
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 22 December 2022
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1095189
This article is part of the Research Topic Calcium and Parathormone: An Update of Clinical Presentation and New Therapies View all 6 articles
Context: The recent American and European guidelines on management of patients with primary hyperparathyroidism (PHPT) did not endorse neurocognitive evaluation as part of standard work-up and did not consider it as a surgery criterion.The neurocognitive deleterious effects of hyperparathyroidism and impact of parathyroidectomy on PHPT patients is yet to be elucidated.
Objective: To evaluate specific neurocognitive functions in PHPT patients prior to parathyroidectomy and describe the changes during follow-up with serial evaluations.
Design: A prospective case-control study including parathyroidectomy candidates evaluated at a tertiary teaching university hospital. Thorough neurocognitive evaluation was conducted before and 1- & 6-months following parathyroidectomy: Rey Auditory Verbal Learning Test (RAVLT), Rey-Osterrieth Complex Figure Test (ROCF), Trail Making Test A, Trail Making Test B, Addenbrooke’s Cognitive Examination-III (ACE), Frontal Assessment Battery (FAB), Beck Depression Inventory (BDI).
Results: 18 consecutive patients underwent successful parathyroidectomy. Various neurocognitive functions improved significantly after successful parathyroidectomy: long term auditory memory (RAVLT, p=0.008), short- and long-term visual memory (ROCF, p=0.006 and p=0.002 respectively), visual attention and complex concentration skills (trail making A, p<0.001) and executive abilities (trail making B, p=0.005). No change was identified in frontal-lobe abilities. Depression symptoms were absent or minimal prior to surgery and no significant change was observed after surgery.
Conclusions: PHPT is associated with significant various neurocognitive dysfunctions when mindfully evaluated before surgery. Successful parathyroidectomy results in several neurocognitive aspect improvements. The data suggest that neurocognitive deterioration may be considered an added parathyroidectomy criterion when surgical decision is not straightforward.
Primary hyperparathyroidism (PHPT), the most frequent cause of hypercalcemia, is a common disease with 0.86% prevalence in the USA, and is more frequent in post-menopausal women (3-4/1 female-male ratio) (1), whereas in different western Europe countries the prevalence varies but a similar pattern of increased incidence specifically in postmenopausal women is observed (2) Nowadays, PHPT is usually diagnosed by laboratory routine tests in asymptomatic patients, but classic symptoms include kidney-related symptoms such as nephrolithiasis (15-20% of PHPT patients) and bone-related symptoms (<2% of PHPT patients) (1, 3). Non-classic symptoms include muscle weakness, fatigue, rheumatological, neuromuscular manifestations, and neurocognitive and psychiatric symptoms (4–6). Parathyroidectomy is the only definitive treatment for PHPT in patients who meet at least one of specific criterion (7, 8).
Based on the association between primary hyperparathyroidism and several impaired neurocognitive functions as well as quality of life, with improvement in the latter after successful parathyroidectomy (9–12), the Association of Endocrine Surgeons’ guidelines endorsed consideration of neurocognitive impairment for parathyroidectomy (7). However, neurocognitive disorders were not considered an indication for surgery by the recently-updated Fifth International Parathyroid Workshop guidelines (13). On the other hand, the recent 2021 European Expert Consensus on practical management of specific aspects of parathyroid disorders did not even mention neurocognitive impairment in PHPT patients as a relevant topic (14). This is due to controversies and questions that remain unanswered: the extent of altered neurocognitive functions in patients with hyperparathyroidism remains unclear, as well as which of them can improve after successful parathyroidectomy, and the timeline for maximal improvement achievement. Moreover, since the incidence of PHPT peaks during the sixth decade, it is often hard to isolate its effect on neurocognitive function, as some changes may be attributed to aging or comorbidities (15). Finally, the mechanisms leading to impaired neurocognitive functions are still unclear, and may be related to either hypercalcemia, decreased 25 hydroxy vitamin D (25OHD) or elevated PTH levels; all may impact brain activity (16).
The aim of this study was to evaluate the baseline neurocognitive performance of patients with PHPT and to prospectively evaluate the changes after parathyroidectomy via a wide-range battery of validated tests at 1- & 6-months following surgery. In addition, the study aims to determine the time period for symptom improvement and its association to serum calcium and parathyroid hormone (PTH) levels.
This prospective study was approved by the IRB committee (HMO-18-0558).
Participants in this study were PHPT patients who were candidates for parathyroidectomy and later completed the procedure. Participants completed a battery of validated neurocognitive tests and questionnaires before, and 1- & 6-months following surgery. In addition, clinical data were collected from patients’ medical records – demographics, symptoms, and lab results (including PTH and calcium levels before and after surgery).
Patients were recruited between 1.1.2018 and 31.12.2020 from both the General Surgery and Ear, Nose and Throat Departments at Hadassah-Hebrew University Medical Center in Jerusalem. All patients gave informed consent to participate in the study. All tests, before and after surgery, were conducted at patients’ homes (by the same interviewer (NT) over the study period) in order to ensure a quiet and comfortable environment as well as homogeneity. Total time for completing the battery of test was between 50 and 120 minutes, depending on the subjects’ performance and cooperation.
The battery of tests included the following tests in this order of execution:
examines short- and long- term verbal memory alongside learning abilities (17). It consists of a list of 15 words that are read out loud by the examiner and is recalled by the subject from memory alone. This process is repeated five times, then a different list of 15 words is read to and recalled by the subject. In the 7th trial, the subject is required to recall the first list without hearing it again. A 20-minute break is held before the 8th trial (recall of the first list is done), also without hearing the list again. In this time, other tests are performed. Points are given per word that is recalled correctly in each attempt, so that the score for each trial is 0-15, with 15 being the best possible score.
examines short- and long- term visual-spatial memory, attention, planning, and working memory (18). In this test, a large, complex image is required to be drawn in three stages: First, the image is copied by the subject. Second, the image is taken from the subject and he/she must draw it from memory. A 20-minute break is held before the second recall drawing of the image, without seeing the image again. In this time, other tests are performed. Points are given for each part of the image that is drawn, according to three parameters: whether elements are drawn correctly, whether they are drawn in the right location and whether they are the same size. Each component can be scored 0, 0.5, 1, or 2 points. The total scoring of the image is on a scale of 0-36, 36 being the highest score possible.
examines visual attention, mental flexibility, and executive functioning (19). Trail Making Test A requires drawing a line between numbers in an ascending pattern from 1 to 25 as quickly as possible. Trail Making Test B is similar but the connection should be from a number to a letter and back to a number, also in an ascending pattern, with numbers being from 1-13 and letters A-L. Score for each test is the time taken for subjects to complete them and the number of errors.
examines various frontal-lobe related functions (20). It consists of six sections with six specific tasks scored 0-3 points each, with a maximum of 18 points being perfect performance.
a comprehensive test which examines various neurocognitive functions including orientation, attention, memory, fluency, language, praxis and visuospatial functions (21). Each task in the test is scored with rules specific to that task, with total scoring of the ACE test being between 0 to 100 points, with higher score indicating better performance.
assesses depression (22). It is a questionnaire consisting of 21 questions that the subject answers alone without the interference of the examiner. Each question has four possible answers scored between 0-3 points, so the total of the BDI test is a sum of them and ranges between 0 to 63 points. Higher score indicates greater depression.
For each patient, scores were compared before and after (1 & 6 months) parathyroidectomy and therefore they served as their own control.
Data analysis was performed using SPSS version 25 software (SPSS, Inc, Chicago, IL). Descriptive statistics are presented using prevalence and percentage values for categorical variables, while continuous variables are presented with means and standard deviation. Non-continuous variables are presented by median and range. Generalized linear model was used to analyze the differences between each session and the others.
This study included 18 participants. Their demographic and clinical are detailed in Table 1. The mean age of participants was 67.9 ± 7.6 years and 14 (77.8%) were females. Mean serum calcium level prior parathyroidectomy was 11.1 ± 0.7 mg/dl (normal 8.5-10.5 mg/dl) and mean serum PTH level was 15.4 ± 7.6 pmol/l (normal 1.8-8.3 pmol/l). Intraoperative findings included 13 (72.2%) patients with a single adenoma, 1 (5.6%) patient with double adenomas, and 2 (11.1%) patients with four-gland hyperplasia. The mean weight of the glands removed was 683.7 ± 661 mg. All included patients were cured and completed two sessions of neurocognitive tests: one pre-operative and another 1-month post-operatively. Of these 18 participants, 13 (72.2%) also completed a third session 6-months post-operatively. Table 2 presents the different tests scores, where improvement is defined by score elevation. Significant improvement in short- and long- term verbal memory alongside learning abilities were seen after curative parathyroidectomy (ACE 5, p=0.004; RAVLT 8, p=0.002). short- and long- term visual memory, attention, planning and working memory also improved significantly as the patients were able to draw the complex figure more precisely after observing it immediately and after a 20 minutes delay (ROCF 2 and ROCF 3 – p. value <0.001 for each test).
Another improvement was seen on the patients’ performance of Trail Making Tests A and B wherein they both completed the tasks faster and more accurately (p value of <0.001 and =0.006, respectively). Thus, visual attention, mental flexibility, and executive functioning all improved after curative surgery. Interestingly, the performances of the patients in the Trail Making Test B were normalized since the standard deviation narrowed from one session to the next, as seen in Table 3.
Nevertheless, no change was seen in frontal lobe functions as measured via the FAB (mean score was ~16 – near normal - in all three sessions and with a non-significant p-value of 0.082). Although depression scores were improved after surgery (BDI), this did not reach statistical significance (p=0.697). It is important to note that the preoperative BDI score did not reach the lower limit of the depression range in any of the patients. (A score up to 10 is considered normal).
Regarding improvement rates for each test, 89% improved in the RCFT3; 78% improved in the RCFT 2, RAVLT 8 and Trail Making Test A; 67% improved in the Trail Making B test; 61% improved in the ACE 5 and BDI tests; 50% improved in the ACE 5 DELAY and FAB tests. All patients showed some improvement. The average number of tests improved was 6.9 ± 1.4 tests per patient.
No correlation was found between patients’ preoperative PTH nor calcium levels and their change in neurocognitive tests. Other variables that were tested for correlation with change in neurocognitive tests including age, total weight of adenomas removed, pre-operative serum 25OHD levels, failed to demonstrate significant correlation.
In this prospective study evaluating 18 PHPT patients for neurocognitive disorders before and 1 month after successful parathyroidectomy, a follow-up over 6 months in 13 of these patients was completed. The baseline impaired neurocognitive functions were: [1] the short- and long-term verbal memory with learning abilities assessed by RAVLT, [2] the short- and long-term visual memory, attention, planning, and working memory assessed by ROCF test, [3] visual attention, mental flexibility, and executive functioning evaluated by TMTs, and [4] complex neurocognitive functions (attention, memory, fluency, language, and visuospatial functions) assessed by ACE-III tests. This study demonstrated that successful parathyroidectomy resulted in a significant improvement in short- and long-term verbal memory and learning abilities (ACE5, RAVLT8). Moreover, a significant improvement in short- and long-term visual memory, attention, planning and working memory (ROCF2, ROCF3) was identified. Lastly, it demonstrated a significant improvement in visual attention, mental flexibility and executive function (TMT A, TMT B). The study did not identify an improvement in depressive symptoms (BDI) nor in frontal lobe-associated abilities (FAB). This is probably because all our patients had low baseline scores for depressive symptoms and near-normal baseline assessment of frontal capacities before surgery (mean FAB test results 16/18) making it difficult to identify any improvement of these functions. Neurocognitive impairment, mood and psychiatric disorders in PHPT patients were described as part of the general clinical symptoms in mid-twentieth century (usually retrospective) studies and were found in up to 65% of cases (23–26).
A review of the English-language literature concerning prospective, comprehensive evaluation of neurocognitive function before and after parathyroidectomy (5, 9–12, 27–37, 38), demonstrates that our results are in accordance with previous studies, but not with all, and add new data in terms of neurocognitive assessment before surgery, observed improvement after parathyroidectomy, and timing of improvement (Table 4). The tests used in prior studies were recurrent from one study to the other although not exactly similar, and could show a wide variability of both baseline range of impairment in verbal and visual memory, attention, complex neurocognitive functions, depression or anxiety symptoms, as well as post-surgery extent of improvement Table 4).
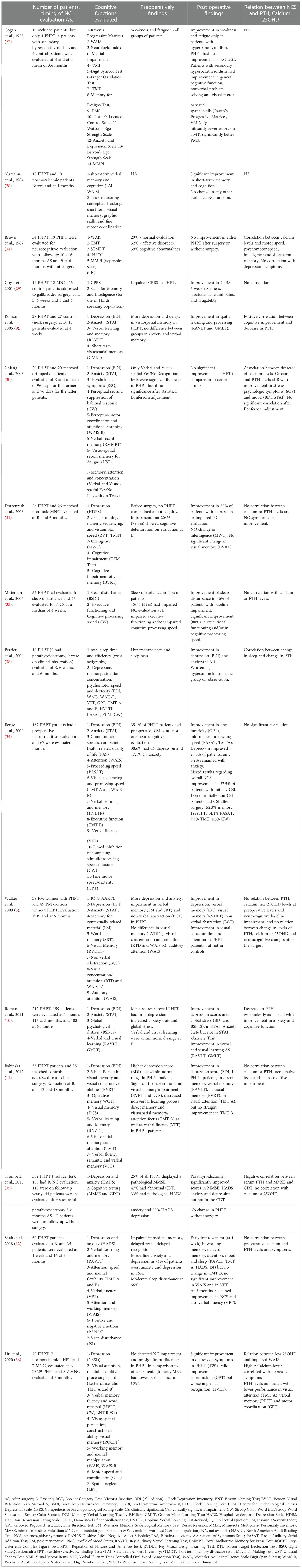
Table 4 Review of studies with neurocognitive evaluation of primary hyperparathyroidism patients at baseline and after parathyroidectomy.
Some subtle differences in retrieval verbal memory versus identification verbal memory capacity or phonetic and semantic verbal flux warrant further investigation for better characterization in patients with hyperparathyroidism. Of note, our study is the first to show a significant improvement in the TMT B related to amelioration of visual attention dysfunction, whereas Babinska et al. (11) as well as Shah et al. (12) failed to demonstrate such improvement. Moreover, difference in the timing of improvement from one study to the other can be observed, which may be due to the different recruited populations and the heterogeneous design of the studies.
The recently published European expert consensus on practical management of specific aspects of parathyroid disorders as part of the recommendations of the European Society of Endocrinology Program of Parathyroid Disorders (PARAT2021) did not assess at all the issue of neurocognitive impairment in PHPT patients (14). However, the recently-updated Fifth International Workshop on the Evaluation and Management of Primary Hyperparathyroidism recommended avoiding performing routine neurocognitive evaluation and to avoid using baseline neurocognitive impairment to decide on surgery (8). The main argument was about evaluation of quality of life (QoL) in PHPT patients (39): non-specific and subjective QoL questionnaires as well as short form 36-item (SF-36) have been used in the past and showed decreased QoL in PHPT patients, with improvement after successful parathyroidectomy (40–42); however, a recent well-designed Scandinavian prospective study showed no significant difference in QoL in PHPT patients during 10 years of follow-up after parathyroidectomy in comparison with those without surgery (43). Similarly, the 2019 first European workshop on parathyroid only raised the ambiguous results concerning potential benefit of parathyroidectomy on QoL (44). However, the debate is not closed as a recent large review showed that despite heterogenous designs in the 31 included studies, PHPT was associated with reduced QoL and several parameters (vitality, mental health, mood swings) improved significantly after parathyroidectomy when appropriately assessed either by the Parathyroidectomy Assessment of Symptoms Scale (PAS) or the 36-item Short Form Survey (SF-36) (45). Moreover these positions were actually based on a very limited aspect of impaired cognition in PHPT patients, and did not consider the studies which described extended neurocognitive assessment in PHPT patients.
Thus, we believe that performing a neurocognitive assessment beyond simple evaluation of QoL, but rather focusing on verbal, visual, visuospatial, attention, and executive functioning assessment, as observed in our study and many others, may reveal the potential benefit of parathyroid surgery in those patients for whom there is no clear-cut decision based on the classical surgical criteria as updated by the Fifth International Workshop on Parathyroidectomy. Indeed, the observed improvement following parathyroidectomy may have a significant impact on the day-to-day life of patients with PHPT, for example due to improved capacity to remember a list of words or to plan and execute simple tasks.
Finally, despite some studies demonstrating a correlation between baseline PTH levels and impaired neurocognitive assessment, or extent of decrease in PTH levels after parathyroidectomy and neurocognitive improvement, many other studies, including the present one, found no correlation between PTH, calcium or 25OHD levels and pre-operative neurocognitive function deterioration or post-surgery improvement (Table 4). However, the correlation between 25OHD levels and cognitive impairment was rarely evaluated.
A systematic review included 27 studies of low to moderate quality with evaluation of relation between PTH and dementia or cognitive impairment, found mixed results and potential improvement in memory after parathyroidectomy (46); A more recent systematic review (47) identified two studies that demonstrated potential correlation between elevated PTH levels and alteration in the Mini Mental State Examination. However, the quality of evidence was low overall, with heterogenous results for other neurocognitive evaluations. Another study (which did not focus on patients with primary hyperparathyroidism) did not show any relation between elevated PTH levels and progressive cognitive decline over 20 years as evaluated by three tests (Delayed Word Recall, the Digit Symbol Substitution, and the Word Fluency tests) and a compound Z score (48). On the other hand, analyses of calcium and monoamine in the cerebral spine fluid were found to correlate with neurocognitive impairment and improvement after surgery (49, 50); data from a recent review did not demonstrate that changes in CSF could be related to neurocognitive symptoms (51).
These observations underscore the fact that we are still far from understanding the pathophysiology underlying neurocognitive symptoms in PHPT patients. However there is a physiologic basis to support the association between PHPT and neurocognitive symptoms, as co-distribution in specific regions of the brain of parathyroid hormone 2 receptor (PTH2R) and an endogenous peptide (tuberoinfundibular peptide of 39 residues, able to locally activate PTH2R) suggested the existence of a neuromodulatory system in specific regions of the brain (52) which can be involved in auditory and limbic functions as well as stress regulation or anxiety levels. Several studies (32, 36, 38, 53, 54) examined dysfunction in specific cerebral zones using functional resonance magnetic imaging, alteration in vertebral vascular flow using transcranial doppler (36) or single photon emission computed tomography (55), or perioperative changes in cortical excitability with transcranial magnetic stimulation (56) in patients with PHPT. However, these studies have not yet reach a systematic and homogeneous conclusion (16).
The findings of our study are concordant with those of previously studies (Table 4), and show that specific subtle neurocognitive function are repeatedly found to be altered in PHPT patients and may improve after successful parathyroidectomy.
Our study has several limitations. First, it includes a relatively small sample of patients, which limits the extension of our results to larger cohorts. Unfortunately, the coronavirus pandemic prevented us from continuing to perform in-home interviews and testing. Second, the study design did not include a control group of patients who did not undergo parathyroidectomy. Yet, each patient is compared to his/her own results before surgery, and the intention of this study was to focus on the effect of successful surgery. Third, patients’ pre-surgery neurocognitive assessments demonstrated only mild impairment in most of the tests, and were normal in some, which of course limits the possibility to identify any improvement after surgery.
Despite these important limitations, our study did demonstrate that in patients with PHPT, parathyroidectomy leads to rapid (within one month) improvement in several neurocognitive functions such as short- and long-term verbal memory, short- and long-term visual memory, visual attention, and concentration. Other functions such as complex cognitive functions and global management capacity improve later (at 6 months). This study suggests that parathyroidectomy may positively impact daily neurocognitive functions of PHPT patients, with important improvement in several day-to-day activities. A practical approach to identify PHPT patients without straight indication for parathyroidectomy but who could benefit from surgery, would be to evaluate QoL and to screen neurocognitive impairment by asking during a consultation a simple question to the patient as: “Do you feel you have any problems with your thinking or memory?” and separately to family members: “Do you feel he/she has any problems with his/her thinking or memory?”; according to the answers, a decision can be taken to address or not the patient for further specific neurocognitive evaluation as described in our study.
As recommended in the research agenda published by the Fifth International Workshop on Primary Hyperparathyroidism (13), larger prospective randomized controlled trials are essential to determine the optimal neurocognitive evaluation battery tests before parathyroidectomy and to evaluate the benefit of the latter. A systematic approach and further obtention of appropriate data will determine if altered neurocognitive function may be considered a potential new surgery criterion for patients with primary hyperparathyroidism who do not meet the strict consensual criteria for parathyroidectomy.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Hadassah Medical Center Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
AS - Design, writing and reviewing. NT - Retrieving data, analysis of data, writing. HM - Design, retrieving data, writing, reviewing JN - Design, reviewing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bilezikian JP. Primary hyperparathyroidism. J Clin Endocrinol Metab (2018) 103(11):3993–4004. doi: 10.1210/jc.2018-01225
2. Minisola S, Arnold A, Belaya Z, Brandi ML, Clarke BL, Hannan FM, et al. Epidemiology, pathophysiology, and genetics of primary hyperparathyroidism. J Bone Miner Res (2022) 37(11):2315–29. doi: 10.1002/jbmr.4665
3. Bilezikian JP, Bandeira L, Khan A, Cusano NE. Hyperparathyroidism. Lancet. (2018) 391(10116):168–78. doi: 10.1016/S0140-6736(17)31430-7
4. Repplinger D, Schaefer S, Chen H, Sippel RS. Neurocognitive dysfunction: a predictor of parathyroid hyperplasia. Surgery. (2009) 146(6):1138–43. doi: 10.1016/j.surg.2009.09.009
5. Walker MD, McMahon DJ, Inabnet WB, Lazar RM, Brown I, Vardy S, et al. Neuropsychological features in primary hyperparathyroidism: a prospective study. J Clin Endocrinol Metab (2009) 94(6):1951–8. doi: 10.1210/jc.2008-2574
7. Wilhelm SM, Wang TS, Ruan DT, Lee JA, Asa SL, Duh QY, et al. The American association of endocrine surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg (2016) 151(10):959–68. doi: 10.1001/jamasurg.2016.2310
8. Bilezikian JP, Khan AA, Clarke BL, Mannstadt M, Potts JT, Brandi ML, et al. The fifth international workshop on the evaluation and management of primary hyperparathyroidism. J Bone Miner Res (2022) 37(11):2290–92. doi: 10.1002/jbmr.4671
9. Roman SA, Sosa JA, Mayes L, Desmond E, Boudourakis L, Lin R, et al. Parathyroidectomy improves neurocognitive deficits in patients with primary hyperparathyroidism. Surgery. (2005) 138(6):1121–8. doi: 10.1016/j.surg.2005.08.033
10. Roman SA, Sosa JA, Pietrzak RH, Snyder PJ, Thomas DC, Udelsman R, et al. The effects of serum calcium and parathyroid hormone changes on psychological and cognitive function in patients undergoing parathyroidectomy for primary hyperparathyroidism. Ann Surg (2011) 253(1):131–7. doi: 10.1097/SLA.0b013e3181f66720
11. Babinska D, Barczynski M, Stefaniak T, Oseka T, Babinska A, Babinski D, et al. Evaluation of selected cognitive functions before and after surgery for primary hyperparathyroidism. Langenbecks Arch Surg (2012) 397(5):825–31. doi: 10.1007/s00423-011-0885-5
12. Shah-Becker S, Derr J, Oberman BS, Baker A, Saunders B, Carr MM, et al. Early neurocognitive improvements following parathyroidectomy for primary hyperparathyroidism. Laryngoscope. (2018) 128(3):775–80. doi: 10.1002/lary.26617
13. Bilezikian JP, Khan AA, Silverberg SJ, Fuleihan GE, Marcocci C, Minisola S, et al. Evaluation and management of primary hyperparathyroidism: Summary statement and guidelines from the fifth international workshop. J Bone Miner Res (2022) 37(11):2293–314. doi: 10.1002/jbmr.4677
14. Bollerslev J, Rejnmark L, Zahn A, Heck A, Appelman-Dijkstra NM, Cardoso L, et al. European Expert consensus on practical management of specific aspects of parathyroid disorders in adults and in pregnancy: Recommendations of the ESE educational program of parathyroid disorders. Eur J Endocrinol (2022) 186(2):R33–63. doi: 10.1530/EJE-21-1044
15. Grant P, Velusamy A. What is the best way of assessing neurocognitive dysfunction in patients with primary hyperparathyroidism? J Clin Endocrinol Metab (2014) 99(1):49–55. doi: 10.1210/jc.2013-3115
16. Chandran M, Yeh LTL, de Jong MC, Bilezikian JP, Parameswaran R. Cognitive deficits in primary hyperparathyroidism - what we know and what we do not know: A narrative review. Rev Endocr Metab Disord (2022) 23(5):1079–87. doi: 10.1007/s11154-022-09750-9
17. Khosravi Fard E, LK J, Akbarzadeh Bagheban A, R WK. Comparison of the rey auditory verbal learning test (RAVLT) and digit test among typically achieving and gifted students. Iran J Child Neurol (2016) Spring, 10(2):26–37.
18. Shin MS, Park SY, Park SR, Seol SH, Kwon JS. Clinical and empirical applications of the rey-osterrieth complex figure test. Nat Protoc (2006) 1(2):892–9. doi: 10.1038/nprot.2006.115
19. Strauss ES, Sherman EMMS, Spreen OSTrail making test (TMT). a compendium of neuropsychological tests: Administration, norms, and commentary, 3rd ed, New York: Oxford University Press. (2006) 655–677.
20. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. (2000) 55(11):1621–6. doi: 10.1212/WNL.55.11.1621
21. Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, Hodges JR. A brief cognitive test battery to differentiate alzheimer's disease and frontotemporal dementia. Neurology. (2000) 55(11):1613–20. doi: 10.1212/01.wnl.0000434309.85312.19
22. Strauss EM, Sherman EMMS, Spreen OS. Beck depression inventory-second edition (BDI-II). In: Press OU, editor Compendium of neuropsychological tests, 3rd ed, (New York: Oxford University Press). (2006) p.1084–9.
23. Karpati G, Frame B. Neuropsychiatric disorders in primary hyperparathyroidism. clinical analysis with review of the literature.Arch Neurol (1964) 10:387–97. doi: 10.1001/archneur.1964.00460160057005
24. Henson RA. The neurological aspects of hypercalcaemia: With special reference to primary hyperparathyroidism. J R Coll Physicians Lond (1966) 1(1):41–50.
25. Petersen P. Psychiatric disorders in primary hyperparathyroidism. J Clin Endocrinol Metab (1968) 28(10):1491–5. doi: 10.1210/jcem-28-10-1491
26. Joborn C, Hetta J, Palmer M, Akerstrom G, Ljunghall S. Psychiatric symptomatology in patients with primary hyperparathyroidism. Ups J Med Sci (1986) 91(1):77–87. doi: 10.3109/03009738609178493
27. Cogan MG, Covey CM, Arieff AI, Wisniewski A, Clark OH, Lazarowitz V, et al. Central nervous system manifestations of hyperparathyroidism. Am J Med (1978) 65(6):963–70. doi: 10.1016/0002-9343(78)90748-9
28. Numann PJ, Torppa AJ, Blumetti AE. Neuropsychologic deficits associated with primary hyperparathyroidism. Surgery. (1984) 96(6):1119–23.
29. Goyal A, Chumber S, Tandon N, Lal R, Srivastava A, Gupta S. Neuropsychiatric manifestations in patients of primary hyperparathyroidism and outcome following surgery. Indian J Med Sci (2001) 55(12):677–86.
30. Chiang CY, Andrewes DG, Anderson D, Devere M, Schweitzer I, Zajac JD. A controlled, prospective study of neuropsychological outcomes post parathyroidectomy in primary hyperparathyroid patients. Clin Endocrinol (Oxf) (2005) 62(1):99–104. doi: 10.1111/j.1365-2265.2004.02180.x
31. Dotzenrath CM, Kaetsch AK, Pfingsten H, Cupisti K, Weyerbrock N, Vossough A, et al. Neuropsychiatric and cognitive changes after surgery for primary hyperparathyroidism. World J Surg (2006) 30(5):680–5. doi: 10.1007/s00268-005-0444-8
32. Perrier ND, Coker LH, Rorie KD, Burbank NS, Kirkland KA, Passmore LV, et al. Preliminary report: functional MRI of the brain may be the ideal tool for evaluating neuropsychologic and sleep complaints of patients with primary hyperparathyroidism. World J Surg (2006) 30(5):686–96. doi: 10.1007/s00268-005-0361-x
33. Mittendorf EA, Wefel JS, Meyers CA, Doherty D, Shapiro SE, Lee JE, et al. Improvement of sleep disturbance and neurocognitive function after parathyroidectomy in patients with primary hyperparathyroidism. Endocr Pract (2007) 13(4):338–44. doi: 10.4158/EP.13.4.338
34. Benge JF, Perrier ND, Massman PJ, Meyers CA, Kayl AE, Wefel JS. Cognitive and affective sequelae of primary hyperparathyroidism and early response to parathyroidectomy. J Int Neuropsychol Soc (2009) 15(6):1002–11. doi: 10.1017/S1355617709990695
35. Trombetti A, Christ ER, Henzen C, Gold G, Brandle M, Herrmann FR, et al. Clinical presentation and management of patients with primary hyperparathyroidism of the Swiss primary hyperparathyroidism cohort: a focus on neuro-behavioral and cognitive symptoms. J Endocrinol Invest (2016) 39(5):567–76. doi: 10.1007/s40618-015-0423-3
36. Liu M, Sum M, Cong E, Colon I, Bucovsky M, Williams J, et al. Cognition and cerebrovascular function in primary hyperparathyroidism before and after parathyroidectomy. J Endocrinol Invest (2020) 43(3):369–79. doi: 10.1007/s40618-019-01128-0
37. Brown GG, Preisman RC, Kleerekoper M. Neurobehavioral symptoms in mild primary hyperparathyroidism: related to hypercalcemia but not improved by parathyroidectomy. Henry Ford Hosp Med J (1987) 35(4):211–5.
38. Perrier ND, Balachandran D, Wefel JS, Jimenez C, Busaidy N, Morris GS, et al. Prospective, randomized, controlled trial of parathyroidectomy versus observation in patients with "asymptomatic" primary hyperparathyroidism. Surgery. (2009) 146(6):1116–22. doi: 10.1016/j.surg.2009.09.034
39. Bilezikian JP, Silverberg SJ, Bandeira F, Cetani F, Chandran M, Cusano NE, et al. Management of primary hyperparathyroidism. J Bone Miner Res (2022) 37(11):2391–403. doi: 10.1002/jbmr.4682
40. Talpos GB, Bone HG 3rd, Kleerekoper M, Phillips ER, Alam M, Honasoge M, et al. Randomized trial of parathyroidectomy in mild asymptomatic primary hyperparathyroidism: patient description and effects on the SF-36 health survey. Surgery (2000) 128(6):1013–20. doi: 10.1067/msy.2000.110844
41. Vestergaard P, Mosekilde L. Cohort study on effects of parathyroid surgery on multiple outcomes in primary hyperparathyroidism. BMJ. (2003) 327(7414):530–4. doi: 10.1136/bmj.327.7414.530
42. Weber T, Keller M, Hense I, Pietsch A, Hinz U, Schilling T, et al. Effect of parathyroidectomy on quality of life and neuropsychological symptoms in primary hyperparathyroidism. World J Surg (2007) 31(6):1202–9. doi: 10.1007/s00268-007-9006-6
43. Pretorius M, Lundstam K, Hellstrom M, Fagerland MW, Godang K, Mollerup C, et al. Effects of parathyroidectomy on quality of life: 10 years of data from a prospective randomized controlled trial on primary hyperparathyroidism (the SIPH-study). J Bone Miner Res (2021) 36(1):3–11. doi: 10.1002/jbmr.4199
44. Bollerslev J, Schalin-Jantti C, Rejnmark L, Siggelkow H, Morreau H, Thakker R, et al. MANAGEMENT OF ENDOCRINE DISEASE: Unmet therapeutic, educational and scientific needs in parathyroid disorders. Eur J Endocrinol (2019) 181(3):P1–P19. doi: 10.1530/EJE-19-0316
45. Livschitz J, Yen TWF, Evans DB, Wang TS, Dream S. Long-term quality of life after parathyroidectomy for primary hyperparathyroidism: A systematic review. JAMA Surg (2022). doi: 10.1001/jamasurg.2022.4249
46. Lourida I, Thompson-Coon J, Dickens CM, Soni M, Kuzma E, Kos K, et al. Parathyroid hormone, cognitive function and dementia: a systematic review. PloS One (2015) 10(5):e0127574. doi: 10.1371/journal.pone.0127574
47. Jiang W, Hu CY, Li FL, Hua XG, Huang K, Zhang XJ. Elevated parathyroid hormone levels and cognitive function: A systematic review. Arch Gerontol Geriatr (2020) 87:103985. doi: 10.1016/j.archger.2019.103985
48. Kim SM, Zhao D, Schneider ALC, Korada SK, Lutsey PL, Guallar E, et al. Association of parathyroid hormone with 20-year cognitive decline: The ARIC study. Neurology. (2017) 89(9):918–26. doi: 10.1212/WNL.0000000000004290
49. Joborn C, Hetta J, Rastad J, Agren H, Akerstrom G, Ljunghall S. Psychiatric symptoms and cerebrospinal fluid monoamine metabolites in primary hyperparathyroidism. Biol Psychiatry (1988) 23(2):149–58. doi: 10.1016/0006-3223(88)90085-6
50. Joborn C, Hetta J, Niklasson F, Rastad J, Wide L, Agren H, et al. Cerebrospinal fluid calcium, parathyroid hormone, and monoamine and purine metabolites and the blood-brain barrier function in primary hyperparathyroidism. Psychoneuroendocrinology. (1991) 16(4):311–22. doi: 10.1016/0306-4530(91)90017-N
51. Kaleem I, Alexander J, Hisbulla M, Kannichamy V, Mishra V, Banerjee A, et al. A review of the relationship of the cerebrospinal fluid changes during the dysregulation of parathyroid hormone with psychiatric or neurological manifestations. Cureus. (2021) 13(1):e12679. doi: 10.7759/cureus.12679
52. Dobolyi A, Dimitrov E, Palkovits M, Usdin TB. The neuroendocrine functions of the parathyroid hormone 2 receptor. Front Endocrinol (Lausanne) (2012) 3:121. doi: 10.3389/fendo.2012.00121
53. Gong X, Zou L, Wu H, Shan Y, Liu G, Zheng S, et al. Altered brain structural and cognitive impairment in end-stage renal disease patients with secondary hyperparathyroidism. Acta Radiol (2020) 61(6):796–803. doi: 10.1177/0284185119878360
54. Gazes Y, Liu M, Sum M, Cong E, Kuo J, Lee JA, et al. Functional magnetic resonance imaging in primary hyperparathyroidism. Eur J Endocrinol (2020) 183(1):21–30. doi: 10.1530/EJE-20-0123
55. Cermik TF, Kaya M, Ugur-Altun B, Bedel D, Berkarda S, Yigitbasi ON. Regional cerebral blood flow abnormalities in patients with primary hyperparathyroidism. Neuroradiology. (2007) 49(4):379–85. doi: 10.1007/s00234-006-0198-5
56. Hermsen A, Eienbroker A, Haag A, Mylius V, Hamer HM, Menzler K, et al. Perioperative changes in cortical excitability, mood, and quality of life in patients with primary hyperparathyroidism: a pilot study using transcranial magnetic stimulation. Eur J Endocrinol (2014) 170(2):201–9. doi: 10.1530/EJE-13-0552
Keywords: parathyroid hormone, parathyroidectomy, cognitive performance, memory, primary hyperpaparathyroidism
Citation: Szalat A, Tamir N, Mazeh H and Newman JP (2022) Successful parathyroidectomy improves cognition in patients with primary hyperparathyroidism: A prospective study in a tertiary medical center and comprehensive review of the literature. Front. Endocrinol. 13:1095189. doi: 10.3389/fendo.2022.1095189
Received: 18 November 2022; Accepted: 05 December 2022;
Published: 22 December 2022.
Edited by:
Mara Carsote, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Claudio Casella, University of Brescia, ItalyCopyright © 2022 Szalat, Tamir, Mazeh and Newman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Auryan Szalat, YXVyeWFuc0BoYWRhc3NhaC5vcmcuaWw=
†These authors have contributed equally to this work and share first authorship
‡ORCID: Auryan Szalat, orcid.org/0000-0003-3339-4408
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.