- 1Department of Orthopedic Surgery, Fujian Medical University Union Hospital, Fuzhou, Fujian, China
- 2The School of Health, Fujian Medical University, Fuzhou, Fujian, China
- 3Department of Emergency, Zhaotong Traditional Chinese Medicine Hospital, Zhaotong, Yunnan, China
Purpose: Recently, the effects of paraspinal muscle degeneration on osteoporotic vertebral fractures (OVFs) have attracted the attention of researchers; however, studies are limited, and their results vary. Hence, this study aimed to determine the role of paraspinal muscle degeneration in the occurrence and recurrence of OVF.
Methods: Following the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guideline, the PubMed, Embase, Web of Science, Wanfang Data, China National Knowledge Infrastructure, and ClinicalTrials.gov databases were comprehensively searched for relevant studies. Studies comparing the cross-sectional area (CSA) or fatty infiltration (FI) of the paraspinal muscles (including the psoas (PS), erector spinae plus multifidus (ES+MF), quadratus lumborum) in patients with and without initial OVF, or with and without recurrent OVF were included and analyzed.
Results: Eleven studies were included in the meta-analysis. Seven studies investigated the effects of paraspinal muscles on initial OVF, and the overall results revealed significantly lower CSAES+MF (SMD: -0.575, 95% CI: -0.866 to -0.285) and CSAPS (SMD: -0.750, 95% CI: -1.274 to -0.226), and higher FI (SMD: 0.768, 95% CI: 0.475 to 1.062) in the fracture group. Meanwhile, four studies evaluated the effects of the paraspinal muscles on recurrent OVF, and the pooled results demonstrated significantly higher FI (SMD:0.720, 95% CI: 0.258 to 1.182) in the refracture group, although no significant difference in CSAES+MF (SMD: -0.103, 95% CI: -0.395 to 0.189) was observed between the two groups.
Conclusions: Paraspinal muscle degeneration plays a role in the occurrence and recurrence of OVF. Assessing the paraspinal muscles may be useful for identifying high-risk populations.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier (CRD42021276681).
Introduction
With the aging of the global population, the prevalence of osteoporotic vertebral fractures (OVFs) has been increasing annually (1). OVFs not only causes serious pain and spinal kyphosis but also leads to disability and decreased quality of life (2). Although various measures have been implemented to prevent their occurrence, their prevalence remains constant at 3.2-46% among individuals aged >50 years (3). Moreover, regardless of treatment regimen taken (vertebral augmentation or conservative treatment), approximately 5.4-51.8% of the patients still suffer from recurrent fractures (4, 5). Certainly, OVFs remain a major public health issue requiring urgent resolution.
OVFs were previously considered as a skeletal disease, and most of previous studies have focused on the influence of bone mineral density, intravertebral cleft, and spine sagittal alignment (6, 7). Although the paraspinal muscles and spine are tightly connected at both the anatomical and functional levels, the association between paraspinal muscle degeneration and OVF has long been disregarded (8). Recently, several studies have reported a higher prevalence of sarcopenia in patients with OVFs than in those without OVFs (9, 10). Some researchers have also revealed a higher incidence of OVFs in patients with sarcopenia (11). Based on these findings, some scholars have proposed an association between paraspinal muscle degeneration and OVFs (12), whereas others have reported otherwise (13). Due to the lack of convincing evidence, we performed a meta-analysis to determine the effects of paraspinal muscle degeneration on the occurrence and recurrence of OVFs.
Materials and methods
Literature search
This meta-analysis was registered in the PROSPERO (CRD42021276681) and conducted in accordance with the PRISMA statement (Supplementary Material 1) (14). A comprehensive literature search was performed on the PubMed, Embase, Web of Science, Wanfang Data, China National Knowledge Infrastructure, and ClinicalTrials.gov databases on September 2, 2021 to identify relevant studies. The following search terms were used: ‘spinal fracture’ OR ‘spine fracture’ OR ‘vertebral fracture’; and ‘paraspinal muscle’ OR ‘paravertebral muscle’. Additionally, the references of relevant reviews and studies were also searched to identify potential eligible studies. All searches were limited to clinical studies published in Chinese or English.
Inclusion/exclusion criteria
The defined inclusion criteria were as follows: (1) studies comparing the characteristics of paraspinal muscles in patients with and without initial OVF, or with and without recurrent OVF; (2) the cross-sectional area (CSA) or fatty infiltration (FI) of at least one of the paraspinal muscles, including the psoas (PS), erector spinae plus multifidus (ES+MF), and quadratus lumborum was measured using magnetic resonance imaging (MRI) or computerized tomography (CT).
And the exclusion criteria were as follows: (1) studies comparing the characteristics of paraspinal muscles in other diseases, such as low back pain, lumbar disc herniation, and lumbar spinal stenosis; (2) studies comparing the characteristics of paraspinal muscles using other imaging modalities (ultrasound or dual-energy X-ray absorptiometry); (3) studies with insufficient data for calculating the results; (4) review articles, conference abstracts, commentaries, letters, case reports and animal studies.
Data extraction and quality assessment
Two reviewers independently screened the titles and abstracts of initially identified studies, and the full-text of such studies were evaluated according to the inclusion criteria. Any disagreements were resolved through discussion with a third reviewer. The following data were extracted for each included study: author name, publication year, study location, fracture type, measuring method and level, age, sex, sample size and duration of follow-up. Additionally, the CSA and FI of each paraspinal muscle were also collected. Regarding quality assessment, the Newcastle-Ottawa Scale (NOS) (15) was used to evaluate the quality of each included cohort/case-control study. A high-quality study was defined as having a NOS score of > 6.
Statistical analysis
This meta-analysis was performed using the STATA software (version 12.0). We calculated the standardized mean difference (SMD) with 95% confidence interval (CI) for continuous variables, and significance was set at P<0.05. The I-squared (I2) test was used to assess heterogeneity. A random-effects model was applied when I2>50%. Otherwise, a fixed-effects model was used.
Results
Search results
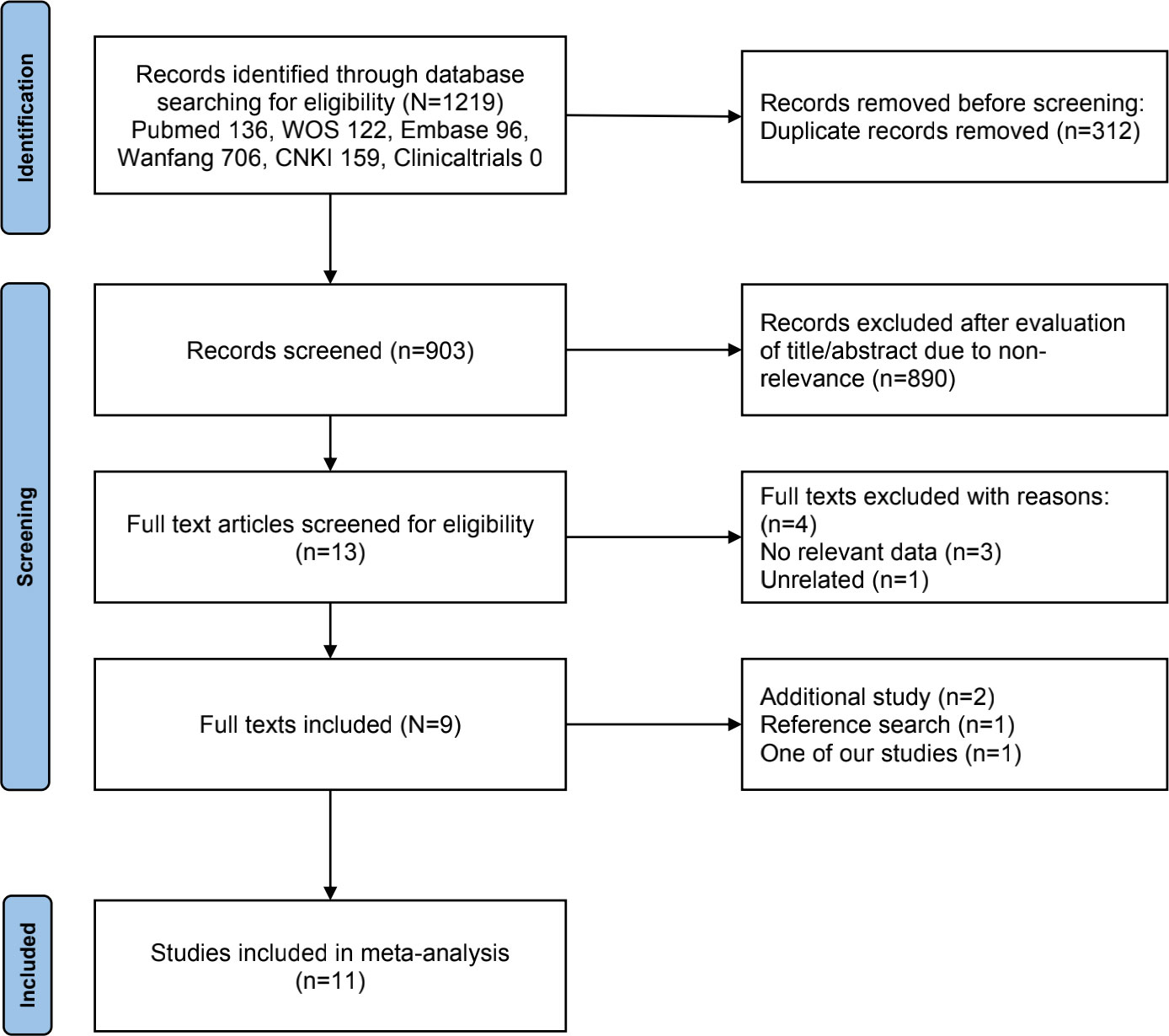
A flowchart of our study selection processes is presented in Figure 1. The initial search identified 1219 relevant studies, and 312 duplicates were removed. After screening titles and abstracts, 890 studies were eliminated. Following a full-text assessment, four studies were also excluded. Subsequently, two additional studies were included; one was identified by the reference search, and the other was our study that met the inclusion criteria. Finally, 11 eligible studies were included in the meta-analysis (12, 13, 16–23).
Study characteristics and quality evaluation
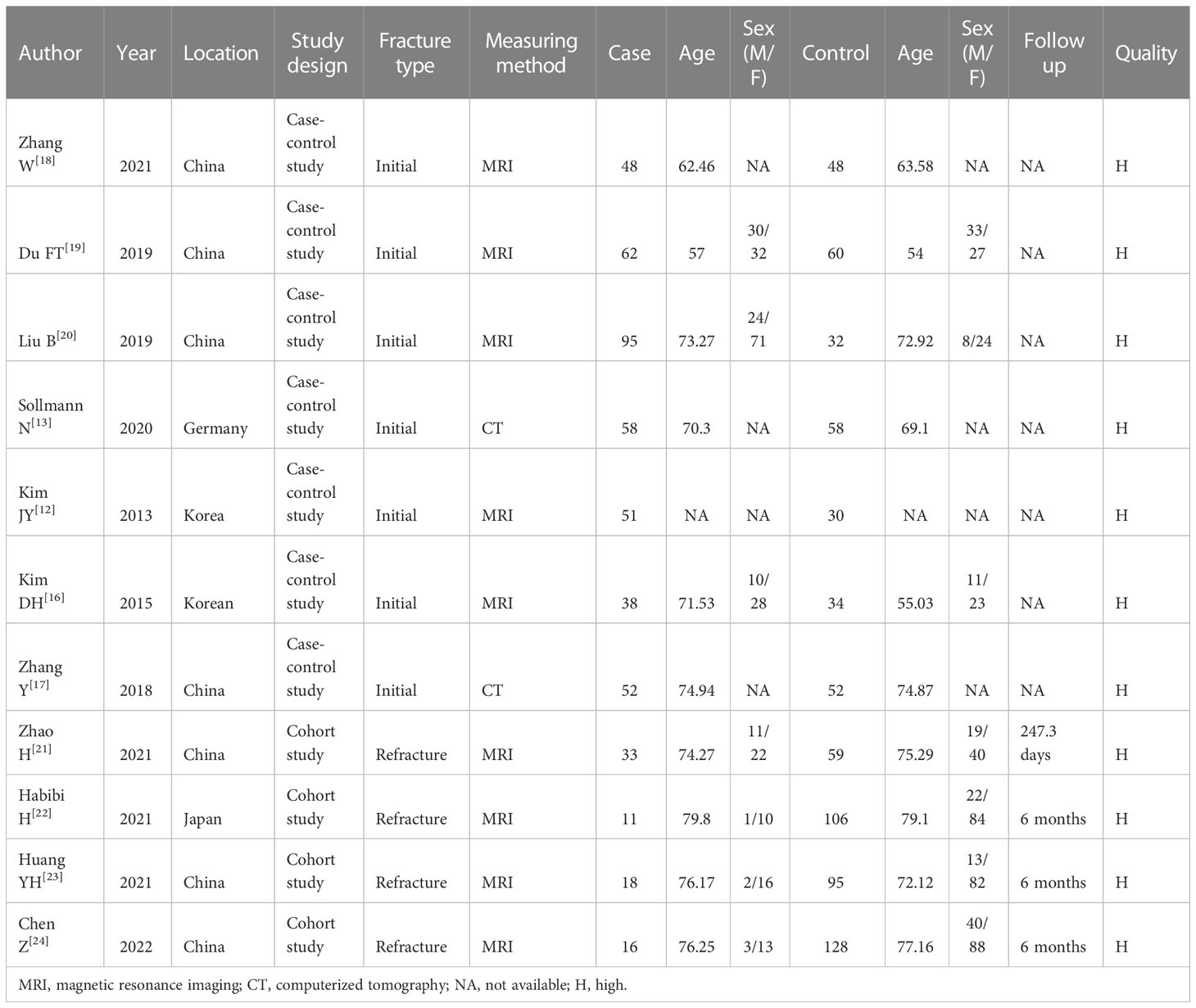
The characteristics of the included studies are summarized in Table 1. Seven studies compared the characteristics of the paraspinal muscles in patients with and without initial OVF (12, 13, 16–20), and four studies compared the characteristics of the paraspinal muscles in patients with and without recurrent OVF (21–24). Overall, 482 patients were in the case group (fracture/refracture), and 702 patients were in the control group (non-fracture/non-refracture), with the mean age ranging from 54 to 79.8 years. Most studies measured the CSA and FI of the paraspinal muscles using MRI, except for two studies that used CT to measure the CSA. According to NOS scores, all included studies were considered high-quality.
Effects of the paraspinal muscles on initial fracture
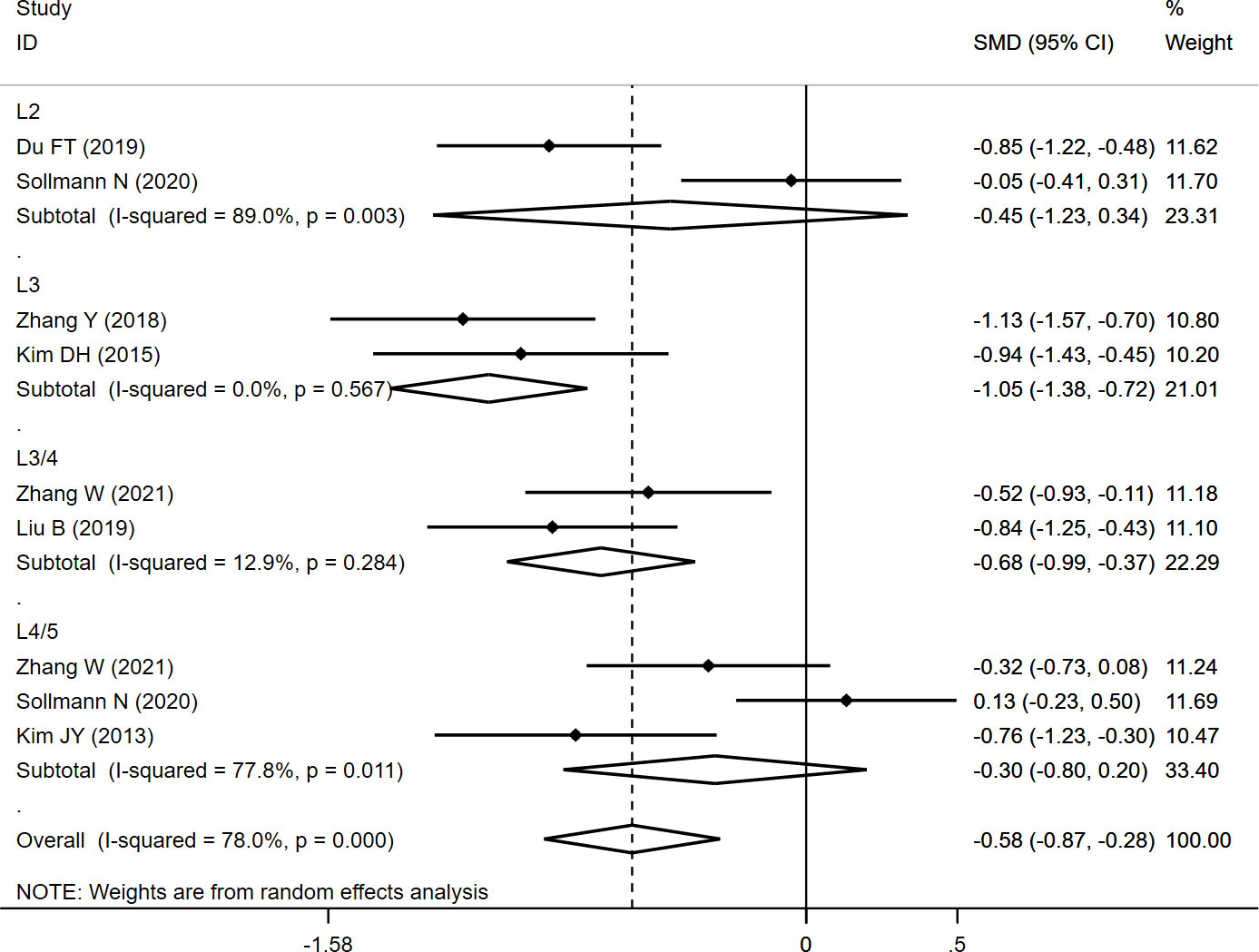
The CSAES+MF was measured at different levels (L2, L3, L3/4, and L4/5), and the overall result revealed significantly lower CSAES+MF (SMD: -0.575, 95% CI: -0.866 to -0.285, P: 0.000) in the fracture group than in the non-fracture group. In the subgroup analysis, the results revealed significantly lower CSAES+MF at L3 (SMD: -1.049, 95% CI: -1.376 to -0.723, P: 0.000) and L3/4 (SMD: -0.678, 95% CI: -0.989 to -0.367, P: 0.000) in the fracture group than in the non-fracture group, whereas no significant differences were observed at L2 (SMD: -0.449, 95% CI: -1.233 to 0.336, P: 0.262) and L4/5 (SMD: -0.301, 95% CI: -0.802 to 0.201, P: 0.239) between both the groups (Table 2; Figure 2).
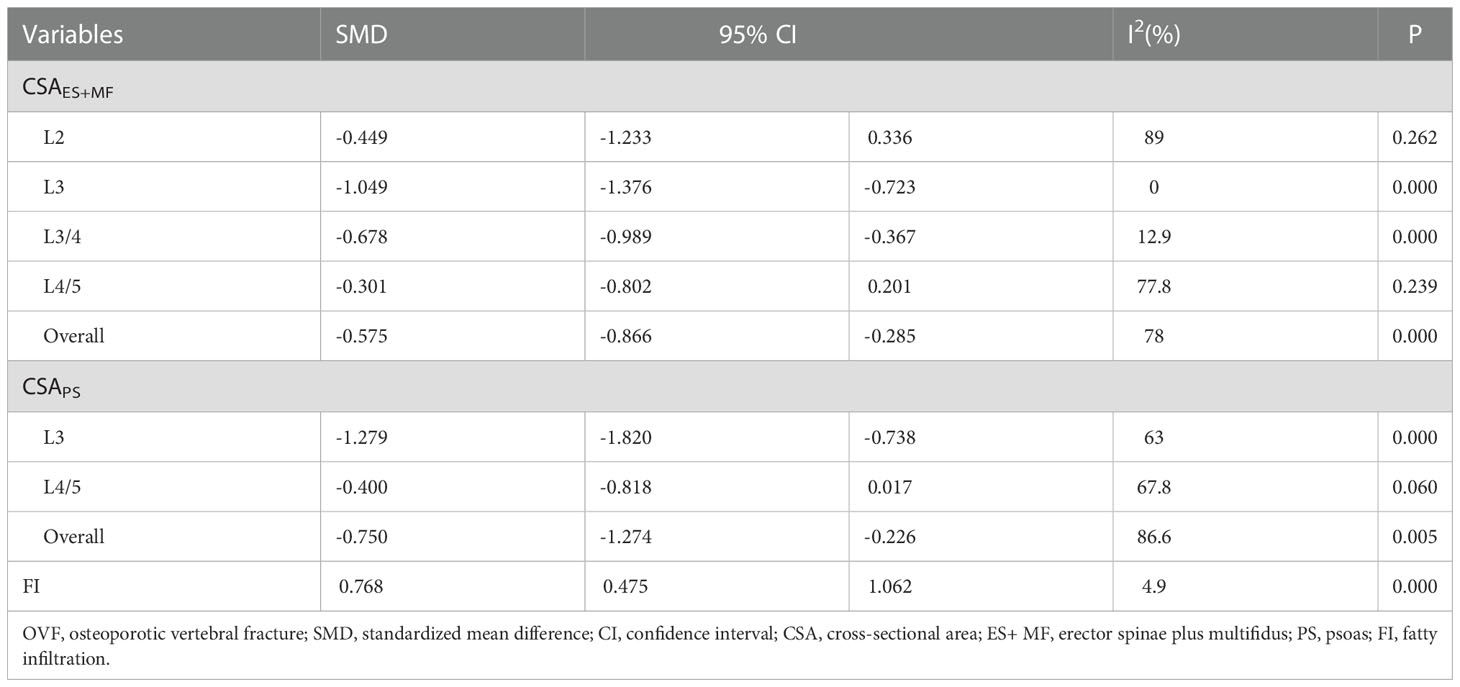
Table 2 The pooled analysis of paraspinal muscle characteristics in patients with and without initial OVF.
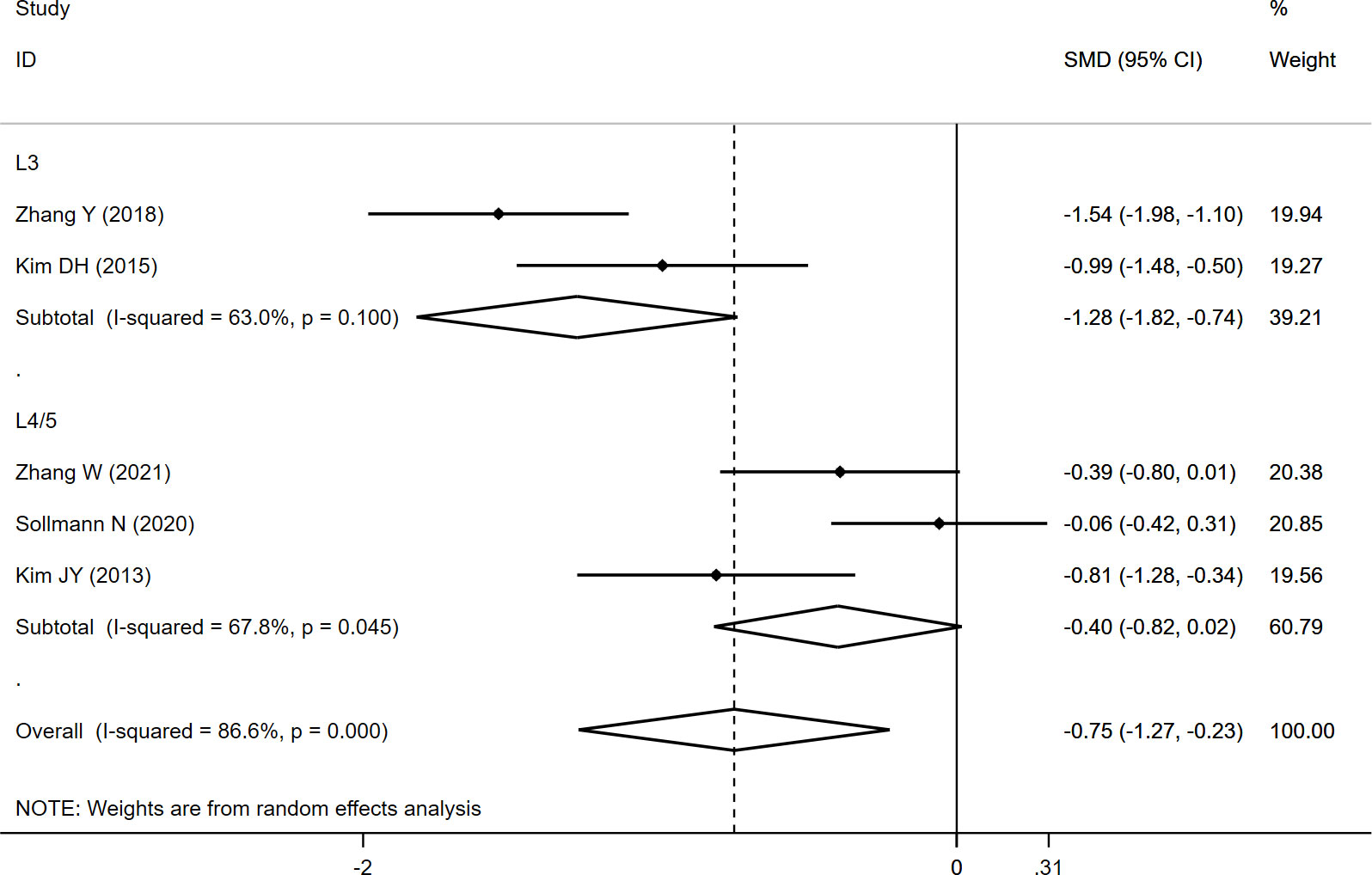
The CSAPS was measured at L3 and L4/5, and the overall results indicated significantly lower CSAPS (SMD: -0.750, 95% CI: -1.274 to -0.226, P: 0.005) in the fracture group than in the non-fracture group. In the subgroup analysis, a significantly lower CSAPS at L3 (SMD: -1.279, 95% CI: -1.820 to -0.738, P: 0.000) was noted in fracture group than in the non-fracture group, whereas no significant difference at L4/5 (SMD: -0.400, 95% CI: -0.818 to 0.017, P: 0.060) was observed between both the groups (Table 2; Figure 3).
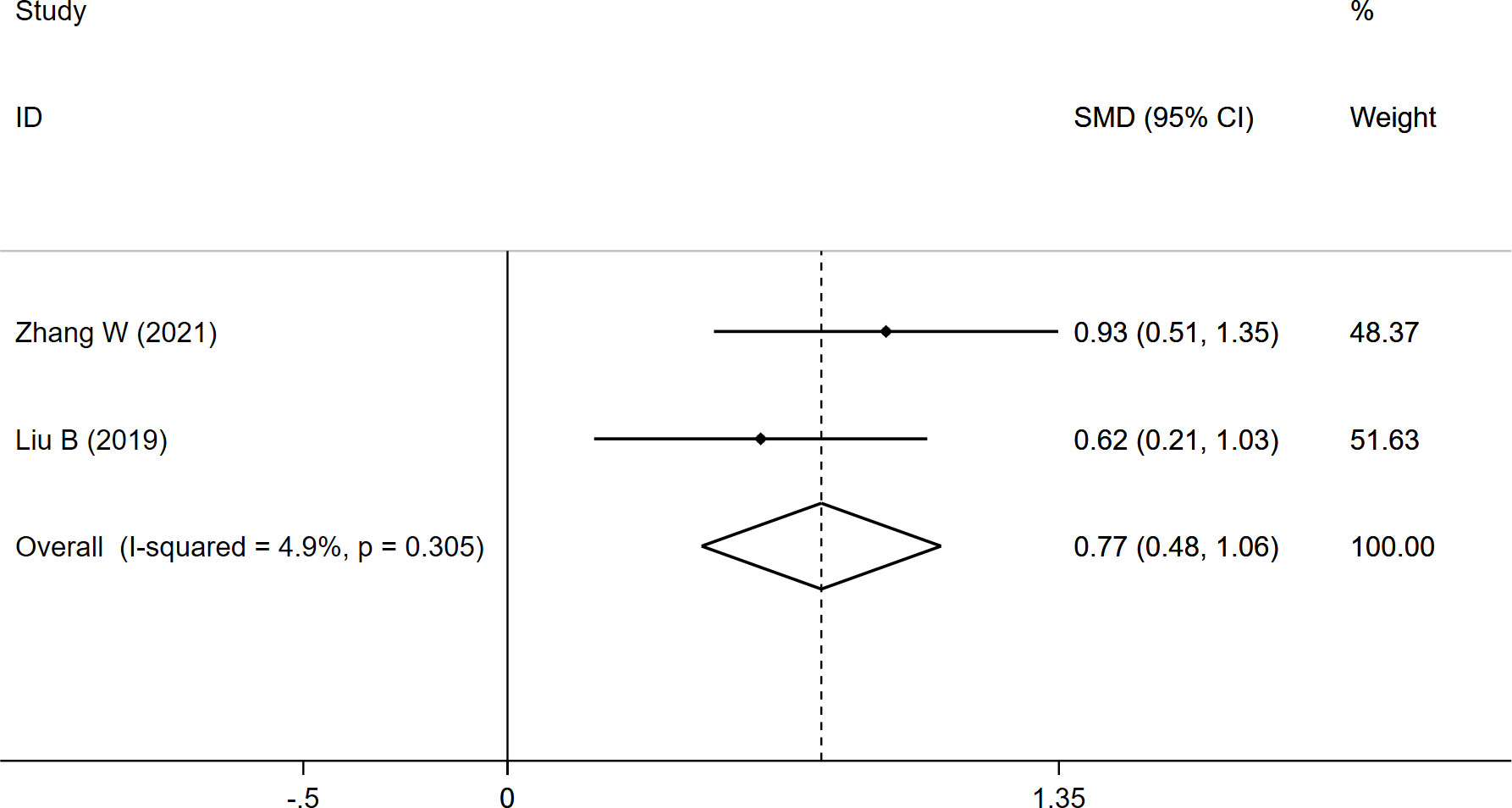
The FI data were pooled from two studies, and the results demonstrated a significantly higher FI (SMD: 0.768, 95% CI: 0.475 to 1.062, P: 0.000) in the fracture group than in the non-fracture group (Table 2; Figure 4).
Effects of paraspinal muscles on recurrent fracture
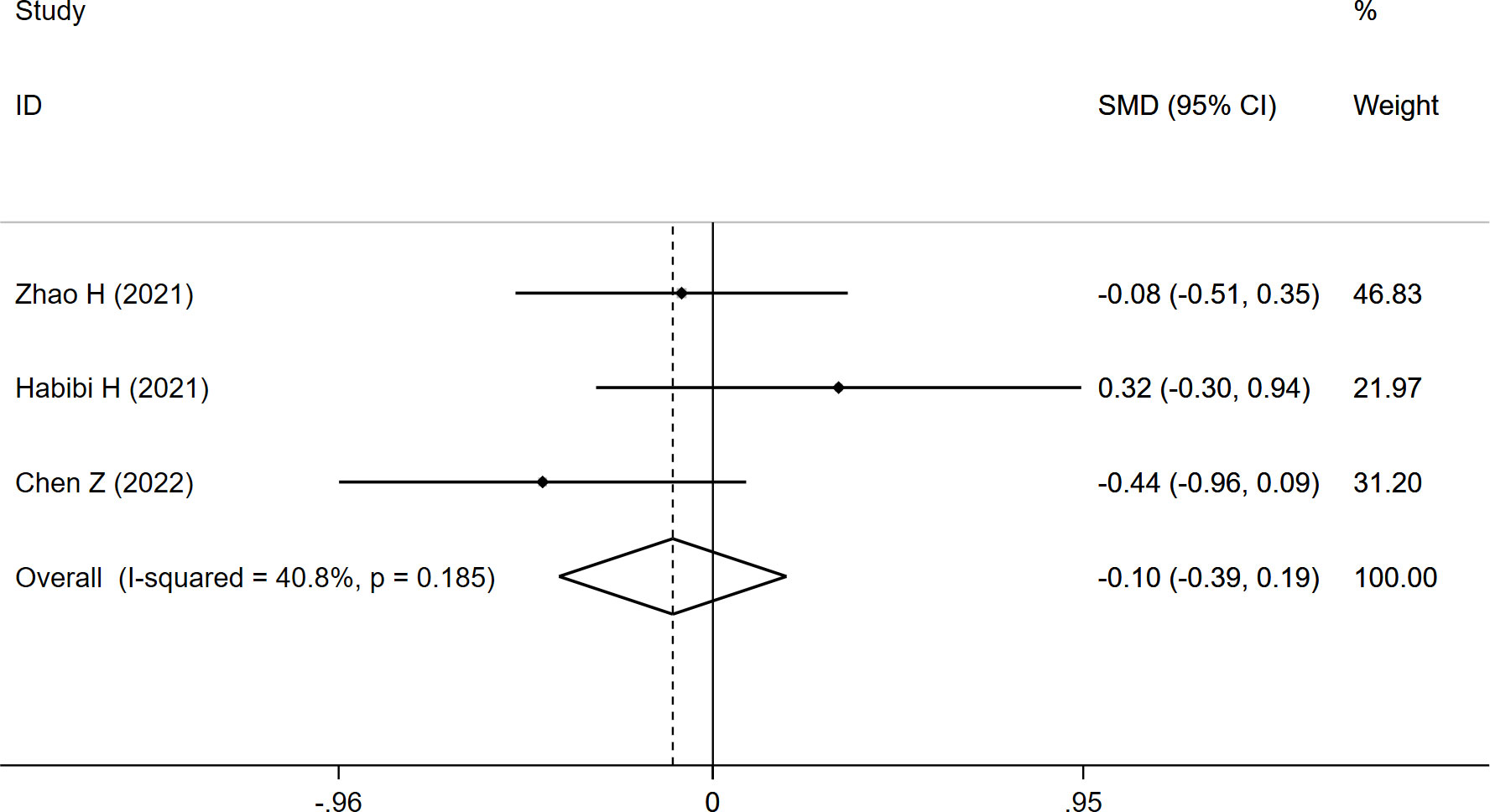
CSAES+MF was reported in three studies, and the overall result revealed no significant difference between the two groups (SMD: -0.103, 95% CI: -0.395 to 0.189, P: 0.489) (Table 3; Figure 5).
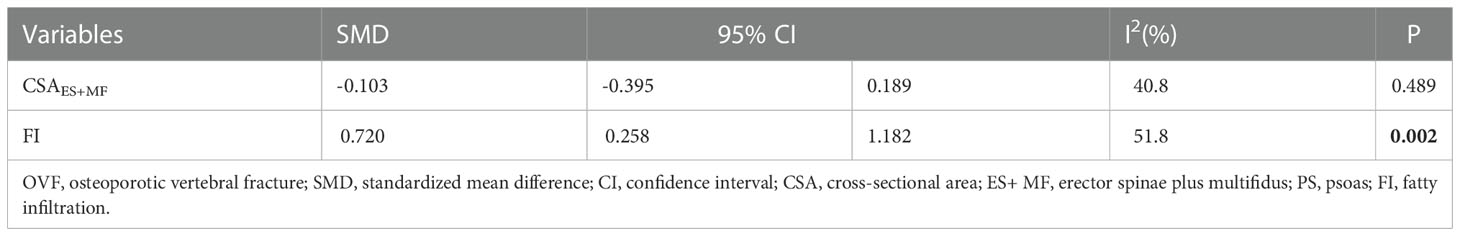
Table 3 The pooled analysis of paraspinal muscle characteristics in patients with and without recurrent OVF.
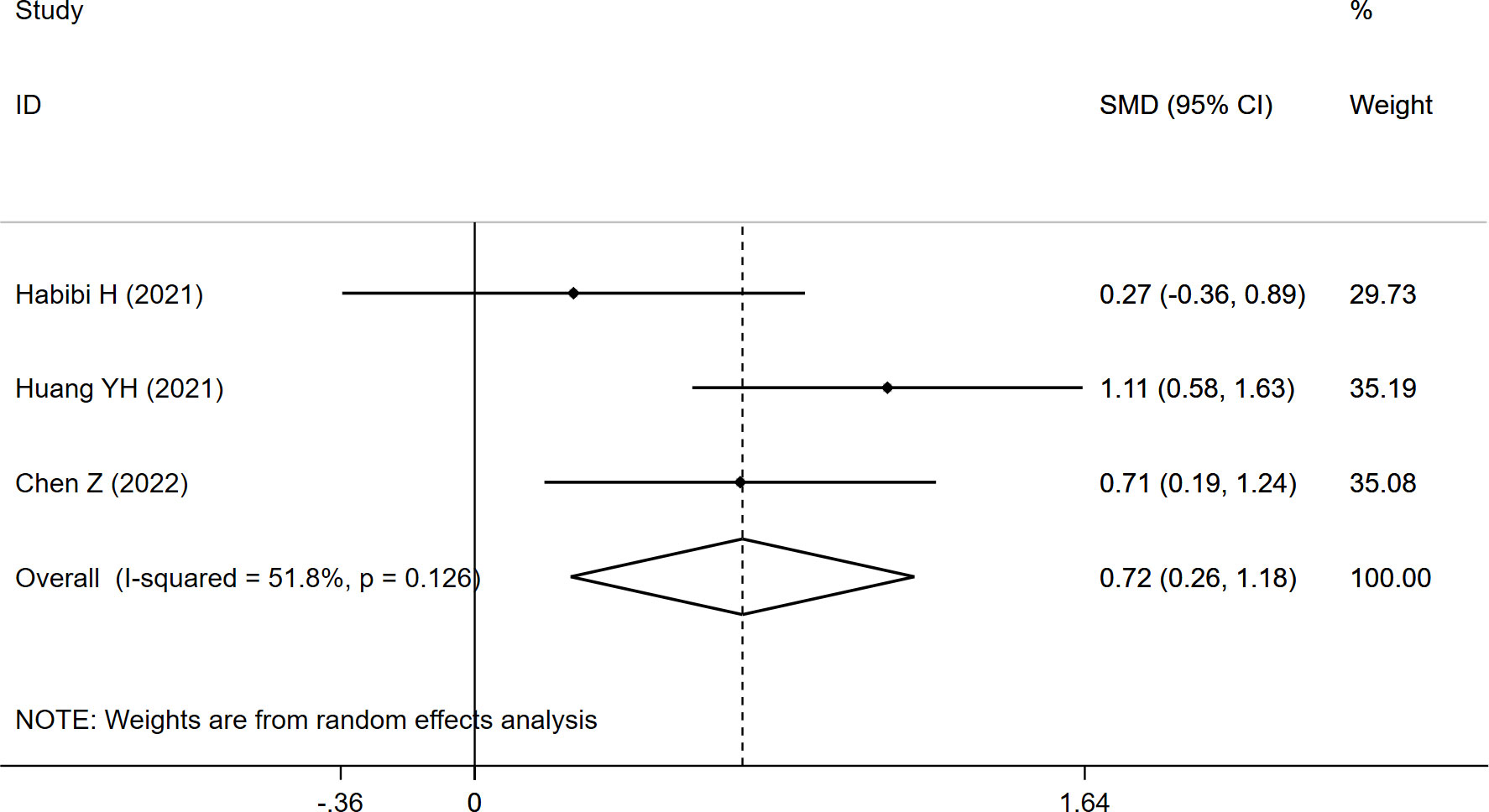
Considering FI, the pooled results of the three studies revealed significantly higher FI (SMD:0.720, 95% CI: 0.258 to 1.182, P: 0.002) in the refracture group than in the non-fracture group (Figure 6).
Discussion
Concomitant with the progressive bone loss, aging also results in the deterioration of muscle quantity and quality (25). According to epidemiological investigations, the prevalence of sarcopenia ranges from 4.1% to 11.5% among the general older population (26), and the incidence is even higher in patients with OVF (10, 11). Recently, the effects of paraspinal muscle degeneration on OVF have attracted increasing attention among researchers, although the studies are limited, and their results remain controversial. Our meta-analysis suggests that paraspinal muscle degeneration is associated with the occurrence and recurrence of OVF.
Paraspinal muscle degeneration is generally characterized by decreased muscle CSA, reduced muscle strength, and increased FI (27). Previous studies have identified a higher prevalence of sarcopenia in patients with OVF (9). In a case-control study, the authors have reported significantly lower CSAES+MF in the fracture group (2,170 mm2) than in the non-fracture group (3,040 mm2) (16). In another study, Jeon et al. have suggested that fatty degeneration of the paraspinal muscle is associated with vertebral collapse (28). Furthermore, Kim et al. have demonstrated both lower CSA and greater FI in patients with OVF than those without OVF (12). However, Sollmann et al. have indicated that OVF is independent of the characteristics of the paraspinal muscles (13). The results of our meta-analysis revealed significantly lower CSAES+MF and CSAPS, and higher FI in the fracture group than in the non-fracture group, which suggested that paraspinal muscle degeneration might contribute to the occurrence of initial OVF.
Regarding the causes of recurrent OVF, the effect of the paraspinal muscles was also disregarded. Only recently have studies attempted to investigate the links between paraspinal muscle degeneration and recurrent OVF. Takahashi et al. have suggested that paraspinal muscle atrophy was significantly correlated with delayed union after OVF (29). Similarly, Katsu et al. have reported a significantly smaller CSA of the paraspinal muscle in patients with insufficient union after OVF (30). Additionally, Huang et al. have found a significantly lower CSA and higher FI in the refracture group than in the non-refracture group (23). However, Habibi et al. have revealed that only the FI of the paraspinal muscles, but not the CSA or relative-CSA, was significantly correlated with recurrent fracture (22). Furthermore, Zhao et al. observed no significant differences in the CSA and FI of the paraspinal muscles between the refracture and non-refracture groups (21). Our results indicated significantly higher FI in the refracture group than in the non-refracture group, although CSA was not significantly different between the groups, thus clarifying the previous controversial findings.
Although the findings of our study indicate a possibly close association between paraspinal muscle degeneration and the occurrence and recurrence of OVF. Presently, the causality between paraspinal muscle degeneration and vertebral fractures remains largely unknown. Muscle atrophy and fatty degeneration may likely reduce the muscle strength and force generation capacity, which may decrease bone mineral density and affect spinal balance, thus leading to an increased risk of fracture (17, 28, 30).
Limitation
This meta-analysis had some limitation. First, the number of included studies was relatively small, and some studies have reported only a part of the outcome. Second, we only assessed the CSA and FI of the paraspinal muscles; other muscle characteristics, such as strength, endurance, and activation, were not available in the included studies. Finally, the imaging modality used was not completely consistent; although both MRI and CT could accurately define the CSA of the paraspinal muscles, inconsistent methods might still lead to some potential bias.
Conclusion
The current evidence suggests that paraspinal muscle degeneration plays a role in the occurrence and recurrence of OVF. Assessing the paraspinal muscles may be useful for identifying high-risk populations. Further studies are needed to explore the possibility of reducing the incidence of OVF by preventing paraspinal muscle degeneration.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ZC wrote the manuscript. TS, WWL, JS and ZY collected the data and conducted analyses. WGL revised the manuscript. All authors approved the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1073013/full#supplementary-material
Abbreviations
- OVF, Osteoporotic vertebral fractures; CSA, Cross-sectional area; FI, Fatty infiltration; PS, psoas; ES+MF, Erector spinae plus multifidus; MRI, Magnetic resonance imaging; CT, Computerized tomography; NOS, Newcastle-Ottawa Scale; SMD, standardized mean difference; CI, confidence interval.
References
1. Odén A, McCloskey EV, Kanis JA, Harvey NC, Johansson H. Burden of high fracture probability worldwide: secular increases 2010-2040. Osteoporos Int (2015) 26:2243–8. doi: 10.1007/s00198-015-3154-6
2. Sosa DD, Eriksen EF. Reduced bone material strength is associated with increased risk and severity of osteoporotic fractures. an impact microindentation study. Calcif Tissue Int (2017) 101:34–42. doi: 10.1007/s00223-017-0256-5
3. Ballane G, Cauley JA, Luckey MM, El-Hajj Fuleihan G. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int (2017) 28:1531–42. doi: 10.1007/s00198-017-3909-3
4. Lee HM, Park SY, Lee SH, Suh SW, Hong JY. Comparative analysis of clinical outcomes in patients with osteoporotic vertebral compression fractures (OVCFs): conservative treatment versus balloon kyphoplasty. Spine J (2012) 12:998–1005. doi: 10.1016/j.spinee.2012.08.024
5. Ma X, Xing D, Ma J, Wang J, Chen Y, Xu W, et al. Risk factors for new vertebral compression fractures after percutaneous vertebroplasty: qualitative evidence synthesized from a systematic review. Spine (2013) 38:E713–722. doi: 10.1097/BRS.0b013e31828cf15b
6. Zvekic-Svorcan J, Aleksic J, Jankovic T, Filipovic K, Cvetkovic M, Vuksanovic M, et al. Capture the vertebral fracture: Risk factors as a prediction. J Bback musculoskeletal Rehabil (2019) 32:269–76. doi: 10.3233/bmr-170898
7. Ji C, Rong Y, Wang J, Yu S, Yin G, Fan J, et al. Risk factors for refracture following primary osteoporotic vertebral compression fractures. Pain physician (2021) 24:E335–e340. doi: 10.1016/j.bas.2021.100253
8. Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int (2017) 28:2781–90. doi: 10.1007/s00198-017-4151-8
9. Hida T, Shimokata H, Sakai Y, Ito S, Matsui Y, Takemura M, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J (2016) 25:3424–31. doi: 10.1007/s00586-015-3805-5
10. Iolascon G, Giamattei MT, Moretti A, Di Pietro G, Gimigliano F, Gimigliano R. Sarcopenia in women with vertebral fragility fractures. Aging Clin Exp Res (2013) 25(Suppl 1):S129–131. doi: 10.1007/s40520-013-0102-1
11. Wang WF, Lin CW, Xie CN, Liu HT, Zhu MY, Huang KL, et al. The association between sarcopenia and osteoporotic vertebral compression refractures. Osteoporos Int (2019) 30:2459–67. doi: 10.1007/s00198-019-05144-x
12. Kim JY, Chae SU, Kim GD, Cha MS. Changes of paraspinal muscles in postmenopausal osteoporotic spinal compression fractures: magnetic resonance imaging study. J Bone Metab (2013) 20:75–81. doi: 10.11005/jbm.2013.20.2.75
13. Sollmann N, Franz D, Burian E, Löffler MT, Probst M, Gersing A, et al. Assessment of paraspinal muscle characteristics, lumbar BMD, and their associations in routine multi-detector CT of patients with and without osteoporotic vertebral fractures. Eur J Radiol (2020) 125:108867. doi: 10.1016/j.ejrad.2020.108867
14. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clin Res ed) (2009) 339:b2535. doi: 10.1136/bmj.b2535
15. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
16. Kim DH, Choi DH, Park JH, Lee JH, Choi YS. What is the effect of spino-pelvic sagittal parameters and back muscles on osteoporotic vertebral fracture? Asian Spine J (2015) 9:162–9. doi: 10.4184/asj.2015.9.2.162
17. Zhang Y, Guo J, Duanmu Y, Zhang C, Zhao W, Wang L, et al. Quantitative analysis of modified functional muscle-bone unit and back muscle density in patients with lumbar vertebral fracture in Chinese elderly men: A case-control study. Aging Clin Exp Res (2019) 31:637–44. doi: 10.1007/s40520-018-1024-8
18. Zhang W, Qin WY, Zhu Y. Lumbar paraspinal muscle quantity and quality in postmenopausal women with osteoporotic vertebral compression fractures: a case-control study based on MRI. Orthopaedics (2021) 12:8–13. doi: 10.3969/j.issn.1674-8573.2021.01.002
19. Du FT, Yu DG, Fang JF. Clinical significance of cross-sectional area of paravertebral muscles in patients with thoracolumbar spine fracture. China Med (2019) 14:408–11. doi: 10.3760/j.issn.1673-4777.2019.03.023
20. Liu B, Liu X, Wang G, Shen X, Chang L, Peng S, et al. Measurement of paravertebral muscle indicators on MRI in the patients with osteoporotic vertebral fracture and its clinical significance. Chin J Tissue Eng Res (2019) 23:578–83. doi: 10.3969/j.issn.2095-4344.1040
21. Zhao H, He Y, Yang JS, Bao W, Chen J, Liu JJ, et al. Can paraspinal muscle degeneration be a reason for refractures after percutaneous kyphoplasty? a magnetic resonance imaging observation. J orthop Surg Res (2021) 16:476. doi: 10.1186/s13018-021-02623-y
22. Habibi H, Takahashi S, Hoshino M, Takayama K, Sasaoka R, Tsujio T, et al. Impact of paravertebral muscle in thoracolumbar and lower lumbar regions on outcomes following osteoporotic vertebral fracture: a multicenter cohort study. Arch osteoporos (2021) 16:2. doi: 10.1007/s11657-020-00866-6
23. Huang YH, Liu X, Shang XW. Risk factors for adjacent vertebral fractures after PKP treatment in patients with osteoporotic vertebral compression fractures. Shandong Med J (2021) 61:72–6. doi: 10.3969/j.issn.1002-266X.2021.23.018
24. Chen Z, Yao Z, Wu C, Wang G, Liu W. Assessment of clinical, imaging, surgical risk factors for subsequent fracture following vertebral augmentation in osteoporotic patients. Skeletal Radiol (2022) 51(8):1623-30. doi: 10.1007/s00256-022-04009-5
25. So KY, Kim DH, Choi DH, Kim CY, Kim JS, Choi YS. The influence of fat infiltration of back extensor muscles on osteoporotic vertebral fractures. Asian Spine J (2013) 7:308–13. doi: 10.4184/asj.2013.7.4.308
26. Chen LK, Lee WJ, Peng LN, Liu LK, Arai H, Akishita M. Recent advances in sarcopenia research in Asia: 2016 update from the Asian working group for sarcopenia. J Am Med Dir Assoc (2016) 17:767.e761–767. doi: 10.1016/j.jamda.2016.05.016
27. Crawford RJ, Filli L, Elliott JM, Nanz D, Fischer MA, Marcon M, et al. Age- and level-dependence of fatty infiltration in lumbar paravertebral muscles of healthy volunteers. AJNR Am J neuroradiol (2016) 37:742–8. doi: 10.3174/ajnr.A4596
28. Jeon I, Kim SW, Yu D. Paraspinal muscle fatty degeneration as a predictor of progressive vertebral collapse in osteoporotic vertebral compression fractures. Spine J (2021) 22(2):313-20. doi: 10.1016/j.spinee.2021.07.020
29. Takahashi S, Hoshino M, Takayama K, Sasaoka R, Tsujio T, Yasuda H, et al. The natural course of the paravertebral muscles after the onset of osteoporotic vertebral fracture. Osteoporos Int (2020) 31:1089–95. doi: 10.1007/s00198-020-05338-8
Keywords: osteoporotic vertebral compression fracture, refracture, paraspinal muscles, meta-analysis, degeneration
Citation: Chen Z, Shi T, Li W, Sun J, Yao Z and Liu W (2023) Role of paraspinal muscle degeneration in the occurrence and recurrence of osteoporotic vertebral fracture: A meta-analysis. Front. Endocrinol. 13:1073013. doi: 10.3389/fendo.2022.1073013
Received: 19 October 2022; Accepted: 09 December 2022;
Published: 04 January 2023.
Edited by:
Giacomina Brunetti, University of Bari Aldo Moro, ItalyReviewed by:
Xiaoqian Dang, The Second Affiliated Hospital of Xi’an Jiaotong University, ChinaWei Nan Zeng, Sichuan University, China
Copyright © 2023 Chen, Shi, Li, Sun, Yao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenge Liu, bHdnc3BpbmVAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Zhi Chen
Zhi Chen Tengbin Shi
Tengbin Shi Wenwen Li2†
Wenwen Li2† Wenge Liu
Wenge Liu