, Liu, Zhang, Ren, Liu, Li, Zhang and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yichun Guan, Z3VhbnlpY2h1bm1heUAxNjMuY29t
†These authors have contributed equally to this work
 Wenjuan Zhang
Wenjuan Zhang Zhaozhao Liu†
Zhaozhao Liu† Wen Zhang
Wen Zhang