
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 03 February 2022
Sec. Pituitary Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.819330
This article is part of the Research TopicQuality of Life, Clinical Approach, and Long-Term Follow-Up for AcromegalyView all 6 articles
 Naoki Yamamoto1†
Naoki Yamamoto1† Shin Urai1†
Shin Urai1† Hidenori Fukuoka2*
Hidenori Fukuoka2* Masaaki Yamamoto2
Masaaki Yamamoto2 Kenichi Yoshida1
Kenichi Yoshida1 Masaki Suzuki1
Masaki Suzuki1 Hiroki Shichi1
Hiroki Shichi1 Yasunori Fujita1
Yasunori Fujita1 Keitaro Kanie1
Keitaro Kanie1 Genzo Iguchi1,3,4
Genzo Iguchi1,3,4 Yutaka Takahashi1,5
Yutaka Takahashi1,5 Wataru Ogawa1
Wataru Ogawa1Context: With the increasing number of older patients with acromegaly, it is important to understand the effects of aging on the quality of life (QoL) in acromegaly.
Objective: To investigate the factors associated with the QoL of older acromegaly patients.
Design: This was a single-center, retrospective, cross-sectional study conducted between 2014 and 2019.
Methods: Among 90 acromegaly patients at Kobe University Hospital, 74 who had completed the QoL evaluation under treatment were enrolled (age = 62.0 [50.7–70.0], female 52%). SF-36 and the AcroQoL questionnaire were used to quantify QoL. The patients were divided into two groups: the young and middle-aged group, aged <65 years (51.0 [46.0–59.2], n =42), and the older group, aged ≥65 years (70.5 [69.0–73.0], n =32). The factors associated with the QoL scores were analyzed using univariate and multivariate regression analyses.
Results: The scores for the physical component summary of SF-36 were negatively associated with age (P <0.01), while those for the mental or role/social component summary were positively associated (P <0.01, P =0.03, respectively). In contrast, AcroQoL scores were not associated with age. However, the different factors were associated with lower AcroQoL scores; arthropathy and higher BMI in the older group (P <0.01, and P =0.01, respectively), and treatment modalities and size of pituitary tumor in the young and middle-aged group (P <0.01, P =0.04, respectively). Replacement of hydrocortisone was commonly associated both in young and middle-aged group (P =0.04), and in older group (P =0.02).
Conclusion: We showed that the factors associated with impaired QoL differed in the young and middle-aged, and older patients with acromegaly. In older patients, arthropathy and higher BMI were associated with poor QoL. These suggest the importance of early diagnosis and appropriate treatment in preventing arthropathy in acromegaly.
Acromegaly is a chronic condition characterized by the persistent excess of growth hormone (GH) and insulin-like growth factor-I (IGF-I) mainly caused by GH-secreting pituitary adenomas (1). This hormonal excess leads to multiple systemic complications, including metabolic disorders, cardiovascular disease, respiratory disease, osteoarthropathy, neoplasms, and a distinctive appearance. Moreover, the local mass effect around the sella turcica can cause several dysfunctions, such as visual impairment, ocular motility disorder, headache, and continuing hypopituitarism (2, 3). These complications lead to increased mortality, if not properly treated.
Advances in diagnostic approaches, surgical techniques, and pharmacological therapies for acromegaly have improved mortality in recent decades (4, 5). Therefore, the proportion of older patients with acromegaly is steadily increasing, as expected (6). However, the quality of life (QoL) of patients with acromegaly is still impaired even in a biochemically cured state (7). Improving QoL is considered the next step in managing this chronic disease and a major concern, especially for older patients. Older patients frequently have more complications, partly due to the lack of physiological reserves, which impair their daily living activity (8, 9) and lead to adverse treatment effects (10, 11). In a study of a healthy population using Short From-36 (SF-36), a widely used general health-related QoL questionnaire, QoL declined with age as a result of declines in physical, mental, and social functions (12). In a study using the AcroQoL questionnaire, a disease-specific questionnaire for acromegaly, some factors, including therapeutic modalities such as radiotherapy and drug therapy, symptoms such as headache and joint pain, and poor body image, contributed to poor QoL scores (13, 14). We have previously developed a Japanese version of AcroQoL and showed that acromegaly patients with surgical remission have better QoL scores than young and middle-aged patients receiving medical treatment or radiotherapy (15). However, the difference in QoL between young and middle-aged, and older patients remains unclear.
In the present study, to clarify the impact of aging on the QoL of patients with acromegaly under treatment, we compared the QoL scores in older patients with those in young and middle-aged patients and investigated the factors associated with QoL.
This was a single-center, retrospective, cross-sectional study. Ninety patients with acromegaly who were visiting the Division of Diabetes and Endocrinology at Kobe University Hospital between March 2014 and September 2019 were included. Eighty-one patients completed the AcroQoL questionnaire. Among them, untreated patients (n =7) were excluded and, finally, 74 patients receiving treatment were enrolled in the present study. Among them, 61 patients completed the SF-36. The diagnosis of acromegaly was based on the diagnostic guidelines (16). Briefly, clinical signs, impaired serum GH suppression to less than 1.0 ng/mL during a 75 g oral glucose tolerance test, elevated serum IGF-I levels over the age- and sex-matched normal range, and the presence of pituitary tumors were confirmed in all the patients. The duration of the disease was defined as the period from the first diagnosis of the disease to the date the QoL questionnaire was administered. The clinical data of the patients were obtained from their medical records when the questionnaire was performed. The random GH (<1.0 ng/mL) and IGF-I standard deviation score (SDS) (<2.0) were used as the criteria for biochemical disease control as described previously (17). Only the IGF-I SDS, but not GH, was used in two patients treated with a growth hormone receptor antagonist (GHA). Hormone deficiency such as adrenal insufficiency, hypothyroidism, and hypogonadism was defined when the patients had been received hormone replacement therapy due to symptoms related to hormone deficiency, and low associated hormone levels. For the diagnose of GH deficiency, among the 36 patients who had met the biochemical disease control of acromegaly, 5 (14%) had undergone insulin tolerance test and 6 (17%) had undergone GH-releasing peptides (GHRP)-2 test (18). The number of patients receiving hormone replacement therapy and the total number of replacement therapy for deficient hormones were reviewed using medical records. Hydrocortisone [7 patients (9%)], levothyroxine [11 patients (14%)], and gonadal hormones [3 patients (4%)] were each replaced in patients who had deficient symptoms and corresponding low hormone levels. Only one patient had been diagnosed with GH deficiency and received recombinant GH replacement therapy. Therefore, the IGF-I levels of this one patient were excluded from the present analysis. We were able to include the size of the pituitary tumor before the initial treatment in 64 patients based on imaging data of MRI scans, while 10 patients could not be included due to lack of original data.
The present study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Kobe University Hospital (approval #1363).
The serum GH and IGF-I levels were measured using an enzyme-linked immunosorbent assay (ELISA, Tosoh Co. Ltd, Tokyo, Japan) and an immunoradiometric assay (IRMA, Daiichi Radioisotope Laboratories, Tokyo, Japan), respectively. IGF-I SDS was calculated based on age- and sex-matched healthy Japanese individuals, as previously described (19).
In this study, we used the Japanese version of AcroQoL developed from the original Spanish version in collaboration with Dr. S. Webb, a disease-specific QoL assessment (15). The AcroQoL consists of 22 items and two major categories: physical (8 items) and psychological (14 items) scales. The psychological scale is further divided into two subscales; appearance (7 items) and personal relationship (7 items). Each item is answered on a 1–5 Likert scale measuring either the frequency of occurrence or the degree of agreement with the items. The results are quoted as scores in the range of 0–100%, with higher scores indicating better QoL.
The SF-36 was used to measure general health-related QoL, as previously described (20). The SF-36 consists of 36 items evaluating general wellbeing using a 1–5 Likert scale. For the SF-36 Version 2, the results of the items are formulated into two component summaries: the physical component summary (PCS) and the mental component summary (MCS). However, as the factor structure underlying these two-component summaries does not apply to the Japanese population, three-component summaries, including the role/social component summary (RCS), are employed in the Japanese version (21), which was used in the present study. The response for each item was converted to a score within the range of 0–100, with a higher score indicating better QoL.
We divided all the patients into two age groups based on the World Health Organization definition of older adults (22). The young and middle-aged group included patients younger than 65 years old, and the older group included patients aged 65 years and older. We compared the AcroQoL scores of the groups and investigated the factors associated with QoL impairment.
We further divided the patients into biochemically controlled or uncontrolled subgroups, depending on the criteria described in patients and designs, to determine the effects of disease control status on the AcroQoL score for each age group.
To investigate the association between treatment modalities and the AcroQoL score, we divided the patients into the following three subgroups: surgery only, medical therapy without radiotherapy, and radiotherapy in both age groups.
All statistical analyses were performed using SPSS ver. 27.0 software (IBM Corporation, Armonk, NY, USA). All continuous variables were analyzed for graphical statistics, and the Shapiro-Wilk normality test was performed to determine the normality of their distribution. The normally or non-normally distributed data for the two groups were compared using the unpaired Student’s t-test or the Mann–Whitney U test, respectively. A one-way analysis of variance (ANOVA) or Kruskal-Wallis tests with post-hoc test was used to compare the intergroup differences of more than three groups in normally or non-normally distributed data, respectively. The χ2 test or Fisher’s exact test was used for categorical data. Univariate regression analyses were performed to estimate the variables associated with the impairment of the total and subscale AcroQoL scores. Furthermore, variables with a P-value of less than 0.05 in the univariate analyses, were selected as candidates for stepwise multivariate regression analyses. In terms of collinearity between variables, either the total number of hormone replacement or the type of hormone replacement was used as the candidate in the multivariate regression analyses. For the univariate or multivariate regression analyses, the categorical data were converted to dummy variables: absence =0 or presence =1. Similarly, the treatment modalities were coded as dummy variables: surgery only =1, medical therapy without radiotherapy =2, and radiotherapy =3. The results are presented as mean ± standard deviation for normally distributed data and as median [interquartile range] for non-normally distributed data. Statistical significance was set at P <0.05.
The median age at the time of evaluation was 62.0 [50.7–70.0] years. The male-to-female ratio was approximately 1:1, and the median disease duration was 10.0 [3.0–16.0] years. Since these participants have already been treated, the median random GH and IGF-I SDS were 0.9 [0.3–1.8] ng/mL and 0.1 [-0.8–1.4], respectively. The treatment modalities were as follows: transsphenoidal surgery (TSS) only (n =34, 46%), medical therapy without radiotherapy (n =31, 42%), and radiotherapy (n =9, 12%). Of the 31 patients receiving medical therapy without radiotherapy, 22 were treated with previous TSS, while nine were treated with primary medical therapy. In the radiotherapy subgroup, eight of nine patients were still on medical therapy, while one patient was not receiving drugs for acromegaly. Of all the patients, 36 (48%) achieved biochemical control. The symptoms related to acromegaly were shown in Table 1.
In the AcroQoL (n =74), all the scores, including the total, physical, and psychological, were low (63.4 ± 19.8%, 61.1 ± 23.9%, and 64.8 ± 19.1%, respectively) (Table 2), as in previous report (23). In the SF-36 (n =61), the PCS score was negatively associated with age (adjusted R2 =0.24, P <0.01), while both MCS and RCS scores were positively associated with age (adjusted R2 = 0.13, P <0.01, adjusted R2 =0.05, P =0.03, respectively) (Figures 1A–C). However, no association between age and each AcroQoL score was detected (Figures 1D–F).
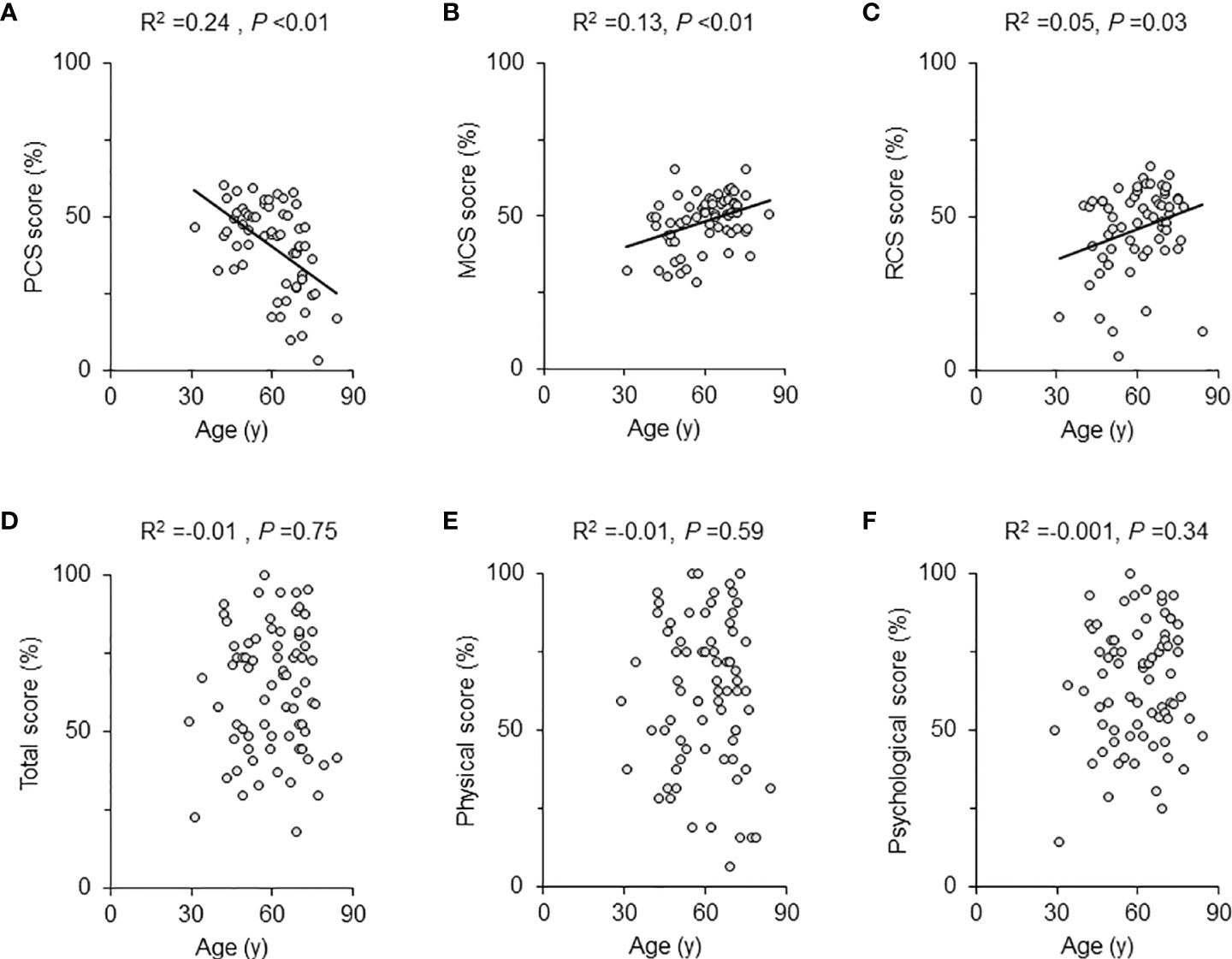
Figure 1 Scatter plot and univariate regression analysis for QoL scores and age. The univariate regression analysis results for the SF-36 scores and age of all patients were shown with scattered plots for each scale: (A) physical component summary (PCS) score, (B) mental component summary (MCS) score, and (C) role/social component summary (RCS) score. The AcroQoL scores of all patients are presented as follows: (D) total score, (E) physical score, and (F) psychological score. The solid lines are approximate straight lines. The adjusted R2 and P-values are shown in each figure.
Next, we divided the patients into two groups, namely the young and middle-aged, and older groups, as shown in the Materials and Methods section. As expectedly, both age (at QoL questionnaires) and age at diagnosis were significantly younger in the young and middle-aged group than in the older (P <0.01, P <0.01, respectively). Their female to male ratio, and serum GH, were not different. Although IGF-I levels were higher in young and middle-aged group, there was no difference in IGF-I SDS. BMI was higher in the young and middle-aged group than in the older (P =0.02). Moreover, duration of illness was shorter in the young and middle-aged group than in the older (P =0.02). The prevalence of macroglossia, arthropathy, and diabetes mellitus was higher in the older group (P <0.01, P <0.01, P <0.01, respectively). The proportion of treatment modalities did not differ in these groups (Table 3).
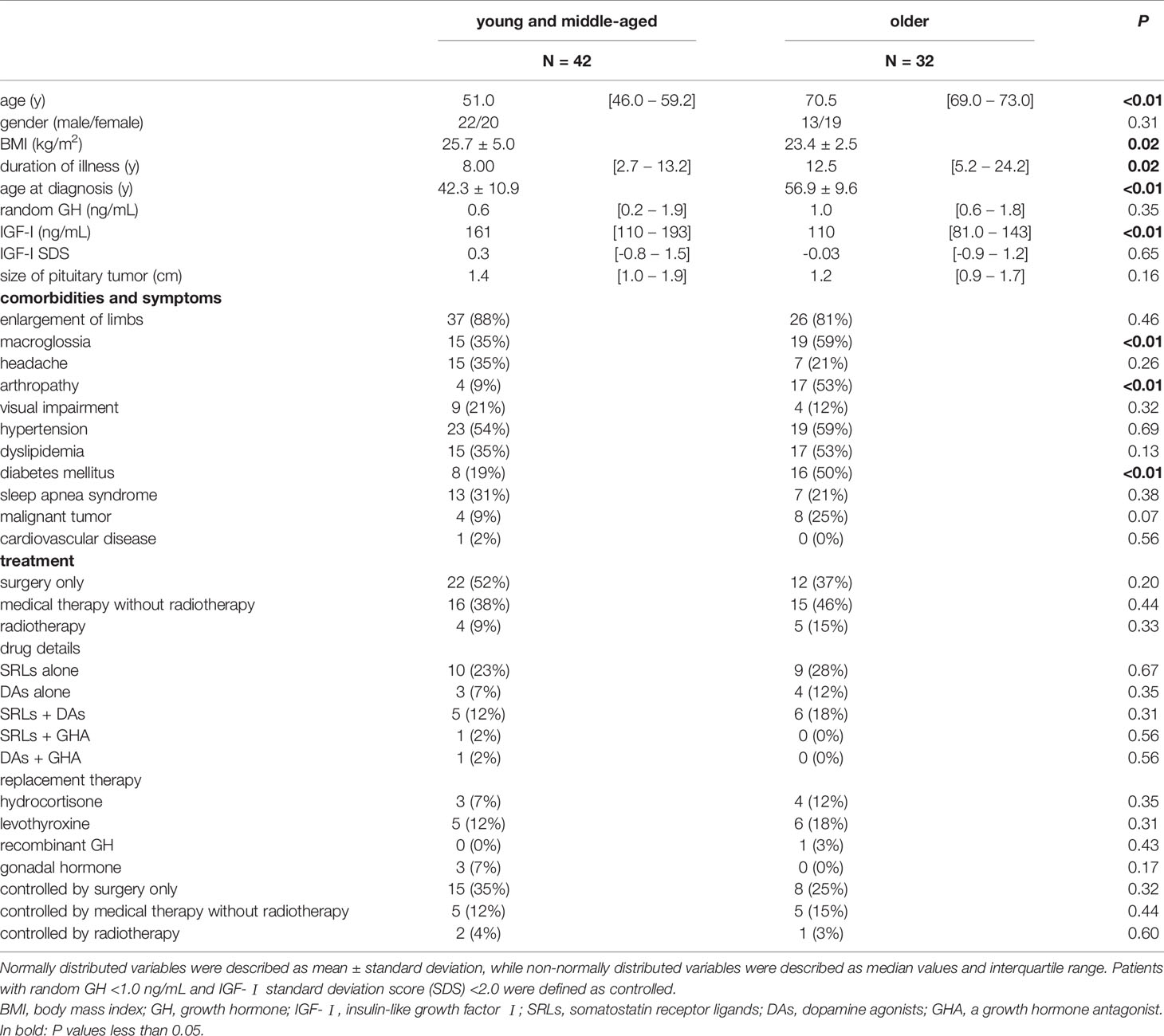
Table 3 Comparison of clinical characteristics between the young and middle-aged, and the older groups.
A comparison of the AcroQoL scores of the patients with and without biochemical disease control showed no difference in the scores for the young and middle-aged (Figure 2A) or older groups (Figure 2B). Next, the AcroQoL scores for the following three subgroups were compared based on the treatment modalities: surgery only, medical therapy without radiotherapy, and radiotherapy. In the young and middle-aged group, all the subscale scores of the AcroQoL were higher for patients treated with surgery than for those treated with medical therapy without radiotherapy (Figure 3A). The patients who received radiotherapy had the lowest total and psychological QoL scores, as shown previously (15). On the contrary, no difference in the AcroQoL scores was observed among the treatment modality subgroups in the older group (Figure 3B).
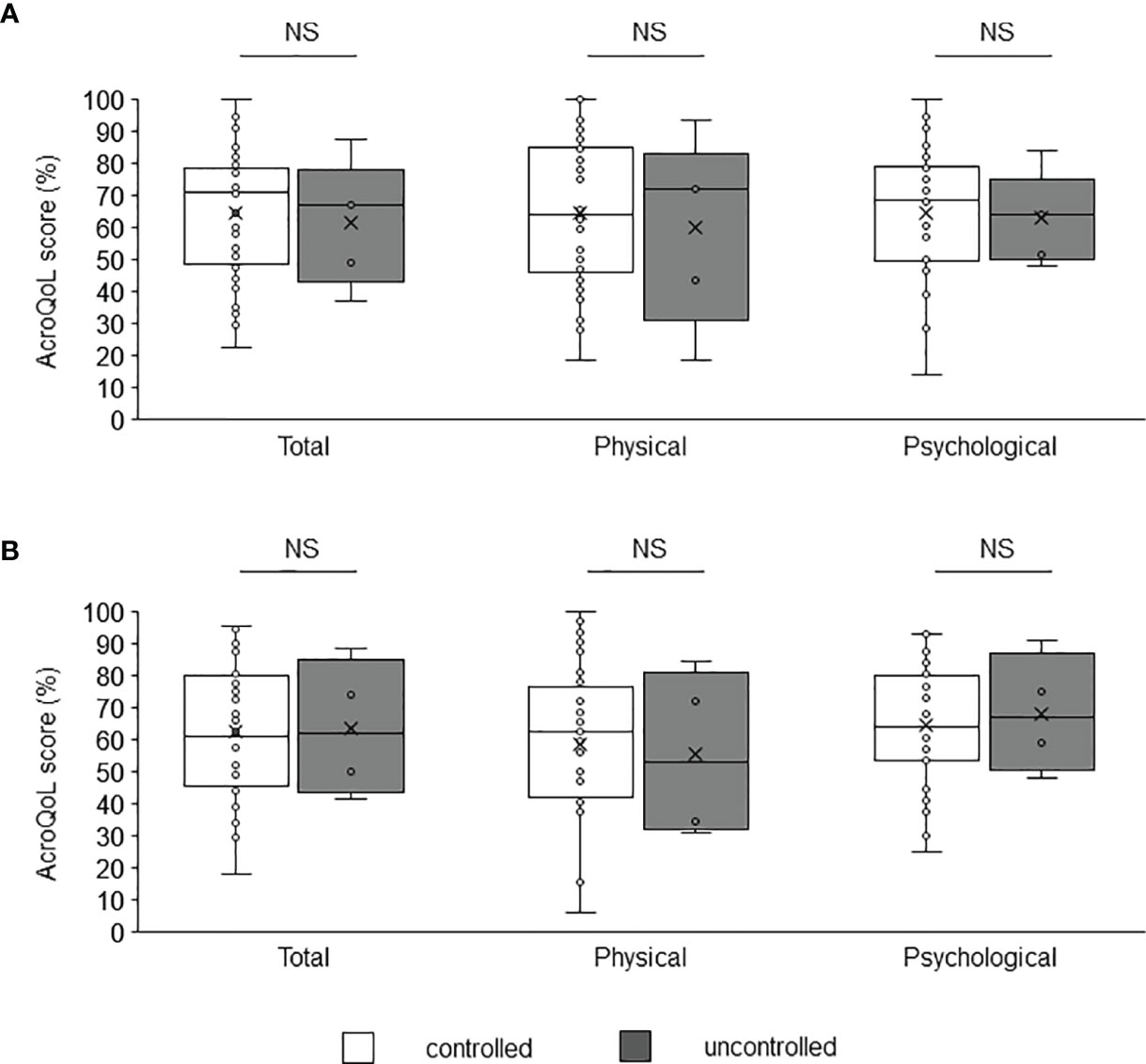
Figure 2 Comparison of AcroQoL scores of the subgroups based on the disease control status. Comparison of AcroQoL scores of the subgroups based on the disease control status in the (A) young and middle-aged, and (B) older groups. Patients with random GH levels of <1.0 ng/mL and IGF-I SDS of <2.0 are considered controlled, and others are considered uncontrolled. Individual scores have been plotted, and median values and interquartile ranges are shown in the box plot. NS, not significant.
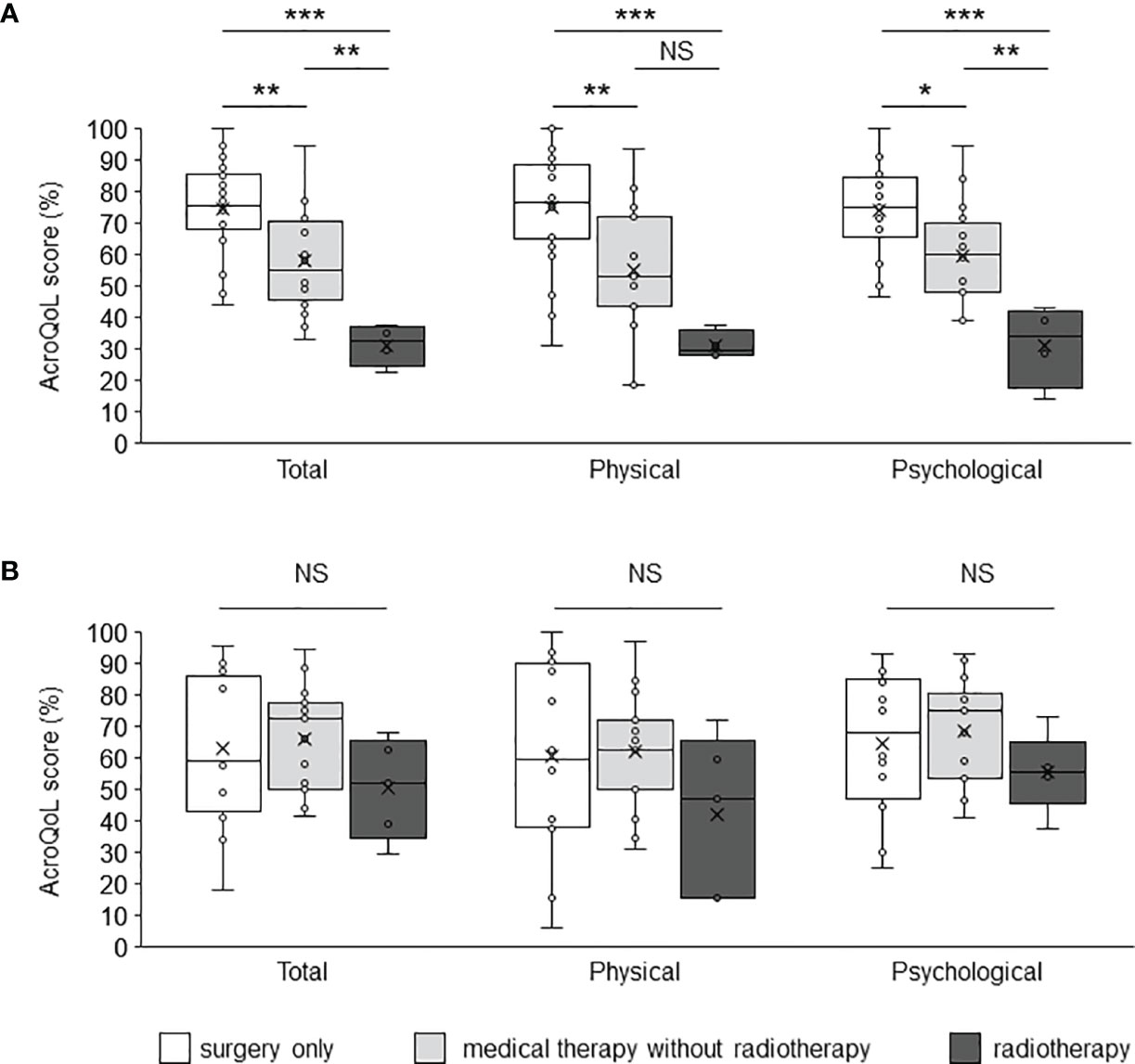
Figure 3 Comparison of the AcroQoL scores of the subgroups based on treatment modalities. Comparison of the AcroQoL scores of the subgroups treated with surgery only, medical therapy without radiotherapy, and radiotherapy in the (A) young and middle-aged, and (B) older groups. Individual scores have been plotted, and the median values and interquartile ranges are presented in a box plot. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Stepwise multivariate regression analyses were performed to determine the factors associated with impaired QoL for each age group. In the young and middle-aged group, the treatment modalities (defined in Material and Methods), size of pituitary tumor, hydrocortisone replacement therapy, and the total number of hormone replacement therapy were selected as candidate for independent variables by the univariate regression analysis (Tables S1–S3). When hydrocortisone replacement therapy, in addition to the treatment modalities and size of pituitary tumor, was included for the multivariate analysis, all the variables were found to be independent factors associated with impaired AcroQoL scores in the young and middle-aged group (Table 4A). When the total number of hormone replacement therapy was included in place of hydrocortisone replacement therapy for multivariate analysis, the treatment modalities and the total number of hormone replacement therapy were independently associated with poor AcroQoL scores in total scales (adjusted R2 =0.43, P <0.01, P =0.03, respectively).
In the older group, BMI, complications of dyslipidemia, arthropathy, and hydrocortisone replacement therapy were the independent variables. Interestingly, higher BMI, complications of arthropathy, and hydrocortisone replacement therapy were identified as independent factors contributing to impaired AcroQoL scores in the older group (Table 4B).
In recent years, many people over the age of 65 years maintain their physical and mental health and participate in social activities, and some countries including Japan have proposed redefining “older people” as over the age of 75 years (24). Therefore, to clarify the effect of the aging on QoL, all the patients were further divided into the following four groups: younger than 55, 55-64, 65-74, and 75 years and older (Table S4). Multivariate regression analysis showed that only the treatment modalities were associated with impaired total AcroQoL scores in both groups <55 and 55-64 years (adjusted R2 =0.40, P <0.01, adjusted R2 =0.35, P <0.01, respectively). In the 65-74 years group, higher BMI and complication of arthropathy were associated with poor AcroQoL scores in total scale (adjusted R2 =0.47, P <0.01, P =0.02, respectively). Moreover, only complication of arthropathy was associated in the 75 years and older group (adjusted R2 =0.59, P =0.02).
This is the first study of the QoL focused on older patients with acromegaly. We demonstrated differences in the factors associated with impaired QoL for different age groups. To the best of our knowledge, most QoL studies focus on young or middle-aged patients with acromegaly, but not on older acromegaly patients. Because of the increasing number of older patients with acromegaly, it is important to adjust the strategy with consideration of QoL.
Age-dependent QoL decline has been observed in healthy populations (12). In this study, age was negatively associated with QoL scores for the physical component of the SF-36; however, a decrease in the QoL for the mental and role/social components was observed in younger patients. These data suggest that acromegaly can have a greater impact on younger patients, especially mentally and socially, rather than physically. On the other hand, AcroQoL showed no association with age, suggesting that age has little effect on the disease-specific QoL for acromegaly. Moreover, the scores of all the patients were impaired.
In this study, we showed that the factors associated with impaired AcroQoL were significantly different for various age groups. For young and middle-aged patients, treatment modalities including current medical therapy and past radiotherapy impaired the QoL score, as shown in previous reports (13, 14). In contrast, treatment modalities did not affect the AcroQoL of older patients.
In the present study, 31 (79%) of 39 patients receiving medical therapy used somatostatin analogs; however, there was no significant difference in the proportion of patients using somatostatin analogs between the younger and older groups. It has been reported that treatment-related burdens such as gastrointestinal side effects, injection site reactions, and the need for periodic drug injections interfere with their daily and work activities, even for the biochemically controlled status of somatostatin analogs (25). The treatment-related burden can lead to QoL impairment for younger patients because of their social status. Moreover, patients who require radiotherapy generally have aggressive tumors, which are often associated with hypopituitarism and may result in impaired QoL.
In contrast, arthropathy and higher BMI were associated with poor QoL for older patients. Motor disability and joint symptoms, mainly due to arthropathy, contribute to reduced AcroQoL scores, even after long-term disease remission (26, 27). Cartilage hypertrophy caused by a chronic excess of GH/IGF-I is partially reversible during the early stages; however, the subsequent deterioration of the joint architecture is irreversible (3, 28). The risk factors for acromegalic arthropathy include female sex, higher BMI, and older age at diagnosis, which are common to primary osteoarthritis (29). In addition, higher GH/IGF-I levels at diagnosis and longer duration of the disease have been demonstrated as disease-specific risk factors for arthropathy (30, 31). In this study, there was no difference in the female ratio between the younger and older groups, and BMI was lower in the older group. It is considered that a longer disease duration in the older group can result in the deterioration of arthropathy. These data suggest the importance of early diagnosis and appropriate treatment of acromegaly to prevent the development of arthropathy.
Interestingly, it has already been reported that a higher BMI is associated with poor QoL in acromegaly (32, 33). A higher BMI is a risk factor for arthropathy and can worsen the symptoms; however, we did not observe an association between the prevalence of arthropathy and BMI. High GH/IGF-I levels in acromegaly can lead to increased muscle mass and extracellular fluid volume, resulting in increased BMI. In contrast, decreased GH/IGF-I levels can promote body mass via an increase in fat mass (34). Therefore, the association between chronic GH/IGF-I excess and normalized hormonal levels and an increase in BMI has not been established and remains controversial. The reason for the high BMI contribution to reduced QoL remains unclear. It was speculated that the burden of these comorbidities was more severe in older patients with a higher BMI than in younger patients.
Excess GH/IGF-I in acromegaly causes insulin resistance and glucose intolerance, increasing the risk of frailty or sarcopenia, especially in older patients (35). The presence of sarcopenia is associated with decrease in health-related QoL (36). Moreover, a decrease in IGF-I after treatment for acromegaly has been shown to increase fat accumulation and decrease lean body mass (37). Multiple factors, including changes in body composition and sarcopenia, may affect health-related QoL in older acromegaly patients under treatment. Further studies focused on the effect of these metabolic factors on QoL in older patients are needed.
In the present study, the number of hormone replacement therapy was associated with poor AcroQoL scores in young and middle-aged group. It was reported that patients with multiple pituitary hormone deficiencies had worse QoL than patients with isolated GH deficiency (38). However, there was no association between the number of hormone replacement therapy and the AcroQoL scores in older patients in this study. Further investigation is needed to clarify whether impairment of QoL due to multiple pituitary hormone deficiencies varies with age.
In contrast, the AcroQoL scores were lower for patients who received hydrocortisone replacement therapy in both age groups in this study. It is well-known that patients with adrenal insufficiency, compared with the general population, have impaired health-related QoL due to exhaustion, social disability, and anxiety irrespective of age, even with hydrocortisone replacement therapy (39). Therefore, it is not surprising that hydrocortisone replacement therapy was associated with lower AcroQoL scores in both age groups in the present study.
It was interesting that the size of pituitary tumor before the initial treatment was associated with poor AcroQoL scores in young and middle-aged patients, while no association was found in older group in this study. It was reported that macroadenoma impaired health-related QoL in the patients with non-functional pituitary tumor (40). Although pathological findings were not reviewed in the present study, it was possible that several patients in the young and middle-aged group with macroadenoma had sparsely granulated adenoma, which was resistant to treatment, leading to decreased QoL.
The limitation of the present study is that it was a retrospective cross-sectional study with a relatively small sample. Therefore, improvements in QoL associated with early diagnosis and appropriate treatment of arthropathy remain to be elucidated, although arthropathy is generally irreversible. Moreover, in this study, we only investigated the acromegaly patients without a control study using healthy subjects. To conclude the effect of aging on QoL with acromegaly patients under treatment, we needed further study including control subjects of young, middle-aged, and older people.
In conclusion, we have shown that the factors differently affected QoL across the age groups. Arthropathy, higher BMI, and hydrocortisone replacement therapy are associated with impaired disease-specific QoL of older patients with acromegaly. These data suggest that early diagnosis and appropriate treatment are important to prevent impaired QoL of older patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Kobe University Hospital (approval #1363). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
NY, SU, HF, and YT conceived and designed the study. NY, SU, KY, MY, and HF contributed to collect the data. NY, SU, MY, HF, and GI contributed to analyze data. NY, SU, HF, MY, MS, HS, YF, KK, GI, YT, and WO interpreted data and contributed to discussion. NY, SU, HF, and YT drafted the manuscript.
This work was supported by Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology 19K09003.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Dr. H Bando, Dr. Y Ooi, and Dr. Y Sasaki for their insightful advice. We are grateful to Ms. K Imura, Ms. Y Shindo, Ms. M Nishime, and Ms. M Sakoda for their excellent assistance. We also thank our biostatisticians of the Clinical and Translational Research Center at Kobe University Hospital for their helpful suggestions in statistics.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.819330/full#supplementary-material
1. Ben-Shlomo A, Melmed S. Acromegaly. Endocrinol Metab Clin North Am (2008) 37:101–22. doi: 10.1016/j.ecl.2007.10.002
2. Melmed S, Bronstein MD, Chanson P, Klibanski A, Casanueva FF, Wass J, et al. A Consensus Statement on Acromegaly Therapeutic Outcomes. Nat Rev Endocrinol (2018) 14:552–61. doi: 10.1038/s41574-018-0058-5
3. Giustina A, Barkan A, Beckers A, Biermasz N, Biller B, Boguszewski C, et al. A Consensus on the Diagnosis and Treatment of Acromegaly Comorbidities: An Update. J Clin Endocrinol Metab (2020) 105:dgz096. doi: 10.1210/clinem/dgz096
4. Bolfi F, Neves AF, Boguszewski CL, Nunes-Nogueira VS. Mortality in Acromegaly Decreased in the Last Decade: A Systematic Review and Meta-Analysis. Eur J Endocrinol (2018) 179:59–71. doi: 10.1530/EJE-18-0255
5. Arosio M, Reimondo G, Malchiodi E, Berchialla P, Borraccino A, De Marinis L, et al. Predictors of Morbidity and Mortality in Acromegaly: An Italian Survey. Eur J Endocrinol (2012) 167:189–98. doi: 10.1530/EJE-12-0084
6. Matsubayashi K, Kawakami K. Prevalence, Incidence, Comorbidities, and Treatment Patterns Among Japanese Patients With Acromegaly: A Descriptive Study Using a Nationwide Claims Database. Endocr J (2020) 67:997–1006. doi: 10.1507/endocrj.EJ20-0129
7. Kyriakakis N, Lynch J, Gilbey SG, Webb SM, Murray RD. Impaired Quality of Life in Patients With Treated Acromegaly Despite Long-Term Biochemically Stable Disease: Results From a 5-Years Prospective Study. Clin Endocrinol (Oxf) (2017) 86:806–15. doi: 10.1111/cen.13331
8. Gill TM, Allore HG, Gahbauer EA, Murphy TE. Change in Disability After Hospitalization or Restricted Activity in Older Persons. JAMA (2010) 304:1919–28. doi: 10.1001/jama.2010.1568
9. van den Akker M, Buntinx F, Metsemakers JF, Roos S, Knottnerus JA. Multimorbidity in General Practice: Prevalence, Incidence, and Determinants of Co-Occurring Chronic and Recurrent Diseases. J Clin Epidemiol (1998) 51:367–75. doi: 10.1016/s0895-4356(97)00306-5
10. Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, et al. Incidence and Preventability of Adverse Drug Events Among Older Persons in the Ambulatory Setting. JAMA (2003) 289:1107–16. doi: 10.1001/jama.289.9.1107
11. Akishita M, Teramoto S, Arai H, Arai H, Mizukami K, Morimoto S, et al. Incidence of Adverse Drug Reactions in Geriatric Wards of University Hospitals. Nihon Ronen Igakkai Zasshi (2004) 41:303–6. doi: 10.3143/geriatrics.41.303
12. Walters SJ, Munro JF, Brazier JE. Using the SF-36 With Older Adults: A Cross-Sectional Community-Based Survey. Age Ageing (2001) 30:337. doi: 10.1093/ageing/30.4.337
13. Gadelha MR, Kasuki L, Lim D, Fleseriu M. Systemic Complications of Acromegaly and the Impact of the Current Treatment Landscape: An Update. Endocr Rev (2019) 40:268–332. doi: 10.1210/er.2018-00115
14. Guo X, Wang K, Yu S, Gao L, Wang Z, Zhu H, et al. Quality of Life and its Determinants in Patients With Treated Acromegaly: A Cross-Sectional Nationwide Study in China. J Clin Endocrinol Metab (2021) 106:211–25. doi: 10.1210/clinem/dgaa750
15. Yoshida K, Fukuoka H, Matsumoto R, Bando H, Suda K, Nishizawa H, et al. The Quality of Life in Acromegalic Patients With Biochemical Remission by Surgery Alone is Superior to That in Those With Pharmaceutical Therapy Without Radiotherapy, Using the Newly Developed Japanese Version of the AcroQoL. Pituitary (2015) 18:876–83. doi: 10.1007/s11102-015-0665-2
16. Katznelson L, Laws ER Jr., Melmed S, Molitch ME, Murad MH, Utz A, et al. Acromegaly: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2014) 99:3933–51. doi: 10.1210/jc.2014-2700
17. Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S, Casanueva FF, et al. A Consensus on Criteria for Cure of Acromegaly. J Clin Endocrinol Metab (2010) 95:3141–8. doi: 10.1210/jc.2009-2670
18. Chihara K, Shimatsu A, Hizuka N, Tanaka T, Seino Y, Katofor Y, et al. A Simple Diagnostic Test Using GH-Releasing Peptide-2 in Adult GH Deficiency. Eur J Endocrinol (2007) 157:19–27. doi: 10.1530/EJE-07-0066
19. Isojima T, Shimatsu A, Yokoya S, Chihara K, Tanaka T, Hizuka N, et al. Standardized Centile Curves and Reference Intervals of Serum Insulin-Like Growth Factor-I (IGF-I) Levels in a Normal Japanese Population Using the LMS Method. Endocr J (2012) 59:771–80. doi: 10.1507/endocrj.ej12-0110
20. Hawthorne G, Osborne RH, Taylor A, Sansoni J. The SF36 Version 2: Critical Analyses of Population Weights, Scoring Algorithms and Population Norms. Qual Life Res (2007) 16:661–73. doi: 10.1007/s11136-006-9154-4
21. Suzukamo Y, Fukuhara S, Green J, Kosinski M, Gandek B, Ware JE. Validation Testing of a Three-Component Model of Short Form-36 Scores. J Clin Epidemiol (2011) 64:301–8. doi: 10.1016/j.jclinepi.2010.04.017
22. World Health Organization. Global Health and Aging(2011). Available at: https://www.who.int/ageing/publications/global_health.pdf (Accessed November 7, 2021).
23. Badia X, Trainer P, Biermasz NR, Tiemensma J, Carreño A, Roset M, et al. Mapping AcroQoL Scores to EQ-5D to Obtain Utility Values for Patients With Acromegaly. J Med Econ (2018) 21:382–9. doi: 10.1080/13696998.2017.1419960
24. Ouchi Y, Rakugi H, Arai H, Akishita M, Ito H, Toba K, et al. Redefining the Elderly as Aged 75 Years and Older: Proposal From the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr Gerontol Int (2017) 17:1045–7. doi: 10.1111/ggi.13118
25. Fleseriu M, Molitch M, Dreval A, Biermasz NR, Gordon MB, Crosby RD, et al. Disease and Treatment-Related Burden in Patients With Acromegaly Who Are Biochemically Controlled on Injectable Somatostatin Receptor Ligands. Front Endocrinol (Lausanne) (2021) 12:627711. doi: 10.3389/fendo.2021.627711
26. Fatti LM, Cangiano B, Vitale G, Persani L, Mantovani G, Sala E, et al. Arthropathy in Acromegaly: A Questionnaire-Based Estimation of Motor Disability and its Relation With Quality of Life and Work Productivity. Pituitary (2019) 22:552–60. doi: 10.1007/s11102-019-00966-8
27. Biermasz NR, Pereira AM, Smit JW, Romijn JA, Roelfsema F. Morbidity After Long-Term Remission for Acromegaly: Persisting Joint-Related Complaints Cause Reduced Quality of Life. J Clin Endocrinol Metab (2005) 90:2731–9. doi: 10.1210/jc.2004-2297
28. Okazaki K, Jingushi S, Ikenoue T, Urabe K, Sakai H, Ohtsuru A, et al. Expression of Insulin-Like Growth Factor I Messenger Ribonucleic Acid in Developing Osteophytes in Murine Experimental Osteoarthritis and in Rats Inoculated With Growth Hormone-Secreting Tumor. Endocrinology (1999) 140:4821–30. doi: 10.1210/endo.140.10.7053
29. Kropf LL, Madeira M, Vieira Neto L, Gadelha MR, de Farias ML. Functional Evaluation of the Joints in Acromegalic Patients and Associated Factors. Clin Rheumatol (2013) 32:991–8. doi: 10.1007/s10067-013-2219-1
30. Biermasz NR, Wassenaar MJ, van der Klaauw AA, Pereira AM, Smit JW, Roelfsema F, et al. Pretreatment Insulin-Like Growth Factor-I Concentrations Predict Radiographic Osteoarthritis in Acromegalic Patients With Long-Term Cured Disease. J Clin Endocrinol Metab (2009) 94:2374–9. doi: 10.1210/jc.2008-2393
31. Layton MW, Fudman EJ, Barkan A, Braunstein EM, Fox IH. Acromegalic Arthropathy. Characteristics and Response to Therapy. Arthritis Rheumatol (1988) 31:1022–7. doi: 10.1002/art.1780310813
32. T’Sjoen G, Bex M, Maiter D, Velkeniers B, Abs R. Health-Related Quality of Life in Acromegalic Subjects: Data From AcroBel, the Belgian Registry on Acromegaly. Eur J Endocrinol (2007) 157:411–7. doi: 10.1530/EJE-07-0356
33. Kauppinen-Mäkelin R, Sane T, Sintonen H, Markkanen H, Välimäki MJ, Löyttyniemi E, et al. Quality of Life in Treated Patients With Acromegaly. J Clin Endocrinol Metab (2006) 91:3891–6. doi: 10.1210/jc.2006-0676
34. Zhang S, Li Y, Guo X, Gao L, Lian W, Yao Y, et al. Body Mass Index and Insulin-Like Growth Factor 1 as Risk Factors for Discordant Growth Hormone and Insulin-Like Growth Factor 1 Levels Following Pituitary Surgery in Acromegaly. J Formos Med Assoc (2018) 117:34–41. doi: 10.1016/j.jfma.2017.02.014
35. Wang M, Tan Y, Shi Y, Wang X, Liao Z, Wei P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front Endocrinol (Lausanne) (2020) 11:568. doi: 10.3389/fendo.2020.00568
36. Rizzoli R, Reginster JY, Arnal JF, Bautmans I, Beaudart C, Bischoff-Ferrari H, et al. Quality of Life in Sarcopenia and Frailty. Calcif Tissue Int (2013) 93:101–20. doi: 10.1007/s00223-013-9758-y
37. Wolf P, Salenave S, Durand E, Young J, Kamenicky P, Chanson P, et al. Treatment of Acromegaly has Substantial Effects on Body Composition: A Long-Term Follow-Up Study. Eur J Endocrinol (2021) 186:173–81. doi: 10.1530/EJE-21-0900
38. Rosilio M, Blum WF, Edwards DJ, Shavrikova EP, Valle D, Lamberts SW, et al. Long-Term Improvement of Quality of Life During Growth Hormone (GH) Replacement Therapy in Adults With GH Deficiency, as Measured by Questions on Life Satisfaction-Hypopituitarism (QLS-H). J Clin Endocrinol Metab (2004) 89:1684–93. doi: 10.1210/jc.2003-030134
39. Hahner S, Loeffler M, Fassnacht M, Weismann D, Koschker AC, Quinkler M, et al. Impaired Subjective Health Status in 256 Patients With Adrenal Insufficiency on Standard Therapy Based on Cross-Sectional Analysis. J Clin Endocrinol Metab (2007) 92:3912–22. doi: 10.1210/jc.2007-0685
Keywords: acromegaly, quality of life, arthropathy, older patients, AcroQoL
Citation: Yamamoto N, Urai S, Fukuoka H, Yamamoto M, Yoshida K, Suzuki M, Shichi H, Fujita Y, Kanie K, Iguchi G, Takahashi Y and Ogawa W (2022) The Effect of Aging on Quality of Life in Acromegaly Patients Under Treatment. Front. Endocrinol. 13:819330. doi: 10.3389/fendo.2022.819330
Received: 21 November 2021; Accepted: 10 January 2022;
Published: 03 February 2022.
Edited by:
Alessandro Peri, University of Florence, ItalyReviewed by:
Luiz Augusto Casulari, University of Brasilia, BrazilCopyright © 2022 Yamamoto, Urai, Fukuoka, Yamamoto, Yoshida, Suzuki, Shichi, Fujita, Kanie, Iguchi, Takahashi and Ogawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hidenori Fukuoka, ZnVrdW9rYWhAbWVkLmtvYmUtdS5hYy5qcA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.