
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 15 August 2022
Sec. Cellular Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.834627
This article is part of the Research Topic GnRH Agonist Triggering of Final Oocyte Maturation: Improving Safety without Compromising Success View all 5 articles
 Bella Martazanova*
Bella Martazanova* Nona Mishieva
Nona Mishieva Irina Vedikhina
Irina Vedikhina Anastasia Kirillova
Anastasia Kirillova Irina Korneeva
Irina Korneeva Tatyana Ivanets
Tatyana Ivanets Aydar Abubakirov
Aydar Abubakirov Gennady T. Sukhikh
Gennady T. SukhikhThe major limitations associated with gonadotropin-releasing hormone agonist (GnRHa) triggering are inferior clinical outcomes in fresh embryo transfer cycles caused by luteal phase insufficiency following the GnRHa triggering. We included 153 high-risk patients in this study. In group I, the patients received gonadotropin-releasing hormone agonist (GnRHa) trigger + 1,500 IU human chorionic gonadotropin (hCG) support on the oocyte pick-up (OPU) day; in group II, the patients had a dual trigger (GnRHa + 1,500 IU hCG); and in group III (control), 10,000 IU hCG trigger was prescribed for the final oocyte maturation. The levels of LH, estradiol, and progesterone were evaluated in serum on the stimulation starting day, day 6 of stimulation, on the day of the trigger administration, OPU day, days 3 and 5 post-OPU, and day 14 post-ET, as well as in follicular fluid. Progesterone concentration was significantly lower in group I on OPU+5 compared to the hCG group (I vs. III, р = 0.0065). Progesterone levels were significantly lower in group II in serum on OPU+5 compared to groups I and III (I vs. II, р = 0.0068; II vs. III, р = 1.76 × 108). The progesterone levels were significantly higher in follicular fluid in group III compared to the study groups (I vs. III, р = 0.002; II vs. III, p = 0.009). However, no significant differences in clinical outcomes were found between the groups. Then, we divided all women into pregnant and non-pregnant groups and found that estradiol (p = 0.00009) and progesterone (p = 0.000036) on the day of the pregnancy test were significantly higher in the pregnant women group. Also, progesterone on OPU day was significantly higher in the non-pregnant group (p = 0.033). Two cases of moderate ovarian hyperstimulation syndrome (OHSS) late-onset occurred in group I (3.5%, 2/56), no case of moderate/severe OHSS late-onset in group II, and three cases of moderate late-onset in group III (5.7%, 3/53). The low-dose hCG supplementation improves the luteal phase insufficiency after GnRHa triggering, which is confirmed by the comparable pregnancy rates in fresh transfer cycles between the groups. However, low-dose hCG carries a similar risk of OHSS as the full dose of hCG in high-responder patients.
The luteal phase insufficiency is leading to inferior clinical outcomes in fresh embryo transfer cycles after using gonadotropin-releasing hormone agonist (GnRHa) for oocyte maturation. This became the main limitation for GnRH triggering implementation in fresh cycles despite an effective avoidance of ovarian hyperstimulation syndrome (OHSS) (1). This negative outcome is the result of a luteal phase defect caused by the reduced luteinizing hormone (LH) surge and fast luteolysis (2). The GnRH agonist-induced surge lasts less than 48 h, which is crucial for corpus luteum support (3).
A recent Cochrane review demonstrated that GnRHa triggering is associated with low clinical pregnancy rates and high rates of early pregnancy loss (4, 5) despite good embryological outcomes (6–8). It is still an open question regarding the optimal protocol for luteal phase support after GnRHa triggering (9, 10). The main approaches that are used to overcome luteal phase insufficiency are i) luteal support with high doses of estradiol (E2) and progesterone (P) or ii) application of a single low-dose hCG bolus for the luteal phase rescue (10, 11).
According to current data, intensive luteal phase support is not enough for luteal phase insufficiency correction after GnRHa trigger in patients with peak E2 <4,000 pg/ml compared with those with peak E2 <4,000 pg/ml (11). The serum LH on the day of a trigger is one of the strictest predictors of pregnancy. The authors conclude that some form of LH supplementation after the GnRHa trigger may be necessary for corpus luteum support and in-vitro fertilization (IVF) success in high-risk patients with peak E2 <4,000 pg/ml (11). However, the LH/hCG supplementation in high-responder patients could increase the risk of OHSS development (12, 13).
This prospective observational study aimed to evaluate the levels of estradiol and progesterone and a low-dose bolus on OPU or dual trigger in high-risk patients after GnRHa agonist triggering versus the standard hCG trigger in fresh embryo transfer cycles.
This study was a prospective observational study and was approved by the Ethics Committee and Institutional Review Board of Kulakov National Medical Research Centre of Obstetrics, Gynecology, and Perinatology, protocol no. 2, 15 February 2013. Written informed consent was provided by all participants. The patients were recruited from February 2013 to December 2014.
This is a secondary analysis of a study that was held in the years 2013–2014 in the Kulakov National Medical Research Centre of Obstetrics, Gynecology, and Perinatology. A total of 258 high-risk, high-responder patients were included. The inclusion criteria were as follows: age <40 years, anti-Müllerian hormone (AMH) level >2.5 ng/ml, antral follicle count (AFC) >14, and not using additional methods for OHSS prevention (such as dopamine agonist, GnRH antagonist in the luteal phase, etс.). The exclusion criteria were as follows: severe endometriosis, uterine abnormalities, subserosal fibroids, intramural fibroids >4 cm, hydrosalpinx, and severe male infertility. We excluded patients with an estradiol concentration >4,000 pg/ml (14,685.366 pmol/L) and patients that did not receive a fresh embryo transfer (ET). Thus, only 153 women were enrolled in the hormonal profile analysis (Supplementary Materials).
The power analysis was performed according to the data reported by Engmann et al. who demonstrated a 0% OHSS rate after GnRHa triggering and 31% in the hCG triggering control group (14). The 80% power of detection with a 31% difference between the group proportions was achieved with the group sample size of 20 with L = 0.025 (three-arm design).
Furthermore, a post-hoc power analysis was performed according to the results from Datta et al., who reported a 16.2% OHSS rate after GnRHa triggering and hCG bolus on OPU day and 31% in the hCG triggering group (12). The group sample size of 125 in the study groups with L = 0.025 (three-arm design) achieved 80% power of 31% difference between the group detection.
Power analysis was performed using the Statistica software first followed by using R. However, based on the totality of outcomes in the interim analysis, we decided to discontinue further recruitment.
The primary outcomes were the serum and follicular concentrations of LH, estradiol, and progesterone and late-onset OHSS rate after different ways of ovulation triggering. The secondary outcomes were the number of mature metaphase II (MII) oocytes, the number of top-quality embryos per cycle, and the ongoing pregnancy rate.
The treatment description was accurately reported in our previous manuscript (13). All women underwent the IVF program using a flexible GnRH antagonist protocol. The patients were divided into three groups to receive one of the three types of ovulation trigger when at least three ovarian follicles had reached 17 mm in diameter.
In group I (n = 56), patients received the GnRHa trigger triptorelin 0.2 mg (Diferelin, Ipsen Pharma Biotech, Les Ulis, France) subcutaneously, and on the day of oocyte retrieval, 1,500 IU hCG (Pregnyl, Organon, Oss, the Netherlands) administered intramuscularly was added (15). In group II (n = 44), patients received a dual trigger (triptorelin 0.2 mg + 1,500 IU hCG) (16). In group III (n = 53), the control group, patients received a full-dose hCG trigger (10,000 IU Pregnyl, Organon, Oss, the Netherlands). The oocyte trigger was chosen by chance by a physician involved in the study.
All patients in groups I and II received luteal phase support with micronized P 600 mg/day (Utrogestan; Olvera, Spain; Besins Manufacturing, Belgium) and estradiol valerate 4 mg/day (Proginova; Lanno, France; Bayer Pharma AG, Germany) starting on the day after OPU. In group III, patients received micronized P 600 mg/day only, starting on the day after OPU.
In the study, we considered the late-onset forms of OHSS. Because all patients that were enrolled for analysis were allowed fresh ET and did not have early OHSS, the severity of OHSS was graded using the Golan classification (17). In the study, we considered moderate and severe forms of OHSS as they are clinically relevant.
The fertilization of mature oocytes (MII) was performed via standard IVF in case of normal sperm parameters according to World Health Organization recommendations (18) or by ICSI in case of male infertility. Embryos were cultured in 6% CO2 and 5% O2 in sequential media (ISM1, BlastAssist media; Origio, Denmark). Embryo transfer was performed either on day 3 (OPU+3) or day 5 (OPU+5). Blastocysts’ score was assigned according to the Gardner and Schoolcraft classification (19). Blastocysts graded ≥3BB on day 5 were classified as top-quality embryos. One or two embryos were used for the ET on OPU+3 or OPU+5.
The hormonal level assessment was accurately reported in our previous manuscript (13). The serum concentrations of LH (IU/L), E2 (pmol/L), and P (nmol/L) were measured in real-time using an IMMULITE 2000 immunoassay system (Siemens AG, Flanders, NJ, USA). Plasma samples were obtained on the stimulation starting day, day 6 of stimulation, the day of ovulation triggering, OPU day, days 3 and 5 post-OPU, and the day of the pregnancy test.
Follicular fluid (FF) samples for hormonal level measurement were collected on the day of OPU. A total of 108 FF samples were obtained from 54 patients.
Statistical analysis was performed with the IBM SPSS v 22.0. Continuous variables were tested for normality. For non-normally distributed data, we used the Kruskal–Wallis test and then the Mann–Whitney test. For the multiplicity of statistical testing, Bonferroni correction was applied. Categorical variables were compared using Fisher’s exact test. p-values <0.05 were considered statistically significant. Spearman’s rank correlation testing with p-values was defined as <0.05.
Patients’ characteristics such as race, age, duration and causes of infertility, the starting and total doses of rFSH, mean basal E2 level, and mean AMH concentration did not differ significantly between the groups (Table 1).
The LH levels were significantly lower on the OPU day in the hCG group (I vs. III, р = 4.61 × 108; II vs. III, p = 1.71 × 107), and the same result was observed in follicular fluid, too (I vs. III, р = 0.002; II vs. III, p = 0.009). The estradiol concentration was similar in all groups during ovarian stimulation and was significantly lower in the dual trigger group on OPU+5 (I vs. II, р = 0.004; II vs. III, p = 0.001). Progesterone levels were significantly lower in the dual trigger group in serum on OPU+3 compared to the hCG group (II vs. III, р = 0.045); however, after the Bonferroni correction was applied, the significance was not confirmed (Тable 2).
However, on OPU+5, progesterone concentrations were significantly lower in the dual trigger group compared to groups I and III (I vs. II, р = 0.0068; II vs. III, р = 1.76 × 108) (Table 2). In group I, progesterone concentration was significantly lower compared to the control group on OPU+5 (I vs. III, р = 0.0065). The P levels were significantly higher in follicular fluid in the hCG group (I vs. III, р = 0.002; II vs. III, p = 0.009) (Table 3). Then, we divided all women into pregnant and non-pregnant groups and found that E (p = 0.00009) and P (p = 0.000036) on the day of the pregnancy test were significantly higher in the pregnant women group. Also, P on OPU day was significantly higher in the non-pregnant group (p = 0.033). The E2 on OPU+3 (p = 0.045) and OPU+5 (p = 0.047) was significantly higher in the pregnant women group; however, the p-value was near the 0.05 threshold (Table 4). The LH, estradiol, and progesterone concentrations in follicular fluid were comparable between the pregnant and non-pregnant women (Table 5). The LH, estradiol, and progesterone concentrations in blood serum and follicular fluid in non-pregnant and pregnant women per group are described in Table 6. The estradiol level was significantly higher in the blood serum of pregnant women compared to that of non-pregnant patients in all groups (group I = 0.042, group II = 0.028, group III = 0.022), and the progesterone concentrations were higher in the blood serum in the hCG group (p > 0.0001).
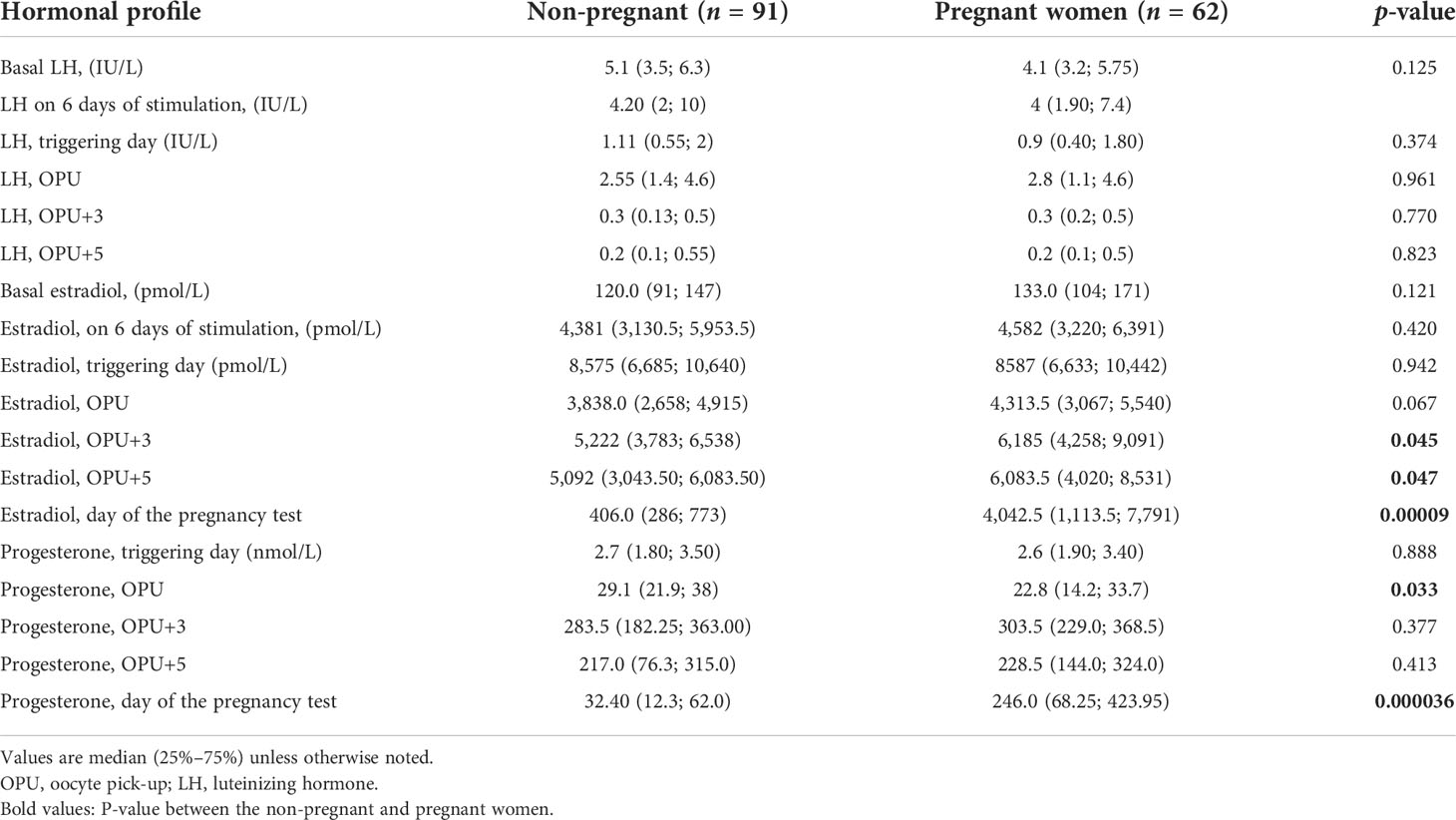
Table 4 LH, estradiol, and progesterone concentrations in blood serum in pregnant and non-pregnant women.
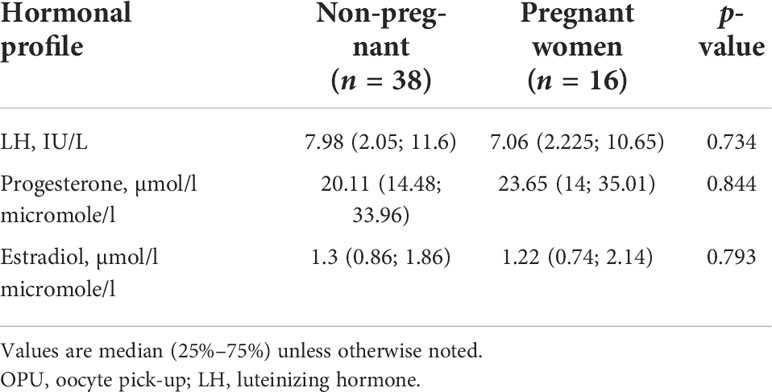
Table 5 LH, estradiol, and progesterone concentrations in follicular fluid in pregnant and non-pregnant women.
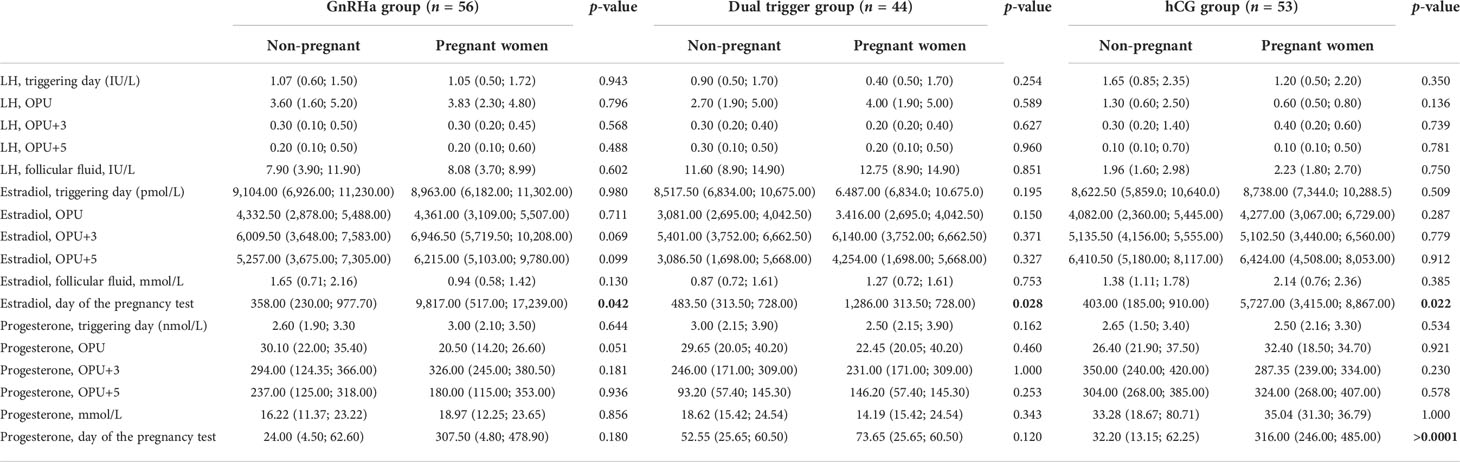
Table 6 LH, estradiol, and progesterone concentrations in blood serum and follicular fluid in non-pregnant and pregnant women per group.
There were no statistically significant differences in the main embryological (Table 7) or clinical outcomes between the groups (Table 8). No cases of fetal malformations were detected in this study. Three cases of premature birth on the 27–31 gestation weeks (one in group I and two in the hCG group) were observed. No significant difference was observed between the groups in moderate late-onset OHSS (Table 8).
In this study, we compared the levels of estradiol, progesterone, and LH in the early luteal phase and the pregnancy test day after GnRHa triggering plus 1,500 IU hCG on OPU day (group I) and dual triggering (group II) approach with a full dose of hCG.
Our study indicates that estradiol and progesterone concentrations elicited by the modified luteal support including a small dose of hCG resulted in comparable pregnancy rates. However, low-dose hCG carries a similar risk of OHSS as the full dose of hCG in high-risk patients.
In the present study, we found a comparable late-onset OHSS in group I and the control group (with a full dose of hCG). Our previous results demonstrated that any dose of hCG caused a similar VEGF concentration in the blood, even when the GnRH agonist had been added for oocyte triggering, which could have resulted in OHSS (13) and supported the present conclusion.
According to previous data, the GnRHa triggering administration effectively eliminated OHSS in high-risk patients despite the hCG low-dose timing (20). Thus, no cases of OHSS were observed in the GnRHa group with the hCG support on OPU day in women at risk of OHSS with an E2 peak of 7,936 pmol/ml (20); only one case of late-onset OHSS was reported (n = 182) using the dual trigger approach despite the inclusion of high responders (a mean peak E2 level of 4,748 ± 1,493 pg/ml) (16). According to the latest publication, there was no difference in live birth rates and OHSS between patients who received low-dose hCG at the time of GnRH agonist trigger (dual trigger) and those who received low-dose hCG at the time of oocyte retrieval; however, the authors show that the cases of OHSS in the group who received low-dose hCG at the time of oocyte retrieval were moderate, while the one case of OHSS in the dual trigger group was mild (21).
However, some studies demonstrated a 16.2%–26% incidence of mild to moderate OHSS after GnRHa triggering following low-dose hCG luteal support on OPU day (12, 22) and a 9% incidence after the dual triggering in the high-responder patients (23).
After hormonal level analysis, we found that the LH concentrations were significantly lower on the OPU day in the hCG group (Table 2), and the same result was observed in follicular fluid, too (Table 3), which is the result of induced endogenous LH surge.
The progesterone levels were significantly lower on OPU+5 in the study groups compared to the hCG group (Table 2). We supposed that it is the result of corpus luteum luteolysis, even when low doses of hCG were added. However, the progesterone concentrations required for pregnancy in the fresh ET cycle were not known.
As the ovarian stimulation by itself and hCG as a trigger are not natural processes and induce superphysiological hormonal levels and multiple follicular growth with unnaturally long hCG corpus luteum support [the hCG surge lasts for 7–10 days after administration reaching a peak after 24 h (24) with a mean half-life of 2.32 days (55 h) (25)], the GnRH agonist approach is more natural for oocyte triggering, as it induces the FSH and LH surge which lasts only 24–36 h (24, 26).
The progesterone levels were significantly higher in follicular fluid in group III compared to the study groups (Table 3), which could be the result of the “truncated” endogenous LH surge and complement previous data on the importance of luteal phase modification after GnRHa triggering.
Then, we compared the LH, estradiol, and progesterone concentrations in blood serum and follicular fluid in non-pregnant and pregnant women per group (Table 6). No correlation was found between the studied parameters and the IVF outcomes during the early luteal phase. Only on the day of the pregnancy test, the estradiol level was significantly higher in the blood serum of pregnant women compared to that of non-pregnant patients in all groups (Table 6), while the progesterone concentrations were significantly higher in the blood serum in the hCG group than those in pregnant women (Table 6).
Thus, high-responder patients who had GnRH agonist triggering with further modified luteal support (groups I and II) had sufficient concentrations of estradiol and progesterone for pregnancy support, and even the levels of progesterone were significantly different between the groups on OPU+5 (Table 2).
Then, we divided all women into pregnant and non-pregnant groups, and as expected, we found that estradiol (p = 0.00009) and progesterone (p = 0.000036) on the day of the pregnancy test were significantly higher in the pregnant women group (Table 4). It is complementary to previously reported data (27) and could be the result of the endogenous hCG corpus luteum support.
Also, progesterone on OPU day was significantly higher in the non-pregnant group (p = 0.033). Despite that we did not find premature luteinization during stimulation in the present study, it is known that progesterone receptor expression in stimulated cycle endometria is similar to the one described during the first days of the luteal phase in natural cycles, and supraphysiological concentrations of steroid hormones might cause accentuated maturation of the endometrium in IVF cycles (28). So, we supposed that higher progesterone levels in the OPU phase could affect the endometrium.
The estradiol levels on OPU+3 (p = 0.045) and OPU+5 (p = 0.047) were significantly higher in the pregnant women group; however, the p-value was near the 0.05 threshold, so further studies are needed to evaluate this tendency (Table 4). The progesterone levels during this period did not show any difference between pregnant and non-pregnant women (Table 4).
All measured hormones in follicular fluid were comparable between pregnant and non-pregnant women (Table 5). We supposed that further investigations are needed to clarify the role of estradiol and progesterone in pregnancy prediction.
An interesting result of the influence of luteal serum progesterone levels on live birth rates was shown by Thomsen et al. (2018). The authors reported that serum progesterone levels of 60–100 nmol/L in the early luteal phase and 150–250 nmol/L during the mid-luteal phase correlated with the high chances of pregnancy in fresh embryo transfer cycles. Furthermore, mid-luteal progesterone levels >400 nmol/L led to a significant reduction in the chance of a positive hCG test, and patients with progesterone >100 nmol/L had a lower risk of an early pregnancy loss compared to the reference group (27). Also, according to a 2014 review, the minimum mid-luteal progesterone threshold is approximately 80–100 nmol/L, which correlates with an early pregnancy loss reduction and an increased live birth rate (29).
As a consequence, we ranged our results according to progesterone levels (1: progesterone levels of 60–100 nmol/L, 2: progesterone levels of 150–250 nmol/L, and 3: progesterone levels >400 nmol/L) on OPU+3 and OPU+5 and measured the correlation between pregnancy rates and early pregnancy loss; however, no difference was observed.
In line with the present study, the recent data from Kaye et al. demonstrated that corpus luteum function was higher when low-dose hCG was given on the OPU day compared with adjuvant hCG given on the ovulation trigger day. Though both methods of hCG support effectively improved the luteal phase insufficiency and led to high pregnancy rates, the authors concluded that the potential for OHSS risk with increased corpus luteum activity after hCG on the OPU day should be considered (30). Thus, they reported about three cases of mild–moderate OHSS in the group receiving adjuvant hCG on the OPU day (15%) and one in the group receiving the dual trigger (10%) (30). The authors supposed that the difference in hormonal profile between the groups with different timing of hCG supplementation could be the result of differences in corpus luteum age as the hCG-stimulated steroidogenic response is dependent on the age of the corpus luteum and steroidogenic acute regulatory gene expression (30, 31).
In the present study, we did not find any difference in the treatment success rates between the GnRHa (groups I and II) and the hCG triggering groups, and the reproductive outcomes of the study groups were similar to those of previous studies.
According to previous data, the ongoing pregnancy rate after GnRHa triggering plus the administration of a low dose of hCG on OPU day was reported to be 28%–37.1% (12, 20). Also, previous studies demonstrated the ongoing pregnancy rate of 57.7%–58.8% after dual triggering in fresh ET cycles (11, 16).
The current prospective randomized double-blind research that compared the timing of hCG (on ovulation triggering day or on the OPU day) supplementation after GnRHa triggering reported comparable live birth rates between the dual trigger group and the GnRHa group with hCG on OPU day (14/34, 41.2% vs. 21/37, 56.8%, p = 0.19), with OHSS rates of 9.7% and 3.8%, respectively (21).
The major limitations of this work are the small subset of patients and the non-randomization design. Also, another study limitation is that for the initial same size calculation, we used the early OHSS rate and not the serum hormonal concentrations and late-onset OHSS rate because late OHSS is not a common complication. In addition, in the study of Datta et al., no late-onset OHSS occurred in the GnRH agonist triggering and hCG groups; in contrast, the incidence of early mild-to-moderate OHSS was 16.2% with the GnRHa trigger and 31.0% with the HCG trigger (12). In the study of Humaidan et al., only two cases of moderate late-onset OHSS occurred in the hCG group, and there were no cases of late-onset OHSS in GnRH + 1,500 hCG in the high-risk patients (20).
However, our study is unique as it is one of the last studies that received approval from the ethics committee for hCG administration to high-risk patients. Moreover, the strength of the present study is that it involves good prognosis patients in terms of patient characteristics: young women with normal body mass index; high ovarian reserve without any burdened anamnesis, which reduces the effect of patient-dependent risk factors on the effectiveness of the IVF program; and the close monitoring of the patient’s hormonal response during ovarian stimulation and early luteal phase.
We believe that more studies are needed on luteal support avoiding low-dose hCG administration, with high doses of estradiol and progesterone only (10), because a single bolus hCG support for luteal phase rescue does not eliminate OHSS.
Based on the present data, we concluded that modified luteal support including a small dose of hCG effectively supports corpus luteum function, which results in similar pregnancy and OHSS rates compared to full-dose hCG in high-responder patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics committee and Institutional Review Board of Kulakov National Medical Research Centre of Obstetrics, Gynecology, and Perinatology protocol no. 2 from 15 of February 2013. The patients/participants provided their written informed consent to participate in this study.
BM collected, analyzed, and interpreted the data and drafted the manuscript. NM was responsible for the conception, design, and data collection. IV was responsible for the collection and hormonal setting of the data. AK performed the embryological procedures and edited the manuscript. IK performed the OHSS treatment and reviewed and discussed the results. TI, AA, and GS reviewed and discussed the results. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.834627/full#supplementary-material
1. Fatemi HM, Garcia-Velasco J. Avoiding ovarian hyperstimulation syndrome with the use of gonadotropin-releasing hormone agonist trigger. Fertil Steril (2015) 103:870–3. doi: 10.1016/j.fertnstert.2015.02.004
2. Kol S. Luteolysis induced by a gonadotropin-releasing hormone agonist is the key to prevention of ovarian hyperstimulation syndrome. Fertil Steril (2004) 81:1–5. doi: 10.1016/j.fertnstert.2003.05.032
3. Chandrasekher YA, Hutchison JS, Zelinski-Wooten MB, Hess DL, Wolf DP, Stouffer RL. Initiation of periovulatory events in primate follicles using recombinant and native human luteinizing hormone to mimic the midcycle gonadotropin surge. J Clin Endocrinol Metab (1994) 79:298–306. doi: 10.1210/jcem.79.1.8027245
4. Youssef MA, van der Veen F, Al-Inany HG, Griesinger G, Mochtar MH, Aboulfoutouh I, et al. “Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist assisted reproductive technology cycles,”. In: Youssef MA, editor. Cochrane database of systematic reviews (2011) 19;(1):CD008046. Chichester, UK: John Wiley & Sons, Ltd. doi: 10.1002/14651858.CD008046.pub3
5. Youssef MAFM, van der Veen F, Al-Inany HG, Mochtar MH, Griesinger G, Nagi Mohesen M, et al. Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist-assisted reproductive technology. Cochrane Database Syst Rev (2014) 10:CD008046. doi: 10.1002/14651858.CD008046.pub4
6. Reddy J, Turan V, Bedoschi G, Moy F, Oktay K. Triggering final oocyte maturation with gonadotropin-releasing hormone agonist (GnRHa) versus human chorionic gonadotropin (hCG) in breast cancer patients undergoing fertility preservation: An extended experience. J Assist Reprod Genet (2014) 31:927–32. doi: 10.1007/s10815-014-0248-6
7. Bodri D, Guillén JJ, Galindo A, Mataró D, Pujol A, Coll O. Triggering with human chorionic gonadotropin or a gonadotropin-releasing hormone agonist in gonadotropin-releasing hormone antagonist-treated oocyte donor cycles: findings of a large retrospective cohort study. Fertil Steril (2009) 91:365–71. doi: 10.1016/j.fertnstert.2007.11.049
8. Papanikolaou EG, Verpoest W, Fatemi H, Tarlatzis B, Devroey P, Tournaye H. A novel method of luteal supplementation with recombinant luteinizing hormone when a gonadotropin-releasing hormone agonist is used instead of human chorionic gonadotropin for ovulation triggering: A randomized prospective proof of concept study. Fertil Steril (2011) 95:1174–7. doi: 10.1016/j.fertnstert.2010.09.023
9. Engmann L, Benadiva C, Humaidan P. GnRH agonist trigger for the induction of oocyte maturation in GnRH antagonist IVF cycles: A SWOT analysis. Reprod BioMed Online (2016) 32:274–85. doi: 10.1016/j.rbmo.2015.12.007
10. Humaidan P, Engmann L, Benadiva C. Luteal phase supplementation after gonadotropin-releasing hormone agonist trigger in fresh embryo transfer: The American versus European approaches. Fertil Steril (2015) 103:879–85. doi: 10.1016/j.fertnstert.2015.01.034
11. Griffin D, Benadiva C, Kummer N, Budinetz T, Nulsen J, Engmann L. Dual trigger of oocyte maturation with gonadotropin-releasing hormone agonist and low-dose human chorionic gonadotropin to optimize live birth rates in high responders. Fertil Steril (2012) 97(6):1316–20. doi: 10.1016/j.fertnstert.2012.03.015
12. Datta AK, Eapen A, Birch H, Kurinchi-Selvan A, Lockwood G. Retrospective comparison of GnRH agonist trigger with HCG trigger in GnRH antagonist cycles in anticipated high-responders. Reprod BioMed Online (2014) 29:552–8. doi: 10.1016/j.rbmo.2014.08.006
13. Martazanova B, Mishieva N, Vtorushina V, Vedikhina I, Levkov L, Korneeva I, et al. Angiogenic cytokine and interleukin 8 levels in early luteal phase after triggering ovulation with gonadotropin-releasing hormone agonist in high-responder patients. Am J Reprod Immunol (2021) 85:1–9. doi: 10.1111/aji.13381
14. Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C. The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective rando. Fertil Steril (2008) 89:84–91. doi: 10.1016/j.fertnstert.2007.02.002
15. Humaidan P, Ejdrup Bredkjær H, Westergaard LG, Yding Andersen C. 1,500 IU human chorionic gonadotropin administered at oocyte retrieval rescues the luteal phase when gonadotropin-releasing hormone agonist is used for ovulation induction: a prospective, randomized, controlled study. Fertil Steril (2010) 93:847–54. doi: 10.1016/j.fertnstert.2008.12.042
16. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Comparison of “triggers” using leuprolide acetate alone or in combination with low-dose human chorionic gonadotropin. Fertil Steril (2011) 95:2715–7. doi: 10.1016/j.fertnstert.2011.03.109
17. Golan A, Weissman A. Symposium: Update on prediction and management of OHSS - a modern classification of OHSS. Reprod Biomed Online (2009):28–32. doi: 10.1016/S1472-6483(10)60042-9
18. World Health Organization. Laboratory manual for the examination and processing of human semen. Switzerland.:Cambridge Cambridge Univ Press (2010). Available at: https://apps.who.int/iris/handle/10665/44261
19. Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol (1999) 11:307–11. doi: 10.1097/00001703-199906000-00013
20. Humaidan P, Polyzos NP, Alsbjerg B, Erb K, Mikkelsen AL, Elbaek HO, et al. GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: Two prospective randomized controlled multi-centre studies in IVF patients. Hum Reprod (2013) 28:2511–21. doi: 10.1093/humrep/det249
21. Engmann LL, Maslow BS, Kaye LA, Griffin DW, Diluigi AJ, Schmidt DW, et al. Low dose human chorionic gonadotropin administration at the time of gonadotropin releasing-hormone agonist trigger versus 35 h later in women at high risk of developing ovarian hyperstimulation syndrome - a prospective randomized double-blind clinical tri. J Ovarian Res (2019) 12:8. doi: 10.1186/s13048-019-0483-7
22. Seyhan A, Ata B, Polat M, Son WY, Yarali H, Dahan MH. Severe early ovarian hyperstimulation syndrome following GnRH agonist trigger with the addition of 1500 IU hCG. Hum Reprod (2013) 28:2522–8. doi: 10.1093/humrep/det124
23. O’Neill KE, Senapati S, Maina I, Gracia C, Dokras A. GnRH agonist with low-dose hCG (dual trigger) is associated with higher risk of severe ovarian hyperstimulation syndrome compared to GnRH agonist alone. J Assist Reprod Genet (2016) 33:1175–84. doi: 10.1007/s10815-016-0755-8
24. Fauser BC, De Jong D, Olivennes F, Wramsby H, Tay C, Itskovitz-Eldor J, et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab (2002) 87:709–15. doi: 10.1210/jc.87.2.709
25. Damewood MD, Shen W, Zacur HA, Schlaff WD, Rock JA, Wallach EE. Disappearance of exogenously administered human chorionic gonadotropin. Fertil Steril (1989) 52:398–400. doi: 10.1016/S0015-0282(16)60906-8
26. Itskovitz J, Boldes R, Levron J, Erlik Y, Kahana L, Brandes JM. Induction of preovulatory luteinizing hormone surge and prevention of ovarian hyperstimulation syndrome by gonadotropin-releasing hormone agonist. Fertil Steril (1991) 56:213–20. doi: 10.1016/S0015-0282(16)54474-4
27. Thomsen LH, Kesmodel US, Erb K, Bungum L, Pedersen D, Hauge B, et al. The impact of luteal serum progesterone levels on live birth rates-a prospective study of 602 IVF/ICSI cycles. Hum Reprod (2018) 33:1506–16. doi: 10.1093/humrep/dey226
28. Papanikolaou EG, Bourgain C, Kolibianakis E, Tournaye H, Devroey P. Steroid receptor expression in late follicular phase endometrium in GnRH antagonist IVF cycles is already altered, indicating initiation of early luteal phase transformation in the absence of secretory changes. Hum Reprod (2005) 20:1541–7. doi: 10.1093/humrep/deh793
29. Yding Andersen C, Vilbour Andersen K. Improving the luteal phase after ovarian stimulation: Reviewing new options. Reprod BioMed Online (2014) 28:552–9. doi: 10.1016/j.rbmo.2014.01.012
30. Kaye L, Griffin D, Thorne J, Neuber E, Nulsen J, Benadiva C, et al. Independent serum markers of corpora lutea function after gonadotropin-releasing hormone agonist trigger and adjuvant low dose human chorionic gonadotropin in in vitro fertilization. Fertil Steril (2019) 112:534–44. doi: 10.1016/j.fertnstert.2019.04.034
31. Kohen P, Castro O, Palomino A, Muñoz A, Christenson LK, Sierralta W, et al. The steroidogenic response and corpus luteum expression of the steroidogenic acute regulatory protein after human chorionic gonadotropin administration at different times in the human luteal phase. J Clin Endocrinol Metab (2003) 88:3421–30. doi: 10.1210/jc.2002-021916
Keywords: GnRH agonist trigger, dual trigger, modified luteal support, progesterone, OHSS
Citation: Martazanova B, Mishieva N, Vedikhina I, Kirillova A, Korneeva I, Ivanets T, Abubakirov A and Sukhikh GT (2022) Hormonal profile in early luteal phase after triggering ovulation with gonadotropin-releasing hormone agonist in high-responder patients. Front. Endocrinol. 13:834627. doi: 10.3389/fendo.2022.834627
Received: 13 December 2021; Accepted: 19 July 2022;
Published: 15 August 2022.
Edited by:
Ralf Jockers, Université de Paris, FranceReviewed by:
Jaana Seikkula, Central Finland Central Hospital, FinlandCopyright © 2022 Martazanova, Mishieva, Vedikhina, Kirillova, Korneeva, Ivanets, Abubakirov and Sukhikh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bella Martazanova, ZHIuYmVsbGEuaXZmQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.