- Department of Medical Oncology, Cancer Centre, West China Hospital, Sichuan University, Chengdu, China
Metaplastic breast carcinoma (MBC) is an aggressive subtype of breast cancer, accounting for <1%. The clinical outcome is unknown due to the lack of treatment options. Here, we present the case of a 58-year-old woman with advanced MBC, in which standard adjuvant chemotherapy was unsuccessful. In the second-line therapy, she received anti-angiogenic(anlotinib) therapy plus chemotherapy. Finally, she was subsequently treated with immunotherapy (toripalimab) combined anlotinib and achieved partial response (PR); thus, immunotherapy plus anti-angiogenic therapy might be a novel option for advanced MBC patients.
Introduction
Metaplastic breast carcinoma (MBC) is a rare and aggressive subtype of breast cancer (about 1%) (1). MBC occurs commonly in women over the age of 60 years, typically presenting as a larger tumor size (2, 3). According to the WHO breast tumor classification in 2019, MBC was divided into five subtypes including adenosquamous carcinoma, pure squamous cell carcinomas, pure spindle cell carcinoma, metaplastic carcinoma with mesenchymal differentiation, and mixed metaplastic carcinoma (1). More than 80% of MBC did not express estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2 receptor (HER2) (4). Advanced MBC has a poor prognosis compared to non-MBC triple-negative breast cancer (TNBC) due to rapid tumor growth and insensitivity to standard chemotherapy (5, 6). Immune checkpoint inhibitors (ICIs) block the PD-L1/PD-1 and CTLA-4/B7 signaling pathways, thereby preventing effector T cells from being inactivated and maintaining/keeping them to be able to kill tumor cells. In the past decade, immunotherapy improved survival benefits for patients with TNBC; whether immunotherapy is effective for MBC is still unknown (7–12). Here, we report an advanced MBC patient who failed with standard chemotherapy in the first-line therapy and anlotinib plus chemotherapy in the second-line therapy. She was subsequently treated with toripalimab plus anlotinib and achieved partial response (PR). Thus, immunotherapy combined with anti-angiogenic therapy might be a novel option for advanced MBC patients in later-line treatment.
Case Report
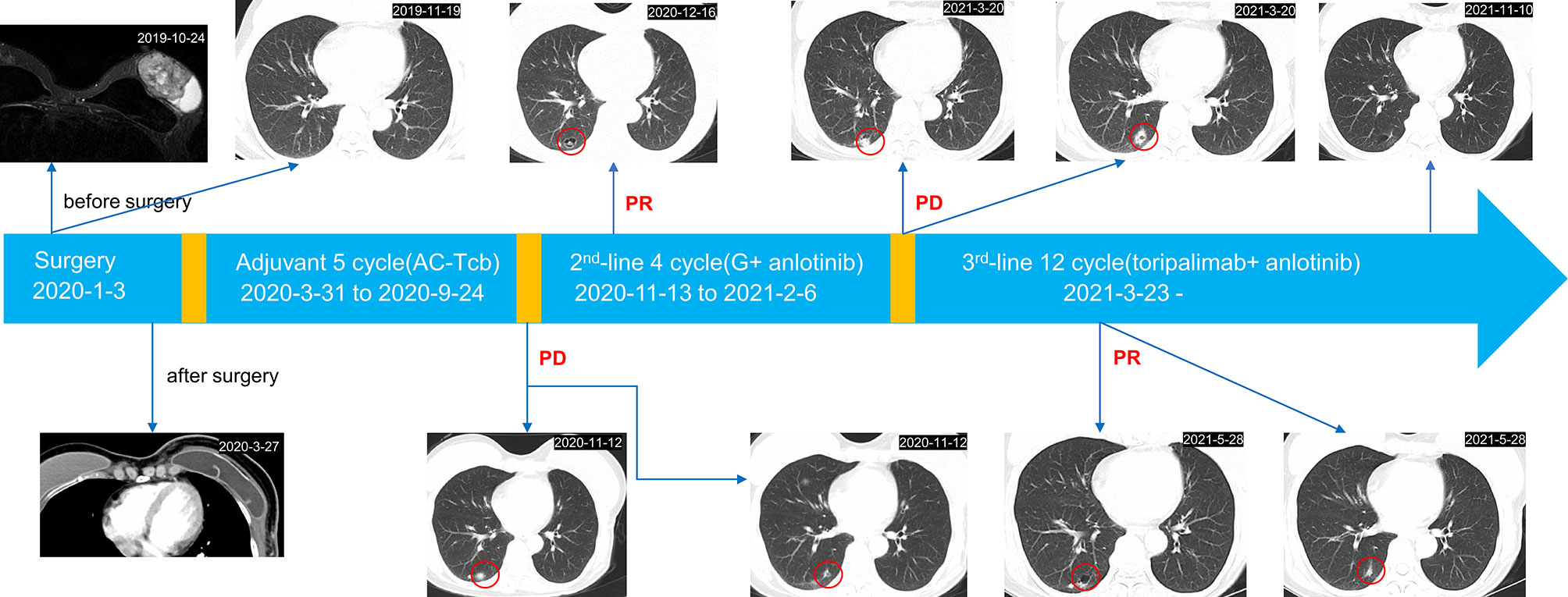
A 58-year-old female came to our hospital with a chief complaint of finding a left breast mass. Breast ultrasound showed a left BI-RADs 4c breast mass and enlarged left axillary lymph nodes (Figure 1). The tumor biomarkers such as carcinoembryonic antigen (CEA) (1.2 ng/ml) and cancer antigen 153 (CA 153) (8.63 U/ml) were in the normal range. She underwent modified radical mastectomy, axillary lymph node dissection, and breast reconstruction. MBC (squamous cell carcinoma and sarcomatoid components) was established by pathological examination and confirmed by immunohistochemistry (IHC) staining, which demonstrated ER (−), PR (−), Her2 (0), Ki67(+, 20%), GATA3 (+), PCK (+), P63 (+), CK5/6 (+), SMA (+), P53(−), Desmin (−), Myogenin (−), and STAB2 (−). CD31 stain was negative in the tumor cell and positive in the tumor vasculature. PD-L1 expression in the tumor cell and tumor vasculature was assessed using antibody 22C3 (Agilent Technologies, USA) and a combined positive score of <1% (Figure 2). All of the three excised lymph nodes were free of tumor cells (T3N0M0, stage IIB). Adjuvant chemotherapy was prescribed after surgery, and chemotherapy regimen consisted of anthracycline plus cyclophosphamide followed by paclitaxel plus carboplatin (AC-TCb). After four cycles of AC and one cycle of TCb, multiple pulmonary metastases (>5, Supplementary Figure S1 in the Supplementary Appendix) in the lung were shown in the following chest CT scan. The efficacy was evaluated as progression disease (PD). Anlotinib (10 mg, qd, days 1–14) combined with gemcitabine (1,400 mg, every 3 weeks) was prescribed for the second-line therapy. After two cycles of combined therapy, the metastases in the lung achieved PR, while after four cycles, we rechecked the enhanced CT images, and the efficacy evaluation was PD (Supplementary Figure S2). After multi-disciplinary treatment, we changed the original scheme, and toripalimab (an anti-PD1 antibody, Junshi Inc., China, Shanghai) at a dose of 160 mg(3 mg/kg) combined with anlotinib (10 mg, qd, days 1–14) was given every 3 weeks. After two cycles of combined therapy, the size of pulmonary metastases became smaller with no treatment-related adverse events. Up to now, the patient sustained remission more than 8 months without further complaints and side effects (sustained PR) and continue to receive anlotinib plus toripalimab regularly.

Figure 2 Pathological examination showed spindle cell morphology (A, H&E). (B–N) Immunohistochemistry data: ER (−), PR (−), Her2(−), Ki-67 (+, positive proportion about 20%), GATA3 (+), PCK (+), PD-L1(−, positive proportion about <1%), CD5/6 (+), SMA (+), P53(−), Desmin (−), P63(+), and CD31 (−) supported the diagnosis. Original magnification: (A–N), 200×.
Discussion
MBC is a rare and aggressive subtype of breast cancer. Reddy et al. showed that MBC has a lower OS than non-MBC (64.4 vs. 159.2 m, p < 0.001) (13). No standard of care for this disease is established, while the current therapy often contains chemotherapy. Our patients received immunotherapy plus anti-angiogenic therapy, which has been shown to improve the prognosis of MBC.
Previous studies showed that MBC had a unique tumor environment. Several studies observed a high level of PD-L1 expression and high density of CD8+ tumor infiltrating lymphocytes (TILs) in this tumor. Upasana et al. showed that MBC has the highest PD-L1 expression and CD8+ TILs than the other subtypes of breast cancer (14). Lien et al. observed that the positivity for TILs, combined positive score (CPS), and tumor proportion score (TPS) were 34.1%, 47.6%, and 17.1%, respectively. In addition, squamous cell carcinoma components in MBC had the highest positivity rates of TILs and CPS (15). Kalaw et al. enrolled 146 MBC patients, and 73% of them had PD-L1 expression in tumor ≥5% (16). In addition, Joneja et al. tested the expression of PD-L1 in 72 MBC patients, and the results showed positive PD-L1 of tumor and immune cells at 46% and 43%, respectively (14). High PD-L1 expression and TILs are generally associated with good response to ICIs.
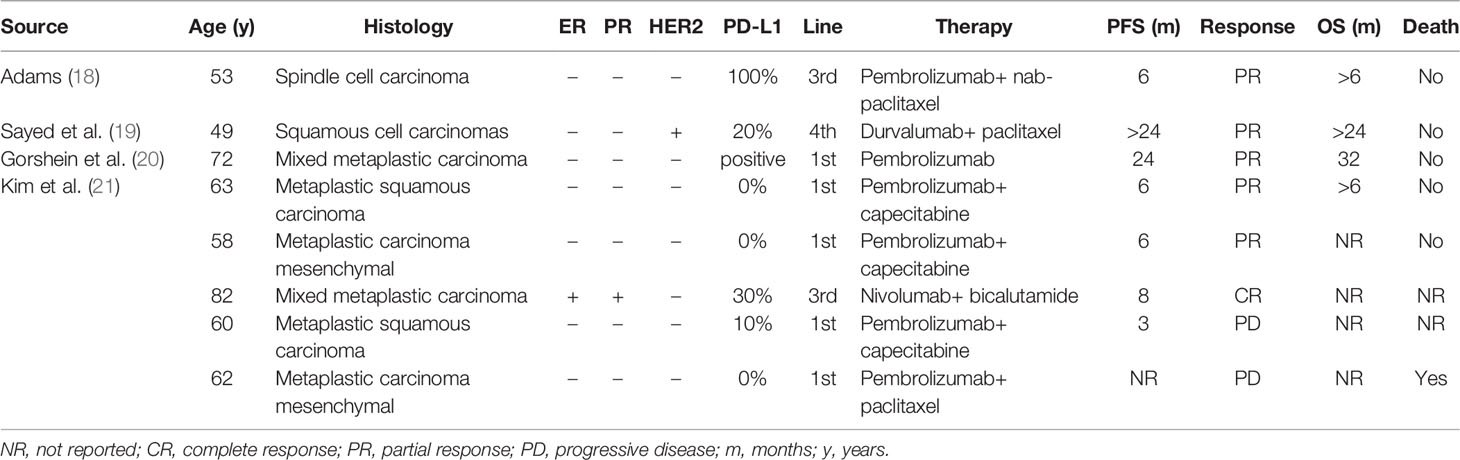
ICIs have changed the treatment landscape of advanced breast cancer. Impassion 130 was a randomized phase III study that tested the efficiency of atezolizumab combined with nab-paclitaxel in the first-line treatment. The chemo-immunotherapy showed benefit in both median progression-free survival (PFS) (7.5 m vs. 5.3 m, p<0.001) and OS (21.0 m vs. 18.7 m, p = 0.078) compared with placebo plus nab-paclitaxel (8). A similar good outcome was also achieved by pembrolizumab. In the phase III KEYNOTE-355 study, the combination of pembrolizumab and chemotherapy achieved an mPFS of 9.7m (CPS > 10) (7). In a single-arm, phase 2 trial (DART trial), 17 MBC patients received the nivolumab and ipilimumab combination therapy. The ORR, median PFS, and median OS were 18%, 2 months, and 12 months, respectively (17). Previously, four case reports observed the efficacy of ICIs in advanced MBC (Table 1). Six of eight patients showed a good prognosis after immunotherapy (18–21).
Anlotinib is a Chinese multitarget tyrosine kinase inhibitor (TKI), which can inhibit VEGFR1, VEGFR2, VEGFR3, c-Kit, and PDGFR, and is approved by the Chinese National Medical Products Administration for the treatment of advanced non-small-cell lung carcinoma (NSCLC), SCLC, soft-tissue sarcoma, and medullary thyroid carcinoma in the later line (22–25). Hu et al. investigate anlotinib for HER2-negative breast cancer in later-line therapy. The mPFS was 5.22 m, and the disease control rate (DCR) was 80.8%. Meanwhile, severe adverse events (AEs, ≥G3) were hypertension (26.92%) and hand–foot syndrome (3.85%). These results showed that anlotinib had good efficacy and limited toxicity with HER2-negative breast cancer (26). Only one case of advanced MBC treated with anlotinib has been reported. This MBC patient underwent anlotinib (12 mg/day, 2 weeks on, 1 week off), and achieved a durable PR for more than 25 months (27).
Several studies demonstrated that anti-angiogenic agents have synergistic effects with ICIs. Antiangiogenic therapy can make abnormal tumor vessels normalization, which increases the infiltration of immune effector cells in TME (28). In three phase 3 trials (IMBrave150, KEYNOTE-426, and IMpower150), atezolizumab combined with bevacizumab, pembrolizumab combined with axitinib, and atezolizumab combined with bevacizumab+ chemotherapy were shown to bring survival benefit to advanced hepatocellular carcinoma, advanced renal cell carcinoma, and advanced NSCLC, respectively (29–32). No case report described the efficacy of these combination therapy in advanced MBC. However, there were no clinical studies that reported the anlotinib plus ICI in earlier line treatment for MBC. We look forward to observing prospective clinical trials to explore the efficacy of the combined scheme on MBC in the future. In addition, it is necessary to explore the relationship of PD-L1 expression and vascularization for the efficacy of anlotinib and toripalimab.
In conclusion, we described a case of advanced MBC treated with toripalimab plus anlotinib after failure of standard chemotherapy and chemotherapy plus anti-angiogenic therapy. Immuno-combined anti-angiogenic therapy might be a useful candidate for advanced MBC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author. The full original source data can access in https://www.jianguoyun.com/p/DcmPFKQQ7oeDChiYyJsE.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee on Biomedical Research, West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
YJ and JL contributed to conception of the study. YF drafted the manuscript. YJ reviewed the manuscript. JL edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.810747/full#supplementary-material
Supplementary Figure 1 | The CT showed that mutiple metastases of lung after adjuvant chemotherapy.
Supplementary Figure 2 | The CT showed that multiple pulmonary metastases after anlotinib plus gemcitabine.
References
1. Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, et al. The 2019 World Health Organization Classification of Tumours of the Breast. Histopathology (2020) 77:181–5. doi: 10.1111/his.14091
2. Pezzi CM, Patel-Parekh L, Cole K, Franko J, Klimberg VS, Bland K, et al. Characteristics and Treatment of Metaplastic Breast Cancer: Analysis of 892 Cases From the National Cancer Data Base. Ann Surg Oncol (2007) 14:166–73. doi: 10.1245/s10434-006-9124-7
3. Honma N, Ogata H, Yamada A, Matsuda Y, Kontani K, Miyashita M, et al. Clinicopathological Characteristics and Prognostic Marker of Triple-Negative Breast Cancer in Older Women. Hum Pathol (2021) 111:10–20. doi: 10.1016/j.humpath.2021.01.005
4. Lee H, Jung SY, Ro JY, Kwon Y, Sohn JH, Park IH, et al. Metaplastic Breast Cancer: Clinicopathological Features and its Prognosis. J Clin Pathol (2012) 65:441–6. doi: 10.1136/jclinpath-2011-200586
5. Moreno AC, Lin YH, Bedrosian I, Shen Y, Babiera GV, Shaitelman SF. Outcomes After Treatment of Metaplastic Versus Other Breast Cancer Subtypes. J Cancer (2020) 11:1341–50. doi: 10.7150/jca.40817
6. Polamraju P, Haque W, Cao K, Verma V, Schwartz M, Klimberg VS, et al. Comparison of Outcomes Between Metaplastic and Triple-Negative Breast Cancer Patients. Breast (2020) 49:8–16. doi: 10.1016/j.breast.2019.10.003
7. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab Plus Chemotherapy Versus Placebo Plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer (KEYNOTE-355): A Randomised, Placebo-Controlled, Double-Blind, Phase 3 Clinical Trial. Lancet (2020) 396:1817–28. doi: 10.1016/S0140-6736(20)32531-9
8. Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab Plus Nab-Paclitaxel as First-Line Treatment for Unresectable, Locally Advanced or Metastatic Triple-Negative Breast Cancer (IMpassion130): Updated Efficacy Results From a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol (2020) 21:44–59. doi: 10.1016/S1470-2045(19)30689-8
9. Adams S, Othus M, Patel SP, Chae YK, Kurzrock R. Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Metaplastic Carcinoma of the Breast : Dart (Swog S1609, Cohort 36). J Clin Oncol (2020) 38(15_suppl):1073. doi: 10.1200/JCO.2020.38.15_suppl.1073
10. Winer EP, Lipatov O, Im SA, Goncalves A, Muñoz-Couselo E, Lee KS, et al. Pembrolizumab Versus Investigator-Choice Chemotherapy for Metastatic Triple-Negative Breast Cancer (KEYNOTE-119): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2021) 22:499–511. doi: 10.1016/S1470-2045(20)30754-3
11. Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol (2016) 34:2460–7. doi: 10.1200/JCO.2015.64.8931
12. Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab Monotherapy for Previously Treated Metastatic Triple-Negative Breast Cancer: Cohort A of the Phase II KEYNOTE-086 Study. Ann Oncol (2019) 30:397–404. doi: 10.1093/annonc/mdy517
13. Reddy TP, Rosato RR, Li X, Moulder S, Piwnica-Worms H, Chang JC. A Comprehensive Overview of Metaplastic Breast Cancer: Clinical Features and Molecular Aberrations. Breast Cancer Res (2020) 22:121. doi: 10.1186/s13058-020-01353-z
14. Joneja U, Vranic S, Swensen J, Feldman R, Chen W, Kimbrough J, et al. Comprehensive Profiling of Metaplastic Breast Carcinomas Reveals Frequent Overexpression of Programmed Death-Ligand 1. J Clin Pathol (2017) 70:255–9. doi: 10.1136/jclinpath-2016-203874
15. Lien HC, Lee YH, Chen IC, Lin CH, Chen TW, Lu YT, et al. Tumor-Infiltrating Lymphocyte Abundance and Programmed Death-Ligand 1 Expression in Metaplastic Breast Carcinoma: Implications for Distinct Immune Microenvironments in Different Metaplastic Components. Virchows Arch (2021) 478:669–78. doi: 10.1007/s00428-020-02954-x
16. Kalaw E, Lim M, Kutasovic JR, Sokolova A, Taege L, Johnstone K, et al. Metaplastic Breast Cancers Frequently Express Immune Checkpoint Markers FOXP3 and PD-L1. Br J Cancer (2020) 123:1665–72. doi: 10.1038/s41416-020-01065-3
17. Adams S, Othus M, Patel SP, Miller KD, Chugh R, Schuetze SM, et al. A Multicenter Phase II Trial of Ipilimumab and Nivolumab in Unresectable or Metastatic Metaplastic Breast Cancer: Cohort 36 of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART, SWOG S1609). Clin Cancer Res (2021) 28(2):271–8. doi: 10.1158/1078-0432.CCR-21-2182.
18. Adams S. Dramatic Response of Metaplastic Breast Cancer to Chemo-Immunotherapy. NPJ Breast Cancer (2017) 3:8. doi: 10.1038/s41523-017-0011-0
19. Al Sayed AD, Elshenawy MA, Tulbah A, Al-Tweigeri T, Ghebeh H. Complete Response of Chemo-Refractory Metastatic Metaplastic Breast Cancer to Paclitaxel-Immunotherapy Combination. Am J Case Rep (2019) 20:1630–5. doi: 10.12659/AJCR.918770
20. Gorshein E, Matsuda K, Riedlinger G, Sokol L, Rodriguez-Rodriguez L, Eladoumikdachi F, et al. Durable Response to PD1 Inhibitor Pembrolizumab in a Metastatic, Metaplastic Breast Cancer. Case Rep Oncol (2021) 14:931–7. doi: 10.1159/000515510
21. Kim I, Rajamanickam V, Bernard B, Chun B, Wu Y, Martel M, et al. A Case Series of Metastatic Metaplastic Breast Carcinoma Treated With Anti-PD-1 Therapy. Front Oncol (2021) 11:635237. doi: 10.3389/fonc.2021.635237
22. Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol (2018) 4:1569–75. doi: 10.1001/jamaoncol.2018.3039
23. Wu D, Nie J, Hu W, Dai L, Zhang J, Chen X, et al. A Phase II Study of Anlotinib in 45 Patients With Relapsed Small Cell Lung Cancer. Int J Cancer (2020) 147:3453–60. doi: 10.1002/ijc.33161
24. Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: A Novel Multi-Targeting Tyrosine Kinase Inhibitor in Clinical Development. J Hematol Oncol (2018) 11:120. doi: 10.1186/s13045-018-0664-7
25. Li D, Chi Y, Chen X, Ge M, Zhang Y, Guo Z, et al. Anlotinib in Locally Advanced or Metastatic Medullary Thyroid Carcinoma: A Randomized, Double-Blind Phase IIB Trial. Clin Cancer Res (2021) 27:3567–75. doi: 10.1158/1078-0432.CCR-20-2950
26. Hu N, Si Y, Yue J, Sun T, Wang X, Jia Z, et al. Anlotinib has Good Efficacy and Low Toxicity: A Phase II Study of Anlotinib in Pre-Treated HER-2 Negative Metastatic Breast Cancer. Cancer Biol Med (2021) 18(3):849–59. doi: 10.20892/j.issn.2095-3941.2020.0463.
27. Zou J, Yang X, Duan J, Wang J, Yang Z, Luo D, et al. A Case Report of Targeted Therapy With Anlotinib in a Patient With Advanced Breast Metaplastic Carcinoma. Onco Targets Ther (2021) 14:4599–607. doi: 10.2147/OTT.S318645
28. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat Rev Clin Oncol (2018) 15:325–40. doi: 10.1038/nrclinonc.2018.29
29. Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, et al. Patient-Reported Outcomes With Atezolizumab Plus Bevacizumab Versus Sorafenib in Patients With Unresectable Hepatocellular Carcinoma (IMbrave150): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol (2021) 22:991–1001. doi: 10.1016/S1470-2045(21)00151-0
30. Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab Plus Axitinib Versus Sunitinib Monotherapy as First-Line Treatment of Advanced Renal Cell Carcinoma (KEYNOTE-426): Extended Follow-Up From a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2020) 21:1563–73. doi: 10.1016/S1470-2045(20)30436-8
31. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med (2018) 378:2288–301. doi: 10.1056/NEJMoa1716948
Keywords: metaplastic breast carcinoma, immunotherapy, anti-angiogenic, outcome, PR
Citation: Fu Y, Liu J and Jiang Y (2022) Partial Response After Toripalimab Plus Anlotinib for Advanced Metaplastic Breast Carcinoma: A Case Report. Front. Endocrinol. 13:810747. doi: 10.3389/fendo.2022.810747
Received: 07 November 2021; Accepted: 17 February 2022;
Published: 23 March 2022.
Edited by:
Veronica Vella, University of Catania, ItalyReviewed by:
Shengchun Liu, First Affiliated Hospital of Chongqing Medical University, ChinaElisabeth Huijbers, VU Medical Center, Netherlands
Shengjun Ji, The Affiliated Suzhou Hospital of Nanjing Medical University, China
Copyright © 2022 Fu, Liu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Jiang, amlhbmdfeXVAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Yang Fu
Yang Fu Jie Liu
Jie Liu Yu Jiang
Yu Jiang