- 1Human Cancer Genomic Research, Research Center, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- 2Department of Radiology, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- 3Department of Surgery, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- 4Department of Pathology, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
Background: Papillary thyroid microcarcinomas (PTMCs) have been attributed to the recent increased incidence of thyroid cancer. Although indolent, a subset of PTMC could potentially develop distant metastasis (DM). This study aimed to evaluate the clinico-pathological features and molecular characteristics of PTMC and identify the risk factors for DM in PTMC patients from Middle Eastern ethnicity.
Methods: We retrospectively analyzed 210 patients with histologically confirmed PTMC. Clinico-pathological associations for DM, BRAF mutation and TERT mutation were analyzed successfully in 184 patients. Multivariate analysis was performed using Cox proportional hazards model and logistic regression analysis.
Results: Among the PTMC patients included in this cohort, DM was noted in 6.0% (11/184), whereas tumor relapse occurred in 29/184 (15.8%). Of the 11 cases with DM, lung metastasis occurred in 8 cases, bone metastasis in 2 cases and brain metastasis in 1 case. Presence of extrathyroidal extension and male sex were significantly associated with DM. Molecular analysis showed BRAF V600E mutations to be the most frequent, being detected in 45.7% (84/184). TERT promoter mutations were detected in 16 (8.7%) cases and were significantly associated with DM and shorter metastasis-free survival in multivariate analysis.
Conclusions: Our study indicates a surprisingly high frequency of TERT promoter mutation in Saudi patients with PTMC. Identifying TERT promoter mutations as an independent predictor of DM in patients with microcarcinoma could explain the inherent aggressive nature of PTMC from Middle Eastern ethnicity and magnify its role in patient risk stratification, which might help in improving therapeutic strategy for these patients.
Introduction
Papillary Thyroid Carcinoma (PTC) is the commonest subtype of thyroid cancer (1). The incidence of PTC has increased over the past decades (2–4). Papillary thyroid microcarcinoma (PTMC) is defined as PTC measuring ≤ 1cm along the largest diameter (5). Important advancements in diagnostic imaging and increased thoroughness of pathological examination have led to increased prevalence of PTMC among newly diagnosed PTC (6–8). Despite the increasing incidence of PTMC, it has an indolent clinical course, with good prognosis and favorable patient outcome (4, 9–11). Given the indolent nature of PTMC, it is anticipated the majority of PTMC will not cause clinically significant disease during patients’ lifetime and distant metastasis (DM) from PTMC has been considered a rare occurrence (10, 12, 13).
Several clinico-pathological features such as extrathyroidal extension, multifocality, lymph node metastasis, vascular invasion, and aggressive histological subtypes have been proposed as prognostic factors for PTMC recurrence (14–17). However, incidence and risk factors for distant metastasis remain to be further illustrated, especially in ethnicities where thyroid cancer is very common, such as the Middle Eastern ethnicity. In fact, PTC is very common in Saudi Arabia and ranks as the second most common cancer affecting females, after breast cancer (18). Therefore, identifying the incidence and risk factors for PTMC DM in Middle Eastern ethnicity is urgently needed. Indeed, it is noteworthy that PTC from Middle eastern ethnicity is unique with regards to being inherently more aggressive, as evidenced by other studies from this region, which also found a relatively high rate of aggressive variants (19, 20), multifocality (21, 22), extrathyroidal extension (19, 23), distant metastasis (19, 23) and a low median age at diagnosis (24, 25). A study by Gul et al. (26) in a Middle Eastern (Turkish) population assessed the difference in clinico-pathological characteristics between PTMC and PTC. They found that the incidence of capsular invasion, vascular invasion, extrathyroidal extension, lymph node involvement and multifocality was significantly lower in PTMC compared to PTC.
In this retrospective study, we evaluated the various clinico-pathological features to discriminate which PTMC will have more aggressive behavior. We then deepen the analysis of those PTMC with DM, focusing on identifying molecular markers for DM from PTMC. It is known that the initiation of PTC involves primary driver mutations, of which the most common are mitogen-activated protein (MAP) kinase mutations (BRAF V600E and RAS mutations) (27, 28). However, the development of more aggressive PTC is suggested to be associated with additional late driver mutations like telomerase reverse transcriptase (TERT) promoter mutations (29, 30). Previous studies have evaluated the mutational profile of PTMC with aggressive features with contrasting results (31–33). BRAF is the most studied gene in PTMC, being detected in about 50% of cases and was associated with aggressive clinical features and risk of recurrence (32, 34, 35). However, incidence of TERT promoter mutations and their clinical impact on PTMC have shown contradicting results. While the presence of TERT promoter mutations have been reported at a low rate in some studies (36, 37), others failed to find any TERT mutations in PTMC (38). The conflicting results about the clinical impact of TERT mutations in PTMC also exist. A previous report has shown close correlation between TERT mutations and unfavorable outcomes such as recurrence and DM (37), whereas others could not identify the correlation between TERT promoter mutations and PTMC patients’ outcome (36, 39).
Therefore, we have conducted this study to analyze the clinico-pathological and molecular characteristics of Middle Eastern PTMC patients. The aim of this study was to report the clinical outcomes in PTMC and to identify factors that can be predictive of distance metastasis in these patients. If predictive markers could be identified at presentation using pre-surgical fine-needle aspiration specimens, the management of PTMC patients will improve, thereby leading to better surgical and therapeutic interventions.
Materials and Methods
Patient Selection
Two hundred and ten PTMC patients diagnosed between 1988 and 2018 at King Faisal Specialist Hospital and Research Centre (Riyadh, Saudi Arabia) were available to be included in the study. However, molecular data (TERT, RAS and BRAF mutation) were successfully analyzed in 184 cases and hence were included in the study. Cases were identified based on clinical history followed by fine needle aspiration cytology for confirmation. The Institutional Review Board of the hospital approved this study and the Research Advisory Council (RAC) provided waiver of consent under project RAC # 2211168 and # 2110031.
Clinico-Pathological Data
Baseline clinico-pathological data were collected from case records and have been summarized in Table 1. Staging of PTC was performed using the eighth edition of American Joint Committee on Cancer (AJCC) staging system. Based on the 2015 American Thyroid Association (ATA) guidelines, tall cell, hobnail, columnar cell, diffuse sclerosing and insular variants were classified as aggressive variants, whereas classical and follicular variants were classified as non-aggressive variants (40), for multivariate analysis. DM was either synchronous (at the time of diagnosis) or metachronous (developed during follow-up). Of the 11 cases showing DM, four cases were diagnosed by computed tomography (CT) scan and further confirmed by histopathological examination, whereas the remaining seven cases were diagnosed by 131-I whole body scan, which showed iodine absorption in distant metastatic lesions. Only structural recurrence (local, regional or distant) was considered for analysis. Recurrence was defined as any newly detected tumor (local or distant) or metastatic regional lymph node (LN), based on ultrasound and/or imaging studies in patients who had been previously free of disease following initial treatment. Risk stratification was done based on 2015 ATA guidelines, as low-, intermediate- and high-risk PTC.
DNA Isolation
DNA was isolated from formalin-fixed, paraffin-embedded (FFPE) tumor tissues using Gentra DNA isolation kit (Gentra, Minneapolis, MN, USA), following the manufacturer’s recommendations.
PCR and Sanger Sequencing
Primer 3 software was used to design the primers for the two hotspot mutations (C228T and C250T) in promoter region of TERT gene along with entire coding and splicing regions of MAPK genes exons 2 and 3 in HRAS, KRAS and NRAS and exon 15 in BRAF (Supplementary Table 1). PCR was performed in a total volume of 25 µl with 20 ng of genomic DNA, 2.5 µl 10 x Taq buffer, 2.3 mM dNTPs, 1 unit Taq polymerase and 0.2 µM each primer and de-ionized water. The efficiency and quality of the amplified PCR products was confirmed by loading them on a 2% agarose gel.
The PCR products were subsequently subjected to direct sequencing with BigDye terminator V 3.1 cycle sequencing reagents and analyzed on an ABI 3730XL DNA analyzer (Applied Biosystems, Foster City, CA). Reference sequences were downloaded from NCBI GenBank. Sequencing traces were analyzed with the Mutation Surveyor v4.04 (Soft Genetics, LLC, State College, PA).
Follow-Up and Study Endpoint
Patients were regularly followed-up by both physical examinations and imaging studies to identify tumor recurrence. The median follow-up was 9.3 years (range 1.0 – 28.6 years). Metastasis-free survival (MFS) was defined as the time (in months) from date of initial surgery to the occurrence of any DM. In case of no DM, date of last follow-up was the study endpoint for MFS.
Statistical Analysis
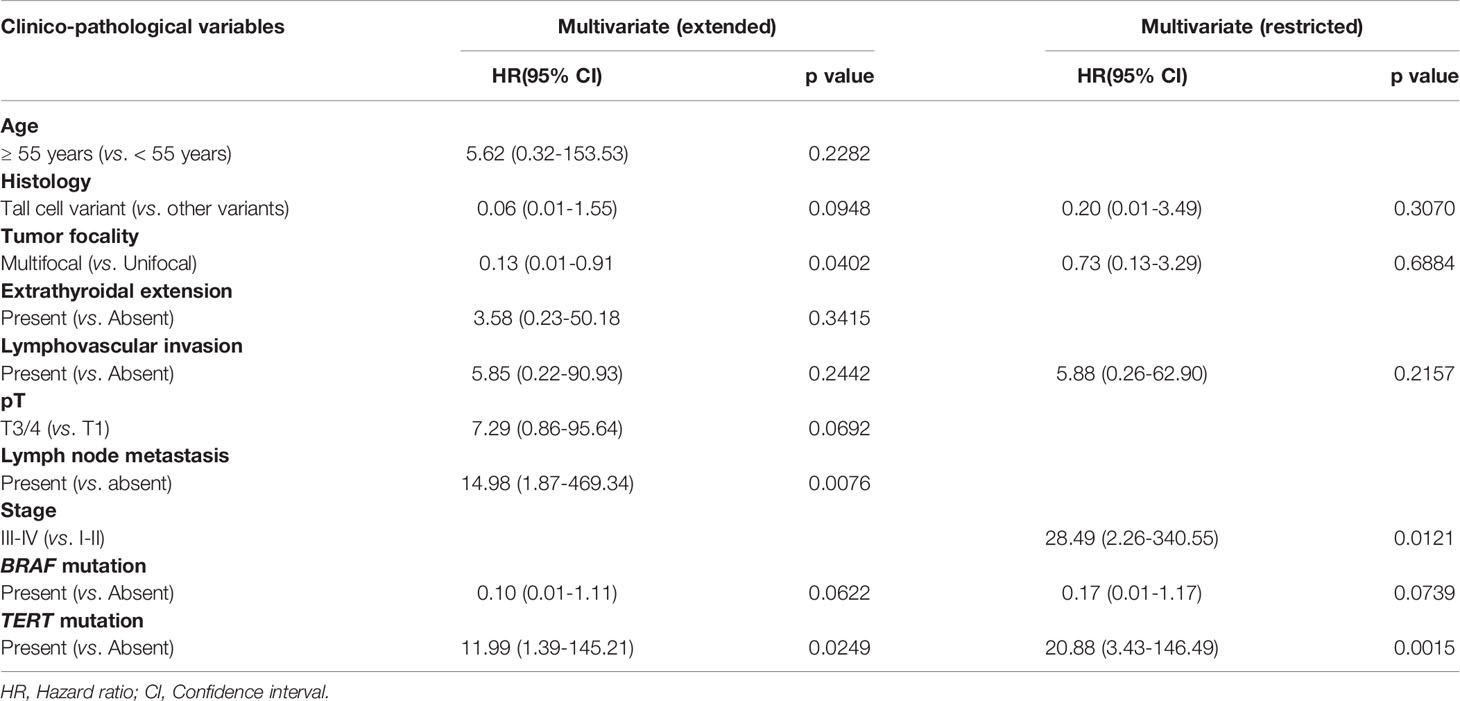
The associations between clinico-pathological variables were performed using contingency table analysis and Chi square tests. Mantel-Cox log-rank test was used to evaluate MFS. Survival curves were generated using the Kaplan-Meier method. Cox proportional hazards model (extended and restricted) and logistic regression was used for multivariate analysis. Since stage is a composite variable (consisting of age, tumor size, extrathyroidal extension, LN metastasis and DM), we performed multivariate Cox proportional hazards by dividing it into two models. The extended model included age, histology, tumor focality, extrathyroidal extension, lymphovascular invasion, pT, lymph node metastasis, BRAF mutation and TERT mutation as co-variates, whereas the restricted model consisted of histology, tumor focality, lymphovascular invasion, stage, BRAF mutation and TERT mutation as co-variates. Backward elimination was applied to select variables for inclusion in the multivariate Cox proportional hazards model. Two-sided tests were used for statistical analyses with a limit of significance defined as p value < 0.05. Data analyses were performed using the JMP14.0 (SAS Institute, Inc., Cary, NC) software package.
Results
Patient and Tumor Characteristics
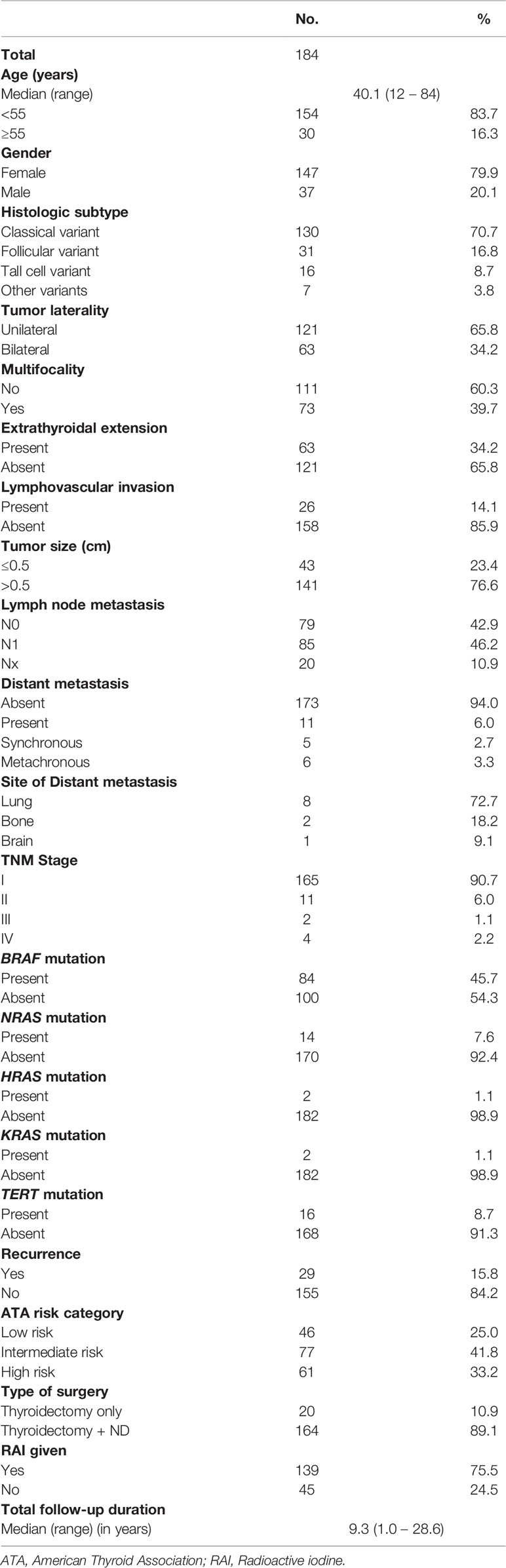
Median age of the study population was 40.1 years (range: 12 – 84 years), with a male to female ratio of 1:4. The majority of tumors were classical variant of PTC (70.7%; 130/184). 34.2% (63/184) of tumors were bilateral and 39.7% (73/184) were multifocal. 34.2% (63/184) of tumors exhibited extrathyroidal extension and 14.1% (26/184) showed lymphovascular invasion. Tumor recurrence was seen in 15.8% (29/184). DM was noted in 6.0% (11/184), with 2.7% (5/184) of them being synchronous and 3.3% (6/184) being metachronous. DM to the lung was most common, accounting for 72.7% (8/11), followed by bone (18.2%; 2/11) and brain (9.1%; 1/11) (Figure 1 and Table 1).
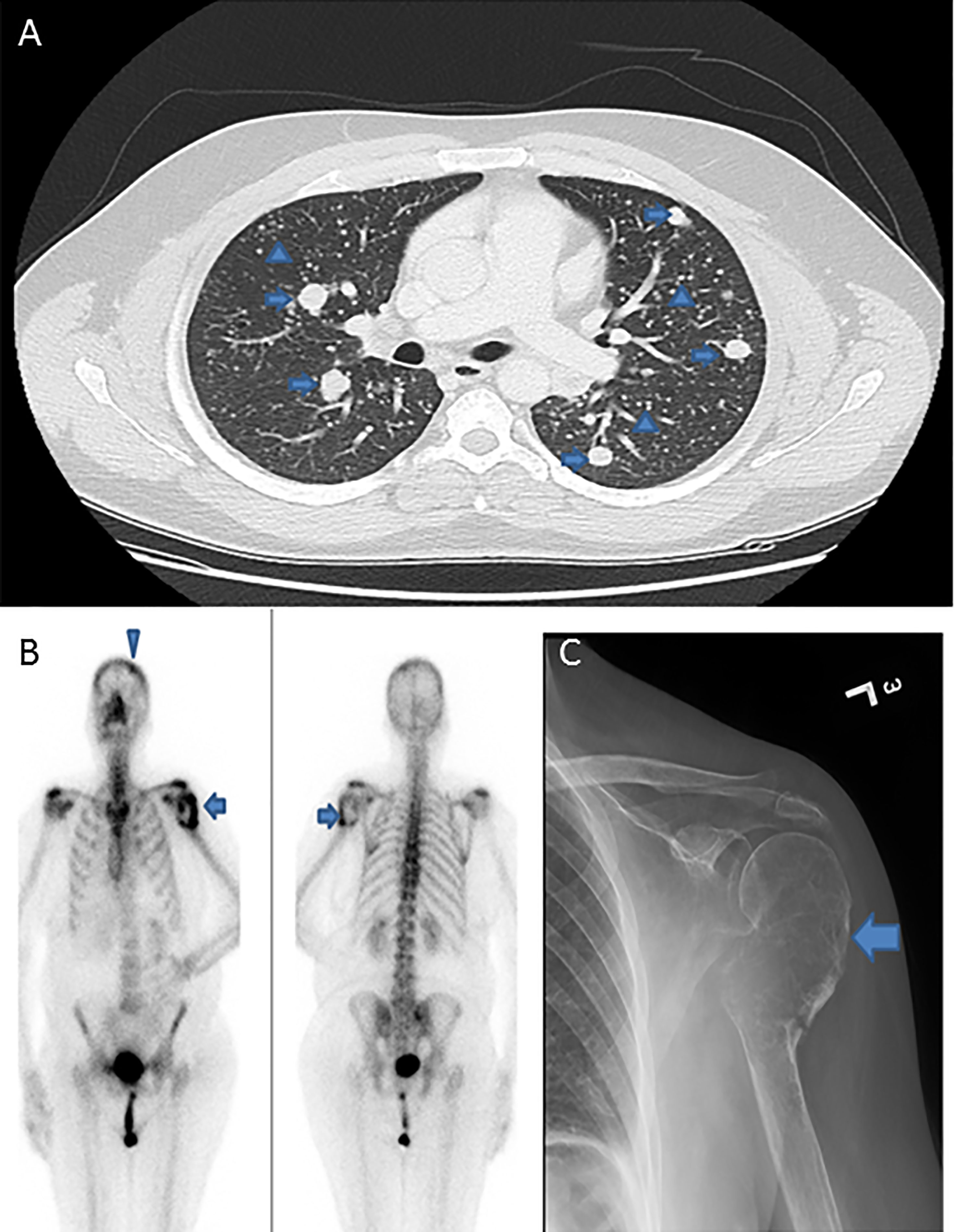
Figure 1 Radiologic images of distant metastasis in papillary thyroid microcarcinoma cases. (A) Computed tomography of the lung showing extensive pulmonary metastases with classic canon-ball metastases (arrows) and miliary metastatic nodules (arrow heads). (B) Positron emission tomography (PET) scan and (C) X-ray, showing expansile lytic metastatic lesion in the left humerus (arrows) with pathologic fracture related to metastasis. PET scan also shows a metastatic focus in the skull bone (arrow head).
Clinico-Pathological Associations of Distant Metastasis in PTMC
DM was noted in 6.0% (11/184) of PTMCs and was significantly associated with male sex (p = 0.0088) and extrathyroidal extension (p = 0.0008). On further analysis with molecular markers, we found that DM in PTMC was associated with TERT mutation (p < 0.0001) but not MAP kinase gene mutations (BRAF, p = 0.1969; NRAS, p = 0.8522; HRAS, p = 0.0801; KRAS, p = 1.0000) (Table 2).
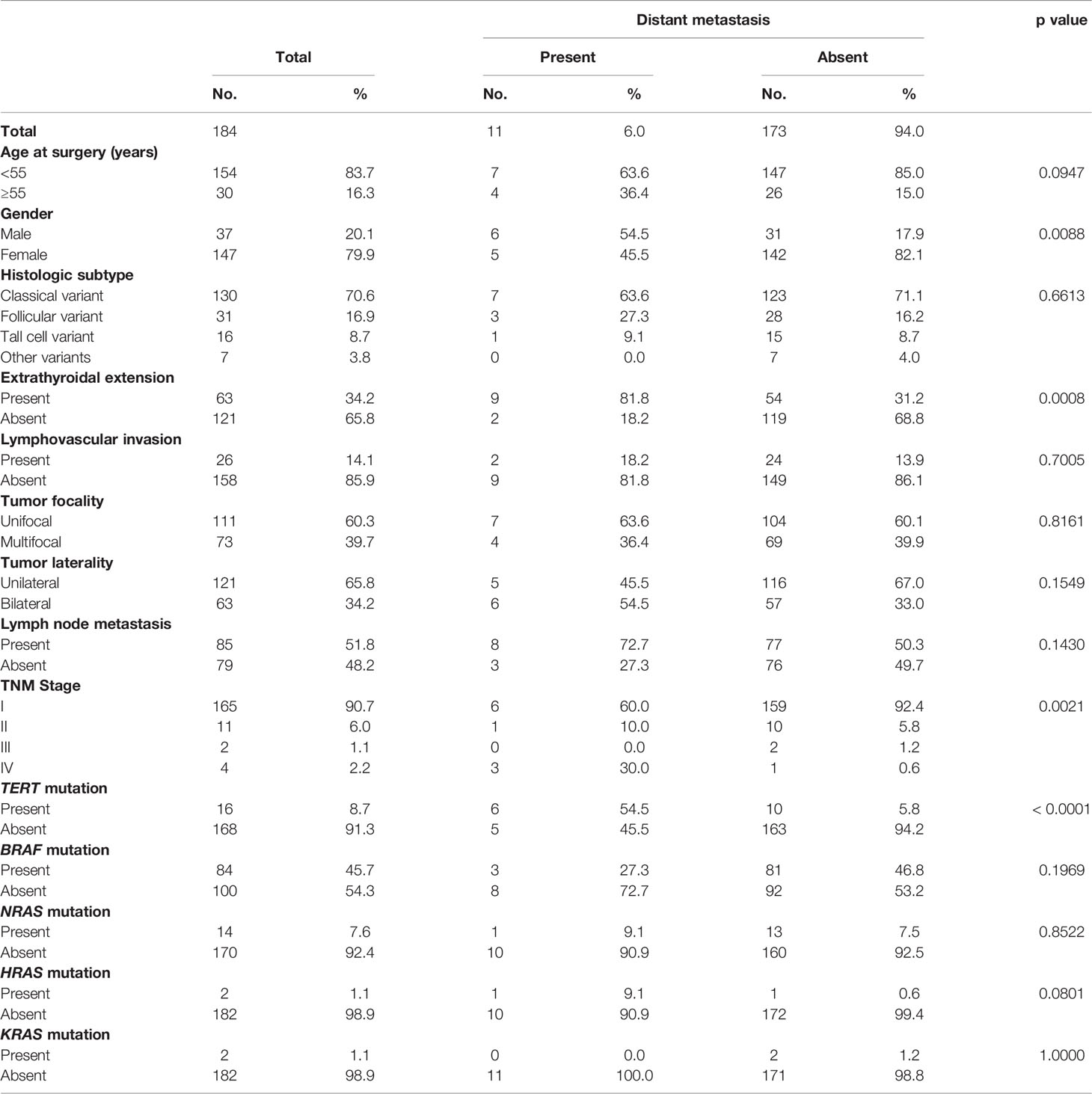
Table 2 Clinico-pathological associations of distant metastasis in papillary thyroid microcarcinoma.
Clinico-Pathological Associations of BRAF and TERT Mutations in PTMC
We next analyzed the clinico-pathological associations of BRAF and TERT mutations in our cohort. BRAF mutation was seen in 45.7% (84/184) of PTMCs. BRAF mutations were significantly associated with tall cell variant of PTC (P = 0.0140), extrathyroidal extension (p = 0.0014), multifocality (p = 0.0004), bilaterality (p = 0.0039) and presence of lymph node metastasis (p = 0.0067). However, no association was seen with DM (p = 0.1969) or tumor recurrence (p = 0.4753) (Supplementary Table 2).
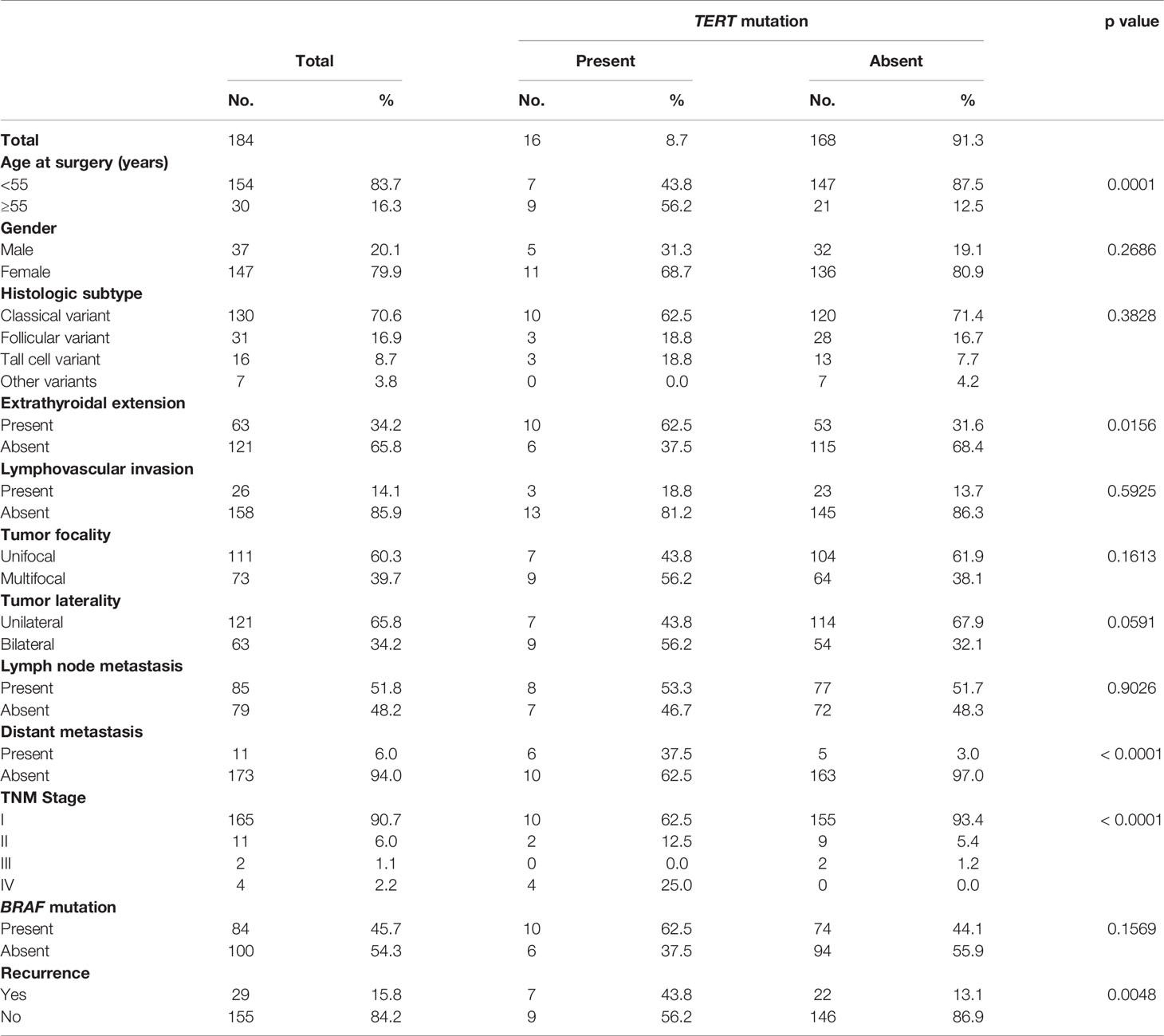
TERT promoter mutations were seen in 8.7% (16/184) of PTMCs and were significantly associated with adverse clinico-pathological characteristics, such as older age (p = 0.0001), extrathyroidal extension (p = 0.0156), DM (p < 0.0001), stage IV tumors (p < 0.0001) and tumor recurrence (p = 0.0048) (Table 3).
TERT Promoter Mutations Are Independent Predictors for Distant Metastasis and Metastasis-Free Survival in PTMC
Given the fact that the frequency of both DM and TERT promoter mutations was relatively high in our cohort and also were significantly associated with each other, we sought to further analyze the relationship between them. Using Mantel Cox log rank test, we found that TERT mutations were associated with shorter metastasis-free survival (p = 0.0002; Figure 2A). Next, we sought to analyze whether this prognostic effect was due to co-existing BRAF mutation. In our PTMC series, coexistence of BRAF and TERT promoter mutations was found in 5.4% (10/184) of the cases. We found that patients with TERT mutation alone had a significantly shorter metastasis-free survival compared to patients with TERT+BRAF mutations (p = 0.0364; Figure 2B), suggesting that TERT mutations had a significantly greater impact on clinical outcome in PTMC.
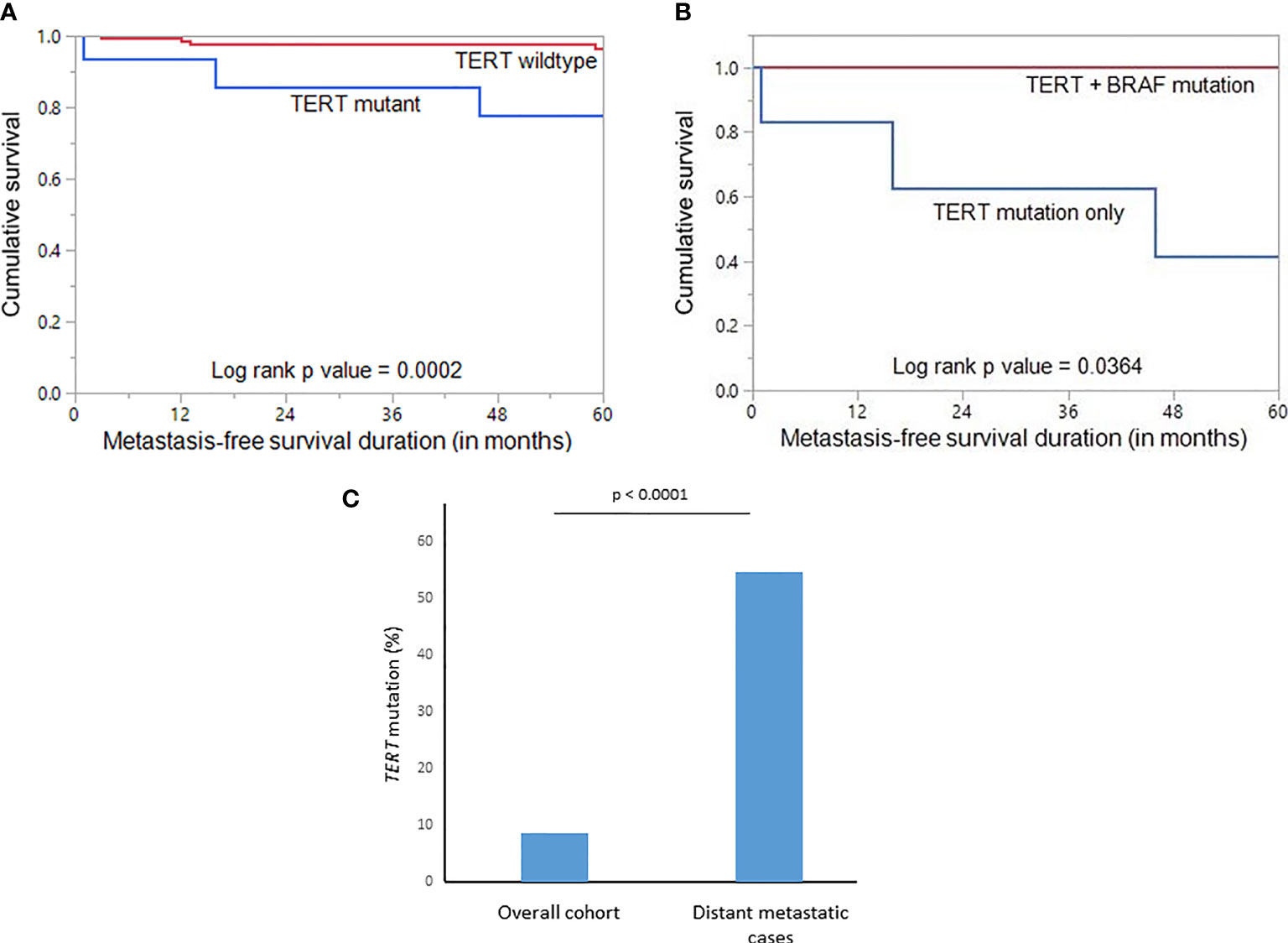
Figure 2 (A) Kaplan-Meier survival curve showing shorter metastasis-free survival in TERT mutant cases compared to TERT wildtype (p = 0.0002). (B) Kaplan-Meier survival curve showing shorter metastasis-free survival in TERT mutant only cases compared to TERT+BRAF mutant cases (p = 0.0364). (C) Significantly higher frequency of TERT mutation was noted in the distant metastatic cases compared to the overall cohort of papillary thyroid microcarcinomas (p < 0.0001).
In multivariate Cox proportional hazards analysis using the extended model, LN metastasis (p = 0.0076) and TERT mutation (p = 0.0249) were found to be significant, whereas in multivariate analysis of the restricted model, only TERT mutation was an independent predictor for metastasis-free survival (Hazard ratio = 20.88, 95% confidence interval = 3.43 – 146.49, p = 0.0015) (Table 4).
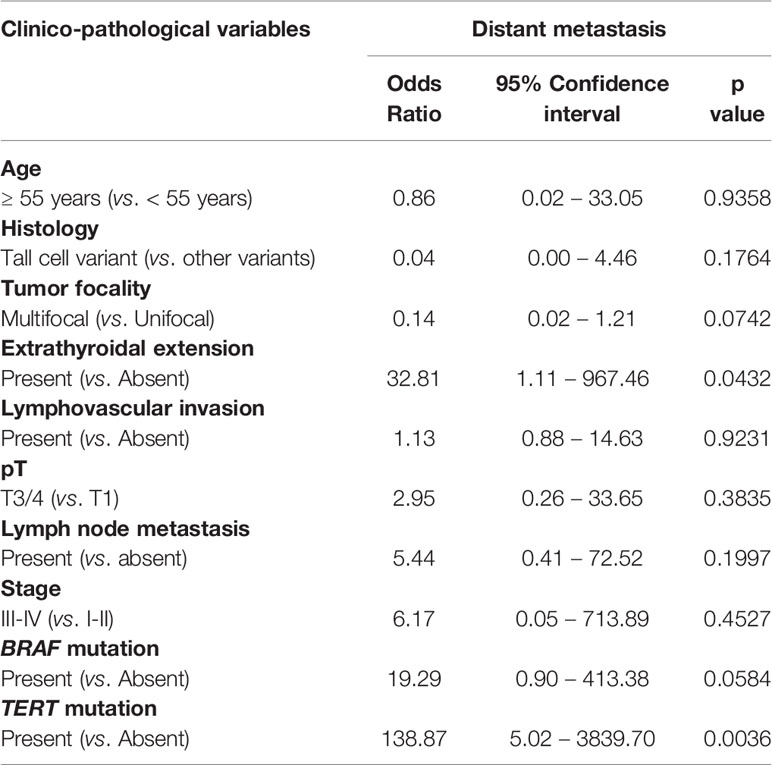
Using multivariate logistic regression analysis, TERT mutation was also found to be an independent predictor for DM (Odds ratio = 138.87, 95% confidence interval = 5.02 – 3839.70, p = 0.0036) (Table 5). To further support the predictive power of TERT mutation for DM, we analyzed the frequency of TERT mutations in the overall cohort and distant metastatic cases alone. We found that the frequency of TERT mutations in the distant metastatic cases was more than 6-fold higher compared to the overall cohort (54.5% vs. 8.7%, p < 0.0001; Figure 2C).
Discussion
PTMC has shown a sharp rise in incidence in recent decades (2–4, 41). Current therapeutic approaches range from active surveillance to surgery and RAI therapy depending on risk stratification of PTC tumors (42–44). Although PTMC is frequently reported to have an excellent outcome (9–11), identifying aggressive PTMC is still challenging. Unfortunately, identifying a way to accurately predict the outcome of PTMC patients is still debatable and has not reached a consensus. We have conducted this current study to identify the incidence, clinical, pathological, and molecular features of Middle Eastern PTMC and detect if any of these characteristics could help to predict tumor behavior.
We analyzed 184 Middle Eastern patients affected by PTMC who were followed for a median duration of 9.2 years. All patients had undergone thyroidectomy, with 75.5% (139/184) of patients receiving RAI therapy. Although we observed aggressive clinical features in 63.6% of the PTMCs, our cohort had a favorable prognosis as a whole (5-year cancer-specific survival was 100% and 5-year disease-free survival was 80.3%). Nevertheless, persistent disease was found in 12.1% of PTMC patients and recurrence was observed in 15.8%, mostly loco-regional, with a mean duration of 4.2 years. This relatively high incidence of recurrence could be explained by the fact that nearly one-third of patients presented with ATA high risk disease, which is not seen in most modern studies from Western population and may be partially attributed to genetics or differences in presentation and access to health care.
Interestingly, incidence of DM in this cohort was 6% among PTMC patients (3.3% patients developed DM after a median of 3.2 years of initial diagnosis while 2.7% presented with DM at diagnosis). This relatively high incidence rate among Middle Eastern PTMC patients is in contrast to previous reports from other ethnicities, where DM from PTMC was very rare (10, 12, 45–48). We were intrigued by the occurrence of distance metastasis at this relatively high rate. Therefore, we further characterized the clinico-pathological and molecular factors that are associated with DM in PTMC. We identified male sex and extrathyroidal extension as risk factors for DM in PTMC. The majority of DM (72.7%; 8/11) were seen in the lungs. This is of clinical importance since it suggests that PTMC should be considered in the differential diagnosis for primary cancer when metastatic lesions from an unknown origin are seen.
Furthermore, genetic profile analysis of PTMCs showed that the most common mutations were MAP kinase pathway gene alterations (BRAF mutation in 45.7%, followed by NRAS mutation in 7.6%). BRAF mutation in PTC from the Middle East has been reported to range from 40% - 71% (49–51), with the corresponding frequency in PTMC ranging from 18% - 72% (52–54) in published literature from this region, which is in concordance with our findings. Our study demonstrated a significant correlation between BRAF mutations and aggressive histopathological features such as tall cell variant, bilaterality, multifocality, extrathyroidal extension and LN metastasis. However, no association with DM or clinical outcome was found in this cohort. Previous studies have rarely analyzed genes other than MAP kinase pathway genes (31, 33). Several studies have reported that mutations in TERT promoter are associated with aggressive clinico-pathological features in PTC, especially if they co-exist with BRAF mutations (55–57). However, the significance of TERT promoter mutations in predicting aggressiveness of PTMC has not been fully illustrated and requires further investigation.
In the present study, mutations of TERT promoter were successfully analyzed in 184 PTMCs and the prevalence was 8.7%. This finding is surprising, considering the fact that TERT mutations are late events in thyroid carcinogenesis and their presence is usually seen in large and more advanced PTCs. The prevalence of TERT promoter mutations was relatively high compared to previous studies in the literature from PTMC patients of different ethnic backgrounds. De Biase et al. (36) have reported the prevalence of TERT promoter mutations in 4.7% (19/404) PTMC from Italy, whereas Sama et al. (45) found the prevalence to be 2% (2/100) in PTMC from the same population. Three other studies from Asian population reported a frequency of 3.2% (16/504) from Korea (39), 1.2% (1/86) from China (37) and 0% (0/26) from Japan (38). We have previously reported a frequency of 18.0% for TERT promoter mutations in a large cohort of PTCs (n = 927) (55). However, data on incidence of TERT mutations in PTMC from Middle eastern ethnicity is lacking. These differences in the mutation frequency could be attributed to various factors, including cohort size, environmental factors as well as geographical and ethnic variations.
None of the above studies have shown significant association between TERT promoter mutation and aggressive clinico-pathological characteristics in PTMC. In contrast, the present study showed TERT promoter mutations were significantly associated with aggressive clinico-pathological features, such as older age, extrathyroidal extension, tumor recurrence and DM. In fact, we demonstrated using Cox regression analysis that TERT promoter mutations were an independent prognostic marker for MFS. TERT mutations were significant in both the extended and restricted models, which confirms the prognostic value of TERT promoter mutations as an independent marker for MFS. In addition, we also found that TERT promoter mutations were an independent predictive marker for DM in PTMC using logistic regression analysis. Supporting this novel finding in this ethnicity, we further analyzed the frequency of TERT promoter mutations in distant metastatic PTMC. Upon comparing the frequency of TERT promoter mutations in the original unselected cohort and PTMC with documented DM, we found TERT promoter mutations frequency increased by more than 6-fold in the latter group (54.5% vs. 8.7% p < 0.0001). Similar findings have been reported in PTC by Melo et al. (58) and others (37, 55, 59), where they show that TERT promoter mutations are a marker for DM. Studies by Povoa et al. (60) and Melo et al. (61) analyzed the association of TERT and BRAF mutations with distant metastasis in PTC. They found that while TERT mutations were associated with distant metastasis, BRAF mutations were not, which is in concordance with our findings. We also found that patients with TERT mutation alone had a significantly shorter metastasis-free survival compared to patients with coexisting TERT and BRAF mutations. Taken together, these data suggest that presence of TERT promoter mutations in PTMC might reflect true aggressive biological subtype of PTC in this ethnicity. Additional larger studies are required to confirm this assumption.
Despite the limitations of this study being a retrospective single institute study, and the relatively small number of cases, it has several strengths, including the unique PTMC cohort from Middle Eastern ethnicity, long follow-up time and comprehensive follow-up information on recurrence as well as detailed histopathological and molecular information. However, owing to the aggressive behavior of PTMCs in our cohort, the finding may not be applicable to general PTMCs. Further confirmation with a larger sample size is encouraged.
In conclusion, patients with PTMC from this ethnicity harbor aggressive features and may develop distance metastasis, in particular lung metastases. Screening for distant metastasis should be considered, especially in patients harboring TERT promoter mutations. The available evidence indicates TERT promoter mutations may have great value in improving the risk stratification and predication DM and MFS of patients with aggressive PTMC. Therefore, mutation in the TERT promoter may be an important factor in the genetic background, driving aggressiveness of PTMC from this ethnicity and detection of such mutations may help in the accurate identification and management of PTMC in this population.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Advisory Council, King Faisal Specialist Hospital and Research Centre. Written informed consent for participation was not provided by the participants’ legal guardians/next of kin because: Since only retrospective clinical data was utilized and patients were de-identified, a waiver of written consent was granted for this study.
Author Contributions
Study concept and design: KA-K, SP, and AS. Executed the study: SP, AS, KI, SA, MA-R, AA, SA-S, and FA-D. Statistical analysis: ZQ. Drafting the article: KA-K, SA, AS, and SP. Critical revision of the article for important intellectual content, writing of the article, and approval of the final version: KA-K, SP, AS, KI, ZQ, SA, MA-R, AA, SA-S, and FA-D. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Felisa DeVera and Padmanaban Annaiyappanaidu for their technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.808298/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Pereira M, Williams VL, Hallanger Johnson J, Valderrabano P. Thyroid Cancer Incidence Trends in the United States: Association With Changes in Professional Guideline Recommendations. Thyroid (2020) 30(8):1132–40. doi: 10.1089/thy.2019.0415
3. Kitahara CM, Sosa JA. The Changing Incidence of Thyroid Cancer. Nat Rev Endocrinol (2016) 12(11):646–53. doi: 10.1038/nrendo.2016.110
4. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. Jama (2017) 317(13):1338–48. doi: 10.1001/jama.2017.2719
5. Asa SL. The Current Histologic Classification of Thyroid Cancer. Endocrinol Metab Clinics (2019) 48(1):1–22. doi: 10.1016/j.ecl.2018.10.001
6. Chen AY, Jemal A, Ward EM. Increasing Incidence of Differentiated Thyroid Cancer in the United States, 1988–2005. Cancer: Interdiscip Int J Am Cancer Soc (2009) 115(16):3801–7. doi: 10.1002/cncr.24416
7. Brito JP, Davies L. Is There Really an Increased Incidence of Thyroid Cancer? Curr Opin Endocrinology Diabetes Obes (2014) 21(5):405–8. doi: 10.1097/MED.0000000000000094
8. Franceschi S, Vaccarella S. Thyroid Cancer: An Epidemic of Disease or an Epidemic of Diagnosis? Int J Cancer (2015) 136(11):2738–9. doi: 10.1002/ijc.29311
9. Kuo EJ, Roman SA, Sosa JA. Patients With Follicular and Hurthle Cell Microcarcinomas Have Compromised Survival: A Population Level Study of 22,738 Patients. Surgery (2013) 154(6):1246–54. doi: 10.1016/j.surg.2013.04.033
10. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient Age is Significantly Related to the Progression of Papillary Microcarcinoma of the Thyroid Under Observation. Thyroid (2014) 24(1):27–34. doi: 10.1089/thy.2013.0367
11. Wang TS, Goffredo P, Sosa JA, Roman SA. Papillary Thyroid Microcarcinoma: An Over-Treated Malignancy? World J Surg (2014) 38(9):2297–303. doi: 10.1007/s00268-014-2602-3
12. Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three Distinctly Different Kinds of Papillary Thyroid Microcarcinoma Should be Recognized: Our Treatment Strategies and Outcomes. World J Surg (2010) 34(6):1222–31. doi: 10.1007/s00268-009-0359-x
13. Shimizu T, Oba T, Chino T, Soma A, Ono M, Ito T, et al. Papillary Thyroid Microcarcinoma With Lung Metastases: A Case Report and Review of the Literature. Thyroid Res (2021) 14(1):1–7. doi: 10.1186/s13044-021-00106-0
14. Kim K-E, Kim E-K, Yoon JH, Han KH, Moon HJ, Kwak JY. Preoperative Prediction of Central Lymph Node Metastasis in Thyroid Papillary Microcarcinoma Using Clinicopathologic and Sonographic Features. World J Surg (2013) 37(2):385–91. doi: 10.1007/s00268-012-1826-3
15. Mercante G, Frasoldati A, Pedroni C, Formisano D, Renna L, Piana S, et al. Prognostic Factors Affecting Neck Lymph Node Recurrence and Distant Metastasis in Papillary Microcarcinoma of the Thyroid: Results of a Study in 445 Patients. Thyroid (2009) 19(7):707–16. doi: 10.1089/thy.2008.0270
16. Noguchi S, Yamashita H, Uchino S, Watanabe S. Papillary Microcarcinoma. World J Surg (2008) 32(5):747–53. doi: 10.1007/s00268-007-9453-0
17. Pazaitou-Panayiotou K, Capezzone M, Pacini F. Clinical Features and Therapeutic Implication of Papillary Thyroid Microcarcinoma. Thyroid (2007) 17(11):1085–92. doi: 10.1089/thy.2007.0005
18. Alrawaji, Alshahrani, Alzahrani, Alomran, Almadouj, Alshehri, et al. Cancer Incidence Report Saudi Arabia 2015. Council SH, editor. Riyadh: Saudi Cancer Registry (2018).
19. Alzahrani AS, Alomar H, Alzahrani N. Thyroid Cancer in Saudi Arabia: A Histopathological and Outcome Study. Int J Endocrinology (2017) 2017. doi: 10.1155/2017/8423147
20. Alhozali A, Al-Ghamdi A, Alahmadi J. Pattern of Thyroid Cancer at King Abdulaziz University Hospital, Jeddah: A 10-Year Retrospective Study. Open J Endocrine Metab Dis (2016) 6(3):121–5. doi: 10.4236/ojemd.2016.63016
21. Geron Y, Benbassat C, Shteinshneider M, Or K, Markus E, Hirsch D, et al. Multifocality Is Not an Independent Prognostic Factor in Papillary Thyroid Cancer: A Propensity Score–Matching Analysis. Thyroid (2019) 29(4):513–22. doi: 10.1089/thy.2018.0547
22. Hirshoren N, Kaganov K, Weinberger JM, Glaser B, Uziely B, Mizrahi I, et al. Thyroidectomy Practice After Implementation of the 2015 American Thyroid Association Guidelines on Surgical Options for Patients With Well-Differentiated Thyroid Carcinoma. JAMA Otolaryngology–Head Neck Surg (2018) 144(5):427–32. doi: 10.1001/jamaoto.2018.0042
23. Al-Qahtani KH, Tunio MA, Al Asiri M, Bayoumi Y, Balbaid A, Aljohani NJ, et al. Comparative Clinicopathological and Outcome Analysis of Differentiated Thyroid Cancer in Saudi Patients Aged Below 60 Years and Above 60 Years. Clin Interventions Aging (2016) 11:1169. doi: 10.2147/CIA.S107881
24. Al-Zaher N, Al-Salam S, El Teraifi H. Thyroid Carcinoma in the United Arab Emirates: Perspectives and Experience of a Tertiary Care Hospital. Hematology/Oncology Stem Cell Ther (2008) 1(1):14–21. doi: 10.1016/S1658-3876(08)50055-0
25. Keinan-Boker L, Silverman BG. Trends of Thyroid Cancer in Israel: 1980–2012. Rambam Maimonides Med J (2016) 7(1):e0001. doi: 10.5041/RMMJ.10228
26. Gul K, Ozdemir D, Ersoy R, Aydin C, Erkan A, Ersoy PE, et al. Comparison of Papillary Thyroid Microcarcinoma and Carcinoma/Papiller Tiroid Mikrokarsinom Ve Karsinomun Karsilastirilmasi. Turkish J Endocrinol Metab (2009) 13:47–52.
27. Zaballos MA, Santisteban P. Key Signaling Pathways in Thyroid Cancer. J Endocrinol (2017) 235(2):R43–61. doi: 10.1530/JOE-17-0266
28. Fukushima T, Takenoshita S. Roles of RAS and BRAF Mutations in Thyroid Carcinogenesis. Fukushima J Med Sci (2005) 51(2):67–75. doi: 10.5387/fms.51.67
29. Agrawal N, Akbani R, Aksoy BA, Ally A, Arachchi H, Asa SL, et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell (2014) 159(3):676–90. doi: 10.1016/j.cell.2014.09.050
30. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and Transcriptomic Hallmarks of Poorly Differentiated and Anaplastic Thyroid Cancers. J Clin Invest (2016) 126(3):1052–66. doi: 10.1172/JCI85271
31. Jeon MJ, Chun SM, Lee J-Y, Choi KW, Kim D, Kim TY, et al. Mutational Profile of Papillary Thyroid Microcarcinoma With Extensive Lymph Node Metastasis. Endocrine (2019) 64(1):130–8. doi: 10.1007/s12020-019-01842-y
32. Li F, Chen G, Sheng C, Gusdon AM, Huang Y, Lv Z, et al. BRAFV600E Mutation in Papillary Thyroid Microcarcinoma: A Meta-Analysis. Endocrine-related Cancer (2015) 22(2):159. doi: 10.1530/ERC-14-0531
33. Castro B, Rodrigues E. Molecular Biology of Papillary Thyroid Microcarcinomas: What Is New? Rev Portuguesa Endocrinologia Diabetes e Metabolismo (2016) 11(2):287–95. doi: 10.1016/j.rpedm.2016.04.003
34. Huang K, Gao N, Bian D, Zhai Q, Yang P, Zhang Y. Associations of BRAF V600E, Clinical Pathology and Imaging Factors With the Recurrence Rate of Papillary Thyroid Microcarcinoma. Exp Ther Med (2020) 20(6):1–. doi: 10.3892/etm.2020.9373
35. Lin K-L, Wang O-C, Zhang X-H, Dai X-X, Hu X-Q, Qu J-M. The BRAF Mutation Is Predictive of Aggressive Clinicopathological Characteristics in Papillary Thyroid Microcarcinoma. Ann Surg Oncol (2010) 17(12):3294–300. doi: 10.1245/s10434-010-1129-6
36. De Biase D, Gandolfi G, Ragazzi M, Eszlinger M, Sancisi V, Gugnoni M, et al. TERT Promoter Mutations in Papillary Thyroid Microcarcinomas. Thyroid (2015) 25(9):1013–9. doi: 10.1089/thy.2015.0101
37. Liu R, Li Y, Chen W, Cong J, Zhang Z, Ma L, et al. Mutations of the TERT Promoter Are Associated With Aggressiveness and Recurrence/Distant Metastasis of Papillary Thyroid Carcinoma. Oncol Lett (2020) 20(4):1–. doi: 10.3892/ol.2020.11904
38. Yabuta T, Matsuse M, Hirokawa M, Yamashita S, Mitsutake N, Miyauchi A. TERT Promoter Mutations Were Not Found in Papillary Thyroid Microcarcinomas That Showed Disease Progression on Active Surveillance. Thyroid (2017) 27(9):1206–7. doi: 10.1089/thy.2016.0645
39. Lee J, Ha EJ, Roh J, Kim HK. Presence of TERT ± BRAF V600E Mutation is Not a Risk Factor for the Clinical Management of Patients With Papillary Thyroid Microcarcinoma. Surgery (2021) 170(3):743–7. doi: 10.1016/j.surg.2021.03.056
40. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
41. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide Increasing Incidence of Thyroid Cancer: Update on Epidemiology and Risk Factors. J Cancer Epidemiol (2013) 2013. doi: 10.1155/2013/965212
42. Brito JP, Hay ID. Management of Papillary Thyroid Microcarcinoma. Endocrinol Metab Clinics (2019) 48(1):199–213. doi: 10.1016/j.ecl.2018.10.006
43. Miyauchi A, Ito Y, Oda H. Insights Into the Management of Papillary Microcarcinoma of the Thyroid. Thyroid (2018) 28(1):23–31. doi: 10.1089/thy.2017.0227
44. Walgama E, Sacks WL, Ho AS. Papillary Thyroid Microcarcinoma: Optimal Management Versus Overtreatment. Curr Opin Oncol (2020) 32(1):1–6. doi: 10.1097/CCO.0000000000000595
45. Samà MT, Grosso E, Mele C, Laurora S, Monzeglio O, Marzullo P, et al. Molecular Characterisation and Clinical Correlation of Papillary Thyroid Microcarcinoma. Endocrine (2021) 71(1):149–57. doi: 10.1007/s12020-020-02380-8
46. Al-Qurayshi Z, Nilubol N, Tufano RP, Kandil E. Wolf in Sheep’s Clothing: Papillary Thyroid Microcarcinoma in the US. J Am Coll Surgeons (2020) 230(4):484–91. doi: 10.1016/j.jamcollsurg.2019.12.036
47. Yu X-M, Wan Y, Sippel RS, Chen H. Should All Papillary Thyroid Microcarcinomas Be Aggressively Treated? An Analysis of 18,445 Cases. Ann Surg (2011) 254(4):653–60. doi: 10.1097/SLA.0b013e318230036d
48. Jeon MJ, Kim WG, Choi YM, Kwon H, Lee Y-M, Sung T-Y, et al. Features Predictive of Distant Metastasis in Papillary Thyroid Microcarcinomas. Thyroid (2016) 26(1):161–8. doi: 10.1089/thy.2015.0375
49. Zarkesh M, Zadeh-Vakili A, Azizi F, Fanaei SA, Foroughi F, Hedayati M. The Association of BRAF V600E Mutation With Tissue Inhibitor of Metalloproteinase-3 Expression and Clinicopathological Features in Papillary Thyroid Cancer. Int J Endocrinol Metab (2018) 16(2):e56120. doi: 10.5812/ijem.56120
50. Al-Salam S, Sharma C, Afandi B, Al Dahmani K, Al-Zahrani AS, Al Shamsi A, et al. BRAF and KRAS Mutations in Papillary Thyroid Carcinoma in the United Arab Emirates. PloS One (2020) 15(4):e0231341. doi: 10.1371/journal.pone.0231341
51. Al-Masri M, Al-Shobaki T, Al-Najjar H, Iskanderian R, Younis E, Abdallah N, et al. BRAF V600E Mutation in Papillary Thyroid Carcinoma: It’s Relation to Clinical Features and Oncologic Outcomes in a Single Cancer Centre Experience. Endocrine connections (2021) 10(12):1531–7. doi: 10.1530/EC-21-0410
52. Schulten H-J, Salama S, Al-Mansouri Z, Alotibi R, Al-Ghamdi K, Al-Hamour OA, et al. BRAF Mutations in Thyroid Tumors From an Ethnically Diverse Group. Hereditary Cancer Clin Pract (2012) 10(1):1–7. doi: 10.1186/1897-4287-10-10
53. Kurtulmus N, Duren M, Ince U, Yakicier MC, Peker O, Aydın O, et al. BRAF V600E Mutation in Turkish Patients With Papillary Thyroid Cancer: Strong Correlation With Indicators of Tumor Aggressiveness. Endocrine (2012) 42(2):404–10. doi: 10.1007/s12020-012-9651-x
54. Fakhruddin N, Jabbour M, Novy M, Tamim H, Bahmad H, Farhat F, et al. BRAF and NRAS Mutations in Papillary Thyroid Carcinoma and Concordance in BRAF Mutations Between Primary and Corresponding Lymph Node Metastases. Sci Rep (2017) 7(1):1–11. doi: 10.1038/s41598-017-04948-3
55. Bu R, Siraj AK, Divya SP, Kong Y, Parvathareddy SK, Al-Rasheed M, et al. Telomerase Reverse Transcriptase Mutations are Independent Predictor of Disease-Free Survival in M Iddle E Astern Papillary Thyroid Cancer. Int J Cancer (2018) 142(10):2028–39. doi: 10.1002/ijc.31225
56. Liu T, Wang N, Cao J, Sofiadis A, Dinets A, Zedenius J, et al. The Age-and Shorter Telomere-Dependent TERT Promoter Mutation in Follicular Thyroid Cell-Derived Carcinomas. Oncogene (2014) 33(42):4978–84. doi: 10.1038/onc.2013.446
57. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT Promoter Mutations Cooperatively Identify the Most Aggressive Papillary Thyroid Cancer With Highest Recurrence. J Clin Oncol (2014) 32(25):2718. doi: 10.1200/JCO.2014.55.5094
58. Melo M, da Rocha AG, Vinagre J, Batista R, Peixoto J, Tavares C, et al. TERT Promoter Mutations Are a Major Indicator of Poor Outcome in Differentiated Thyroid Carcinomas. J Clin Endocrinol Metab (2014) 99(5):E754–E65. doi: 10.1210/jc.2013-3734
59. Liu C, Liu Z, Chen T, Zeng W, Guo Y, Huang T. TERT Promoter Mutation and Its Association With Clinicopathological Features and Prognosis of Papillary Thyroid Cancer: A Meta-Analysis. Sci Rep (2016) 6(1):1–9. doi: 10.1038/srep36990
60. Póvoa AA, Teixeira E, Bella-Cueto MR, Batista R, Pestana A, Melo M, et al. Genetic Determinants for Prediction of Outcome of Patients With Papillary Thyroid Carcinoma. Cancers (2021) 13(9):2048. doi: 10.3390/cancers13092048
Keywords: papillary thyroid microcarcinoma, TERT promoter mutations, distant metastasis, metastasis-free survival, predictor
Citation: Parvathareddy SK, Siraj AK, Iqbal K, Qadri Z, Ahmed SO, Al-Rasheed M, AlQatie AA, Al-Sobhi SS, Al-Dayel F and Al-Kuraya KS (2022) TERT Promoter Mutations Are an Independent Predictor of Distant Metastasis in Middle Eastern Papillary Thyroid Microcarcinoma. Front. Endocrinol. 13:808298. doi: 10.3389/fendo.2022.808298
Received: 03 November 2021; Accepted: 11 February 2022;
Published: 11 March 2022.
Edited by:
Jing Yang, Sichuan University, ChinaCopyright © 2022 Parvathareddy, Siraj, Iqbal, Qadri, Ahmed, Al-Rasheed, AlQatie, Al-Sobhi, Al-Dayel and Al-Kuraya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khawla S. Al-Kuraya, a2t1cmF5YUBrZnNocmMuZWR1LnNh
†These authors have contributed equally to this work
 Sandeep Kumar Parvathareddy1†
Sandeep Kumar Parvathareddy1† Abdul K. Siraj
Abdul K. Siraj Khawla S. Al-Kuraya
Khawla S. Al-Kuraya