
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 10 March 2022
Sec. Cancer Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.830760
This article is part of the Research Topic The Role of Genetic Alterations in Thyroid Cancer View all 6 articles
Purpose: Anaplastic thyroid carcinoma (ATC) and primary squamous cell carcinoma of the thyroid (PSCCTh) have similar histological findings and are currently treated using the same approaches; however, the characteristics and prognosis of these cancers are poorly researched. The objective of this study was to determine the differences in characteristics between ATC and PSCCTh and establish prognostic models.
Patients and Methods: All variables of patients with ATC and PSCCTh, diagnosed from 2004–2015, were retrieved from the Surveillance, Epidemiology, and End Results Program (SEER) database. Percentage differences for categorical data were compared using the Chi-square test. Kaplan-Meier curves, log-rank test, and Cox-regression for survival analysis, and C-index value was used to evaluate the performance of the prognostic models.
Results: After application of the inclusion and exclusion criteria, a total of 1164 ATC and 124 PSCCTh patients, diagnosed from 2004 to 2015, were included in the study. There were no differences in sex, ethnicity, age, marital status, or percentage of proximal metastases between the two cancers; however, radiotherapy, chemotherapy, incidence of surgical treatment, and presence of multiple primary tumors were higher in patients with ATC than those with PSCCTh. Further cancer-specific survival (CSS) of patients with PSCCTh was better than that of patients with ATC. Prognostic factors were not identical for the two cancers. Multivariate Cox model analysis indicated that age, sex, radiotherapy, chemotherapy, surgery, multiple primary tumors, marital status, and distant metastasis status are independent prognostic factors for CSS in patients with ATC, while for patients with PSCCTh, the corresponding factors are age, radiotherapy, multiple primary tumors, and surgery. The C-index values of the two models were both > 0.8, indicating that the models exhibited good discriminative ability.
Conclusion: Prognostic factors influencing CSS were not identical in patients with ATC and PSCCTh. These findings indicate that different clinical treatment and management plans are required for patients with these two types of thyroid cancer.
Anaplastic thyroid cancer (ATC) is the most aggressive type of primary thyroid cancer, and accounts for approximately 1%–2% of all thyroid tumors, most of which occur in elderly adults. Giant cells, spindle cells, and squamous cells show obvious pleomorphism, while anaplastic tumors have no clear differentiation (1, 2). Primary squamous cell carcinoma of the thyroid (PSCCTh) is an extremely rare and highly aggressive malignant tumor, which comprises < 1% of all primary thyroid cancers and has poor prognosis. In terms of cytology, PSCCTh consists of large pleiomorphic epithelial cells, with frequent keratinization and necrotic components (3, 4). The most common symptom of PSCCTh is an enlarged anterior neck mass, followed by difficulty breathing or swallowing, and a change in voice. Both ATC and PSCCTh present with abundant squamous cell-like cells; hence, the two conditions are easy to confuse during clinical diagnosis and are treated similarly (5).
The similarities and differences between ATC and PSCCTh have become a focus of increased research attention. Given the rarity of these two diseases, conducting prospective studies with large samples is very difficult. Both types of primary thyroid cancer are highly aggressive and have poor prognosis. At present, combined treatments, including surgery, radiotherapy, and chemotherapy, are often used in the clinic (6); however, the role of adjuvant therapy for these two cancers remains unclear. In addition, differences in cancer-specific survival (CSS) between patients with ATC and PSCCTh have not been extensively studied, and understanding the factors that influence CSS prognosis in ATC and PSCCTh is crucial.
With the aim of better understanding the demographic and clinical treatment-related indicators that differentiate ATC and PSCCTh, and exploring factors influencing CSS and prognosis in patients with these tumors, we conducted a retrospective study based on data from the Surveillance, Epidemiology and End Results Program (SEER) database (SEER 18 registry grouping) (7).
We extracted patient data from the updated SEER database (https://seer.cancer.gov), and downloaded using SEER*Stat software (version 8.3.6; https://seer.cancer.gov/), this database (Incidence -SEER 18 Regs Custom Data with additional treatment fields, Nov 2018 Sub, 1975-2016 varying) includes radiotherapy and chemotherapy information. Since patient identifying information has been removed from this data set, local ethics committee review and patient informed consent were not required.
The cases included in this study were diagnosed with ATC or PSCCTh from 2004–2015. Samples were selected based International Classification of Diseases for Oncology third edition (ICD-O-3) organizational behavior codes, issued by the World Health Organization (WHO) in 2013. The histology codes for ATC are 8012, 8020, 8021, and 8030–8032, and those for PSCCTh are 8070–8076; the primary thyroid location code for all samples was C73.9. Demographic and clinical data, including year of diagnosis, age at diagnosis, ethnicity, sex, primary tumor, marital status at diagnosis, surgery of the primary site, N stage, M stage, radiotherapy, chemotherapy, survival time, and SEER cause-specific death classification-related variables, were retrieved for analysis. Samples with unknown ethnicity or survival time, or where the surgical status or N stage data field was blank, were excluded from the analyses.
Categorical data are expressed as rates or percentages and differences were analyzed using the chi-square test. In this study, CSS of patients with ATC and PSCCTh was the primary research endpoint. The Kaplan-Meier method was used to evaluate patient CSS, and the log-rank method was used to assess differences in CSS between patients with the two types of thyroid cancer. Single-factor and multi-factor Cox regression models were used to assess the impact of demographic and clinical factors on CSS in patients with ATC and PSCCTh; variables with p < 0.2 on univariate test and important variables, recognized by clinical experts (such as treatments), were included in the multivariate model. Harrel’s Consistency Index (C-Index) was used to evaluate the models. Mapping and statistical analyses were conducted using R Language 3.6.0 (http://www.r-project.org/). All p-values were two-sided and p < 0.05 was considered significant.
Of data in the SEER database from 2004 to 2015, 1288 patients with primary thyroid cancer (ATC = 1164, PSCCTh = 124) met the inclusion and exclusion criteria and were finally included in this study. Analysis of differences in demographic and clinical data of patients with ATC and PSCCTh are presented in Table 1. There were no differences in age distribution, sex, ethnicity, marital status, or distribution of proximal metastasis between the two cancers. A higher percentage of patients with ATC underwent radiotherapy, chemotherapy, or surgery, and had multiple primary tumors and distant metastasis (p < 0.05).
To evaluate the CSS prognosis of patients with ATC and PSCCTh, we visualized and analyzed CSS in patients from these two groups. Kaplan-Meier curves indicated that the median CSS of patients with ATC was 4 months, while that of patients with PSCCTh was almost 10 months. Log-rank test analysis demonstrated that CSS was longer in patients with PSCCTh than in those with ATC (p < 0.01) (Figure 1).
Univariate Cox regression analysis of all variables was conducted to investigate the influence of different factors on CSS prognosis in patients with ATC, and showed that ‘white’ ethnicity, radiotherapy, chemotherapy, surgery, and marital status were prognostic factors influencing CSS in patients with ATC. In multivariate Cox analysis, age ≥ 65 years, male sex, radiotherapy, chemotherapy, surgery, multiple primary tumors, and distant metastasis were independent prognostic factors affecting CSS in patients with ATC (Table 2). The C-index of the model was 0.803 (95% confidence interval (CI): 0.787–0.819), demonstrating that it has a good discriminative ability.
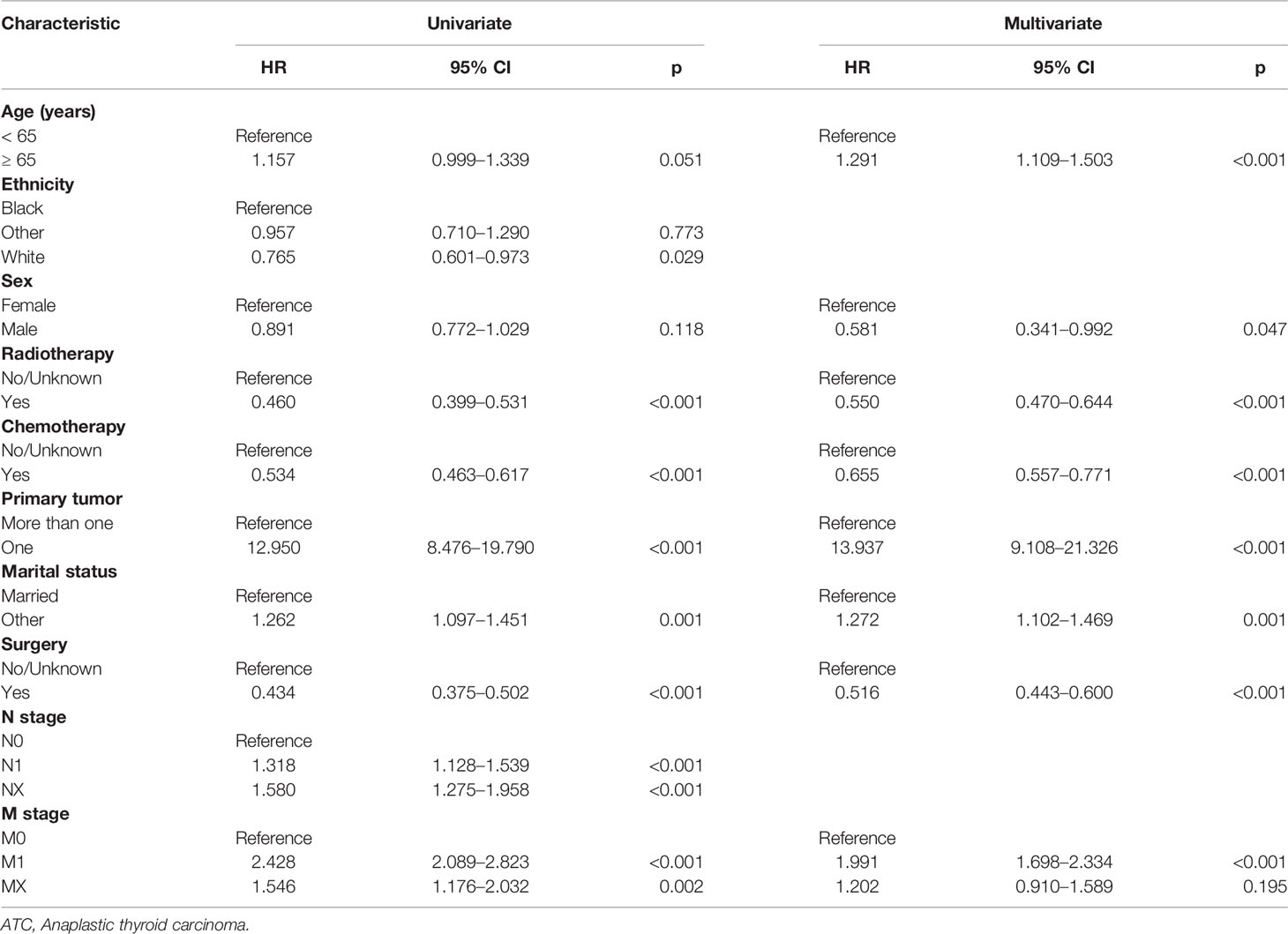
Table 2 Univariate and multivariate Cox regression models of factors associated with CSS in patients with ATC.
To study influences on CSS in patients with PSCCTh, we conducted univariate and multivariate Cox model analyses of various potential prognostic factors. Univariate Cox analysis found that age, sex, and multiple primary tumors were factors influencing CSS in patients with PSCCTh. Although radiotherapy and chemotherapy were not significantly associated according to univariate tests, they are routine treatments for PSCCTh; therefore, we included them in the multivariate model. The multi-factor Cox model indicated that age, radiotherapy, multiple primary tumors, and surgery were independent prognostic factors for CSS in patients with PSCCTh. Distant metastasis did not retain significance in the multivariate model; however, its test value was close to the significance level (Table 3). The C-index of the model was 0.829 (95% CI: 0.786–0.872), indicating that it has good discrimination ability for patients with PSCCTh.
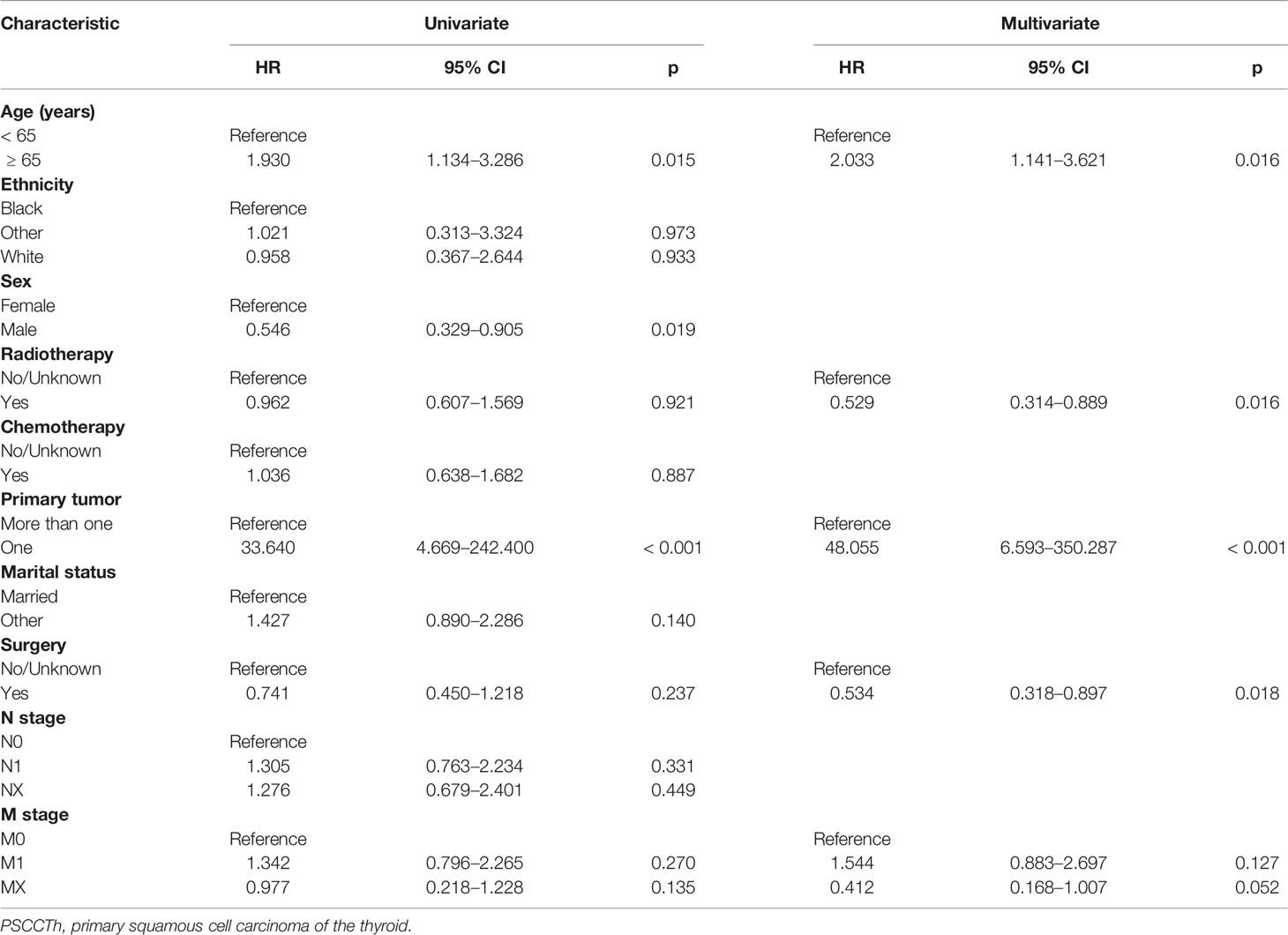
Table 3 Univariate and multivariate Cox regression model of factors associated with CSS in patients with PSCCTh.
Both ATC and PSCCTh are rare malignant thyroid tumors. The data analyzed in this study were all retrieved from the SEER database. The study period was from 2004 to 2015, and the study included 1164 and 124 patients with ATC and PSCCTh, respectively. Our results indicate that median CSS time was longer in patients with PSCCTh than in those with ATC, consistent with previous studies (8). We also found that a higher proportion of patients with ATC had distant metastases, consistent with the results reported by Glaser et al. (9).
In line with previous studies, we found that women comprise a higher proportion of patients with ATC and PSCCTh than men, which may be related to the biological properties of these cancers (10, 11). Since PSCCTh is a rare type of thyroid cancer histology and clinical experience of this disease is limited, appropriate treatment for PSCCTh is controversial. At present, the treatment methods applied for PSCCTh and ATC are similar, and primarily comprise chemotherapy, radiotherapy, and surgery; however, treatment rates for ATC were higher than those for PSCCTh, because compared with PSCCTh, ATC has a clear, well-defined structure (8).
Age was a prognostic factor for CSS in patients with ATC. The survival rate of patients ≥ 65 years old was less than that of patients < 65 years old, confirming the findings of Gui et al. (12). Smallridge et al. (13) demonstrated that, for patients diagnosed with early ATC, surgical treatment, chemotherapy, radiotherapy, and systemic treatment can achieve optimal survival outcomes, while active palliative and clinical care are crucial for individuals with advanced ATC (14). Relevant studies (15, 16) have reported that sex, surgical treatment, radiotherapy, and chemotherapy are all prognostic factors for CSS in patients with ATC, consistent with the results of this study. Here, we found that marital status was a prognosis factor for CSS in patients with ATC. Several studies have demonstrated that people with differing marital status have varying levels of home care and psychological support, resulting in differences in their living conditions (17, 18).
Our research demonstrates that PSCCTh is more likely to occur in elderly female patients. Age is an independent factor influencing CSS in patients with PSCCTh, whereas sex and ethnicity are not associated with prognosis. Radiotherapy is a protective factor for CSS in patients with PSCCTh, and related studies (19) have reached the same conclusion. In a case report, Shimaoka and Tsukada (20) proposed that chemotherapy has an active role in the treatment of PSCCTh; however, in this study we did not detect chemotherapy as a prognostic factor for CSS in patients with PSCCTh. Surgical treatment was also an independent factor which affected CSS in patients with PSCCTh. Published evidence indicates that the best treatment for PSCCTh is early diagnosis, followed by aggressive surgery to prevent bleeding or suffocation caused by obstruction (21). Unlike CSS of patients with ATC, marital status was not a prognostic factor for CSS in patients with PSCCTh. In the PSCCTh CSS prognostic model, the statistical test value of M stage is close to the threshold for statistical significance, and the hazard ratio (HR) value of Primary tumor is very large. In addition to the real role of the variables, the reason for this phenomenon may be caused by the sample size.
According to the American Thyroid Cancer Association guidelines, surgery is suitable for low-risk patients with small tumors (< 1 cm), without clinical lymph node metastases, because of its manageable risk (22). Conzo et al. found that patients who undergo hemithyroidectomy for thyroid follicular neoplasms had a lower incidence of hypothyroidism and shorter hospital stay than those receiving total thyroidectomy; however, total thyroidectomy should be the first choice in patients with high cancer risk (23). For patients with papillary thyroid carcinoma without lymph node involvement, prophylactic neck dissection is inappropriate, and there is controversy over whether radioactive iodine treatment is necessary (24). Pezzolla et al. suggested that incidental thyroid carcinomas with a predominant papillary pattern can be treated with endocrine surgery after clear follow-up by a multidisciplinary follow-up team (25). For ATC, Conzo et al. (26) proposed that surgery should be the basis of treatment, with multimodal treatment, including surgery, chemotherapy, and radiotherapy, having potential to improve survival of patients with locally advanced ATC, and our study also reached similar conclusions. Research by Au et al. (27) found that surgery is not a prognostic factor for CSS in patients with PSCCTh; this differs from our results, as our findings indicate that surgical treatment is protective factor for CSS in patients with PSCCTh, possibly due to variations in the surgical and radiological methods used.
Lin et al. (15) found that, although the overall survival of patients with multiple primary thyroid tumors was much lower than that of patients with a single primary thyroid cancer, there was no difference in CSS between patients with multiple and single primary thyroid tumors. Amer (28) compared the survival rates of patients with single and multiple primary tumors, and found that the overall survival rate of patients with multiple primary tumors was higher than that of patients with single primary tumors. This may be because the survival time of patients with many types of cancer is not sufficiently long to develop a second origin. In this study, multiple primary tumors was a protective factor for CSS in patients with ATC and PSCCTh; this does not indicate that the mortality rate of patients with single primary thyroid tumors is lower and may reflect that the mortality rate from other cancers in patients with several primary tumors is higher than that caused by thyroid cancer.
This study has some shortcomings. The limited number of patients with PSCCTh included in the study (N = 124) may have contributed to possible false negative results. In particular, some factors had large HR values in the model, and comprehensive validation methods could not be applied, with only C-index calculation used. In addition, this was a retrospective study and some samples with incomplete data were removed, with only patients with complete information included, which may have led to bias.
This study included analysis of CSS in a large population cohort of patients with ATC and PSCCTh. Our findings show that, although both ATC and PSCCTh are aggressive thyroid malignancies, the prognosis for CSS is better in patients with PSCCTh than in those with ATC. We have established prognostic models for CSS of patients with both ATC and PSCCTh, and the prognostic factors for the two diseases were not identical, indicating that specific clinical treatment and management plans need to be developed for these two types of thyroid cancer.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
SJ and Y-NY contributed to the conception and design of the study. SJ and XML collected and analyzed data. DHL and DDP wrote the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by funds from the National Natural Science Foundation of China (61803112, 32160151), the Ministry of Education Industry-Academia Cooperation Collaborative Education Project (202101311012, 202102576027), the Science and Technology Foundation of Guizhou Province (2019-2811, 2018-1133), NSFC Incubation Program by Guizhou Medical University (20NSP033).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.830760/full#supplementary-material
1. Are C, Shaha AR. Anaplastic Thyroid Carcinoma: Biology, Pathogenesis, Prognostic Factors, and Treatment Approaches. Ann Surg Oncol (2006) 13:453–64. doi: 10.1245/ASO.2006.05.042
2. Giuffrida D, Gharib H. Anaplastic Thyroid Carcinoma: Current Diagnosis and Treatment. Ann Oncol (2000) 11:1083–9. doi: 10.1023/a:1008322002520
3. Goldman RL. Primary Squamous Cell Carcinoma of the Thyroid Gland: Report of a Case and Review of the Literature. Am Surg (1964) 30:247.
4. Korovin GS, Kuriloff DB, Cho HT, Sobol SM. Squamous Cell Carcinoma of the Thyroid: A Diagnostic Dilemma. Ann Otol Rhinol Laryngol (1989) 98:59–65. doi: 10.1177/000348948909800113
5. Bolfi F, Domingues MA, Sobrinho-Simoes M, Soares P, Celestino R, Castilho EC, et al. Primary Squamous Cell Carcinoma of the Thyroid Diagnosed as Anaplastic Carcinoma: Failure in Fine-Needle Aspiration Cytology? Case Rep Pathol (2014) 2014:301780. doi: 10.1155/2014/301780
6. Cho JK, Woo SH, Park J, Kim MJ, Jeong HS. Primary Squamous Cell Carcinomas in the Thyroid Gland: An Individual Participant Data Meta-Analysis. Cancer Med (2014) 3:1396–403. doi: 10.1002/cam4.287
7. Surveillance, Epidemiology, and End Results Program. (2018). Available at: https://www.seer.cancer.gov (Accessed Nov. 2018).
8. Limberg J, Ullmann TM, Stefanova D, Finnerty BM, Beninato T, Fahey TJ 3rd, et al. Prognostic Characteristics of Primary Squamous Cell Carcinoma of the Thyroid: A National Cancer Database Analysis. World J Surg (2020) 44:348–55. doi: 10.1007/s00268-019-05098-5
9. Glaser SM, Mandish SF, Gill BS, Balasubramani GK, Clump DA, Beriwal S. Anaplastic Thyroid Cancer: Prognostic Factors, Patterns of Care, and Overall Survival. Head Neck (2016) 38(Suppl 1):E2083–90. doi: 10.1002/hed.24384
10. Arora S, Christos P, Pham A, Desai P, Wernicke AG, Nori D, et al. Comparing Outcomes in Poorly-Differentiated Versus Anaplastic Thyroid Cancers Treated With Radiation: A Surveillance, Epidemiology, and End Results Analysis. J Cancer Res Ther (2014) 10:526–30. doi: 10.4103/0973-1482.138207
11. Zhou XH. Primary Squamous Cell Carcinoma of the Thyroid. Eur J Surg Oncol (2002) 28:42–5. doi: 10.1053/ejso.2001.1180
12. Gui W, Zhu W, Lu W, Shang C, Zheng F, Lin X, et al. Development and Validation of a Prognostic Nomogram to Predict Overall Survival and Cancer-Specific Survival for Patients With Anaplastic Thyroid Carcinoma. PeerJ (2020) 8:e9173. doi: 10.7717/peerj.9173
13. Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, et al. American Thyroid Association Guidelines for Management of Patients With Anaplastic Thyroid Cancer. Thyroid (2012) 22:1104–39. doi: 10.1089/thy.2012.0302
14. Tiedje V, Stuschke M, Weber F, Dralle H, Moss L, Führer D. Anaplastic Thyroid Carcinoma: Review of Treatment Protocols. Endocr Relat Cancer (2018) R153-61. doi: 10.1530/ERC-17-0435
15. Lin JD, Lin KJ, Chao TC, Hseuh C, Tsang NM, Huang BY. Clinical Presentations of Thyroid Cancer Patients With Multiple Primary Cancers. J Endocrinol Invest (2011) 34:824–30. doi: 10.3275/7747
16. Chen J, Tward JD, Shrieve DC, Hitchcock YJ. Surgery and Radiotherapy Improves Survival in Patients With Anaplastic Thyroid Carcinoma: Analysis of the Surveillance, Epidemiology, and End Results 1983-2002. Am J Clin Oncol (2008) 31:460–4. doi: 10.1097/COC.0b013e31816a61f3
17. Shi RL, Qu N, Lu ZW, Liao T, Gao Y, Ji QH. The Impact of Marital Status at Diagnosis on Cancer Survival in Patients With Differentiated Thyroid Cancer. Cancer Med (2016) 5:2145–54. doi: 10.1002/cam4.778
18. Shi LY, Liu J, Yu LJ, Lei YM, Leng SX, Zhang HY. Clinic-Pathologic Features and Prognostic Analysis of Thyroid Cancer in the Older Adult: A SEER Based Study. J Cancer (2018) 9:2744–50. doi: 10.7150/jca.24625
19. Chen KH, Chou YH, Cheng AL. Primary Squamous Cell Carcinoma of the Thyroid With Cardiac Metastases and Right Ventricle Outflow Tract Obstruction. J Clin Oncol (2012) 30:e260–3. doi: 10.1200/jco.2011.39.9808
20. Shimaoka K, Tsukada Y. Squamous Cell Carcinomas and Adenosquamous Carcinomas Originating From the Thyroid Gland. Cancer (1980) 46:1833–42. doi: 10.1002/1097-0142(19801015)46:8<1833::aid-cncr2820460822>3.0.co;2-g
21. Booya F, Sebo TJ, Kasperbauer JL, Fatourechi V. Primary Squamous Cell Carcinoma of the Thyroid: Report of Ten Cases. Thyroid (2006) 16:89–93. doi: 10.1089/thy.2006.16.89
22. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association Management Guidelines for Patients With Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2009) 19:1167–214. doi: 10.1089/thy.2009.0110
23. Conzo G, Calò PG, Gambardella C, Tartaglia E, Mauriello C, Della Pietra C, et al. Controversies in the Surgical Management of Thyroid Follicular Neoplasms. Retrospective Analysis of 721 Patients. Int J Surg (2014) 12(Suppl 1):S29–34. doi: 10.1016/j.ijsu.2014.05.013
24. Conzo G, Docimo G, Ruggiero R, Napolitano S, Palazzo A, Gambardella C, et al. Surgical Treatment of Papillary Thyroid Carcinoma Without Lymph Nodal Involvement. G Chir (2012) 33:339–42.
25. Pezzolla A, Docimo G, Ruggiero R, Monacelli M, Cirocchi R, Parmeggiani D, et al. Incidental Thyroid Carcinoma: A Multicentric Experience. Recenti Prog Med (2010) 101:194–8. doi: 10.1701/493.5851
26. Conzo G, Polistena A, Calò PG, Bononi P, Gambardella C, Mauriello C, et al. Efficacy of Combined Treatment for Anaplastic Thyroid Carcinoma: Results of a Multinstitutional Retrospective Analysis. Int J Surg (2014) 12(Suppl 1):S178–82. doi: 10.1016/j.ijsu.2014.05.015
27. Au JK, Alonso J, Kuan EC, Arshi A, St John MA. Primary Squamous Cell Carcinoma of the Thyroid: A Population-Based Analysis. Otolaryngol Head Neck Surg (2017) 157:25–9. doi: 10.1177/0194599817698436
Keywords: ATC, PSCCTh, SEER database, prognosis factors, cox model, cancer-specific survival
Citation: Jin S, Liu X, Peng D, Li D and Ye Y-N (2022) Differences Between Cancer-Specific Survival of Patients With Anaplastic and Primary Squamous Cell Thyroid Carcinoma and Factors Influencing Prognosis: A SEER Database Analysis. Front. Endocrinol. 13:830760. doi: 10.3389/fendo.2022.830760
Received: 20 December 2021; Accepted: 11 February 2022;
Published: 10 March 2022.
Edited by:
Jing Yang, Sichuan University, ChinaReviewed by:
Carlotta Giani, University of Pisa, ItalyCopyright © 2022 Jin, Liu, Peng, Li and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan-Nong Ye, eXluQGdtYy5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.