
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol., 22 November 2022
Sec. Adrenal Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1075785
This article is part of the Research TopicA Year in Review: Discussions in Adrenal EndocrinologyView all 7 articles
Despite the current screening approach for Cushing syndrome (CS), delayed diagnosis is common due to broad spectrum of presentation, poor discriminant symptoms featured in diabetes and obesity, and low clinical index of suspicion. Even if initial tests are recommended to screen CS, divergent results are not infrequent. As global prevalence of type 2 diabetes and obesity increases, CS may not be frequent enough to back routine screening to avoid false-positive results. This represents a greater challenge in countries with limited health resources. The development of indexes incorporates clinical features and biochemical data that are largely used to provide a tool to predict the presence of disease. In clinical endocrinology, indexes have been used in Graves’ ophthalmology, hirsutism, and hypothyroidism. The use of clinical risk scoring system may assist clinicians in discriminating CS in the context of at-risk populations and, thus, may provide a potential intervention to decrease time to diagnosis. Development and validation of clinical model to estimate pre-test probability of CS in different geographic source population may help to establish regional prediction model for CS. Here, we review on the latest progress in clinical risk scoring system for CS and attempt to raise awareness for the use, validation, and/or development of clinical risk scores in CS.
Cushing syndrome (CS) is caused by continued exposure to excess of either endogenous cortisol or exogenous glucocorticoids. Endogenous CS results from improper hypercortisolism due to either adrenocorticotropin (ACTH) hypersecretion or autonomous adrenal cortisol hypersecretion (1, 2). It is an infrequent endocrine disease that poses diagnostic challenges even by attendant endocrinologists (3, 4). To complicate matters, patients may present with few clinical or nonspecific features (1, 5). Therefore, it is very challenging to diagnose CS in the early stages or in cyclic CS (6–8).
The Endocrine Society’s Clinical Practice Guideline for the diagnosis of CS recommends that exogenous glucocorticoids intake should be rule out before performing screening tests (1, 9). It also states that hypercortisolism should be tested in patients in whom a diagnosis is most likely with signs and symptoms that include weight gain, abnormal glucose tolerance, and hypertension (1, 9, 10). Global prevalence of type 2 diabetes (T2D) and obesity is becoming more common, and these, in turn, are commonly associated with mild increased cortisol levels (11, 12). Moreover, other conditions with secondarily activated hypothalamic–pituitary–adrenal such as depression, alcohol dependence, and glucocorticoid resistance offer even more difficulties in discriminating CS (1, 13). Even in highly specialized center, half of the patients submitted for screening of CS did not belong to the recommended groups for screening (9). As the lifetime experience to diagnose CS for a physician is very low (14, 15), we can only speculate that the screening tests for hypercortisolism might be unnecessary used, leading to false-positive results. Unaided clinical diagnosis may be difficult even for seasoned endocrinologists, especially in patients with no clear-cut features of CS, no use of exogenous glucocorticoids, and associated with mild hypercortisolism conditions.
To reduce unnecessary screening and shorten the time of diagnosis, numerous attempts have been made including clinical scores (16–18), automated face recognition (19–21), radiological or clinical assessments of skin thickness (22, 23), the quantification of facial plethora with near-infrared imaging (24), and, more recently, changes in white blood cell count (25, 26). This mini-review focuses on the latest progress in clinical risk scoring system for CS. We attempt to raise awareness for the use, validation, and/or development of clinical risk scores in CS.
A systematic search was conducted in PubMed, Medline, EBSCO, Web of Science, ScienceDirect, Scopus, and OVID, for articles that developed and/or validated clinical risk score for the pre-test probability of CS. All epidemiological, cross-sectional, cohort, retrospective, longitudinal, observational, comparative, case-control, and case-report studies were considered, which contained the following keywords or MeSH terms: “cortisol”, “ACTH”, “corticotropin”, “pituitary adenoma”, and “Cushing syndrome”. Articles written in English or Spanish were included.
Simple screening tests to discriminate CS are needed as early diagnosis can prevent associated comorbidities and mortality (27, 28). Moreover, the appropriate use of testing to screen patient-at-risk is crucial because delayed diagnosis may be associated with symptoms after treatment (29). CS is an infrequent disease that has a low prevalence so its screening test must have a high specificity to avoid false-positive results (30, 31). Current clinical guidelines outlined by the evidence-based 2008 Endocrine Society suggest testing with one of the following first-line tests: late-night salivary cortisol (LNSC) (two measurements), 24-h urinary free cortisol (UFC) excretion (two measurements), or the overnight 1-mg dexamethasone suppression test (DST). The performance of these first-line screening tests in usual clinical practice is uncertain as none of them have ideal sensitivity or specificity (32, 33). Table 1 shows the diagnostic criteria and sensitivities and specificities for these tests. Consequently, clinicians must individualize the choice for the initial test to reduce false-positive results. There is a need to increase clinician awareness concerning the need of complementary and individualized tests to determine CS diagnosis. LNSC assay usefulness for screening is based on the lack of circadian rhythm in CS (1, 44). Its use has increased overtime (45). One of the challenges of LNSC is on its cutoff to interpretate the results. Thresholds and accuracy vary widely, perhaps, due to assay and/or collection differences (40, 41). Sensitivities and specificities ranges from 83% to 100% and 84% to 97%, respectively (39–43). Despite its advantage to be non-invasiveness, LNSC is not useful to diagnose CS in patients with irregular sleep schedule, night worker, and in older men with comorbidities such as T2D and/or hypertension (1, 46). Appropriate assay-specific, age-specific values, sex, age, and other medical conditions on LNSC have not been fully characterized (1, 47).
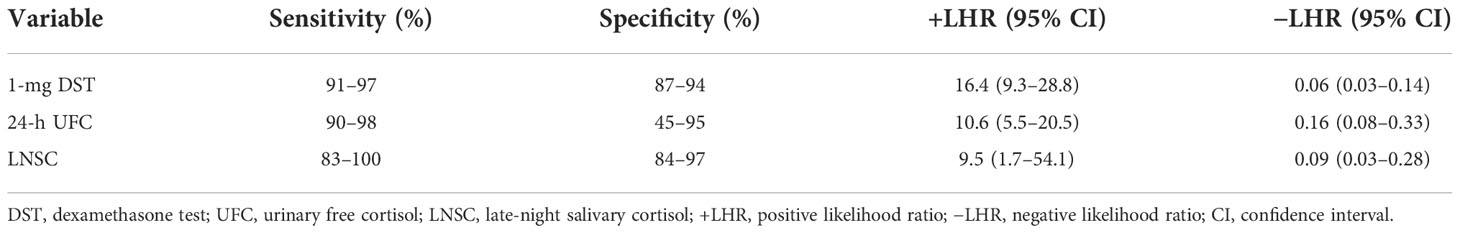
Table 1 Sensitivity and specificity and likelihood ratio of screening tests for Cushing syndrome (34–43).
Twenty-four–hour UFC is a reliable first-line screening test with sensitivity and specificity of up to 98% and 92%, respectively (37, 38). Current guidelines suggest performing at least 24-h UFC determinations, and patients can be considered to have CS if UFC is above more than three times the upper limit of normal together with another abnormal first-line screening test (1). One of the main issues with UFC is its limited utility in subclinical hypercortisolism, resulting in a false-negative result (1). Regardless of some limitation for its interpretation, UFC has a superior diagnostic performance with a likelihood ratio of 10.6 (95% CI, 5.5–20.5) for an abnormal result and a likelihood ratio for CS of 0.16 (95% CI, 0.08–0.33) for a normal result (36).
Overnight 1-mg DST is used to discriminate patients if the patients have CS of any etiology from patients who do not have it (1). Broadly speaking, overnight 1-mg DST remains the screening test of choice, but its sensitivity and specificity depend on the threshold of plasma cortisol used (37, 38, 48). False-positive results can occur in patients taking oral estrogens that increase in corticosteroid-binding globulin or any medications that modify the metabolism of dexamethasone (1, 49). False-negative results can be seen on patients with decreased metabolism of dexamethasone secondary to drugs, liver, or kidney failure (50).
CS is an infrequent disease, and few centers see sufficiently large numbers of patients to collect sufficient data to obtain reliable estimates of predictors within a short period of time (30, 31). Correspondingly, there are few published studies that attempt to weight each clinical variable (16–18). Until now, few diagnostic scores for CS have been developed to identify which patients should be screened for CS (16–18). First developed in 1964 by Nugent et al., a clinical score based on 19 simple clinical and laboratory data associated with hypercortisolism was used to increase the diagnostics usefulness (16). However, only half of the patients with suspected CS could be diagnosed, correctly lacking its clinical usefulness (Table 2). León-Justel et al. proposed a risk score based on clinical signs and one biochemical parameter that included 353 at-risk patients from 13 different hospitals across Spain (17). Screening was considered having at least two of the risk factors for CS: high blood pressure (defined as taking two or more drugs and having a systolic blood pressure over 140 mmHg and/or a diastolic blood pressure over 90 mmHg), obesity (body mass index >30), uncontrolled T2D (HbA1c >7.0%), osteoporosis (T-score ≥−2.5 SD), and virilization syndrome (hirsutism) with menstrual disorders. Biochemical data including LNSC and the low-dose DST were used. Risk score for CS included osteoporosis, dorsocervical fat pad, and muscular atrophy and LNSC levels. The researchers developed an equation to determine the risk of CS with sensitivity and specificity for this model being 96% and 83%, respectively. Although this study was a good start to identify key predictors for CS, it included a biochemical test, making it inappropriate for its applicability in an exclusive clinical estimate of the probability of CS before testing (Table 2). Moreover, the included clinical signs are already well known to be associated with endogenous hypercortisolism, and LNSC (included in the equation) is already a screening test for CS (1). Finally, several methodological flaws limit its reliability (51).
Parasiliti-Caprino et al. developed and internally validated the “Cushing score” to discriminate between a low- and high-pretest probability of CS (18). The researchers included 150 CS cases and 300 controls from five endocrinology centers in Italy. Baseline characteristics associated with CS were collected, including muscle wasting (proximal muscle atrophy and proximal muscle weakness), skin changes (easy bruisability, facial plethora, hirsutism, purple striae, and/or seborrhea), atypical fat distribution (central adiposity, dorsocervical fat pad, and facial fullness), bone mineral loss (osteopenia or osteoporosis), cardiometabolic alterations (diabetes, dyslipidemia, hypertension, and obesity), and psychiatric symptoms. The prediction model for CS was built from a multivariable logistic regression that included key features associated with clinical impression of hypercortisolism. Predictive variables included age [odds ratio (OR), 3.15; 95% CI, 1.34–7.42; P = 0.009 for age 40-59 years; OR, 7.35; 95% CI, 2.79–19.37; P < 0.001 for age < 40 years], facial fullness (OR, 2.13; 95% CI, 1.16–3.93; P = 0.015), facial plethora (OR, 1.98; 95% CI, 1.04–3.77; P = 0.038), proximal muscle atrophy (OR, 2.46; 95% CI, 1.24–4.88; P = 0.010), hirsutism and/or seborrhea (OR, 1.91; 95% CI, 1.06–3.41; P = 0.030), absence of obesity (OR, 5.93; 95% CI, 3.27–10.73; P < 0.001), hypertension (OR, 3.36; 95% CI, 1.81–6.21; P < 0.001), diabetes (OR, 1.87; 95% CI, 0.98–3.57; P = 0.059), and bone mineral density (OR, 2.35; 95% CI, 1.14–4.86, P = 0.021 for osteopenia; OR, 5.13; 95% CI, 2.39–11.02; P < 0.001 for osteoporosis). Receiver operating characteristic curve analysis and area under the curve (AUC) were used, showing a high predictive performance (0.873). In addition, internal validation was conducted, showing an AUC of 0.841. The prediction model “Cushing score” was a 17.5-point scale classified as follows: low-risk class (score value, ≤5.5; probability of disease, 0.8%), an intermediate-low-risk class (score value, 6–8.5; probability of disease, 2.7%), an intermediate-high-risk class (score value, 9–11.5; probability of disease, 18.5%), and a high-risk class (score value, 12.0–17.5; probability of disease, 72.5%).
Given the heterogenous nature of CS (1, 16), whether it is possible to accurately identify patients at risk using a model remains to be determined. As global prevalence of T2D and obesity increases (52, 53). CS may not be frequent enough to back routine screening to avoid false-positive results. Those clinical scores are summarized in Table 2.
Risk scores are useful to stratify a population for targeted screening. Data from risk factors are used to calculate an individual’s score (54). In clinical endocrinology, risk scores have been used in Graves’ ophthalmology (55), hirsutism (56), hypothyroidism (57), and even in CS (16). Clinically relevant information derived from research assists clinicians to avoid unnecessary diagnosis tests or treatment (58). Discrimination of CS in the absence of clear-cut features is challenging, especially in the context of the ever-increasing prevalence of T2D, obesity, and depression (52, 53, 59). Time to diagnosis of CS remains to be delayed as its signs and symptoms overlap with common diseases including metabolic syndrome and polycystic ovary syndrome (15, 27). Moreover, the time taken to diagnose CS differs according to its subtypes and geographic regions (27). A standardized approach with a clinical score system based on applied evidence might decrease the time of diagnosis, and a timely diagnosis of CS decreases its morbidity and chronic sequalae (27, 28, 59).
One of the current challenges of CS is it rareness and the absence of lead symptoms (13, 30, 31). Moreover, different definitions of first symptoms of CS might explain why time diagnosis varies (27). There are several well-established characteristic phenotypes associated with chronic hypercortisolism such as weight gain, menstrual irregularities, hirsutism, muscle weakness, bruisability, skin atrophy, and buffalo hump; however, the independent contribution of each factor is not well understood or defined (60). In addition, comorbidities including hypertension, hypercholesterolemia, T2D, and osteoporosis are likely to be present in patients with hypercortisolism, but systematic screening recommendation for CS is questioned (9, 61–64). The combination of these clinical data into tools to estimate the risk and, therefore, to screen for CS, is still in its infancy (15). The development of an algorithm for predicting whom to screen CS could assist clinicians to consider it with the ultimate goal of reducing morbidity and mortality.
Because the prevalence of endogenous hypercortisolism disease is increasing because of metabolic and chronic diseases such as T2D, obesity, metabolic syndrome, and depression, there might be a need for the use of diagnostic scores (preferably based only on clinical signs) to guide physicians the use of initial screening test. One of the objectives in this review is to raise awareness in the medical community regarding the use, validation, and/or development of clinical risk scores in CS.
CS diagnoses are still delayed, increasing the danger of poor prognosis. Because of a high prevalence of T2D, obesity, and other comorbidities, it is important to build and validate a simple risk score prediction for CS based on easily acquired clinical variables to achieve potential risk reduction and healthcare costs, diagnostic tests, and treatment of comorbidities.
Both authors did data collection, writing, and critical revision of the article. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2008) 93(5):1526–40. doi: 10.1210/jc.2008-0125
2. Hasenmajer V, Sbardella E, Sciarra F, Minnetti M, Isidori AM, Venneri MA. The immune system in cushing’s syndrome. Trends Endocrinol metabolism: TEM. (2020) 31(9):655–69. doi: 10.1016/j.tem.2020.04.004
3. Findling JW, Raff H. DIAGNOSIS OF ENDOCRINE DISEASE: Differentiation of pathologic/neoplastic hypercortisolism (Cushing’s syndrome) from physiologic/non-neoplastic hypercortisolism (formerly known as pseudo-cushing’s syndrome). Eur J Endocrinol (2017) 176(5):R205–r16. doi: 10.1530/EJE-16-0946
4. Kreitschmann-Andermahr I, Psaras T, Tsiogka M, Starz D, Kleist B, Siegel S, et al. From first symptoms to final diagnosis of cushing’s disease: experiences of 176 patients. Eur J endocrinol (2015) 172(3):285–9. doi: 10.1530/EJE-14-0766
5. Sharma ST, Nieman LK, Feelders RA. Cushing’s syndrome: epidemiology and developments in disease management. Clin Epidemiol (2015) 7:281–93. doi: 10.2147/CLEP.S44336
6. Cipoli DE, Martinez EZ, Castro M, Moreira AC. Clinical judgment to estimate pretest probability in the diagnosis of cushing’s syndrome under a Bayesian perspective. Arquivos brasileiros endocrinologia e metabologia. (2012) 56(9):633–7. doi: 10.1590/S0004-27302012000900006
7. Savas M, Mehta S, Agrawal N, van Rossum E, Feelders RA. Approach to the patient: Diagnosis of cushing’s syndrome. J Clin Endocrinol Metab (2022) dgac492. doi: 10.1210/clinem/dgac492
8. Świątkowska-Stodulska R, Berlińska A, Stefańska K, Kłosowski P, Sworczak K. Cyclic cushing’s syndrome - a diagnostic challenge. Front endocrinol (2021) 12:658429. doi: 10.3389/fendo.2021.658429
9. Braun LT, Vogel F, Zopp S, Marchant Seiter T, Rubinstein G, Berr CM, et al. Whom should we screen for cushing syndrome? the endocrine society practice guideline recommendations 2008 revisited. J Clin Endocrinol Metab (2022) 107(9):e3723–e30. doi: 10.1210/clinem/dgac379
10. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet (London England) (9996) 2015:913–27:386. doi: 10.1016/S0140-6736(14)61375-1
11. Scaroni C, Albiger NM, Palmieri S, Iacuaniello D, Graziadio C, Damiani L, et al. Approach to patients with pseudo-cushing’s states. Endocrine connections (2020) 9(1):R1–r13. doi: 10.1530/EC-19-0435
12. Li D, El Kawkgi OM, Henriquez AF, Bancos I. Cardiovascular risk and mortality in patients with active and treated hypercortisolism. Gland surgery (2020) 9(1):43–58. doi: 10.21037/gs.2019.11.03
13. Debono M, Newell-Price JD. Cushing’s syndrome: Where and how to find it. Front hormone Res (2016) 46:15–27. doi: 10.1159/000443861
14. Aron DC. Cushing’s syndrome: why is diagnosis so difficult? Rev endocrine Metab Disord (2010) 11(2):105–16. doi: 10.1007/s11154-010-9127-3
15. Braun LT, Riester A, Oßwald-Kopp A, Fazel J, Rubinstein G, Bidlingmaier M, et al. Toward a diagnostic score in cushing’s syndrome. Front endocrinol (2019) 10:766. doi: 10.3389/fendo.2019.00766
16. Nugent CA, Warner HR, Dunn JT, Tyler FH. Probability theory in the diagnosis of cushing’s syndrome. J Clin Endocrinol Metab (1964) 24:621–7. doi: 10.1210/jcem-24-7-621
17. León-Justel A, Madrazo-Atutxa A, Alvarez-Rios AI, Infantes-Fontán R, Garcia-Arnés JA, Lillo-Muñoz JA, et al. A probabilistic model for cushing’s syndrome screening in At-risk populations: A prospective multicenter study. J Clin Endocrinol Metab (2016) 101(10):3747–54. doi: 10.1210/jc.2016-1673
18. Parasiliti-Caprino M, Bioletto F, Frigerio T, D’Angelo V, Ceccato F, Ferraù F, et al. A new clinical model to estimate the pre-test probability of cushing’s syndrome: The cushing score. Front endocrinol (Lausanne) (2021) 12:747549. doi: 10.3389/fendo.2021.747549
19. Learned-Miller E, Lu Q, Paisley A, Trainer P, Blanz V, Dedden K, et al. Detecting acromegaly: screening for disease with a morphable model. Med image computing computer-assisted intervention: MICCAI Int Conf Med Image Computing Computer-Assisted Intervention (2006) 9(Pt 2):495–503. doi: 10.1007/11866763_61
20. Schneider HJ, Kosilek RP, Günther M, Roemmler J, Stalla GK, Sievers C, et al. A novel approach to the detection of acromegaly: accuracy of diagnosis by automatic face classification. J Clin Endocrinol Metab (2011) 96(7):2074–80. doi: 10.1210/jc.2011-0237
21. Kosilek RP, Schopohl J, Grunke M, Reincke M, Dimopoulou C, Stalla GK, et al. Automatic face classification of cushing’s syndrome in women - a novel screening approach. Exp Clin Endocrinol diabetes: Off journal German Soc Endocrinol [and] German Diabetes Assoc (2013) 121(9):561–4. doi: 10.1055/s-0033-1349124
22. Ferguson JK, Donald RA, Weston TS, Espiner EA. Skin thickness in patients with acromegaly and cushing’s syndrome and response to treatment. Clin endocrinol (1983) 18(4):347–53. doi: 10.1111/j.1365-2265.1983.tb00578.x
23. Corenblum B, Kwan T, Gee S, Wong NC. Bedside assessment of skin-fold thickness. a useful measurement for distinguishing cushing’s disease from other causes of hirsutism and oligomenorrhea. Arch Internal Med (1994) 154(7):777–81. doi: 10.1001/archinte.1994.00420070099011
24. Afshari A, Ardeshirpour Y, Lodish MB, Gourgari E, Sinaii N, Keil M, et al. Facial plethora: Modern technology for quantifying an ancient clinical sign and its use in cushing syndrome. J Clin Endocrinol Metab (2015) 100(10):3928–33. doi: 10.1210/jc.2015-2497
25. Paja M, Merlo I, Rodríguez-Soto J, Cruz-Iglesias E, Moure MD, Elías C, et al. White blood cell count: a valuable tool for suspecting cushing’s syndrome. J Endocrinol Invest (2022). doi: 10.1007/s40618-022-01892-6
26. Detomas M, Altieri B, Chifu I, Remde H, Zhou X, Landwehr LS, et al. Subtype-specific pattern of white blood cell differential in endogenous hypercortisolism. Eur J endocrinol (2022) 187(3):439–49. doi: 10.1530/EJE-22-0211
27. Rubinstein G, Osswald A, Hoster E, Losa M, Elenkova A, Zacharieva S, et al. Time to diagnosis in cushing’s syndrome: A meta-analysis based on 5367 patients. J Clin Endocrinol Metab (2020) 105(3):e12–e22. doi: 10.1210/clinem/dgz136
28. Javanmard P, Duan D, Geer EB. Mortality in patients with endogenous cushing’s syndrome. Endocrinol Metab Clinics North America (2018) 47(2):313–33. doi: 10.1016/j.ecl.2018.02.005
29. Valassi E, Chiodini I, Feelders RA, Andela CD, Abou-Hanna M, Idres S, et al. Unmet needs in cushing’s syndrome: the patients’ perspective. Endocr Connect (2022) 11(7):e220027. doi: 10.1530/EC-22-0027
30. Steffensen C, Bak AM, Rubeck KZ, Jørgensen JO. Epidemiology of cushing’s syndrome. Neuroendocrinology. (2010) 92 Suppl 1:1–5. doi: 10.1159/000314297
31. Valassi E, Santos A, Yaneva M, Tóth M, Strasburger CJ, Chanson P, et al. The European registry on cushing’s syndrome: 2-year experience. Baseline demographic Clin characteristics Eur J endocrinol (2011) 165(3):383–92. doi: 10.1530/EJE-11-0272
32. Ceccato F, Boscaro M. Cushing’s syndrome: Screening and diagnosis. High Blood Pressure Cardiovasc prevention: Off J Ital Soc Hypertension (2016) 23(3):209–15. doi: 10.1007/s40292-016-0153-4
33. Baid SK, Rubino D, Sinaii N, Ramsey S, Frank A, Nieman LK. Specificity of screening tests for cushing’s syndrome in an overweight and obese population. J Clin Endocrinol Metab (2009) 94(10):3857–64. doi: 10.1210/jc.2008-2766
34. Isidori AM, Kaltsas GA, Mohammed S, Morris DG, Jenkins P, Chew SL, et al. Discriminatory value of the low-dose dexamethasone suppression test in establishing the diagnosis and differential diagnosis of cushing’s syndrome. J Clin Endocrinol Metab (2003) 88(11):5299–306. doi: 10.1210/jc.2003-030510
35. Meikle AW. Dexamethasone suppression tests: usefulness of simultaneous measurement of plasma cortisol and dexamethasone. Clin endocrinol (1982) 16(4):401–8. doi: 10.1111/j.1365-2265.1982.tb00733.x
36. Elamin MB, Murad MH, Mullan R, Erickson D, Harris K, Nadeem S, et al. Accuracy of diagnostic tests for cushing’s syndrome: a systematic review and metaanalyses. J Clin Endocrinol Metab (2008) 93(5):1553–62. doi: 10.1210/jc.2008-0139
37. Ceccato F, Barbot M, Zilio M, Frigo AC, Albiger N, Camozzi V, et al. Screening tests for cushing’s syndrome: Urinary free cortisol role measured by LC-MS/MS. J Clin Endocrinol Metab (2015) 100(10):3856–61. doi: 10.1210/jc.2015-2507
38. Isidori AM, Graziadio C, Paragliola RM, Cozzolino A, Ambrogio AG, Colao A, et al. The hypertension of cushing’s syndrome: controversies in the pathophysiology and focus on cardiovascular complications. J hypertension (2015) 33(1):44–60. doi: 10.1097/HJH.0000000000000415
39. Antonelli G, Ceccato F, Artusi C, Marinova M, Plebani M. Salivary cortisol and cortisone by LC-MS/MS: validation, reference intervals and diagnostic accuracy in cushing’s syndrome. Clinica chimica acta; Int J Clin Chem (2015) 451(Pt B):247–51. doi: 10.1016/j.cca.2015.10.004
40. Ceccato F, Barbot M, Zilio M, Ferasin S, Occhi G, Daniele A, et al. Performance of salivary cortisol in the diagnosis of cushing’s syndrome, adrenal incidentaloma, and adrenal insufficiency. Eur J endocrinol (2013) 169(1):31–6. doi: 10.1530/EJE-13-0159
41. Deutschbein T, Broecker-Preuss M, Flitsch J, Jaeger A, Althoff R, Walz MK, et al. Salivary cortisol as a diagnostic tool for cushing’s syndrome and adrenal insufficiency: improved screening by an automatic immunoassay. Eur J endocrinol (2012) 166(4):613–8. doi: 10.1530/EJE-11-0945
42. Erickson D, Singh RJ, Sathananthan A, Vella A, Bryant SC. Late-night salivary cortisol for diagnosis of cushing’s syndrome by liquid chromatography/tandem mass spectrometry assay. Clin endocrinol (2012) 76(4):467–72. doi: 10.1111/j.1365-2265.2011.04239.x
43. Raff H. Cushing’s syndrome: diagnosis and surveillance using salivary cortisol. Pituitary. (2012) 15(1):64–70. doi: 10.1007/s11102-011-0333-0
44. Boscaro M, Arnaldi G. Approach to the patient with possible cushing’s syndrome. J Clin Endocrinol Metab (2009) 94(9):3121–31. doi: 10.1210/jc.2009-0612
45. Bäcklund N, Brattsand G, Israelsson M, Ragnarsson O, Burman P, Edén Engström B, et al. Reference intervals of salivary cortisol and cortisone and their diagnostic accuracy in cushing’s syndrome. Eur J endocrinol (2020) 182(6):569–82. doi: 10.1530/EJE-19-0872
46. Liu H, Bravata DM, Cabaccan J, Raff H, Ryzen E. Elevated late-night salivary cortisol levels in elderly male type 2 diabetic veterans. Clin endocrinol (2005) 63(6):642–9. doi: 10.1111/j.1365-2265.2005.02395.x
47. Fleseriu M. Salivary cortisol in the diagnosis of cushing syndrome, always more than one! J Endocrine Soc (2020) 4(10):bvaa109. doi: 10.1210/jendso/bvaa109
48. Pecori Giraldi F, Ambrogio AG, De Martin M, Fatti LM, Scacchi M, Cavagnini F. Specificity of first-line tests for the diagnosis of cushing’s syndrome: assessment in a large series. J Clin Endocrinol Metab (2007) 92(11):4123–9. doi: 10.1210/jc.2007-0596
49. Valassi E, Swearingen B, Lee H, Nachtigall LB, Donoho DA, Klibanski A, et al. Concomitant medication use can confound interpretation of the combined dexamethasone-corticotropin releasing hormone test in cushing’s syndrome. J Clin Endocrinol Metab (2009) 94(12):4851–9. doi: 10.1210/jc.2009-1500
50. Gilbert R, Lim EM. The diagnosis of cushing’s syndrome: an endocrine society clinical practice guideline. Clin biochemist Rev (2008) 29(3):103–6. doi: 10.1210/jc.2008-0125
51. Collins GS, Le Manach Y. Letter to the Editor: Models developed using small datasets should be appropriately evaluated. J Clin Endocrinol Metab (2016) 101(11):L104–L5. doi: 10.1210/jc.2016-2934
52. Cooper AJ, Gupta SR, Moustafa AF, Chao AM. Sex/Gender differences in obesity prevalence, comorbidities, and treatment. Curr Obes Rep (2021) 10(4):458–66. doi: 10.1007/s13679-021-00453-x
53. Ferrari AJ, Santomauro DF, Herrera AMM, Shadid J, Ashbaugh C, Erskine HE, et al. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Psychiatry (2022) 9(2):137–50. doi: 10.1016/S2215-0366(21)00395-3
54. Davies MJ, Gray LJ, Troughton J, Gray A, Tuomilehto J, Farooqi A, et al. Developing the risk score. 2017. In: A community-based primary prevention programme for type 2 diabetes mellitus integrating identification and lifestyle intervention for prevention: a cluster randomised controlled trial. Available at: https://www.ncbi.nlm.nih.gov/books/NBK409312/.
55. Stan MN, Garrity JA, Bahn RS. The evaluation and treatment of graves ophthalmopathy. Med Clinics North America (2012) 96(2):311–28. doi: 10.1016/j.mcna.2012.01.014
56. Sachdeva S. Hirsutism: evaluation and treatment. Indian J Dermatol (2010) 55(1):3–7. doi: 10.4103/0019-5154.60342
57. Kalra S, Khandelwal SK, Goyal A. Clinical scoring scales in thyroidology: A compendium. Indian J Endocrinol Metab (2011) 15(Suppl 2):S89–94. doi: 10.4103/2230-8210.83332
58. Galvao MC, Ricarte IL, Grad RM, Pluye P. The clinical relevance of information index (CRII): assessing the relevance of health information to the clinical practice. Health Inf libraries J (2013) 30(2):110–20. doi: 10.1111/hir.12021
59. Sharma ST, Nieman LK, Feelders RA. Comorbidities in cushing’s disease. Pituitary. (2015) 18(2):188–94. doi: 10.1007/s11102-015-0645-6
60. Nieman LK. Cushing’s syndrome: update on signs, symptoms and biochemical screening. Eur J Endocrinol (2015) 173(4):M33–8. doi: 10.1530/EJE-15-0464
61. Tabarin A, Perez P. Pros and cons of screening for occult cushing syndrome. Nat Rev Endocrinol (2011) 7(8):445–55. doi: 10.1038/nrendo.2011.51
62. Shimon I. Screening for cushing’s syndrome: is it worthwhile? Pituitary (2015) 18(2):201–5. doi: 10.1007/s11102-015-0634-9
63. Aresta C, Soranna D, Giovanelli L, Favero V, Parazzoli C, Gennari L, et al. When to suspect hidden hypercortisolism in type 2 diabetes: A meta-analysis. Endocrine practice: Off J Am Coll Endocrinol Am Assoc Clin Endocrinologists (2021) 27(12):1216–24. doi: 10.1016/j.eprac.2021.07.014
Keywords: cortisol, ACTH, corticotropin, pituitary adenoma, obesity
Citation: Lam-Chung CE and Cuevas-Ramos D (2022) The promising role of risk scoring system for Cushing syndrome: Time to reconsider current screening recommendations. Front. Endocrinol. 13:1075785. doi: 10.3389/fendo.2022.1075785
Received: 20 October 2022; Accepted: 01 November 2022;
Published: 22 November 2022.
Edited by:
Giuseppe Reimondo, University of Turin, ItalyReviewed by:
Mara Carsote, Carol Davila University of Medicine and Pharmacy, RomaniaCopyright © 2022 Lam-Chung and Cuevas-Ramos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: D. Cuevas-Ramos, ZGFuaWVsLmN1ZXZhc3JAaW5jbW5zei5teA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.