
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 29 April 2022
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.828993
 Hui Ji1†
Hui Ji1† Yan Su1,2†
Yan Su1,2† Mianqiu Zhang1
Mianqiu Zhang1 Xin Li1
Xin Li1 Xiuling Li1
Xiuling Li1 Hui Ding1
Hui Ding1 Li Dong1
Li Dong1 Shanren Cao1
Shanren Cao1 Chun Zhao1
Chun Zhao1 Junqiang Zhang1
Junqiang Zhang1 Rong Shen1,2*
Rong Shen1,2* Xiufeng Ling1*
Xiufeng Ling1*Objectives: To investigate the incidence of functional ovarian cysts, its influence on clinical rates, and proper management after depot gonadotropin-releasing hormone (GnRH) agonist pretreatment in artificial frozen-thawed embryo transfer cycles (AC-FET).
Methods: This retrospective cohort study involved 3375 AC-FET cycles with follicular-phase depot GnRH agonist administration between January 2017 and December 2020. Subjects were divided into a study group (cycles with cyst formation) and a control group (cycles without cyst formation). The study group was matched by propensity scoring matching with the control group at a ratio of 1:2. For patients with ovarian cyst formation, two major managements were used: a conservative approach (i.e., expectant treatment) and a drug approach (i.e., continued agonist administration). The primary outcome was live birth rate (LBR).
Results: The incidence of functional ovarian cysts following pituitary downregulation is 10.1% (341/3375). The study group exhibited a LBR similar to the control group (54.5% vs. 50.1%, adjusted odds ratio [aOR] 1.17, 95% confidence interval [CI] 0.88–1.56, P = 0.274). Patients with a lower body mass index and anti-Müllerian hormone, and a higher basal estradiol level were more susceptible to developing functional ovarian cysts. The LBR decreased after the drug approach compared with the conservative approach, but not significantly (aOR 0.63, 95% CI 0.35–1.14, P = 0.125). Following the conservative approach, cycles arrived at live births had a significantly shorter duration from the detection of functional cysts to the start of endometrium preparation (15.7 ± 5.1 days vs. 17.4 ± 5.3 days, P = 0.009) and a significantly higher proportion of ovarian cysts on the initial day of exogenous hormone supplementation (51.4% vs. 30.3%, P = 0.001). After controlling for all confounders, the differences remained statistically significant.
Conclusions: It is unnecessary to cancel cycles that experience functional ovarian cyst formation. Conservative management and further agonist suppression protocol had similar pregnancy rates. However, a conservative approach was recommended due to its lower cost and fewer side effects. Our findings support a shorter waiting period when choosing the conservative protocol.
The key step in frozen-thawed embryo transfer (FET) treatment is preparing good-receptivity endometrium. Among all the FET cycle regimens, artificial cycles (AC) administer exogenous estrogen and progesterone to mimic natural cycles but do not always guarantee complete pituitary suppression (1). GnRH agonists are introduced in artificial FET cycles (AC-FET) mainly to suppress gonadotropin secretion, avoid spontaneous ovulation, and reduce cycle cancellation (2). Consequently, AC-FET protocols with gonadotropin-releasing hormone (GnRH) agonists offer the most control over cycle timing and provide less monitoring than other endometrial preparation regimens. Nevertheless, these cycles are more expensive and can have adverse effects. Current literature does not have sufficient evidence to support the use of agonist in AC-FET treatment (3).
Despite the disadvantages, a recent randomized controlled study suggested that a depot GnRH agonist applied in the follicular phase improves the implantation rate and pregnancy rates by enhancing endometrial receptivity (4). In addition, the pregnancy rates increased in women diagnosed with endometriosis (5, 6) or adenomyosis (7, 8) after GnRH agonist application before IVF or intracytoplasmic sperm injection. Irrespective of the two diseases, previous studies have shown that GnRH agonist pretreatment could increase pregnancy rates in women with polycystic ovary syndrome (PCOS) (9, 10) or repeated implantation failure (11, 12). All these findings show that agonist pretreatment is a favorable and feasible option in AC-FET cycles.
Applying a GnRH agonist can lead to a functional ovarian cyst during the treatment. No consensus has been reached yet on the exact mechanism of ovarian cyst formation. Firouzabadi et al. (13) have addressed several possible rationales: the effect of primary flare-up induced by the agonists affecting gonadotropins; inadequate inhibition of circulating gonadotropins following pituitary suppression; the direct effect of agonist on the ovaries and subsequent steroidogenesis; the quantity of progesterone at the time of agonist administration. Although such ovarian cysts have been constantly discussed in controlled ovarian stimulation (COS), there are no data on their influence on FET outcomes or what should be done when encountering these cysts.
For patients undergoing COS procedures, the ovarian cyst formation rate ranges from 5.5% (14) to 52.9% (15), mainly due to different definitions of functional cyst, administration timing, and agonist type and dose. Several publications have considered it a frustrating event that results in lower IVF outcomes (16–18). However, some authors have found that ovarian cysts after downregulation do not negatively impact the ensuing pregnancy rates (19, 20). In addition, three major managements have encountered this undesired event: cyst aspiration, continuous use of a GnRH agonist, and conservative treatment until the cyst has resolved. Still, no consensus has been reached on the optimal or proper protocol for an unexpected ovarian cyst.
Of particular interest, we conducted this retrospective study to determine the incidence and effect of a functional ovarian cyst on FET outcomes following GnRH agonist administration and provide new evidence for IVF providers and infertility couples in the management of this specific event.
This retrospective cohort study was conducted at the reproductive center of Women’s Hospital of Nanjing Medical University from January 2017 to December 2020. Participants younger than 41 years who underwent AC-FET with GnRH agonist pretreatment were recruited. The exclusion criteria were: uterus malformation, fallopian hydrosalpinx, endometrial thickness (EMT) < 6 mm on the initial day of progesterone (P) exposure (21), cycles of preimplantation genetic testing, oocyte donation or vitrified oocyte, and incomplete cycle data.
All FETs were performed in an artificial cycle with depot GnRH agonist downregulation (22). A baseline ultrasound was performed to detect any sign of an ovarian cyst on the second or third day of the natural or progestin-induced menstrual cycle. After excluding the presence of a functional ovarian cyst, women were intramuscularly administered a dose of 1.875 mg long-acting GnRH agonist (Diphereline, Ipsen Pharma Biotech, Signes, France). We revaluated serum hormone levels (FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol) and transvaginal ultrasonography 14 days later. Once pituitary downregulation criteria were achieved (EMT ≤ 5 mm, FSH < 5 mIU/mL, LH < 5 mIU/mL, and E2 < 50 pg/ml), the participants took 4–6 mg oral estrogen (estradiol valerate, Progynova, DELPHARM Lille SAS., Lille, France) per day for one week. Some cycles were detected with functional ovarian cysts, defined as serum E2 levels ≥ 50 pg/ml and a mean cyst diameter ≥ 15 mm (23). Different strategies were used to deal with these ovarian cysts, including expectant management (a conservative approach), continued GnRH agonist suppression (a drug approach), or transvaginal cyst aspiration (a surgical approach). The decision was based on the patient’s medical history and the doctor’s preference. When applying the conservative approach, some doctors preferred full resolution of ovarian cysts (cyst mean diameter < 10 mm) and fulfillment of downregulation criteria before exogenous hormone supplementation. At the same time, other clinicians waited until the downregulation criteria were achieved. Then, 4–6 mg exogenous estrogen was administered daily for a week. Regarding the drug approach, patients were administered another half or a whole shot of the agonist and carefully monitored. Since dealing with an ovarian cyst is obscure in the current literature, the decision of a half or a whole shot was based on the choice of both clinicians and patients. If patients want to have ET earlier or doctors manage things more conservatively, a half shot of agonist was administered and patients returned to the hospital 14 days later. Otherwise, patients were applied with a whole shot and reevaluated 28 days thereafter. If the baseline hormone levels were normal and the cyst did not persist, 4–6 mg/d oral estrogen was started for one week. The same estrogen dose was commenced on the second or third day of bleeding after aspiration in patients who underwent surgical aspiration.
The estrogen dose was adjusted to 6–12 mg once a day according to the EMT and serum E2 level in all FET cycles. After adequate endometrial proliferation with an EMT ≥ 7 mm and a serum E2 concentration ≥ 200 pg/ml, along with a serum level of P ≤ 1.5 ng/ml, luteal phase support (LPS) was initiated via vaginal administration of 90 mg progesterone (Crinone 8% gel, Fleet Laboratories Ltd., Watford, United Kingdom) once and 10 mg of dydrogesterone (Abbott Biologicals B.V., Weesp, the Netherlands) thrice every day. A total of 21 patients could only achieve a maximum EMT between ≥6mm and <7mm after long estrogen exposure but was the thickest EMT they could achieve. Such cycles were included in the final analysis, accounting for about 2.2% of the whole population (22/935). Cycles were canceled if the serum P level was >1.5 ng/ml prior to LPS, or a prolonged period of estrogen priming (more than 24 days) was required. In the case of pregnancy, LPS was continued until 10–12 weeks of gestation.
After surviving the thawing procedure, day (D) 3 cleavage-stage embryos were cultured for another 16 hours before the transfer based on our work schedule. For D5 or D6 blastocysts, additional 2–6 h incubation was performed before transfer. Cryopreserved cleavage-stage embryos or blastocysts were transferred 3 and 5 days after progesterone initiation, respectively. The D3 embryos reaching the morula stage which containing 16–32 blastomeres with > 90% of its cell mass compacted were good-quality embryos (24). According to Gardner’s scoring system, the blastocysts were graded on the basis of three parameters: cavity expansion, inner cell mass (ICM), and trophectoderm (TE) (25). Each blastocyst was scored according to the degree of cavity expansion to obtain 1–6 grades. Once the embryo reached the expansion level 3 or above, ICM and TE were graded according to the cell size and density (A, B, or C). A blastocyst graded ≥ 3 with A or B for both ICM and TE was defined as a good-quality blastocyst (grades 3–6 AA/AB/BA/BB); otherwise, it was considered low quality.
The primary outcome of this study was the live birth rate (LBR). Live birth was defined as the delivery of a viable infant after 28 weeks of gestation. The secondary outcomes included the implantation rate (IR), clinical pregnancy rate (CPR), and abortion rate (AR). The IR was calculated from the number of gestational sacs per number of transferred embryos. The clinical pregnancy was determined by gestational sac ultrasound at 6–7 weeks of gestation. Abortion was defined as a pregnancy loss during the first and second trimesters.
All data were analyzed using the SPSS software version 26 (IBM Corp., Armonk, NY, USA). We used the Student’s t-test or Mann-Whitney U test (if data were not normally distributed) for quantitative variables and Pearson’s χ2 test or Fisher’s exact test for categorical variables. To compare pregnancy rates between the cyst-positive and cyst-negative groups, we used the propensity scoring matching (PSM) method to alleviate potential selection bias. The final variables included in the PSM analysis model were patient age, infertility type, duration and cause, body mass index (BMI), baseline FSH and anti-Müllerian hormone (AMH) levels, EMT, embryo developmental stage, number of transferred embryos, and good-quality embryo number. After calculating the propensity score of each subject, patients in the cyst-positive group were matched in a 1:2 ratio with patients in the cyst-negative group with a 0.1 caliper width using the nearest neighbor matching. Moreover, multivariate regression analysis was conducted to identify the factors that had a significant effect on the occurrence of functional cysts. The multiple regression model contained variables that showed significant differences on univariate analysis at P < 0.1. Additionally, the confounders with a P-value < 0.1 or had a significant influence on LBR were included in the multivariable logistic regression to estimate the independent effect of ovarian cyst formation and different solutions on LBR.
Continuous data are presented as the mean ± SD following the t-test and median (Inter-Quartile Range, IQR) derived from the U test. Results were expressed as the adjusted odds ratio (aOR) and 95% confidence intervals (95% CI) in the multivariate regression analysis. The statistical significance was accepted at P-value < 0.05.
The patient selection flow chart is shown in Figure 1. Initially, we conducted 3375 frozen-thawed autologous embryo transfer cycles using downregulation protocol between January 01, 2017, and December 31, 2020. The FET cycles were divided into two groups according to the occurrence of functional ovarian cysts after GnRH agonist: the study group (cycles with ovarian cyst formation, n = 341) and the control group (cycles without ovarian cyst formation, n = 3034). After excluding 370 cycles, a total of 3005 FET cycles were included (314 cycles in the study group and 2691 in the control group). The number of participants after PSM in the study and control groups was 312 and 623, respectively.
The demographic parameters of the unpaired and paired participants in the two groups are summarized in Table 1. There were significant differences in terms of BMI, serum levels of basal E2 and AMH, and EMT within groups before PSM analysis (P < 0.01). After PSM, the demographics of the matched groups were comparable (P > 0.05).
We applied the multivariable logistic regression analysis to predict the possible confounders for the formation of ovarian cysts (Table 2). Patients with a higher BMI (aOR 0.87, 95% CI 0.84–0.91, P < 0.001) and AMH value (aOR 0.97, 95% CI 0.95–0.99, P = 0.007) were less susceptible to ovarian cysts after GnRH agonist supplementation, whereas a higher basal E2 level increased their incidence (aOR 1.01, 95% CI 1.00–1.01, P = 0.002).
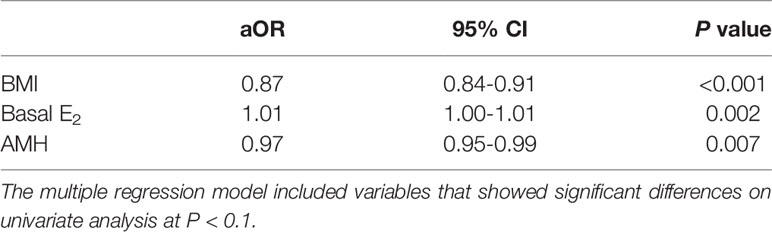
Table 2 Variables associated with the functional ovarian cyst formation in artificial frozen-thawed embryo transfer cycles with GnRH agonist: multivariate logistic regression analysis.
Clinical outcomes after PSM are shown in Table 3. The IR (44.4% vs. 41.2%, P = 0.217), CPR (60.3% vs. 58.1%, P = 0.529), AR (9.6% vs. 13.8%, P = 0.152), and LBR (54.5% vs. 50.1%, P = 0.204) were comparable in the study group than those in the control group. We further analyzed the influence of functional cysts on LBR in the multivariate logistic regression. An initial cohort of 935 FET cycles was divided into two groups: the LB group (cycles reaching live births) and the non-LB group (cycles failing to have live births) (Table 4). FET cycles with ovarian cyst formation had a higher LBR than those without ovarian cyst, but with no statistical difference (aOR 1.17, 95% CI 0.88–1.56, P = 0.274) (Table 5).
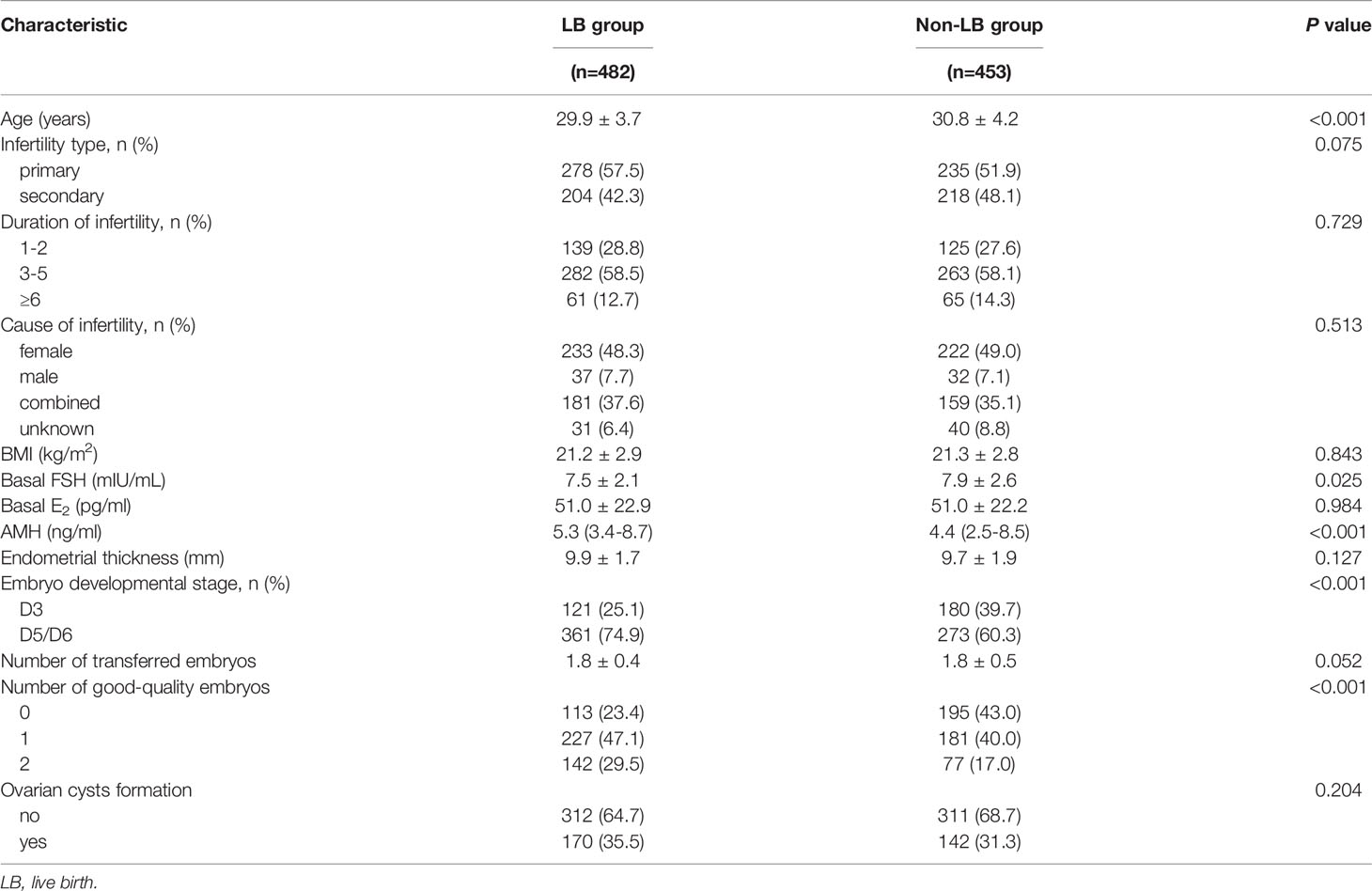
Table 4 Characteristics between cycles reaching live births or failing to have live births in the overall population after propensity score matching.
A total of 314 patients had functional cysts after pituitary suppression and were treated with three different protocols: the conservative approach (n = 251), the drug approach (n = 59), and the surgical approach (n = 4). Due to the small sample size of cyst aspiration, we only analyzed the effectiveness of the conservative versus the drug approach. The baseline demographic characteristics of the two cohorts are listed in Table 6. The LB group yielded a higher proportion of the conservative approach than the non-LB group, although the difference was not statistically significant (84.5% vs. 76.8%, P = 0.083). With the different strategies as the main exposure of interest, the logistic regression analysis revealed that the drug approach had no statistically negative influence on LBR compared with the conservative protocol (aOR 0.63, 95% CI 0.35–1.14, P = 0.125) (Table 5).
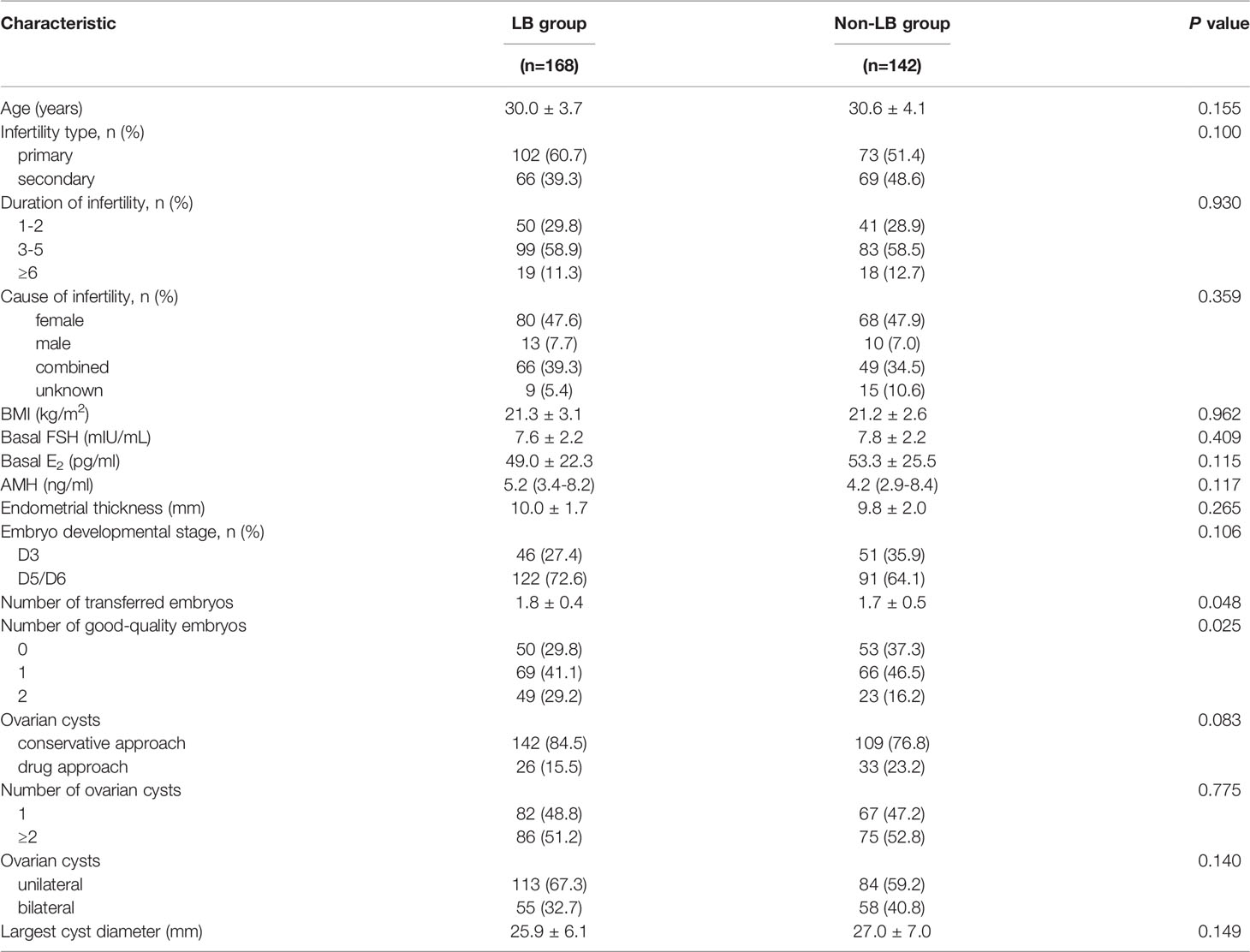
Table 6 Characteristics between cycles reaching live births or failing to have live births under conservative and drug approach.
If we focused on live births only in the drug approach, the LBR was associated with a shorter waiting period between the detection of functional cysts and the start of endometrium preparation (15.7 ± 5.1 days vs. 17.4 ± 5.3 days, aOR 0.94, 95% CI 0.89–0.99, P = 0.025) and a higher rate of ovarian cysts on estrogen starting day (51.4% vs. 30.3%, aOR 2.11, 95% CI 1.19–3.74, P = 0.010), both in univariate and crude analysis (Tables 5, 7).
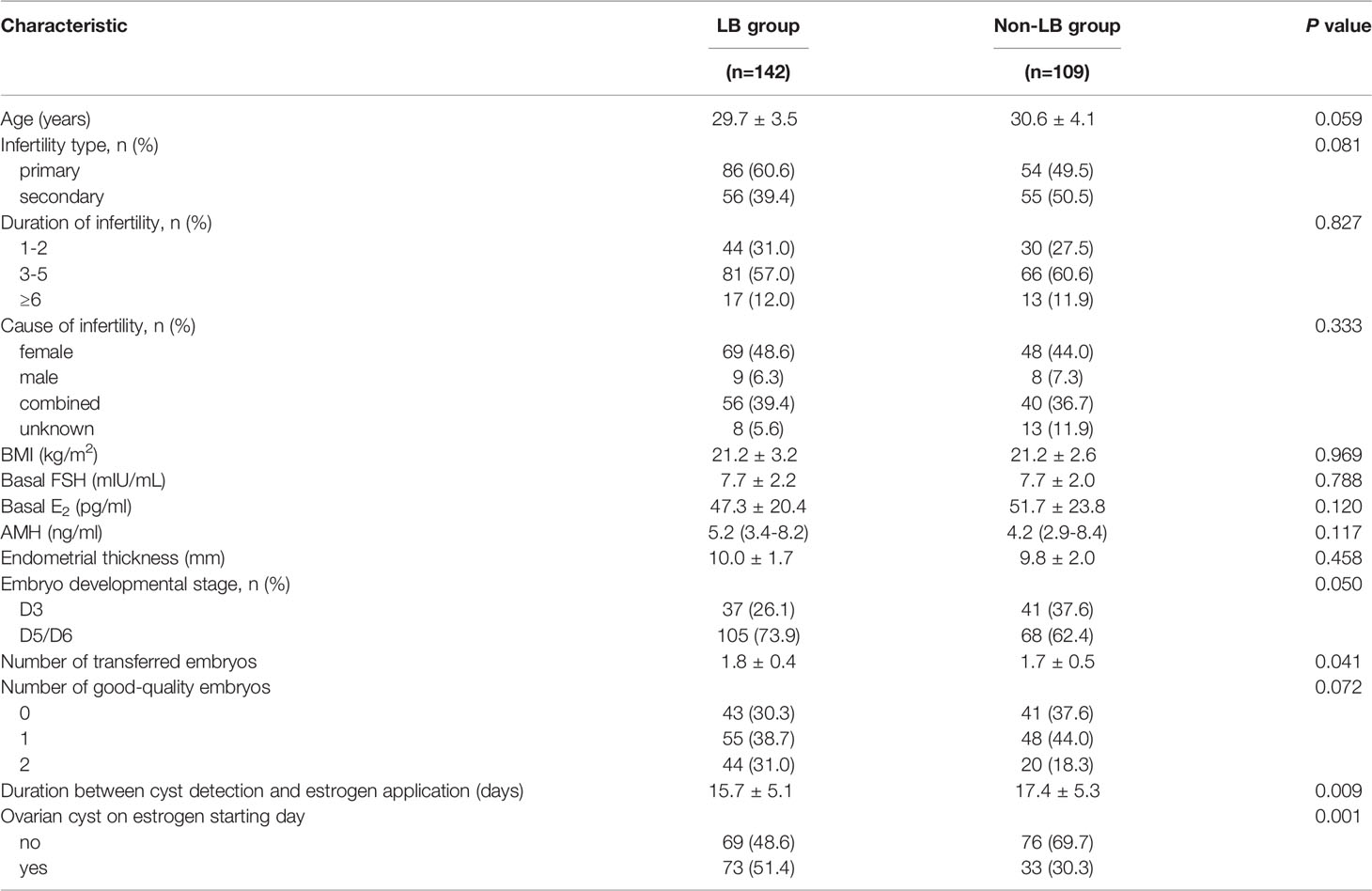
Table 7 Characteristics between cycles reaching live births or failing to have live births under the conservative approach.
One side effect of AC-FET cycles with GnRH agonist co-treatment is the unexpected formation of functional ovarian cysts. Although their incidence and management have raised constant debate in the COS process, there is a lack of data regarding the event’s influence on pregnancy outcomes in FET cycles. Our findings confirmed that the LBR was not compromised in FET cycles with ovarian cysts. Neither conservative management nor further agonist suppression improved clinical outcomes. However, a conservative approach was recommended due to its lower cost and fewer side effects. Data also supports a shorter waiting period when choosing the conservative protocol.
In this study, the formation rate of functional ovarian cysts was 10.1% (341/3375) in patients treated with follicular-phase depot GnRH agonist, similar to the 9.3% after the luteal-phase suppression as reported by Qublan et al. (23). Other investigators found that agonist injection in the follicular phase was associated with a higher rate of cyst formation (14, 26). However, their findings contradict a previous study in which the event incidence was higher when administered at mid-luteal phase than menstruation (15.4% vs. 13.6%) (27). Meanwhile, the occurrence of the ovarian cyst had no apparent connection with the type or route of a specific agonist when injected at menstruation (28). In general, cyst formation will always happen no matter what, when, or how the agonist is used. In that case, it is logical and meaningful to focus on this specific issue.
Cyst formation does not happen at random and might indicate patients with a poor ovarian response according to published data (13, 16, 17, 23). The levels of lower AMH correlating with higher basal E2 are significant predictors for poor ovarian reserve (29, 30). Additionally, E2 increases pituitary sensitivity to GnRH by stimulating an increase in the expression of the gene encoding the GnRH receptor (31). Due to the primary flare-up caused by the agonist, a relatively high serum E2 and low AMH may thus have a promoted effect on the induction of cyst formation. Our results concur with findings that ovarian cysts were significantly associated with patients with higher basal E2 and lower AMH levels. However, none of these papers reported patient BMI as part of their data. Women diagnosed with PCOS are considered high ovarian responders (32) and generally display a higher BMI than normal population (33–35). This finding would partially explain why participants with a higher BMI in our study are less likely to have functional cysts; however, the underlying biological mechanisms require further study.
The effect of the ovarian cyst presence on pregnancy outcomes has been discussed frequently in COS treatment and fresh ET cycles, but the findings are conflicting. Some investigations point out that the existence of ovarian cysts increased the cycle cancellation rate, lowered oocyte number and quality, and compromised the pregnancy results (14, 16, 23, 36). In such cases, the cysts inside the ovary would impede the final stages of the pre-ovulatory follicles, reduce the space for other follicles to develop, and damage the blood supply to the growing follicles (14, 36), all contributing to poor oocyte results. However, other studies failed to show any significant difference between cyst-positive and cyst-negative cycles after GnRH agonist therapy (27, 37). Our results demonstrate that functional cysts have no significant detrimental effect on LBR following the transfer of vitrified embryos (54.5% vs. 50.1%, P = 0.204). Since the major focus is endometrium growth rather than follicle development in FET cycles, it is understandable that patients with functional cysts do not experience a decline in clinical rates.
Different approaches have been used in managing these functional cysts following GnRH agonist administration. The most beneficial protocol before COS in IVF cycles has been the subject of several retrospective papers. There were comparable pregnancy rates between patients who underwent ovarian cyst aspiration and those who chose the conservative approach (13, 14, 23, 36). Considering the extra cost, additional risk of surgical complications, and emotional burden related to the surgery, a systematic review did not provide supportive evidence for cyst drainage before COS (38). In consistent with these reports, most physicians in our center do not consider cyst aspiration a patient-friendly option. Therefore, only four women underwent cyst aspiration surgery in the present study, a number too small for effective statistical analysis. Yet, in light of the absence of existing data between the effectiveness of expectant management and continuous suppression with GnRH agonist, we compared the FET outcomes between the two protocols. The LBR in the drug approach was lower than that in the conservative approach, although the difference did not reach statistical significance (aOR 0.63, 95% CI 0.35–1.14, P = 0.125). A tendency was observed in favor of the conservative approach than the drug approach. However, the most prominent side effect of continued depot GnRH agonist is estrogen deficiency, which will cause a menopause-like state (39). Therefore, the findings of our study recommend the conservative approach as the first choice for women with an ovarian cyst undergoing FET, particularly in terms of lower cost and more safety. Notwithstanding, one should be cautious about the interpretation of this finding because the decision-making of two available strategies is not randomly controlled and mainly at the physician’s discretion. Data supported endometriosis (40) or adenoma (41) were associated with a worse prognosis versus other infertility factors. When dealing with functional cysts in these women, doctors might continue agonist suppression at a higher odd.
More specifically, we investigated the confounders affecting LBR in patients undergoing a conservative approach. The shorter duration between the day of ovarian cyst occurrence and exogenous hormone initiation, and the presence of persistent cysts are both protective factors for live births. The data suggest that there may be an advantage in shortening the waiting period instead of considering a fully diminished follicular cyst. Once the serum hormone levels and EMT reached the downregulation criteria, exogenous estrogen could be initiated to proliferate the endometrium to achieve optimal outcomes. This referred result is in line with Segal et al. (18) and Zeyneloglu et al. (42), revealing that longer suppression with a GnRH agonist ended with lower pregnancy rates.
The body of literature indicates that a half-dose injection of depot GnRH agonist (1.875 mg) is equally effective for pituitary desensitization compared with a full-dose (3.75 mg) or a long multiple-dose (43–45). Furthermore, pituitary desensitization usually occurs approximately 14 days after agonist supplementation and continues until the eighth week after the injection (46). The depot GnRH agonist administration in our study was used at a dose of 1.875 mg during menstruation, and the hormone levels and ultrasound were reassessed in the subsequent two weeks. The incidence is about 10.1%, which is not a rare but a common event. Our study is the first to focus on the subject of functional ovarian cysts in AC-FET cycles following GnRH agonist suppression. We believe that our findings are valuable in clinical practice and could provide crucial evidence for both physicians and patients.
Despite our efforts, the present research has some limitations that need to be taken into consideration. First, it was conducted at a single institution. Second, the retrospective nature of this study and the inherent selection biases therein. For instance, the choice to proceed with the conservative protocol or further agonist administration was based on the doctor’s preference, which could interfere with the final results. Lastly, the limited sample size was not large enough to arrive at sufficiently convincing conclusions. Future studies that include a larger sample size are needed to validate the findings of this retrospective study and provide more information.
In conclusion, our study suggests that functional ovarian cysts do not pose any detrimental effect on pregnancy rates following FET treatment. The prevalence of cyst formation increased with increasing basal E2 levels and lower AMH and BMI values. Patients who underwent a conservative approach had similar clinical outcomes than those with further agonist suppression. To avoid medical costs and potential side effects, it may be wise to conservatively treat women with ovarian cysts until further evidence is available. Under the conservative strategy, it is unnecessary to initiate exogenous estrogen administration until the cyst has fully resolved; a short waiting period can obtain better pregnancy results once downregulation has been achieved.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Ethics Committee of Women’s Hospital of Nanjing Medical University (NJFY-2020-KY-070). The ethics committee waived the requirement of written informed consent for participation.
HJ collected data, performed the analysis, and wrote the manuscript. YS participated in the study design and drafted the article. MZ and XLi participated in the acquisition and analysis of data. XLLi, HD, LD, SC, CZ, and JZ reviewed the final article and made important intellectual contents. RS and XLing were corresponding authors and they participated in the study design, did the final proof reading and confirmed the final version. All authors contributed to the article and approved the submitted version.
The study was funded by National Natural Science Foundation of China (grant no. 81771536, 81871210), Program for the Top Innovative Talents of “Six Major Projects” of Jiangsu Province (grant no. LGY2018004), and Open fund of State Key Laboratory of Reproductive Medicine, Nanjing Medical University (grant no. SKLRM-K201806). The open access publication fees are covered by National Natural Science Foundation of China (grant no 81871210).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Su Y, Ji H, Jiang W, Xu L, Lu J, Zhao C, et al. Effect of Unplanned Spontaneous Follicular Growth and Ovulation on Pregnancy Outcomes in Planned Artificial Frozen Embryo Transfer Cycles: A Propensity Score Matching Study. Hum Reprod (2021) 36(6):1542–51. doi: 10.1093/humrep/deab059
2. Dal Prato L, Borini A, Cattoli M, Bonu MA, Sciajno R, Flamigni C. Endometrial Preparation for Frozen-Thawed Embryo Transfer With or Without Pretreatment With Gonadotropin-Releasing Hormone Agonist. Fertil Steril (2002) 77(5):956–60. doi: 10.1016/s0015-0282(02)02960-6
3. Ghobara T, Gelbaya TA, Ayeleke RO. Cycle Regimens for Frozen-Thawed Embryo Transfer. Cochrane Database Syst Rev (2017) 7:CD003414. doi: 10.1002/14651858.CD003414.pub3
4. Xu B, Geerts D, Hu S, Yue J, Li Z, Zhu G, et al. The Depot GnRH Agonist Protocol Improves the Live Birth Rate Per Fresh Embryo Transfer Cycle, But Not the Cumulative Live Birth Rate in Normal Responders: A Randomized Controlled Trial and Molecular Mechanism Study. Hum Reprod (2020) 35(6):1306–18. doi: 10.1093/humrep/deaa086
5. Mohamed AM, Chouliaras S, Jones CJ, Nardo LG. Live Birth Rate in Fresh and Frozen Embryo Transfer Cycles in Women With Endometriosis. Eur J Obstet Gynecol Reprod Biol (2011) 156(2):177–80. doi: 10.1016/j.ejogrb.2011.01.020
6. Sallam HN, Garcia-Velasco JA, Dias S, Arici A. Long-Term Pituitary Down-Regulation Before In Vitro Fertilization (IVF) for Women With Endometriosis. Cochrane Database Syst Rev (2006) 1):CD004635. doi: 10.1002/14651858.CD004635.pub2
7. Niu Z, Chen Q, Sun Y, Feng Y. Long-Term Pituitary Downregulation Before Frozen Embryo Transfer Could Improve Pregnancy Outcomes in Women With Adenomyosis. Gynecol Endocrinol (2013) 29(12):1026–30. doi: 10.3109/09513590.2013.824960
8. Lan J, Wu Y, Wu Z, Wu Y, Yang R, Liu Y, et al. Ultra-Long GnRH Agonist Protocol During IVF/ICSI Improves Pregnancy Outcomes in Women With Adenomyosis: A Retrospective Cohort Study. Front Endocrinol (Lausanne) (2021) 12:609771. doi: 10.3389/fendo.2021.609771
9. Aghahoseini M, Alyasin A, Rashidi S, Samaei-Nouroozi A, Saeidi H, Shabani-Nashtaei M. The Efficacy of Gonadotropin-Releasing Hormone (GNRH) Agonist Before Frozen Embryo Transfer in Improving Pregnancy Outcome and Decreasing Miscarriage Rate in Hyperandrogenic Polycystic Ovary Syndrome Women: A Randomized Clinical Trial. Minerva Ginecol (2020) 72(4):212–8. doi: 10.23736/S0026-4784.20.04467-6
10. Tsai HW, Wang PH, Lin LT, Chen SN, Tsui KH. Using Gonadotropin-Releasing Hormone Agonist Before Frozen Embryo Transfer may Improve Ongoing Pregnancy Rates in Hyperandrogenic Polycystic Ovary Syndrome Women. Gynecol Endocrinol (2017) 33(9):686–9. doi: 10.1080/09513590.2017.1307961
11. Yang X, Huang R, Wang YF, Liang XY. Pituitary Suppression Before Frozen Embryo Transfer is Beneficial for Patients Suffering From Idiopathic Repeated Implantation Failure. J Huazhong Univ Sci Technol Med Sci (2016) 36(1):127–31. doi: 10.1007/s11596-016-1554-2
12. Davar R, Dashti S, Omidi M. Endometrial Preparation Using Gonadotropin-Releasing Hormone Agonist Prior to Frozen-Thawed Embryo Transfer in Women With Repeated Implantation Failure: An RCT. Int J Reprod BioMed (2020) 18(5):319–26. doi: 10.18502/ijrm.v13i5.7150
13. Firouzabadi RD, Sekhavat L, Javedani M. The Effect of Ovarian Cyst Aspiration on IVF Treatment With GnRH. Arch Gynecol Obstet (2010) 281(3):545–9. doi: 10.1007/s00404-009-1195-9
14. Fiszbajn GE, Lipowicz RG, Elberger L, Grabia A, Papier SD, Brugo Olmedo SP, et al. Conservative Management Versus Aspiration of Functional Ovarian Cysts Before Ovarian Stimulation for Assisted Reproduction. J Assist Reprod Genet (2000) 17(5):260–3. doi: 10.1023/a:1009406315729
15. Biljan MM, Lapensee L, Mahutte NG, Bissonnette F, Hemmings R, Tan SL. Effects of Functional Ovarian Cysts Detected on the 7th Day of Gonadotropin-Releasing Hormone Analog Administration on the Outcome of IVF Treatment. Fertil Steril (2000) 74(5):941–5. doi: 10.1016/s0015-0282(00)01555-7
16. Keltz MD, Jones EE, Duleba AJ, Polcz T, Kennedy K, Olive DL. Baseline Cyst Formation After Luteal Phase Gonadotropin-Releasing Hormone Agonist Administration is Linked to Poor In Vitro Fertilization Outcome. Fertil Steril (1995) 64(3):568–72. doi: 10.1016/s0015-0282(16)57794-2
17. Jenkins JM, Anthony FW, Wood P, Rushen D, Masson GM, Thomas E. The Development of Functional Ovarian Cysts During Pituitary Down-Regulation. Hum Reprod (1993) 8(10):1623–7. doi: 10.1093/oxfordjournals.humrep.a137902
18. Segal S, Shifren JL, Isaacson KB, Leykin L, Chang Y, Pal L, et al. Effect of a Baseline Ovarian Cyst on the Outcome of In Vitro Fertilization-Embryo Transfer. Fertil Steril (1999) 71(2):274–7. doi: 10.1016/s0015-0282(98)00449-x
19. Penzias AS, Jones EE, Seifer DB, Grifo JA, Thatcher SS, DeCherney AH. Baseline Ovarian Cysts do Not Affect Clinical Response to Controlled Ovarian Hyperstimulation for In Vitro Fertilization. Fertil Steril (1992) 57(5):1017–21. doi: 10.1016/s0015-0282(16)55019-5
20. Hornstein MD, Barbieri RL, Ravnikar VA, McShane PM. The Effects of Baseline Ovarian Cysts on the Clinical Response to Controlled Ovarian Hyperstimulation in an In Vitro Fertilization Program. Fertil Steril (1989) 52(3):437–40. doi: 10.1016/s0015-0282(16)60914-7
21. Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N. The Impact of a Thin Endometrial Lining on Fresh and Frozen-Thaw IVF Outcomes: An Analysis of Over 40 000 Embryo Transfers. Hum Reprod (2018) 33(10):1883–8. doi: 10.1093/humrep/dey281
22. Ji H, Zhou Y, Cao S, Zhang J, Ling X, Zhao C, et al. Effect of Embryo Developmental Stage, Morphological Grading, and Ploidy Status on Live Birth Rate in Frozen Cycles of Single Blastocyst Transfer. Reprod Sci (2021) 28(4):1079–91. doi: 10.1007/s43032-020-00381-6
23. Qublan HS, Amarin Z, Tahat YA, Smadi AZ, Kilani M. Ovarian Cyst Formation Following GnRH Agonist Administration in IVF Cycles: Incidence and Impact. Hum Reprod (2006) 21(3):640–4. doi: 10.1093/humrep/dei371
24. Tsai NC, Su YT, Lin YJ, Chiang HJ, Huang FJ, Kung FT, et al. Developmental Potential of Surplus Morulas With Delayed and/or Incomplete Compaction After Freezing-Thawing Procedures. Reprod Biol Endocrinol (2019) 17(1):87. doi: 10.1186/s12958-019-0535-2
25. Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst Score Affects Implantation and Pregnancy Outcome: Towards a Single Blastocyst Transfer. Fertil Steril (2000) 73(6):1155–8. doi: 10.1016/s0015-0282(00)00518-5
26. Feldberg D, Ashkenazi J, Dicker D, Yeshaya A, Goldman GA, Dicker D, et al. Ovarian Cyst Formation: A Complication of Gonadotropin-Releasing Hormone Agonist Therapy. Fertil Steril (1989) 51(1):42–5. doi: 10.1016/s0015-0282(16)60425-9
27. Herman A, Ron-El R, Golan A, Nahum H, Soffer Y, Caspi E. Follicle Cysts After Menstrual Versus Midluteal Administration of Gonadotropin-Releasing Hormone Analog in In Vitro Fertilization. Fertil Steril (1990) 53(5):854–8. doi: 10.1016/s0015-0282(16)53521-3
28. Tarlatzis BC, Bili H, Bontis J, Lagos S, Vatev I, Mantalenakis S. Follicle Cyst Formation After Administration of Different Gonadotrophin-Releasing Hormone Analogues for Assisted Reproduction. Hum Reprod (1994) 9(11):1983–6. doi: 10.1093/oxfordjournals.humrep.a138378
29. Smotrich DB, Widra EA, Gindoff PR, Levy MJ, Hall JL, Stillman RJ. Prognostic Value of Day 3 Estradiol on In Vitro Fertilization Outcome. Fertil Steril (1995) 64(6):1136–40. doi: 10.1016/S0015-0282(16)57974-6
30. Patrelli TS, Gizzo S, Sianesi N, Levati L, Pezzuto A, Ferrari B, et al. Anti-Mullerian Hormone Serum Values and Ovarian Reserve: Can it Predict a Decrease in Fertility After Ovarian Stimulation by ART Cycles? PloS One (2012) 7(9):e44571. doi: 10.1371/journal.pone.0044571
31. Nett TM, Turzillo AM, Baratta M, Rispoli LA. Pituitary Effects of Steroid Hormones on Secretion of Follicle-Stimulating Hormone and Luteinizing Hormone. Domest Anim Endocrinol (2002) 23(1-2):33–42. doi: 10.1016/s0739-7240(02)00143-1
32. Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, et al. GnRH Antagonist Versus Long Agonist Protocols in IVF: A Systematic Review and Meta-Analysis Accounting for Patient Type. Hum Reprod Update (2017) 23(5):560–79. doi: 10.1093/humupd/dmx017
33. Yuan C, Liu X, Mao Y, Diao F, Cui Y, Liu J. Polycystic Ovary Syndrome Patients With High BMI Tend to Have Functional Disorders of Androgen Excess: A Prospective Study. J BioMed Res (2016) 30(3):197–202. doi: 10.7555/JBR.30.20140111
34. Wang F, Dai W, Yang XH, Guo YH, Sun YP. Analyses of Optimal Body Mass Index for Infertile Patients With Either Polycystic or Non-Polycystic Ovary Syndrome During Assisted Reproductive Treatment in China. Sci Rep (2016) 6:34538. doi: 10.1038/srep34538
35. Kakoly NS, Earnest A, Moran LJ, Teede HJ, Joham AE. Group-Based Developmental BMI Trajectories, Polycystic Ovary Syndrome, and Gestational Diabetes: A Community-Based Longitudinal Study. BMC Med (2017) 15(1):195. doi: 10.1186/s12916-017-0957-7
36. Eryilmaz OG, Sarikaya E, Aksakal FN, Hamdemir S, Dogan M, Mollamahmutoglu L. Ovarian Cyst Formation Following Gonadotropin-Releasing Hormone-Agonist Administration Decreases the Oocyte Quality in IVF Cycles. Balkan Med J (2012) 29(2):197–200. doi: 10.5152/balkanmedj.2011.019
37. Owj M, Ashrafi M, Baghestani AR. Ovarian Cyst Formation and In Vitro Fertilization Outcome. Int J Gynaecol Obstet (2004) 87(3):258–9. doi: 10.1016/j.ijgo.2004.08.004
38. McDonnell R, Marjoribanks J, Hart RJ. Ovarian Cyst Aspiration Prior to In Vitro Fertilization Treatment for Subfertility. Cochrane Database Syst Rev (2014) 12):CD005999. doi: 10.1002/14651858.CD005999.pub2
39. Garner C. Uses of GnRH Agonists. J Obstet Gynecol Neonatal Nurs (1994) 23(7):563–70. doi: 10.1111/j.1552-6909.1994.tb01922.x
40. Li A, Zhang J, Kuang Y, Yu C. Analysis of IVF/ICSI-FET Outcomes in Women With Advanced Endometriosis: Influence on Ovarian Response and Oocyte Competence. Front Endocrinol (Lausanne) (2020) 11:427. doi: 10.3389/fendo.2020.00427
41. Sharma S, Bathwal S, Agarwal N, Chattopadhyay R, Saha I, Chakravarty B. Does Presence of Adenomyosis Affect Reproductive Outcome in IVF Cycles? A Retrospective Analysis of 973 Patients. Reprod BioMed Online (2019) 38(1):13–21. doi: 10.1016/j.rbmo.2018.09.014
42. Zeyneloglu HB, Isik AZ, Kara S, Senoz S, Ozcan U, Gokmen O. Impact of Baseline Cysts at the Time of Administration of Gonadotropin-Releasing Hormone Analog for In Vitro Fertilization. Int J Fertil Womens Med (1998) 43(6):300–5.
43. Balasch J, Gomez F, Casamitjana R, Carmona F, Rivera F, Vanrell JA. Pituitary-Ovarian Suppression by the Standard and Half-Doses of D-Trp-6-Luteinizing Hormone-Releasing Hormone Depot. Hum Reprod (1992) 7(9):1230–4. doi: 10.1093/oxfordjournals.humrep.a137832
44. Hsieh Y, Tsai H, Chang C, Lo H. Comparison of a Single Half-Dose, Long-Acting Form of Gonadotropin-Releasing Hormone Analog (GnRH-A) and a Short-Acting Form of GnRH-A for Pituitary Suppression in a Controlled Ovarian Hyperstimulation Program. Fertil Steril (2000) 73(4):817–20. doi: 10.1016/s0015-0282(99)00608-1
45. Isikoglu M, Ozdem S, Berkkanoglu M, Jamal H, Senturk Z, Ozgur K. Single-Dose Depot Leuprolide is as Efficient as Daily Short-Acting Leuprolide in ICSI Cycles. Hum Reprod (2007) 22(6):1657–61. doi: 10.1093/humrep/dem054
Keywords: frozen-thawed embryo transfer, artificial cycle, functional ovarian cyst, gonadotropin-releasing hormone agonist, pregnancy outcome
Citation: Ji H, Su Y, Zhang M, Li X, Li X, Ding H, Dong L, Cao S, Zhao C, Zhang J, Shen R and Ling X (2022) Functional Ovarian Cysts in Artificial Frozen-Thawed Embryo Transfer Cycles With Depot Gonadotropin-Releasing Hormone Agonist. Front. Endocrinol. 13:828993. doi: 10.3389/fendo.2022.828993
Received: 04 December 2021; Accepted: 29 March 2022;
Published: 29 April 2022.
Edited by:
Gedis Grudzinskas, Independent researcher, London, United KingdomReviewed by:
Remzi Atılgan, Firat University, TurkeyCopyright © 2022 Ji, Su, Zhang, Li, Li, Ding, Dong, Cao, Zhao, Zhang, Shen and Ling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Shen, cm9uZ3NoZW4xNjNAMTYzLmNvbQ==; Xiufeng Ling, bGluZ3hpdWZlbmdfbmpmeUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.