- 1Department of Medical Imaging, the First Affiliated Hospital of Kunming Medical University, Kunming, China
- 2Department of Radiology, Beijing Jishuitan Hospital, Beijing, China
- 3GE Healthcare, Magnetic Resonance Field Application Team, Chengdu, China
- 4GE Healthcare, Magnetic Resonance Research China, Beijing, China
There is an interaction between the lumbar spine and paraspinal muscles, which may play a role in the development of intervertebral disc (IVD) degeneration and may affect CLBP. The study aims to assess the relationship between IVD degeneration and paraspinal muscle fat infiltration in CLBP patients by quantitative MR imaging, and to evaluate the influence of sex and age on CLBP muscle fat infiltration. Sixty CLBP patients (46.3 years ±17.0) and thirty-two healthy subjects (44.9 years ±17.6) were recruited for this study. 3.0 T MRI was used to perform the sagittal and axial T1, T2 of the lumbar spine, and axial paraspinal muscle IDEAL imaging at the L4/5 and L5/S1 levels. Proton density fat fraction (PDFF) of the multifidus and erector spinae at two IVD levels were measured. The Pfirrmann grades of IVD degeneration, Oswestry Disability Index (ODI), and Visual Analog Scale (VAS) were also evaluated. Compare the cross-sectional area (CSA) and PDFF of the paraspinal muscles between CLBP patients and healthy subjects, and analyze the relationship between the muscle PDFF and Pfirrmann grades, gender, and age of CLBP patients. Compared with healthy subjects, the CSA of the multifidus muscle in CLBP patients decreased (1320.2±188.1mm2 vs. 1228.7±191.0 mm2, p<0.05) at the L4/5 level, the average PDFF increased, (7.7±2.6% vs. 14.79±5.3%, 8.8±4.2% vs. 16.03±5.3%, all p<0.05) at both L4/5 and L5/S1 levels. The PDFF of paraspinal muscles were correlated with adjacent IVD degeneration, ODI and VSA in CLBP patients (all p<0.05). After using age and body mass index (BMI) as control variables, significance was retained (all p<0.05). Multiple regression analysis revealed sex and age also were significantly associated with multifidus PDFF (all p < 0.05). This study confirmed that the CSA decreased and the PDFF increased of the paraspinal muscles in CLBP patients. It reveals a significant correlation between the PDFF of CLBP paraspinal muscles and the grade of IVD degeneration. Sex and age are also important factors influencing CLBP paraspinal muscle infiltration.
Introduction
Low back pain (LBP) has become a global challenge with tremendous economic burden for society and public health systems (1, 2). The lifetime prevalence of LBP is reported to be as high as 84%, with chronic low back pain (CLBP) accounting for approximately 23% of LBP (3). Furthermore, more than 10% of patients with LBP develop severe disabilities (4). The diversity and complexity of etiology limit the prevention and treatment strategies of LBP. Intervertebral disc (IVD) degeneration refers to the physiological and pathological process of natural degeneration and aging of the IVD, in which structural damage causes the degeneration of the disc and the surrounding area (5). IVD degeneration is the basis of various clinical spinal diseases, for example, annulus tears, instability of the spine, degeneration in the facet joints, disc herniation, spinal stenosis and CLBP (5, 6). And IVD degeneration is usually considered as the leading cause of CLBP, especially at the L4-S1 level, but the treatments are mainly limited to partial symptomatic relief (7, 8).
The paraspinal muscles (multifidus, erector spinae, and psoas) are essential determinants of the structural stability and functions of the lumbar spine (9). Previous animal and human studies suggested that increased myoelectric activity and structural remodeling of muscles (e.g. muscle atrophy, fat infiltration, and fiber type changes) were associated with CLBP (10–14). Given the important role of paraspinal muscles on the lumbar spine, muscle lesions may worsen CLBP. It is crucial to study the interactions between paraspinal muscle changes and CLBP, but they are often underestimated. In addition, it is unclear whether the degeneration of the lumbar IVD is related to increased fatty infiltration within the paraspinal muscles in CLBP patients.
Previous studies had reported the muscle cross-sectional area (CSA) and fat content of the paraspinal muscles in CLBP patients (15–18). Based on anatomical imaging, the CSA variable of muscles is routinely preferred (19), which can be used as a structural measure of muscle hypertrophy or atrophy. The assessments of fat infiltration were mainly based on the decrease of CT attenuation values (14–16, 20, 21) or the increase of the relative signal intensity on the conventional MRI T1 and T2 images (17, 22, 23). There is no published literature on CLBP related to the intramuscular adipose tissue in CLBP study. In recent years, an advanced chemical shift encoding-based water-fat MRI has been used for non-invasive quantitative assessment of fat and water signals in various parts of the human body (24–26), such as available Iterative Decomposition of water and fat with Echo Asymmetry and Least Square Estimation (IDEAL-IQ). Proton density fat fraction (PDFF) outcomes can be obtained with high resolution and high accuracy from IDEAL-IQ (25). This method is considered to be a reliable measurement method comparable to MR spectroscopy (the gold standard method in vivo) for quantifying fat infiltration in muscles (27–29). Sollmann et al. showed that the PDFF measurement after paraspinal muscle segmentation is a potential biomarker for muscle changes in the future (30). Furthermore, Zhao and Patzelt et al. found a negative correlation between the PDFF of paraspinal muscles and the bone mineral density of the lumbar spine, and the progress and severity of tumor cachexia can be monitored through the PDFF of the paraspinal muscles (31, 32). Therefore, PDFF help accurately quantify the fat content, especially intramuscular lipids in the paraspinal muscles of CLBP, and further explore the relationship between IVD degeneration and paraspinal muscle remodeling in CLBP patients. Furthermore, results of previous studies reveal that a decrease in the multifidus CSA, a decrease of muscle density, and a decrease in the size of Type I and Type II/MHC-2X fibers and interstitial fibrosis in patients with intervertebral herniation. And the fat infiltration may be associated with these muscle changes (14, 33, 34). We hypothesized that paraspinal muscle CSA and fat content are changed in CLBP patients compared to healthy subjects, significantly, and are associated with the degeneration of the adjacent IVD.
The purpose of our study was to compare the CSA and PDFF of paraspinal muscles in patients with CLBP and healthy subjects using novel quantitative MRI, investigate the relationship between IVD degeneration and paraspinal muscle fat infiltration. Furthermore, we compared the age-related and sex-related changes in CSA and PDFF of the paraspinal muscles in patients with CLBP.
Materials and Methods
Participants
In this retrospective study, sixty patients with CLBP and thirty-two healthy subjects were selected in this study from January 2019 to December 2020 (46 males, 46 females; mean age: 45.82 years; age range: 24-72 years). Patients with CLBP and healthy subjects were matched for age and sex. Informed consent forms were signed by each participant, and ethical committee approval was obtained. The inclusion criteria were as follows: the untreated patient has symptoms of LBP for more than 3 months; Healthy subjects have no symptoms of LBP;BMI ranged from 18.5 to 23.9 kg/m2. The exclusion criteria were visceral LBP (such as urinary tract stones); spinal trauma, fracture, tumor, infection, deformity, spondylolisthesis, surgery, and other musculoskeletal diseases; pregnancy; and contraindications for MRI. Except that the subjects in the control group had no low back pain, the other inclusion and exclusion criteria were the same as those in the low back pain group. The control group did not have any clinical symptoms, and the other inclusion and exclusion criteria were identical to those described previously. The selected subjects also completed the Oswestry Disability Index (ODI) (35) and Visual Analog Scale (VAS) (36) to assess the level of back pain and dysfunction. The ODI covers 10 items (pain, lifting, walking, social life, personal care, sitting, standing, sleeping, travelling and sex life), and each scored from 0 to 5. Total ODI score = score of each item × 2, the total ODI score ranges from 0 to 100. A higher total ODI score reflects higher disability. According to the median age of the included participants, participants under 45 years old are classified as the young group, and 45 years old or older are classified as the elderly group. Table 1 shows the baseline clinical characteristics of the participants.
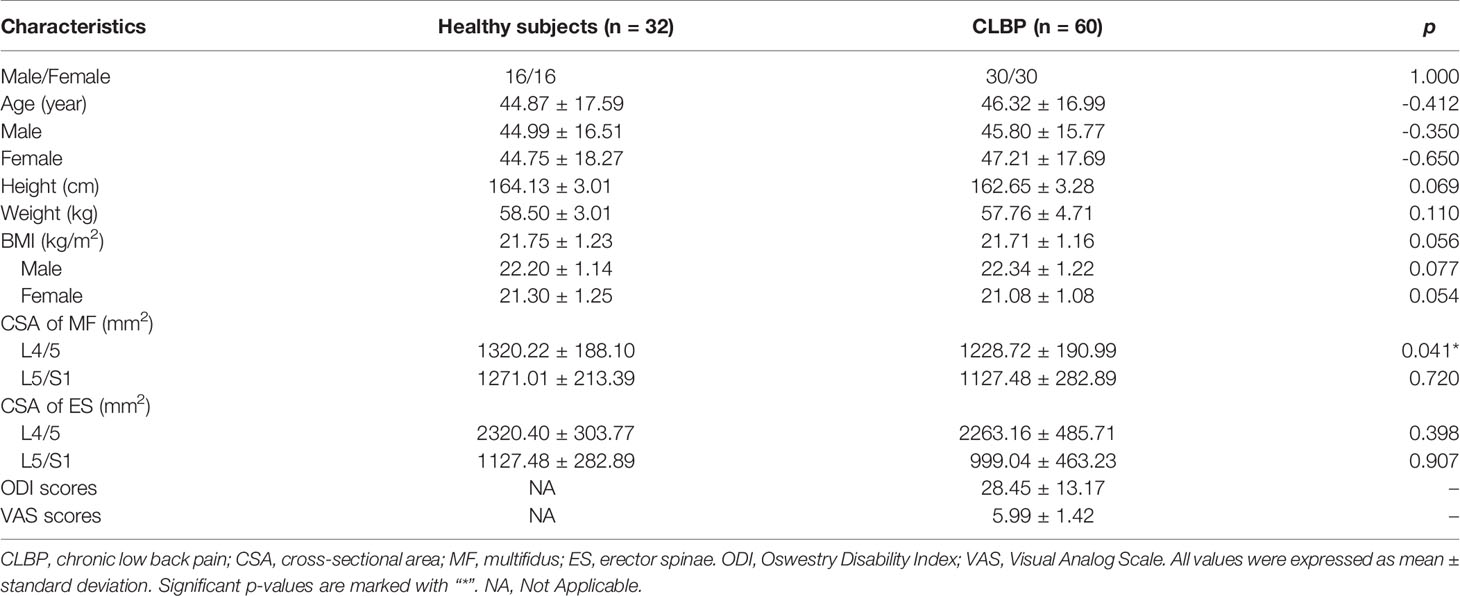
Table 1 Comparison of clinical characteristics and CSA of paraspinal muscle between healthy subjects and CLBP patients.
MR Data Acquisition
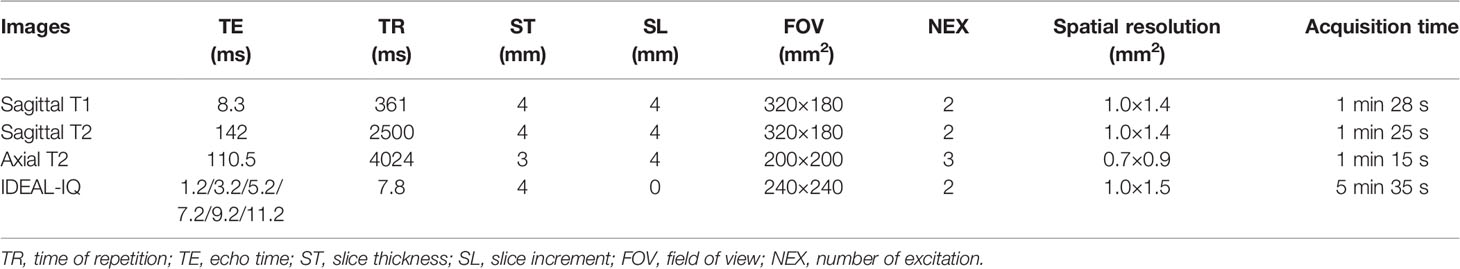
All MRI experiments were performed using a 3.0T MR system (Discovery 750w, GE Healthcare, USA). A 32-channel the phased array spine coil was used for CLBP patients and healthy subjects. To reduce motion artifacts, an abdominal bandage was used to compress the abdomen and a wedge-shaped foam pad was placed under the lower limbs of participants in a standard supine position. MRI scanning for participants included sagittal T1-weighted imaging (T1), T2-weighted imaging (T2) of the lumbar spine, and axial T2, IDEAL-IQ of paraspinal muscles. The MRI protocols of participants are summarized in Table 2.
Image Analyses
All raw MR images were processed on a commercially available workstation (Advantage Windows 4.6, GE Medical Systems, USA). The degeneration degree of IVD at L4/5 and L5/S1 was assessed by two blinded experienced radiologists according to Pfirrmann grading system (I-V) by MRI T2 (37). The Pfirrmann grading system was divided into five grades to evaluate the homogeneity of intervertebral disc structure, signal strength, discrimination between nucleus and anulus, and disc height. When there was a disagreement, both radiologists discussed to achieve a consensus. Muscle cross-sectional area (CSA) and PDFF values of the bilateral paraspinal muscles were obtained on a region of interest (ROI) basis at the central level of L4/5 and L5/S1. The CSA and PDFF of the paraspinal muscles were measured at two-disc levels for each participant. The two radiologists manually delineated the shape of the bilateral multifidus and erector spinae (Figure 1). The muscle CSA was measured by manually delineating the ROI on the axial T2 images, then the same ROI was automatically copied by the workstation to the fat fraction map to obtain the PDFF value. The average of the two measurements was calculated and used for later analysis.
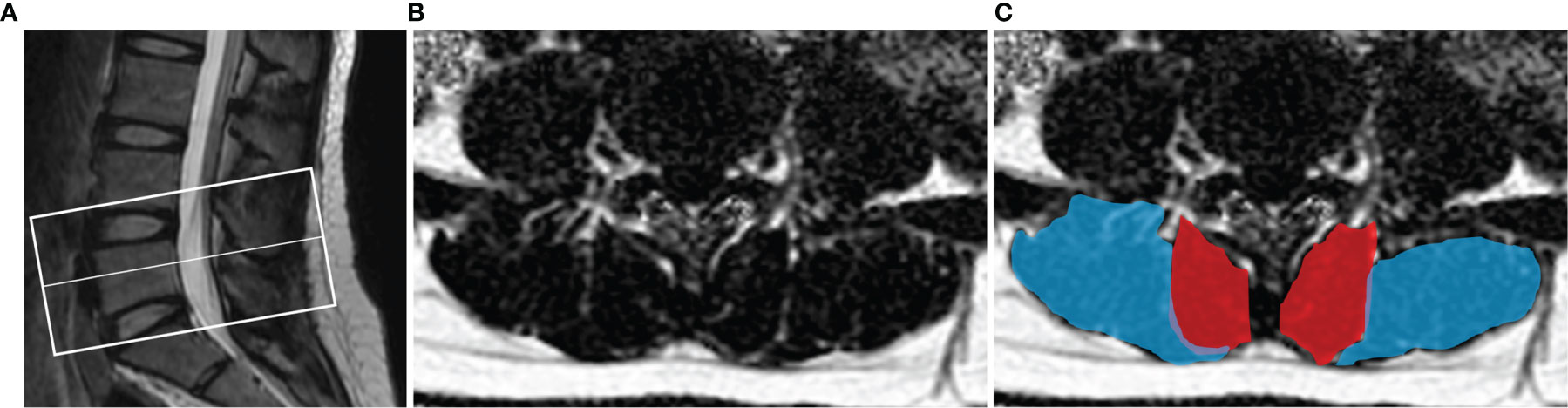
Figure 1 Demonstration of paraspinal muscle segmentation. (A) The center level of the scan was at the midline of L5. (B) Processed PDFF maps of paraspinal muscles; (C) manual segmentation of paraspinal muscles, multifidus (red) and erector spinae (blue).
Statistical Analysis
SPSS 22.0 was performed for the statistical analysis. Mean ± SD was used to express data. Comparisons between patients with CLBP and healthy subjects were determined using the independent-sample t-test. Pearsons correlations and Spearman’s rank correlations were computed between paraspinal muscles CSA, PDFF and Pfirrmann grade, ODI, VSA. One-way analysis of variance (ANOVA) was employed for the comparisons among multiple groups, and Tukey’s multiple comparisons test was utilized for the post hoc test after ANOVA. Analysis of covariance was used with age as a covariate to ensure that there was no effect of age on the differences of muscle PDFF. The Cochran-Armitage trend test was used between Pfirrmann grade and other variables. A p-value <0.05 was reported statistically significant.
Result
Comparison of CSA and PDFF in the Paraspinal Muscle Between Healthy Subjects and Patients With CLBP
There were no differences in gender, age, height, weight, and BMI between healthy subjects and CLBP patients (Table 1). The inter-observer agreement of measured CSA and PDFF between two radiologists was good (ICC=0.964, p<0.001). The CSAs of the multifidus and erector spinae of CLBP were smaller than those of healthy subjects, but the difference was statistically significant only in the multifidus at the L4/5 level (p<0.05, Table 1). The PDFF maps showed that the paraspinal muscle PDFFs were increased in patients with CLBP (Figure 2). At the level of L4/5 and L5/S1, the multifidus and erector spinae PDFF of CLBP patients were significantly increased than those of healthy subjects, and the differences were statistically significant (all p <0.05, Figure 2).
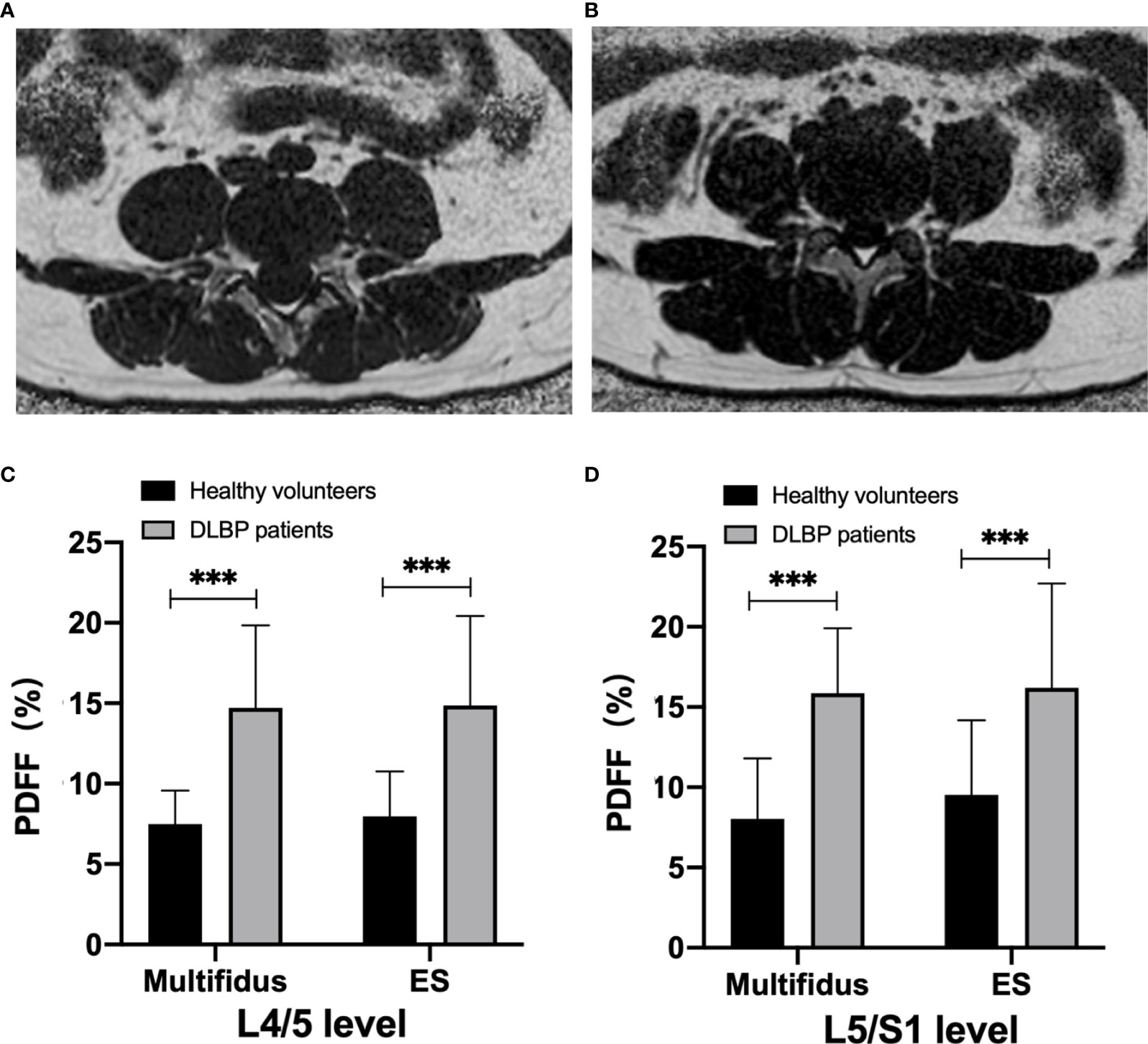
Figure 2 MRI PDFF of lumbar paraspinal muscle in patients with CLBP. (A) Patient with CLBP, Male, 29 years old, CLBP for 9 years, PDFF left multifidus = 10.9%, PDFF right multifidus =11.9%, PDFF left erector spinae =10.8%, PDFF right erector spinae = 10.1%. (B) Healthy Volunteer, male, 30 years old, PDFF left multifidus= 7.2%, PDFF right multifidus = 7.1%, PDFF left erector spinae = 5.1%, PDFF right erector spinae = 4.8%. (C, D) Bar chart of paraspinal muscle PDFF at L4/5 and L5/S1 levels. Data are reported as mean ± standard deviation of mean. ***p < 0.001.
Correlation Between Paraspinal Muscles CSA, PDFF and Pfirrmann Grade of IVDs
The multifidus CSA was weakly correlated to Pfirrmann grade of IVD degeneration (r=-0.265, p =0.004), but there was no significant correlation between the CSA of erector spinae and the Pfirrmann grade (r=-0.305, p =0.708). With the increase of Pfirrmann grade of IVDD, the PDFF values of the multifidus and erector spinae in CLBP patients gradually increased, in the order of Grade V>Grade IV>Grade III>Grade II>Grade I (Table 3). In the multifidus muscle, the PDFFs of Grade V and Grade IV were higher than that of Grade III and Grade II, and the difference was statistically significant (p<0.05, Table 3). There were differences in the age of CLBP patients with different Pfirrmann grades, but there was no statistically significant difference in BMI among the groups. After adjusting for age, comparing the multifidus and erector spinae PDFF among different Pfirrmann grades, the results showed that the PDFF of the high-grade Pfirrmann grade were higher than that of the low-grade Pfirrmann grade (Table 3). Figures 3 and 4 show the relationships between multifidus and erector spinae age-adjusted PDFF with Pfirrmann grade at the L4/5 and L5/S1 levels. There was a significant correlation (r = 0.717 and 0.744, all p < 0.05) between PDFF of MF and Pfirrmann grade at the IVD levels. In addition, the correlation between erector spinae PDFFs and Pfirrmann grade was lower than that of multifidus (r=0.651 and 0.658, all p <0.05). The Pfirrmann grade of IVD degeneration in the control group, 5, 44, 13, 2, 0 discs had Grade I- V, respectively.
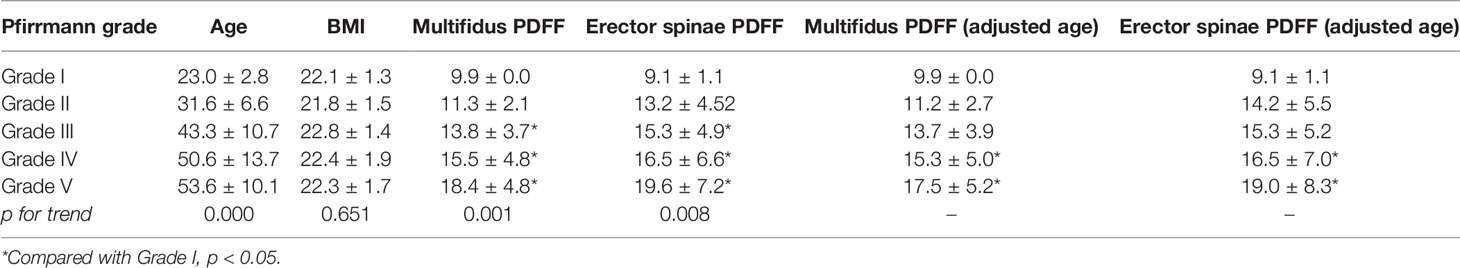
Table 3 Differences in PDFF and age-adjusted PDFF values of paraspinal muscles between different Pfirrmann grades of two intervertebral discs in CLBP patients .
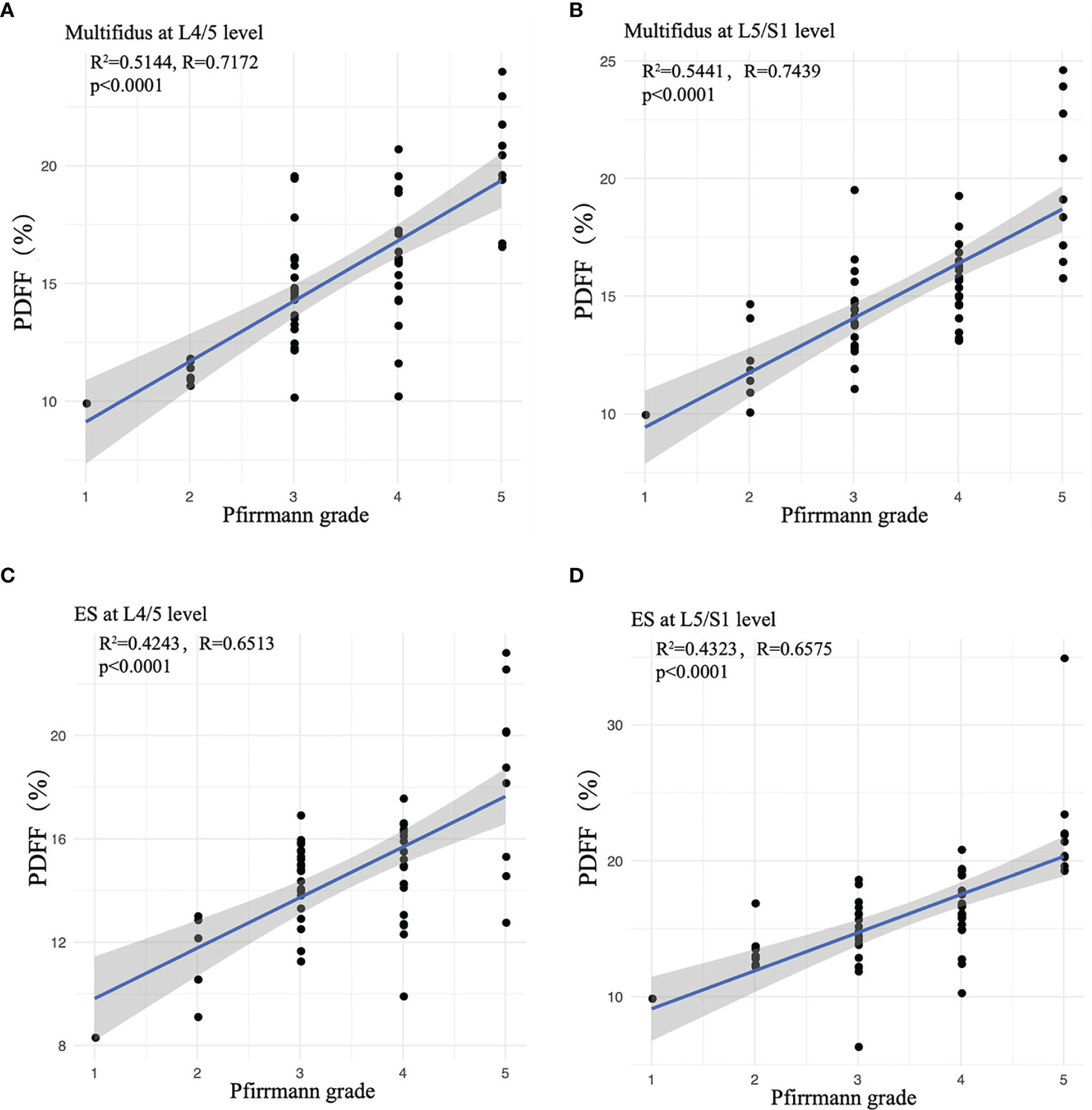
Figure 3 Correlation between age-adjusted PDFF of paraspinal muscles and Pfirrmann grade. (A) Multifidus at L4/5 level; (B) Multifidus at L5/S1 level; (C) ES at L4/5 level; (D) ES at L5/S1 level. ES, erector spinae. Spearman’s Rank-Order Correlation was used.
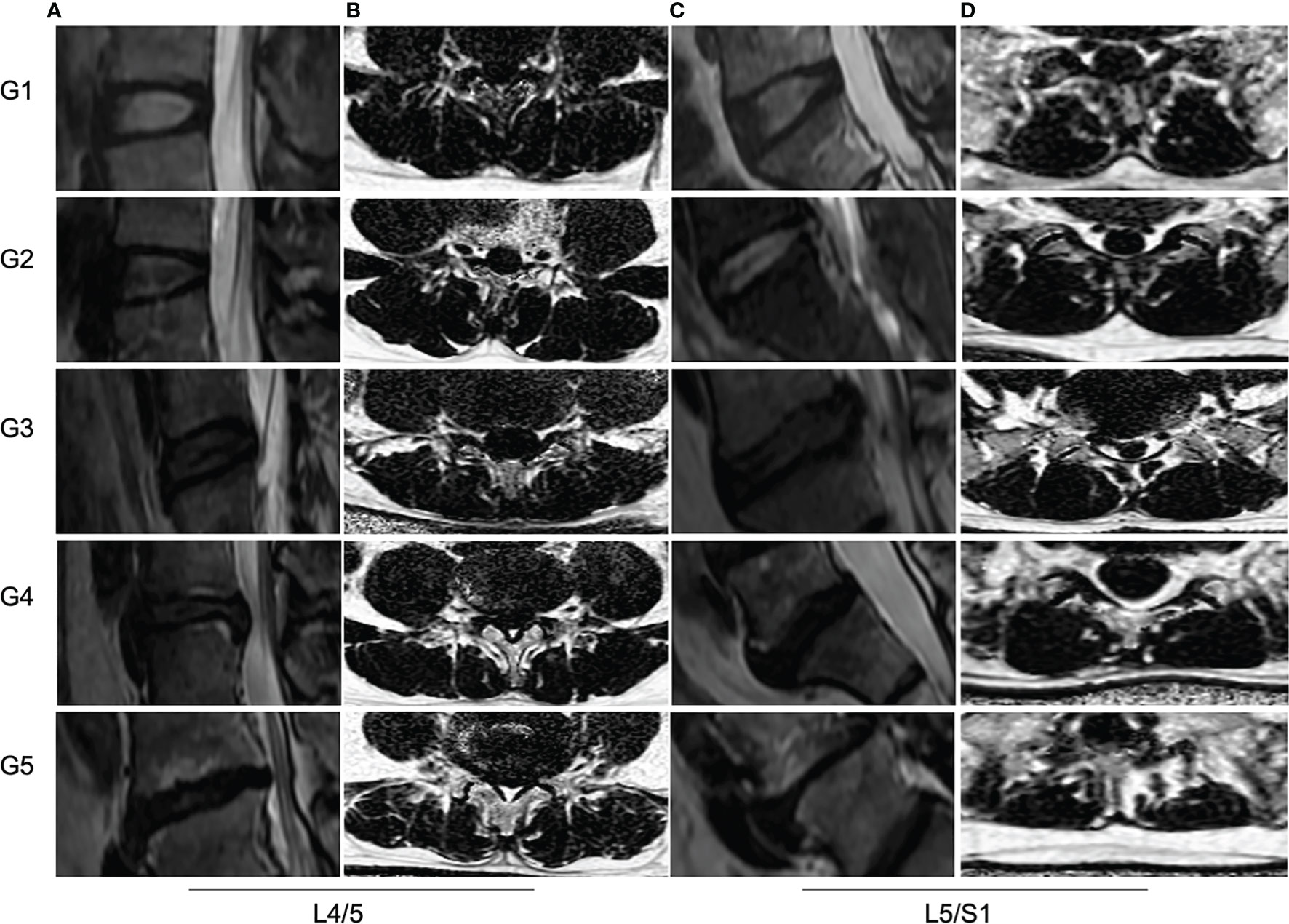
Figure 4 MRI PDFF of lumbar paraspinal muscles at different Pfirrmann grade of IVDs degeneration. (A, C) Sagittal T2 images of IVDs at L4/5 and L5/S1 levels. The Pfirrmann grade of IVDs degeneration from left to right are for G1, G2 G3, G4 and G5, respectively. (B, D) The PDFF of paraspinal muscles at L4/5 and L5/S1 levels. The mean PDFF at the L4/5 from up to bottom are for 9.1%, 10.5%, 14.2%, 16.3% and 22.3%, respectively. The mean PDFF at L5/S1 from up to bottom are for 10.8%, 12.1%, 14.0%, 16.5% and 24.0%, respectively.
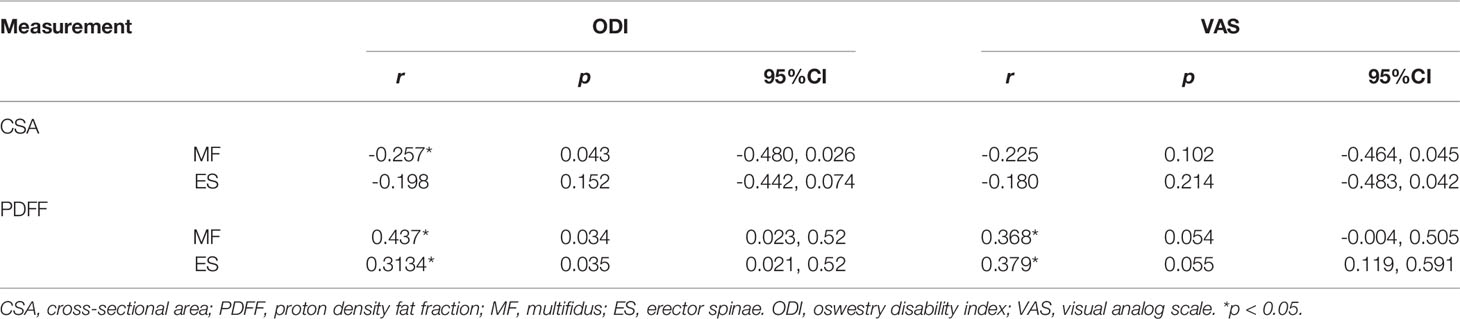
Correlation Between Paraspinal Muscles CSA, PDFF and the ODI, VSA of Patients With CLBP
Table 4 shows the overall relationships between CSA, PDFF of paraspinal muscles and ODI, VSA of CLBP patients. There was a moderate correlation between PDFF, ODI and VSA, and higher than CSA.
Analysis of the Difference of CSA and PDFF Regarding Sex and Age
In healthy subjects and CLBP patients, male paraspinal muscle CSAs at the L4/5 and L5/S1 were larger than females, and the difference was statistically significant (Figure 5, all p <0.05). In addition, the CSAs of paraspinal muscles in female CLBP patients were lower than that of the healthy subjects, and the difference was statistically significant (Figure 5, all p <0.05). Regardless of male or female, the PDFFs of the paraspinal muscles showed a significant increase in the CLBP patients. And female CLBP patients were higher than males in the PDFFs at the level of L4/5 (Figure 5, all p<0.05). The paraspinal muscle CSAs of the old were slightly smaller than that of the young in the both healthy subjects and CLBP patients, but the difference was not statistically significant(Figure 6, all p>0.05). Whether the old group or young group, CLBP patients were significantly higher than healthy subjects in the PDFFs of the paraspinal muscles (Figure 6, all p<0.05). And the PDFFs of the paraspinal muscles of the elderly CLBP patients were higher than that of the young at the level of L4/5 (Figure 6, p<0.05).
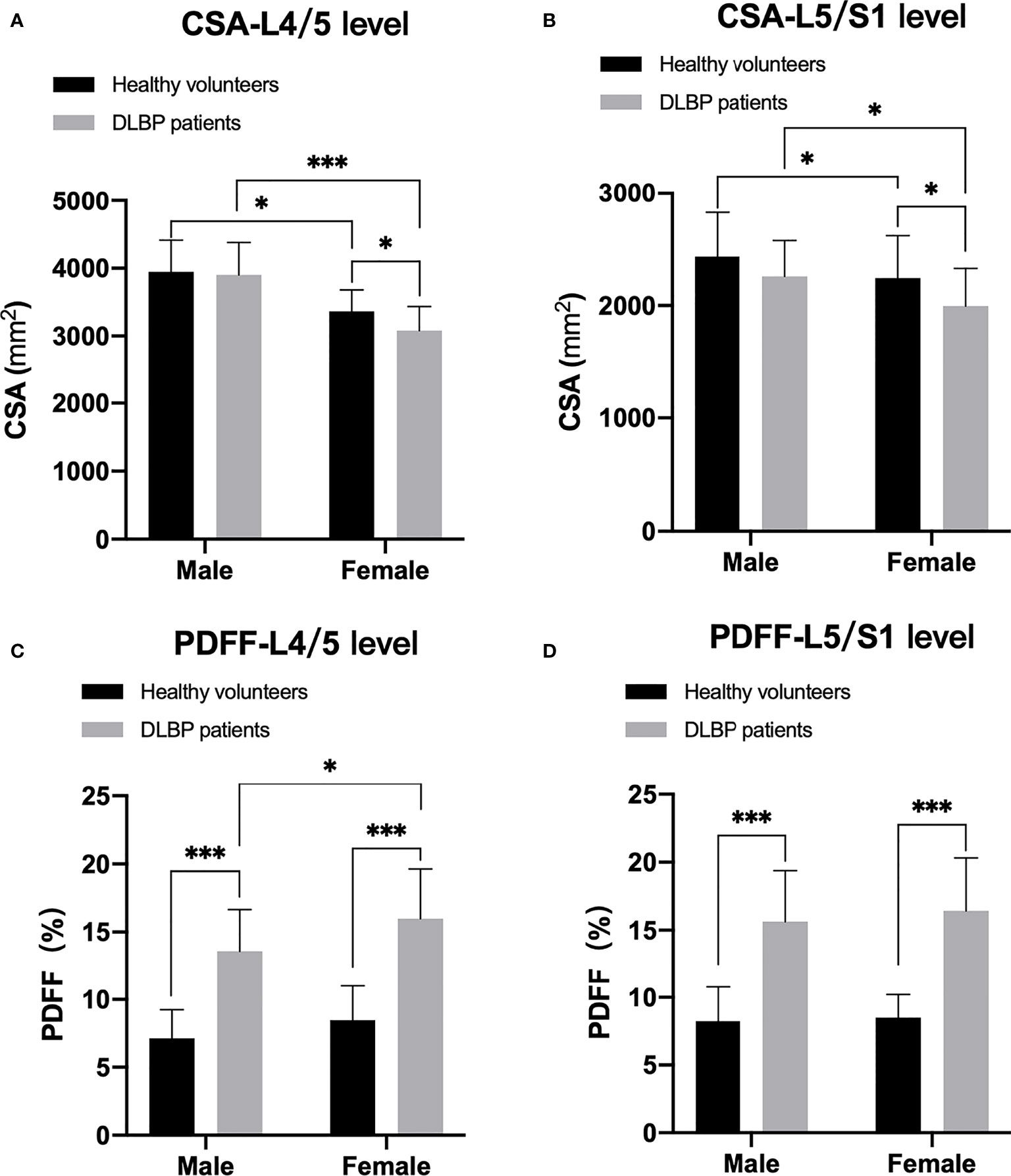
Figure 5 Difference of CSA and PDFF regarding sex. (A) CSA at L4/5 level; (B) CSA at L5/S1 level; (C) PDFF at L4/5 level; (D) PDFF at L5/S1 level. *p < 0.05, ***p < 0.001.
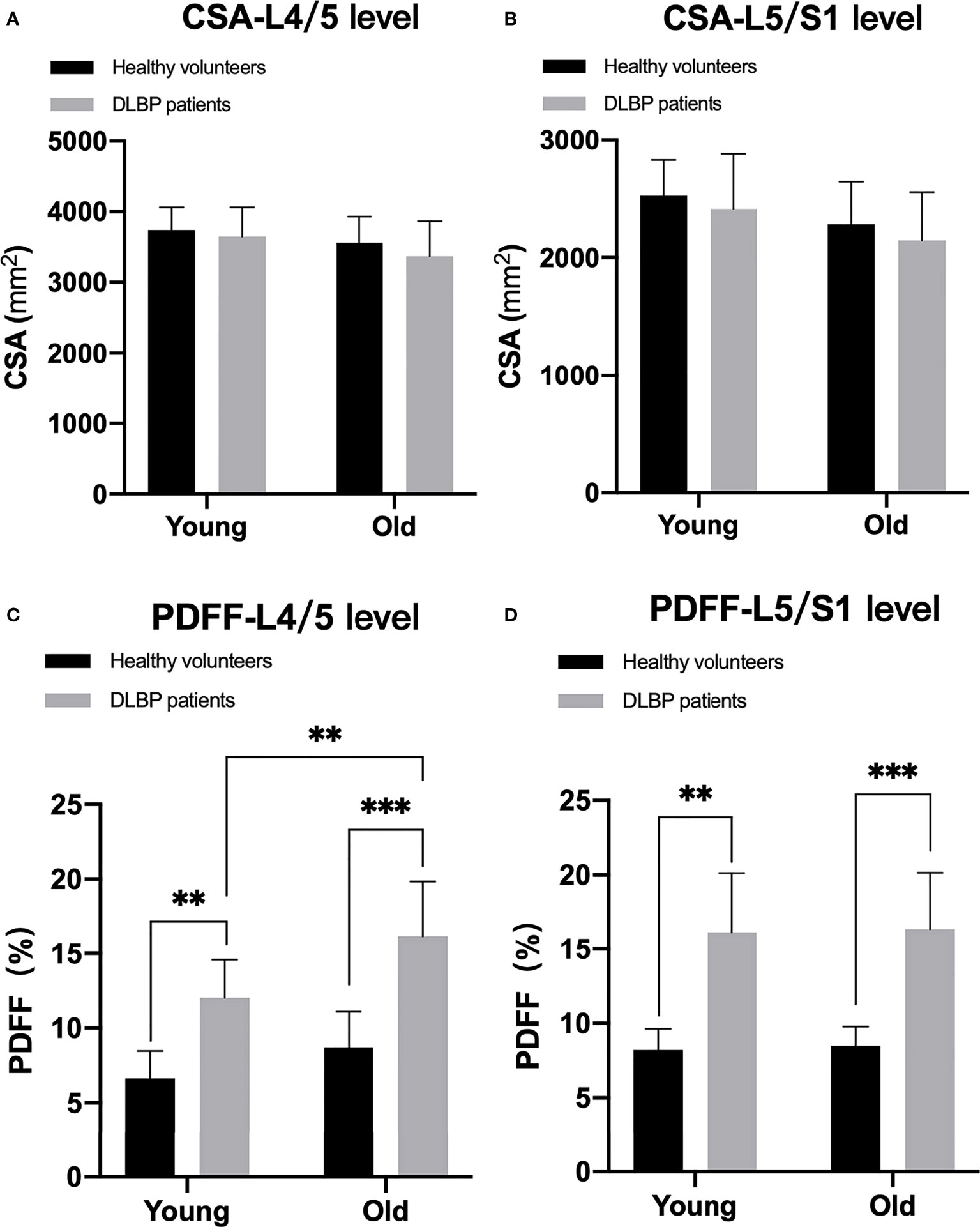
Figure 6 Difference of CSA and PDFF regarding age. (A) CSA at L4/5 level; (B) CSA at L5/S1 level; (C) PDFF at L4/5 level; (D) PDFF at L5/S1 level. **p< 0.05, ***p < 0.001.
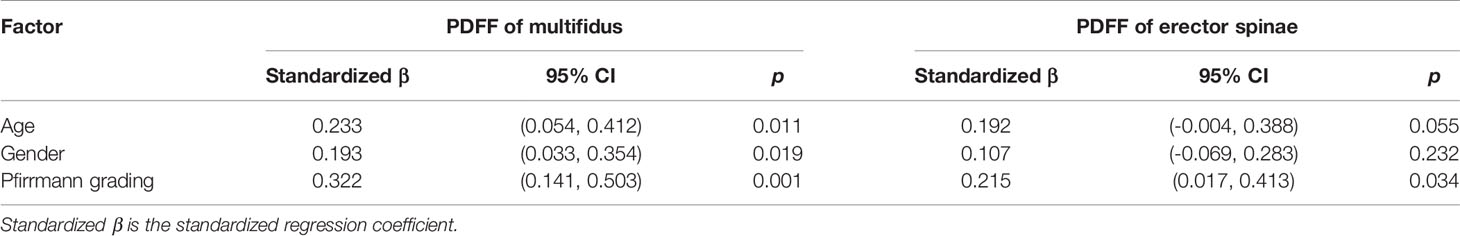
Multiple Linear Regression Analysis
Table 5 shows the multiple linear regression analysis of paraspinal muscle PDFFs. Age, gender, and Pfirrmann grade of IVDs were independent factors of multifidus FF value (p <0.05), and Pfirrmann grade of IVDs was an independent factor of erector spinae PDFF value (p <0.05).
Discussion
Using quantitative MR imaging, our study showed that the paraspinal muscles atrophy and fat content increase in patients with CLBP compared to healthy subjects. And a significant correlation was observed between the degeneration of the lumbar spine IVD and the PDFF of adjacent paraspinal muscle in patients with CLBP. Furthermore, sex and age were also independently associated with the paraspinal muscle fat.
Compared with healthy subjects, the CSA of the paraspinal muscles of CLBP patients were decreased only in the multifidus muscle at the L4/5 level. Although the muscle CSA is the most studied, paraspinal muscle atrophy in CLBP remains controversial (38–40). Barker et al. used conventional MRI to compare the multifidus CSA of patients with unilateral pain CLBP, and the results showed that the multifidus CSA of the painful side was lower than that of the asymptomatic side (41). The strength of the paraspinal muscles measured during maximal isometric trunk flexion and trunk extension contractions is decreased in patients with CLBP (42). Some studies have found that muscle CSA was reduced but not significant (38). This may be related to changes in the composition of muscles. When muscle tissue is atrophy, fat infiltration occurs in the paraspinal muscles (29, 43). To some extent, due to the filling and replacement of adipose tissue, the overall muscle CSA has not changed significantly. Our findings in this study further support this view.
In our study, compared with the control group, the PDFF of the paraspinal muscles of CLBP patients was significantly increased. Yanik et al. quantified the fat content of multifidus muscle in patients with CLBP and asymptomatic subjects by conventional MRI, and the results were consistent with this study (33, 44, 45). On the basis of the previous research, we manually delineated the edge of the muscle as the ROI, and further applied the multi-echo Dixon method, which corrected the main magnetic field inhomogeneity effect, T2* effect, T1 effect and other confounding factors, in order to make the quantification of paraspinal muscle fat content is more accurate, better repeatability and reliability (46–48). In order to minimize the possible impact of the slightly differences in spatial resolutions of T2 and PDFF images, we selected the layers at the center of the L4/5 and L5/S1 intervertebral discs as much as possible to delineate the ROI of the paraspinal muscles. In addition, we artificially removed cases with obvious motion artifacts. In this study, we further found the correlation between PDFF and IVD degeneration is higher than correlations between CSA and IVD degeneration. It indicated that the paraspinal muscles of CLBP patients had muscle tissue atrophy and fat replacement.
Recently, much attention has focused on lumbar IVD degeneration in CLBP patients and it is related to the Oswestry Disability Index (41, 49). Paraspinal muscle may play an important role in elucidating and treating lumbar spine dysfunction and spinal imbalance (50, 51). Indeed, we found significant correlations between IVD degeneration of the lumbar spines and the PDFF of adjacent paraspinal muscles in our cohort. Sato et al. revealed that muscle CSA changes were more correlated with pressure pain sensitivity in CLBP patients (52). This difference may be related to the different pain measurement methods we use. Furthermore, the multifidus muscle is more significantly affected. The anatomical relationships between the multifidus muscle and lumbar spine would be relevant to this interpretation. In the lumbar spine, the multifidus muscle is the most developed and important. The multifidus contributes to side bending (tilting) and rotation (twisting) (53). Compared with the erector spinae, the multifidus muscle is more closely related to the lamina and spinous process (54). And the multifidus is a short muscle which makes it more local and prone to changes at the L4/5 and L5/S1levels. The stability of the spine is reduced after CLBP, and muscle changes may be used as a compensatory strategy to cause long-term paraspinal muscle fatigue. When the muscle is decompensated, it will promote the recurrence or exacerbation of CLBP (55). This underlines the importance of the muscles PDFF in the context of IVD degradation. The increased fat infiltration in the paraspinal muscles may be related to the inflammatory disorders found in the multifidus muscles of patients with degenerative spine (56). In animal experiments, James et al. found an increase in macrophages and TNF in the multifidus muscle of a sheep model of IVD degeneration (57). The increased inflammation is likely to be an important factor in promoting fat infiltration of skeletal muscle (58). At the same time, we found that the level of pain and dysfunction in CLBP patients were higher, but the direct relationship between IVD degeneration, paraspinal muscle remodeling, pain, and dysfunction still needs further exploration.
In this study, we account for the potential effects of sex, age, and BMI on muscle fat. In the normal population, males have a larger CSA of paraspinal muscles and lower fat content than females. This is consistent with the results of previous studies (59). We found that both males and females with CLBP have muscle fat infiltration. And it seems to be more pronounced in females, the underlying mechanism is the decline in muscle performance caused by hormone deficiency after menopause in women (43, 60). Age is an important factor in the fat infiltration of paraspinal muscles. Our results show that paraspinal muscle fat increases with age (59, 61), indicating that paraspinal muscle are gradually deteriorating, even in healthy individuals. Therefore, it is necessary to consider the relationship between paraspinal muscles and spinal degeneration with age as a covariate. The PDFFs of the paraspinal muscles in both young and old CLBP patients were significantly increased, the increase was more significant in the elderly. This may be related to the poorer basic muscle strength and performance of the elderly. Fat infiltration of paravertebral muscles in CLBP patients can only be supported if age and sex effects are fully clear. In this study, through the ODI scale test, we observed that the activity level of CLBP patients was reduced. Hodges and Goubert et al. believes that pain leads to disuse muscle atrophy caused by reduced multifidus muscle activity (45, 62). But several previous researches conclude that as to the assumption that patients with CLBP suffer from disuse and physical deconditioning empirical evidence is still lacking (63, 64). In future studies, the activity level of CLBP patients deserves further consideration.
There are several limitations of this study. First, cross-sectional design with relatively small subjects is considered the main limitation. Subsequent longitudinal cohort studies are warranted to further investigate and confirm the relationship between IVD degeneration and paraspinal muscle fat infiltration. Furthermore, the current intervertebral degeneration grading system is qualitative and subjective, and a quantitative method is a better choice. Moreover, the L4/5 and L5/S1 IVDs are regarded as the level of interest because they are the most degradable levels. However, it is unclear whether the paraspinal muscles at the level of the L1-4 IVDs have changed. The post-processing software used in this study can obtain the PDFF of muscle, but not the PDFF of muscle tissue. So, the intermuscular and intramuscular fat cannot be completely distinguished in this study. At last, it will be interesting to further explore the distribution of fat in the paraspinal muscles and clearly quantify the levels of lipids within and outside muscle cells.
Using quantitative MRI to measure CSA and PDFF, this study confirmed the changes in CSA and PDFF of the paraspinal muscles in CLBP patients and found a significant correlation between lumbar IVD degeneration and the PDFF of paraspinal muscles. Sex and age are important factors also considered influencing factors for the paraspinal muscles in CLBP patients. Our findings clearly highlighted the assessment of fat content within paraspinal muscles in CLBP patients and might trigger a paradigm shift in the intervention strategy to CLBP. Paraspinal muscle fat infiltration should also be evaluated as treatment outcome, and its use as a treatment endpoint for therapies should be further investigated.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Kunming Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
BH and XC: Designed the study and conceived the report. YH and LW: Wrote the draft of the manuscript and revised it critically. XZ, JC, ZZ and YJ: Data acquisition and processing. BH and YH: Analyzed and interpreted the results of MRI. LN: Technical support for MRI scanning. YH, LW and XZ: Statistical analysis, and created the figures and tables. All authors had read and approved the final manuscript.
Funding
This work is supported by the Applied Basic Research Project of Yunnan Province- Kunming Medical University Joint Fund (202001AY070001-038), Beijing Hospitals Authority Youth Programme (QMS20200402), and Yunnan Provincial Bone and Joint Disease Clinical Medicine Center Project (ZX2019-03-04).
Conflict of Interest
XZ and LN were employed by GE Healthcare.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Buchbinder R, van Tulder M, Öberg B, Costa LM, Woolf A, Schoene M, et al. Low Back Pain: A Call for Action. Lancet (2018) 391(10137):2384–8. doi: 10.1016/s0140-6736(18)30488-4
2. Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, et al. What Low Back Pain is and Why We Need to Pay Attention. Lancet (2018) 391(10137):2356–67. doi: 10.1016/s0140-6736(18)30480-x
3. Balagué F, Mannion AF, Pellisé F, Cedraschi C. Non-Specific Low Back Pain. Lancet (2012) 379(9814):482–91. doi: 10.1016/s0140-6736(11)60610-7
4. Côté P, Cassidy JD, Carroll L. The Saskatchewan Health and Back Pain Survey. The Prevalence of Neck Pain and Related Disability in Saskatchewan Adults. Spine (Phila Pa 1976) (1998) 23(15):1689–98. doi: 10.1097/00007632-199808010-00015
5. Wang F, Cai F, Shi R, Wang XH, Wu XT. Aging and Age Related Stresses: A Senescence Mechanism of Intervertebral Disc Degeneration. Osteoarthr Cartil (2016) 24(3):398–408. doi: 10.1016/j.joca.2015.09.019
6. Silagi ES, Shapiro IM, Risbud MV. Glycosaminoglycan Synthesis in the Nucleus Pulposus: Dysregulation and the Pathogenesis of Disc Degeneration. Matrix Biol (2018) 71-72:368–79. doi: 10.1016/j.matbio.2018.02.025
7. Berman BM, Langevin HM, Witt CM, Dubner R. Acupuncture for Chronic Low Back Pain. N Engl J Med (2010) 363(5):454–61. doi: 10.1056/NEJMct0806114
8. Cherkin D, Balderson B, Wellman R, Hsu C, Sherman KJ, Evers SC, et al. Effect of Low Back Pain Risk-Stratification Strategy on Patient Outcomes and Care Processes: The MATCH Randomized Trial in Primary Care. J Gen Intern Med (2018) 33(8):1324–36. doi: 10.1007/s11606-018-4468-9
9. Hodges PW, Danneels L. Changes in Structure and Function of the Back Muscles in Low Back Pain: Different Time Points, Observations, and Mechanisms. J Orthop Sports Phys Ther (2019) 49(6):464–76. doi: 10.2519/jospt.2019.8827
10. Geisser ME, Ranavaya M, Haig AJ, Roth RS, Zucker R, Ambroz C, et al. A Meta-Analytic Review of Surface Electromyography Among Persons With Low Back Pain and Normal, Healthy Controls. J Pain (2005) 6(11):711–26. doi: 10.1016/j.jpain.2005.06.008
11. Solomonow M, Hatipkarasulu S, Zhou BH, Baratta RV, Aghazadeh F. Biomechanics and Electromyography of a Common Idiopathic Low Back Disorder. Spine (Phila Pa 1976) (2003) 28(12):1235–48. doi: 10.1097/01.Brs.0000065568.47818.B9
12. Ranger TA, Cicuttini FM, Jensen TS, Heritier S, Urquhart DM. Paraspinal Muscle Cross-Sectional Area Predicts Low Back Disability But Not Pain Intensity. Spine J (2019) 19(5):862–8. doi: 10.1016/j.spinee.2018.12.004
13. Agten A, Stevens S, Verbrugghe J, Timmermans A, Vandenabeele F. Biopsy Samples From the Erector Spinae of Persons With Nonspecific Chronic Low Back Pain Display a Decrease in Glycolytic Muscle Fibers. Spine J (2020) 20(2):199–206. doi: 10.1016/j.spinee.2019.09.023
14. Kalichman L, Carmeli E, Been E. The Association Between Imaging Parameters of the Paraspinal Muscles, Spinal Degeneration, and Low Back Pain. BioMed Res Int (2017) 2017:2562957. doi: 10.1155/2017/2562957
15. Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, De Cuyper HJ. CT Imaging of Trunk Muscles in Chronic Low Back Pain Patients and Healthy Control Subjects. Eur Spine J (2000) 9(4):266–72. doi: 10.1007/s005860000190
16. Kamaz M, Kireşi D, Oğuz H, Emlik D, Levendoğlu F. CT Measurement of Trunk Muscle Areas in Patients With Chronic Low Back Pain. Diagn Interv Radiol (2007) 13(3):144–8.
17. Wan Q, Lin C, Li X, Zeng W, Ma C. MRI Assessment of Paraspinal Muscles in Patients With Acute and Chronic Unilateral Low Back Pain. Br J Radiol (2015) 88(1053):20140546. doi: 10.1259/bjr.20140546
18. Lee CH. Quantitative T2-Mapping Using MRI for Detection of Prostate Malignancy: A Systematic Review of the Literature. Acta Radiol (2019) 60(9):1181–9. doi: 10.1177/0284185118820058
19. Peng X, Li X, Xu Z, Wang L, Cai W, Yang S, et al. Age-Related Fatty Infiltration of Lumbar Paraspinal Muscles: A Normative Reference Database Study in 516 Chinese Females. Quant Imaging Med Surg (2020) 10(8):1590–601. doi: 10.21037/qims-19-835
20. Wang L, Yin L, Zhao Y, Su Y, Sun W, Liu Y, et al. Muscle Density Discriminates Hip Fracture Better Than Computed Tomography X-Ray Absorptiometry Hip Areal Bone Mineral Density. J Cachexia Sarcopenia Muscle (2020) 11(6):1799–812. doi: 10.1002/jcsm.12616
21. Wang L, Yin L, Zhao Y, Su Y, Sun W, Chen S, et al. Muscle Density, But Not Size, Correlates Well With Muscle Strength and Physical Performance. J Am Med Dir Assoc (2021) 22(4):751–9. doi: 10.1016/j.jamda.2020.06.052
22. Baum T, Cordes C, Dieckmeyer M, Ruschke S, Franz D, Hauner H, et al. MR-Based Assessment of Body Fat Distribution and Characteristics. Eur J Radiol (2016) 85(8):1512–8. doi: 10.1016/j.ejrad.2016.02.013
23. Lee JH, Yoon YC, Kim HS, Kim JH, Choi BO. Texture Analysis Using T1-Weighted Images for Muscles in Charcot-Marie-Tooth Disease Patients and Volunteers. Eur Radiol (2021) 31(5):3508–17. doi: 10.1007/s00330-020-07435-y
24. Ma Q, Cheng X, Hou X, Yang Z, Ma D, Wang Z. Bone Marrow Fat Measured by a Chemical Shift-Encoded Sequence (Ideal-IQ) in Patients With and Without Metabolic Syndrome. J Magn Reson Imaging (2021) 54(1):146–53. doi: 10.1002/jmri.27548
25. Sollmann N, Dieckmeyer M, Schlaeger S, Rohrmeier A, Syvaeri J, Diefenbach MN, et al. Associations Between Lumbar Vertebral Bone Marrow and Paraspinal Muscle Fat Compositions-An Investigation by Chemical Shift Encoding-Based Water-Fat Mri. Front Endocrinol (Lausanne) (2018) 9:563. doi: 10.3389/fendo.2018.00563
26. Naarding KJ, van der Holst M, van Zwet EW, van de Velde NM, de Groot IJM, Verschuuren J, et al. Association of Elbow Flexor MRI Fat Fraction With Loss of Hand-To-Mouth Movement in Patients With Duchenne Muscular Dystrophy. Neurology (2021) 97(17):e1737–42. doi: 10.1212/wnl.0000000000012724
27. Agten CA, Rosskopf AB, Gerber C, Pfirrmann CW. Quantification of Early Fatty Infiltration of the Rotator Cuff Muscles: Comparison of Multi-Echo Dixon With Single-Voxel MR Spectroscopy. Eur Radiol (2016) 26(10):3719–27. doi: 10.1007/s00330-015-4144-y
28. Marty B, Reyngoudt H, Boisserie JM, Le Louër J, CAA E, Fromes Y, et al. Water-Fat Separation in MR Fingerprinting for Quantitative Monitoring of the Skeletal Muscle in Neuromuscular Disorders. Radiology (2021) 300(3):652–60. doi: 10.1148/radiol.2021204028
29. Mengiardi B, Schmid MR, Boos N, Pfirrmann CW, Brunner F, Elfering A, et al. Fat Content of Lumbar Paraspinal Muscles in Patients With Chronic Low Back Pain and in Asymptomatic Volunteers: Quantification With MR Spectroscopy. Radiology (2006) 240(3):786–92. doi: 10.1148/radiol.2403050820
30. Sollmann N, Zoffl A, Franz D, Syväri J, Dieckmeyer M, Burian E, et al. Regional Variation in Paraspinal Muscle Composition Using Chemical Shift Encoding-Based Water-Fat MRI. Quant Imaging Med Surg (2020) 10(2):496–507. doi: 10.21037/qims.2020.01.10
31. Zhao Y, Huang M, Serrano Sosa M, Cattell R, Fan W, Li M, et al. Fatty Infiltration of Paraspinal Muscles is Associated With Bone Mineral Density of the Lumbar Spine. Arch Osteoporos (2019) 14(1):99. doi: 10.1007/s11657-019-0639-5
32. Patzelt L, Junker D, Syväri J, Burian E, Wu M, Prokopchuk O, et al. Mri-Determined Psoas Muscle Fat Infiltration Correlates With Severity of Weight Loss During Cancer Cachexia. Cancers (Basel) (2021) 13(17):4433. doi: 10.3390/cancers13174433
33. Yanik B, Keyik B, Conkbayir I. Fatty Degeneration of Multifidus Muscle in Patients With Chronic Low Back Pain and in Asymptomatic Volunteers: Quantification With Chemical Shift Magnetic Resonance Imaging. Skeletal Radiol (2013) 42(6):771–8. doi: 10.1007/s00256-012-1545-8
34. Yoshihara K, Shirai Y, Nakayama Y, Uesaka S. Histochemical Changes in the Multifidus Muscle in Patients With Lumbar Intervertebral Disc Herniation. Spine (Phila Pa 1976) (2001) 26(6):622–6. doi: 10.1097/00007632-200103150-00012
35. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry Low Back Pain Disability Questionnaire. Physiotherapy (1980) 66(8):271–3. doi: 10.1037/t04205-000
36. Chiarotto A, Maxwell LJ, Ostelo RW, Boers M, Tugwell P, Terwee CB. Measurement Properties of Visual Analogue Scale, Numeric Rating Scale, and Pain Severity Subscale of the Brief Pain Inventory in Patients With Low Back Pain: A Systematic Review. J Pain (2019) 20(3):245–63. doi: 10.1016/j.jpain.2018.07.009
37. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic Resonance Classification of Lumbar Intervertebral Disc Degeneration. Spine (Phila Pa 1976) (2001) 26(17):1873–8. doi: 10.1097/00007632-200109010-00011
38. Ranger TA, Cicuttini FM, Jensen TS, Peiris WL, Hussain SM, Fairley J, et al. Are the Size and Composition of the Paraspinal Muscles Associated With Low Back Pain? A Systematic Review. Spine J (2017) 17(11):1729–48. doi: 10.1016/j.spinee.2017.07.002
39. Fortin M, Macedo LG. Multifidus and Paraspinal Muscle Group Cross-Sectional Areas of Patients With Low Back Pain and Control Patients: A Systematic Review With a Focus on Blinding. Phys Ther (2013) 93(7):873–88. doi: 10.2522/ptj.20120457
40. Fortin M, Gibbons LE, Videman T, Battie MC. Do Variations in Paraspinal Muscle Morphology and Composition Predict Low Back Pain in Men? Scandinavian J Med Sci sports (2015) 25(6):880–7. doi: 10.1111/sms.12301
41. Barker KL, Shamley DR, Jackson D. Changes in the Cross-Sectional Area of Multifidus and Psoas in Patients With Unilateral Back Pain: The Relationship to Pain and Disability. Spine (Phila Pa 1976) (2004) 29(22):E515–9. doi: 10.1097/01.brs.0000144405.11661.eb
42. Moreno Catalá M, Schroll A, Laube G, Arampatzis A. Muscle Strength and Neuromuscular Control in Low-Back Pain: Elite Athletes Versus General Population. Front Neurosci (2018) 12:436. doi: 10.3389/fnins.2018.00436
43. Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C. Are MRI-Defined Fat Infiltrations in the Multifidus Muscles Associated With Low Back Pain? BMC Med (2007) 5:2. doi: 10.1186/1741-7015-5-2
44. Lee SK, Jung JY, Kang YR, Jung JH, Yang JJ. Fat Quantification of Multifidus Muscle Using T2-Weighted Dixon: Which Measurement Methods are Best Suited for Revealing the Relationship Between Fat Infiltration and Herniated Nucleus Pulposus. Skeletal Radiol (2020) 49(2):263–71. doi: 10.1007/s00256-019-03270-5
45. Goubert D, De Pauw R, Meeus M, Willems T, Cagnie B, Schouppe S, et al. Lumbar Muscle Structure and Function in Chronic Versus Recurrent Low Back Pain: A Cross-Sectional Study. Spine J (2017) 17(9):1285–96. doi: 10.1016/j.spinee.2017.04.025
46. Yoo YH, Kim HS, Lee YH, Yoon CS, Paek MY, Yoo H, et al. Comparison of Multi-Echo Dixon Methods With Volume Interpolated Breath-Hold Gradient Echo Magnetic Resonance Imaging in Fat-Signal Fraction Quantification of Paravertebral Muscle. Korean J Radiol (2015) 16(5):1086–95. doi: 10.3348/kjr.2015.16.5.1086
47. Han G, Jiang Y, Zhang B, Gong C, Li W. Imaging Evaluation of Fat Infiltration in Paraspinal Muscles on MRI: A Systematic Review With a Focus on Methodology. Orthop Surg (2021) 13(4):1141–8. doi: 10.1111/os.12962
48. Fischer MA, Nanz D, Shimakawa A, Schirmer T, Guggenberger R, Chhabra A, et al. Quantification of Muscle Fat in Patients With Low Back Pain: Comparison of Multi-Echo MR Imaging With Single-Voxel MR Spectroscopy. Radiology (2013) 266(2):555–63. doi: 10.1148/radiol.12120399
49. Liu ZZ, Wen HQ, Zhu YQ, Zhao BL, Kong QC, Chen JY, et al. Short-Term Effect of Lumbar Traction on Intervertebral Discs in Patients With Low Back Pain: Correlation Between the T2 Value and ODI/VAS Score. Cartilage (2021) 13(1_suppl):1947603521996793. doi: 10.1177/1947603521996793
50. Hori Y, Hoshino M, Inage K, Miyagi M, Takahashi S, Ohyama S, et al. Issls PRIZE in CLINICAL SCIENCE 2019: Clinical Importance of Trunk Muscle Mass for Low Back Pain, Spinal Balance, and Quality of Life-A Multicenter Cross-Sectional Study. Eur Spine J (2019) 28(5):914–21. doi: 10.1007/s00586-019-05904-7
51. Owen PJ, Miller CT, Mundell NL, Verswijveren S, Tagliaferri SD, Brisby H, et al. Which Specific Modes of Exercise Training are Most Effective for Treating Low Back Pain? Network Meta-Analysis. Br J Sports Med (2020) 54(21):1279–87. doi: 10.1136/bjsports-2019-100886
52. Goubert D, Meeus M, Willems T, De Pauw R, Coppieters I, Crombez G, et al. The Association Between Back Muscle Characteristics and Pressure Pain Sensitivity in Low Back Pain Patients. Scand J Pain (2018) 18(2):281–93. doi: 10.1515/sjpain-2017-0142
53. Kalimo H, Rantanen J, Viljanen T, Einola S. Lumbar Muscles: Structure and Function. Ann Med (1989) 21(5):353–9. doi: 10.3109/07853898909149220
54. Macintosh JE, Valencia F, Bogduk N, Munro RR. The Morphology of the Human Lumbar Multifidus. Clin Biomech (Bristol Avon) (1986) 1(4):196–204. doi: 10.1016/0268-0033(86)90146-4
55. Bailey JF, Fields AJ, Ballatori A, Cohen D, Jain D, Coughlin D, et al. The Relationship Between Endplate Pathology and Patient-Reported Symptoms for Chronic Low Back Pain Depends on Lumbar Paraspinal Muscle Quality. Spine (Phila Pa 1976) (2019) 44(14):1010–7. doi: 10.1097/brs.0000000000003035
56. James G, Chen X, Diwan A, Hodges PW. Fat Infiltration in the Multifidus Muscle is Related to Inflammatory Cytokine Expression in the Muscle and Epidural Adipose Tissue in Individuals Undergoing Surgery for Intervertebral Disc Herniation. Eur Spine J (2021) 30(4):837–45. doi: 10.1007/s00586-020-06514-4
57. James G, Sluka KA, Blomster L, Hall L, Schmid AB, Shu CC, et al. Macrophage Polarization Contributes to Local Inflammation and Structural Change in the Multifidus Muscle After Intervertebral Disc Injury. Eur Spine J (2018) 27(8):1744–56. doi: 10.1007/s00586-018-5652-7
58. Wu H, Ballantyne CM. Skeletal Muscle Inflammation and Insulin Resistance in Obesity. J Clin Invest (2017) 127(1):43–54. doi: 10.1172/jci88880
59. Crawford RJ, Filli L, Elliott JM, Nanz D, Fischer MA, Marcon M, et al. Age- and Level-Dependence of Fatty Infiltration in Lumbar Paravertebral Muscles of Healthy Volunteers. AJNR Am J Neuroradiol (2016) 37(4):742–8. doi: 10.3174/ajnr.A4596
60. Sions JM, Elliott JM, Pohlig RT, Hicks GE. Trunk Muscle Characteristics of the Multifidi, Erector Spinae, Psoas, and Quadratus Lumborum in Older Adults With and Without Chronic Low Back Pain. J Orthop Sports Phys Ther (2017) 47(3):173–9. doi: 10.2519/jospt.2017.7002
61. Lee SH, Park SW, Kim YB, Nam TK, Lee YS. The Fatty Degeneration of Lumbar Paraspinal Muscles on Computed Tomography Scan According to Age and Disc Level. Spine J (2017) 17(1):81–7. doi: 10.1016/j.spinee.2016.08.001
62. Hodges P, Holm AK, Hansson T, Holm S. Rapid Atrophy of the Lumbar Multifidus Follows Experimental Disc or Nerve Root Injury. Spine (Phila Pa 1976) (2006) 31(25):2926–33. doi: 10.1097/01.brs.0000248453.51165.0b
63. Bousema EJ, Verbunt JA, Seelen HAM, Vlaeyen JWS, Knottnerus AJ. Disuse and Physical Deconditioning in the First Year After the Onset of Back Pain. Pain (2007) 130(3):279–86. doi: 10.1016/j.pain.2007.03.024
Keywords: chronic low back pain, quantitative MRI, paraspinal muscles, fatty infiltration, lumbar intervertebral disk degeneration
Citation: Huang Y, Wang L, Zeng X, Chen J, Zhang Z, Jiang Y, Nie L, Cheng X and He B (2022) Association of Paraspinal Muscle CSA and PDFF Measurements With Lumbar Intervertebral Disk Degeneration in Patients With Chronic Low Back Pain. Front. Endocrinol. 13:792819. doi: 10.3389/fendo.2022.792819
Received: 11 October 2021; Accepted: 21 April 2022;
Published: 26 May 2022.
Edited by:
Giuseppe Guglielmi, University of Foggia, ItalyReviewed by:
Maria Pilar Aparisi Gomez, Auckland District Health Board, New ZealandWenmin Guan, Capital Medical University, China
Copyright © 2022 Huang, Wang, Zeng, Chen, Zhang, Jiang, Nie, Cheng and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo He, aGVib195ZHl5QHFxLmNvbQ==; Xiaoguang Cheng, eGlhbzY1QDI2My5uZXQ=
†These authors have contributed equally to this work
 Yilong Huang1†
Yilong Huang1† Ling Wang
Ling Wang Lisha Nie
Lisha Nie Xiaoguang Cheng
Xiaoguang Cheng Bo He
Bo He