
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 22 February 2022
Sec. Bone Research
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.833485
This article is part of the Research Topic Assessment of Osteoporotic Fractures and Risk Prediction View all 12 articles
 Qing-Chang Chen1,2
Qing-Chang Chen1,2 Yan Zhang3*
Yan Zhang3*Bone diseases are the leading causes of disability and severely compromised quality of life. Neuropeptide Y (NPY) is a multifunctional neuropeptide that participates in various physiological and pathological processes and exists in both the nerve system and bone tissue. In bone tissue, it actively participates in bone metabolism and disease progression through its receptors. Previous studies have focused on the opposite effects of NPY on bone formation and resorption through paracrine modes. In this review, we present a brief overview of the progress made in this research field in recent times in order to provide reference for further understanding the regulatory mechanism of bone physiology and pathological metabolism.
The mammalian skeleton is a vital organ formed by several bone types, and it is also the place for hematopoiesis and mineral storage, with powerful self-repair ability and mineralized extracellular matrix. The traditional view of factors affecting bone metabolism such as endocrine, paracrine, and mechanical stimulation has long been discussed. Recent findings reported that bone tissue (including the periosteum, cortical and trabecular bone, bone marrow) was abundantly innervated by autonomic nerve terminals, which is one of the key factors regulating bone metabolism and remodeling through direct or indirect manner (1, 2), making the autonomic nerve system and bone metabolism closely linked.
When neuropeptide Y (NPY) was first discovered in 1983, the awareness of its function in energy balance, obesity, and bone metabolism has gradually increased (3, 4). As a 36-amino acid peptide belonging to the pancreatic polypeptide family, NPY is most abundantly produced and expressed in the nervous system (5). In the central nervous system, NPY is distributed in the amygdala, locus coeruleus, and cerebral cortex, with the highest expression level in the hypothalamus. It acts to coordinate signals from a wide variety of sources to participate in appetite, circadian rhythm, and energy utilization regulation (6, 7). In the periphery, NPY was found to be co-stored and co-released with neurotransmitter noradrenaline (NA) in postganglionic sympathetic nerves (8). Recent studies have reported that NPY and its receptors have also been identified in bone tissue, such as in osteoblasts, osteocytes, and adipocytes (2, 9, 10), indicating the potential role of NPY on bone remodeling in local sites. Moreover, it can also act as a mediator of the autonomic nervous system to mediate bone marrow mesenchymal cell (BMSC) differentiation fate by constructing a mouse model that lacks osteocyte-specific NPY (2). Even though various physiological conditions and pathophysiological processes such as obesity (11), anxiety (12), food intake (13), chronic pain (14), neurodegenerative disorders (15), and bone disease (2) have been proven to require NPY to participate, its effect on bone metabolism is still poorly understood.
In this review, we focus on the effects of NPY on bone metabolism in some physiological and pathological states. The aims of this article are to review the regulatory effects and to achieve a comprehensive understanding of NPY on bone metabolism.
Bone remodeling involves mineralized bone removal by osteoclasts followed by bone matrix formation through osteoblasts that subsequently become mineralized (16). It is a key process for maintaining bone mass in a dynamic balance and continues throughout life. Previous studies have proven the vital role of NPY in the regulation of food intake and energy homeostasis, and its role in bone metabolism has gradually become a hot topic in recent years.
NPY is a highly conserved endogenous peptide and multifunctional neurotransmitter acting via five G-protein-coupled receptor subtypes named Y1R, Y2R, Y4R, Y5R, and Y6R, of which Y1R and Y2R modulate bone mass at differing sites and through different ways (2, 14, 17). The arcuate nucleus of the hypothalamus exhibited the greatest expression level of NPY, and Y2R is the most abundant subtype in the central nervous system (18), which is also peripherally found in the liver, intestine, spleen, muscle, and adipose tissue, suggesting Y2R may have local effects in these tissues (19). Y2 antagonist treatment resulted in reduced bone resorption level and greater bone mineral density in ovariectomized (OVX) mice (20). Hypothalamic Y2R knockout mice exhibited increased osteoblast activity, mineralization rate, and bone mass, indicating a catabolic role of Y2R in stimulating cortical and cancellous bone formation (Table 1) (28, 29).
Y1R has also been reported to be involved in many physiological activities, such as mitogenic activity, macrophage migration, and pulpal development (17, 21, 22). In bone tissue, Y1R is highly expressed in BMSCs, osteoblast, osteocyte, monocyte/macrophage, and osteoclast (2), prompting it to play a regulatory role in the local area. Y1R germline deletion resulted in elevated osteoblast activity and mineral apposition rate, together with increased formation of highly multinucleated osteoclasts and enhanced surface area, demonstrating a negative role of Y1R on bone mass maintenance (23, 24). Furthermore, the Y1R antagonist regulated gut microbiota and exhibited an anti-osteoporotic effect in OVX rats (25), revealing that Y1R may affect bone mass through multiple ways.
To date, little is known about the role of Y4R, Y5R, and Y6R in bone mass maintenance. Y4R was reported to mainly affect body weight, fat mass, energy expenditure, and anxiety-like and depression-related behavior (31, 32). Interestingly, male mice lacking both Y2R and Y4R displayed a synergistic effect in trabecular bone volume upregulation compared with Y2R knockout mice, but female double knockout mice did not show this bone phenotype, suggesting a synergy between Y2 and Y4 receptor pathways (33). Igura et al. reported that Y5R expression level in bone marrow cells declined with age and Y5R overexpression strengthened the proliferation effect induced by NPY, indicating that Y5R may take part in bone metabolism by affecting the self-renewal ability of bone marrow cells (34). Y6R, which is restricted to the suprachiasmatic nucleus (SCN) of the hypothalamus, is required for the maintenance of bone mass in mice. Mice lacking Y6R displayed reduced numbers of osteoblast precursors and increased osteoclast activity (37).
As seed cells in bone marrow, BMSCs are able to commit to osteogenic lineage and differentiate into mature osteoblasts. Intensive studies in recent years have demonstrated that a number of transcription factors are involved in this process. Among them, runt-related transcription factor 2 (runx2) and osterix are considered as master transcription factors in osteogenic differentiation and they control bone formation (39). Zhang et al. found that runx2 level and mineralized nodules were decreased after NPY treatment in osteogenic differentiation of BMSCs, confirming that NPY inhibits osteogenesis by inhibiting runx2, and this effect may be achieved through Y1R (2). Germline deletion of Y1R and knockout of NPY produce anabolic responses in bone, with upregulated runx2 and osterix level, resulting in a generalized increase in bone mass owing to stimulated osteoblast activity and an increased bone formation rate (40, 41). Besides, dorsomedial nucleus NPY knockdown mice showed increased basal and obesity-induced decrease in bone mineral density (BMD) together with reduced activating transcription factor 4 (ATF4) expression level (42). Activator protein 1 (AP1) antagonists targeted to NPY neurons resulted in increased trabecular bone formation and mass (43). In glucocorticoid-induced osteoporotic skeleton, NPY expression and marrow adipogenesis were upregulated, together with increased post-translational modification of peroxisome proliferator-activated receptor gamma (PPARγ) (44).
Paradoxically, several studies have reported that NPY acts as a promoting factor in the process of bone formation and fracture repair. Liu et al. found that low doses of NPY stimulate BMSC osteogenic differentiation and mineralization while a high NPY concentration had the opposite effect (45). In patients with combined injuries, NPY levels were increased than in those with simple fractures, and further experiment demonstrated that NPY directly promotes BMSC osteogenic differentiation (46). Y1R antagonist-treated mice or Y1R-deficient mice exhibited a delay in fracture repair and cartilage removal, as evidenced by reduced calcified nodule area and decreased bone callus volume and strength (47, 48). Researchers recently used overexpression plasmids and small interfering RNA (siRNA) targeting NPY transfected into the MC3T3−E1 osteoblastic cell line and found that NPY overexpression markedly enhanced the osteogenic ability by an autocrine mechanism, together with the upregulation of osterix and runx2 level (49). Knockdown of the Y1R induced alkaline phosphatase (ALP) activity and mineralization together with upregulated mRNA expression of specific genes that characterize osteoblastic differentiation in MC3T3−E1 cells (50).
As an anxiolytic factor, NPY was reported to protect against chronic stress‐induced bone loss specifically through Y2R, evidenced by increased bone mass and bone formation rate (51). Also, NPY can regulate bone formation through an indirect manner. Ma et al. found that NPY stimulated human osteoblast osteogenic activity by enhancing gap junction intercellular communication (52). The Y1R antagonist upregulated serum Ca2+ concentration, changed the gut microflora community composition, and improved bone mass in OVX rats (25). Although the studies mentioned above seem inconsistent, it is certain that bone formation is strongly influenced by NPY.
Bone resorption was mediated by mature osteoclast, which is a tissue-specific multinuclear giant cell derived from hematopoietic stem cells through the myelomonocytic precursor cells/macrophage lineage. In brief, hematopoietic stem cells are committed to macrophage colony-forming units (CFU-M) in the presence of macrophage colony-stimulating factor (M-CSF). When the receptor activator of nuclear factor-kappa B ligand (RANKL) binds RANK on the surface of osteoclast precursors, osteoclastogenesis is immediately triggered. CFU-M is further differentiated into mononucleated osteoclasts and subsequently fused to multinucleated osteoclasts, then fully matured upon a cognate interaction with osteoblasts (53). Wu et al. reported that NPY greatly increased the amount of RAW264.7 cell (mouse leukemic monocyte macrophage cell line) migration at different concentrations, and this effect can be diminished by the Y1R antagonist and ERK1/2 inhibitor, which suggest that NPY promotes osteoclast migration through Y1R and ERK1/2 activation (22). NPY has also been shown to exhibit an inhibitory effect on isoprenaline-induced osteoclastogenesis by suppressing RANKL expression in mouse bone marrow cells (54). In addition, an in-vitro experiment confirmed that the regulator of osteoclastogenesis RANKL/OPG ratio was higher in NPY-treated BMSCs, and this effect can be reversed with Y1R antagonist treatment, making evidence that NPY may facilitate bone resorption through Y1R (55).
On the contrary, Park et al. found that NPY can mobilize hematopoietic stem/progenitor cells (HSPCs) from the bone marrow to the peripheral blood and ameliorated low bone density in an ovariectomy-induced osteoporosis mouse model by reducing osteoclast number (56). Seldeen et al. used an osteoporotic mouse model injected once daily with JNJ-31020028, a brain-penetrant Y2R small molecule antagonist. Then, primary bone cell cultures were isolated from the tibiae, and it was found that bone marrow cultures obtained from the Y2R antagonist-treated mice exhibited significantly more osteoclasts and greater areal coverage with in-vitro osteoclast differentiation induction, which means that central NPY inhibited osteoclastogenesis through Y2R (20).
In our study, osteoclast number and activity seem not be significantly influenced by bone-specific deficiency of NPY in young and aged mice (2). Matic et al. generated a mouse model where NPY was overexpressed specifically in mature osteoblasts and osteocytes and characterized the bone phenotype of 3-month-old mice. It was found that bone volume was reduced; however, bone formation rate and osteoclast activity were not significantly changed (57). The direct and indirect effects of NPY on bone resorption need further exploration.
In addition to participating in bone metabolism through affecting bone turnover, NPY may also affect bone mass through other ways. Blood vessels play an irreplaceable important role in the metabolic balance of bones. Several studies have confirmed that NPY-immunoreactive fibers were predominantly localized alongside with blood vessel walls in bone; moreover, Y1R, Y2R, and Y5R were confirmed to be expressed on endothelial cells (ECs), providing a material basis for the vasoregulatory role of NPY in addition to directly regulating bone tissue cells (58). It has been observed that BMSC migration and VEGF expression were upregulated after NPY treatment (45) and increased levels of VEGF stimulate angiogenesis and osteoblastic differentiation of BMSCs (59). Besides, Y1R signaling disruption is responsible for enhancing the deposition and maturity of collagen and mineral hydroxyapatite layers in the skeletal muscle, and bone mechanical property was furthered improved (60) (Figure 1).
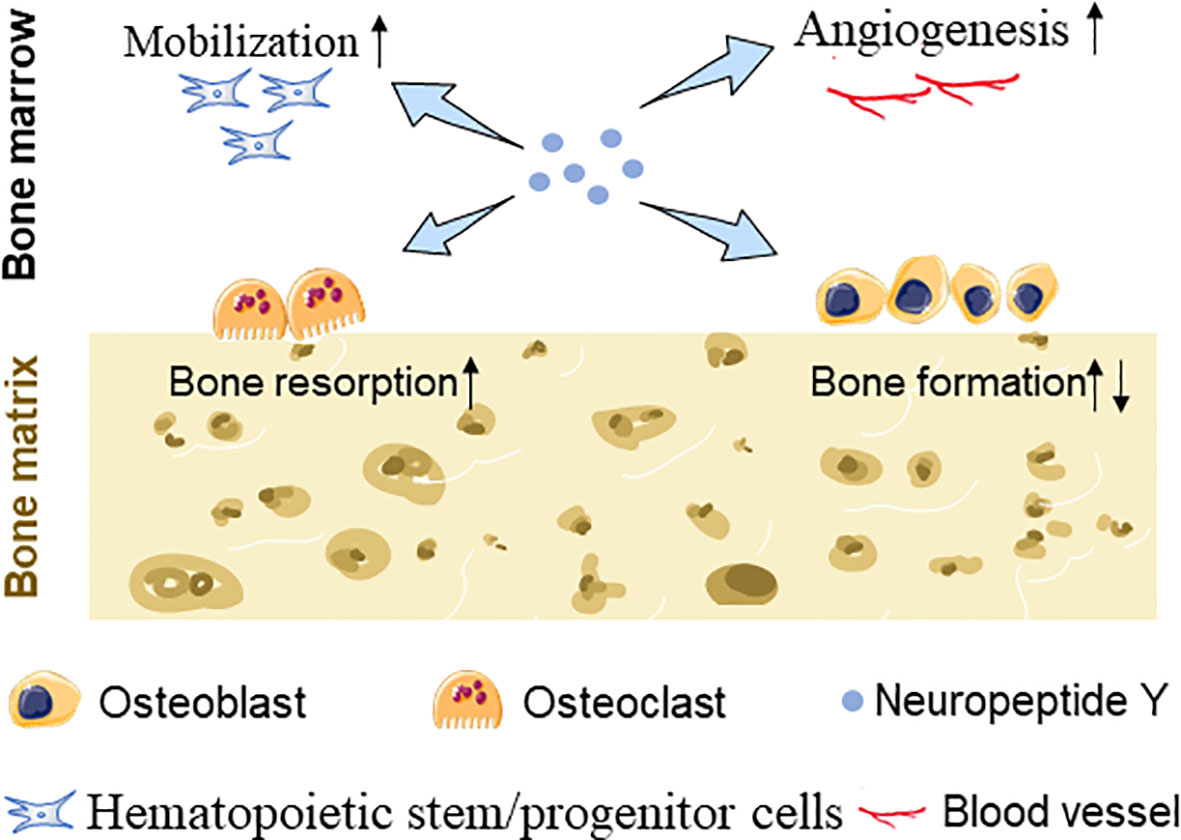
Figure 1 Schematic diagram showing NPY-mediated BMSC mobilization, EC angiogenesis, and bone turnover changes.
Osteoporosis (OP) is a common skeletal disorder characterized by compromised bone mass and degraded bone microarchitecture, often resulting in fragility fractures and severely compromised quality of life in elderly people. Increasing age and postmenopausal state are proven to be associated with this condition. Zhang et al. reported that ovariectomy induced NPY upregulation in bone tissue after constructing a model of OP in adult female mouse. γ‐Oryzanol (ORZ), a functional substance extracted from rice bran, alleviated the severity of postmenopausal and senile OP through the autonomic nervous system by inhibiting osteocytic-NPY secretion (2). In glucocorticoid-mediated bone loss, NPY mRNA expression and protein concentration were elevated, while BMD and bone microstructure were significantly reduced (44). Xie et al. reported that the OP group exhibited deteriorated bone microstructure and more microdamage than the osteoarthritis (OA) group, and they also measured NPY and Y1R expression levels in patients after constructing a postmenopausal osteoporotic rat model and found these to be both upregulated in OP groups. Y1R antagonist treatment in vivo for OVX rats could improve bone microstructure and decrease bone microdamage, and this may be achieved via the cAMP/PKA/CREB signaling pathway (10). Also, NPY is increased in the rat spinal cord after nerve injury in the model of peripheral nerve trauma (61). Above all, it is possible that NPY participates in the pathogenesis of osteoporosis. In detail, NPY plays a negative role in the process of osteoporosis.
Bone fracture healing is a multistep and overlapping process involving inflammation, osteogenesis, and angiogenesis (62). Among these processes, the formation of primary bone is a crucial one since it is the key process of fracture healing. Gu et al. focused on patients with traumatic brain injury–fracture-combined injuries and found that the NPY level was increased, accomplished with an increase of bone formation markers, indicating an active role of NPY in fracture healing (46). Sousa et al. generated germline (Y1−/−) and osteoblastic-specific Y1R knockout mice to characterize whether Y1R plays a role in fracture healing. The fracture healing process was delayed in the global deletion of Y1R in mice, and this delay is independent from osteoblast-specific Y1R. In Y1R-specific deficient mice, delayed endochondral fracture healing seems to be the result of impaired inflammatory response and cartilage removal since Y1R is widely expressed in neuronal but also in non-neuronal cells, such as immune cells (47). However, Long et al. established an angular fracture rat model and found that regenerating NPY fibers were increased in the early stages and then reduced between 21 and 56 days on the concave side compared with the convex side, suggesting that NPY innervation appears to correlate with the loss of callus thickness in angular fractures (63). Based on the evidence mentioned above, the authors hypothesized that NPY plays an important role in fracture healing, and this role may not be achieved through Y1R. Further study is needed to clarify the underlying mechanism.
NPY is produced not only by the central and peripheral nervous system but also by immune cells such as macrophages, B cells, neutrophils, and lymphocytes (64). It can cause the activation of immune cell response and induce the release of proinflammatory cytokines including TNF-α or interleukin-6, acting as a potent modulator of the immune responses during inflammation, infection, and autoimmunity (65–67). In animal models of systemic inflammation such as endotoxemia, the expression of NPY in the hypothalamus was slightly increased and positively correlated with the severity of inflammation (68, 69). A cross-sectional design of rheumatoid arthritis (RA) patients found that serum levels of NPY are significantly related to TNF-α levels and disease activity in RA independently of IL-6, TNF-α, or leptin levels (67). In patients with knee osteoarthritis, concentrations of NPY in synovial fluid were gradually upregulated with the severity of pain, suggesting a role for NPY as a putative regulator of joint homeostasis (66). This suggested that NPY plays a crucial role in both systematic and local sites, and often reflected the severity of inflammation.
As the most common joint disease worldwide, OA is characterized by cartilage degradation, synovial inflammation, subchondral bone remodeling, and osteophyte formation and primarily identified as a non-inflammatory musculoskeletal degeneration (70). Several studies suggest the involvement of NPY in the pathogenesis of OA, and it has already been identified as the major peptide involved both in the generation of pain. NPY concentration in synovial fluid was significantly higher in OA patients compared with controls and positively correlated with pain intensity (66, 71). Kang et al. reported that NPY was overexpressed in human OA cartilage accompanied with increased Y2R expression. Stress stimulus resulted in the sympathetic release of NPY, which in turn promoted the upregulation of NPY and Y2R in articular cartilage and participated in chondrocyte hypertrophy together with cartilage matrix degradation (30). Hernanz et al. demonstrated a significant stimulatory activity of NPY on inflammatory factors such as IL-1β, IL-6, and TNF-α production by whole blood leukocytes from OA patients in vitro, which play critical roles in pain in the early stage of OA, indicating a positive effect of NPY in inflammation (72, 73).
Mood disorders such as chronic stress and depression often have adverse consequences on many organs, including the bone. In view of the negative effects of NPY signaling on bone metabolism mentioned above, NPY activity associated with chronic stress and depression would predict a deleterious influence on bone homeostasis. In multiple sclerosis (MS) patients, autonomic nervous system dysfunction and low BMD are intertwined with some mood disorders such as depression, fatigue, and migraine (74). Higher levels of depression were demonstrated in osteocalcin-deficient mice when compared with wild-type mice, giving evidence to bone signal back to the brain (75). Animal experiments also showed that antidepressants may exhibit clinical efficacy by increasing NPY expression levels (76). However, as a well-described anxiolytic factor, NPY was also reported to exhibit a stress-protective role specifically through Y2 receptors (51). The relationships between NPY and mood disorder and between NPY and bone mass maintenance are intriguing and need further investigations.
Previous studies have verified that NPY is widely present in the brain and bone tissue and strongly influences bone metabolism through direct and indirect manner. In addition to directly regulating bone formation and resorption, NPY may also participate in bone metabolism by affecting gut microbiota and blood vessel formation. Furthermore, NPY has also been reported to play an intermediary role in autonomic nerve regulation on bone metabolism. As a substance synthesized by multiple places, it will be a challenge to clearly clarify the role of NPY on bone turnover and elucidate the pathophysiology of common bone diseases mentioned above. Also, whether NPY derived from sympathetic nerve endings and osteocytes has different physiological effects remains to be explored. Even though previous studies have shown that NPY participates in bone metabolism, especially in the bone formation process and BMSC fate decision, the effect of NPY on osteoclastogenesis and mood disorder is not fully understood.
In spite of NPY being mostly expressed in the central nervous system, the role of NPY secreted by surrounding tissues, organs, and cell types in bone metabolism and cell signal transduction may be an important future research consideration. Future research on NPY and its receptors will be beneficial for new drug development and identifying new treatments for bone diseases.
YZ conceived and designed the manuscript. Q-CC wrote the paper. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Elefteriou F. Impact of the Autonomic Nervous System on the Skeleton. Physiol Rev (2018) 8:1083–112. doi: 10.1152/physrev.00014.2017
2. Zhang Y, Chen CY, Liu YW, Rao SS, Tan YJ, Qian YX, et al. Neuronal Induction of Bone-Fat Imbalance Through Osteocyte Neuropeptide Y. Adv Sci (Weinh) (2021) 8:e2100808. doi: 10.1002/advs.202100808
3. Adrian TE, Allen JM, Bloom SR, Ghatei MA, Rossor MN, Roberts GW, et al. Neuropeptide Y Distribution in Human Brain. Nature (1983) 306:584–6. doi: 10.1038/306584a0
4. Zhang L, Bijker MS, Herzog H. The Neuropeptide Y System: Pathophysiological and Therapeutic Implications in Obesity and Cancer. Pharmacol Ther (2011) 131:91–113. doi: 10.1016/j.pharmthera.2011.03.011
5. Liu S, Wang ZF, Su YS, Ray RS, Jing XH, Wang YQ, et al. Somatotopic Organization and Intensity Dependence in Driving Distinct NPY-Expressing Sympathetic Pathways by Electroacupuncture. Neuron (2020) 108:436–450.e7. doi: 10.1016/j.neuron.2020.07.015
6. Clemenzi MN, Martchenko A, Loganathan N, Tse EK, Brubaker PL, Belsham DD. Analysis of Western Diet, Palmitate and BMAL1 Regulation of Neuropeptide Y Expression in the Murine Hypothalamus and BMAL1 Knockout Cell Models. Mol Cell Endocrinol (2020) 507:110773. doi: 10.1016/j.mce.2020.110773
7. Dyzma M, Boudjeltia KZ, Faraut B, Kerkhofs M. Neuropeptide Y and Sleep. Sleep Med Rev (2010) 14:161–5. doi: 10.1016/j.smrv.2009.09.001
8. Vähätalo LH, Ruohonen ST, Ailanen L, Savontaus E. Neuropeptide Y in Noradrenergic Neurons Induces Obesity in Transgenic Mouse Models. Neuropeptides (2016) 55:31–7. doi: 10.1016/j.npep.2015.11.088
9. Igwe JC, Jiang X, Paic F, Ma L, Adams DJ, Baldock PA, et al. Neuropeptide Y Is Expressed by Osteocytes and Can Inhibit Osteoblastic Activity. J Cell Biochem (2009) 108:621–30. doi: 10.1002/jcb.22294
10. Xie W, Li F, Han Y, Qin Y, Wang Y, Chi X, et al. Neuropeptide Y1 Receptor Antagonist Promotes Osteoporosis and Microdamage Repair and Enhances Osteogenic Differentiation of Bone Marrow Stem Cells via cAMP/PKA/CREB Pathway. Aging (Albany NY) (2020) 12:8120–36. doi: 10.18632/aging.103129
11. Hassan AM, Mancano G, Kashofer K, Fröhlich EE, Matak A, Mayerhofer R, et al. High-Fat Diet Induces Depression-Like Behaviour in Mice Associated With Changes in Microbiome, Neuropeptide Y, and Brain Metabolome. Nutr Neurosci (2019) 22:877–93. doi: 10.1080/1028415X.2018.1465713
12. Yang Z, Han S, Keller M, Kaiser A, Bender BJ, Bosse M, et al. Structural Basis of Ligand Binding Modes at the Neuropeptide Y Y1 Receptor. Nature (2018) 556:520–4. doi: 10.1038/s41586-018-0046-x
13. Sobrino Crespo C, Perianes Cachero A, Puebla Jiménez L, Barrios V, Arilla Ferreiro E. Peptides and Food Intake. Front Endocrinol (Lausanne) (2014) 5:58. doi: 10.3389/fendo.2014.00058
14. Diaz-delCastillo M, Woldbye DPD, Heegaard AM. Neuropeptide Y and Its Involvement in Chronic Pain. Neuroscience (2018) 387:162–9. doi: 10.1016/j.neuroscience.2017.08.050
15. Li C, Wu X, Liu S, Zhao Y, Zhu J, Liu K. Roles of Neuropeptide Y in Neurodegenerative and Neuroimmune Diseases. Front Neurosci (2019) 13:869. doi: 10.3389/fnins.2019.00869
16. Feng X, McDonald JM. Disorders of Bone Remodeling. Annu Rev Pathol (2011) 6:121–45. doi: 10.1146/annurev-pathol-011110-130203
17. Czarnecka M, Lu C, Pons J, Maheswaran I, Ciborowski P, Zhang L, et al. Neuropeptide Y Receptor Interactions Regulate Its Mitogenic Activity. Neuropeptides (2019) 73:11–24. doi: 10.1016/j.npep.2018.11.008
18. Garduño J, Hernández-López S, Rolón DC, de la Cruz L, Hernández-Vázquez F, Reyes-Vaca A, et al. Electrophysiological Characterization of Glucose Sensing Neurons in the Hypothalamic Arcuate Nucleus of Male Rats. Neurosci Lett (2019) 703:168–76. doi: 10.1016/j.neulet.2019.03.041
19. Shi YC, Lin S, Castillo L, Aljanova A, Enriquez RF, Nguyen AD, et al. Peripheral-Specific Y2 Receptor Knockdown Protects Mice From High-Fat Diet-Induced Obesity. Obes (Silver Spring) (2011) 19:2137–48. doi: 10.1038/oby.2011.99
20. Seldeen KL, Halley PG, Volmar CH, Rodríguez MA, Hernandez M, Pang M, et al. Neuropeptide Y Y2 Antagonist Treated Ovariectomized Mice Exhibit Greater Bone Mineral Density. Neuropeptides (2018) 67:45–55. doi: 10.1016/j.npep.2017.11.005
21. Rethnam S, Raju B, Fristad I, Berggreen E, Heyeraas KJ. Differential Expression of Neuropeptide Y Y1 Receptors During Pulpal Inflammation. Int Endod J (2010) 43:492–8. doi: 10.1111/j.1365-2591.2010.01704.x
22. Wu W, Peng S, Shi Y, Li L, Song Z, Lin S. NPY Promotes Macrophage Migration by Upregulating Matrix Metalloproteinase-8 Expression. J Cell Physiol (2021) 236:1903–12. doi: 10.1002/jcp.29973
23. Lee NJ, Nguyen AD, Enriquez RF, Doyle KL, Sainsbury A, Baldock PA, et al. Osteoblast Specific Y1 Receptor Deletion Enhances Bone Mass. Bone (2011) 48:461–7. doi: 10.1016/j.bone.2010.10.174
24. Sousa DM, Conceição F, Silva DI, Leitão L, Neto E, Alves CJ, et al. Ablation of Y1 Receptor Impairs Osteoclast Bone-Resorbing Activity. Sci Rep (2016) 6:33470. doi: 10.1038/srep33470
25. Xie W, Han Y, Li F, Gu X, Su D, Yu W, et al. Neuropeptide Y1 Receptor Antagonist Alters Gut Microbiota and Alleviates the Ovariectomy-Induced Osteoporosis in Rats. Calcif Tissue Int (2020) 106:444–54. doi: 10.1007/s00223-019-00647-5
26. Yang CH, Ann-Onda D, Lin X, Fynch S, Nadarajah S, Pappas EG, et al. Neuropeptide Y1 Receptor Antagonism Protects β-Cells and Improves Glycemic Control in Type 2 Diabetes. Mol Metab (2021) 55:101413. doi: 10.1016/j.molmet.2021.101413
27. Yu W, Chen FC, Xu WN, Ding SL, Chen PB, Yang L, et al. Inhibition of Y1 Receptor Promotes Osteogenesis in Bone Marrow Stromal Cells via cAMP/PKA/CREB Pathway. Front Endocrinol (Lausanne) (2020) 11:583105:583105. doi: 10.3389/fendo.2020.583105
28. Baldock PA, Allison S, McDonald MM, Sainsbury A, Enriquez RF, Little DG, et al. Hypothalamic Regulation of Cortical Bone Mass: Opposing Activity of Y2 Receptor and Leptin Pathways. J Bone Miner Res (2006) 21:1600–7. doi: 10.1359/jbmr.060705
29. Lundberg P, Allison SJ, Lee NJ, Baldock PA, Brouard N, Rost S, et al. Greater Bone Formation of Y2 Knockout Mice Is Associated With Increased Osteoprogenitor Numbers and Altered Y1 Receptor Expression. J Biol Chem (2007) 282:19082–91. doi: 10.1074/jbc.M609629200
30. Kang X, Qian Z, Liu J, Feng D, Li H, Zhang Z, et al. Neuropeptide Y Acts Directly on Cartilage Homeostasis and Exacerbates Progression of Osteoarthritis Through NPY2R. J Bone Miner Res (2020) 35:1375–84. doi: 10.1002/jbmr.3991
31. Kang N, Wang XL, Zhao Y. Discovery of Small Molecule Agonists Targeting Neuropeptide Y4 Receptor Using Homology Modeling and Virtual Screening. Chem Biol Drug Des (2019) 94:2064–72. doi: 10.1111/cbdd.13611
32. Zhang L, Riepler SJ, Turner N, Enriquez RF, Lee IC, Baldock PA, et al. Y2 and Y4 Receptor Signaling Synergistically Act on Energy Expenditure and Physical Activity. Am J Physiol Regul Integr Comp Physiol (2010) 299:R1618–1628. doi: 10.1152/ajpregu.00345.2010
33. Sainsbury A, Baldock PA, Schwarzer C, Ueno N, Enriquez RF, Couzens M, et al. Synergistic Effects of Y2 and Y4 Receptors on Adiposity and Bone Mass Revealed in Double Knockout Mice. Mol Cell Biol (2003) 23:5225–33. doi: 10.1128/MCB.23.15.5225-5233.2003
34. Igura K, Haider HKH, Ahmed RP, Sheriff S, Ashraf M. Neuropeptide Y and Neuropeptide Y Y5 Receptor Interaction Restores Impaired Growth Potential of Aging Bone Marrow Stromal Cells. Rejuvenation Res (2011) 14:393–403. doi: 10.1089/rej.2010.1129
35. Gøtzsche CR, Nikitidou L, Sørensen AT, Olesen MV, Sørensen G, Christiansen SH, et al. Combined Gene Overexpression of Neuropeptide Y and Its Receptor Y5 in the Hippocampus Suppresses Seizures. Neurobiol Dis (2012) 45:288–96. doi: 10.1016/j.nbd.2011.08.012
36. Fukasaka Y, Nambu H, Tanioka H, Obata A, Tonomura M, Okuno T, et al. An Insurmountable NPY Y5 Receptor Antagonist Exhibits Superior Anti-Obesity Effects in High-Fat Diet-Induced Obese Mice. Neuropeptides (2018) 70:55–63. doi: 10.1016/j.npep.2018.05.006
37. Khor EC, Yulyaningsih E, Driessler F, Kovaĉić N, Wee NKY, Kulkarni RN, et al. The Y6 Receptor Suppresses Bone Resorption and Stimulates Bone Formation in Mice via a Suprachiasmatic Nucleus Relay. Bone (2016) 84:139–47. doi: 10.1016/j.bone.2015.12.011
38. Mullins DE, Guzzi M, Xia L, Parker EM. Pharmacological Characterization of the Cloned Neuropeptide Y y(6) Receptor. Eur J Pharmacol (2000) 395:87–93. doi: 10.1016/s0014-2999(00)00255-7
39. Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, et al. Fate Decision of Mesenchymal Stem Cells: Adipocytes or Osteoblasts? Cell Death Differ (2016) 23:1128–39. doi: 10.1038/cdd.2015.168
40. Lee NJ, Doyle KL, Sainsbury A, Enriquez RF, Hort YJ, Riepler SJ, et al. Critical Role for Y1 Receptors in Mesenchymal Progenitor Cell Differentiation and Osteoblast Activity. J Bone Miner Res (2010) 25:1736–47. doi: 10.1002/jbmr.61
41. Baldock PA, Lee NJ, Driessler F, Lin S, Allison S, Stehrer B, et al. Neuropeptide Y Knockout Mice Reveal a Central Role of NPY in the Coordination of Bone Mass to Body Weight. PloS One (2009) 4:e8415. doi: 10.1371/journal.pone.0008415
42. Qin Q, Chen P, Cui Z, Wang J, Xie B, Zhang S, et al. Neuropeptide Y Knockdown in the Dorsomedial Hypothalamus Improved Basal and Obesity-Induced Decrease in Bone Mass Density. Neuro Endocrinol Lett (2019) 40:289–96.
43. Idelevich A, Sato K, Avihai B, Nagano K, Galien A, Rowe G, et al. Both NPY-Expressing and CART-Expressing Neurons Increase Energy Expenditure and Trabecular Bone Mass in Response to AP1 Antagonism, But Have Opposite Effects on Bone Resorption. J Bone Miner Res (2020) 35:1107–18. doi: 10.1002/jbmr.3967
44. Wang FS, Lian WS, Weng WT, Sun YC, Ke HJ, Chen YS, et al. Neuropeptide Y Mediates Glucocorticoid-Induced Osteoporosis and Marrow Adiposity in Mice. Osteoporos Int (2016) 27:2777–89. doi: 10.1007/s00198-016-3598-3
45. Liu S, Jin D, Wu JQ, Xu ZY, Fu S, Mei G, et al. Neuropeptide Y Stimulates Osteoblastic Differentiation and VEGF Expression of Bone Marrow Mesenchymal Stem Cells Related to Canonical Wnt Signaling Activating In Vitro. Neuropeptides (2016) 56:105–13. doi: 10.1016/j.npep.2015.12.008
46. Gu XC, Zhang XB, Hu B, Zi Y, Li M. Neuropeptide Y Accelerates Post-Fracture Bone Healing by Promoting Osteogenesis of Mesenchymal Stem Cells. Neuropeptides (2016) 60:61–6. doi: 10.1016/j.npep.2016.09.005
47. Sousa DM, McDonald MM, Mikulec K, Peacock L, Herzog H, Lamghari M, et al. Neuropeptide Y Modulates Fracture Healing Through Y1 Receptor Signaling. J Orthop Res (2013) 31:1570–8. doi: 10.1002/jor.22400
48. Dong P, Gu X, Zhu G, Li M, Ma B, Zi Y. Melatonin Induces Osteoblastic Differentiation of Mesenchymal Stem Cells and Promotes Fracture Healing in a Rat Model of Femoral Fracture via Neuropeptide Y/Neuropeptide Y Receptor Y1 Signaling. Pharmacology (2018) 102:272–80. doi: 10.1159/000492576
49. Zhang B, Zhang X, Xiao J, Zhou X, Chen Y, Gao C. Neuropeptide Y Upregulates Runx2 and Osterix and Enhances Osteogenesis in Mouse MC3T3−E1 Cells via an Autocrine Mechanism. Mol Med Rep (2020) 22:4376–82. doi: 10.3892/mmr.2020.11506
50. Yahara M, Tei K, Tamura M. Inhibition of Neuropeptide Y Y1 Receptor Induces Osteoblast Differentiation in MC3T3−E1 Cells. Mol Med Rep (2017) 16:2779–84. doi: 10.3892/mmr.2017.6866
51. Baldock PA, Lin S, Zhang L, Karl T, Shi Y, Driessler F, et al. Neuropeptide Y Attenuates Stress-Induced Bone Loss Through Suppression of Noradrenaline Circuits. J Bone Miner Res (2014) 29:2238–49. doi: 10.1002/jbmr.2205
52. Ma WH, Liu YJ, Wang W, Zhang YZ. Neuropeptide Y, Substance P, and Human Bone Morphogenetic Protein 2 Stimulate Human Osteoblast Osteogenic Activity by Enhancing Gap Junction Intercellular Communication. Braz J Med Biol Res (2015) 48:299–307. doi: 10.1590/1414-431X20144226
53. Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells (2020) 9:2073. doi: 10.3390/cells9092073
54. Amano S, Arai M, Goto S, Togari A. Inhibitory Effect of NPY on Isoprenaline-Induced Osteoclastogenesis in Mouse Bone Marrow Cells. Biochim Biophys Acta (2007) 1770:966–73. doi: 10.1016/j.bbagen.2007.02.009
55. Wood J, Verma D, Lach G, Bonaventure P, Herzog H, Sperk G, et al. Structure and Function of the Amygdaloid NPY System: NPY Y2 Receptors Regulate Excitatory and Inhibitory Synaptic Transmission in the Centromedial Amygdala. Brain Struct Funct (2016) 221:3373–91. doi: 10.1007/s00429-015-1107-7
56. Park MH, Kim N, Jin HK, Bae JS. Neuropeptide Y-Based Recombinant Peptides Ameliorate Bone Loss in Mice by Regulating Hematopoietic Stem/Progenitor Cell Mobilization. BMB Rep (2017) 50:138–43. doi: 10.5483/bmbrep.2017.50.3.191
57. Matic I, Matthews BG, Kizivat T, Igwe JC, Marijanovic I, Ruohonen ST, et al. Bone-Specific Overexpression of NPY Modulates Osteogenesis. J Musculoskelet Neuronal Interact (2012) 12:209–18. doi: 10.1055/s-0032-1305278
58. Wu JQ, Jiang N, Yu B. Mechanisms of Action of Neuropeptide Y on Stem Cells and Its Potential Applications in Orthopaedic Disorders. World J Stem Cells (2020) 12:986–1000. doi: 10.4252/wjsc.v12.i9.986
59. Zhang LF, Qi J, Zuo G, Jia P, Shen X, Shao J, et al. Osteoblast-Secreted Factors Promote Proliferation and Osteogenic Differentiation of Bone Marrow Stromal Cells via VEGF/heme-Oxygenase-1 Pathway. PloS One (2014) 9:e99946. doi: 10.1371/journal.pone.009994
60. Sousa DM, Martins PS, Leitão L, Alves CJ, Gomez-Lazaro M, Neto E, et al. The Lack of Neuropeptide Y-Y1 Receptor Signaling Modulates the Chemical and Mechanical Properties of Bone Matrix. FASEB J (2020) 34:4163–77. doi: 10.1096/fj.201902796R
61. Marvizon JC, Chen W, Fu W, Taylor BK. Neuropeptide Y Release in the Rat Spinal Cord Measured With Y1 Receptor Internalization Is Increased After Nerve Injury. Neuropharmacology (2019) 158:107732. doi: 10.1016/j.neuropharm.2019.107732
62. Rather HA, Jhala D, Vasita R. Dual Functional Approaches for Osteogenesis Coupled Angiogenesis in Bone Tissue Engineering. Mater Sci Eng C Mater Biol Appl (2019) 103):109761. doi: 10.1016/j.msec.2019.109761
63. Long H, Ahmed M, Ackermann P, Stark A, Li J. Neuropeptide Y Innervation During Fracture Healing and Remodeling. A Study of Angulated Tibial Fractures in the Rat. Acta Orthop (2010) 81:639–46. doi: 10.3109/17453674.2010.504609
64. Wheway J, Herzog H, Mackay F. NPY and Receptors in Immune and Inflammatory Diseases. Curr Top Med Chem (2007) 7:1743–52. doi: 10.2174/156802607782341046
65. Jana B, Całka J, Palus K. Inflammation Changes the Expression of Neuropeptide Y Receptors in the Pig Myometrium and Their Role in the Uterine Contractility. PloS One (2020) 15:e0236044. doi: 10.1371/journal.pone.0236044
66. Wang L, Zhang L, Pan H, Peng S, Lv M, Lu WW. Levels of Neuropeptide Y in Synovial Fluid Relate to Pain in Patients With Knee Osteoarthritis. BMC Musculoskelet Disord (2014) 15:319. doi: 10.1186/1471-2474-15-319
67. Ramirez-Villafaña M, Saldaña-Cruz AM, Aceves-Aceves JA, Perez-Guerrero EE, Fajardo-Robledo NS, Rubio-Arellano ED, et al. Serum Neuropeptide Y Levels Are Associated With TNF-α Levels and Disease Activity in Rheumatoid Arthritis. J Immunol Res (2020) 2020:8982163. doi: 10.1155/2020/8982163
68. Duan K, Yu W, Lin Z, Tan S, Bai X, Gao T, et al. Insulin Ameliorating Endotoxaemia-Induced Muscle Wasting Is Associated With the Alteration of Hypothalamic Neuropeptides and Inflammation in Rats. Clin Endocrinol (Oxf) (2015) 82:695–703. doi: 10.1111/cen.12610
69. Cheng M, Gao T, Xi F, Cao C, Chen Y, Zhao C, et al. Dexmedetomidine Ameliorates Muscle Wasting and Attenuates the Alteration of Hypothalamic Neuropeptides and Inflammation in Endotoxemic Rats. PloS One (2017) 12:e0174894. doi: 10.1371/journal.pone.0174894
70. Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet (2015) 386:376–87. doi: 10.1016/S0140-6736(14)60802-3
71. Xiao J, Yu W, Wang X, Wang B, Chen J, Liu Y, et al. Correlation Between Neuropeptide Distribution, Cancellous Bone Microstructure and Joint Pain in Postmenopausal Women With Osteoarthritis and Osteoporosis. Neuropeptides (2016) 56:97–104. doi: 10.1016/j.npep.2015.12.006
72. Hernanz A, Medina S, de Miguel E, Martín-Mola E. Effect of Calcitonin Gene-Related Peptide, Neuropeptide Y, Substance P, and Vasoactive Intestinal Peptide on Interleukin-1beta, Interleukin-6 and Tumor Necrosis Factor-Alpha Production by Peripheral Whole Blood Cells From Rheumatoid Arthritis and Osteoarthritis Patients. Regul Pept (2003) 115:19–24. doi: 10.1016/s0167-0115(03)00127-7
73. Li L, Li Z, Li Y, Hu X, Zhang Y, Fan P. Profiling of Inflammatory Mediators in the Synovial Fluid Related to Pain in Knee Osteoarthritis. BMC Musculoskelet Disord (2020) 21(1):99. doi: 10.1186/s12891-020-3120-0
74. Hesse S, Moeller F, Petroff D, Lobsien D, Luthardt J, Regenthal R, et al. Altered Serotonin Transporter Availability in Patients With Multiple Sclerosis. Eur J Nucl Med Mol Imaging (2014) 41:827–35. doi: 10.1007/s00259-013-2636-z
75. Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, et al. Maternal and Offspring Pools of Osteocalcin Influence Brain Development and Functions. Cell (2013) 155:228–41. doi: 10.1016/j.cell.2013.08.042
Keywords: bone disease, NPY, bone formation, bone resorption, osteoporosis
Citation: Chen Q-C and Zhang Y (2022) The Role of NPY in the Regulation of Bone Metabolism. Front. Endocrinol. 13:833485. doi: 10.3389/fendo.2022.833485
Received: 11 December 2021; Accepted: 20 January 2022;
Published: 22 February 2022.
Edited by:
Zhi-Feng Sheng, Central South University, ChinaReviewed by:
Kaiping Zhao, Beijjing Jishuitan Hospital, ChinaCopyright © 2022 Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zhang, eWFuemhhbmcwMjI3QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.