
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 26 May 2022
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.768363
This article is part of the Research Topic The Legacy of Dr. Leonard D. Kohn to Thyroid Pathophysiology View all 11 articles
The regulation of the female reproductive system is one of the most relevant actions of thyroid hormones. Adequate thyroid hormones production is essential for normal menstrual function and fertility as well as for the successful maintenance of pregnancy. The relationship between reproductive failure and thyroid disorders is particularly relevant and attracts attention worldwide. Thyroid autoimmunity (TAI), defined by the presence of circulating antithyroid antibodies targeting thyroid peroxidase (TPOAb) and thyroglobulin (TgAb), is prevalent among women of reproductive age and is the most frequent cause of thyroid dysfunction. Several studies addressed the association between TAI, thyroid function, and fertility as well as pregnancy outcome after spontaneous or assisted conception. Infertility, miscarriages, and fetal-maternal complications are described in overt autoimmune hypothyroidism. More debatable is the role of mild thyroid dysfunction, mainly subclinical hypothyroidism (SCH), and TAI in the absence of thyroid dysfunction in infertility and reproductive outcome. Assisted reproductive technology (ART) has become an integral element of care for infertility. Women with TAI undergoing ART are of particular interest since they carry a higher risk of developing hypothyroidism after the ovarian stimulation but whether TAI, in absence of thyroid dysfunction, adversely affects ART outcome is still controversial. Likewise, the role of levothyroxine (LT4) in improving fertility and the success of ART in euthyroid women with TAI is unclear. This review discusses the role of TAI, in the absence of thyroid dysfunction, in infertility and in ART outcome.
The definition of infertility is the failure to achieve clinical pregnancy after 12 months or more of regular unprotected sexual intercourse (1). Female factors (ovulatory disorders, endometriosis, pelvic adhesions, tubal blockage/abnormalities, and hyperprolactinemia) account for 35%, male factors for 30%, and combined factors for 20%. In 15% of the cases the cause remains unknown (idiopathic or unexplained infertility). The prevalence of infertility varies worldwide and is estimated to affect between 8 and 12% of reproductive-aged couples (2). Multiple elements can contribute to infertility affecting the female, the male, or both partners and many studies have been dedicated to identifying treatable risk factors that contribute to infertility. Among these risk factors are the presence of thyroid dysfunction and/or TAI. Therefore, screening for TSH and TPOAb is generally part of the initial work-up of infertile women. Indeed, thyroid hormones (TH) regulate the hypothalamic-pituitary-ovarian axis. A role of TH on luteinizing hormone (LH) secretion, on granulosa cell functions, LH/hCG receptor expression, and follicle development has been demonstrated (3). Therefore, ovulatory dysfunction and infertility are common in untreated thyroid dysfunction (4). Moreover, TH plays a critical role in implantation and early fetal development through actions on the placenta and endometrium (5). If adequate TH is necessary to avoid reducing the chances of conception and implantation, the same is true for the maintenance and outcome of pregnancy as well as for fetal brain development. In pregnancy significant adaptations of thyroid function are required, and the maternal thyroid must increase the hormone production by approximately 50% (6, 7). These pregnancy related adaptations cannot be accomplished in women with undiagnosed or uncontrolled hypothyroidism. The adaptation is even more difficult in assisted conception since the controlled ovarian stimulation protocols of ART anticipate the strain on thyroid function observed only after conception in spontaneous pregnancy (8). Thyroid disorders are more prevalent in females and are frequently encountered as a new diagnosis in women seeking pregnancy or during pregnancy. TAI is characterized by the presence of circulating antithyroid antibodies targeting thyroid peroxidase (TPOAb) and thyroglobulin (TgAb). Indeed, also circulating antibodies targeting TSH receptor, that are hallmarks of Graves’ disease, have significant implications in reproductive age women and in pregnancy but their discussion is beyond the scope of this work and has been reviewed elsewhere (9). TAI is the leading cause of thyroid dysfunction and comprises a spectrum of conditions ranging from euthyroid thyroiditis to subclinical or overt hypothyroidism and, less frequently, to transient thyrotoxicosis. There is evidence that TAI-related thyroid dysfunction (mainly overt and subclinical hypothyroidism) adversely affects conception and pregnancy outcomes (10). Rather, it is unclear what impact TAI has with normal thyroid function in infertility, and in the outcome of pregnancy especially in women undergoing ART; clinical studies as well as meta-analyses report discrepant results (11). Despite a multitude of studies, a clear pathophysiological link connecting TAI and reproductive failure has not been identified. It is challenging to distinguish the effect of TAI per se from the changes it can induce in thyroid function, that is, TSH levels at the high end of the normal range or compatible with subclinical and overt hypothyroidism. When looking at studies addressing the role of TAI-related thyroid dysfunction in reproductive failure it must be remembered that the serum TSH level cut-off used to define normal thyroid function in pregnancy has been changed over time. The upper limit of serum TSH was set at 2.5 mIU/L according to the American Thyroid Association (ATA) guidelines published in 2011 (12). In the newest guidelines published in 2017, the need for trimester specific and local population TSH reference is strongly reaffirmed and, if this is not available, the upper limit of TSH in the first trimester is set at 4.0 mIU/L (13). Recently, guidelines specifically addressing thyroid dysfunction prior to or during ART have been published by the European Thyroid Association (ETA) (14). In searching a thyroid function independent pathogenic role of TAI in reproductive failure, several studies have been dedicated to potential immune-dependent direct effect on the ovary, on the endometrium, and on the feto-maternal unit. The findings are interesting but merely speculative and need confirmation (15). There remain unanswered questions on the relationship between TAI and reproductive outcome; several fields of research are open (11, 16). Increasing knowledge on the pathophysiological link between TAI and reproductive failure would improve the management and treatment of infertile women with TAI. The treatment of infertile euthyroid women with TAI remains the greatest areas of uncertainty, some studies demonstrate that LT4 reduces the risk of adverse pregnancy outcome while others do not (17). In this work we reviewed the epidemiological, clinical, and pathophysiological data addressing the relationship of TAI, without thyroid dysfunction, with infertility and ART outcome.
The prevalence of TAI differs with races, age, iodine supply, and smoking, and is estimated as high as 5–16% in women aged 20–45 years in Europe (18, 19). Many studies have investigated the prevalence of TAI in infertile women. The studies are heterogeneous for design (frequently cross-sectional or retrospective), sample size, subjects (women with different causes of infertility or with a selected cause) and controls (unselected, age matched fertile, healthy non-pregnant) and for different methods of autoantibodies assay. In studies published until the early 2000s the prevalence of TAI in infertile women ranged from 6.8 to 14.5% but without significant differences compared to controls (20, 21). Only in two studies the prevalence was higher than the control population in women with anovulation (26%), in women with idiopathic infertility, and, mostly, with endometriosis (30%) compared to the unselected population (22, 23). However pooling the result of the studies the prevalence of TAI among women attending infertility clinics has been estimated higher compared to the general population with a relative risk of 2.1 (24). In more recent studies a prevalence of TAI in infertile women between 13% and 19% has been reported (25–27). In a large observational cohort study of 19,213 women with a history of miscarriage or infertility trying for a pregnancy, TPOAb was found in 9.5% of asymptomatic women (28). TAI was found in 5.9% of women at the first intra-uterine insemination and in 25% of women undergoing ART (17, 29). The secondary analysis of data from two multicenter, randomized, controlled trials reported that 8.6% infertile women had TPOAb (30). In one study not showing significant difference of TAI prevalence between the whole infertile population and the controls, higher prevalence was observed in women with female causes and, the highest, in women with endometriosis (29%) (31). This association was not confirmed in a subsequent study showing TPOAb in 14.9% of women with endometriosis and in 22.2% of the control group (32). An increased prevalence of TAI has also been reported in women with polycystic ovary syndrome (PCOS). In a prospective study TPOAb/TgAb or hypoechoic pattern at thyroid ultrasound was significantly higher in PCOS women compared to controls (26.9 vs. 8.3% and 42.3% vs. 6.5% respectively). PCOS patients had a higher mean TSH level and a higher incidence of TSH levels above the upper limit of normal (22). Again these findings were not confirmed in a recent study on 210 women with PCOS and 343 age matched controls: no differences were found in the prevalence of TPOAb and/or hypohecoic pattern at thyroid ultrasound between patient and controls (4.8% and 7.6% and 9.3% and 12.3%, respectively) but subjects with TAI showed significantly higher adiposity and insulin resistance index than those without (33). Finally, in a meta-analysis pooling four studies, TAI was more prevalent in euthyroid women with idiopathic infertility with an odds ratio of 1.5 (34). It is worth remembering that in most of the cited studies TAI is defined based on the presence of TPOAb. In a prospective study both TPOAb and TgAb were present in 8% of the cases but isolated TgAb prevalence was close to that of isolated TPOAb (4% vs. 5%). Interestingly women with TgAb had significantly higher serum TSH levels compared with women without TAI. The study also showed a higher prevalence of TAI in infertile patients (35). In 436 women attending a fertility center, TPOAb and TgAb were detected in 10.6% and 9.2%, respectively, overlap was found in 4.6% (36). Therefore, up to 5% of TAI women can be missed if only TPOAb is measured. On the other hand, it is well known that circulating thyroid autoantibodies could wane in pregnancy or be absent, outside pregnancy, in the so-called “seronegative chronic autoimmune thyroiditis” (37). Table 1 summarizes some of the studies on the prevalence of TAI in infertile women.
In conclusion, a higher prevalence of TAI cannot be demonstrated in all infertile women, but it is plausible in women with PCOS and with unexplained infertility. In the case of PCOS TAI might further affect fertility, being also associated to higher TSH levels and to a worse metabolic phenotype.
A clear pathophysiological link connecting TAI to infertility and to pregnancy outcome after spontaneous conception or ART has not been identified. There are several hypotheses and potential points of action have been proposed. Thyroid hormone-dependent as well as thyroid hormone-independent immunological effect of TAI on the ovary, on the uterus, and on fetoplacental unit have all been implicated. Moreover, TAI could represent a peripheral marker of a general immune imbalance affecting fertilization, implantation, and pregnancy maintenance (15–17). The most relevant hypotheses are briefly illustrated.
In searching a potential target of TAI in infertility and ART outcome, antithyroid antibodies direct binding and damage to the reproductive organs has been investigated. Interestingly it has been demonstrated that antithyroid antibodies can pass through the blood–follicle barrier during the maturation period. In a study of 31 women undergoing IVF, TPOAb and TgAb were measurable in the follicular fluid, on the day of oocyte retrieval, in 14 patients with TAI and in none of the negative control. The follicular fluid concentrations of antithyroid antibodies were approximately half with respect to those in the serum thus indicating that they pass the blood-follicle barrier and reach concentration proportional to their blood levels. In this study oocyte fertilization, good quality embryos, and pregnancy rates were lower in women with TAI than in negative controls, while early miscarriage rate was higher. This “follicle hypothesis” suggests that presence of antithyroid antibodies may create a cytotoxic environment that damages the maturing oocyte reducing its quality and fertilization potential (38). A recent study confirmed the association of antithyroid antibodies in follicular fluid and ART outcome. In 52 women undergoing ART a statistically significant correlation was found between the levels of TPOAb and TgAb in serum and follicular fluid. Pregnancy rates per initiated cycle and per embryo transfer cycle were lower in TAI women thus suggesting a negative effect on the post-implantation embryo development (39). Although only in three patients, thyroid peroxidase has been demonstrated, by immunocytochemistry, in the granulosa cumulus cells of the human ovarian follicle, thereby supporting the hypothesis that TPOAb could target their antigen directly at the level of the ovary (40). In an autoimmune thyroiditis animal model antithyroid antibodies were evidenced on the surface of pre-implantation embryos (41). Also, it has been shown that human anti-zona pellucida antibodies recognize antigens within the murine thyroid tissue. It can be speculated that the zona pellucida, which has an important functional role in the interaction between the oocyte and the sperm cell and in pre-implantation, may be a target for antithyroid antibodies (42). Although the evidence is limited, a role of a hostile immune environment at the level of the ovary, with TPO as the direct antigen, has been proposed as one of the non-thyroid hormone dependent mechanisms at least in the early stage of autoimmune process in infertility (16). These antibodies may generate an inflammatory response that alters the milieu of the maturing oocyte affecting ovarian reserve and embryo quality.
An alternative pathogenetic hypothesis considers that TAI might be a marker of both generalized humoral and cellular immune dysfunction. Polyclonal lymphocyte B activation is more frequent in TAI and is associated with the increased levels of non-organ-specific antibodies, such as antinuclear antibodies (ANA), anti-dsDNA, anti-ssDNA, and antiphospholipid antibodies (aPL). Indeed, most of these autoantibodies are associated with reproductive failure; aPL can cross-react with trophoblast–placental tissue thus reducing trophoblast viability, syncytialization and invasion (43). An increased rate of recurrent miscarriage is reported in patients with poly-autoimmune disorders as compared with patients affected by isolated TAI (44). An increased levels of antibodies against laminin-1 (LN-1), that have been associated with reproductive failure, have been demonstrated in serum and follicular fluid of infertile women with TAI. The serum levels of these antibodies were inversely correlated with oocyte count, along with a significantly reduced implantation rate and pregnancy rate (45). The concurrent presence of anti-ovarian autoantibodies, hallmarks of premature ovarian insufficiency (POF) obviously can affect the immune homeostasis of oocytes and impair ovarian reserve. POF can occur in isolation but is often associated with other autoimmune conditions. Hypothyroidism, TAI, and Graves’ disease are the most seen associated disorders (46). Lymphocyte T cells, T helper (Th)1/Th2 balance, and regulatory T (Treg) cells play an important role in pregnancy; an altered cellular immune status and increased secretion of inflammatory cytokines is considered to contribute to adverse pregnancy outcome such as miscarriage and pre-term birth (PBT) (47). IL-2 and INF-γ, produced by Th1 cells, play important roles for the induction of implantation failure and abortion while the proinflammatory cytokine IL-17 is involved in the pathogenesis of abortion and PTB (48). Animal models have shown that an increased incidence of fetal loss and enhanced Th1 cell proliferation in Tg-immunized mice (49). Also natural killer (NK) cells dysregulation in the peripheral blood, with a prevalence of cytotoxic over immunoregulatory is closely related to reproductive failures, including recurrent miscarriage (RM) and infertility (50). Indeed, phenotypic and functional analysis on peripheral blood mononuclear cells from healthy donors and from TAI patients showed Th1 oriented changes of innate immunity, elevated NK, and NKT-like cells ratios, and enhanced natural cytotoxicity in TAI positive euthyroid women (51). Proinflammatory cytokine such as IL-2 and IL-17 as well as interferon gamma have been shown increased in the serum and/or in the follicular fluid of patients with TAI along with a quantitative and qualitative changes in endometrial T cells with reduction of secretion of IL-4 and IL 10 (52–54). Also, an increase in the percentage of cytotoxic NK cells in women with TAI was associated with reproductive failures (51). These data, although merely descriptive, suggest that cellular immune dysfunction, with a proinflammatory Th1 immune response and excessive activation of NK cells and NKT-like cells might induce the occurrence of infertility, miscarriage, and PTB in women with TAI (15).
The natural history of chronic autoimmune thyroiditis is a progressive decline in the functional capacity of the thyroid gland, leading to subclinical and, ultimately, to overt hypothyroidism. A decreased functional capacity of the thyroid gland may become apparent during early pregnancy since this condition constitutes a state of extra demand of thyroid hormone production. Indeed, it is well known that TAI increases the risk of developing subclinical or overt hypothyroidism during pregnancy. In the first half of spontaneous pregnancy women with TPOAb had an increased prevalence of subclinical and overt hypothyroidism compared to controls (20.1 vs. 2.4% and 3.3 vs. 0.1%, respectively) (55). In women undergoing ART the ovarian stimulation anticipates the extra demand of thyroid hormone, which occurs only after conception during spontaneous pregnancy, and therefore TSH levels can increase significantly above 2.5 mIU/L before pregnancy in the presence of TAI (8). In early pregnancy the human chorionic gonadotropin (hCG), through its high homology with TSH, stimulates the thyroid, thus helping to maximize thyroid hormone secretion (6). An inappropriate thyroidal response to hCG stimulation has been considered an early marker of abnormal thyroid functional capacity in women with TAI. A study gathering data from two large population-based prospective cohorts showed that there was a positive association of hCG with FT4 as well as a negative association of hCG with TSH in TPOAb-negative women but not in TPOAb-positive women during early pregnancy. Women with TPOAb and lower FT4 than expected had a higher risk of premature delivery. This study therefore links the adverse pregnancy outcome to changes in thyroid function in TAI (56). It is believed that women with TAI may not benefit from the stimulation due to hCG aimed to meet the increased demand of thyroid hormones in early pregnancy. A recent study showed that TgAb also interferes with the thyroidal response to hCG. In 822 women at 7-20 weeks of gestation, when TSH was within the pregnancy-specific reference range, high concentrations of TPOAb and TgAb attenuated the FT4 stimulation and TSH suppression induced by hCG. This effect was more pronounced when both TPOAb and TgAb were present (57). Whether these data can be extrapolated for the dose of hCG used for the ovulation induction remains unknown and deserves further investigation (11). The antithyroid antibodies-induced impaired response of thyroid to hCG is not in contrast with their action on the ovary. Indeed, a two-stage mechanism has been recently proposed: at early stages of autoimmunity, when TPOAb levels are low and thyroid function is normal, the main effect of the antibodies is the creation of a hostile immune environment at the level of the ovary; as the autoimmune process progresses, the impaired response to hCG also becomes apparent (16).
Several studies addressed the effect of TAI on pregnancy outcomes in euthyroid women who had conceived spontaneously (58). Euthyroid women with TAI carried twofold risk of miscarriage and were found to have slightly higher age and TSH levels (59). A triple or double risk of miscarriage or preterm birth, respectively, has also been reported in women with TPOAb (60). In a recent study the presence of TPOAb was also associated with preterm birth; the association did not appear to be related to differences in thyroid function, although the highest risk of preterm birth was observed in women with TPOAb and TSH >4.0 mIU/L. No association of TgAb positivity with preterm birth was found (61). In infertile women the presence of TPOAb increased the risk of miscarriage while a preconceptional TSH ≥ 2.5 mIU/L did not (30). Likewise, there has been intense interest in whether TAI may affect the success of the infertility treatments. Whenever possible primary approaches to female infertility are targeted to the identified cause and include ovulation induction for women with ovulatory dysfunction; endoscopic or surgical procedures to treat tubal obstruction or endometriosis. Intrauterine insemination (IUI) with donor or partner sperm is the first line treatment in couples with unexplained infertility, cervical factor infertility, and male infertility. When all these approaches fail ART is indicated. ART comprises both in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). Although the two main indicators of ART success are the proportion of clinically pregnant women and the proportion of miscarried women, there are several parameters used to evaluate each step of the in vitro and in vivo ART outcomes starting from the ovarian response to the controlled hyperstimulation (COH), and the number of oocytes retrieved (NOR) and going further to the fertilization rate (FR) and to the implantation rate (IR). The most relevant outcome is the pregnancy rate (PR), and, better, the clinical pregnancy rate (CPR). Pregnancy indeed can be defined as either biochemical (i.e., a transient rise in hCG concentration) or clinical (the formation of a gestational sac and fetal heartbeat) and the outcome can be defined as live birth rate (LBR), ending with a miscarriage, miscarriage rate (MR), or with a pre-term birth (PTBR). Also, infant/neonatal complications are of interest, among these, above all, low birth weight (LBW).
The term ovarian reserve (OR), that is, the complete follicle pool and, mostly, the functional ovarian reserve (the number of maturing growing follicles after recruitment), expresses the woman’s reproductive potential. Several studies addressed the effect of TAI on OR by evaluating its predictive markers such as high basal follicle stimulating hormone (FSH) levels, antral follicle count (AFC), and anti-Müllerian hormone concentrations (AMH). In 30 adolescent patients with TAI, AMH levels were comparable to controls. Serum AMH was negatively correlated with TSH but not with TPOAb or TgAb levels (62). Also in a case-control study 30 adolescent girls, newly diagnosed with TAI, had normal ovarian reserve based on measurements of AMH, inhibin B, FSH, LH/FSH ratio, estradiol, and AFC (63). In 775 reproductive age women, without any thyroid or ovarian dysfunction, those with AMH levels in the lower quartile for age had higher levels of TPOAb at baseline while there was no statistically significant difference in thyroid hormones compared to women with AMH in the higher quartiles. Over a 12-year follow up FT4 was decreased in all quartiles whereas TPOAb increased only in women with AMH in the low quartile (64). In a study involving 108 euthyroid TAI patients with regular menstrual cycles, either treated or not with LT4, lower AMH levels were found in patients compared to the age-matched healthy women (65). OR is one of the most important determinants of downstream ART outcomes since it can affect the ovarian response to the COH protocols. Indeed, in 288 infertile women (55 euthyroid with TAI and 233 without) undergoing their first ART, higher levels of AMH were associated with better COH outcome as reflected by the estradiol/recombinant FSH ratio (E2/rFSH) and by the number of oocytes reaching metaphase II (M II oocytes). Women with TAI had lower AMH and higher FSH levels compared to TAI negative, but a poorer COH outcome was observed also in women with TAI and higher AMH levels. Thus, although diminished OR impairs COH outcome independently from TAI, the latter reduces ovarian response in women with preserved OR (66). The same group had previously also shown the effect of thyroid function on the above-mentioned markers of COH. In 262 women undergoing ART, those with TAI and TSH levels below 2.5 mIU/L had better ovarian response demonstrated by higher serum estradiol levels, higher E2/rFSH ratio, and a greater number of M II oocytes compared with those with TSH >2.5 mIU/L (67). In a study involving 1044 infertile women eligible for IUI/IVF, TSH levels, TPOAb positivity, and TgAb positivity were comparable between patients with variable ovarian reserve according to AMH levels. However, after patients with known causes of diminished ovarian reserve (i.e., genetic) were excluded, TPOAb positivity was higher in patients with low ovarian reserve thus indicating an association of TPOAb with idiopathic low ovarian reserve in unexplained infertility (68). In a study group of 436 women seeking fertility treatment, thyroid function or TPOAb positivity was not associated with AFC, while TgAb positivity was associated with a higher AFC. However, among women with diminished ovarian reserve or unexplained infertility, TPOAb and not TgAb, as well as lower FT3, were associated with a lower AFC (36). On the contrary in a large study including 5000 women no differences were found in the prevalence of TAI as well as of overt or subclinical hypothyroidism in women with low, normal, or high ovarian reserve expressed by age-specific AMH values (69). Also in 225 infertile women TSH <3.0 mIU/L was associated with significantly higher AMH compared to those with TSH ≥3.0 mIU/L after adjustment for thyroid autoimmunity and age, thus suggesting that thyroid function, even in the euthyroid range, has a more significant impact than TAI in the ovarian reserve (70).
Multiple clinical studies, systematic reviews, and metanalyses have evaluated the impact of TAI in ART in vitro and in vivo outcomes with conflicting conclusions. Indeed, the studies differ for sample size, design, causes of infertility, and type of ART, TSH level cut-off used to define euthyroidism as well as for the endpoints. Embryo quality was assessed in 431 embryos in euthyroid women with low ovarian reserve undergoing IVF. Comparable embryo quality was observed between women with TSH low-normal or high normal TSH (cut off 2.5 mIU/L) but impaired embryo quality was observed in women with TPOAb and TSH ≤ 2.5 mIU/L. Increasing TSH affected embryo quality and a trend toward the same effect was observed in women with TPOAb (71). Also, in a retrospective cohort study of 123 euthyroid women with or without TAI undergoing ART, embryo quality was significantly impaired in women with at least one autoantibody while no differences were found in AMH, FSH, and in the number of oocytes picked up and fertilized as well as in implantation rate and in pregnancy rate (72). Several prospective cohort studies releveled lower LBR and increased MR in euthyroid women with TPOAb undergoing IVF/ICSI. In 234 euthyroid women undergoing the first cycle of ART no differences in pregnancy rate were observed but the TAI women had threefold higher risk of miscarriage (73). No differences in pregnancy rate were observed between 412 TPOAb negative women and the 72 TPOAb positive that had been randomly assigned to receive LT4 or placebo. Nevertheless, a twofold risk of miscarriage was observed in euthyroid TPOAb positive women not subjected to LT4 treatment. The risk of miscarriage was not reduced by LT4 treatment in TPOAb positive women. This links the miscarriage to an abnormal immune response rather than to subsequent mild thyroid failure (74). Also, in a prospective study involving 590 infertile hypothyroid women treated with LT4, to maintain TSH levels below 2.5 μIU/mL, higher TPOAb titer was one of the risk factors for miscarriage, after IUI and IVF despite appropriate treatment (75). Among 194 euthyroid women undergoing IVF, the 60 with TPOAb/Tg Ab showed lower clinical pregnancy and live birth rate when confronted with controls. The same parameters were improved in 30 antithyroid antibodies-positive women treated with prednisone (76). On the contrary no effect on pregnancy outcome was observed in several retrospective studies published in the last 20 years. In 416 euthyroid women no differences in pregnancy and delivery rates were observed between women with and without TPOAb. However, women with TPOAb who failed to become pregnant or miscarried had high-normal TSH compared to the ones who delivered and compared to women without TPOAb (77). In a study enrolling 2406 women, analysis of cumulative delivery did not show differences between TAI positive and negative women after 6 IVF/ICSI cycles (78). Also, in more recent studies no impact of TPOAb and TgAb was observed in the MR and on the in vitro ART outcome such as NOR, FR, and embryo quality (26, 79, 80). In 584 women undergoing IVF/ICSI with TSH between 0.45 and 4.5 μIU/ml, NOR, FR, and CPR did not significantly differ between TAI positive and TAI negative. Subgroup analysis for only primary infertility patients showed a statistically significant lower CPR in TAI positive compared to TAI negative (29).
Also looking at meta-analysis studies conflicting results are reported. A meta-analysis of prospective studies reporting data on 1098 subfertile women undergoing IVF reported high miscarriage risk in euthyroid TAI women undergoing IVF but did not find significant difference in FR, CPR, and delivery rates (81). Comparable results were reported in a further study showing that TAI increased the risk of MR while it did not affect NOR, implantation, and clinical pregnancy rate. The effect persisted after meta-regression analysis of age and TSH serum levels although the authors do not exclude possible modifying effects of these two variables (82). A more recent metanalysis including 14 studies performed on euthyroid TAI women did not show differences in in vitro and in vivo ART outcomes (CPR, MR, LBR per cycle, number of embryos transferred, NOR) neither for maternal age nor TSH levels when compared to women without TAI (83). Thus, heterogeneous results are reported and, also, the risk for adverse outcome is reported lower in the more recent metanalysis studies than in the previous ones (11). The explanation for the heterogeneity of these results can be found in the study design, in the TSH threshold used to define euthyroidism and as well as in the type of ART treatment and ovulation induction protocols. The effect of preconception TSH levels in the ART outcomes has been the subject of a recent meta-analysis. The study reported that when the TSH cut-off value for SCH was set at 2.5 mIU/L, no significant differences were observed in ART-related outcomes between SCH patients and normal women. On the contrary there was an increased risk of MR in women with SCH when a TSH cut-off value of 3.5–5.0 mIU/L was used (84). Regarding the studies on ART procedure, some have shown that the ICSI outcome was not affected by TAI. Indeed, in the last years ICSI has become the most popular insemination method worldwide. In a meta-analysis of studies including 1855 ICSI cycles, women with and without TAI, not differing for age, had comparable CPR, MR, and LBR. The authors propose that the presence of TAI may become a new indication for ICSI, independently of the cause of infertility, as it may overcome the detrimental impact of autoimmunity on fertilization and embryo quality (85). Indeed, previous observations reported that ICSI, which requires no interaction between the sperm cell and the zona pellucida, resulted in similar PR in women with or without TAI undergoing ART for male infertility (86). If this is true different outcomes are expected between IVF/ICSI and IUI. In a retrospective cohort study, enrolling 3143 patients, no significant different outcomes (PR, MR, LBR) were observed after IUI in euthyroid women with and without TAI also when comparing subgroups according to TSH level (TSH ≥2.5 mIU/L vs. TSH <2.5 mIU/L) (87). This latter finding was confirmed in a study enrolling 726 euthyroid women undergoing IUI for unexplained infertility. In this study, cycle characteristics and pregnancy outcomes (CPR, MR, LBR) of patients with serum TSH levels between 0.3-2.5 mIU/L and 2.5-4.5 mIU/L were compared and no statistically significant differences could be detected (88). In conclusion, although ART is an ideal model to analyze the effect of TAI on each stage of the reproductive process, compared to spontaneous conception, the multitude of variables present in the studies (age, causes of infertility, protocols for ovarian stimulation, the type of ART treatment, thyroid antibody levels, thyroid function) make it difficult to attribute to TAI per se a role on adverse outcomes and also to identify the TSH level threshold at which a negative impact on ART is expected, keeping in mind that TSH above 2.5 mIU/L is easily encountered after ovarian stimulation and even more so in the presence of TAI (78, 84). In Table 2 are illustrated the above-mentioned studies addressing the effect of TAI on the prediction markers of ovarian reserve/response as well as on the pregnancy outcome.
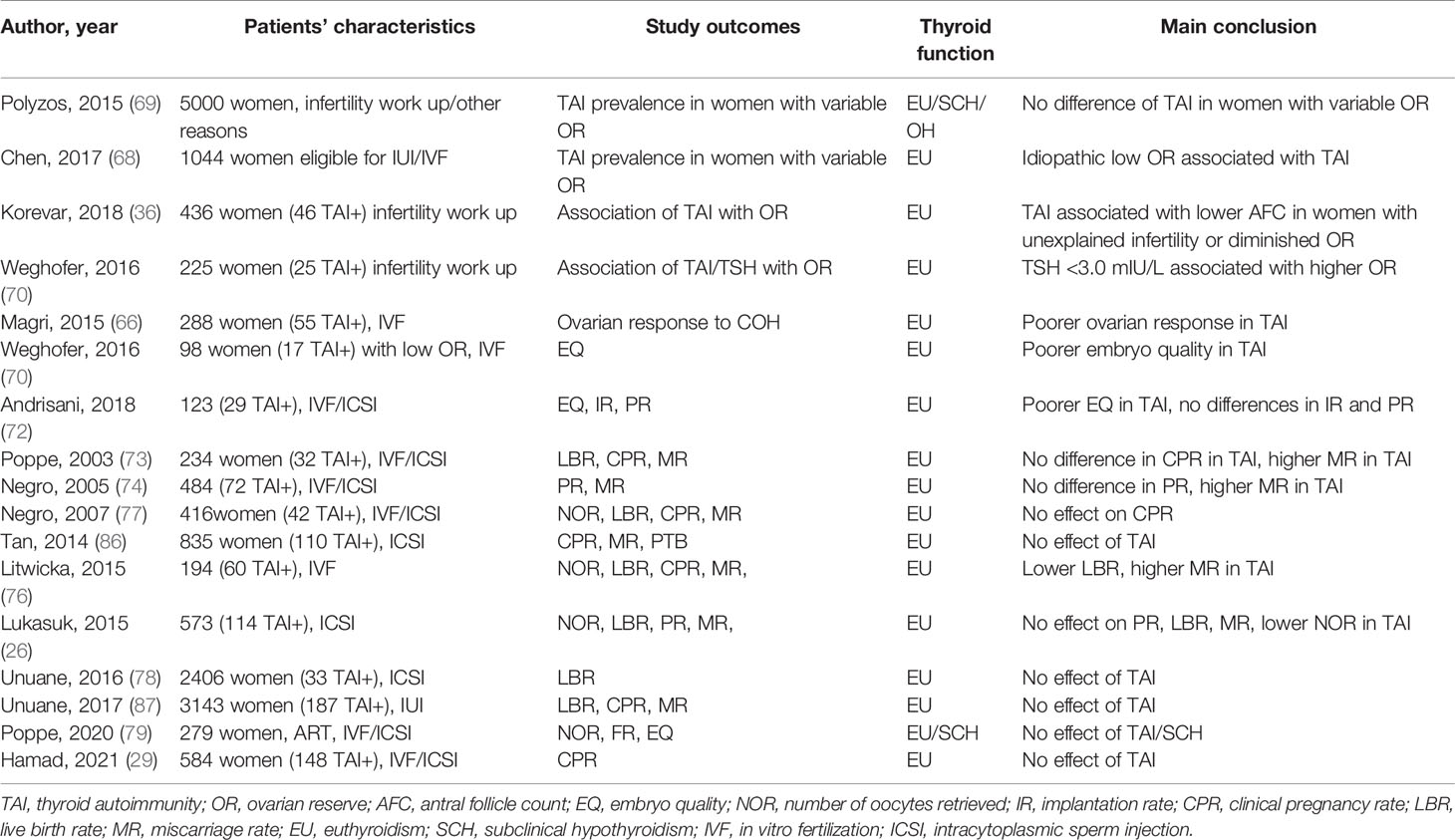
Table 2 Studies addressing the effect of TAI on ovarian reserve/response and on assisted reproductive technology (ART) outcome.
Universal thyroid screening in pregnancy fulfills most criteria for a beneficial and cost-effective screening program, aimed to reduce fetal and maternal complications, but it is still a matter of debate (89). Despite the discordant findings of the studies regarding the association of antithyroid antibodies with infertility and adverse outcome after ART, infertile women constitute a selected group of patients for whom specific recommendations for screening and management come from the guidelines. According to ATA, women with infertility or with a history of miscarriage are “at high risk” of developing thyroid dysfunction and for them serum TSH concentration is recommended as soon pregnancy is confirmed with reflex TPOAb measurement if TSH is >2.5-10 mIU/L. Women undergoing ART, instead, fulfill the criteria for TSH universal screening (13). As per the very recent ETA guidelines, specifically referring to ART, TSH and TPOAb should be measured in women seeking medical advice for infertility; “the TgAb can be added systematically according to the local regulatory authority rules” while it should be measured when TSH levels are higher than 2.5 mIU/L and TPOAb are absent (14). Thyroid ultrasound, which is useful to calculate the gland volume as well as to detect features compatible with autoimmune thyroiditis, is not mentioned in ATA guidelines while is recommended by ETA if TSH is >2.5 mIU/L and TPOAb is negative. Regarding the treatment, there is not debate about the need to start LT4 in women with overt hypothyroidism who undergo ART or become pregnant spontaneously independently from their thyroid antibodies status. For ATA the decision to treat women with TSH >2.5 and 10 mIU/L is based on TSH level and on the TPOAb status. Treatment is recommended in TPOAb-positive women when TSH is above 4 mUI/mL (if trimester specific ranges are not available) while it can be considered in TPOAb positive women with TSH >2.5 < 4 mUI/L and in TPOAb negative women with TSH >4 < 10 mUI/L. According to ATA, for euthyroid women undergoing ART LT4 treatment, at low starting dose of 25-50 μg “may be considered given its potential benefits in comparison to its minimal risk”. Indeed the 2.5 mUI/L TSH threshold still has value in women undergoing ART since ATA states that it is prudent to recommend treatment for “any TSH elevation over 2.5 mUI/L” before the procedure. On the contrary, also in case of ART, for some authors, a TSH level of 4 mIU/L is the cut-off for treatment since LT4 seems to increase LBR only when TSH is >4.0 mIU/L and also taking into account potential harmful effects of overtreatment (17, 90). The ETA guidelines recommend that women with serum TSH >4.0 mIU/L planning ART should be treated with LT4 independently of their TPOAb status, Table 3. In women with TAI and TSH levels >2.5<4 mIU/L, LT4 treatment at low starting dose of 25-50 μg LT4, before ovarian stimulation, could be “initiated in a case-by-case manner” such as in women with recurrent miscarriage, in women over 35 years of age, and with ovarian causes of infertility (14). According to ETA, LT4 treatment of TAI women with TSH levels >2.5<4 mIU/L could optimize ovarian reserve and improve embryo quality while there is no definitive evidence of benefits on pregnancy outcome. Several studies have addressed the benefit of LT4 on ART outcome in euthyroid women with TAI. In one randomized clinical trial (RCT) previously mentioned, pregnancy rate was not affected either by presence of TPOAb or by treatment with LT4. Euthyroid women (TSH ≤ 2.5 mIU/L) with TAI had high MR but LT4 treatment did not improve the delivery rate (74). These findings were supported by a recent large RCT. Treatment with LT4, 25-μg/d or 50-μg/d according to the TSH ≤2.5 or >2.5 mIU/L, respectively, did not reduce MR or increase CPR and LBR among 600 Chinese euthyroid women with TAI undergoing IVF. The study excluded women at high risk of miscarriage, and women positive for antinuclear and anticardiolipin antibodies, or lupus anticoagulant to eliminate other autoimmune confounding factors. Moreover, although in a limited number of patients, no benefits of therapy were observed in women with TSH>4 mIU/L (91). This study supports the conservative approach expressed by the recent guidelines (14). Nevertheless, in a meta-analysis, also including some studies, LT4 treatment had no significant impact on live births in women with SCH or TAI undergoing IVF; however, it decreased miscarriage rates. It is worth remembering that in some studies patients characterized by TPO-positivity and TSH levels 4.0 or 4.5 mIU/L were mixed in data synthesis (92). The TABLET study, a randomized, placebo-controlled trial, investigated the effect of a fixed dose of LT4 50 μg daily, on LBR in TPOAb positive euthyroid women. This very large study included women with a history of recurrent miscarriage or receiving treatment for infertility. Prespecified subgroup analyses were completed for the primary outcome according to maternal age, the baseline TSH level (≤2.5 mIU/L or >2.5 mIU/L), and infertility treatment. LT4 treatment did not result in a higher LBR compared with placebo (93). The studies not showing a benefit of LT4 support the hypothesis that the increased risk of miscarriage in TAI is linked to an abnormal immune response rather than to mild thyroid failure. In this regard ETA guidelines suggest ICSI as the preferred fertilization method in women with TAI to overcome potential negative effects of antithyroid antibodies on oocytes and embryos (14). Currently no treatments have been shown to reduce thyroid autoimmunity and this aspect continues to be studied (94). In a previous study the use of glucocorticoids demonstrated an increase in pregnancy rates with treatment compared with placebo (76). In a more recent study it has been demonstrated that glucocorticoid may improve the pregnancy outcomes of ART women with antithyroid antibodies positive, but does not reduce the risk of miscarriage (95). Selenium supplementation decreases thyroid autoantibodies concentration during pregnancy but with no benefit on fetal or maternal outcomes (96).
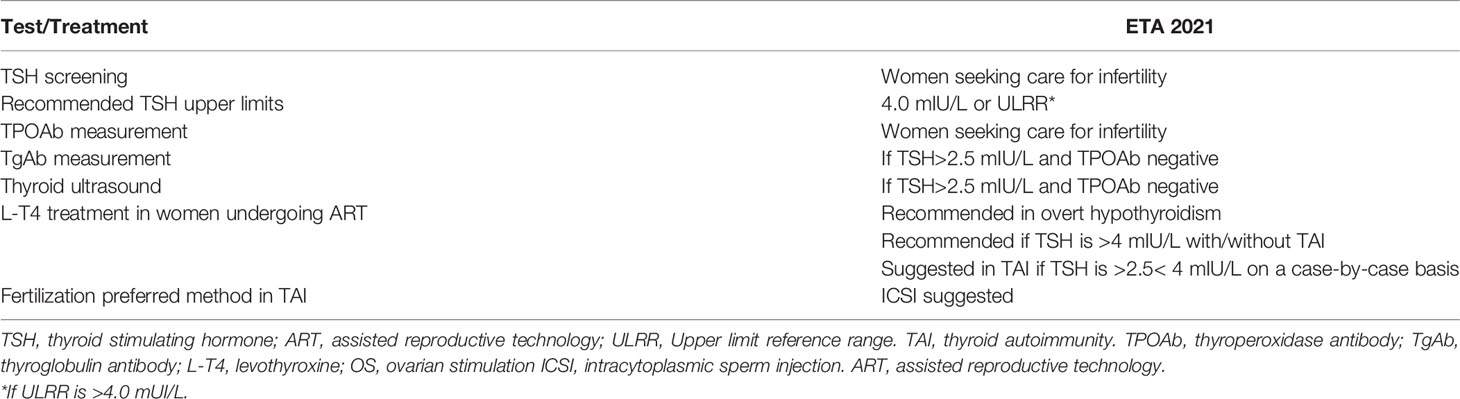
Table 3 Recommendations for women with infertility and/or undergoing assisted reproductive technology (ART) according to the ETA 2021 guidelines (14).
Overt thyroid dysfunction leads to menstrual disturbances, fertility problems, and pregnancy complications. Also, thyroid autoimmunity, even in the setting of euthyroidism, might adversely affect fertility and reproductive outcome by creating a cytotoxic environment that damages the maturing oocyte reducing its quality and fertilization potential and by inducing a subtle dysfunction during early pregnancy. Thyroid autoimmunity is associated with some causes of infertility (ovarian and idiopathic) and with some adverse pregnancy outcome, such as miscarriage, after assisted conception. Women with TAI undergoing ART are an ideal model to analyze the effect of thyroid autoimmunity/dysfunction on each stage of the reproductive process, compared to spontaneous conception. A better understanding of the pathophysiology may have an impact on the therapeutic approach. Although many questions remain unanswered, there is no doubt that it is justified for women seeking medical advice for infertility screening for thyroid dysfunction and autoimmunity. The decision for treatment should be based on the current evidence and recommendations but it cannot omit an individualized clinical judgement on the cause of the infertility as well as on the obstetric history of the women.
IB: substantial contributions to the conception and design of the work; reviewing the literature; drafting the work; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CG, GDD, GF, and GN: contributions to the design of the work; revising the work critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril (2017) 108(3):393–406. doi: 10.1016/j.fertnstert.2017.06.005
2. Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PloS Med (2012) 9(12):e1001356. doi: 10.1371/journal.pmed.1001356
3. Vissenberg R, Manders VD, Mastenbroek S, Fliers E, Afink GB, Ris-Stalpers C, et al. Pathophysiological Aspects of Thyroid Hormone Disorders/Thyroid Peroxidase Autoantibodies and Reproduction. Hum Reprod Update (2015) 21(3):378–87. doi: 10.1093/humupd/dmv004
4. Krassas GE, Poppe K, Glinoer D. Thyroid Function and Human Reproductive Health. Endocr Rev (2010) 31(5):702–55. doi: 10.1210/er.2009-0041
5. Oki N, Matsuo H, Nakago S, Murakoshi H, Laoag-Fernandez JB, Maruo T. Effects of 3,5,3'-Triiodothyronine on the Invasive Potential and the Expression of Integrins and Matrix Metalloproteinases in Cultured Early Placental Extravillous Trophoblasts. J Clin Endocrinol Metab (2004) 89(10):5213–21. doi: 10.1210/jc.2004-0352
6. Glinoer D. The Regulation of Thyroid Function in Pregnancy: Pathways of Endocrine Adaptation From Physiology to Pathology. Endocr Rev (1997) 18(3):404–33. doi: 10.1210/edrv.18.3.0300
7. de Escobar GM, Obregon MJ, del Rey FE. Maternal Thyroid Hormones Early in Pregnancy and Fetal Brain Development. Best Pract Res Clin Endocrinol Metab (2004) 18(2):225–48. doi: 10.1016/j.beem.2004.03.012
8. Mintziori G, Goulis DG, Toulis KA, Venetis CA, Kolibianakis EM, Tarlatzis BC. Thyroid Function During Ovarian Stimulation: A Systematic Review. Fertil Steril (2011) 96(3):780–5. doi: 10.1016/j.fertnstert.2011.06.020
9. Bucci I, Giuliani C, Napolitano G. Thyroid-Stimulating Hormone Receptor Antibodies in Pregnancy: Clinical Relevance. Front Endocrinol (Lausanne) (2017) 8:137. doi: 10.3389/fendo.2017.00137
10. Pearce EN. Thyroid Disorders During Pregnancy and Postpartum. Best Pract Res Clin Obstet Gynaecol (2015) 29(5):700–6. doi: 10.1016/j.bpobgyn.2015.04.007
11. Poppe K. MANAGEMENT OF ENDOCRINE DISEASE: Thyroid and Female Infertility: More Questions Than Answers?! Eur J Endocrinol (2021) 184(4):R123–R35. doi: 10.1530/EJE-20-1284
12. Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and Postpartum. Thyroid (2011) 21(10):1081–125. doi: 10.1089/thy.2011.0087
13. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid (2017) 27(3):315–89. doi: 10.1089/thy.2016.0457
14. Poppe K, Bisschop P, Fugazzola L, Minziori G, Unuane D, Weghofer A. 2021 European Thyroid Association Guideline on Thyroid Disorders Prior to and During Assisted Reproduction. Eur Thyroid J (2021) 9(6):281–95. doi: 10.1159/000512790
15. Zhu Q, Xu QH, Xie T, Wang LL, Liu H, Muyayalo KP, et al. Recent Insights Into the Impact of Immune Dysfunction on Reproduction in Autoimmune Thyroiditis. Clin Immunol (2021) 224:108663. doi: 10.1016/j.clim.2020.108663
16. Dosiou C. Thyroid and Fertility: Recent Advances. Thyroid (2020) 30(4):479–86. doi: 10.1089/thy.2019.0382
17. Unuane D, Velkeniers B. Impact of Thyroid Disease on Fertility and Assisted Conception. Best Pract Res Clin Endocrinol Metab (2020) 34(4):101378. doi: 10.1016/j.beem.2020.101378
18. Valdes S, Maldonado-Araque C, Lago-Sampedro A, Lillo JA, Garcia-Fuentes E, Perez-Valero V, et al. Population-Based National Prevalence of Thyroid Dysfunction in Spain and Associated Factors:RGlAYmV0LmVzStudy. Thyroid (2017) 27(2):156–66. doi: 10.1089/thy.2016.0353
19. Ragusa F, Fallahi P, Elia G, Gonnella D, Paparo SR, Giusti C, et al. Hashimotos' Thyroiditis: Epidemiology, Pathogenesis, Clinic and Therapy. Best Pract Res Clin Endocrinol Metab (2019) 33(6):101367. doi: 10.1016/j.beem.2019.101367
20. Roussev RG, Kaider BD, Price DE, Coulam CB. Laboratory Evaluation of Women Experiencing Reproductive Failure. Am J Reprod Immunol (1996) 35(4):415–20. doi: 10.1111/j.1600-0897.1996.tb00503.x
21. Kutteh WH, Yetman DL, Carr AC, Beck LA, Scott RT. Increased Prevalence of Antithyroid Antibodies Identified in Women With Recurrent Pregnancy Loss But Not in Women Undergoing Assisted Reproduction. Fertil Steril (1999) 71(5):843–8. doi: 10.1016/S0015-0282(99)00091-6
22. Janssen OE, Mehlmauer N, Hahn S, Offner AH, Gartner R. High Prevalence of Autoimmune Thyroiditis in Patients With Polycystic Ovary Syndrome. Eur J Endocrinol (2004) 150(3):363–9. doi: 10.1530/eje.0.1500363
23. Kaider AS, Kaider BD, Janowicz PB, Roussev RG. Immunodiagnostic Evaluation in Women With Reproductive Failure. Am J Reprod Immunol (1999) 42(6):335–46. doi: 10.1111/j.1600-0897.1999.tb00110.x
24. Poppe K, Velkeniers B, Glinoer D. Thyroid Disease and Female Reproduction. Clin Endocrinol (Oxf) (2007) 66(3):309–21. doi: 10.1111/j.1365-2265.2007.02752.x
25. Karacan M, Alwaeely F, Cebi Z, Berberoglugil M, Batukan M, Ulug M, et al. Effect of Antithyroid Antibodies on ICSI Outcome in Antiphospholipid Antibody-Negative Euthyroid Women. Reprod BioMed Online (2013) 27(4):376–80. doi: 10.1016/j.rbmo.2013.07.002
26. Lukaszuk K, Kunicki M, Kulwikowska P, Liss J, Pastuszek E, Jaszczolt M, et al. The Impact of the Presence of Antithyroid Antibodies on Pregnancy Outcome Following Intracytoplasmatic Sperm Injection-ICSI and Embryo Transfer in Women With Normal Thyreotropine Levels. J Endocrinol Invest (2015) 38(12):1335–43. doi: 10.1007/s40618-015-0377-5
27. Sakar MN, Unal A, Atay AE, Zebitay AG, Verit FF, Demir S, et al. Is There an Effect of Thyroid Autoimmunity on the Outcomes of Assisted Reproduction? J Obstet Gynaecol (2016) 36(2):213–7. doi: 10.3109/01443615.2015.1049253
28. Dhillon-Smith RK, Tobias A, Smith PP, Middleton LJ, Sunner KK, Baker K, et al. The Prevalence of Thyroid Dysfunction and Autoimmunity in Women With History of Miscarriage or Subfertility. J Clin Endocrinol Metab (2020) 105(8):2667–77. doi: 10.1210/clinem/dgaa302
29. Hamad A, Alhalabi N, Nmr N, Abbas F, Al-Hammami H, Ibrahim N, et al. Impact of Thyroid Autoimmunity in Euthyroid Women on the Outcomes of In Vitro Fertilization. Ann Med Surg (Lond) (2021) 67:102473. doi: 10.1016/j.amsu.2021.102473
30. Seungdamrong A, Steiner AZ, Gracia CR, Legro RS, Diamond MP, Coutifaris C, et al. Preconceptional Antithyroid Peroxidase Antibodies, But Not Thyroid-Stimulating Hormone, are Associated With Decreased Live Birth Rates in Infertile Women. Fertil Steril (2017) 108(5):843–50. doi: 10.1016/j.fertnstert.2017.08.026
31. Poppe K, Glinoer D, Van Steirteghem A, Tournaye H, Devroey P, Schiettecatte J, et al. Thyroid Dysfunction and Autoimmunity in Infertile Women. Thyroid (2002) 12(11):997–1001. doi: 10.1089/105072502320908330
32. Petta CA, Arruda MS, Zantut-Wittmann DE, Benetti-Pinto CL. Thyroid Autoimmunity and Thyroid Dysfunction in Women With Endometriosis. Hum Reprod (2007) 22(10):2693–7. doi: 10.1093/humrep/dem267
33. Kim JJ, Yoon JW, Kim MJ, Kim SM, Hwang KR, Choi YM. Thyroid Autoimmunity Markers in Women With Polycystic Ovary Syndrome and Controls. Hum Fertil (Camb) (2020) 25(1):128–34. doi: 10.1080/14647273.2019.1709668
34. van den Boogaard E, Vissenberg R, Land JA, van Wely M, Ven der Post JA, Goddijn M, et al. Significance of (Sub)Clinical Thyroid Dysfunction and Thyroid Autoimmunity Before Conception and in Early Pregnancy: A Systematic Review. Hum Reprod Update (2016) 22(4):532–3. doi: 10.1093/humupd/dmw003
35. Unuane D, Velkeniers B, Anckaert E, Schiettecatte J, Tournaye H, Haentjens P, et al. Thyroglobulin Autoantibodies: Is There Any Added Value in the Detection of Thyroid Autoimmunity in Women Consulting for Fertility Treatment? Thyroid (2013) 23(8):1022–8. doi: 10.1089/thy.2012.0562
36. Korevaar TIM, Minguez-Alarcon L, Messerlian C, de Poortere RA, Williams PL, Broeren MA, et al. Association of Thyroid Function and Autoimmunity With Ovarian Reserve in Women Seeking Infertility Care. Thyroid (2018) 28(10):1349–58. doi: 10.1089/thy.2017.0582
37. Rotondi M, Chiovato L, Pacini F, Bartalena L, Vitti P. Management of Subclinical Hypothyroidism in Pregnancy: A Comment From the Italian Society of Endocrinology and the Italian Thyroid Association to the 2017 American Thyroid Association Guidelines-"The Italian Way". Thyroid (2018) 28(5):551–5. doi: 10.1089/thy.2017.0424
38. Monteleone P, Parrini D, Faviana P, Carletti E, Casarosa E, Uccelli A, et al. Female Infertility Related to Thyroid Autoimmunity: The Ovarian Follicle Hypothesis. Am J Reprod Immunol (2011) 66(2):108–14. doi: 10.1111/j.1600-0897.2010.00961.x
39. Medenica S, Garalejic E, Arsic B, Medjo B, Bojovic Jovic D, Abazovic D, et al. Follicular Fluid Thyroid Autoantibodies, Thyrotropin, Free Thyroxine Levels and Assisted Reproductive Technology Outcome. PloS One (2018) 13(10):e0206652. doi: 10.1371/journal.pone.0206652
40. Monteleone P, Faviana P, Artini PG. Thyroid Peroxidase Identified in Human Granulosa Cells: Another Piece to the Thyroid-Ovary Puzzle? Gynecol Endocrinol (2017) 33(7):574–6. doi: 10.1080/09513590.2017.1296424
41. Lee YL, Ng HP, Lau KS, Liu WM, WS O, Yeung WS, et al. Increased Fetal Abortion Rate in Autoimmune Thyroid Disease is Related to Circulating TPO Autoantibodies in an Autoimmune Thyroiditis Animal Model. Fertil Steril (2009) 91(5 Suppl):2104–9. doi: 10.1016/j.fertnstert.2008.07.1704
42. Kelkar RL, Meherji PK, Kadam SS, Gupta SK, Nandedkar TD. Circulating Auto-Antibodies Against the Zona Pellucida and Thyroid Microsomal Antigen in Women With Premature Ovarian Failure. J Reprod Immunol (2005) 66(1):53–67. doi: 10.1016/j.jri.2005.02.003
43. Deroux A, Dumestre-Perard C, Dunand-Faure C, Bouillet L, Hoffmann P. Female Infertility and Serum Auto-Antibodies: A Systematic Review. Clin Rev Allergy Immunol (2017) 53(1):78–86. doi: 10.1007/s12016-016-8586-z
44. Cellini M, Santaguida MG, Stramazzo I, Capriello S, Brusca N, Antonelli A, et al. Recurrent Pregnancy Loss in Women With Hashimoto's Thyroiditis With Concurrent Non-Endocrine Autoimmune Disorders. Thyroid (2020) 30(3):457–62. doi: 10.1089/thy.2019.0456
45. Caccavo D, Pellegrino NM, Nardelli C, Vergine S, Leone L, Marolla A, et al. Anti-Laminin-1 Antibodies in Serum and Follicular Fluid of Women With Hashimoto's Thyroiditis Undergoing In Vitro Fertilization. Int J Immunopathol Pharmacol (2016) 29(2):280–7. doi: 10.1177/0394632015627281
46. Szeliga A, Calik-Ksepka A, Maciejewska-Jeske M, Grymowicz M, Smolarczyk K, Kostrzak A, et al. Autoimmune Diseases in Patients With Premature Ovarian Insufficiency-Our Current State of Knowledge. Int J Mol Sci (2021) 22(5):2594–605. doi: 10.3390/ijms22052594
47. Ghaebi M, Nouri M, Ghasemzadeh A, Farzadi L, Jadidi-Niaragh F, Ahmadi M, et al. Immune Regulatory Network in Successful Pregnancy and Reproductive Failures. BioMed Pharmacother (2017) 88:61–73. doi: 10.1016/j.biopha.2017.01.016
48. Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and Regulatory T-Cell Paradigm in Pregnancy. Am J Reprod Immunol (2010) 63(6):601–10. doi: 10.1111/j.1600-0897.2010.00852.x
49. Imaizumi M, Pritsker A, Kita M, Ahmad L, Unger P, Davies T. Pregnancy and Murine Thyroiditis: Thyroglobulin Immunization Leads to Fetal Loss in Specific Allogeneic Pregnancies. Endocrinology (2001) 142(2):823–9. doi: 10.1210/endo.142.2.7966
50. Seshadri S, Sunkara SK. Natural Killer Cells in Female Infertility and Recurrent Miscarriage: A Systematic Review and Meta-Analysis. Hum Reprod Update (2014) 20(3):429–38. doi: 10.1093/humupd/dmt056
51. Miko E, Meggyes M, Doba K, Farkas N, Bogar B, Barakonyi A, et al. Characteristics of Peripheral Blood NK and NKT-Like Cells in Euthyroid and Subclinical Hypothyroid Women With Thyroid Autoimmunity Experiencing Reproductive Failure. J Reprod Immunol (2017) 124:62–70. doi: 10.1016/j.jri.2017.09.008
52. Turhan Iyidir O, Konca Degertekin C, Sonmez C, Atak Yucel A, Erdem M, Akturk M, et al. The Effect of Thyroid Autoimmunity on T-Cell Responses in Early Pregnancy. J Reprod Immunol (2015) 110:61–6. doi: 10.1016/j.jri.2015.04.002
53. Lu H, Huang Y, Xin H, Hao C, Cui Y. The Expression of Cytokines IFN-Gamma, IL-4, IL-17A, and TGF-Beta1 in Peripheral Blood and Follicular Fluid of Patients Testing Positive for Anti-Thyroid Autoantibodies and its Influence on In Vitro Fertilization and Embryo Transfer Pregnancy Outcomes. Gynecol Endocrinol (2018) 34(11):933–9. doi: 10.1080/09513590.2018.1459546
54. Twig G, Shina A, Amital H, Shoenfeld Y. Pathogenesis of Infertility and Recurrent Pregnancy Loss in Thyroid Autoimmunity. J Autoimmun (2012) 38(2-3):J275–J81. doi: 10.1016/j.jaut.2011.11.014
55. Medici M, de Rijke YB, Peeters RP, Visser W, de Muinck Keizer-Schrama SM, Jaddoe VV, et al. Maternal Early Pregnancy and Newborn Thyroid Hormone Parameters: The Generation R Study. J Clin Endocrinol Metab (2012) 97(2):646–52. doi: 10.1210/jc.2011-2398
56. Korevaar TI, Steegers EA, Pop VJ, Broeren MA, Chaker L, de Rijke YB, et al. Thyroid Autoimmunity Impairs the Thyroidal Response to Human Chorionic Gonadotropin: Two Population-Based Prospective Cohort Studies. J Clin Endocrinol Metab (2017) 102(1):69–77. doi: 10.1210/jc.2016-2942
57. Hou Y, Liu A, Li J, Wang H, Yang Y, Li Y, et al. Different Thyroidal Responses to Human Chorionic Gonadotropin Under Different Thyroid Peroxidase Antibody and/or Thyroglobulin Antibody Positivity Conditions During the First Half of Pregnancy. Thyroid (2019) 29(4):577–85. doi: 10.1089/thy.2018.0097
58. Dhillon-Smith RK, Coomarasamy A. TPO Antibody Positivity and Adverse Pregnancy Outcomes. Best Pract Res Clin Endocrinol Metab (2020) 34(4):101433. doi: 10.1016/j.beem.2020.101433
59. Chen L, Hu R. Thyroid Autoimmunity and Miscarriage: A Meta-Analysis. Clin Endocrinol (Oxf) (2011) 74(4):513–9. doi: 10.1111/j.1365-2265.2010.03974.x
60. Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association Between Thyroid Autoantibodies and Miscarriage and Preterm Birth: Meta-Analysis of Evidence. Bmj (2011) 342:d2616. doi: 10.1136/bmj.d2616
61. The Consortium on Thyroid and Pregnancy—Study Group on Preterm Birth. Association of Thyroid Function Test Abnormalities and Thyroid Autoimmunity With Preterm Birth: A Systematic Review and Meta-Analysis. JAMA (2019) 322(7):632–41. doi: 10.1001/jama.2019.10931
62. Ozalp Akin E, Aycan Z. Evaluation of the Ovarian Reserve in Adolescents With Hashimoto's Thyroiditis Using Serum Anti-Mullerian Hormone Levels. J Clin Res Pediatr Endocrinol (2018) 10(4):331–5. doi: 10.4274/jcrpe.0047
63. Pirgon O, Sivrice C, Demirtas H, Dundar B. Assessment of Ovarian Reserve in Euthyroid Adolescents With Hashimoto Thyroiditis. Gynecol Endocrinol (2016) 32(4):306–10. doi: 10.3109/09513590.2015.1116510
64. Bahri S, Tehrani FR, Amouzgar A, Rahmati M, Tohidi M, Vasheghani M, et al. Overtime Trend of Thyroid Hormones and Thyroid Autoimmunity and Ovarian Reserve: A Longitudinal Population Study With a 12-Year Follow Up. BMC Endocr Disord (2019) 19(1):47. doi: 10.1186/s12902-019-0370-7
65. Ozturk Unsal I, Hepsen S, Akhanli P, Calapkulu M, Sencar ME, Yalcindag A, et al. Evaluation of Serum Anti-Mullerian Hormone Levels in Women With Hashimoto Thyroiditis in the Reproductive Age. Turk J Med Sci (2021) 51(2):716–21. doi: 10.3906/sag-2012-177
66. Magri F, Schena L, Capelli V, Gaiti M, Zerbini F, Brambilla E, et al. Anti-Mullerian Hormone as a Predictor of Ovarian Reserve in ART Protocols: The Hidden Role of Thyroid Autoimmunity. Reprod Biol Endocrinol (2015) 13(1):106. doi: 10.1186/s12958-015-0103-3
67. Magri F, Capelli V, Gaiti M, Brambilla E, Montesion L, Rotondi M, et al. Impaired Outcome of Controlled Ovarian Hyperstimulation in Women With Thyroid Autoimmune Disease. Thyroid (2013) 23(10):1312–8. doi: 10.1089/thy.2013.0022
68. Chen CW, Huang YL, Tzeng CR, Huang RL, Chen CH. Idiopathic Low Ovarian Reserve Is Associated With More Frequent Positive Thyroid Peroxidase Antibodies. Thyroid (2017) 27(9):1194–200. doi: 10.1089/thy.2017.0139
69. Polyzos NP, Sakkas E, Vaiarelli A, Poppe K, Camus M, Tournaye H. Thyroid Autoimmunity, Hypothyroidism and Ovarian Reserve: A Cross-Sectional Study of 5000 Women Based on Age-Specific AMH Values. Hum Reprod (2015) 30(7):1690–6. doi: 10.1093/humrep/dev089
70. Weghofer A, Barad DH, Darmon S, Kushnir VA, Gleicher N. What Affects Functional Ovarian Reserve, Thyroid Function or Thyroid Autoimmunity? Reprod Biol Endocrinol (2016) 14(1):26. doi: 10.1186/s12958-016-0162-0
71. Weghofer A, Himaya E, Kushnir VA, Barad DH, Gleicher N. The Impact of Thyroid Function and Thyroid Autoimmunity on Embryo Quality in Women With Low Functional Ovarian Reserve: A Case-Control Study. Reprod Biol Endocrinol (2015) 13:43. doi: 10.1186/s12958-015-0041-0
72. Andrisani A, Sabbadin C, Marin L, Ragazzi E, Dessole F, Armanini D, et al. The Influence of Thyroid Autoimmunity on Embryo Quality in Women Undergoing Assisted Reproductive Technology. Gynecol Endocrinol (2018) 34(9):752–5. doi: 10.1080/09513590.2018.1442427
73. Poppe K, Glinoer D, Tournaye H, Devroey P, van Steirteghem A, Kaufman L, et al. Assisted Reproduction and Thyroid Autoimmunity: An Unfortunate Combination? J Clin Endocrinol Metab (2003) 88(9):4149–52. doi: 10.1210/jc.2003-030268
74. Negro R, Mangieri T, Coppola L, Presicce G, Casavola EC, Gismondi R, et al. Levothyroxine Treatment in Thyroid Peroxidase Antibody-Positive Women Undergoing Assisted Reproduction Technologies: A Prospective Study. Hum Reprod (2005) 20(6):1529–33. doi: 10.1093/humrep/deh843
75. Inagaki Y, Takeshima K, Nishi M, Ariyasu H, Doi A, Kurimoto C, et al. The Influence of Thyroid Autoimmunity on Pregnancy Outcome in Infertile Women: A Prospective Study. Endocr J (2020) 67(8):859–68. doi: 10.1507/endocrj.EJ19-0604
76. Litwicka K, Arrivi C, Varricchio MT, Mencacci C, Greco E. In Women With Thyroid Autoimmunity, Does Low-Dose Prednisolone Administration, Compared With No Adjuvant Therapy, Improve In Vitro Fertilization Clinical Results? J Obstet Gynaecol Res (2015) 41(5):722–8. doi: 10.1111/jog.12615
77. Negro R, Formoso G, Coppola L, Presicce G, Mangieri T, Pezzarossa A, et al. Euthyroid Women With Autoimmune Disease Undergoing Assisted Reproduction Technologies: The Role of Autoimmunity and Thyroid Function. J Endocrinol Invest (2007) 30(1):3–8. doi: 10.1007/BF03347388
78. Unuane D, Velkeniers B, Deridder S, Bravenboer B, Tournaye H, De Brucker M. Impact of Thyroid Autoimmunity on Cumulative Delivery Rates in In Vitro Fertilization/Intracytoplasmic Sperm Injection Patients. Fertil Steril (2016) 106(1):144–50. doi: 10.1016/j.fertnstert.2016.03.011
79. Poppe K, Autin C, Veltri F, Sitoris G, Kleynen P, Praet JP, et al. Thyroid Disorders and In Vitro Outcomes of Assisted Reproductive Technology: An Unfortunate Combination? Thyroid (2020) 30(8):1177–85. doi: 10.1089/thy.2019.0567
80. Chen X, Mo ML, Huang CY, Diao LH, Li GG, Li YY, et al. Association of Serum Autoantibodies With Pregnancy Outcome of Patients Undergoing First IVF/ICSI Treatment: A Prospective Cohort Study. J Reprod Immunol (2017) 122:14–20. doi: 10.1016/j.jri.2017.08.002
81. Toulis KA, Goulis DG, Venetis CA, Kolibianakis EM, Negro R, Tarlatzis BC, et al. Risk of Spontaneous Miscarriage in Euthyroid Women With Thyroid Autoimmunity Undergoing IVF: A Meta-Analysis. Eur J Endocrinol (2010) 162(4):643–52. doi: 10.1530/EJE-09-0850
82. Busnelli A, Paffoni A, Fedele L, Somigliana E. The Impact of Thyroid Autoimmunity on IVF/ICSI Outcome: A Systematic Review and Meta-Analysis. Hum Reprod Update (2016) 22(6):775–90. doi: 10.1093/humupd/dmw019
83. Venables A, Wong W, Way M, Homer HA. Thyroid Autoimmunity and IVF/ICSI Outcomes in Euthyroid Women: A Systematic Review and Meta-Analysis. Reprod Biol Endocrinol (2020) 18(1):120. doi: 10.1186/s12958-020-00671-3
84. Zhao T, Chen BM, Zhao XM, Shan ZY. Meta-Analysis of ART Outcomes in Women With Different Preconception TSH Levels. Reprod Biol Endocrinol (2018) 16(1):111. doi: 10.1186/s12958-018-0424-0
85. Poppe K, Autin C, Veltri F, Kleynen P, Grabczan L, Rozenberg S, et al. Thyroid Autoimmunity and Intracytoplasmic Sperm Injection Outcome: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab (2018) 103(5):1755–66. doi: 10.1210/jc.2017-02633
86. Tan S, Dieterle S, Pechlavanis S, Janssen OE, Fuhrer D. Thyroid Autoantibodies Per Se Do Not Impair Intracytoplasmic Sperm Injection Outcome in Euthyroid Healthy Women. Eur J Endocrinol (2014) 170(4):495–500. doi: 10.1530/eje-13-0790
87. Unuane D, Velkeniers B, Bravenboer B, Drakopoulos P, Tournaye H, Parra J, et al. Impact of Thyroid Autoimmunity in Euthyroid Women on Live Birth Rate After IUI. Hum Reprod (2017) 32(4):915–22. doi: 10.1093/humrep/dex033
88. Karakis LS, Kiyak H, Okmen B, Ozdemir C, Turkgeldi E. Impact of Preconceptional Serum Thyroid Stimulating Hormone Values Ranging Between 2.5 and 4.5 mIU/L on Live Birth Rates Following Ovulation Induction and Intrauterine Insemination Treatment for Unexplained Infertility. BMC Womens Health (2021) 21(1):162. doi: 10.1186/s12905-021-01299-0
89. Taylor PN, Zouras S, Min T, Nagarahaj K, Lazarus JH, Okosieme O. Thyroid Screening in Early Pregnancy: Pros and Cons. Front Endocrinol (Lausanne) (2018) 9:626. doi: 10.3389/fendo.2018.00626
90. Velkeniers B, Van Meerhaeghe A, Poppe K, Unuane D, Tournaye H, Haentjens P. Levothyroxine Treatment and Pregnancy Outcome in Women With Subclinical Hypothyroidism Undergoing Assisted Reproduction Technologies: Systematic Review and Meta-Analysis of RCTs. Hum Reprod Update (2013) 19(3):251–8. doi: 10.1093/humupd/dms052
91. Wang H, Gao H, Chi H, Zeng L, Xiao W, Wang Y, et al. Effect of Levothyroxine on Miscarriage Among Women With Normal Thyroid Function and Thyroid Autoimmunity Undergoing In Vitro Fertilization and Embryo Transfer: A Randomized Clinical Trial. Jama (2017) 318(22):2190–8. doi: 10.1001/jama.2017.18249
92. Rao M, Zeng Z, Zhao S, Tang L. Effect of Levothyroxine Supplementation on Pregnancy Outcomes in Women With Subclinical Hypothyroidism and Thyroid Autoimmuneity Undergoing In Vitro Fertilization/Intracytoplasmic Sperm Injection: An Updated Meta-Analysis of Randomized Controlled Trials. Reprod Biol Endocrinol (2018) 16(1):92. doi: 10.1186/s12958-018-0410-6
93. Dhillon-Smith RK, Middleton LJ, Sunner KK, Cheed V, Baker K, Farrell-Carver S, et al. Levothyroxine in Women With Thyroid Peroxidase Antibodies Before Conception. N Engl J Med (2019) 380(14):1316–25. doi: 10.1056/NEJMoa1812537
94. De Leo S, Pearce EN. Autoimmune Thyroid Disease During Pregnancy. Lancet Diabetes Endocrinol (2018) 6(7):575–86. doi: 10.1016/s2213-8587(17)30402-3
95. Zhou G, Zhou M, Duan X, Li W. Glucocorticoid Supplementation Improves Reproductive Outcomes in Infertile Women With Antithyroid Autoimmunity Undergoing ART: A Meta-Analysis. Med (Baltimore) (2021) 100(16):e25554. doi: 10.1097/MD.0000000000025554
96. Mantovani G, Isidori AM, Moretti C, Di Dato C, Greco E, Ciolli P, et al. Selenium Supplementation in the Management of Thyroid Autoimmunity During Pregnancy: Results of the "SERENA Study", a Randomized, Double-Blind, Placebo-Controlled Trial. Endocrine (2019) 66(3):542–50. doi: 10.1007/s12020-019-01958-1
Keywords: thyroid autoimmunity, female infertility, assisted conception, assisted reproduction technology, pregnancy outcome, miscarriage, thyroid peroxidase antibodies, thyroglobulin antibodies
Citation: Bucci I, Giuliani C, Di Dalmazi G, Formoso G and Napolitano G (2022) Thyroid Autoimmunity in Female Infertility and Assisted Reproductive Technology Outcome. Front. Endocrinol. 13:768363. doi: 10.3389/fendo.2022.768363
Received: 31 August 2021; Accepted: 21 February 2022;
Published: 26 May 2022.
Edited by:
Vasyl Vasko, Uniformed Services University of the Health Sciences, United StatesReviewed by:
Minho Shong, Chungnam National University, South KoreaCopyright © 2022 Bucci, Giuliani, Di Dalmazi, Formoso and Napolitano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ines Bucci, aW5lcy5idWNjaUB1bmljaC5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.