
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 05 January 2023
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1061766
This article is part of the Research Topic Molecular and Cytogenetic Research Advances in Human Reproduction - Volume II View all 12 articles
Implantation is the first step in human reproduction. Successful implantation depends on the crosstalk between embryo and endometrium. Recurrent implantation failure (RIF) is a clinical phenomenon characterized by a lack of implantation after the transfer of several embryos and disturbs approximately 10% couples undergoing in vitro fertilization and embryo transfer. Despite increasing literature on RIF, there is still no widely accepted definition or standard protocol for the diagnosis and treatment of RIF. Progress in predicting and preventing RIF has been hampered by a lack of widely accepted definitions. Most couples with RIF can become pregnant after clinical intervention. The prognosis for couples with RIF is related to maternal age. RIF can be caused by immunology, thrombophilias, endometrial receptivity, microbiome, anatomical abnormalities, male factors, and embryo aneuploidy. It is important to determine the most possible etiologies, and individualized treatment aimed at the primary cause seems to be an effective method for increasing the implantation rate. Couples with RIF require psychological support and appropriate clinical intervention. Further studies are required to evaluate diagnostic method and he effectiveness of each therapy, and guide clinical treatment.
Implantation is the first step of crosstalk between the embryo and endometrium, which is the key point for a successful pregnancy. The implantation process includes apposition, adhesion, and invasion (Figure 1) (1). Successful implantation is identified as an intrauterine gestational sac seen on ultrasonography. Implantation failure may occur during the attachment and migration process, with a negative urine or blood test for human chorionic gonadotropin (hCG) or failure to form an intrauterine gestational sac with positive hCG. Recurrent implantation failure (RIF) is a clinical phenomenon with no widely accepted definition. The key factors that need to be considered while establishing the definition of RIF are the number of embryos transferred or unsuccessful in vitro fertilization-embryo transfer (IVF-ET) cycles, the quality of embryos, fresh or frozen embryos, and maternal age, which are disputed points. The increase in the cumulative live birth rate with more IVF-ET cycles showed a progressive decline (2). Other analyses showed that after three IVF-ET cycles, cumulative pregnancy rates did not increase significantly, and the pregnancy rate per cycle tended to decrease after three cycles of unsuccessful treatment (3–5). When RIF was defined as two or more implantation failures, the live birth rate was significantly lower than when RIF was defined as three or more implantation failures, which was considered an excessively increased denominator (6). Hence, a blind increase in IVF-ET cycles may not lead to a successful pregnancy, and we need to set a cut-off point for treatment cycles to recognize patients with RIF. Owing to the different quality of embryos, the number of transferred embryos varies from 3 to 10 or more (7). A good-quality embryo has the proper developmental status according to the day of its development (8). A poor-quality embryo implies that patients need to go through more embryos that are transferred to acquire a successful pregnancy. Another factor that should be considered when defining RIF is maternal age. It is well known that pregnancy rates decrease with maternal age (9); older patients required more cycles of blastocyst transfer to reach the same implantation rate as young women (10). Defining RIF without considering maternal age is meaningless. Based on the above considerations, the widely accepted definition of RIF, as presented by Coughlan, is the failure to achieve a clinical pregnancy after the transfer of at least four good-quality embryos in a minimum of three fresh or frozen cycles in a woman under 40 years of age (11). The preimplantation genetic diagnosis consortium of the European Society of Human Reproduction and Embryology (ESHRE) defined RIF as when more than three good-quality embryo transfers or ten embryos in multiple transfer cycles are performed without achieving a clinical pregnancy (12). In clinical practice, an international survey of clinicians and embryologists showed that the majority defined RIF as failed embryo transfer with three cycles, both fresh and frozen, with no agreement on the cutoff upper age (7).
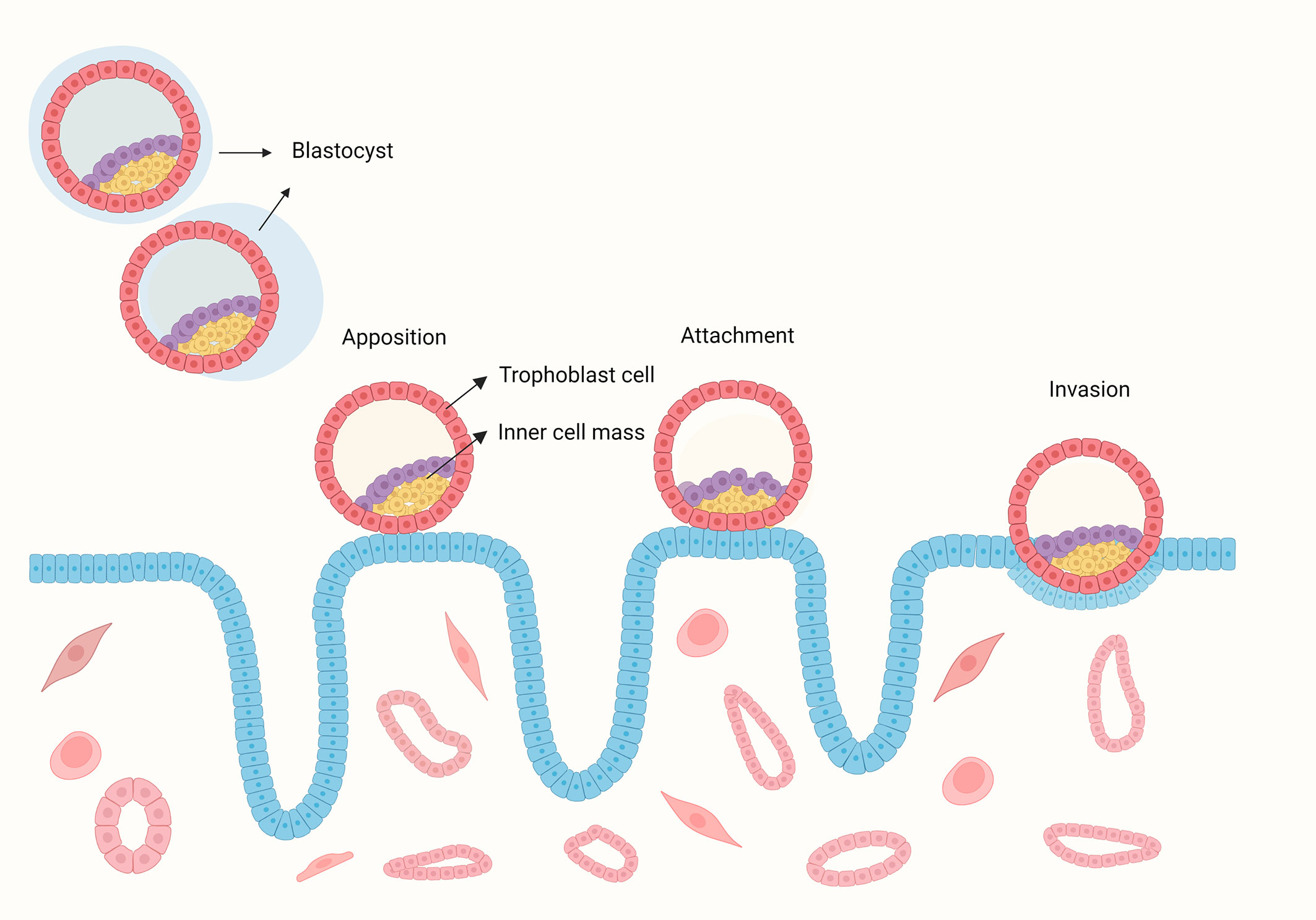
Figure 1 The process of human blastocyst implantation. The blastocyst hatches from the zona pellucida and contact with the endometrium, then the embryo bind to the endometrium during the attachment stage, during with the crosstalk between the embryo and endometrium induces up-regulation of surface receptors and the secretion of signalling molecules and hormones. This signalling directs epithelial withdrawal and trophoblast invading the endometrium.
IVF-ET success rates have improved over the decades due to technical improvements, which affect RIF definition, mainly in the number of embryos transferred. In clinical practice, different centers often adjust their definitions according to their own status (7). Overall, we defined RIF as failure to become clinically pregnant after the transfer of at least three good-quality embryos in three fresh or frozen cycles in women under 40 years of age. Here, a good-quality embryo means day 3 embryo ≥ 8 cells, symmetric, with <10% fragmentation (8), or blastocyst with a grade ≥ 3BB (13). However, further analysis of multiple center clinical data with large sample size is needed to process a more internationally accepted definition of RIF. This review summarizes the etiology of RIF and the current clinical treatment.
Known risk factors for RIF include body mass index (BMI), smoking, alcohol consumption, and stress.
Body mass index is associated with implantation. Obesity affects the female reproductive system. Pre-pregnancy obesity is associated with abnormal menstruation, anovulation, and pregnancy complications (14, 15). In IVF-ET, obese patients tend to have a lower pregnancy rate than normal-weight patients (16). Furthermore, when BMI was ≥ 30 kg/m2, patients undergoing IVF-ET had significantly decreased odds of implantation (17). In addition, obesity can alter the markers of uterine receptivity and decidualization, which may contribute to a decrease in the implantation rate in obese patients (18).
In women undergoing IVF, it is difficult to assess the amount of smoking owing to inaccurate responses to questionnaires, which makes the association between smoking and IVF uncertain. However, for patients who smoked for > 5 years, smoking was associated with fewer oocytes retrieved, a higher cycle cancellation rate, and a lower implantation rate (19). Meanwhile, for male partners, smoking negatively affects sperm motility and counts and increases sperm DNA damage (20).
Alcohol has a negative effect on pregnancy. In developed countries, alcohol use is a risk factor for stillbirth (21) and can also affect the neurocognitive function of the offspring, such as hyperactivity, impulsivity, and lack of awareness of social cues (22). Therefore, couples trying to conceive are advised to quit drinking before pregnancy (23).
Cortisol production increases in response to stress, which is believed to be a risk factor for pregnancy. Maternal stress, measured by the level of cortisol, increased the risk of miscarriage by 2.7-fold (24). However, another study showed that stress did not affect the outcomes of patients undergoing the first cycle. Failure of the last IVF cycle leads to a high risk of stress (25).
RIF is a complex clinical phenomenon with several different etilogies, including maternal factors, paternal factors and embryo factor. There may not be one single cause, but several factors working together lead to RIF. Among the etilogies, maternal factors include different aspects. Though a good quality embryo is foundation for successful implantation, the state of mother is also crucial, which we will focus on,
Successful implantation is a process of maternal-fetal immune tolerance involving various molecules. Trophoblast invasion can activate the maternal immune response to fetal antigens. Local immune cells at the implantation site in the endometrium, which are activated by the embryos, mediate maternal-fetal immune tolerance and promote placental development. They involve in regulating the differentiation of decidual cell, remodeling uterine vascular, promoting epithelial attachment and regulating immune activation. In this stage, immune cells, including innate lymphocytes, T cells, decidual dendritic cells, and macrophages, are activated, and they are also associated with adverse pregnancy outcomes such as RIF (26).
Innate lymphocytes (ILCs) have been proved to exist in human decidua (27). They are divided into two subtypes: natural killer (NK) cells and non-cytotoxic helper ILCs (ILC1s, ILC2s, and ILC3s) (28). NK cells in the uterus (uNk cells) account for over 70% of all endometrial leukocytes in early pregnancy (28, 29) and possess unique functions that differentiate them from peripheral NK cells. They secrete specific chemokines, express unique cell surface markers, and display a large granule morphology. However, they show poor cytotoxicity because they are unable to polarize granules into the immune synapse (30).
NK cells in the decidua stroma secrete cytokines and express receptors mediating maternal-fetal immunity. uNK cells are not directly cytolytic to fetal extravillous trophoblast (EVT) cells (31). They prompt the low cytotoxicity of uNK cells necessary for semi-allogeneic fetus. Specifically, uNK cells express killer cell immunoglobulin-like receptors (KIRs) that can bind to selectively expressed ligands on EVT, such as human leukocyte antigen-C (HLA-C), human leukocyte antigen-G (HLA-G), and human leukocyte antigen-E (HLA-E) (32, 33). The function of uNK cells depends on the balance between inhibitory and activating receptors (34), as KIR genes are highly polymorphic. Each pregnancy involves different maternal/fetal genetic combinations that deliver activating or inhibitory signals to uNK cells. KIR genes can be grouped into two main haplotypes, A and B (35). The maternal KIR genotype could be AA (inhibition of KIR), AB, or BB (activation of KIR). Trophoblast invasion is regulated by interactions between the maternal KIR and fetal HLA-C. Women with the KIR AA genotype have a higher risk of preeclampsia and other pregnancy-related complications (36). About 78% of patients with more than five unsuccessful IVF treatments or embryo transfers lacked three KIR-activating receptors (2DS1, 2DS3, and 3DS5) (37). Moreover, the KIR genotype of Tel AA combined with the HLA-C2C2 genotype was more prevalent in patients with RIF (p/pcorr. = 0.004/0.012, OR = 2.321) (38). This specific combination of polymorphic KIR and HLA-C genotypes can also affect decidual vascular remodeling (39).
Angiogenesis is the foundation for implantation. uNK cells are the main source of angiogenic growth factors such as placental growth factor, vascular endothelial growth factor (VEGF)-A, and angiopoietin, which may direct angiogenesis during embryo implantation (40, 41). In early pregnancy, uNK cells aggregate around spiral arteries, and animal studies have shown that uNK is involved in spiral artery remodeling (42). These findings suggested that uNK cells play a role in mediating vascular changes during implantation. The number of uNK cells, which was no correlation with peripheral NK level, increases in patients with RIF (43, 44).. However, the production of angiogenic factors, such as VEGF, by uNK cells was lower in patients with RIF than in fertile women, which may be attributed to the increased cytotoxicity of CD16+ uNK cells (45, 46). The angiogenic factors produced by uNK cells may be located at the implantation site and move toward the embryo, directing the development of maternal vasculature to the implantation site (47). Hypoxia-inducible factor 1-alpha (HIF-1α) is a transcription factor expressed under hypoxic conditions and can promote angiogenesis by increasing VEGF expression in the tumor tissue. HIF-1α inhibitors can activate the anti-tumor functions of NK cells by elevating interferon-γ (IFN-γ) production (48). In early pregnancy, trophoblasts secrete HIF-1α under hypoxic conditions (49). In the uteri of patients with RIF, both HIF-1α expression and angiogenesis are reduced (50). Therefore, we assume that a decrease in HIF-1α may be involved in RIF via the reduction of VEGF secreted by uNK cells or via an increase in uNK cell cytotoxicity. This may be due to abnormal interactions between trophoblasts and uNK cells (Figure 2). However, further studies are required to support this hypothesis. Other studies suggested that patients with RIF showed more abnormal vascular parameters as estimated by the Doppler test, with more uNK cells producing more IL-12 and IL-18. Dysfunction of cytokine signaling may impair vascular remodeling, leading to excessive or insufficient recruitment of uNK cells (51–53). However, another study reported different conclusions. Analysis of uNK cell numbers using standard immunohistochemistry protocols showed that there was no difference in uNK cell numbers and distribution relative to endometrial arterioles between patients with RIF and women with successful IVF cycles. Furthermore, uNK cell numbers were significantly decreased in women who had successful pregnancies compared with those who did not (54). Overall, uNK cells might impair vascular remodeling via abnormal recruitment of NK cells to endometrium, with dysregulated cytokine signaling.
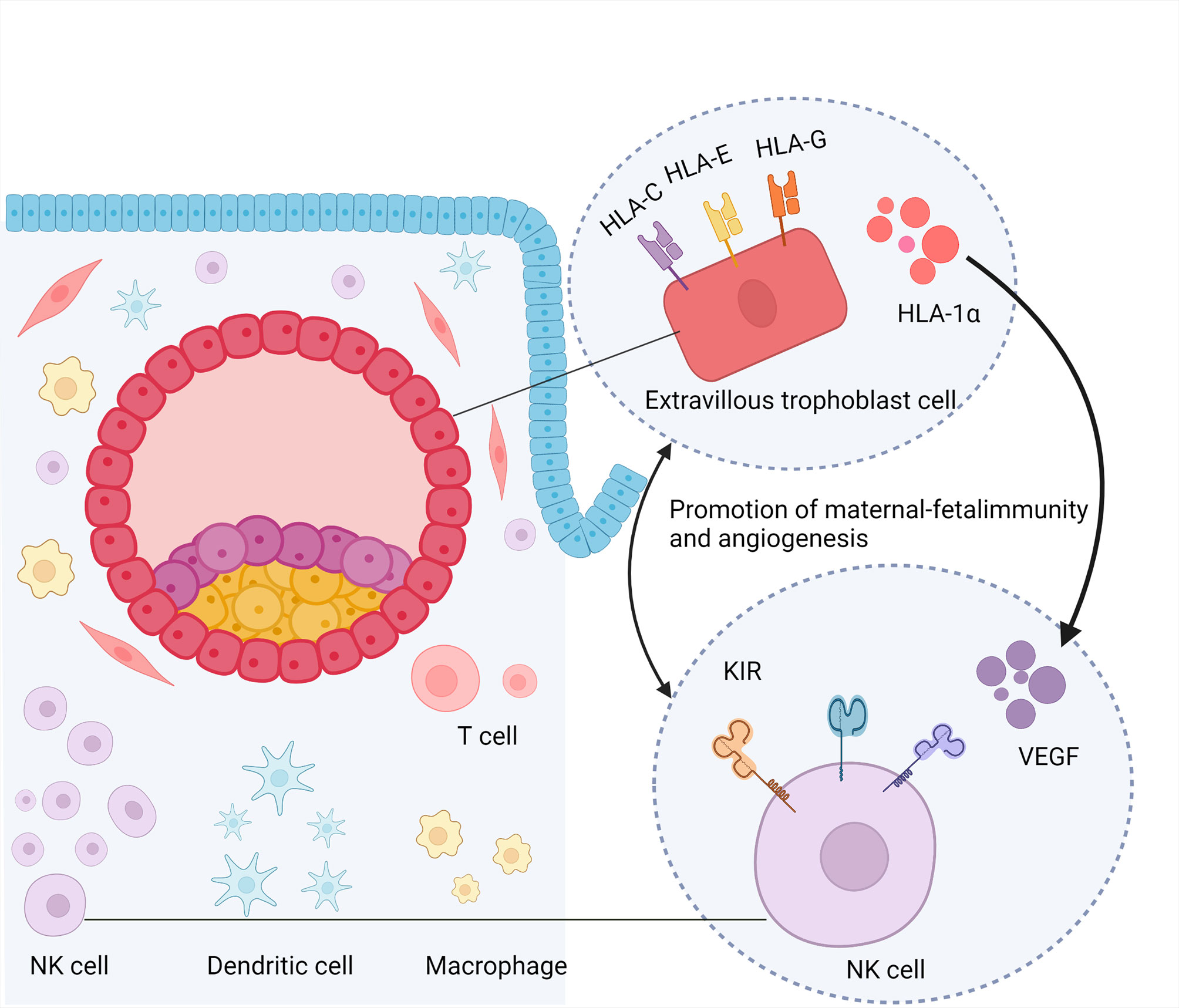
Figure 2 Promotion of maternal-fetal immunity and angiogenesis. NK cell,nature kill cell; DC, dendritic cell; KIR, killer cell immunoglobulin-like receptors; HLA, human leukocyte antigen; VEGF, vascular endothelial growth factor; HIF-1α, hypoxia-inducible factor 1-alpha.
T cells play an important role in immunity during pregnancy. They are divided into four main types: T helper 1 (Th1) cells, Th2, Th17, and regulatory T (Treg) cells. T cells constitute 5–20% of CD45+decidual lymphocytes, which display different functions compared to peripheral blood T cells (55). Th2 cell dominance is essential for normal pregnancy (56). An imbalance of Th1/Th2 is associated with reproductive dysfunction. In patients with RIF, the Th1/Th2 ratio increases in the peripheral blood with an increasing Th1 immune response (57). Meanwhile, anti-inflammatory factors, such as IFN-γ and tumor necrosis factor-α (TNF-α), mainly secreted by Th1 cells, were increased in the peripheral blood of patients with RIF (58).
Th17 cells can produce an anti-inflammatory factor, interleukin-17 (IL-17), which promotes the expression of inflammatory mediators. An abnormal Th17 increase in the peripheral blood and decidua is associated with recurrent miscarriages (59–61). In the peripheral blood of patients with RIF, higher numbers of Th17 cells co-exist with exhausted Treg cells (62). Treg cells are known to mediate pregnancy tolerance, and can potently suppress Th1/Th17-mediated immunity (63). More evidence has shown that exhausted Treg cells may lead to adverse pregnancy outcomes; reduced capability of Treg cells to control over-activated T cells may lead to implantation failure (64). The reduced suppressive capability of Treg cells is associated with CD279/PD-1 expression (65), which may play a role in the RIF mechanism. Moreover, intravenous immunoglobulins (IVIG) can improve the implantation rate by increasing Treg cells in the peripheral blood of patients with RIF, verifying the effect of Treg cells in RIF from another side (66).
DCs account for 10–20% of decidual leukocytes. As antigen-presenting cells, uterine DCs are involved in the recognition of paternal antigens (26). A decrease in immature DCs and an increase in mature DCs were observed in the decidua of women with recurrent spontaneous abortion (67). Few studies have examined the role of DCs in RIF. Depletion of DC in the uterus led to severe impairment of implantation in mice (68). ILT4+ DCs were significantly increased in the peripheral blood and endometrium of patients with RIF compared to that in the fertile control group, probably due to the induction of Treg cells (69). Further studies are required to confirm the relationship between DCs and RIF.
Macrophages regulate implantation, placentation, fetal development, and vascular remodeling at the maternal-fetal surface (70). Macrophages located close to invade trophoblasts and spiral arteriesto promote implantation during early pregnancy (71). The proportion of uterine macrophages was high in patients with RIF with chronic endometritis and adenomyosis, indicating that macrophages are involved in the pathological process of implantation failure in these patients. However, the underlying mechanism remains unknown (72, 73).
Pregnancy is a hypercoagulable condition. Thrombophilias are conditions that predispose individuals to inappropriate blood clot formation (74). Thrombophilia is involved in recurrent pregnancy loss (RPL), but the association between thrombophilia and RIF remains to be elucidated. Thrombophilias are believed to affect implantation by impairing vascularization of the embryo and disturbing blood flow to the decidual vessels (75).
Inherited thrombophilia commonly refers to a condition in which genetic mutations affect the function or quantity of proteins in the coagulation system (76). The common forms of inherited thrombophilias are genetic mutations in factor V Leiden, proteins S and C, prothrombin, and methylenetetrahydrofolate reductase (MTHFR). Mutations in these genes are increased in women with RPL, including RIF (77–79). Moreover, patients with RIF with thrombophilia most commonly harbor the MTHFR C677T variant, which impairs implantation by disturbing vascularization (78, 79). On the other hand, hyperhomocysteinemia caused by the MTHFR C677T variant is also considered a risk factor for RPL (80).
The most prevalent acquired thrombophilia is the antiphospholipid syndrome (APS). It is an autoimmune hypercoagulable state diagnosed by the presence of antiphospholipid antibodies, such as anticardiolipin antibodies, lupus anticoagulant antibodies, and/or anti–2-glycoprotein I antibodies. It has been proven that APS is associated with RPL and patients with previous arterial or venous thromboembolic events have a higher risk of pregnancy complications (81). However, the role of APS in RIF remains unclear. Antiphospholipid antibodies can be detected in patients with RIF (82). In a previous study, the frequency of antiphospholipid antibodies inpatients with RIF was significantly higher than that in the fertile group (83). Nevertheless, other studies have not reported an association between APS and RIF. When APS was analyzed in women with a mean of seven failed IVF-ET cycles, there was no significant association between thrombophilias and RIF (84). Therefore, a clinical practice guideline by the Canadian Fertility and Andrology Society does not recommend testing for thrombophilia in patients with RIF (85).
The endometrium is critical in pregnancy, as it provides an environment for the implantation of developing embryos. Impaired endometrial receptivity is estimated to account for two-thirds of implantation failures (86). Suboptimal endometrial receptivity has been confirmed as a cause of RIF (87). An endometrial biopsy obtained from patients with RIF on the seventh day of progesterone administration revealed 313 genes that were differentially expressed between patients with RIF and the control group (88). Another study revealed differences in several fertility-related genes in cultured endometria of RIF versus patients who became pregnant after IVF-ET (89). Bioinformatical analyses demonstrated that PTGS2, FGB, MUC1, SST, VCAM1, MMP7, ERBB4, FOLR1, and C3 were the key differentia expression genes related to RIF (90). Transcriptomic studies have indicated that patients with RIF express a different endometrial profile compared to the fertile control group on special days of the menstrual cycle. This is assumed to be due to the displacement of the window of implantation (WOI), which affects more than 25% of patients with RIF (87, 91). Furthermore, prostaglandin synthesis appears to be disturbed in patients with RIF and may lead to poor endometrial receptivity (92).
The human microbiome, called “the other human genome,” has been involved in normal physiology and homeostasis, associated with states of health and disease (93, 94). The female reproductive tract contains distinct bacterial communities that form a continuous microbiota changing from the vagina to the ovaries (95). Alterations in the vaginal microbiome are involved in female reproductive system diseases such as bacterial vaginosis, urinary tract infections, and also in pregnancy complications (96–98). Hence, we can assume that microbiota might be involved in several steps of IVF-ET, including gametogenesis, implantation, and delivery.
The vagina is dominated by the Lactobacillus genus, which has a probiotic influence on the vaginal microenvironment (95). It can inhibit the invasion of bacteria by producing high concentrations of lactic acid and short-chain fatty acids, which maintain the acidic environment of the vagina (99, 100). Infertile women display abnormal vaginal microbiota. Ureaplasma spp. in the vagina and Gardnerella spp. in the cervix appeared to be related to women with a history of infertility (101). Investigating the vaginal microbiota in patients with unexplained RIF indicated that vaginal Lactobacillus (found to be positively correlated with pregnancy rates) was significantly decreased compared to patients who became pregnant in the first frozen embryo transfer (FET) cycle. Patients with RIF presented higher microbial α-diversity than the control group (99). Meanwhile, vaginal Lactobacillus in patients with RIF was significantly decreased compared with healthy women, and the vaginal microbiota profiles in patients with RIF had significantly higher levels of five bacterial genera than in healthy women (102). Therefore, the number of vaginal Lactobacillus spp. is assumed to be a predictive biomarker of implantation.
The endometrium contains four orders of magnitude fewer bacteriathan the vagina; the vagina harbors approximately 1010-1011 bacteria (95, 103). High numbers of Lactobacillus spp. in the endometrium during the implantation window were associated with higher successful implantation rates, whereas non-Lactobacillus-dominated microbiota, such as Streptococcus, during the implantation window resulted in negative pregnancy outcomes (104). Bacterial pathogens alter endometrial microbiota, which can result in chronic endometritis. Chronic endometritis is often asymptomatic, leading to inconsistencies in prevalence. The reported prevalence in patients with RIF ranges from 7.7% to 66% (105–109), with a prevalence of 2.8% in patients with general infertility (110). The uterine immune status in chronic endometritis is altered (72). A study on chronic endometritis has shown abundant immune cells in the endometrium and an increase in CD83+ mature DCs, CD68+ macrophages, CD8+ T cells, and Foxp3+ Treg cells; these results might be reasonable for impaired endometrial receptivity and recurrent pregnancy failures (72). Furthermore, microbial alterations in chronic endometritis may also disturb immune status by increasing the synthesis of lipopolysaccharide, an important immunomodulator (111).
During pregnancy, the gut microbiota can change in composition or abundance (112). It may also be involved in embryo implantation by affecting the immune system, coagulation system, and endometriosis pathology (113–115). Patients with RIF display abnormal gut microbiota (116), but the relationship between gut microbiota and implantation failure needs to be further investigated.
Several types of uterine abnormalities can affect implantation rates, including fibroids, polyps, intrauterine adhesions, Mullerian abnormalities, adenomyosis, and hydrosalpinges. The proportion of unidentified intrauterine abnormalities in patients with RIF varied between 14% and 51% (117–120). Most patients are asymptomatic and remain undiagnosed until they undergo transvaginal ultrasound or hysteroscopy.
Fibroids can lead to deformation of the uterine cavity and adhesion, which can prevent the attachment of the embryo to the endometrium. The effect of fibroids on pregnancy outcomes is related to their location. Intramural and subserous fibroids may not have an impact on pregnancy outcomes. Submucosal fibroids can decrease implantation and pregnancy rates in patients undergoing IVF. The mechanism hindering implantation includes increased uterine myometrial contractions, abnormal vascularization, and a disordered cytokine profile (121). A systematic review concluded that patients with submucosal fibroids had lower implantation and live birth rates than the control group. Therefore, the removal of submucosal fibroids before IVF-ET seems to confer benefits (122).
Polyps in the endometrium are the most frequent uterine lesions in patients with RIF that interfere with embryo implantation (121, 123). They not only affect the deformation of the uterine cavity, but also disturb the implantation process by altering cytokines secreted by the endometrium, such as insulin-like growth factor 1 binding protein and TNF-α (124, 125). The removal of endometrial polyps before intrauterine insemination is believed to improve clinical pregnancy rates (126).
Intrauterine adhesion often occurs after the curettage of the gravid uterus to terminate the pregnancy. It impairs the functional layer of the endometrium and prevents embryo attachment for successful implantation. A study of 210 patients with RIF who underwent hysteroscopic evaluation showed that the frequency of intrauterine adhesions was 8.5% (127).
Mullerian abnormalities, such as septate and bicornuate uteri, should be considered in patients with RIF. Compared with other congenital uterine anomalies, partial septate and septate uteri appear to have the poorest reproductive outcomes, such as reduced pregnancy rate, increased risk of first-trimester miscarriage, and preterm birth (128). Among 144 patients undergoing IVF-ET who experienced implantation failure, uterine abnormalities (mainly septate) were found in 14 (9.7%), which led to the assumption that uterine septate may be a factor involved in implantation failure (129). However, a bicornuate uterus is more likely to have less influence on pregnancy. The major risk factors for a bicornuate uterus are mid-trimester abortion and preterm birth (130).
Endometriosis is an estrogen-dependent inflammation with an incidence of up to 50% in infertile women (131, 132). It can affect female IVF-ET in several aspects, including the number of oocytes retrieved, fertilization, and implantation rate (133). The mechanism involves anatomic distortion, oviduct occlusion, abnormal secretion of cytokines involved in endometrial receptivity, and poor oocyte quality (134). In addition, patients with endometriosis-related infertility display different reproductive tract microbiota, which may disturb endometrial receptivity (95). Adenomyosis, defined by the presence of a heterotopic endometrium in the myometrium, is a special form of endometriosis. This can lead to implantation failure in young patients (135). Nevertheless, surgical operation in adenomyosis may not improve clinical outcomes because there is no defined capsule and part of the uterine wall has to be removed (11).
Hydrosalpinges can negatively impact implantation, mainly due to the impairment of embryo development by innutritious fluid (136). Other mechanisms include disturbing endometrial receptivity and physically flushing the embryo out (137). Infertile patients with hydrosalpinges express significantly less αvβ3 integrin, HOXA 10, and leukemia inhibitory factor (LIF) during WOI compared with fertile women (138–140). In IVF-ET, hydrosalpinges are associated with negative outcomes, including lower implantation rates, lower pregnancy rates, and increased spontaneous abortion rates (141, 142). However, the influence of hydrosalpinges on implantation rates appears to be associated with the extent of the hydrosalpinges. One study showed that implantation rates of patients undergoing salpingectomy were not significantly higher than those in the non-intervention group. However, subgroup analysis indicated significantly increased implantation rates when patients with ultrasound-visible hydrosalpinges underwent surgery (143).
Although studies have shown that sperm affects early embryogenesis and placental function, the relationship between male factors and RIF remains poorly understood. Sperm DNA damage is related to poor embryo development, and sperm DNA integrity testing is considered to be associated with reproductive failure (144). However, a prospective study with a small number of patients showed that a high DNA fragmentation index was not correlated with RIF (145), which was consistent with another prospective study (146). Therefore, routine testing for DNA fragmentation is not recommended by the American Society for Reproductive Medicine (ASRM) (147). While sperm aneuploidy rates were evaluated by fluorescence in situ hybridization techniques, there was a significant increase in the incidence of sex chromosome disomies in patients with a previous history of RIF; however, the implantation rates did not significantly increase in patients who underwent subsequent IVF-ET cycles (148).
In addition, protamines are the largest number of nuclear proteins in human sperm, which are divided into protamine 1 (P1) and protamine 2 (P2). They can package compacted chromatin more efficiently and protect sperm from oxidative damage. Recently, the P1/P2 ratio has been identified as a new parameter of sperm function that can partly predict the fertilization outcome of IVF-ET (149, 150). An abnormal P1/P2 ratio is related to infertility (151). A decreased P1/P2 ratio was associated with poor pregnancy outcomes, including a lower fertilization rate of IVF and a lower implantation rate per embryo in patients undergoing IVF-ET (152). Moreover, the sperm of male partners of women with RPL contained significantly higher P1 and P2, and a lower P1/P2 ratio, indicating that protamines are not only important for fertilization, but also play a role in early embryogenesis (153).
In conclusion, there is insufficient evidence for an association between male factors and RIF. We hypothesized that impaired sperm parameters are more likely to be involved in RIF by affecting the chromosomal constitution of embryos, which will be discussed in the following section.
Embryos with abnormal chromosomes are recognized as important factors that cause implantation failure or pregnancy loss (154). The probability of chromosomal aneuploidy in embryos also increases with age. In the first trimester, a spontaneous abortion rate as high as 76% has been attributed to chromosomal abnormalities (155).
Chromosomal abnormalities, including translocations, inversions, deletions, and mosaicism, are more common in patients with RIF than in the general population (156). In cleavage embryos, the incidence of complex chromosomal abnormalities, such as three or more abnormal chromosomes, was independent of age but increased in embryos from patients with a history of RIF (157). This complex abnormality is considered mitotically derived because it is more common in embryos than in retrieved oocytes. However, the exact cause of this remains unknown. Furthermore, embryonic mosaicism is the presence of two or more genetically different cell lineages, usually one with an abnormal chromosome and the other with a normal chromosome, and is common in human preimplantation embryos (158, 159). Due to chromosomal abnormalities in this type of embryo, it is reasonable to suspect that mosaicism can influence the implantation rate. Mosaic embryos have lower implantation rates and live births than euploid embryos, and their implantation potential is affected by the extent of mosaicism (160). Typically, embryos with whole-chromosome aneuploidy display negligible implantation potential (161).
The treatment of patients with RIF presents a challenge to clinicians. Various therapeutic options have been proposed to manage RIF, including lifestyle intervention, immunotherapy, anticoagulant, improving endometrium receptivity and sperm quality and preimplantation genetic testing for aneuploidies (PGT-A). Experienced clinicians and embryologists should discuss therapeutic options with patients to address their questions and offer an individualized treatment plan. We discuss below the different interventions that can be used in the management of RIF.
Patients should be informed that obesity (BMI ≥ 30 kg/m2) or underweight (BMI < 19 kg/m2) can negatively impact reproduction outcomes. Patients should be advised to return to a normal BMI before IVF-ET treatment. Multidisciplinary approaches include low-energy diets, pharmacotherapy, and bariatric surgery (162). Weight loss before clomiphene treatment in patients with PCOS resulted in improved ovulation and live births (163). Moreover, short-term weight loss before IVF-ET was associated with the retrieval of more metaphase II oocytes (164). For safety, some countries do not allow public funding for IVF-ET treatment in obese infertile patients unless their BMI is within a certain level (165).
Women planning pregnancy should stop smoking and avoid secondhand smoke for better IVF-ET outcomes (166). Male partners should also abstain from smoking, as smoking increases the production of reactive oxygen species in seminal plasma, alters sperm microRNA content, and increases DNA fragmentation in sperm (167).
More than one unit of alcohol per day can reduce the efficiency of IVF-ET, including fertilization and pregnancy rates, and excessive alcohol intake can be harmful to semen quality. Therefore, couples with RIF should reduce alcohol intake to one or two units per week or total abstinence from alcohol before IVF-ET.
Stress is also associated with RIF. Lifestyle interventions such as a healthy diet, regular exercise, and even psychological interventions may reduce psychological distress and improve future IVF-ET outcomes (168).
An appropriate controlled ovarian hyperstimulation (COH) protocol should be considered. The stimulation protocol and dose of gonadotrophin require reconsideration if patients have a suboptimal response. Gonadotropin-releasing hormone agonist (GnRHa) combined with human menopausal gonadotropins (HMG) appeared to widen the implantation window compared to a single HMG protocol, resulting in improved IVF-ET success (169). Moreover, the use of long-acting GnRHa for a few months before IVF-ET may increase the pregnancy rate in patients with endometriosis (170). Administration of a single dose of GnRHa in the luteal phase can improve the implantation rate in intracytoplasmic sperm injection (ICSI) cycles (171). This might be partially due to differences in gene expression caused by different luteal support protocols (172). Therefore, it is important to select a specific protocol that includes ovarian stimulation and luteal support in patients with RIF, which may be related to the success rate.
Assisted hatching (AH) is a technique that includes zona thinning and zona drilling/opening, using chemical, mechanical, or laser energy. The effects of AH remain unclear. Embryos that underwent drilling treatment in frozen/thawed embryo transfer displayed a higher implantation rate but no increase in pregnancy rate (173). A recent meta-analysis showed that it was uncertain of the effect of AH on live birth rates (174). In selected patients such as those with RIF, AH might be beneficial. In patients with RIF older than 38 years, AH caused by partial zona dissection led to a significant increase in implantation and clinical pregnancy rates (175). However, ASRM considered that there is insufficient evidence for the benefit of AH in patients with poor prognosis, including poor-quality embryos, more than two previous IVF-ET failures, and advanced maternal age (176). Generally, considering the influence of AH on the embryo and its controversial effect on RIF, AH should be used cautiously.
Abnormally elevated estrogen levels in fresh cycles may influence endometrial morphology and receptivity (177). The endometrium in fresh cycles shows a premature secretory phase followed by dyssynchronous stromal and glandular differentiation in the mid-luteal phase (178). Therefore, the implantation rates in fresh embryo transfer were lower than in frozen-thawed cycles (179). Moreover, the embryo transfer stage is important for successful implantation. Implantation rates were higher in the blastocyst transfer group than in the cleavage embryo transfer group in patients with RIF (180). In patients with RIF with a good ovarian response, the implantation rates of fresh cycles were significantly higher in blastocyst transfer; however, the cycle cancellation rates also increased (181). Thus, transfer of blastocysts in frozen-thawed cycles might be a choice for patients with RIF, and sequential cleavage and blastocyst embryo transfer appeared to be beneficial. It can improve clinical pregnancy rates compared to cleavage embryo transfer. Patients with sufficient embryos may attempt this method (182).
Maternal-fetal immune tolerance is a necessary condition for successful implantation. Several immunological therapies have been explored to increase implantation rates. Endometrial biopsies and peripheral blood sampling for NK cell type and count or Th cell proportion offer a method to assess the maternal immune status and a rationale for immune-modulating therapies (183).
Glucocorticoids are a type of immunomodulator. They can bind to the glucocorticoid receptor on uNK cells and decrease the number of uNK cells (184, 185). A meta-analysis showed that administration of glucocorticoids during routine IVF-ET cycles did not improve live birth rates. However, the use of glucocorticoids in a subgroup of IVF, not ICSI cycles, was related to increasing pregnancy rates with borderline statistical significance, suggesting that specific subgroups of patients might benefit from glucocorticoid therapy (186). Using prednisolone in patients with serum anti-ovarian antibody positivity and at least two previous IVF failures could decrease the serum anti-ovarian antibody level and improve pregnancy outcomes (187). Prednisolone could also improve the implantation rates in patients undergoing ICSI with high-level peripheral CD69+ NK cells (188). However, in a selected group of patients (failure to obtain clinical pregnancy after transfer of at least two embryos in at least two fresh or frozen cycles) with elevated uterine NK cells, prednisolone could decrease uNK cell concentration, but with no significant benefit on pregnancy outcomes (185). Therefore, glucocorticoids should be carefully administered to patients with specific indications, the dosage and time are arbitrary.
Intravenous immunoglobulin (IVIG) is produced by the extraction of IgG fractions from the plasma of healthy donors. It can protect the fetus from the maternal immune system by promoting the expansion of suppressor T cells, inhibiting complement deposition, protecting paternal genes by neutralizing anti-HLA antibodies, and reducing the adhesion of T cells to the human placental extracellular matrix (189, 190). In RPL, IVIG is an efficient therapy for improving pregnancy outcomes by affecting the Th1/Th2 ratio and increasing Treg cells (189, 191). Furthermore, IVIG can improve implantation and pregnancy rates in patients with RIF and immune abnormalities (192). The combined application of IVIG, aspirin, and heparin could increase the pregnancy rates and peripheral blood Treg cell proportion in patients with RIF, compared with patients using only aspirin and heparin (66). In addition, IVIG can decrease NK cell percentage and cytotoxicity, and improve pregnancy and live birth rates in patients with reproductive failure (193, 194). IVIG was administered at 200–500 mg/kg body weight (usually 400 mg/kg) 7 days-24 hours before embryo transfer and lasted until fetal pulse detection or every 3 weeks during pregnancy (192).
Pregnancy is a type of semi-allograft. The maternal immune system treats the fetus as a foreign agent. Excessive immune activation results in implantation failure. Tacrolimus, an immunosuppressant, has been demonstrated to suppress immunological rejection by inhibiting cytotoxic T cell generation, alloantigen-induced lymphocyte proliferation, and the production of IL-2 and IFN-γ (195). It has been used as a plausible treatment for patients with RIF who have an elevated Th1/Th2 ratio and appears to improve pregnancy outcomes (196, 197). However, further evidence is required to support the use of tacrolimus for RIF. Further, its dose and safety need to be carefully assessed.
Cyclosporine is a typical immunosuppressant that induces immune tolerance in patients with autoimmune diseases and organ transplantation. It can promote the invasion and migration of villous trophoblasts, thereby improving implantation (198). The production of IL-4, a Th2 cytokine, and chemokine CXCL12 is increased by cyclosporine at the maternal-fetal surface (199, 200). Cyclosporine could improve pregnancy outcomes in patients with RPL with an elevated Th1/Th2 ratio in peripheral blood (201). Patients with unexplained RIF receiving cyclosporine treatment since the transfer day showed an obvious improvement in implantation rates, especially of non-high-quality embryos (202). However, in patients with only one unsuccessful transfer cycle of high-quality embryos, cyclosporine treatment did not display benefits for clinical pregnancy outcomes in the following FET cycles (203). Therefore, it is not recommended administration of cyclosporine in RIF patients routinely.
Intralipids are fat emulsions containing glycerin, soybean oil, and egg phospholipids, which are used for parenteral nutrition. It can modulate NK cell cytotoxicity and suppress pro-inflammatory cytokine activity (204, 205). In RIF patients with overactivation of NK cells, intralipids can decrease the biomarkers of immune overactivation in the endometrium and increase live birth rates (206). A recent meta-analysis showed that intra-venous intralipid therapy could improve the clinical pregnancy and live birth rates, but the sample sizes of included studies were small, and the treatment protocols were variable (207). However, not all studies showed an improvement after treatment with intralipid. A randomized controlled trial (RCT) in patients with RIF that used 20% (100 mg) intralipid in 500 mL NaCl on the day of embryo transfer demonstrated that the increase in the clinical pregnancy and live birth rates was not significant after intralipid infusion therapy (208). Coulam believed that intralipid is not appropriate for all patients with RIF but for those with some kind of immune abnormality; identifying such patients is essential (209). Overall, there is insufficient evidence regarding the routine use of intralipid therapy in patients with RIF, and a standard treatment protocol is lacking. Large-scale studies are required to explore the effects and safety of intralipids in RIF treatment.
LIT is an active immunotherapy that can modulate maternal fetal interface immune balance by administering lymphocytes obtained from mother’s partner. It was initially conceived to improve immune tolerance and better for implantation. This immunotherapy was first used to treat RPL, but its current application is controversial. The 2017 ESHRE guidelines for RPL do not recommend the use of LIT in affected patients. Meanwhile, some studies have found LIT to be beneficial for RIF (210, 211), but RCTs analyzing the efficiency of LIT in treating this condition are still lacking. In general, there is insufficient evidence to recommend LIT in patients with RIF, and we should be aware of the possible complications such as infections, autoimmune disorders and formation of irregular antibodies.
Aspirin is classified as a non-steroidal anti-inflammatory drug. It can inhibit the activity of cyclooxygenase and is, therefore, used as an antithrombotic agent. In terms of reproduction, aspirin contributed to reduce the inflammation in uterus and improve uterine perfusion, which may improve endometrial receptivity (212, 213). Although aspirin can decrease endometrial and uterine arterial blood flow resistance in patients with unexplained RIF (214), no significant differences were found between the aspirin treatment group and control group with respect to implantation and pregnancy rates (215–218).
LMWH has an activity similar to that of heparin, with an increased half-life and depolymerization. LMWH possess antithrombin or anticoagulation activities. It is speculated that LMWH might prevent placental thrombosis and infarction and modulate decidualization of the endometrium (219, 220). A prospective randomized trial in patients with previous IVF failure and thrombophilia showed a significant increase in implantation and pregnancy rates (221). In RIF patients, LMWH significantly improved live birth rates and reduced miscarriage rates, even though implantation rates were not significantly improved (222). In patients with two or more unexplained failed fresh embryo transfers, LMWH administration from the day after oocyte retrieval led to a tendency of a higher live birth rate with no significant difference, and the implantation rate was also not different (223). Therefore, LMWH may be a potential intervention for patients with RIF, at a dosage of 40mg/day from the day of oocyte retrieval or embryo transfer to 8-12 weeks of gestation.
Generally, hCG can bind to the LH receptor in the endometrium, induce the secretion of cytokines during implantation window and regulate endometrial receptivity and embryo implantation. It is usually administered 0.25–72 hours before embryo transfer at a dosage ranging from 500 to 1000 IU. Administration of hCG appears to regulate embryo implantation among patients with RIF. It can increase the invasion potential of trophoblast cells by modulating the secretion of matrix metalloprotein-2 and tissue inhibitor of metallopeptidase-1 (224). In a previous study, intrauterine injection of hCG before embryo transfer increased the live birth, clinical, and implantation rates of IVF-ET. The effect of 500 IU hCG was better than that of other dosages. However, the outcomes between the first IVF-ET cycle and RIF subgroups did not significantly differ (225). Another study showed that intrauterine injection of hCG before FET improved pregnancy rates in patients with two more implantation failures. Generally, infertile patients may benefit from intrauterine injection of hCG, but further RCTs are needed to confirm these findings.
PBMCs, such as monocytes and T and B lymphocytes, can induce the secretion of interleukins and growth factors, which appear to be beneficial to the endometrial thickness and receptivity (226). In addition, intrauterine PBMCs can promote embryo attachment and invasion by creating a pathway while moving towards the endometrial stroma (227). The immune cells recruited to the implantation site may not induce initial inflammation for successful implantation in RIF patients, which can be improved by PBMCs (228–230). A recent meta-analysis showed that implantation and live birth rates of patients with RIF were significantly increased in the PBMCs group (227). Another clinical trial confirmed the effect of PBMCs on patients with RIF. Intrauterine infusion of PBMCs before embryo transfer significantly increased implantation rates in frozen-thawed cycles (230). Thus, PBMCs are considered an effective treatment for patients with RIF that lacks initial inflammation (Table 1). Blood samples were typically obtained from patients 3 to 5 days before the scheduled embryo transfer, and PBMCs were isolated and cultured with or without hCG, followed by intrauterine infusion. However, larger study populations and more information on the effectiveness and safety of blood products are still needed.
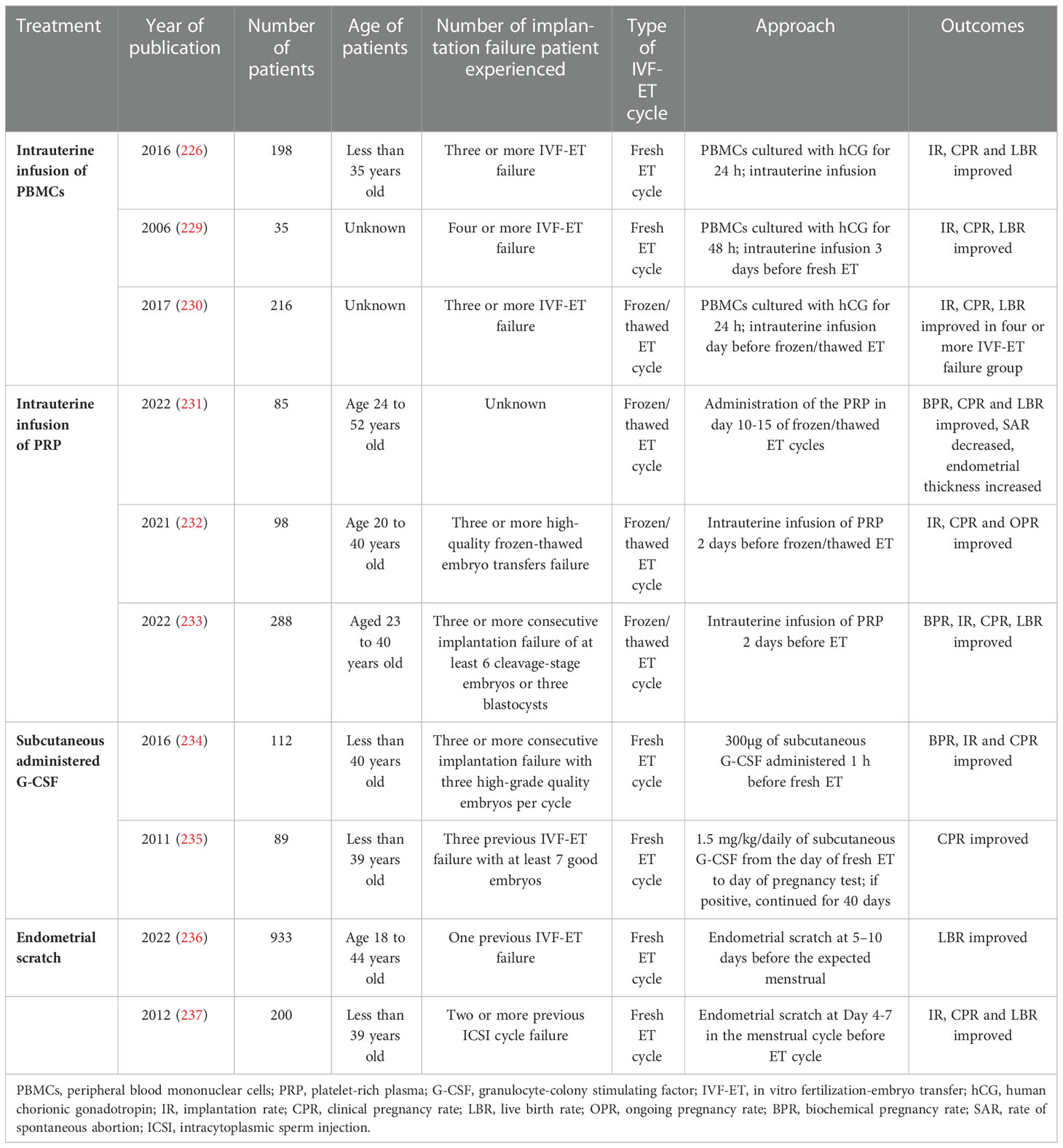
Table 1 Researches of treatment options for improving endometrial receptivity of patients with recurrent implantation failure.
PRP is an autologous blood product containing a high concentration of platelets. The release of platelet-derived growth factors, vascular endothelial growth factors, transforming growth factors, and epidermal growth factors from activated platelets results in angiogenesis, cell proliferation, differentiation, and modification of the local immune response (238–241). It can also promote the expression of tissue remodeling genes and reduce fibrosis in mice with Asherman’s syndrome (242). It has been reported that PRP can improve clinical pregnancy rates and endometrium thickness in patients with RIF (231). The benefit of PRP on implantation rates in patients with RIF has been confirmed by other studies (Table 1) (232, 233, 243). Thus, PRP is a promising treatment option. In addition, more high-quality and large-scale studies are needed to further assess the effects and safety of PRP.
G-CSF is a cytokine produced by endothelial cells, stromal cells, macrophages, and other immune cells (244). It is also produced by decidual cells, which prompted its use as an adjunct treatment (locally or systemically) for patients with a history of RIF or RPL and thin endometrium (85, 245, 246). A meta-analysis showed an increase in clinical rates of intrauterine infusion or subcutaneous injection of G-CSF during both fresh and frozen embryo transfer (247). However, the method of administration and dosage of G-CSF should be carefully selected. G-CSF is typically administered at a dosage ranging from 60 to 300 mg on the day of hCG trigger or embryo transfer. Implantation and pregnancy rates in patients with RIF were not improved by G-CSF intrauterine infusion in two RCTs (248, 249). Furthermore, subcutaneous administration of G-CSF 1 h before fresh embryo transfer resulted in an improvement in clinical pregnancy and implantation rates compared to the control group, which is consistent with the results of other studies (Table 1) (234, 235).
Endometrial scratch before implantation appears to cause decidualization and prepares the endometrium for implantation by increasing cytokines such as LIF and IL-11, which are involved in endometrial receptivity (250), and delaying endometrium maturation caused by COH, which might cause synchronization between the endometrium and embryo (251). Moderate-quality evidence has been demonstrated in previous studies. Endometrial scratch on day 7 of the previous cycle and day 7 of the ET cycle appeared to improve the live birth and pregnancy rates in patients with two previous ET cycles, with no evidence of increasing miscarriage rates or bleeding (251). In patients with one previous IVF cycle failure, higher live birth rates were obtained in the endometrial scratch group, with slightly higher expenditures (236). Endometrial scratches performed during hysteroscopy in the cycle preceding ICSI also improved implantation rates in patients with two or more ICSI cycle failures (237). Therefore, this widely used treatment is safe for improving the IVF-ET outcomes. However, the number and degree of injury and the procedure timing need further investigation.
An ERA is a transcriptomic analysis of gene expression at different stages of the endometrium that detects WOI and can facilitate “personalized” embryo transfer for every patient. Patients with RIF appeared to have a lower receptivity proportion compared to the control group in the ERA test (74.1% vs. 88%). In RIF patients with a “receptive endometrium” diagnosed by ERA, embryo transfer conducted at the receptivity time led to similar clinical pregnancy rates as in general patients undergoing IVF (87). A 5-year multicenter RCT demonstrated that personalized embryo transfer after ERA diagnosis reached higher implantation and live birth rates at the first embryo transfer cycle in infertile patients (252). Thus, ERA is a unique procedure for endometrial evaluation that can improve endometrium-related implantation failure.
Antibiotics can cure infections in most patients with chronic endometritis (253). Chronic endometritis is common in patients with RIF, which can be diagnosed and evaluated by hysteroscopy, and the most frequent infectious agents are bacteria and mycoplasmas (109). Patients with RIF and chronic endometritis received oral antibiotic treatment, and the effect was assessed by hysteroscopy with biopsy. In the cure group, a significant increase in pregnancy and live birth rates was reported compared to the group with continuous chronic endometritis after antibiotic treatment (109). A recent meta-analysis also showed that the implantation and clinical pregnancy rates of patients with RIF with cured chronic endometritis were significantly higher than those of patients with continuous chronic endometritis (254). However, different administration routes have led to different results. Intrauterine antibiotic infusion combined with oral antibiotic administration could not improve clinical pregnancy rates, which may be due to the disturbance of intrauterine infusion in the intrauterine environment (255). In general, chronic endometritis is curable in most patients with RIF, which results in a significant increase in the pregnancy outcomes of IVF-ET performed after treatment.
A few patients with normal hysterosalpingogram results show abnormal hysteroscopy findings (127). Hysteroscopy is a valuable diagnostic and treatment tool that can remove small uterine lesions and restore the shape of the uterine cavity in patients with uterine lesions. Correction of the T-shaped uterus was related to high live birth rates and low miscarriage rates in patients with both primary infertility and recurrent miscarriage (256, 257). Although outpatient hysteroscopy did not improve IVF outcomes in patients with RIF with normal ultrasound of the uterine cavity, which may be due to the high proportion of normal uterine cavity (258). For uterine lesions that affect implantation rates, it is necessary to remove them before the next IVF-ET cycle (122, 126, 259, 260).
Normal sperm has smooth nuclei with normal chromatin content and head shape. Moreover, severe abnormalities in sperm are related to low fertilization, implantation, and pregnancy rates (261). Intracytoplasmic morphologically selected sperm injection (IMSI) is a non-invasive method that examines sperm under 6000× magnification before injection to obtain optimal sperm. The IMSI procedure before ICSI appears to be beneficial for implantation and clinical rates in patients with repeated IVF-ICSI failure (262). However, other studies did not draw the same conclusions (263). Thus, no specific microscopic criterion exists for evaluating sperm morphology, and more studies are needed to assess the effect of IMSI on IVF-ET outcomes.
Aneuploidy accounts for implantation failure and early pregnancy loss, as high as 76% in first-trimester spontaneous abortions (155). PGT-A is a technology that can analyze the chromosomes of embryos in IVF-ET and select euploid embryos for subsequent transfer. Single euploid embryos selected by array comparative genomic hybridization were transferred to patients with RIF, which resulted in implantation rates similar to those in the group without RIF (264). Another retrospective cohort study showed the benefit of using PGT-A in patients with RIF and recurrent miscarriage, leading to a significant increase in implantation rates (265). The cumulative implantation rate of patients with RIF who underwent euploid embryo transfer was 95.2%, which means that most RIFs are due to chromosome aneuploidy and can be improved by transferring euploid embryos (266). Therefore, PGT-A appears to be a considerable treatment option for patients with RIF (12). Furthermore, PGT-A should be administered after a careful assessment of the circumstances of each patient. And the influence of mosaicism must be considered.
RIF remains a complex, growing problem that affects several patients. There are various etiologies, mechanisms, and treatment options (Table 2). Identifying the causes of RIF and providing individualized treatment can improve the implantation rate. However, the treatment of RIF remains challenging, and further research on treatment options is needed to assess the potential of each treatment and establish a standard protocol for each patient.
JM, DL, and WG performed the literature search and data extraction and played a major role in writing the manuscript. DL critically revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by National Natural Science Foundation of China (No. 82071607).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Enders AC, Nelson DM. Pinocytotic activity of the uterus of the rat. Am J Anat (1973) 138:277–99. doi: 10.1002/aja.1001380302
2. Luke B, Brown MB, Wantman E, Lederman A, Gibbons W, Schattman GL, et al. Cumulative birth rates with linked assisted reproductive technology cycles. N Engl J Med (2012) 366:2483–91. doi: 10.1056/NEJMoa1110238
3. Croucher CA, Lass A, Margara R, Winston RM. Predictive value of the results of a first in-vitro fertilization cycle on the outcome of subsequent cycles. Hum Reprod (1998) 13:403–8. doi: 10.1093/humrep/13.2.403
4. Osmanagaoglu K, Tournaye H, Camus M, Vandervorst M, Van Steirteghem A, Devroey P. Cumulative delivery rates after intracytoplasmic sperm injection: 5 year follow-up of 498 patients. Hum Reprod (1999) 14:2651–5. doi: 10.1093/humrep/14.10.2651
5. Sharma V, Allgar V, Rajkhowa M. Factors influencing the cumulative conception rate and discontinuation of in vitro fertilization treatment for infertility. Fertil Steril (2002) 78:40–6. doi: 10.1016/S0015-0282(02)03160-6
6. Sun Y, Zhang Y, Ma X, Jia W, Su Y. Determining diagnostic criteria of unexplained recurrent implantation failure: A retrospective study of two vs three or more implantation failure. Front Endocrinol (Lausanne) (2021) 12:619437. doi: 10.3389/fendo.2021.619437
7. Cimadomo D, Craciunas L, Vermeulen N, Vomstein K, Toth B. Definition, diagnostic and therapeutic options in recurrent implantation failure: an international survey of clinicians and embryologists. Hum Reprod (2021) 36:305–17. doi: 10.1093/humrep/deaa317
8. Medicine ASIR, Embryology ESIG. Istanbul Consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod BioMed Online (2011) 22:632–46. doi: 10.1016/j.rbmo.2011.02.001
9. Immediata V, Patrizio P, Parisen Toldin MR, Morenghi E, Ronchetti C, Cirillo F, et al. Twenty-one year experience with intrauterine inseminations after controlled ovarian stimulation with gonadotropins: maternal age is the only prognostic factor for success. J Assist Reprod Genet (2020) 37:1195–201. doi: 10.1007/s10815-020-01752-3
10. Ata B, Kalafat E, Somigliana E. A new definition of recurrent implantation failure on the basis of anticipated blastocyst aneuploidy rates across female age. Fertil Steril (2021) 116:1320–7. doi: 10.1016/j.fertnstert.2021.06.045
11. Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reprod BioMed Online (2014) 28:14–38. doi: 10.1016/j.rbmo.2013.08.011
12. Thornhill AR, deDie-Smulders CE, Geraedts JP, Harper JC, Harton GL, Lavery SA, et al. Consortium, ESHRE PGD consortium 'Best practice guidelines for clinical preimplantation genetic diagnosis (PGD) and preimplantation genetic screening (PGS)'. Hum Reprod (2005) 20:35–48. doi: 10.1093/humrep/deh579
13. Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril (2004) 81:551–5. doi: 10.1016/j.fertnstert.2003.07.023
14. Aly H, Hammad T, Nada A, Mohamed M, Bathgate S, El-Mohandes A. Maternal obesity, associated complications and risk of prematurity. J Perinatol (2010) 30:447–51. doi: 10.1038/jp.2009.117
15. Bhattacharya S, Campbell DM, Liston WA, Bhattacharya S. Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health (2007) 7:168. doi: 10.1186/1471-2458-7-168
16. Orvieto R, Meltcer S, Nahum R, Rabinson J, Anteby EY, Ashkenazi J. The influence of body mass index on in vitro fertilization outcome. Int J Gynaecol Obstet (2009) 104:53–5. doi: 10.1016/j.ijgo.2008.08.012
17. Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril (2012) 98:102–8. doi: 10.1016/j.fertnstert.2012.04.004
18. Schulte MM, Tsai JH, Moley KH. Obesity and PCOS: the effect of metabolic derangements on endometrial receptivity at the time of implantation. Reprod Sci (2015) 22:6–14. doi: 10.1177/1933719114561552
19. Klonoff-Cohen H, Natarajan L, Marrs R, Yee B. Effects of female and male smoking on success rates of IVF and gamete intra-fallopian transfer. Hum Reprod (2001) 16:1382–90. doi: 10.1093/humrep/16.7.1382
20. Potts RJ, Newbury CJ, Smith G, Notarianni LJ, Jefferies TM. Sperm chromatin damage associated with male smoking. Mutat Res (1999) 423:103–11. doi: 10.1016/S0027-5107(98)00242-5
21. O. American College of, Gynecologists, w. Society for Maternal-Fetal Medicine in collaboration, Metz TD, Berry RS, Fretts RC, Reddy UM, Turrentine MA. Obstetric care consensus #10: Management of stillbirth: (Replaces practice bulletin number 102, march 2009). Am J Obstet Gynecol 222:B2–B20:(2020). doi: 10.1016/j.ajog.2020.01.017
22. Mukherjee RA, Hollins S, Abou-Saleh MT, Turk J. Low level alcohol consumption and the fetus. BMJ (2005) 330:375–6. doi: 10.1136/bmj.330.7488.375
23. National Institute for Healthand Care Excellence. guidance, fertility problems: assessment and treatment. London, National Institute for Health and Care Excellence (2017).
24. Nepomnaschy PA, Welch KB, McConnell DS, Low BS, Strassmann BI, England BG. Cortisol levels and very early pregnancy loss in humans. Proc Natl Acad Sci U.S.A. (2006) 103:3938–42. doi: 10.1073/pnas.0511183103
25. Pasch LA, Gregorich SE, Katz PK, Millstein SG, Nachtigall RD, Bleil ME, et al. Psychological distress and in vitro fertilization outcome. Fertil Steril (2012) 98:459–64. doi: 10.1016/j.fertnstert.2012.05.023
26. Li D, Zheng L, Zhao D, Xu Y, Wang Y. The role of immune cells in recurrent spontaneous abortion. Reprod Sci (2021) 28:3303–15. doi: 10.1007/s43032-021-00599-y
27. Mori M, Bogdan A, Balassa T, Csabai T, Szekeres-Bartho J. The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Semin Immunopathol (2016) 38:635–49. doi: 10.1007/s00281-016-0574-0
28. Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. innate lymphoid cells: a new paradigm in immunology. Science (2015) 348:aaa6566.
29. Kalkunte S, Chichester CO, Gotsch F, Sentman CL, Romero R, Sharma S. Evolution of non-cytotoxic uterine natural killer cells. Am J Reprod Immunol (2008) 59:425–32. doi: 10.1111/j.1600-0897.2008.00595.x
30. Wang F, Qualls AE, Marques-Fernandez L, Colucci F. Biology and pathology of the uterine microenvironment and its natural killer cells. Cell Mol Immunol (2021) 18:2101–13. doi: 10.1038/s41423-021-00739-z
31. King A, Burrows T, Verma S, Hiby S, Loke YW. Human uterine lymphocytes. Hum Reprod Update (1998) 4:480–5. doi: 10.1093/humupd/4.5.480
32. Sharkey AM, Xiong S, Kennedy PR, Gardner L, Farrell LE, Chazara O, et al. Tissue-specific education of decidual NK cells. J Immunol (2015) 195:3026–32. doi: 10.4049/jimmunol.1501229
33. Gamliel M, Goldman-Wohl D, Isaacson B, Gur C, Stein N, Yamin R, et al. Trained memory of human uterine NK cells enhances their function in subsequent pregnancies. Immunity (2018) 48:951–962.e5. doi: 10.1016/j.immuni.2018.03.030
34. Biassoni R, Cantoni C, Falco M, Verdiani S, Bottino C, Vitale M, et al. The human leukocyte antigen (HLA)-c-specific "activatory" or "inhibitory" natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions. J Exp Med (1996) 183:645–50. doi: 10.1084/jem.183.2.645
35. Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest (2010) 120:4102–10. doi: 10.1172/JCI43998
36. Moffett A, Colucci F, Botting RA. Co-evolution of NK receptors and HLA ligands in humans is driven by reproduction. Immunol Rev (2015) 267(1):283–97. doi: 10.1111/imr.12323
37. Wurfel W, Santjohanser C, Hirv K, Buhl M, Meri O, Laubert I, et al. High pregnancy rates with administration of granulocyte colony-stimulating factor in ART-patients with repetitive implantation failure and lacking killer-cell immunglobulin-like receptors. Hum Reprod (2010) 25:2151–2. doi: 10.1093/humrep/deq106
38. Piekarska K, Radwan P, Tarnowska A, Wisniewski A, Radwan M, Wilczynski JR, et al. ERAP, KIR, and HLA-c profile in recurrent implantation failure. Front Immunol (2021) 12:755624. doi: 10.3389/fimmu.2021.755624
39. Chazara O, Xiong S, Moffett A, Maternal KIR. Fetal HLA-c: a fine balance. J Leukoc Biol (2011) 90:703–16. doi: 10.1189/jlb.0511227
40. Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF, et al. Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol (2006) 80:572–80. doi: 10.1189/jlb.0406250
41. Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens (2004) 63:1–12. doi: 10.1111/j.1399-0039.2004.00170.x
42. Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta (2006) 27:939–58. doi: 10.1016/j.placenta.2005.12.006
43. Tuckerman E, Mariee N, Prakash A, Li TC, Laird S. Uterine natural killer cells in peri-implantation endometrium from women with repeated implantation failure after IVF. J Reprod Immunol (2010) 87:60–6. doi: 10.1016/j.jri.2010.07.001
44. Woon EV, Greer O, Shah N, Nikolaou D, Johnson M, Male V. Number and function of uterine natural killer cells in recurrent miscarriage and implantation failure: a systematic review and meta-analysis. Hum Reprod Update (2022) 28:548–82. doi: 10.1093/humupd/dmac006
45. Junovich G, Azpiroz A, Incera E, Ferrer C, Pasqualini A, Gutierrez G. Endometrial CD16(+) and CD16(-) NK cell count in fertility and unexplained infertility. Am J Reprod Immunol (2013) 70:182–9. doi: 10.1111/aji.12132
46. Chen X, Man GCW, Liu Y, Wu F, Huang J, Li TC, et al. Physiological and pathological angiogenesis in endometrium at the time of embryo implantation. Am J Reprod Immunol (2017) 78. doi: 10.1111/aji.12693
47. Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol (2011) 8:1–11. doi: 10.1038/cmi.2010.38
48. Ni J, Wang X, Stojanovic A, Zhang Q, Wincher M, Buhler L, et al. Single-cell RNA sequencing of tumor-infiltrating NK cells reveals that inhibition of transcription factor HIF-1alpha unleashes NK cell activity. Immunity (2020) 52:1075–1087.e8. doi: 10.1016/j.immuni.2020.05.001
49. Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum Reprod Update (2010) 16:415–31. doi: 10.1093/humupd/dmp046
50. Yu X, Gao C, Dai C, Yang F, Deng X. Endometrial injury increases expression of hypoxia-inducible factor and angiogenesis in the endometrium of women with recurrent implantation failure. Reprod BioMed Online (2019) 38:761–7. doi: 10.1016/j.rbmo.2018.12.027
51. Ledee-Bataille N, Dubanchet S, Coulomb-L'hermine A, Durand-Gasselin I, Frydman R, Chaouat G. A new role for natural killer cells, interleukin (IL)-12, and IL-18 in repeated implantation failure after in vitro fertilization. Fertil Steril (2004) 81:59–65. doi: 10.1016/j.fertnstert.2003.06.007
52. Ledee N, Chaouat G, Serazin V, Lombroso R, Dubanchet S, Oger P, et al. Endometrial vascularity by three-dimensional power Doppler ultrasound and cytokines: a complementary approach to assess uterine receptivity. J Reprod Immunol (2008) 77:57–62. doi: 10.1016/j.jri.2007.07.006
53. Ledee-Bataille N, Bonnet-Chea K, Hosny G, Dubanchet S, Frydman R, Chaouat G. Role of the endometrial tripod interleukin-18, -15, and -12 in inadequate uterine receptivity in patients with a history of repeated in vitro fertilization-embryo transfer failure. Fertil Steril (2005) 83:598–605. doi: 10.1016/j.fertnstert.2004.11.021
54. Donoghue JF, Paiva P, Teh WT, Cann LM, Nowell C, Rees H, et al. Endometrial uNK cell counts do not predict successful implantation in an IVF population. Hum Reprod (2019) 34:2456–66. doi: 10.1093/humrep/dez194
55. Tilburgs T, Claas FH, Scherjon SA. Elsevier trophoblast research award lecture: Unique properties of decidual T cells and their role in immune regulation during human pregnancy. Placenta (2010) 31 Suppl:S82–6. doi: 10.1016/j.placenta.2010.01.007
56. Lin Y, Zhang D, Li Y, Li Y, Li B, Du M. Decidual NR2F2-expressing CD4(+) T cells promote TH2 transcriptional program during early pregnancy. Front Immunol (2021) 12:670777. doi: 10.3389/fimmu.2021.670777
57. Kwak-Kim JY, Chung-Bang HS, Ng SC, Ntrivalas EI, Mangubat CP, Beaman KD, et al. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum Reprod (2003) 18:767–73. doi: 10.1093/humrep/deg156
58. Liang PY, Diao LH, Huang CY, Lian RC, Chen X, Li GG, et al. The pro-inflammatory and anti-inflammatory cytokine profile in peripheral blood of women with recurrent implantation failure. Reprod BioMed Online (2015) 31:823–6. doi: 10.1016/j.rbmo.2015.08.009
59. Nakashima A, Ito M, Yoneda S, Shiozaki A, Hidaka T, Saito S. Circulating and decidual Th17 cell levels in healthy pregnancy. Am J Reprod Immunol (2010) 63:104–9. doi: 10.1111/j.1600-0897.2009.00771.x
60. Wang WJ, Hao CF, Yi L, Yin GJ, Bao SH, Qiu LH, et al. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol (2010) 84:164–70. doi: 10.1016/j.jri.2009.12.003
61. Lee SK, Kim JY, Hur SE, Kim CJ, Na BJ, Lee M, et al. An imbalance in interleukin-17-producing T and Foxp3(+) regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum Reprod (2011) 26:2964–71. doi: 10.1093/humrep/der301
62. Ghaebi M, Abdolmohammadi-Vahid S, Ahmadi M, Eghbal-Fard S, Dolati S, Nouri M, et al. T Cell subsets in peripheral blood of women with recurrent implantation failure. J Reprod Immunol (2019) 131:21–9. doi: 10.1016/j.jri.2018.11.002
63. Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, et al. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol (2010) 85:121–9. doi: 10.1016/j.jri.2010.02.006
64. Yang H, Qiu L, Chen G, Ye Z, Lu C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril (2008) 89:656–61. doi: 10.1016/j.fertnstert.2007.03.037
65. Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, et al. PD-L1 negatively regulates CD4+CD25+Foxp3+ tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest (2009) 119:551–64. doi: 10.1172/JCI36604
66. Ahmadi M, Abdolmohammadi-Vahid S, Ghaebi M, Aghebati-Maleki L, Dolati S, Farzadi L, et al. Regulatory T cells improve pregnancy rate in RIF patients after additional IVIG treatment. Syst Biol Reprod Med (2017) 63:350–9. doi: 10.1080/19396368.2017.1390007
67. Qian ZD, Huang LL, Zhu XM. An immunohistochemical study of CD83- and CD1a-positive dendritic cells in the decidua of women with recurrent spontaneous abortion. Eur J Med Res (2015) 20:2. doi: 10.1186/s40001-014-0076-2
68. Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest (2008) 118:3954–65. doi: 10.1172/JCI36682
69. Liu S, Wei H, Li Y, Huang C, Lian R, Xu J, et al. Downregulation of ILT4(+) dendritic cells in recurrent miscarriage and recurrent implantation failure. Am J Reprod Immunol (2018) 80:e12998. doi: 10.1111/aji.12998
70. Sheng YR, Hu WT, Wei CY, Tang LL, Liu YK, Liu YY, et al. Insights of efferocytosis in normal and pathological pregnancy. Am J Reprod Immunol (2019) 82:e13088. doi: 10.1111/aji.13088
71. Ticconi C, Pietropolli A, Di Simone N, Piccione E, Fazleabas A. Endometrial immune dysfunction in recurrent pregnancy loss. Int J Mol Sci 20 (2019) 20:5332. doi: 10.3390/ijms20215332
72. Li Y, Yu S, Huang C, Lian R, Chen C, Liu S, et al. Evaluation of peripheral and uterine immune status of chronic endometritis in patients with recurrent reproductive failure. Fertil Steril (2020) 113:187–196 e1. doi: 10.1016/j.fertnstert.2019.09.001
73. Tremellen KP, Russell P. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. II: adenomyosis macrophages. J Reprod Immunol (2012) 93:58–63. doi: 10.1016/j.jri.2011.12.001
74. Khan S, Dickerman JD. Hereditary thrombophilia. Thromb J (2006) 4:15. doi: 10.1186/1477-9560-4-15
75. Simon A, Laufer N. Assessment and treatment of repeated implantation failure (RIF). J Assist Reprod Genet (2012) 29:1227–39. doi: 10.1007/s10815-012-9861-4
76. Stevens SM, Woller SC, Bauer KA, Kasthuri R, Cushman M, Streiff M, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis (2016) 41:154–64. doi: 10.1007/s11239-015-1316-1
77. Azem F, Many A, Ben Ami I, Yovel I, Amit A, Lessing JB, et al. Increased rates of thrombophilia in women with repeated IVF failures. Hum Reprod (2004) 19:368–70. doi: 10.1093/humrep/deh069
78. Qublan HS, Eid SS, Ababneh HA, Amarin ZO, Smadi AZ, Al-Khafaji FF, et al. Acquired and inherited thrombophilia: Implication in recurrent IVF and embryo transfer failure. Hum Reprod (2006) 21:2694–8. doi: 10.1093/humrep/del203
79. Martinelli I, Taioli E, Ragni G, Levi-Setti P, Passamonti SM, Battaglioli T, et al. Embryo implantation after assisted reproductive procedures and maternal thrombophilia. Haematologica (2003) 88:789–93.
80. Nelen WL, Blom HJ, Steegers EA, den Heijer M, Eskes TK. Hyperhomocysteinemia and recurrent early pregnancy loss: a meta-analysis. Fertil Steril (2000) 74:1196–9. doi: 10.1016/S0015-0282(00)01595-8
81. Bramham K, Hunt BJ, Germain S, Calatayud I, Khamashta M, Bewley S, et al. Pregnancy outcome in different clinical phenotypes of antiphospholipid syndrome. Lupus (2010) 19:58–64. doi: 10.1177/0961203309347794
82. Mahdian S, Pirjani R, Favaedi R, Movahedi M, Moini A, Shahhoseini M. Platelet-activating factor and antiphospholipid antibodies in recurrent implantation failure. J Reprod Immunol (2021) 143:103251. doi: 10.1016/j.jri.2020.103251
83. Jarne-Borras M, Miro-Mur F, Anunciacion-Llunell A, Alijotas-Reig J. Antiphospholipid antibodies in women with recurrent embryo implantation failure: A systematic review and meta-analysis. Autoimmun Rev (2022) 21:103101. doi: 10.1016/j.autrev.2022.103101
84. Steinvil A, Raz R, Berliner S, Steinberg DM, Zeltser D, Levran D, et al. Association of common thrombophilias and antiphospholipid antibodies with success rate of in vitro fertilisation. Thromb Haemost (2012) 108:1192–7. doi: 10.1160/TH12-06-0381
85. Shaulov T, Sierra S, Sylvestre C. Recurrent implantation failure in IVF: A Canadian fertility and andrology society clinical practice guideline. Reprod BioMed Online (2020) 41:819–33. doi: 10.1016/j.rbmo.2020.08.007
86. Craciunas L, Gallos I, Chu J, Bourne T, Quenby S, Brosens JJ, et al. Conventional and modern markers of endometrial receptivity: a systematic review and meta-analysis. Hum Reprod Update (2019) 25:202–23. doi: 10.1093/humupd/dmy044
87. Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Gomez E, Fernandez-Sanchez M, Carranza F, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril (2013) 100:818–24. doi: 10.1016/j.fertnstert.2013.05.004
88. Koler M, Achache H, Tsafrir A, Smith Y, Revel A, Reich R. Disrupted gene pattern in patients with repeated in vitro fertilization (IVF) failure. Hum Reprod (2009) 24:2541–8. doi: 10.1093/humrep/dep193
89. Bersinger NA, Wunder DM, Birkhauser MH, Mueller MD. Gene expression in cultured endometrium from women with different outcomes following IVF. Mol Hum Reprod (2008) 14:475–84. doi: 10.1093/molehr/gan036
90. Feng X, Meng X, Guo S, Li K, Wang L, Ai J. Identification of key genes and immune cell infiltration in recurrent implantation failure: A study based on integrated analysis of multiple microarray studies. Am J Reprod Immunol (2022) 88:e13607. doi: 10.1111/aji.13607
91. Tapia A, Gangi LM, Zegers-Hochschild F, Balmaceda J, Pommer R, Trejo L, et al. Differences in the endometrial transcript profile during the receptive period between women who were refractory to implantation and those who achieved pregnancy. Hum Reprod (2008) 23:340–51. doi: 10.1093/humrep/dem319
92. Achache H, Tsafrir A, Prus D, Reich R, Revel A. Defective endometrial prostaglandin synthesis identified in patients with repeated implantation failure undergoing in vitro fertilization. Fertil Steril (2010) 94:1271–8. doi: 10.1016/j.fertnstert.2009.07.1668
93. Maranduba CM, De Castro SB, de Souza GT, Rossato C, da Guia FC, Valente MA, et al. Intestinal microbiota as modulators of the immune system and neuroimmune system: Impact on the host health and homeostasis. J Immunol Res (2015) 2015:931574. doi: 10.1155/2015/931574
94. Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity (2017) 46:562–76. doi: 10.1016/j.immuni.2017.04.008
95. Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun (2017) 8:875. doi: 10.1038/s41467-017-00901-0
96. Ma B, Forney LJ, Ravel J. Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol (2012) 66:371–89. doi: 10.1146/annurev-micro-092611-150157
97. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med (2000) 342:1500–7. doi: 10.1056/NEJM200005183422007
98. Hyman RW, Fukushima M, Jiang H, Fung E, Rand L, Johnson B, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci (2014) 21:32–40. doi: 10.1177/1933719113488838
99. Fu M, Zhang X, Liang Y, Lin S, Qian W, Fan S. Alterations in vaginal microbiota and associated metabolome in women with recurrent implantation failure. mBio 11 (2020) 11:e03242–19. doi: 10.1128/mBio.03242-19
100. Schoenmakers S, Laven J. The vaginal microbiome as a tool to predict IVF success. Curr Opin Obstet Gynecol (2020) 32:169–78. doi: 10.1097/GCO.0000000000000626
101. Wee BA, Thomas M, Sweeney EL, Frentiu FD, Samios M, Ravel J, et al. A retrospective pilot study to determine whether the reproductive tract microbiota differs between women with a history of infertility and fertile women. Aust N Z J Obstet Gynaecol (2018) 58:341–8. doi: 10.1111/ajo.12754
102. Ichiyama T, Kuroda K, Nagai Y, Urushiyama D, Ohno M, Yamaguchi T, et al. Analysis of vaginal and endometrial microbiota communities in infertile women with a history of repeated implantation failure. Reprod Med Biol (2021) 20:334–44. doi: 10.1002/rmb2.12389
103. Mitchell CM, Haick A, Nkwopara E, Garcia R, Rendi M, Agnew K, et al. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol (2015) 212:611.e1–9. doi: 10.1016/j.ajog.2014.11.043
104. Moreno I, Codoner FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazan J, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol (2016) 215:684–703. doi: 10.1016/j.ajog.2016.09.075
105. Kushnir VA, Solouki S, Sarig-Meth T, Vega MG, Albertini DF, Darmon SK, et al. Systemic inflammation and autoimmunity in women with chronic endometritis. Am J Reprod Immunol (2016) 75:672–7. doi: 10.1111/aji.12508
106. Johnston-MacAnanny EB, Hartnett J, Engmann LL, Nulsen JC, Sanders MM, Benadiva CA. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril (2010) 93:437–41. doi: 10.1016/j.fertnstert.2008.12.131
107. Kitaya K, Tada Y, Hayashi T, Taguchi S, Funabiki M, Nakamura Y. Comprehensive endometrial immunoglobulin subclass analysis in infertile women suffering from repeated implantation failure with or without chronic endometritis. Am J Reprod Immunol (2014) 72:386–91. doi: 10.1111/aji.12277
108. Liu Y, Chen X, Huang J, Wang CC, Yu MY, Laird S, et al. Comparison of the prevalence of chronic endometritis as determined by means of different diagnostic methods in women with and without reproductive failure. Fertil Steril (2018) 109:832–9. doi: 10.1016/j.fertnstert.2018.01.022
109. Cicinelli E, Matteo M, Tinelli R, Lepera A, Alfonso R, Indraccolo U, et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod (2015) 30:323–30. doi: 10.1093/humrep/deu292
110. Kasius JC, Broekmans FJ, Sie-Go DM, Bourgain C, Eijkemans MJ, Fauser BC, et al. The reliability of the histological diagnosis of endometritis in asymptomatic IVF cases: a multicenter observer study. Hum Reprod (2012) 27:153–8. doi: 10.1093/humrep/der341
111. Chen P, Chen P, Guo Y, Fang C, Li T. Interaction between chronic endometritis caused endometrial microbiota disorder and endometrial immune environment change in recurrent implantation failure. Front Immunol (2021) 12:748447. doi: 10.3389/fimmu.2021.748447
112. Yeo E, Brubaker PL, Sloboda DM. The intestine and the microbiota in maternal glucose homeostasis during pregnancy. J Endocrinol (2022) 253:R1–R19. doi: 10.1530/JOE-21-0354
113. Alexander KL, Targan SR, Elson CO. 3rd, microbiota activation and regulation of innate and adaptive immunity. Immunol Rev (2014) 260:206–20. doi: 10.1111/imr.12180
114. Vinchi F. Thrombosis prevention: Let's drug the microbiome! Hemasphere (2019) 3:e165. doi: 10.1097/HS9.0000000000000165
115. Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas (2017) 103:45–53. doi: 10.1016/j.maturitas.2017.06.025
116. Patel N, Patel N, Pal S, Nathani N, Pandit R, Patel M, et al. Distinct gut and vaginal microbiota profile in women with recurrent implantation failure and unexplained infertility. BMC Womens Health (2022) 22:113. doi: 10.1186/s12905-022-01681-6
117. Gao M, Sun Y, Xie H, Fang S, Zhao X. Hysteroscopy prior to repeat embryo transfer may improve pregnancy outcomes for asymptomatic women with repeated implantation failure. J Obstet Gynaecol Res (2015) 41:1569–76. doi: 10.1111/jog.12773
118. Hosseini MA, Ebrahimi N, Mahdavi A, Aleyasin A, Safdarian L, Fallahi P, et al. Hysteroscopy in patients with repeated implantation failure improves the outcome of assisted reproductive technology in fresh and frozen cycles. J Obstet Gynaecol Res (2014) 40:1324–30. doi: 10.1111/jog.12315
119. Lambert M, Hocke C, Jimenez C, Frantz S, Papaxanthos A, Creux H. Repeated in vitro fertilization failure: Abnormalities identified in the diagnostic assessment. Gynecol Obstet Fertil (2016) 44:565–71. doi: 10.1016/j.gyobfe.2016.08.006
120. Pabuccu EG, Yalcin I, Bodur T, Caglar GS, Pabuccu R. Impact of office hysteroscopy in repeated implantation failure: Experience of a single center. J Turk Ger Gynecol Assoc (2016) 17:197–200. doi: 10.5152/jtgga.2016.16166
121. Franasiak JM, Alecsandru D, Forman EJ, Gemmell LC, Goldberg JM, Llarena N, et al. A review of the pathophysiology of recurrent implantation failure. Fertil Steril (2021) 116:1436–48. doi: 10.1016/j.fertnstert.2021.09.014
122. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril (2009) 91:1215–23. doi: 10.1016/j.fertnstert.2008.01.051
123. Richlin SS, Ramachandran S, Shanti A, Murphy AA. Parthasarathy, glycodelin levels in uterine flushings and in plasma of patients with leiomyomas and polyps: implications for implantation. Hum Reprod (2002) 17:2742–7. doi: 10.1093/humrep/17.10.2742
124. Elbehery MM, Nouh AA, Mohamed ML, Alanwar AA, Abd-Allah SH, Shalaby SM. Insulin-like growth factor binding protein-1 and glycodelin levels in uterine flushing before and after hysteroscopic polypectomy. Clin Lab (2011) 57:953–7.
125. Ben-Nagi J, Miell J, Yazbek J, Holland T, Jurkovic D. The effect of hysteroscopic polypectomy on the concentrations of endometrial implantation factors in uterine flushings. Reprod BioMed Online (2009) 19:737–44. doi: 10.1016/j.rbmo.2009.06.011
126. Bosteels J, Weyers S, Puttemans P, Panayotidis C, Van Herendael B, Gomel V, et al. The effectiveness of hysteroscopy in improving pregnancy rates in subfertile women without other gynaecological symptoms: A systematic review. Hum Reprod Update (2010) 16:1–11. doi: 10.1093/humupd/dmp033
127. Demirol A, Gurgan T. Effect of treatment of intrauterine pathologies with office hysteroscopy in patients with recurrent IVF failure. Reprod BioMed Online (2004) 8:590–4. doi: 10.1016/S1472-6483(10)61108-X
128. Chan YY, Jayaprakasan K, Tan A, Thornton JG, Coomarasamy A, Raine-Fenning NJ. Reproductive outcomes in women with congenital uterine anomalies: A systematic review. Ultrasound Obstet Gynecol (2011) 38:371–82. doi: 10.1002/uog.10056
129. Dicker D, Ashkenazi J, Dekel A, Orvieto R, Feldberg D, Yeshaya A, et al. The value of hysteroscopic evaluation in patients with preclinical in-vitro fertilization abortions. Hum Reprod (1996) 11:730–1. doi: 10.1093/oxfordjournals.humrep.a019243
130. Grimbizis GF, Camus M, Tarlatzis BC, Bontis JN, Devroey P. Clinical implications of uterine malformations and hysteroscopic treatment results. Hum Reprod Update (2001) 7:161–74. doi: 10.1093/humupd/7.2.161
131. Lin YH, Chen YH, Chang HY, Au HK, Tzeng CR, Huang YH. Chronic niche inflammation in endometriosis-associated infertility: Current understanding and future therapeutic strategies. Int J Mol Sci 19 (2018) 19:2385. doi: 10.3390/ijms19082385
132. Miller JE, Ahn SH, Monsanto SP, Khalaj K, Koti M, Tayade C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget (2017) 8:7138–47. doi: 10.18632/oncotarget.12577
133. Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril (2002) 77:1148–55. doi: 10.1016/S0015-0282(02)03112-6
134. Sanchez AM, Vanni VS, Bartiromo L, Papaleo E, Zilberberg E, Candiani M, et al. Is the oocyte quality affected by endometriosis? A Rev literature. J Ovarian Res (2017) 10:43. doi: 10.1186/s13048-017-0341-4
135. Vercellini P, Consonni D, Barbara G, Buggio L, Frattaruolo MP, Somigliana E. Adenomyosis and reproductive performance after surgery for rectovaginal and colorectal endometriosis: a systematic review and meta-analysis. Reprod BioMed Online (2014) 28:704–13. doi: 10.1016/j.rbmo.2014.02.006
136. Kodaman PH, Arici A, Seli E. Evidence-based diagnosis and management of tubal factor infertility. Curr Opin Obstet Gynecol (2004) 16:221–9. doi: 10.1097/00001703-200406000-00004
137. Practice Committee of American Society for Reproductive Medicine in collaboration with Society of Reproductive Surgeons. Salpingectomy for hydrosalpinx prior to in vitro fertilization. Fertil Steril (2008) 90(5 Suppl):S66–8. doi: 10.1016/j.fertnstert.2008.08.089
138. Meyer WR, Castelbaum AJ, Somkuti S, Sagoskin AW, Doyle M, Harris JE, et al. Hydrosalpinges adversely affect markers of endometrial receptivity. Hum Reprod (1997) 12:1393–8. doi: 10.1093/humrep/12.7.1393
139. Daftary GS, Kayisli U, Seli E, Bukulmez O, Arici A, Taylor HS. Salpingectomy increases peri-implantation endometrial HOXA10 expression in women with hydrosalpinx. Fertil Steril (2007) 87:367–72. doi: 10.1016/j.fertnstert.2006.06.041
140. Seli E, Kayisli UA, Cakmak H, Bukulmez O, Bildirici I, Guzeloglu-Kayisli O, et al. Removal of hydrosalpinges increases endometrial leukaemia inhibitory factor (LIF) expression at the time of the implantation window. Hum Reprod (2005) 20:3012–7. doi: 10.1093/humrep/dei188
141. Strandell A, Waldenstrom U, Nilsson L, Hamberger L. Hydrosalpinx reduces in-vitro fertilization/embryo transfer pregnancy rates. Hum Reprod (1994) 9:861–3. doi: 10.1093/oxfordjournals.humrep.a138606
142. Camus E, Poncelet C, Goffinet F, Wainer B, Merlet F, Nisand I, et al. Pregnancy rates after in-vitro fertilization in cases of tubal infertility with and without hydrosalpinx: a meta-analysis of published comparative studies. Hum Reprod (1999) 14:1243–9. doi: 10.1093/humrep/14.5.1243
143. Strandell A, Lindhard A, Waldenstrom U, Thorburn J, Janson PO, Hamberger L. Hydrosalpinx and IVF outcome: a prospective, randomized multicentre trial in Scandinavia on salpingectomy prior to IVF. Hum Reprod (1999) 14:2762–9. doi: 10.1093/humrep/14.11.2762
144. Caseiro AL, Regalo A, Pereira E, Esteves T, Fernandes F, Carvalho J. Implication of sperm chromosomal abnormalities in recurrent abortion and multiple implantation failure. Reprod BioMed Online (2015) 31:481–5. doi: 10.1016/j.rbmo.2015.07.001
145. Coughlan C, Clarke H, Cutting R, Saxton J, Waite S, Ledger W, et al. Sperm DNA fragmentation, recurrent implantation failure and recurrent miscarriage. Asian J Androl (2015) 17:681–5. doi: 10.4103/1008-682X.144946
146. Bronet F, Martinez E, Gaytan M, Linan A, Cernuda D, Ariza M, et al. Sperm DNA fragmentation index does not correlate with the sperm or embryo aneuploidy rate in recurrent miscarriage or implantation failure patients. Hum Reprod (2012) 27:1922–9. doi: 10.1093/humrep/des148
147. Practice Committee of the American Society for Reproductive Medicine Practice committee of the American society for reproductive, evaluation and treatment of recurrent pregnancy loss: A committee opinion. Fertil Steril (2012) 98:1103–11. doi: 10.1016/j.fertnstert.2012.06.048
148. Rubio C, Gil-Salom M, Simon C, Vidal F, Rodrigo L, Minguez Y, et al. Incidence of sperm chromosomal abnormalities in a risk population: relationship with sperm quality and ICSI outcome. Hum Reprod (2001) 16:2084–92. doi: 10.1093/humrep/16.10.2084
149. Steger K, Wilhelm J, Konrad L, Stalf T, Greb R, Diemer T, et al. Both protamine-1 to protamine-2 mRNA ratio and Bcl2 mRNA content in testicular spermatids and ejaculated spermatozoa discriminate between fertile and infertile men. Hum Reprod (2008) 23:11–6. doi: 10.1093/humrep/dem363
150. Rogenhofer N, Dansranjavin T, Schorsch M, Spiess A, Wang H, von Schonfeldt V, et al. The sperm protamine mRNA ratio as a clinical parameter to estimate the fertilizing potential of men taking part in an ART programme. Hum Reprod (2013) 28:969–78. doi: 10.1093/humrep/des471
151. Carrell DT, Emery BR, Hammoud S. Altered protamine expression and diminished spermatogenesis: what is the link? Hum Reprod Update (2007) 13:313–27. doi: 10.1093/humupd/dml057
152. de Mateo S, Gazquez C, Guimera M, Balasch J, Meistrich ML, Ballesca JL, et al. Protamine 2 precursors (Pre-P2), protamine 1 to protamine 2 ratio (P1/P2), and assisted reproduction outcome. Fertil Steril (2009) 91:715–22. doi: 10.1016/j.fertnstert.2007.12.047
153. Rogenhofer N, Ott J, Pilatz A, Wolf J, Thaler CJ, Windischbauer L, et al. Unexplained recurrent miscarriages are associated with an aberrant sperm protamine mRNA content. Hum Reprod (2017) 32:1574–82. doi: 10.1093/humrep/dex224
154. Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet (2012) 13:493–504. doi: 10.1038/nrg3245
155. Sciorio R, Dattilo M. PGT-a preimplantation genetic testing for aneuploidies and embryo selection in routine ART cycles: Time to step back? Clin Genet (2020) 98:107–15. doi: 10.1111/cge.13732
156. Raziel A, Friedler S, Schachter M, Kasterstein E, Strassburger D, Ron-El R. Increased frequency of female partner chromosomal abnormalities in patients with high-order implantation failure after in vitro fertilization. Fertil Steril (2002) 78:515–9. doi: 10.1016/S0015-0282(02)03298-3
157. Voullaire L, Collins V, Callaghan T, McBain J, Williamson R, Wilton L. High incidence of complex chromosome abnormality in cleavage embryos from patients with repeated implantation failure. Fertil Steril (2007) 87:1053–8. doi: 10.1016/j.fertnstert.2006.11.043
158. Munne S, Weier HU, Grifo J, Cohen J. Chromosome mosaicism in human embryos. Biol Reprod (1994) 51:373–9. doi: 10.1095/biolreprod51.3.373
159. Taylor TH, Gitlin SA, Patrick JL, Crain JL, Wilson JM, Griffin DK. The origin, mechanisms, incidence and clinical consequences of chromosomal mosaicism in humans. Hum Reprod Update (2014) 20:571–81. doi: 10.1093/humupd/dmu016
160. Spinella F, Fiorentino F, Biricik A, Bono S, Ruberti A, Cotroneo E, et al. Extent of chromosomal mosaicism influences the clinical outcome of in vitro fertilization treatments. Fertil Steril (2018) 109:77–83. doi: 10.1016/j.fertnstert.2017.09.025
161. Tiegs AW, Tao X, Zhan Y, Whitehead C, Kim J, Hanson B, et al. A multicenter, prospective, blinded, nonselection study evaluating the predictive value of an aneuploid diagnosis using a targeted next-generation sequencing-based preimplantation genetic testing for aneuploidy assay and impact of biopsy. Fertil Steril (2021) 115:627–37. doi: 10.1016/j.fertnstert.2020.07.052
162. Cheah S, Gao Y, Mo S, Rigas G, Fisher O, Chan DL, et al. Fertility, pregnancy and post partum management after bariatric surgery: a narrative review. Med J Aust (2022) 216:96–102. doi: 10.5694/mja2.51373
163. Legro RS, Dodson WC, Kunselman AR, Stetter CM, Kris-Etherton PM, Williams NI, et al. Benefit of delayed fertility therapy with preconception weight loss over immediate therapy in obese women with PCOS. J Clin Endocrinol Metab (2016) 101:2658–66. doi: 10.1210/jc.2016-1659
164. Chavarro JE, Ehrlich S, Colaci DS, Wright DL, Toth TL, Petrozza JC, et al. Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertil Steril (2012) 98:109–16. doi: 10.1016/j.fertnstert.2012.04.012
165. Legro RS. Mr. fertility authority, tear down that weight wall! Hum Reprod (2016) 31:2662–4. doi: 10.1093/humrep/dew253
166. Budani MC, Tiboni GM. Ovotoxicity of cigarette smoke: A systematic review of the literature. Reprod Toxicol (2017) 72:164–81. doi: 10.1016/j.reprotox.2017.06.184
167. Fullston T, McPherson NO, Zander-Fox D, Lane M. The most common vices of men can damage fertility and the health of the next generation. J Endocrinol (2017) 234:F1–6. doi: 10.1530/JOE-16-0382
168. Frederiksen Y, Farver-Vestergaard I, Skovgard NG, Ingerslev HJ, Zachariae R. Efficacy of psychosocial interventions for psychological and pregnancy outcomes in infertile women and men: a systematic review and meta-analysis. BMJ Open (2015) 5:e006592. doi: 10.1136/bmjopen-2014-006592
169. Tur-Kaspa I, Confino E, Dudkiewicz AB, Myers SA, Friberg J, Gleicher N. Ovarian stimulation protocol for in vitro fertilization with gonadotropin-releasing hormone agonist widens the implantation window. Fertil Steril (1990) 53:859–64. doi: 10.1016/S0015-0282(16)53522-5
170. Tremellen K, Russell P. Adenomyosis is a potential cause of recurrent implantation failure during IVF treatment. Aust N Z J Obstet Gynaecol (2011) 51:280–3. doi: 10.1111/j.1479-828X.2010.01276.x
171. Oliveira JB, Baruffi R, Petersen CG, Mauri AL, Cavagna M, Franco JG Jr. Administration of single-dose GnRH agonist in the luteal phase in ICSI cycles: a meta-analysis. Reprod Biol Endocrinol (2010) 8:107. doi: 10.1186/1477-7827-8-107
172. Zhao Y, Garcia J, Kolp L, Cheadle C, Rodriguez A, Vlahos NF. The impact of luteal phase support on gene expression of extracellular matrix protein and adhesion molecules in the human endometrium during the window of implantation following controlled ovarian stimulation with a GnRH antagonist protocol. Fertil Steril (2010) 94:2264–71. doi: 10.1016/j.fertnstert.2010.01.068
173. Gabrielsen A, Agerholm I, Toft B, Hald F, Petersen K, Aagaard J, et al. Assisted hatching improves implantation rates on cryopreserved-thawed embryos. a randomized prospective study. Hum Reprod (2004) 19:2258–62. doi: 10.1093/humrep/deh434
174. Lacey L, Hassan S, Franik S, Seif MW, Akhtar MA. Assisted hatching on assisted conception (in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI)). Cochrane Database Syst Rev (2021) 3:CD001894. doi: 10.1002/14651858.CD001894.pub6
175. Stein A, Rufas O, Amit S, Avrech O, Pinkas H, Ovadia J, et al. Assisted hatching by partial zona dissection of human pre-embryos in patients with recurrent implantation failure after in vitro fertilization. Fertil Steril (1995) 63:838–41. doi: 10.1016/S0015-0282(16)57490-1
176. Practice Committee of the American Society for Reproductive Medicine. The role of assistedhatching in in vitro fertilization: a guideline. Fertil Steril (2022) 117:1177–82. doi: 10.1016/j.fertnstert.2022.02.020
177. Thomas K, Thomson AJ, Sephton V, Cowan C, Wood S, Vince G, et al. The effect of gonadotrophic stimulation on integrin expression in the endometrium. Hum Reprod (2002) 17:63–8. doi: 10.1093/humrep/17.1.63
178. Bourgain C, Devroey P. The endometrium in stimulated cycles for IVF. Hum Reprod Update (2003) 9:515–22. doi: 10.1093/humupd/dmg045
179. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril (2011) 96:344–8. doi: 10.1016/j.fertnstert.2011.05.050
180. Guerif F, Bidault R, Gasnier O, Couet ML, Gervereau O, Lansac J, et al. Efficacy of blastocyst transfer after implantation failure. Reprod BioMed Online (2004) 9:630–6. doi: 10.1016/S1472-6483(10)61773-7
181. Levitas E, Lunenfeld E, Har-Vardi I, Albotiano S, Sonin Y, Hackmon-Ram R, et al. Blastocyst-stage embryo transfer in patients who failed to conceive in three or more day 2-3 embryo transfer cycles: a prospective, randomized study. Fertil Steril (2004) 81:567–71. doi: 10.1016/j.fertnstert.2003.08.031
182. Zhang J, Wang C, Zhang H, Zhou Y. Sequential cleavage and blastocyst embryo transfer and IVF outcomes: a systematic review. Reprod Biol Endocrinol (2021) 19:142. doi: 10.1186/s12958-021-00824-y
183. Hviid MM, Macklon N. Immune modulation treatments-where is the evidence? Fertil Steril (2017) 107:1284–93. doi: 10.1016/j.fertnstert.2017.04.009
184. Henderson TA, Saunders PT, Moffett-King A, Groome NP, Critchley HO. Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab (2003) 88:440–9. doi: 10.1210/jc.2002-021174
185. Cooper S, Laird SM, Mariee N, Li TC, Metwally M. The effect of prednisolone on endometrial uterine NK cell concentrations and pregnancy outcome in women with reproductive failure. A retrospective cohort study. J Reprod Immunol (2019) 131:1–6. doi: 10.1016/j.jri.2018.10.001
186. Boomsma CM, Keay SD, Macklon NS. Peri-implantation glucocorticoid administration for assisted reproductive technology cycles. Cochrane Database Syst Rev (2012), CD005996. doi: 10.1002/14651858.CD005996.pub3
187. Forges T, Monnier-Barbarino P, Guillet-May F, Faure GC, Bene MC. Corticosteroids in patients with antiovarian antibodies undergoing in vitro fertilization: a prospective pilot study. Eur J Clin Pharmacol (2006) 62:699–705. doi: 10.1007/s00228-006-0169-0
188. Alhalabi M SS, Taha A, Kafri N, Modi S, Khatib A, Sharif J, et al. Prednisolone improves implantation in ICSI patients with high peripheral CD69 + NK cells. Hum Reprod (2011) 26:i219.
189. Ahmadi M, Abdolmohammadi-Vahid S, Ghaebi M, Aghebati-Maleki L, Afkham A, Danaii S, et al. Effect of intravenous immunoglobulin on Th1 and Th2 lymphocytes and improvement of pregnancy outcome in recurrent pregnancy loss (RPL). BioMed Pharmacother (2017) 92:1095–102. doi: 10.1016/j.biopha.2017.06.001
190. Jerzak M, Rechberger T, Gorski A. Intravenous immunoglobulin therapy influences T cell adhesion to extracellular matrix in women with a history of recurrent spontaneous abortions. Am J Reprod Immunol (2000) 44:336–41. doi: 10.1111/j.8755-8920.2000.440603.x
191. Ahmadi M, Aghdam SA, Nouri M, Babaloo Z, Farzadi L, Ghasemzadeh A, et al. Intravenous immunoglobulin (IVIG) treatment modulates peripheral blood Th17 and regulatory T cells in recurrent miscarriage patients: Non randomized, open-label clinical trial. Immunol Lett (2017) 192:12–9. doi: 10.1016/j.imlet.2017.10.003
192. Abdolmohammadi-Vahid S, Pashazadeh F, Pourmoghaddam Z, Aghebati-Maleki L, Abdollahi-Fard S, Yousefi M. The effectiveness of IVIG therapy in pregnancy and live birth rate of women with recurrent implantation failure (RIF): A systematic review and meta-analysis. J Reprod Immunol (2019) 134-135:28–33. doi: 10.1016/j.jri.2019.07.006
193. Ramos-Medina R, Garcia-Segovia A, Gil J, Carbone J, Aguaron de la Cruz A, Seyfferth A, et al. Experience in IVIg therapy for selected women with recurrent reproductive failure and NK cell expansion. Am J Reprod Immunol (2014) 71:458–66. doi: 10.1111/aji.12217
194. Nyborg KM, Kolte AM, Larsen EC, Christiansen OB. Immunomodulatory treatment with intravenous immunoglobulin and prednisone in patients with recurrent miscarriage and implantation failure after in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril (2014) 102:1650–5.e1. doi: 10.1016/j.fertnstert.2014.08.029
195. Kino T, Hatanaka H, Miyata S, Inamura N, Nishiyama M, Yajima T, et al. FK-506, a novel immunosuppressant isolated from a streptomyces. II. immunosuppressive effect of FK-506 in vitro. J Antibiot (Tokyo) (1987) 40:1256–65. doi: 10.7164/antibiotics.40.1256
196. Nakagawa K, Kwak-Kim J, Ota K, Kuroda K, Hisano M, Sugiyama R, et al. Immunosuppression with tacrolimus improved reproductive outcome of women with repeated implantation failure and elevated peripheral blood TH1/TH2 cell ratios. Am J Reprod Immunol (2015) 73:353–61. doi: 10.1111/aji.12338
197. Nakagawa K, Kuroda K, Sugiyama R, Yamaguchi K. After 12 consecutive miscarriages, a patient received immunosuppressive treatment and delivered an intact baby. Reprod Med Biol (2017) 16:297–301. doi: 10.1002/rmb2.12040
198. Huang W, Lu W, Li Q, Zhang Y, Xie B, Luo S, et al. Effects of cyclosporine a on proliferation, invasion and migration of HTR-8/SVneo human extravillous trophoblasts. Biochem Biophys Res Commun (2020) 533:645–50. doi: 10.1016/j.bbrc.2020.09.072
199. Piao HL, Wang SC, Tao Y, Zhu R, Sun C, Fu Q, et al. Cyclosporine a enhances Th2 bias at the maternal-fetal interface in early human pregnancy with aid of the interaction between maternal and fetal cells. PloS One (2012) 7:e45275. doi: 10.1371/journal.pone.0045275
200. Du MR, Zhou WH, Piao HL, Li MQ, Tang CL, Li DJ. Cyclosporin a promotes crosstalk between human cytotrophoblast and decidual stromal cell through up-regulating CXCL12/CXCR4 interaction. Hum Reprod (2012) 27:1955–65. doi: 10.1093/humrep/des111
201. Azizi R, Ahmadi M, Danaii S, Abdollahi-Fard S, Mosapour P, Eghbal-Fard S, et al. Cyclosporine a improves pregnancy outcomes in women with recurrent pregnancy loss and elevated Th1/Th2 ratio. J Cell Physiol (2019) 234:19039–47. doi: 10.1002/jcp.28543
202. Cheng W, Wu Y, Wu H, Zou Q, Meng Q, Wang F, et al. Improved pregnancy outcomes of cyclosporine a on patients with unexplained repeated implantation failure in IVF/ICSI cycles: A retrospective cohort study. Am J Reprod Immunol (2022) 87:e13525. doi: 10.1111/aji.13525
203. Qu D, Tian X, Ding L, Li Y, Zhou W. Impacts of cyclosporin a on clinical pregnancy outcomes of patients with a history of unexplained transfer failure: a retrospective cohort study. Reprod Biol Endocrinol (2021) 19:44. doi: 10.1186/s12958-021-00728-x
204. Roussev RG, Ng SC, Coulam CB. Natural killer cell functional activity suppression by intravenous immunoglobulin, intralipid and soluble human leukocyte antigen-G. Am J Reprod Immunol (2007) 57:262–9. doi: 10.1111/j.1600-0897.2007.00473.x
205. Roussev RG, Acacio B, Ng SC, Coulam CB. Duration of intralipid's suppressive effect on NK cell's functional activity. Am J Reprod Immunol (2008) 60:258–63. doi: 10.1111/j.1600-0897.2008.00621.x
206. Ledee N, Vasseur C, Petitbarat M, Chevrier L, Vezmar K, Dray G, et al. Intralipid(R) may represent a new hope for patients with reproductive failures and simultaneously an over-immune endometrial activation. J Reprod Immunol (2018) 130:18–22. doi: 10.1016/j.jri.2018.09.050
207. Rimmer MP, Black N, Keay S, Quenby S, Al Wattar BH. Intralipid infusion at time of embryo transfer in women with history of recurrent implantation failure: A systematic review and meta-analysis. J Obstet Gynaecol Res (2021) 47:2149–56. doi: 10.1111/jog.14763
208. Al-Zebeidi J, Agdi M, Lary S, Al-Obaid S, Salim G, Al-Jaroudi D. Effect of empiric intravenous intralipid therapy on pregnancy outcome in women with unexplained recurrent implantation failure undergoing intracytoplasmic sperm injection-embryo transfer cycle: A randomized controlled trial. Gynecol Endocrinol (2020) 36:131–4. doi: 10.1080/09513590.2019.1631280
209. Coulam CB. Intralipid treatment for women with reproductive failures. Am J Reprod Immunol (2021) 85:e13290. doi: 10.1111/aji.13290
210. Kling C, Magez-Zunker J, Jenisch S, Kabelitz D. Experience with allogenic leukocyte immunization (AI) for implantation failure in the in vitro fertilization program. Am J Reprod Immunol (2002) 48:147–50. doi: 10.1034/j.1600-0897.2002.00007.x
211. Gunther V, Alkatout I, Meyerholz L, Maass N, Gorg S, von Otte S, et al. Live birth rates after active immunization with partner lymphocytes. Biomedicines 9 (2021) 9:1350. doi: 10.3390/biomedicines9101350
212. Hanevik HI, Friberg M, Bergh A, Haraldsen C, Kahn JA. Do acetyl salicylic acid and terbutaline in combination increase the probability of a clinical pregnancy in patients undergoing IVF/ICSI? J Obstet Gynaecol (2012) 32:786–9. doi: 10.3109/01443615.2012.717988
213. Dirckx K, Cabri P, Merien A, Galajdova L, Gerris J, Dhont M, et al. Does low-dose aspirin improve pregnancy rate in IVF/ICSI? a randomized double-blind placebo controlled trial. Hum Reprod (2009) 24:856–60. doi: 10.1093/humrep/den476
214. Zhang X, Guo F, Wang Q, Bai W, Zhao A. Low-dose aspirin treatment improves endometrial receptivity in the midluteal phase in unexplained recurrent implantation failure. Int J Gynaecol Obstet (2022) 156:225–30. doi: 10.1002/ijgo.13699
215. Urman B, Mercan R, Alatas C, Balaban B, Isiklar A, Nuhoglu A. Low-dose aspirin does not increase implantation rates in patients undergoing intracytoplasmic sperm injection: a prospective randomized study. J Assist Reprod Genet (2000) 17:586–90. doi: 10.1023/A:1026491426423
216. Stern C, Chamley L, Norris H, Hale L, Baker HW. A randomized, double-blind, placebo-controlled trial of heparin and aspirin for women with in vitro fertilization implantation failure and antiphospholipid or antinuclear antibodies. Fertil Steril (2003) 80:376–83. doi: 10.1016/S0015-0282(03)00610-1
217. Pakkila M, Rasanen J, Heinonen S, Tinkanen H, Tuomivaara L, Makikallio K, et al. Low-dose aspirin does not improve ovarian responsiveness or pregnancy rate in IVF and ICSI patients: A randomized, placebo-controlled double-blind study. Hum Reprod (2005) 20:2211–4. doi: 10.1093/humrep/dei020
218. Duvan CI, Ozmen B, Satiroglu H, Atabekoglu CS, Berker B. Does addition of low-dose aspirin and/or steroid as a standard treatment in nonselected intracytoplasmic sperm injection cycles improve in vitro fertilization success? a randomized, prospective, placebo-controlled study. J Assist Reprod Genet (2006) 23:15–21. doi: 10.1007/s10815-005-9003-3
219. Nelson SM, Greer IA. The potential role of heparin in assisted conception. Hum Reprod Update (2008) 14:623–45. doi: 10.1093/humupd/dmn031
220. Fluhr H, Spratte J, Ehrhardt J, Steinmuller F, Licht P, Zygmunt M. Heparin and low-molecular-weight heparins modulate the decidualization of human endometrial stromal cells. Fertil Steril (2010) 93:2581–7. doi: 10.1016/j.fertnstert.2009.10.025
221. Qublan H, Amarin Z, Dabbas M, Farraj AE, Beni-Merei Z, Al-Akash H, et al. Low-molecular-weight heparin in the treatment of recurrent IVF-ET failure and thrombophilia: A prospective randomized placebo-controlled trial. Hum Fertil (Camb) (2008) 11:246–53. doi: 10.1080/14647270801995431
222. Potdar N, Gelbaya TA, Konje JC, Nardo LG. Adjunct low-molecular-weight heparin to improve live birth rate after recurrent implantation failure: a systematic review and meta-analysis. Hum Reprod Update (2013) 19:674–84. doi: 10.1093/humupd/dmt032
223. Urman B, Ata B, Yakin K, Alatas C, Aksoy S, Mercan R, et al. Luteal phase empirical low molecular weight heparin administration in patients with failed ICSI embryo transfer cycles: a randomized open-labeled pilot trial. Hum Reprod (2009) 24:1640–7. doi: 10.1093/humrep/dep086
224. Tapia-Pizarro A, Argandona F, Palomino WA, Devoto L. Human chorionic gonadotropin (hCG) modulation of TIMP1 secretion by human endometrial stromal cells facilitates extravillous trophoblast invasion in vitro. Hum Reprod (2013) 28:2215–27. doi: 10.1093/humrep/det136
225. Gao M, Jiang X, Li B, Li L, Duan M, Zhang X, et al. Intrauterine injection of human chorionic gonadotropin before embryo transfer can improve in vitro fertilization-embryo transfer outcomes: A meta-analysis of randomized controlled trials. Fertil Steril (2019) 112:89–97.e1. doi: 10.1016/j.fertnstert.2019.02.027
226. Yu N, Zhang B, Xu M, Wang S, Liu R, Wu J, et al. Intrauterine administration of autologous peripheral blood mononuclear cells (PBMCs) activated by HCG improves the implantation and pregnancy rates in patients with repeated implantation failure: a prospective randomized study. Am J Reprod Immunol (2016) 76:212–6. doi: 10.1111/aji.12542
227. Pourmoghadam Z, Abdolmohammadi-Vahid S, Pashazadeh F, Aghebati-Maleki L, Ansari F, Yousefi M. Efficacy of intrauterine administration of autologous peripheral blood mononuclear cells on the pregnancy outcomes in patients with recurrent implantation failure: A systematic review and meta-analysis. J Reprod Immunol (2020) 137:103077. doi: 10.1016/j.jri.2019.103077
228. Nakayama T, Fujiwara H, Maeda M, Inoue T, Yoshioka S, Mori T, et al. Human peripheral blood mononuclear cells (PBMC) in early pregnancy promote embryo invasion in vitro: HCG enhances the effects of PBMC. Hum Reprod (2002) 17:207–12. doi: 10.1093/humrep/17.1.207
229. Yoshioka S, Fujiwara H, Nakayama T, Kosaka K, Mori T, Fujii S. Intrauterine administration of autologous peripheral blood mononuclear cells promotes implantation rates in patients with repeated failure of IVF-embryo transfer. Hum Reprod (2006) 21:3290–4. doi: 10.1093/humrep/del312
230. Li S, Wang J, Cheng Y, Zhou D, Yin T, Xu W, et al. Intrauterine administration of hCG-activated autologous human peripheral blood mononuclear cells (PBMC) promotes live birth rates in frozen/thawed embryo transfer cycles of patients with repeated implantation failure. J Reprod Immunol (2017) 119:15–22. doi: 10.1016/j.jri.2016.11.006
231. Russell SJ, Kwok YSS, Nguyen TTN, Librach C. Autologous platelet-rich plasma improves the endometrial thickness and live birth rate in patients with recurrent implantation failure and thin endometrium. J Assist Reprod Genet (2022) 39:1305–12. doi: 10.1007/s10815-022-02505-0
232. Zamaniyan M, Peyvandi S, Heidaryan Gorji H, Moradi S, Jamal J, Yahya Poor Aghmashhadi F, et al. Effect of platelet-rich plasma on pregnancy outcomes in infertile women with recurrent implantation failure: a randomized controlled trial. Gynecol Endocrinol (2021) 37:141–5. doi: 10.1080/09513590.2020.1756247
233. Xu Y, Hao C, Fang J, Liu X, Xue P, Miao R. Intrauterine perfusion of autologous platelet-rich plasma before frozen-thawed embryo transfer improves the clinical pregnancy rate of women with recurrent implantation failure. Front Med (Lausanne) (2022) 9:850002. doi: 10.3389/fmed.2022.850002
234. Aleyasin A, Abediasl Z, Nazari A, Sheikh M. Granulocyte colony-stimulating factor in repeated IVF failure, a randomized trial. Reproduction (2016) 151:637–42. doi: 10.1530/REP-16-0046
235. Scarpellini F, Sbracia M. The use of G-CSF for implantation failure in IVF: a clinical trial. Fertil Steril (2011) 96:S93. doi: 10.1016/j.fertnstert.2011.07.359
236. van Hoogenhuijze NE, van Eekelen R, Mol F, Schipper I, Groenewoud ER, Traas MAF, et al. Economic evaluation of endometrial scratching before the second IVF/ICSI treatment: a cost-effectiveness analysis of a randomized controlled trial (SCRaTCH trial). Hum Reprod (2022) 37:254–63. doi: 10.1093/humrep/deab261
237. Shohayeb A, El-Khayat W. Does a single endometrial biopsy regimen (S-EBR) improve ICSI outcome in patients with repeated implantation failure? a randomised controlled trial. Eur J Obstet Gynecol Reprod Biol (2012) 164:176–9. doi: 10.1016/j.ejogrb.2012.06.029
238. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg (2004) 114:1502–8. doi: 10.1097/01.PRS.0000138251.07040.51
239. Nazari L, Salehpour S, Hoseini S, Zadehmodarres S, Azargashb E. Effects of autologous platelet-rich plasma on endometrial expansion in patients undergoing frozen-thawed embryo transfer: A double-blind RCT. Int J Reprod BioMed (2019) 17:443–8. doi: 10.18502/ijrm.v17i6.4816
240. Kramer ME, Keaney TC. Systematic review of platelet-rich plasma (PRP) preparation and composition for the treatment of androgenetic alopecia. J Cosmet Dermatol (2018) 17:666–71. doi: 10.1111/jocd.12679
241. Gupta AK, Carviel J. A mechanistic model of platelet-rich plasma treatment for androgenetic alopecia. Dermatol Surg (2016) 42:1335–9. doi: 10.1097/DSS.0000000000000901
242. Kim MK, Yoon JA, Yoon SY, Park M, Lee WS, Lyu SW, et al. Human platelet-rich plasma facilitates angiogenesis to restore impaired uterine environments with asherman's syndrome for embryo implantation and following pregnancy in mice. Cells (2022) 11:1549. doi: 10.3390/cells11091549
243. Maleki-Hajiagha A, Razavi M, Rouholamin S, Rezaeinejad M, Maroufizadeh S, Sepidarkish M. Intrauterine infusion of autologous platelet-rich plasma in women undergoing assisted reproduction: A systematic review and meta-analysis. J Reprod Immunol (2020) 137:103078. doi: 10.1016/j.jri.2019.103078
244. Bussolino F, Wang JM, Defilippi P, Turrini F, Sanavio F, Edgell CJ, et al. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature (1989) 337:471–3. doi: 10.1038/337471a0
245. Scarpellini F, Klinger FG, Rossi G, Sbracia M. Immunohistochemical study on the expression of G-CSF, G-CSFR, VEGF, VEGFR-1, Foxp3 in first trimester trophoblast of recurrent pregnancy loss in pregnancies treated with G-CSF and controls. Int J Mol Sci (2019) 21:285. doi: 10.3390/ijms21010285
246. Tehraninejad E, Davari Tanha F, Asadi E, Kamali K, Aziminikoo E, Rezayof E. G-CSF intrauterine for thin endometrium, and pregnancy outcome. J Family Reprod Health (2015) 9:107–12.
247. Hou Z, Jiang F, Yang J, Liu Y, Zha H, Yang X, et al. What is the impact of granulocyte colony-stimulating factor (G-CSF) in subcutaneous injection or intrauterine infusion and during both the fresh and frozen embryo transfer cycles on recurrent implantation failure: a systematic review and meta-analysis? Reprod Biol Endocrinol (2021) 19:125. doi: 10.1186/s12958-021-00810-4
248. Davari-Tanha F, Shahrokh Tehraninejad E, Ghazi M, Shahraki Z. The role of G-CSF in recurrent implantation failure: A randomized double blind placebo control trial. Int J Reprod BioMed (2016) 14:737–42. doi: 10.29252/ijrm.14.12.737
249. Kalem Z, Namli Kalem M, Bakirarar B, Kent E, Makrigiannakis A, Gurgan T. Intrauterine G-CSF administration in recurrent implantation failure (RIF): An rct. Sci Rep (2020) 10:5139. doi: 10.1038/s41598-020-61955-7
250. Zeyneloglu HB, Onalan G. Remedies for recurrent implantation failure. Semin Reprod Med (2014) 32:297–305. doi: 10.1055/s-0034-1375182
251. Nastri CO, Lensen SF, Gibreel A, Raine-Fenning N, Ferriani RA, Bhattacharya S, et al. Endometrial injury in women undergoing assisted reproductive techniques. Cochrane Database Syst Rev (2015), CD009517. doi: 10.1002/14651858.CD009517.pub3
252. Simon C, Gomez C, Cabanillas S, Vladimirov I, Castillon G, Giles J, et al. A 5-year multicentre randomized controlled trial comparing personalized, frozen and fresh blastocyst transfer in IVF. Reprod BioMed Online (2020) 41:402–15. doi: 10.1016/j.rbmo.2020.06.002
253. Vitagliano A, Saccardi C, Litta PS, Noventa M. Chronic endometritis: Really so relevant in repeated IVF failure? Am J Reprod Immunol 78 (2017) 78. doi: 10.1111/aji.12758
254. Vitagliano A, Saccardi C, Noventa M, Di Spiezio Sardo A, Saccone G, Cicinelli E, et al. Effects of chronic endometritis therapy on in vitro fertilization outcome in women with repeated implantation failure: a systematic review and meta-analysis. Fertil Steril (2018) 110:103–112.e1. doi: 10.1016/j.fertnstert.2018.03.017
255. Pantos K, Simopoulou M, Maziotis E, Rapani A, Grigoriadis S, Tsioulou P, et al. Introducing intrauterine antibiotic infusion as a novel approach in effectively treating chronic endometritis and restoring reproductive dynamics: a randomized pilot study. Sci Rep (2021) 11:15581. doi: 10.1038/s41598-021-95072-w
256. Garzon S, Lagana AS, Di Spiezio Sardo A, Alonso Pacheco L, Haimovich S, Carugno J, et al. Hysteroscopic metroplasty for T-shaped uterus: A systematic review and meta-analysis of reproductive outcomes. Obstet Gynecol Surv (2020) 75:431–44. doi: 10.1097/OGX.0000000000000807
257. Bilgory A, Shalom-Paz E, Atzmon Y, Aslih N, Shibli Y, Estrada D, et al. Diode laser hysteroscopic metroplasty for dysmorphic uterus: a pilot study. Reprod Sci (2022) 29:506–12. doi: 10.1007/s43032-021-00607-1
258. El-Toukhy T, Campo R, Khalaf Y, Tabanelli C, Gianaroli L, Gordts SS, et al. Hysteroscopy in recurrent in-vitro fertilisation failure (TROPHY): A multicentre, randomised controlled trial. Lancet (2016) 387:2614–21. doi: 10.1016/S0140-6736(16)00258-0
259. Homer HA, Li TC, Cooke ID. The septate uterus: a review of management and reproductive outcome. Fertil Steril (2000) 73:1–14. doi: 10.1016/S0015-0282(99)00480-X
260. March CM. Management of asherman's syndrome. Reprod BioMed Online (2011) 23:63–76. doi: 10.1016/j.rbmo.2010.11.018
261. Lo Monte G, Murisier F, Piva I, Germond M, Marci R. Focus on intracytoplasmic morphologically selected sperm injection (IMSI): a mini-review. Asian J Androl (2013) 15:608–15. doi: 10.1038/aja.2013.54
262. Shalom-Paz E, Anabusi S, Michaeli M, Karchovsky-Shoshan E, Rothfarb N, Shavit T, et al. Can intra cytoplasmatic morphologically selected sperm injection (IMSI) technique improve outcome in patients with repeated IVF-ICSI failure? a comparative study. Gynecol Endocrinol (2015) 31:247–51. doi: 10.3109/09513590.2014.982085
263. Teixeira DM, Hadyme Miyague A, Barbosa MA, Navarro PA, Raine-Fenning N, Nastri CO, et al. Regular (ICSI) versus ultra-high magnification (IMSI) sperm selection for assisted reproduction. Cochrane Database Syst Rev (2020) 2:CD010167. doi: 10.1002/14651858.CD010167.pub3
264. Greco E, Bono S, Ruberti A, Lobascio AM, Greco P, Biricik A, et al. Comparative genomic hybridization selection of blastocysts for repeated implantation failure treatment: a pilot study. BioMed Res Int (2014) 2014:457913. doi: 10.1155/2014/457913
265. Pantou A, Mitrakos A, Kokkali G, Petroutsou K, Tounta G, Lazaros L, et al. The impact of preimplantation genetic testing for aneuploidies (PGT-a) on clinical outcomes in high risk patients. J Assist Reprod Genet (2022) 39:1341–9. doi: 10.1007/s10815-022-02461-9
Keywords: recurrent implantation failure, immunology, thrombophilias, endometrial receptivity, microbiome, anatomical abnormalities, aneuploidy
Citation: Ma J, Gao W and Li D (2023) Recurrent implantation failure: A comprehensive summary from etiology to treatment. Front. Endocrinol. 13:1061766. doi: 10.3389/fendo.2022.1061766
Received: 06 October 2022; Accepted: 13 December 2022;
Published: 05 January 2023.
Edited by:
David Seifer, Yale University, United StatesReviewed by:
Xiaoyan Chen, Baoan Women’s and Children’s Hospital, ChinaCopyright © 2023 Ma, Gao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Da Li, bGVlZGFAeW1haWwuY29t; Wenyan Gao, d3lnYW9AY211LmVkdS5jbg==
†ORCID: Wenyan Gao, https://orcid.org/0000-0002-4625-9443
Da Li, https://orcid.org/0000-0002-4895-1185
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.