- 1Department of Radiation, the Affiliated Hospital of Xuzhou Medical University, Jiangsu, China
- 2Department of Cancer Institute, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 3Department of Radiation, the Second Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
Objectives: Androgen deprivation therapy combined with radiotherapy for intermediate-risk prostate cancer is still a matter of debate. We conducted a meta-analysis to evaluate the necessity of androgen deprivation therapy combined with radiotherapy for intermediate-risk prostate cancer patients.
Methods: A comprehensive literature search of articles was performed in PubMed, Embase, Cochrane library, Web of Science, Chinese National Knowledge Infrastructure, Chinese Biological Medicine, Wanfang, and VIP Databases published between February 1988 and April 2022. Studies comparing the survival of patients diagnosed with intermediate-risk prostate cancer who were treated with androgen deprivation therapy combined with radiotherapy or radiotherapy alone were included. Data were extracted and analyzed with the RevMan software (version 5.3) and the Stata software (version 17).
Results: Six randomized controlled trials and nine retrospective studies, including 6853 patients (2948 in androgen deprivation therapy combined with radiotherapy group and 3905 in radiotherapy alone group) were enrolled. Androgen deprivation therapy combined with radiotherapy did not provide an overall survival (HR 1.12, 95% CI 1.01-1.12, p=0.04) or biochemical recurrence-free survival (HR 1.23, 95% CI 1.09-1.39, P=0.001) advantage to intermediate-risk prostate cancer patients.
Conclusion: Androgen deprivation therapy combined with radiotherapy did not show some advantages in terms of overall survival and biochemical recurrence-free survival and radiotherapy alone may be the effective therapy for intermediate-risk prostate cancer patients.
Systematic review registration: https://inplasy.com/inplasy-2022-8-0095/, identifier 202280095.
1. Introduction
Prostate cancer (PC) is the second most common cancer and the fifth leading cause of cancer death among men worldwide. According to relevant epidemiological statistics, there were 1.4 million new cases of PC and 375,000 deaths in 2020 (1). In 2021, PC has the highest 5-year relative survival rate(98%) in the United States, where PC is the most frequent cancer which accounted for 26% of all incident cases in men (2). The 5-year survival rate of early PC can be close to 100% after radical surgery, but PC often has no obvious symptoms in the early stage, and most patients have distant metastasis when diagnosed, and the 5-year survival rate drops to 32% (3). It follows then that today PC still constitutes a global health problem.
At present, the screening and diagnosis of PC mainly rely on prostate-specific antigen (PSA), digital rectal examination, and prostate needle biopsy (4). However, the poor specificity of PSA leads to the over-diagnosis and treatment of PC and the invasive examination brings physical and mental pain to patients as well as increases the economic burden (5). Anti-cancer therapy is rarely based on a single drug and almost always requires combinative approaches since simultaneously attacking more than one target usually achieves greater efficacy (6). It is suspicious whether over-diagnosis contribute to over-treatment. However, radiotherapy (RT) alone may not be effective for treating PC, and RT combined with androgen deprivation therapy (ADT) is often a requisite (7–9). For low-risk PC, RT alone has shown high clinical response but for intermediate and high-risk PC, ADT is necessary for clinical effects.
Current studies are almost based on intermediate and high-risk PC and rarely aim at intermediate-risk PC alone (10, 11). Intermediate risk PC is the most heterogeneous of the three D’Amico risk stratification groups, and because of this, it brings considerable treatment challenges for clinical workers (12). The current National Comprehensive Cancer Network (NCCN) guidelines reclassify intermediate-risk PC patients into favorable intermediate risk (FIR) and unfavorable intermediate risk (UIR) (13). For FIR patients, some trials indicated RT alone is adequate but for UIR patients, ADT is warranted for patients receiving RT and considered for patients receiving RT with brachytherapy boost (14). However, the use of ADT in FIR patients is still controversial (15–17). Considering the potential side effects of over-treatment for patients, we should better define subgroups of patients who may benefit from the combined therapy. Therefore, in this study, we compared the efficacy and safety of RT alone with RT plus ADT in intermediate PC through meta-analysis, to provide evidence-based evidence for the treatment of intermediate-risk PC patients.
2. Materials and methods
This article was written in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and the protocol for this systematic review was registered on INPLASY (202280095) and is available in full on inplasy.com (https://inplasy.com/inplasy-2022-8-0095/) (18).
2.1. Search strategy and study selection
The articles published in PubMed, Embase, Cochrane library, Web of Science, Chinese National Knowledge Infrastructure (CNKI), Chinese Biological Medicine (CBM), Wanfang, and VIP Databases between February 1988 and April 2022 were systematically searched and meanwhile, the related trials in the International Clinical Trial Registry Platform (ICTRP) and the Chinese Clinical Registry up to April 2022 and some conference summaries were also searched and selected. The search terms included (‘Prostatic Neoplasms’ or ‘Prostate Cancer’), (‘intermediate risk’), (‘androgen deprivation therapy’ or ‘endocrine therapy’), and (‘Radiotherapy’ or ‘radiation’).
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows: clinical trials and retrospective studies directly comparing RT alone with ADT plus RT, all patients were confirmed as PC by histopathological or cytological examination and met the diagnostic criteria of intermediate-risk PC, and sufficient data could be extracted for analysis.
The exclusion criteria were as follows: review articles, systematic evaluations, animal basic experiments or case reports; repetitive articles, studies including no relevant or incomplete data; some ongoing clinical trials with no published results; and violation of any of the above inclusion criteria.
2.3. Quality assessment and risk of bias
Randomized controlled trials (RCTs) and retrospective studies were all enrolled in our systematic review and meta-analysis which we assessed the quality of through the Cochrane Collaboration's tool and the Newcastle–Ottawa scale (NOS) respectively. The scoring standard of the Cochrane Collaboration's tool contained the random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. The NOS mainly included the selection (0–4 stars), comparability (0–2 stars), and outcome (0–3 stars). If studies’ scores ≥ 6 stars, it would be regarded as high quality and enrolled in our meta-analysis.
2.4. Data extraction and collection
Data extraction and paper collection were independently and carefully performed by two authors (JC and YY), including the first author’s name, year of publication, number of patients, age of patients, interventions, ADT regimen, radiotherapy dose, and so on. If they had differences, a third author (MF) would resolve the discrepancy and determined whether the article would be included. The primary outcome was OS. The secondary endpoints were BCRFS. The hazard ratio (HR) for OS and BCRFS of patients undergoing combined was used for meta-analysis. We used two methods for data extraction of HR. For one thing, we directly extracted HR and its 95% CI from the enrolled articles. For another, by utilizing the methods of Tierney et al, we calculated HR as well as its 95% CI through the figures from the text and data given in the articles.
2.5. Statistical analysis
We used the random-effects model through the inverse variance method to calculate the pooled HR and evaluated the heterogeneity of all studies through the Cochrane’s Q test and I2 statistic, of which, significant heterogeneity was P<0.1, and I2>50.0%. We evaluated the potential publication bias by funnel plots, Egger’s test, and Begg’s test. All P values were two-sided, and P<0.05 was considered to manifest statistical significance. All statistical analyses were performed using the RevMan software (version 5.3) and the Stata software (version 17).
3. Results
3.1. Characteristics of studies
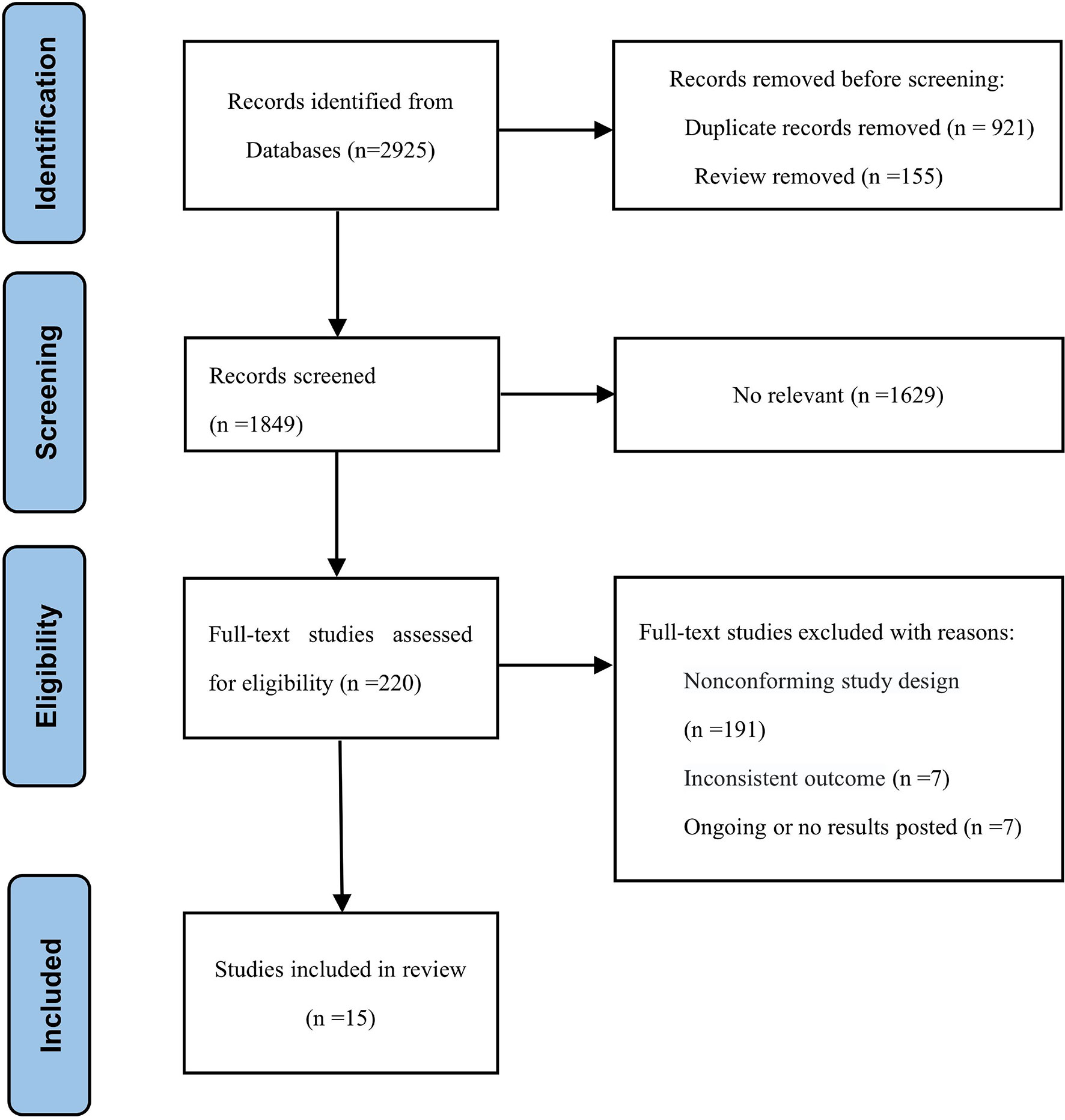
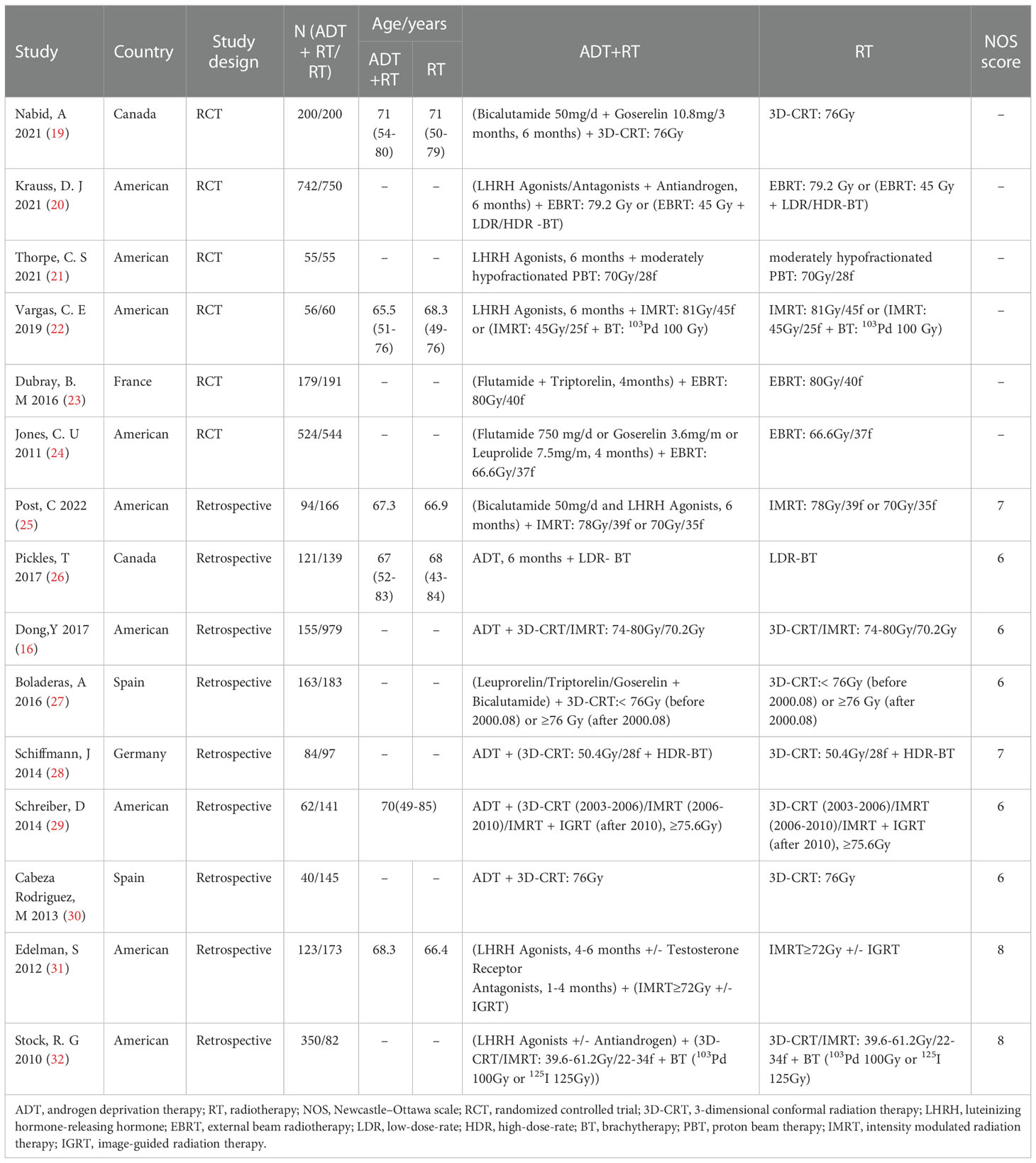
The search strategy identified 2925 citations. We reviewed the titles and abstracts and retrieved the full text of potential eligible research for inclusion. Finally, our systematic review and meta-analysis included 15 studies (6 RCTs and 9 retrospective studies). A flow chart of the literature screening is shown in Figure 1. A total of 6853 patients were included in our analysis, of which, 2948 were in the experimental group and 3905 were in the control group. The detailed characteristics of each research were summed up in Table 1.
3.2. Quality assessment
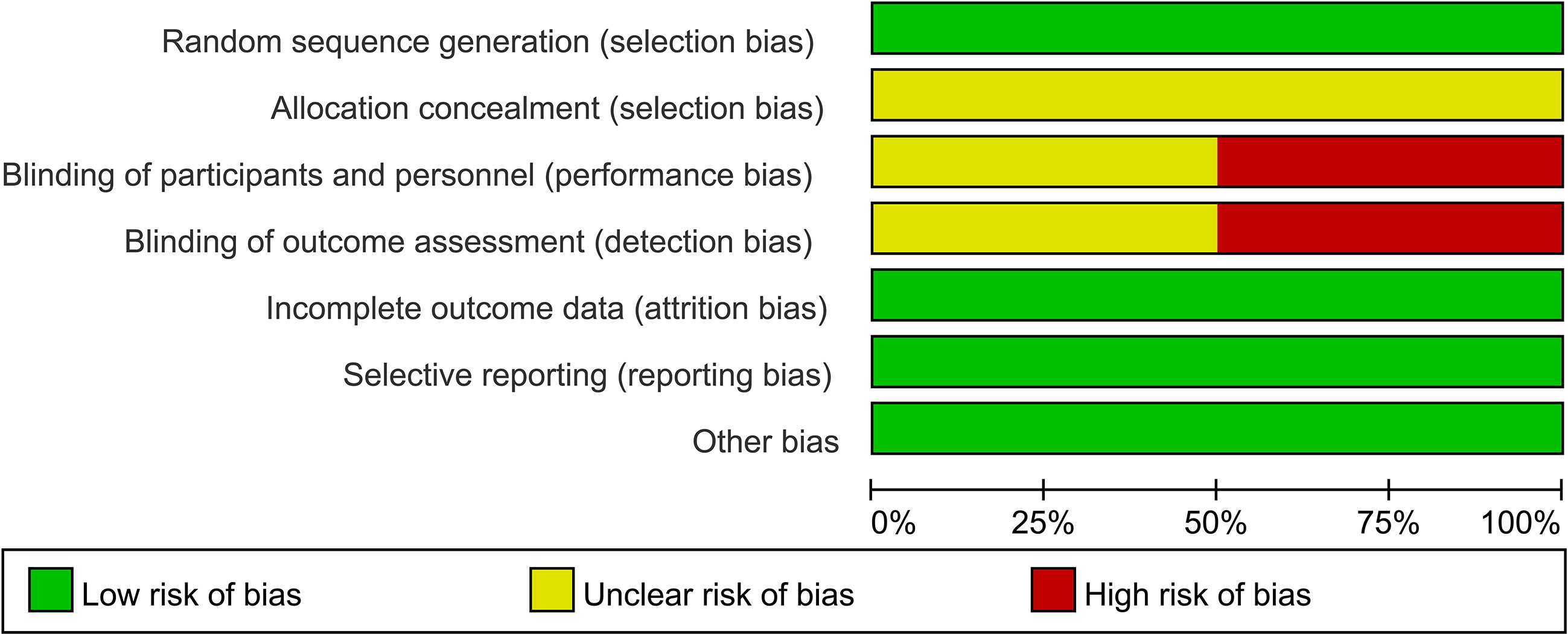
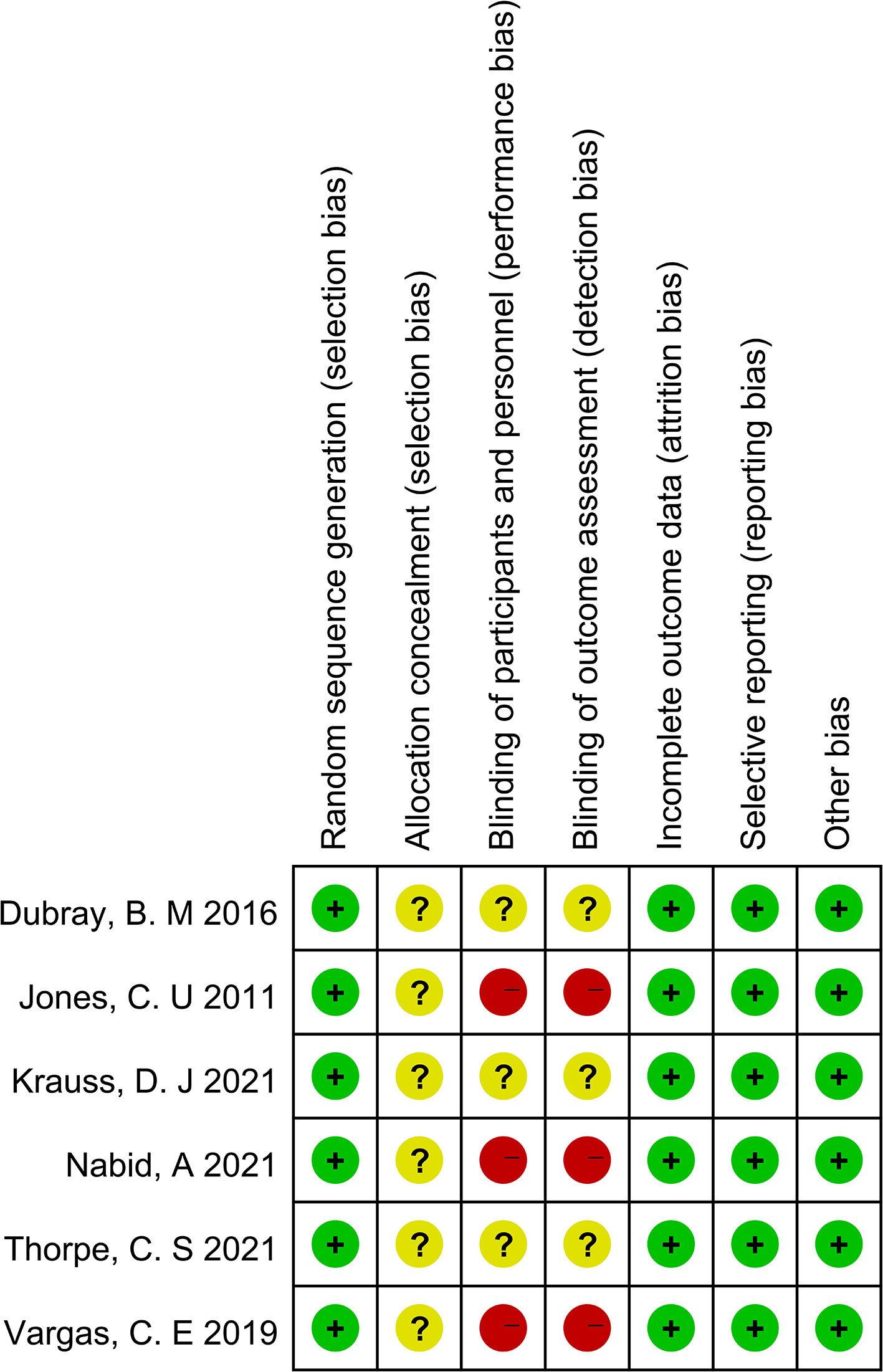
As shown in Figures 2 and 3, we evaluated the quality of the six final included RCTs using the Cochrane Collaboration’s tool. One RCT used the permuted-block randomization Method for random assignment, one used the hierarchical random assignment method, and the remaining four did not describe any particular randomization methods. None of the RCTs included provided sufficient information to evaluate the adequacy of allocation concealment. Three RCTs obtained informed consent from all included patients and were considered unblinded. The remaining three RCTs did not mention information about blindness. The NOS was also used to assess the included retrospective studies. The data and results reported in all included RCTs included were complete and without selective reporting or other bias. At the same time, the NOS was used to evaluate the retrospective studies, and the scores were all greater than or equal to 6. The evaluation results are shown in Table 1. Overall, all the studies included in this meta-analysis were considered to be of high quality.
3.3. Efficiency
3.3.1. OS
There were 4 RCTs and 6 retrospective studies of the included articles reporting OS.
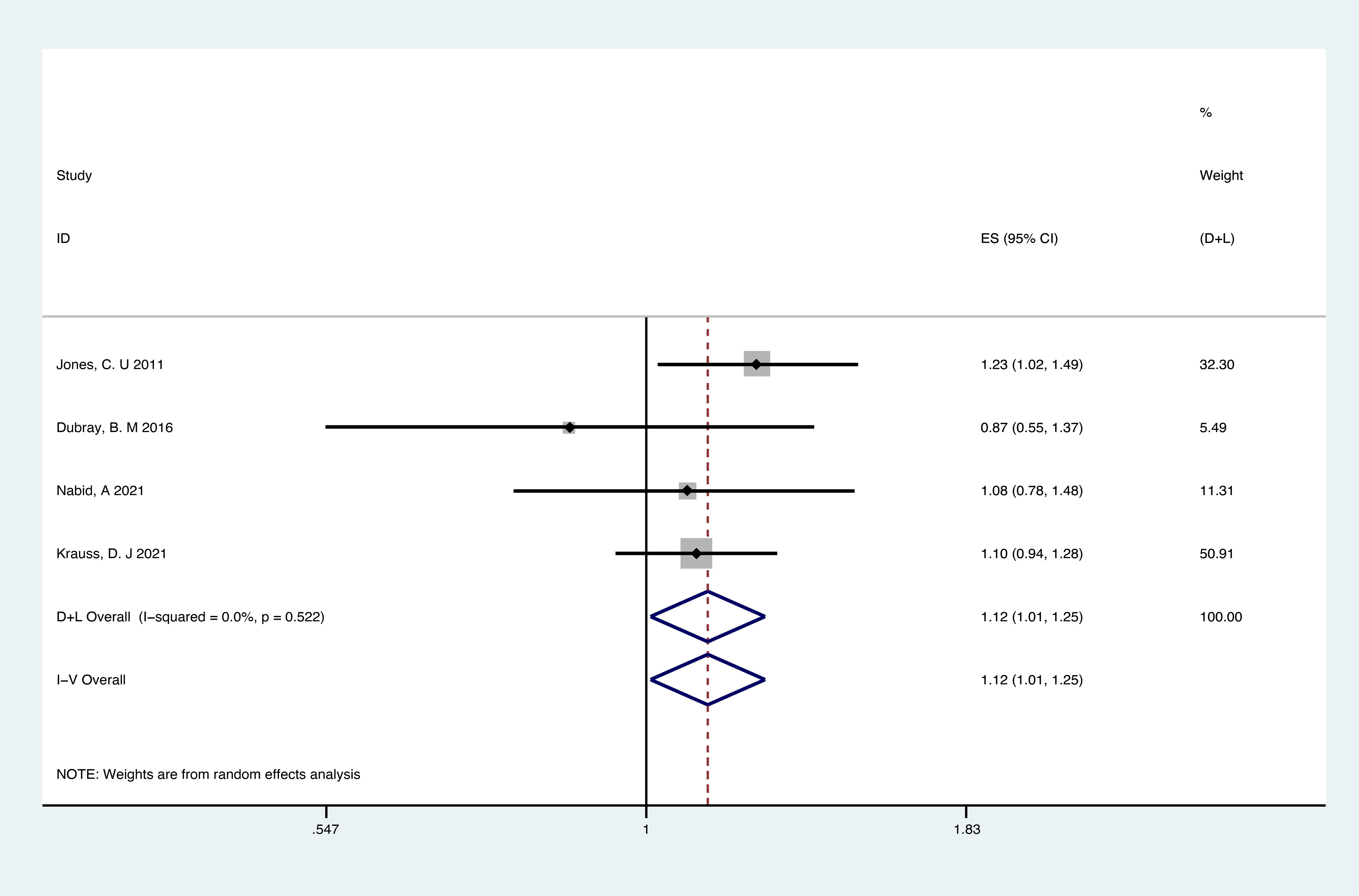
Analyses of RCTs for OS showed that RT plus ADT was associated with a 12% increase in the risk for all-cause mortality (ACM) (HR 1.12, 95% CI 1.01-1.12, p=0.04) with no heterogeneity between studies (P =0.522, I2 = 0%) and the results were statistically significant (Figure 4).
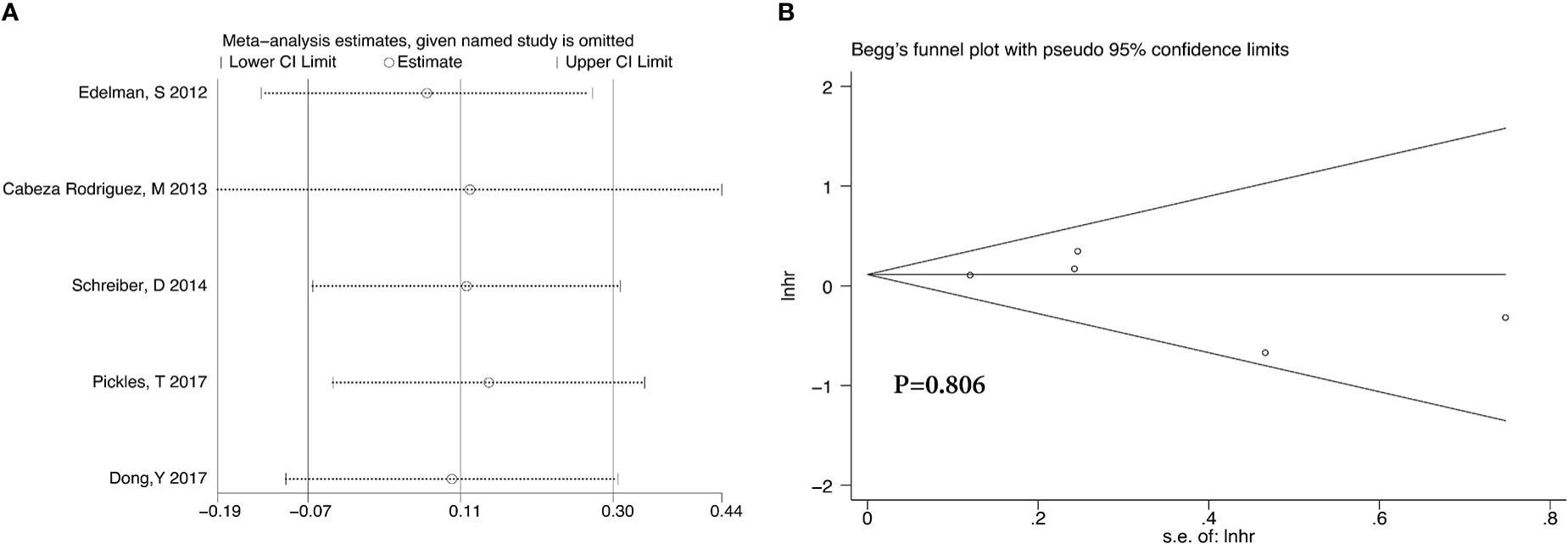
Analyses of the retrospective studies for OS showed that RT plus ADT was associated with a 2% increase in the risk for ACM (HR 1.02, 95% CI 0.86-1.22, P=0.79) with significant heterogeneity between studies (P =0.033, I2 =58.7%) (Figure 5A). Because of this, we tried to conduct the sensitivity analysis, which was performed by excluding one study at a time to assess the influence of each study on overall results. The results showed that the deletion of Post,C’s study had a significant effect on the results (Figure 6), so we exclude Post,C’s study and reanalyzed the remaining studies, which showed that RT plus ADT was associated with a 12% increase in ACM (HR 1.12, 95% CI 0.93-1.35, P=0.24) with no heterogeneity between studies (P =0.389, I2 =3.1%) (Figure 5B) but the results were no statistically significant.
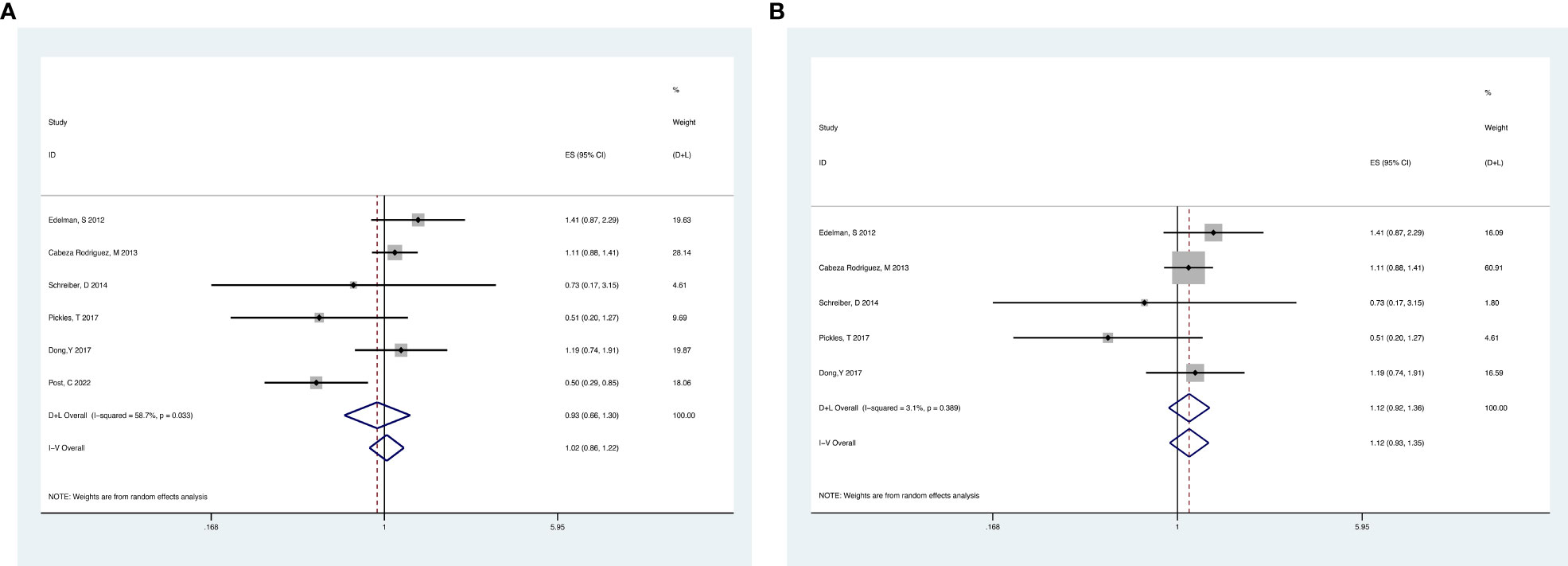
Figure 5 Forest plot for OS of 6 retrospective studies before the sensitivity analysis (A), after the sensitivity analysis (B).
3.3.2. BCRFS
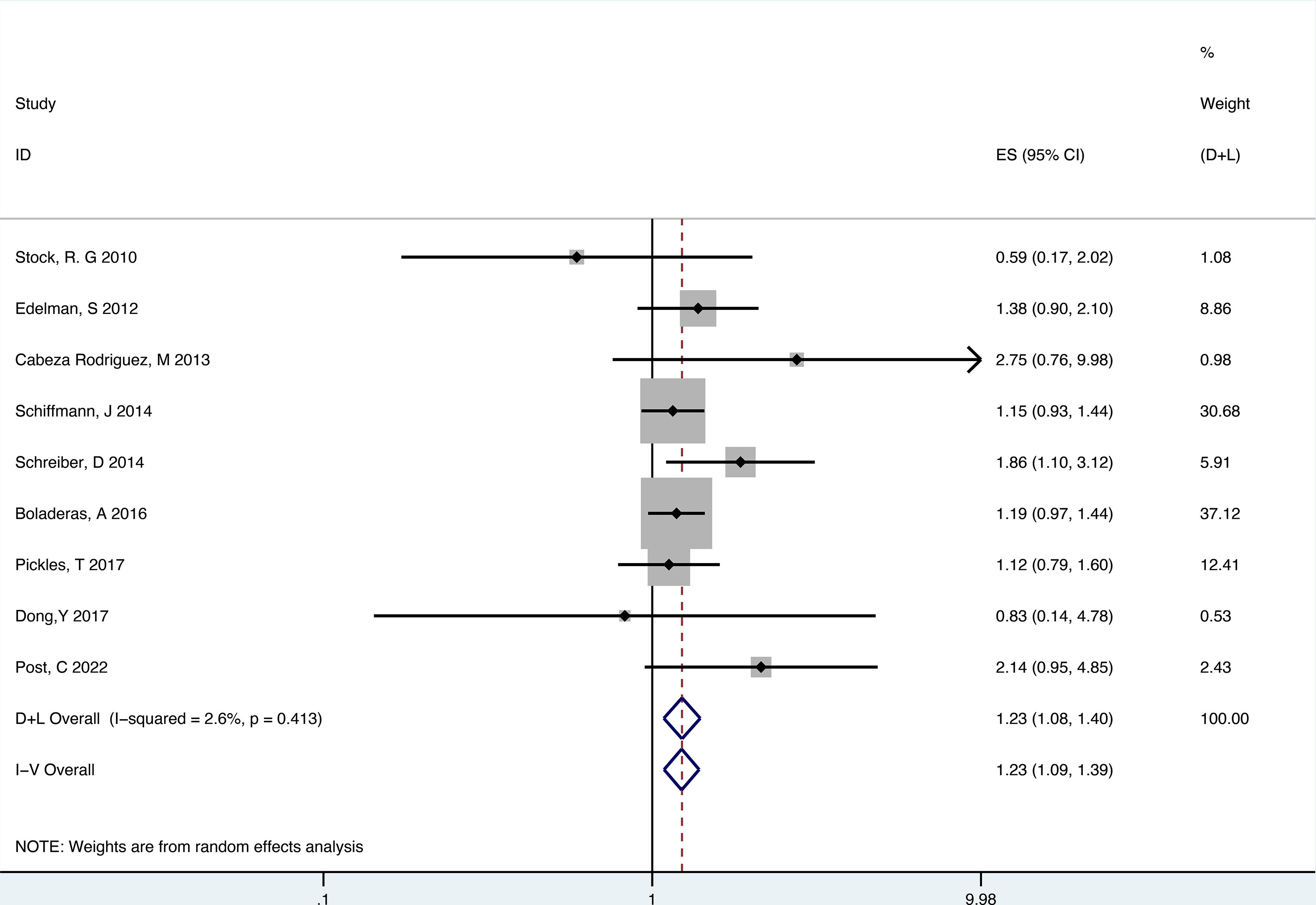
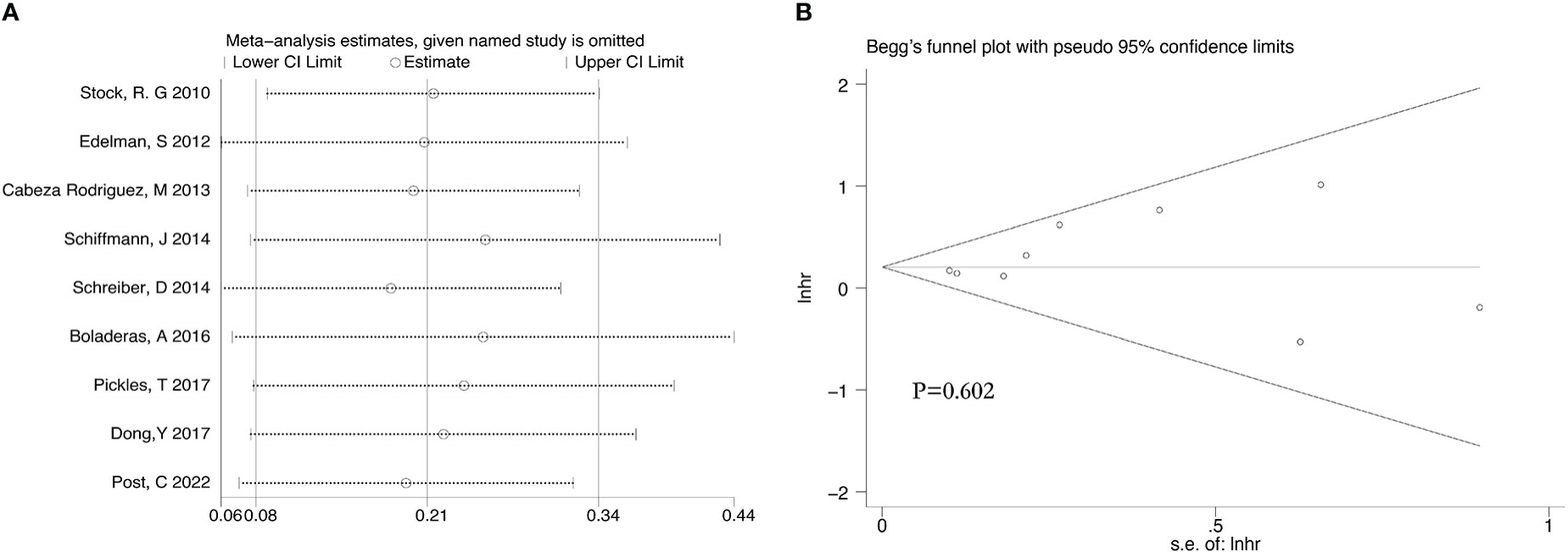
There were 9 retrospective studies of the included articles reporting BCRFS. The results showed that RT plus ADT was associated with a 23% increase in the risk for ACM (HR 1.23, 95% CI 1.09-1.39, P=0.001) with no heterogeneity between studies (P =0.413, I2 = 2.6%) and the results were statistically significant (Figure 7).
3.4. Sensitivity and publication Bias evaluation
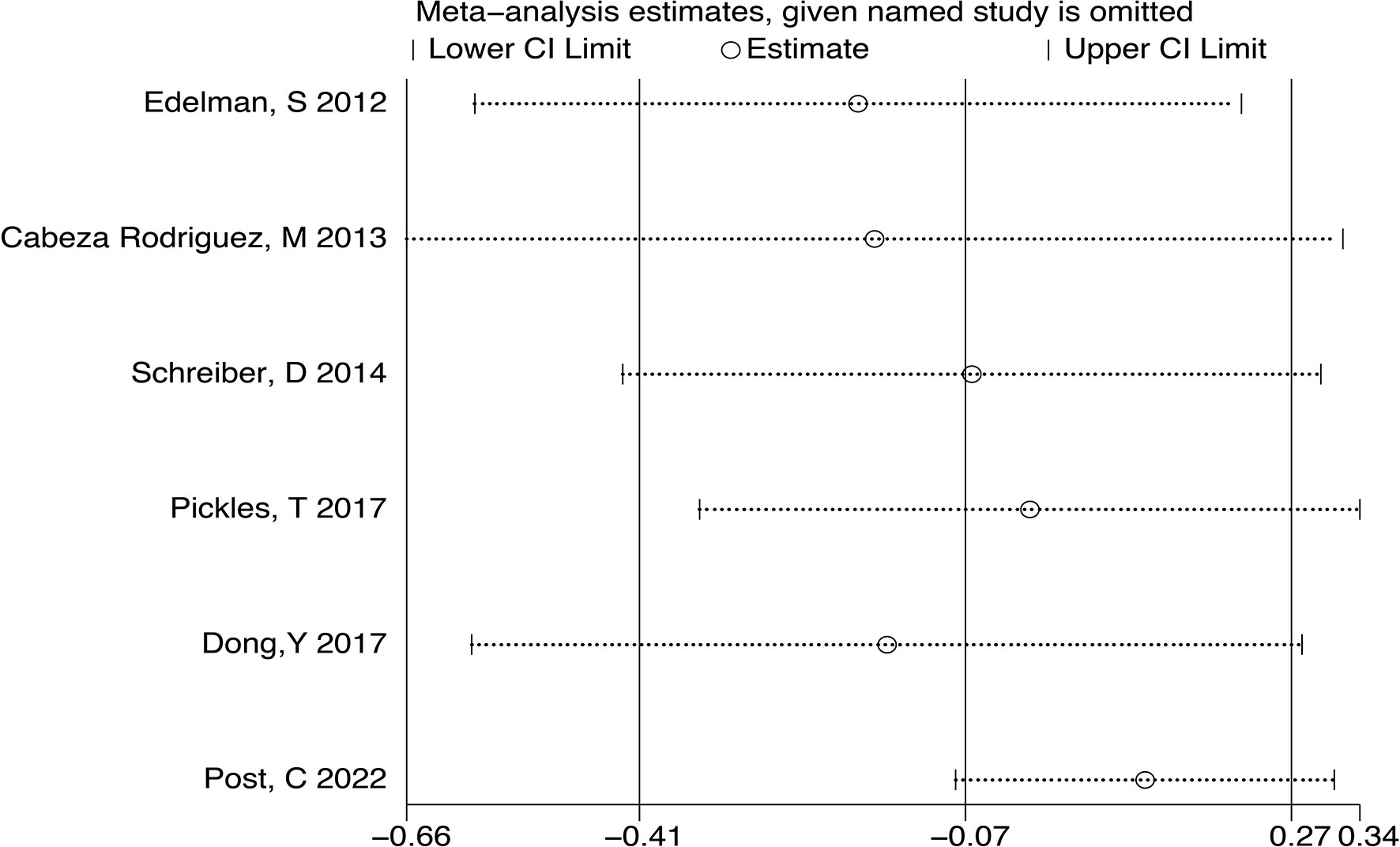
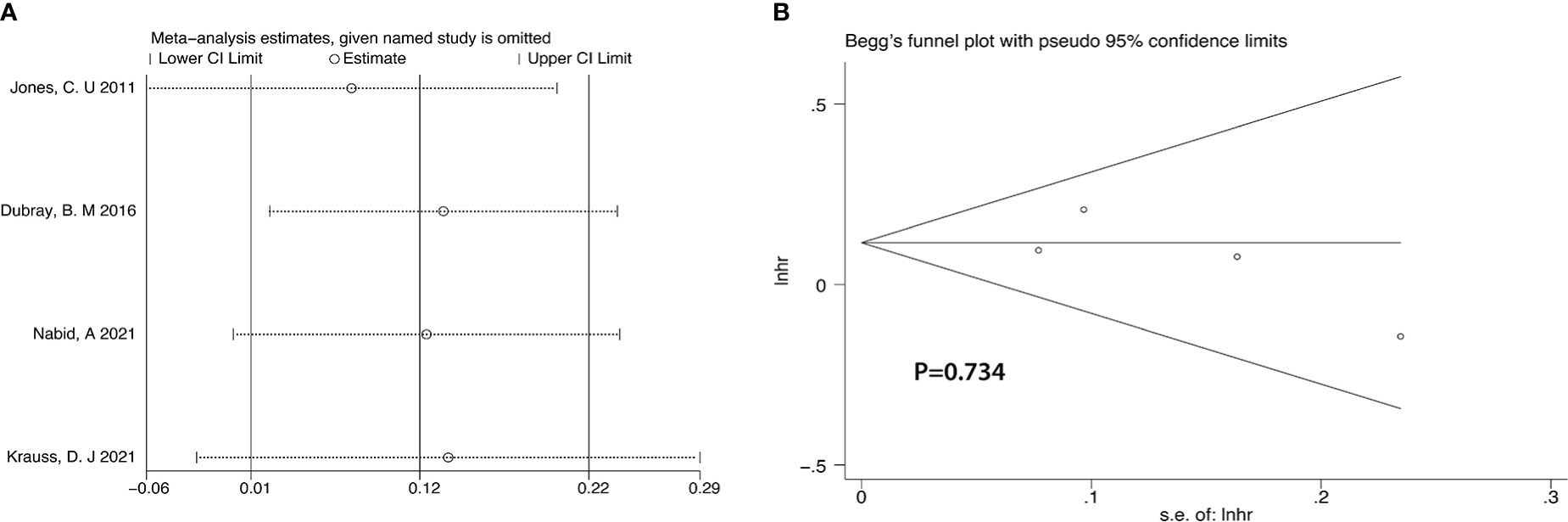
After we analyzed the OS and BCRFS of the included articles, we carried out the sensitivity analyses and the deletion of any one study had no significant effect on the results which showed our meta-analysis is relatively stable (Figure 8A-10A). We also conducted the publication bias evaluation and no evidence of publication bias was found based on Begg’s funnel plot (Figure 8B-10B).
4. Discussion
PC originates from prostatic intraepithelial neoplasia (PIN), which develops into high-grade PIN (HG-PIN), and eventually becomes adenocarcinoma (33, 34). At this point, the cancer cells have penetrated the epithelium and invaded the basal cells, and metastases will soon appear. The growth and function of prostate cells are highly dependent on androgen and inhibition of androgen production or androgen receptor(AR) can play an anticancer effect in all stages of PC (35). However, there are many complications including hyperlipidemia, abdominal obesity, diabetes mellitus type 2, cardiovascular disease, osteoporosis, and so on due to the decrease of androgen levels after ADT treatment (36–40). Therefore, it is important to recognize the hazards of ADT and select the most suitable patients to achieve the purpose of precision treatment.
PC is a malignant men’s tumor with high morbidity and mortality and has become an important public health issue (41). As mentioned above, ADT is the basic treatment for PC but the need for ADT combined with RT is controversial, especially in intermediate PC patients (42, 43). Early two prospective clinical research involving primarily intermediate PC patients showed the benefit of OS when added to 4 to 6 months of ADT before RT (12, 24). On the contrary, a randomized phase 3 study(PMH 9907) showed that the addition of ADT to RT did not significantly affect BF and OS (44). The reason for this difference may be the included patients were not all intermediate PC. As a result, it is needed for the clinical study aimed at intermediate PC patients accurately. Our meta-analysis included 15 studies, which were all based on the patients categorized as the intermediate risk and these targeted choices could make our research more significant.
The original intention of our study was to further divide intermediate-risk PC into FIR and UIR. However, after analyzing the included studies, we found that few studies conducted this subgroup analysis. Nabid A et al. carried out a randomized phase III trial (19), and there was no significant difference in OS between patients receiving DERT 76Gy alone, ADT plus RT 70Gy, and ADT plus DERT 76Gy. Then, they conducted the secondary analysis of the trial and found that UIR patients had significantly worse distant metastases-free survival (DMFS) and OS from ADT but for FIR patients, OS was not significantly different between arms (45). Zachary S et al. also conducted a secondary analysis of the RTOG 9408 randomized clinical trial,which showed that in patients with UIR, ADT improved DM and PCMS but in patients with FIR, ADT did not improve DM, PCMS, and ACM (46). The above two studies indicated that therapeutic optimization may appear possible in intermediate-risk PC patients, FIR patients seem to benefit from RT alone and UIR patients seem to ADT plus RT. Because of most of studies didn’t do these subgroup analyses from intermediate risk patient and intermediate-risk PC was a heterogeneous group which had variable prognoses, our study should have some certain reference significance for the treatment of this grey zone in clinical works.
This systematic review included 6 RCTs and 9 retrospective studies and we analyzed the data separately to improve the accuracy of the analysis result. The results of our study showed that ADT plus RT treatment did not improve the OS and BCRFS, on the contrary, it increased the risk for ACM for the patients of intermediate-risk PC. The most intriguing finding of our meta-analysis was that it was nearly reported in the early study that ADT plus RT treatment showed a clinical benefit in terms of OS and BCRFS. However, some recent studies did not show ADT plus RT treatment’s benefit in OS and BCRFS. It may have something to do with the lack of intensity-modulated radiotherapy (IMRT) and relative low doses therapy. With the improvement of radiotherapy technology and the increase in radiation dose, the absolute advantage of ADT decreased. RT alone, compared to ADT plus RT, maybe the more suitable therapy for intermediate-risk PC patients. However, further personalized therapy should be performed through more detailed studies.
5. Limitations
There are still certain limitations of our meta-analysis. First, there were inconsistencies in the patients’ RT and ADT regimens and it may cause some degree of bias. Second, although we separately analyzed the RCTs and retrospective studies and got the same result that ADT plus RT was not improving OS and BCRFS compared with RT alone, the number of RCTs we enrolled was still small (only 4 studies). Third, the increased risk of ACM in patients receiving ADT may be directly attributed to the use of hormonal therapy but also other co-factors such as comorbidities or incorrect patient selection, which may be the bias. Finally, we used different approaches to extract the data we needed, especially the data of OS and which may bring some bias.
6. Conclusions
Our present meta-analysis revealed that ADT plus RT did not provide OS or BCRFS advantage to intermediate-risk PC patients. RT alone was an adequate therapeutic regimen for intermediate-risk PC patients. However, more detailed therapy methods, whether the favorable risk group or the unfavorable risk group patients should be treated with the same therapy regimen remain need some large clinical trials to confirm.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
JC, FZ and YX were responsible for comprehensive study design, paper revision, and submission. YY, MF and YZ contributed to the search of articles and data extraction. JC, XS and YL performed the statistical analysis. All authors contributed and approved the submitted version.
Funding
This work was supported by a key program from the National Natural Science Foundation of China under Grant 81972845; Introduction of Specialist Team in Clinical Medicine of Xuzhou under Grant 2019TD003; Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX22_1273).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1074540/full#supplementary-material.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Fillon M. Rates of advanced prostate cancer continue to increase. CA: Cancer J For Clin (2020) 70(6):427–9. doi: 10.3322/caac.21641
4. Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. Eau-Eanm-Estro-Esur-Siog guidelines on prostate cancer-2020 update. part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol (2021) 79(2):243–62. doi: 10.1016/j.eururo.2020.09.042
5. Catalona WJ. Prostate cancer screening. Med Clin North Am (2018) 102(2):199–214. doi: 10.1016/j.mcna.2017.11.001
6. Davies A, Lum C, Raju R, Ansell E, Webber K, Segelov E. Anti-cancer therapy made easier: A 25-year update. Intern Med J (2021) 51(4):473–80. doi: 10.1111/imj.14878
7. Miyamoto H, Messing EM, Chang C. Androgen deprivation therapy for prostate cancer: Current status and future prospects. Prostate (2004) 61(4):332–53. doi: 10.1002/pros.20115
8. Denham JW, Steigler A, Lamb DS, Joseph D, Mameghan H, Turner S, et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: Results from the trans-Tasman radiation oncology group 96.01 randomised controlled trial. Lancet Oncol (2005) 6(11):841–50. doi: 10.1016/S1470-2045(05)70348-X
9. Singer EA, Golijanin DJ, Miyamoto H, Messing EM. Androgen deprivation therapy for prostate cancer. Expert Opin Pharmacother (2008) 9(2):211–28. doi: 10.1517/14656566.9.2.211
10. Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma–Long-Term results of phase iii rtog 85-31. Int J Radiat Oncol Biol Phys (2005) 61(5):1285–90. doi: 10.1016/j.ijrobp.2004.08.047
11. Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff R-O, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an eortc randomised study. Lancet Oncol (2010) 11(11):1066–73. doi: 10.1016/S1470-2045(10)70223-0
12. D'Amico AV, Chen M-H, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: A randomized trial. JAMA (2008) 299(3):289–95. doi: 10.1001/jama.299.3.289
13. Schaeffer E, Srinivas S, Antonarakis ES, Armstrong AJ, Bekelman JE, Cheng H, et al. Nccn guidelines insights: Prostate cancer, version 1.2021. J Natl Compr Canc Netw (2021) 19(2):134–43. doi: 10.6004/jnccn.2021.0008
14. Tsumura H, Tanaka N, Oguchi T, Owari T, Nakai Y, Asakawa I, et al. Comparative effectiveness of low-Dose-Rate brachytherapy with or without external beam radiotherapy in favorable and unfavorable intermediate-risk prostate cancer. Sci Rep (2022) 12(1):11023. doi: 10.1038/s41598-022-15028-6
15. Zumsteg ZS, Zelefsky MJ. Short-term androgen deprivation therapy for patients with intermediate-risk prostate cancer undergoing dose-escalated radiotherapy: The standard of care? Lancet Oncol (2012) 13(6):e259–e69. doi: 10.1016/S1470-2045(12)70084-0
16. Dong Y, Ruth KJ, Churilla TM, Viterbo R, Sobczak ML, Smaldone MC, et al. The need for androgen deprivation therapy in patients with intermediate-risk prostate cancer treated with dose-escalated external beam radiation therapy. Can J Urol (2017) 24(1):8656–62.
17. Kollmeier MA, McBride S, Gorovets D, Zelefsky MJ. Role of androgen-deprivation therapy remains uncertain for intermediate-risk patients when using dose-escalated radiotherapy. J Clin Oncol (2020) 38(32):3821–2. doi: 10.1200/JCO.20.02105
18. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (Prisma-p) 2015 statement. Syst Rev (2015) 4:1. doi: 10.1186/2046-4053-4-1
19. Nabid A, Carrier N, Vigneault E, Thu VN, Vavassis P, Brassard M-A, et al. Androgen deprivation therapy and radiotherapy in intermediate-risk prostate cancer: A randomised phase iii trial. Eur J OF Cancer (2021) 143:64–74. doi: 10.1016/j.ejca.2020.10.023
20. Krauss DJ, Karrison TG, Martinez AA, Morton G, Yan D, Bruner DW, et al. Dose escalated radiotherapy alone or in combination with short-term androgen suppression for intermediate risk prostate cancer: Outcomes from the nrg Oncology/Rtog 0815 randomized trial. Int J OF Radiat Oncol Biol Phys (2021) 111(3):S1–S. doi: 10.1016/j.ijrobp.2021.07.039
21. Thorpe CS, DeWees TA, Bhangoo RS, Petersen M, Chang JHC, Hartsell WF, et al. Randomized phase iii study of moderately hypofractionated radiation therapy with or without androgen suppression for intermediate risk adenocarcinoma of the prostate: Analysis of quality of life and toxicity. Pcg Gu (2021) 111(3):e295–. doi: 10.1016/j.ijrobp.2021.07.932
22. Vargas CE, Alam NB, Terk M, Niska JR, Cesareti J, Swartz D, et al. Initial results of a randomized phase iii trial of high dose image guided radiation with or without androgen deprivation therapy for intermediate-risk prostate cancer. Cancer Treat Res Commun (2019) 19. doi: 10.1016/j.ctarc.2019.100119
23. Dubray BM, Salleron J, Guerif SG, Prise EL, Reynaud-Bougnoux A, Hannoun-Levi JM, et al. Does short-term androgen depletion add to high dose radiotherapy (80 gy) in localized intermediate risk prostate cancer? final analysis of getug 14 randomized trial (Eu-20503/ Nct00104741). Journal of Clinical Oncology (2016) 34:. doi: 10.1200/JCO.2016.34.15_suppl.5021
24. Jones CU, Hunt D, McGowan DG, Amin MB, Chetner MP, Bruner DW, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med (2011) 365(2):107–18. doi: 10.1056/NEJMoa1012348
25. Post CM, Kahn JM, Turina CB, Beer TM, Hung AY. Short-term adt and dose-escalated imrt in patients with intermediate-risk prostate cancer: Benefit or caution? Am J Clin Oncol (2022) 45(5):190–5. doi: 10.1097/COC.0000000000000908
26. Pickles T, Morris WJ, Keyes M. High–intermediate prostate cancer treated with low-Dose-Rate brachytherapy with or without androgen deprivation therapy. Brachytherapy (2017) 16(6):1101–5. doi: 10.1016/j.brachy.2017.08.003
27. Boladeras A, Martinez E, Ferrer F, Gutierrez C, Villa S, Pera J, et al. Localized prostate cancer treated with external beam radiation therapy: Long-term outcomes at a European comprehensive cancer centre. Rep Pract Oncol Radiother J Greatpoland Cancer Center Poznan Polish Soc Radiat Oncol (2016) 21(3):181–7. doi: 10.1016/j.rpor.2015.12.002
28. Schiffmann J, Lesmana H, Tennstedt P, Beyer B, Boehm K, Platz V, et al. Additional androgen deprivation makes the difference: Biochemical recurrence-free survival in prostate cancer patients after hdr brachytherapy and external beam radiotherapy. Strahlenther Und Onkol (2014) 191(4):330–7. doi: 10.1007/s00066-014-0794-y
29. Schreiber D, Rineer J, Surapaneni A, Navo E, Agarwal M, Nwokedi E, et al. Dose-escalated radiation therapy with and without short-course androgen deprivation for intermediate-risk prostate cancer. Anticancer Res (2014) 34(8):4189–93.
30. Cabeza Rodriguez M, Olivera J, Samper Ots P, Cascales Garcia A, Diaz C, Zapatero J, et al. Short-Term-Androgen-Deprivation therapy reduces disease recurrence in intermediate-Risk-Prostate-Cancer patients receiving high-dose radiotherapy? Rep Pract Oncol Radiother (2013) 18:S334. doi: 10.1016/j.rpor.2013.03.520
31. Edelman S, Liauw SL, Rossi PJ, Cooper S, Jani AB. High-dose radiotherapy with or without androgen deprivation therapy for intermediate-risk prostate cancer: Cancer control and toxicity outcomes. Int J Radiat Oncol Biol Phys (2012) 83(5):1473–9. doi: 10.1016/j.ijrobp.2011.10.036
32. Stock RG, Yamalachi S, Hall SJ, Stone NN. Impact of hormonal therapy on intermediate risk prostate cancer treated with combination brachytherapy and external beam irradiation. J OF Urol (2010) 183(2):546–50. doi: 10.1016/j.juro.2009.10.006
33. Morse MD, McNeel DG. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum Immunol (2010) 71(5):496–504. doi: 10.1016/j.humimm.2010.02.007
34. Thompson IM, Leach R. Prostate cancer and prostatic intraepithelial neoplasia: True, true, and unrelated? J Clin Oncol (2013) 31(5):515–6. doi: 10.1200/JCO.2012.46.6151
35. Desai K, McManus JM, Sharifi N. Hormonal therapy for prostate cancer. Endocr Rev (2021) 42(3):354–73. doi: 10.1210/endrev/bnab002
36. Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med (2005) 352(2):154–64. doi: 10.1056/NEJMoa041943
37. Braga-Basaria M, Dobs AS, Muller DC, Carducci MA, John M, Egan J, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol (2006) 24(24):3979–83. doi: 10.1200/JCO.2006.05.9741
38. Bosco C, Bosnyak Z, Malmberg A, Adolfsson J, Keating NL, Van Hemelrijck M. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: A meta-analysis. Eur Urol (2015) 68(3):386–96. doi: 10.1016/j.eururo.2014.11.039
39. Crawley D, Garmo H, Rudman S, Stattin P, Häggström C, Zethelius B, et al. Association between duration and type of androgen deprivation therapy and risk of diabetes in men with prostate cancer. Int J Cancer (2016) 139(12):2698–704. doi: 10.1002/ijc.30403
40. Rachner TD, Coleman R, Hadji P, Hofbauer LC. Bone health during endocrine therapy for cancer. Lancet Diabetes Endocrinol (2018) 6(11):901–10. doi: 10.1016/S2213-8587(18)30047-0
41. Sandhu S, Moore CM, Chiong E, Beltran H, Bristow RG, Williams SG. Prostate cancer. Lancet (2021) 398(10305):1075–90. doi: 10.1016/S0140-6736(21)00950-8
42. Serrano NA, Anscher MS. Favorable vs unfavorable intermediate-risk prostate cancer: A review of the new classification system and its impact on treatment recommendations. Oncol (Williston Park) (2016) 30(3):229–36.
43. Beck M, Böhmer D, Aebersold DM, Albrecht C, Flentje M, Ganswindt U, et al. Role of combined radiation and androgen deprivation therapy in intermediate-risk prostate cancer : Statement from the degro working group on prostate cancer. Strahlenther Onkol (2020) 196(2):109–16. doi: 10.1007/s00066-019-01553-3
44. McPartlin AJ, Glicksman R, Pintilie M, Tsuji D, Mok G, Bayley A, et al. Pmh 9907: Long-term outcomes of a randomized phase 3 study of short-term bicalutamide hormone therapy and dose-escalated external-beam radiation therapy for localized prostate cancer. CANCER (2016) 122(16):2595–603. doi: 10.1002/cncr.30093
45. Nabid A, Carrier N, Vigneault E, Van Nguyen T, Vavassis P, Brassard MA, et al. Optimizing treatment in intermediate-risk prostate cancer: Secondary analysis of a randomized phase 3 trial. Int J Radiat Oncol Biol Phys (2021) 111(3):732–40. doi: 10.1016/j.ijrobp.2021.04.013
46. Zumsteg ZS, Spratt DE, Daskivich TJ, Tighiouart M, Luu M, Rodgers JP, et al. Effect of androgen deprivation on long-term outcomes of intermediate-risk prostate cancer stratified as favorable or unfavorable: A secondary analysis of the rtog 9408 randomized clinical trial. JAMA Netw Open (2020) 3(9):e2015083. doi: 10.1001/jamanetworkopen.2020.15083
Keywords: prostate cancer, androgen deprivation therapy, radiotherapy, intermediate-risk, systematic review and meta-analysis
Citation: Chen J, Yuan Y, Fang M, Zhu Y, Sun X, Lou Y, Xin Y and Zhou F (2023) Androgen deprivation therapy and radiotherapy in intermediate-risk prostate cancer: A systematic review and meta-analysis. Front. Endocrinol. 13:1074540. doi: 10.3389/fendo.2022.1074540
Received: 19 October 2022; Accepted: 28 December 2022;
Published: 17 January 2023.
Edited by:
Xiaolan Deng, City of Hope National Medical Center, United StatesReviewed by:
Octavian Sabin Tataru, Sciences and Technology of Târgu Mureş, RomaniaMaurizio Valeriani, Sapienza University of Rome, Italy
Copyright © 2023 Chen, Yuan, Fang, Zhu, Sun, Lou, Xin and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Xin, ZGVlcDM2OUAxNjMuY29t; Fengjuan Zhou, OTU0ODExNzc2QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jiuzhou Chen
Jiuzhou Chen Yan Yuan
Yan Yuan Miao Fang
Miao Fang Youqi Zhu1,2
Youqi Zhu1,2 Yufei Lou
Yufei Lou