
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 06 December 2022
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1080108
This article is part of the Research Topic Levothyroxine Therapy in Patients with Hypothyroidism: Volume II View all 12 articles
 Elisa Gatta
Elisa Gatta Francesca Bambini
Francesca Bambini Caterina Buoso
Caterina Buoso Maria Gava
Maria Gava Virginia Maltese
Virginia Maltese Valentina Anelli
Valentina Anelli Andrea Delbarba
Andrea Delbarba Ilenia Pirola
Ilenia Pirola Carlo Cappelli*
Carlo Cappelli*Purpose: To describe the current knowledge on thyroid hormonal profile in patients on liquid L-T4 therapy and drugs known to interfere with L-T4 absorption.
Methods: A PubMed/MEDLINE, Web of Science, and Scopus research was performed. Case reports, case series, original studies and reviews written in English and published online up to 31 August 2022 were selected and reviewed. The final reference list was defined based on the relevance of each paper to the scope of this review.
Results: The available data showed that novel levothyroxine formulations circumvent gastric pH impairment due to multiple interfering drugs such as proton pump inhibitors, calcium or iron supplements, sevelamer, aluminum/magnesium hydroxide and sodium alginate.
Conclusion: New formulations can be taken simultaneously with drugs interfering with L-T4 absorption, in particular liquid formulations. Softgel capsules need more studies to support these data.
Thyroid hormones (TH) have a critical role in human homeostasis, having an impact on a wide array of tissues including the brain, heart, muscle, and bone and, if they are reduced or absent, they must be replaced in order to quickly restore euthyroidism.
Natural thyroid preparations, such as desiccated or thyroid extract, were the only available treatment until 1950, and contained both thyroxine (T4) and triiodothyronine (T3). After levothyroxine (L-T4) synthesizing, tablet formulations were distributed worldwide (1). At the beginning of the 2000s, liquid formulations started to be produced as drops, unit-dose ampoules or softgel capsules containing L-T4 dissolved in different solutions (2).
L-T4 absorption mainly occurs in the jejunum and ileum (3) and is maximal when the stomach is empty, demonstrating how gastric acidity has a key role in this process (4); for this reason, L-T4 tablets have to be administered with an empty stomach at least one hour before breakfast or at bedtime (5, 6). It is well known that higher daily doses of L-T4 are required in patients with jejunoileal bypass surgery or other bowel resection after surgery (7–9), gastrointestinal disorders such as coeliac disease, Helicobacter pylori infection and atrophic gastritis (4). Additionally, also dietary fiber (10) and espresso coffee (11) can reduce the bioavailability of L-T4. More important, many drugs, such as proton pump inhibitors (PPI) (12), calcium carbonate and ferrous sulphate supplementation (13–15), bile acid sequestrants, cholestyramine (16, 17), sucralfate (18–20), aluminum hydroxide (21) and phosphate binders (22), can significatively reduce L-T4 tables absorption when concomitantly administered.
L-T4 has a narrow therapeutic index (23); a tailored therapy is advocated in order to maintain serum thyroid stimulating hormone (TSH) levels in the normal range, avoiding iatrogenic complications or hypothyroidism symptoms (24). However, cross-sectional studies show that 30–50% of L-T4 users have an abnormal serum TSH level, mainly in patients taking multiple drugs (25–27).
This is a “Giano bifronte” aspect. On the one hand, the daily L-T4 dose must be increased to run behind serum TSH levels, on the other hand, withdrawing interfering drugs can develop iatrogenic hyperthyroidism. This is of particular interest in older patients (≥ 65 years old) for the well-documented increased risk of developing heart disease, osteoporosis, bone fracture and cognitive impairment (28–32).
Recently, it has been demonstrated that new formulations (liquid and softgel capsules) can circumvent the problem of incomplete absorption of L-T4 caused by changes in gastric pH due to their administration at breakfast (33–39) or after bariatric surgery (40). In addition, an increasing number of reports have also shown that these drugs can be administered with many of the aforementioned interfering drugs (33, 41–51).
The present review aimed to evaluate the thyroid hormonal profile in patients on liquid L-T4 therapy and drugs known to interfere with L-T4 absorption.
The review was conducted according to the PRISMA statement, and the checklist is reported as Supplementary file 1.
A PubMed/MEDLINE, Web of Science and Scopus research was performed, for free-text words and terms related to “levothyroxine”, “liquid”, “oral solution”, “softgel capsules”, “drug interaction”, “drug interfering with L-T4 absorption”, “L-T4 malabsorption”, “proton-pump inhibitors”, “sucralfate”, “bile acid sequestrant”, “cation exchange inhibitors”, “calcium salts”, “ferrous sulfate”, “oral bisphosphonates”, “phosphate binders”. Case reports, case series, original studies and reviews written in English and published online up to 31 August 2022 were selected and reviewed.
The final reference list was defined based on the relevance of each paper to the scope of this review.
In the preliminary search, 165 studies were identified through the literature search and 64 remained after the duplicates were removed. A title and abstract review was performed on the remaining studies, with 52 excluded at this first stage. A total of 12 articles were eligible for full-text screening and 12 full-text publications were included in the analysis. A PRISMA flow diagram of the screening and selection process can be found in Figure 1. 10 studies included in the analysis were observational, 7 of them had a prospective design and 3 were retrospective; 1 study was a double-blind crossover clinical trial; 1 was a case report. The studies were published between 2012 and 2019, all studies were from Italy.
Finally, the studies were divided according to the formulation used (liquid or softgel L-T4).
Ten articles regarding liquid L-T4 formulations and interfering absorption drugs were retrieved, for a total amount of 1626 patients, as shown in Table 1.
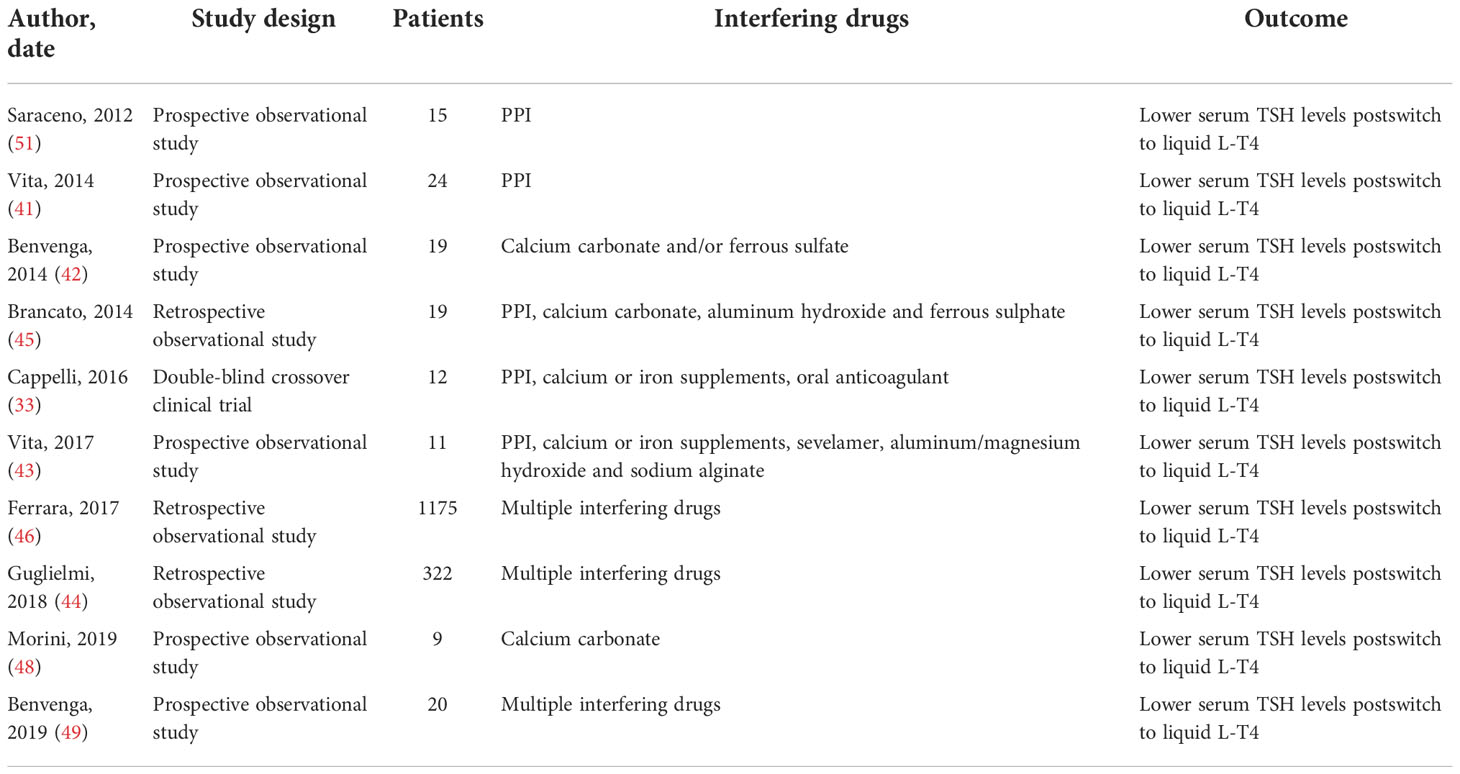
Table 1 Summary of clinical studies and case reports about liquid L-T4 formulations and interfering absorption drugs.
In a prospective non-randomized study, Vita R et al. enrolled 24 patients in PPI and L-T4 tablets therapy. The patients were switched to liquid oral solution maintaining the same L-T4 dosage and interval before the PPI; after 8 and 16 weeks, TSH values were checked. The study showed, for the first time, that patients in whom LT4 tablets failed to normalize or suppress the TSH serum because of the concomitant ingestion of PPI benefited from the switch from tablet to oral solution (41).
Data were confirmed by Saraceno G et al., demonstrating that liquid formulation can solve the problem of incomplete absorption caused by PPI in 15 patients on therapy with different PPIs (51).
The same Authors provided the same results in 19 patients taking calcium carbonate, ferrous sulfate or both (42).
In 2017, Vita R et al. demonstrated the capability of liquid solution to circumvent gastric pH impairment due to multiple interfering drugs (IPP, calcium or iron supplements, sevelamer, aluminum/magnesium hydroxide and sodium alginate) in 11 patients. The switch from L-T4 tablets to liquid permitted reaching the TSH target already after 8 weeks. In detail, mean TSH values under tablet L-T4 were 4-fold higher compared to those under liquid L-T4 (4.3 ± 3.1 vs. 1.1 ± 1.3 mU/L, P< 0.0001) (43).
Guglielmi V et al. conducted a real-life study in primary care, identifying 3965 patients that were taking drugs which interacted with L-T4 absorption, as recorded in the Italian general practice Health Search IMS Health Longitudinal Patients Database (HSD). 322 patients (8%) took liquid L-T4, whereas 3643 (92%) tablets. Serum TSH levels were higher in the latters (2.15 mU/L versus 1.82 mU/L). The Authors concluded that, in clinical practice, the use of oral liquid LT4 is not associated with the increase in daily dosage of L-T4 compared with tablet formulation during exposure to interfering drugs (44).
An observational retrospective study enrolled 53 patients on L-T4 replacement therapy who switched from L-T4 tablets to liquid formulation without changing the daily dose. Among them, 18 (34%) were taking drugs that interfered with LT4 absorption (PPI, calcium carbonate, aluminum hydroxide and ferrous sulphate). The ratio between serum TSH levels 60-90 days postswitch and preswitch were 0.50 (0.31-0.72; 95% CI 0.33-0.69) (45).
Ferrara R et al. conducted a longitudinal real-world study collecting data from 56,354 patients in L-T4 treatment. Among them, 19044 (34%) subjects were also taking drugs which interacted with L-T4 absorption; 501 switched to oral drops and 674 to pre-dosed vials. The Authors demonstrated a significant TSH reduction with respect to preswitch, both in drops and pre-dosed vials group (46).
Our group conducted a double-blind placebo-controlled trial, enrolling 77 naive hypothyroid patients in order to demonstrate if an oral solution of L-T4 could be ingested during breakfast. We observed no influence of breakfast composition and co-treatment with other drugs (in 11 patients, including PPI) on TSH levels (33).
Four articles regarding softgel L-T4 formulation capsules and interfering absorption drugs were retrieved, for a total amount of 47 patients, as shown in Table 2.
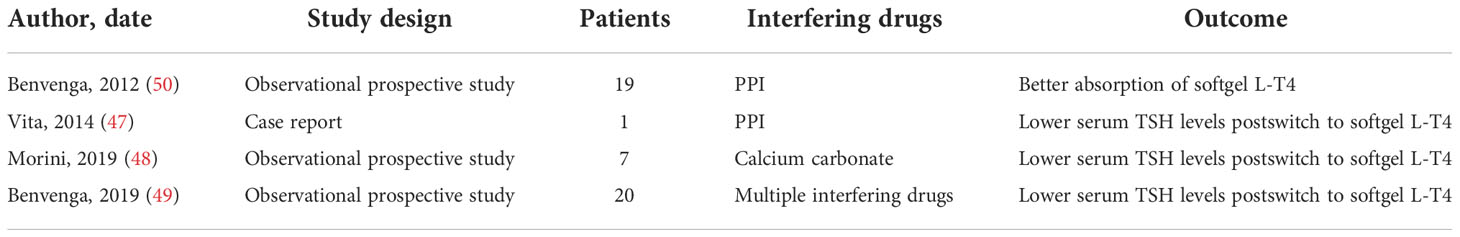
Table 2 Summary of clinical studies and case reports about softgel capsules L-T4 formulations and interfering absorption drugs.
Vita R et al. described a 48-years old woman in whom the impaired absorption of the levothyroxine (L-T4) tablet due to a proton pump inhibitor (PPI) use was corrected by switching the patient to the softgel capsules. Intestinal absorption of L-T4 was later evaluated by administering 600 mg of LT4 as a tablet or softgel capsule while maintaining pantoprazole; pharmacokinetic indices showed better and faster absorption of the softgel capsules versus tablets, at least in this patient (47).
This was confirmed in a randomized crossover study including 16 healthy volunteers. An acute loading with 600 μg of L-T4 (tablets versus softgel capsules) repeated after an acute infusion of 80 mg esomeprazole was conducted. The absorption of L-T4 softgel capsule was significantly increased at baseline and not affected by overload of PPI (50).
Several pathological conditions and endogenous and exogenous factors are well known to interfere with tablet L-T4 pharmacokinetics (52). In particular, various frequently used medications, such as proton pump inhibitors (PPIs), ferrous sulphate, sucralfate, raloxifene, bile acid sequestrants, calcium carbonate, phosphate binders, and aluminum-containing antiacids have been demonstrated to interfere with LT4 absorption, either by increasing gastric pH (the case of PPIs) or binding LT4 into insoluble complexes (the case of calcium or iron salts) (4).
This is well documented for tablet formulation, which needs to be dissolved in the acid gastric pH prior to its absorption at the levels of the duodenum and jejunum. In fact, gastric acidity plays an important role in the absorption of L-T4, as has also been shown by Centanni et al. (53). The mechanism by which intestinal absorption of L-T4 may be impaired in patients with altered gastric pH is still unclear. However, it is possible that hydrophilic sodium salt (pharmaceutical L-T4) remains undissociated in hypochlorydic gastric conditions, thus being absorbed less (54). For these reasons, current guidelines for the treatment of hypothyroidism by a Task Force of the American Thyroid Association recommend that, for optimal and consistent absorption, L-T4 should be taken in a fasting state away from interfering drugs (55).
In the last 20 years, pharmaceutical Companies have developed new L-T4 formulations in liquid solutions. Liquid formulations don’t need to dissolve, as they do not require a gastric acid environment. For these reasons, it reaches plasmatic maximum concentrations (Cmax) quicker and the median Cmax is higher than tablets with a higher gastrointestinal absorption (40, 56–58). This is well evidenced by Brancato et al. and Negro et al. that observed a significant decrease in TSH serum levels in patients previously on therapy with L-T4 tablets after switching to liquid L-T4 (45, 59). These data were confirmed in patients taking liquid L-T4 concomitantly with breakfast (33, 34, 36, 37, 40) and this can improve patients’ compliance and quality of life (38, 60). In addition, it has been demonstrated that novel L-T4 formulations are more effective in reaching normal serum TSH levels in patients who underwent bariatric surgery (40, 61) or with autoimmune gastritis (62), lactose intolerance (63), Helicobacter pylori infection (64), giardiasis (65), diabetic gastroparesis (66), esophageal complications of systemic sclerosis (67) or liver cirrhosis (68), all conditions that somehow impair gastric acidity.
Softgel capsules may represent combinations of practicality of the tablets formulations and pharmacokinetics qualities of liquid ones, resulting in a better gastrointestinal absorption than tablets (56, 57, 69). In fact, changes in intraluminal gastric pH have a negligible effect on liquid and softgel capsules L-T4 absorption (70, 71). In agreement we previously showed that softgel formulations of L-T4 can be taken with breakfast (35), data confirmed by few authors (72, 73).
Levothyroxine is the second prescribed drug in the United States (74). It is important to underline that most of the molecules known to interfere with gastric L-T4 absorption are, at the same time, among those most prescribed (75). For this reason, it is quite common for a patient to be on concomitant treatment with L-T4 and drugs interfering with its absorption. This was well evidenced by Benvenga et al., that showed PPIs as the most frequent molecules either alone (74.8%) or in combination (25.2%) with other interfering drugs (76). Trifirò and colleagues confirmed and extended this result in a large set of patients who required higher L-T4 daily doses when PPIs were co-administered (77). On the other side, data obtained in a large series of patients on liquid L-T4 have clearly demonstrated that this formulation circumvents the co-administration of interfering drugs (41–46, 51). This was confirmed in a subset of patients enrolled in our prospective double-blind placebo study (33). Promising data are available also for softgel capsules (47, 50). Particularly, Benvenga conducted a prospective study showing that 95% of patients taking softgel capsules reached euthyroidism, even in presence of interfering drugs (49). This data were confirmed by Morini et al. (48). More studies in a larger cohort of patients are needed to confirm this important point on softgel capsules.
We summarized the available reports and studies regarding liquid and softgel L-T4 formulations and drugs known to interfere with levothyroxine absorption.
New formulations can be taken simultaneously with drugs interfering with L-T4 absorption, in particular liquid formulations. Softgel capsules need more studies to support these data.
CC conceived the project. CC, AD, and EG provided supervision and project administration. EG, FB, MG, CB, VM, and VA did the literature search. EG wrote the original draft. CC and IP reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1080108/full#supplementary-material
1. McAninch EA, Bianco AC. The history and future of treatment of hypothyroidism. Ann Intern Med (2016) 164(1):50–6. doi: 10.7326/M15-1799
2. Colucci P, D'Angelo P, Mautone G, Scarsi C, Ducharme MP. Pharmacokinetic equivalence of a levothyroxine sodium soft capsule manufactured using the new food and drug administration potency guidelines in healthy volunteers under fasting conditions. Ther Drug Monit (2011) 33(3):355–61. doi: 10.1097/FTD.0b013e318217b69f
3. Benvenga S, Bartolone L, Squadrito S, Lo Giudice F, Trimarchi F. Delayed intestinal absorption of levothyroxine. Thyroid (1995) 5(4):249–53. doi: 10.1089/thy.1995.5.249
4. Liwanpo L, Hershman JM. Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab (2009) 23(6):781–92. doi: 10.1016/j.beem.2009.06.006
5. Bach-Huynh TG, Nayak B, Loh J, Soldin S, Jonklaas J. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metab (2009) 94(10):3905–12. doi: 10.1210/jc.2009-0860
6. Pang X, Pu T, Xu L, Sun R. Effect of l-thyroxine administration before breakfast vs at bedtime on hypothyroidism: A meta-analysis. Clin Endocrinol (Oxf) (2020) 92(5):475–81. doi: 10.1111/cen.14172
7. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med (1979) 90(6):941–2. doi: 10.7326/0003-4819-90-6-941
8. Topliss DJ, Wright JA, Volpé R. Increased requirement for thyroid hormone after a jejunoileal bypass operation. Can Med Assoc J (1980) 123(8):765–6.
9. Bevan JS, Munro JF. Thyroxine malabsorption following intestinal bypass surgery. Int J Obes (1986) 10(3):245–6.
10. Liel Y, Harman-Boehm I, Shany S. Evidence for a clinically important adverse effect of fiber-enriched diet on the bioavailability of levothyroxine in adult hypothyroid patients. J Clin Endocrinol Metab (1996) 81(2):857–9. doi: 10.1210/jcem.81.2.8636317
11. Benvenga S, Bartolone L, Pappalardo MA, Russo A, Lapa D, Giorgianni G, et al. Altered intestinal absorption of l-thyroxine caused by coffee. Thyroid (2008) 18(3):293–301. doi: 10.1089/thy.2007.0222
12. Guzman-Prado Y, Vita R, Samson O. Concomitant use of levothyroxine and proton pump inhibitors in patients with primary hypothyroidism: a systematic review. J Gen Intern Med (2021) 36(6):1726–33. doi: 10.1007/s11606-020-06403-y
13. Singh N, Singh PN, Hershman JM. Effect of calcium carbonate on the absorption of levothyroxine. JAMA (2000) 283(21):2822–5. doi: 10.1001/jama.283.21.2822
14. Campbell NR, Hasinoff BB, Stalts H, Rao B, Wong NC. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med (1992) 117(12):1010–3. doi: 10.7326/0003-4819-117-12-1010
15. Shakir KM, Chute JP, Aprill BS, Lazarus AA. Ferrous sulfate-induced increase in requirement for thyroxine in a patient with primary hypothyroidism. South Med J (1997) 90(6):637–9. doi: 10.1097/00007611-199706000-00011
16. Phillips WA, Schultz JR, Stafford WW. Effects of colestipol hydrochloride on drug absorption in the rat. i. aspirin, l-thyroxine, phenobarbital, cortisone, and sulfadiazine. J Pharm Sci (1974) 63(7):1097–103. doi: 10.1002/jps.2600630714
17. Weitzman SP, Ginsburg KC, Carlson HE. Colesevelam hydrochloride and lanthanum carbonate interfere with the absorption of levothyroxine. Thyroid (2009) 19(1):77–9. doi: 10.1089/thy.2008.0312
18. Havrankova J, Lahaie R. Levothyroxine binding by sucralfate. Ann Intern Med (1992) 117(5):445–6. doi: 10.7326/0003-4819-117-5-445_3
19. Sherman SI, Tielens ET, Ladenson PW. Sucralfate causes malabsorption of l-thyroxine. Am J Med (1994) 96(6):531–5. doi: 10.1016/0002-9343(94)90093-0
20. Campbell JA, Schmidt BA, Bantle JP. Sucralfate and the absorption of l-thyroxine. Ann Intern Med (1994) 121(2):152. doi: 10.7326/0003-4819-121-2-199407150-00024
21. Liel Y, Sperber AD, Shany S. Nonspecific intestinal adsorption of levothyroxine by aluminum hydroxide. Am J Med (1994) 97(4):363–5. doi: 10.1016/0002-9343(94)90303-4
22. Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. Effect of phosphate binders upon TSH and l-thyroxine dose in patients on thyroid replacement. Int Urol Nephrol (2007) 39(2):599–602. doi: 10.1007/s11255-006-9166-6
23. Shah RB, Collier JS, Sayeed VA, Bryant A, Habib MJ, Khan MA. Tablet splitting of a narrow therapeutic index drug: a case with levothyroxine sodium. AAPS PharmSciTech (2010) 11(3):1359–67. doi: 10.1208/s12249-010-9515-8
24. Del Duca SC, Santaguida MG, Brusca N, Gatto I, Cellini M, Gargano L, et al. Individually-tailored thyroxine requirement in the same patients before and after thyroidectomy: a longitudinal study. Eur J Endocrinol (2015) 173(3):351–7. doi: 10.1530/EJE-15-0314
25. Okosieme OE. Thyroid hormone replacement: current status and challenges. Expert Opin Pharmacother (2011) 12(15):2315–28. doi: 10.1517/14656566.2011.600307
26. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med (2000) 160(4):526–34. doi: 10.1001/archinte.160.4.526
27. Parle JV, Franklyn JA, Cross KW, Jones SR, Sheppard MC. Thyroxine prescription in the community: serum thyroid stimulating hormone level assays as an indicator of undertreatment or overtreatment. Br J Gen Pract (1993) 43(368):107–9.
28. Rehman SU, Cope DW, Senseney AD, Brzezinski W. Thyroid disorders in elderly patients. South Med J (2005) 98(5):543–9. doi: 10.1097/01.SMJ.0000152364.57566.6D
29. Biondi B. Natural history, diagnosis and management of subclinical thyroid dysfunction. Best Pract Res Clin Endocrinol Metab (2012) 26(4):431–46. doi: 10.1016/j.beem.2011.12.004
30. Collet TH, Gussekloo J, Bauer DC, den Elzen WP, Cappola AR, Balmer P, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med (2012) 172(10):799–809. doi: 10.1001/archinternmed.2012.402
31. Moon JH, Park YJ, Kim TH, Han JW, Choi SH, Lim S, et al. Lower-but-normal serum TSH level is associated with the development or progression of cognitive impairment in elderly: Korean longitudinal study on health and aging (KLoSHA). J Clin Endocrinol Metab (2014) 99(2):424–32. doi: 10.1210/jc.2013-3385
32. Cappelli C, Pirola I, Daffini L, Gandossi E, Agosti B, Castellano M. Thyroid hormonal profile in elderly patients treated with two different levothyroxine formulations: A single institute survey. Eur Geriatric Med (2014) 5(6):382–5. doi: 10.1016/j.eurger.2014.09.006
33. Cappelli C, Pirola I, Daffini L, Formenti A, Iacobello C, Cristiano A, et al. A double-blind placebo-controlled trial of liquid thyroxine ingested at breakfast: Results of the TICO study. Thyroid (2016) 26(2):197–202. doi: 10.1089/thy.2015.0422
34. Cappelli C, Pirola I, Gandossi E, Formenti A, Castellano M. Oral liquid levothyroxine treatment at breakfast: a mistake? Eur J Endocrinol (2014) 170(1):95–9. doi: 10.1530/EJE-13-0693
35. Cappelli C, Pirola I, Gandossi E, Cristiano A, Daffini L, Agosti B, et al. Thyroid hormone profile in patients ingesting soft gel capsule or liquid levothyroxine formulations with breakfast. Int J Endocrinol (2016) 2016:9043450. doi: 10.1155/2016/9043450
36. Morelli S, Reboldi G, Moretti S, Menicali E, Avenia N, Puxeddu E. Timing of breakfast does not influence therapeutic efficacy of liquid levothyroxine formulation. Endocrine (2016) 52(3):571–8. doi: 10.1007/s12020-015-0788-2
37. Pirola I, Gandossi E, Brancato D, Marini F, Cristiano A, Delbarba A, et al. TSH evaluation in hypothyroid patients assuming liquid levothyroxine at breakfast or 30 min before breakfast. J Endocrinol Invest (2018) 41(11):1301–6. doi: 10.1007/s40618-018-0867-3
38. Bernareggi A, Grata E, Pinorini MT, Conti A. Oral liquid formulation of levothyroxine is stable in breakfast beverages and may improve thyroid patient compliance. Pharmaceutics (2013) 5(4):621–33. doi: 10.3390/pharmaceutics5040621
39. Pirola I, Daffini L, Gandossi E, Lombardi D, Formenti A, Castellano M, et al. Comparison between liquid and tablet levothyroxine formulations in patients treated through enteral feeding tube. J Endocrinol Invest (2014) 37(6):583–7. doi: 10.1007/s40618-014-0082-9
40. Pirola I, Formenti AM, Gandossi E, Mittempergher F, Casella C, Agosti B, et al. Oral liquid l-thyroxine (L-t4) may be better absorbed compared to l-T4 tablets following bariatric surgery. Obes Surg (2013) 23(9):1493–6. doi: 10.1007/s11695-013-1015-y
41. Vita R, Saraceno G, Trimarchi F, Benvenga S. Switching levothyroxine from the tablet to the oral solution formulation corrects the impaired absorption of levothyroxine induced by proton-pump inhibitors. J Clin Endocrinol Metab (2014) 99(12):4481–6. doi: 10.1210/jc.2014-2684
42. Benvenga S, Di Bari F, Vita R. Undertreated hypothyroidism due to calcium or iron supplementation corrected by oral liquid levothyroxine. Endocrine (2017) 56(1):138–45. doi: 10.1007/s12020-017-1244-2
43. Vita R, Di Bari F, Benvenga S. Oral liquid levothyroxine solves the problem of tablet levothyroxine malabsorption due to concomitant intake of multiple drugs. Expert Opin Drug Deliv (2017) 14(4):467–72. doi: 10.1080/17425247.2017.1290604
44. Guglielmi V, Bellia A, Bianchini E, Medea G, Cricelli I, Sbraccia P, et al. Drug interactions in users of tablet vs. oral liquid levothyroxine formulations: a real-world evidence study in primary care. Endocrine (2018) 59(3):585–92. doi: 10.1007/s12020-017-1412-4
45. Brancato D, Scorsone A, Saura G, Ferrara L, Di Noto A, Aiello V, et al. Comparison of TSH levels with liquid formulation versus tablet formulations of levothyroxine in the treatment of adult hypothyroidism. Endocr Pract (2014) 20(7):657–62. doi: 10.4158/EP13418.OR
46. Ferrara R, Ientile V, Arcoraci V, Ferrajolo C, Piccinni C, Fontana A, et al. Treatment pattern and frequency of serum TSH measurement in users of different levothyroxine formulations: a population-based study during the years 2009-2015. Endocrine (2017) 58(1):143–52. doi: 10.1007/s12020-017-1242-4
47. Vita R, Benvenga S. Tablet levothyroxine (L-T4) malabsorption induced by proton pump inhibitor; a problem that was solved by switching to l-T4 in soft gel capsule. Endocr Pract (2014) 20(3):e38–41. doi: 10.4158/EP13316.CR
48. Morini E, Catalano A, Lasco A, Morabito N, Benvenga S. In thyroxine-replaced hypothyroid postmenopausal women under simultaneous calcium supplementation, switch to oral liquid or softgel capsule l-thyroxine ensures lower serum TSH levels and favorable effects on blood pressure, total cholesterolemia and glycemia. Endocrine (2019) 65(3):569–79. doi: 10.1007/s12020-019-01908-x
49. Benvenga S. Liquid and softgel capsules of l-thyroxine results lower serum thyrotropin levels more than tablet formulations in hypothyroid patients. J Clin Transl Endocrinol (2019) 18:100204. doi: 10.1016/j.jcte.2019.100204
50. Benvenga S, Ducharme MP. Effect of proton-pump inhibitors (PPIS) on the relative bioavailability of levothyroxine (LT4) in soft gel capsules and in tablets. Thyroid (2012) 22(s1):a50.
51. Saraceno G, Vita R, Trimarchi F, Benvenga S. Interference of l-T4 absorption by proton-pump inhibitors (PPIS) can be solved by a liquid formulation of l-thyroxine (L-T4). Thyroid (2012) 22(s1):a50.
52. Formenti AM, Daffini L, Pirola I, Gandossi E, Cristiano A, Cappelli C. Liquid levothyroxine and its potential use. Hormones (Athens) (2015) 14(2):183–9. doi: 10.14310/horm.2002.1579
53. Centanni M, Gargano L, Canettieri G, Viceconti N, Franchi A, Delle Fave G, et al. Thyroxine in goiter, helicobacter pylori infection, and chronic gastritis. N Engl J Med (2006) 354(17):1787–95. doi: 10.1056/NEJMoa043903
54. Sherman SI, Malecha SE. Absorption and malabsorption of levothyroxine sodium. Am J Ther (1995) 2(10):814–8. doi: 10.1097/00045391-199510000-00014
55. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid (2014) 24(12):1670–751. doi: 10.1089/thy.2014.0028
56. Yue CS, Scarsi C, Ducharme MP. Pharmacokinetics and potential advantages of a new oral solution of levothyroxine vs. other available dosage forms. Arzneimittelforschung (2012) 62(12):631–6. doi: 10.1055/s-0032-1329951
57. Antonelli A, Elia G, Ragusa F, Paparo SR, Cavallini G, Benvenga S, et al. The stability of TSH, and thyroid hormones, in patients treated with tablet, or liquid levo-thyroxine. Front Endocrinol (Lausanne) (2021) 12:633587. doi: 10.3389/fendo.2021.633587
58. Fallahi P, Ferrari SM, Materazzi G, Ragusa F, Ruffilli I, Patrizio A, et al. Oral l-thyroxine liquid versus tablet in patients submitted to total thyroidectomy for thyroid cancer (without malabsorption): A prospective study. Laryngoscope Investig Otolaryngol (2018) 3(5):405–8. doi: 10.1002/lio2.186
59. Negro R, Valcavi R, Agrimi D, Toulis KA. Levothyroxine liquid solution versus tablet for replacement treatment in hypothyroid patients. Endocr Pract (2014) 20(9):901–6. doi: 10.4158/EP13378.OR
60. Guglielmi R, Grimaldi F, Negro R, Frasoldati A, Misischi I, Graziano F, et al. Shift from levothyroxine tablets to liquid formulation at breakfast improves quality of life of hypothyroid patients. Endocr Metab Immune Disord Drug Targets (2018) 18(3):235–40. doi: 10.2174/1871530318666180125155348
61. Fallahi P, Ferrari SM, Camastra S, Politti U, Ruffilli I, Vita R, et al. TSH normalization in bariatric surgery patients after the switch from l-thyroxine in tablet to an oral liquid formulation. Obes Surg (2017) 27(1):78–82. doi: 10.1007/s11695-016-2247-4
62. Fallahi P, Ferrari SM, Ruffilli I, Antonelli A. Reversible normalisation of serum TSH levels in patients with autoimmune atrophic gastritis who received l-T4 in tablet form after switching to an oral liquid formulation: a case series. BMC Gastroenterol (2016) 16:22. doi: 10.1186/s12876-016-0439-y
63. Fallahi P, Ferrari SM, Marchi S, De Bortoli N, Ruffilli I, Antonelli A. Patients with lactose intolerance absorb liquid levothyroxine better than tablet levothyroxine. Endocrine (2017) 57(1):175–8. doi: 10.1007/s12020-016-1090-7
64. Ribichini D, Fiorini G, Repaci A, Castelli V, Gatta L, Vaira D, et al. Tablet and oral liquid l-thyroxine formulation in the treatment of naïve hypothyroid patients with helicobacter pylori infection. Endocrine (2017) 57(3):394–401. doi: 10.1007/s12020-016-1167-3
65. Tortora A, La Sala D, Vitale M. Switch from tablet levothyroxine to oral solution resolved reduced absorption by intestinal parasitosis. Endocrinol Diabetes Metab Case Rep (2019) 2019. doi: 10.1530/EDM-19-0026
66. Reardon DP, Yoo PS. Levothyroxine tablet malabsorption associated with gastroparesis corrected with gelatin capsule formulation. Case Rep Endocrinol (2016) 2016:1316724. doi: 10.1155/2016/1316724
67. Lobasso A, Nappi L, Barbieri L, Peirce C, Ippolito S, Arpaia D, et al. Severe hypothyroidism due to the loss of therapeutic efficacy of l-thyroxine in a patient with esophageal complication associated with systemic sclerosis. Front Endocrinol (Lausanne) (2017) 8:241. doi: 10.3389/fendo.2017.00241
68. Benvenga S, Capodicasa G, Perelli S, Ferrari SM, Fallahi P, Antonelli A. Increased requirement of replacement doses of levothyroxine caused by liver cirrhosis. Front Endocrinol (Lausanne) (2018) 9:150. doi: 10.3389/fendo.2018.00150
69. Pabla D, Akhlaghi F, Zia H. A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur J Pharm Biopharm (2009) 72(1):105–10. doi: 10.1016/j.ejpb.2008.10.008
70. Vita R, Saraceno G, Trimarchi F, Benvenga S. A novel formulation of l-thyroxine (L-T4) reduces the problem of l-T4 malabsorption by coffee observed with traditional tablet formulations. Endocrine (2013) 43(1):154–60. doi: 10.1007/s12020-012-9772-2
71. Trimboli P, Mouly S. Pharmacokinetics and clinical implications of two non-tablet oral formulations of l-thyroxine in patients with hypothyroidism. J Clin Med (2022) 11(12). doi: 10.3390/jcm11123479
72. Trimboli P, Scappaticcio L, De Bellis A, Maiorino MI, Knappe L, Esposito K, et al. Different formulations of levothyroxine for treating hypothyroidism: A real-life study. Int J Endocrinol (2020) 2020:4524759. doi: 10.1155/2020/4524759
73. Ducharme M, Scarsi C, Bettazzi E, Mautone G, Lewis Y, Celi FS. A novel levothyroxine solution results in similar bioavailability whether taken 30 or just 15 minutes before a high-fat high-calorie meal. Thyroid (2022) 32(8):897–904. doi: 10.1089/thy.2021.0604
74. Fuentes AV, Pineda MD, Venkata KCN. Comprehension of top 200 prescribed drugs in the US as a resource for pharmacy teaching, training and practice. Pharm (Basel) (2018) 6(2). doi: 10.3390/pharmacy6020043
75. Medical Expenditure Panel Survey (MEPS). ClinCalc DrugStats database 2020. Available at: https://meps.ahrq.gov/mepsweb/.
76. Benvenga S, Pantano R, Saraceno G, Lipari L, Alibrando A, Inferrera S, et al. A minimum of two years of undertreated primary hypothyroidism, as a result of drug-induced malabsorption of l-thyroxine, may have metabolic and cardiovascular consequences. J Clin Transl Endocrinol (2019) 16:100189. doi: 10.1016/j.jcte.2019.100189
Keywords: levothyroxine, liquid levothyroxine, softgel capsules, interfering drugs, levothyroxine malabsorption
Citation: Gatta E, Bambini F, Buoso C, Gava M, Maltese V, Anelli V, Delbarba A, Pirola I and Cappelli C (2022) Liquid levothyroxine formulations in patients taking drugs interfering with L-T4 absorption. Front. Endocrinol. 13:1080108. doi: 10.3389/fendo.2022.1080108
Received: 25 October 2022; Accepted: 21 November 2022;
Published: 06 December 2022.
Edited by:
Alessandro Antonelli, University of Pisa, ItalyReviewed by:
Elisavet Fotiadou, Medicover Berlin-Charlottenburg MVZ, GermanyCopyright © 2022 Gatta, Bambini, Buoso, Gava, Maltese, Anelli, Delbarba, Pirola and Cappelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlo Cappelli, Y2FybG8uY2FwcGVsbGlAdW5pYnMuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.