
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 04 January 2023
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1060301
 Sandeep Kumar Parvathareddy1†
Sandeep Kumar Parvathareddy1† Abdul K. Siraj1†
Abdul K. Siraj1† Padmanaban Annaiyappanaidu1
Padmanaban Annaiyappanaidu1 Nabil Siraj1
Nabil Siraj1 Saif S. Al-Sobhi2
Saif S. Al-Sobhi2 Fouad Al-Dayel3
Fouad Al-Dayel3 Khawla S. Al-Kuraya1*
Khawla S. Al-Kuraya1*Background: Tumor multifocality is frequently seen in Papillary thyroid carcinoma (PTC). However, few studies have analysed the impact of bilateral multifocality in PTC. The incidence of bilateral multifocality, its clinico-pathological associations and prognostic impact in PTC from Middle Eastern ethnicity remains unestablished.
Methods: We retrospectively evaluated 1283 patients who underwent total thyroidectomy for PTC. Bilateral and unilateral multifocality were decided based on the final pathology result. Primary outcome was recurrence free survival (RFS). Risk factors for bilateral multifocality were analyzed by multivariate logistic regression analysis.
Results: Multifocal PTC was found in 54.3% (697/1283) of patients. Among the 697 multifocal PTCs, 210 patients (30.1%) had unilateral multifocal PTC and 487 patients (69.9%) had bilateral multifocality. Bilateral multifocality was significantly associated with older age at diagnosis (p = 0.0263), male gender (p = 0.0201), gross extrathyroidal extension (p = 0.0332), larger primary tumor size (>4cm; p = 0.0002), lateral lymph node metastasis (p = 0.0008), distant metastasis at diagnosis (p = 0.0195) and recurrence (p = 0.0001). Bilateral multifocality was also found to be an independent predictor of RFS (Hazard ratio = 1.60; 95% Confidence Interval = 1.05 – 2.55; p = 0.0300). Multivariate logistic regression analysis demonstrated tumor diameter >4cm to be the only independent risk factors for bilaterality in multifocal PTC (Odds ratio = 1.86; 95% Confidence Interval = 1.13 – 3.07; p = 0.0155).
Conclusions: Incidence of bilateral multifocality is high in Middle Eastern PTC. Tumor diameter >4cm can be considered as a predictive factor for bilateral multifocal PTC. Bilateral multifocality appears to be an important prognostic factor for PTC and an independent predictor of RFS. Therefore, patients with bilateral multifocal PTC may benefit from more frequent follow-up to identify recurrences earlier.
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy (1). Its incidence has increased rapidly over the last two decades (2–4). PTC has become the second most prevalent cancer (after breast cancer) among women in Saudi Arabia (5). Although the prognosis of PTC is excellent, up to 20% experience loco-regional recurrence, which might lead to worse clinical outcomes (6–8). The standard treatment of PTC is thyroidectomy followed by selective radioiodine ablation and/or thyroxine therapy (9, 10). However, recent evidence suggests that use of more conservative approaches as well as radioiodine ablation, especially in low risk PTC, are equally effective (11–14). Hence, these treatment approaches, when used in risk defined patients, can reduce the disease recurrence and improve patients’ outcome. There are several clinico-pathological factors affecting the risk of recurrence and prognosis in PTC patients. Most of these factors have been included in the risk stratification system proposed by the American Thyroid Association (ATA) (9). Moreover, accurate risk stratification is vital in helping define the appropriate therapeutic approach of PTC.
PTC with multifocality frequently arise in thyroid gland and has been considered as prognostic marker in PTC (15–17). Several risk stratification systems have addressed the prognostic role of multifocality in PTC including the latest ATA, the European Thyroid Association Guidelines (ETA) and consensus report of European Society of Endocrine Surgeons (9, 18, 19). However, several recent reports debate the impact of multifocality on PTC prognosis and recurrence (20–22). Although many studies have investigated bilateral multifocality, association with clinicopathological factors and prognostic value, the results are still controversial (23–25), which contributes to uncertainty in managing PTC patients with bilaterality multifocality. In addition, the incidence, clinico-pathological associations and prognostic impact of multifocality and bilaterality remained unexplored in PTC of Middle Eastern ethnicity.
Identifying predictive markers of bilateral multifocality and its clinico-pathological associations as well as clinical impact on patients’ prognosis could guide surgeons and oncologists to determine the appropriate treatment for PTC patients. In this retrospective study we investigated the incidence of multifocality in PTC from Middle Eastern ethnicity and its clinico-pathological associations. Moreover, we aimed to determine whether bilateral multifocality can predict patients’ prognosis in this ethnicity.
One thousand five-hundred and fifteen consecutive unselected PTC patients diagnosed between 1988 and 2018 at King Faisal Specialist Hospital and Research Centre (Riyadh, Saudi Arabia) were available to be included in the study. Only those patients with information on tumor focality who underwent total thyroidectomy were included in the study. A total of 1283 PTC cases were available to be included in the study. Of these, 697 patients had multifocal PTCs and were included for final analysis. Cases were identified based on clinical history followed by fine needle aspiration cytology for confirmation. The Institutional Review Board of the hospital approved this study and the Research Advisory Council (RAC) provided waiver of consent under project RAC # 221 1168 and # 2110 031.
Baseline clinico-pathological data were collected from case records and have been summarized in Table 1. All patients included in this study underwent total thyroidectomy. Central neck dissection alone was performed in 9.2% (64/697), lateral neck dissection alone was performed in 2.0% (14/697) and both central as well as lateral neck dissection was performed in 81.5% (568/697) of patients. Central and lateral lymph node dissections were performed in accordance with the 2015 American Thyroid Association guidelines (9), which recommends central and lateral lymph node dissection when clinically positive lymph nodes are found pre-operatively (i.e. by ultrasound or fine needle aspiration cytology). Tumor focality was assessed post-operatively on histopathological examination. Presence of two or more tumor foci was classified as multifocal. Multifocal tumors were further sub-grouped as unilateral multifocal and bilateral multifocal PTCs, depending on whether all tumor foci were located in a single lobe or they were seen in both lobes. Based on the 2015 American Thyroid Association (ATA) guidelines, tall cell, hobnail, columnar cell, diffuse sclerosing and insular variants were classified as aggressive variants, whereas classical and follicular variants were classified as non-aggressive variants (9) for the purpose of multivariate analysis. Staging of PTC was performed using the eighth edition of American Joint Committee on Cancer (AJCC) staging system (26). Only structural recurrence (local, regional or distant) was considered for analysis. Recurrence was defined as any newly detected tumor (local or distant) or metastatic regional lymph node (LN) based on ultrasound and/or imaging studies in patients who had been previously free of disease following initial treatment. Hashimoto’s thyroiditis was diagnosed when the typical histopathological features of lymphoplasmacytic infiltration, a germinal center formation, follicular destruction, a Hurthle cell change, and variable degrees of fibrosis were noted (27).
BRAF mutation data was assessed in our laboratory by Sanger sequencing and has been published by us previously (28).
Radioactive iodine (I-131) therapy was given to 83.2% (1068/1283) of PTC patients in our cohort. All ATA high- and intermediate-risk patients were offered I-131 therapy, whereas low-risk patients with lymph node metastasis also received I-131 therapy. Post-operative serum thyroglobulin levels were analyzed at 6 months and 1 year after surgery. 6.5% (84/1283) of patients showed an elevated post-operative serum thyroglobulin level (> 2ng/ml). Tumor recurrences were detected by serum thyroglobulin levels, whole body scan, ultrasound or CT scans performed during regular follow-up visits. Recurrence-free survival (RFS) was defined as the time (in months) from date of initial surgery to the occurrence of any tumor recurrence (local, regional or distant). In case of no recurrence, date of last follow-up was the study endpoint for RFS.
The associations between clinico-pathological variables and tumor focality was performed using contingency table analysis and Chi square tests. Mantel-Cox log-rank test was used to evaluate RFS. Survival curves were generated using the Kaplan-Meier method. Cox proportional hazards model and logistic regression were used for multivariate analysis. Two-sided tests were used for statistical analyses with a limit of significance defined as p value < 0.05. Data analyses were performed using the JMP14.0 (SAS Institute, Inc., Cary, NC) software package.
Median age of the entire cohort was 38.2 years (range: 6 – 89 years), with a male: female ratio of 1:3. Majority of the tumors were of classical variant (63.8%; 445/697). 24.0% (167/697) of multifocal PTCs were larger than 4cm in largest diameter. Regional LN metastasis was noted in 58.1% (405/697) of cases and distant metastasis was present at diagnosis in 6.5% (45/697). BRAF mutation data was available in 576 cases, with mutation being present in 62.7% (361/576) of multifocal PTCs. 20.5% (143/697) of patients developed tumor recurrence. (Table 1). The median follow-up was 8.2 years (range = 1.0 – 30.1 years). Recurrences were noted at a median of 3.2 years after initial diagnosis (range = 1 – 17.4 years).
Of the 697 multifocal PTCs included in our study, 69.9% (487/697) were bilateral and 30.1% (210/697) were unilateral. Bilateral multifocality was significantly associated with older age at diagnosis (p = 0.0263), male gender (p = 0.0201), gross extrathyroidal extension (p = 0.0332), larger primary tumor size (>4cm; p = 0.0002), lateral lymph node metastasis (p = 0.0008) and distant metastasis at diagnosis (p = 0.0195) (Table 2). Importantly, we found bilateral multifocality to be significantly associated with tumor recurrence (p = 0.0001), with 24.2% (118/487) of bilateral multifocal tumors showing recurrence compared to 11.9% (25/210) of unilateral multifocal PTCs (Table 2).
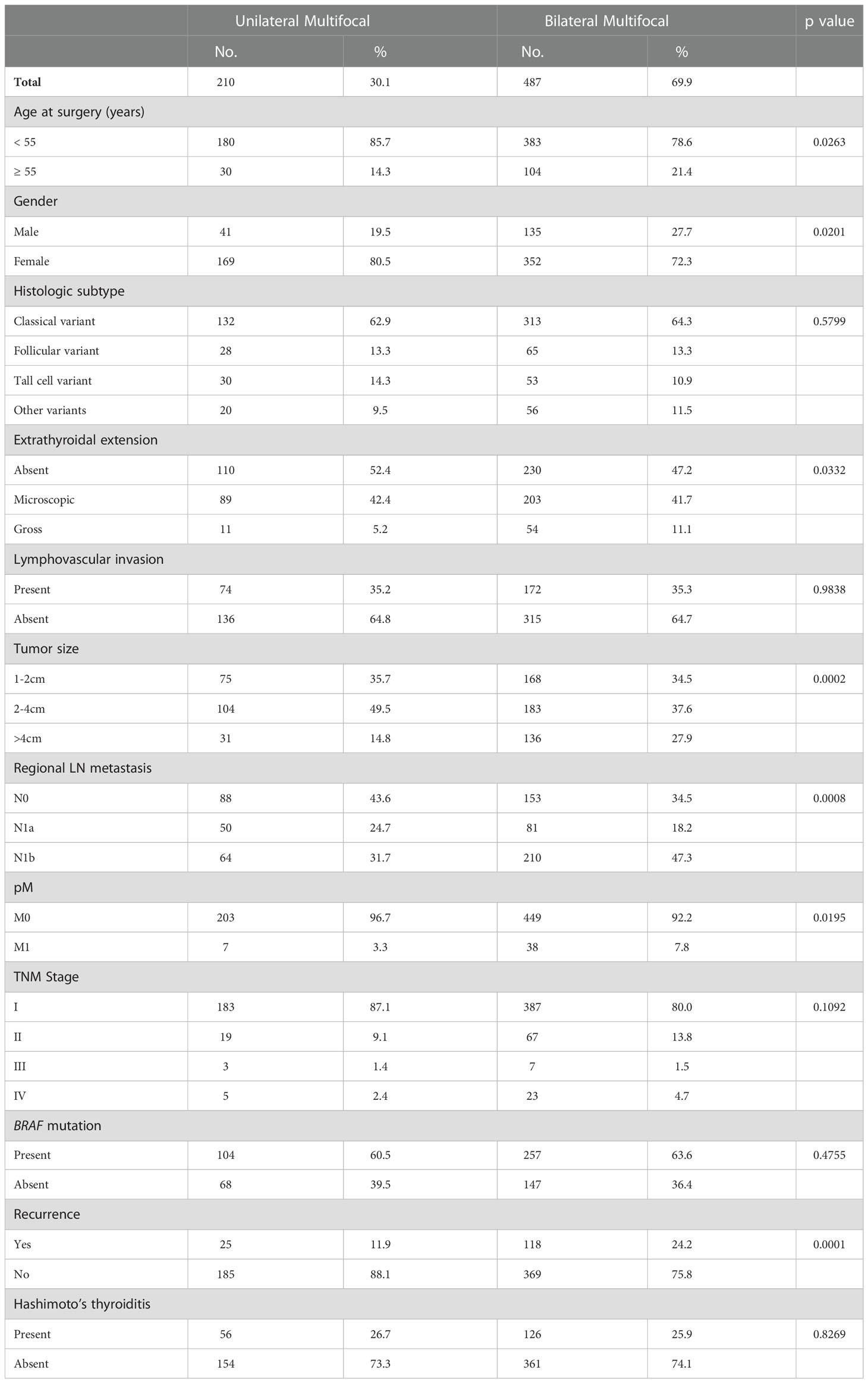
Table 2 Clinico-pathological associations of unilateral and bilateral multifocal papillary thyroid carcinomas.
Since we found bilateral multifocal PTCs to be associated with tumor recurrence, we analysed the prognostic significance of bilateral multifocality with regards to RFS. Using Mantel Cox log rank test, we found that bilateral multifocality was associated with shorter RFS (p = 0.0006) (Figure 1). On multivariate analysis using Cox proportional hazards model, bilateral multifocality was found to be an independent predictor of RFS (Hazard ratio = 1.60; 95% Confidence Interval = 1.05 – 2.55; p = 0.0300) (Table 3).
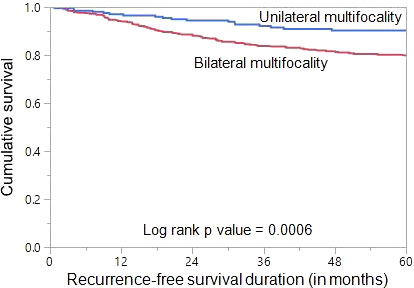
Figure 1 Recurrence-free survival (RFS) in multifocal papillary thyroid carcinoma (PTC). Kaplan-Meier survival curve showing shorter RFS in bilateral multifocal PTC patients compared to those with unilateral multifocal PTC (p = 0.0006).
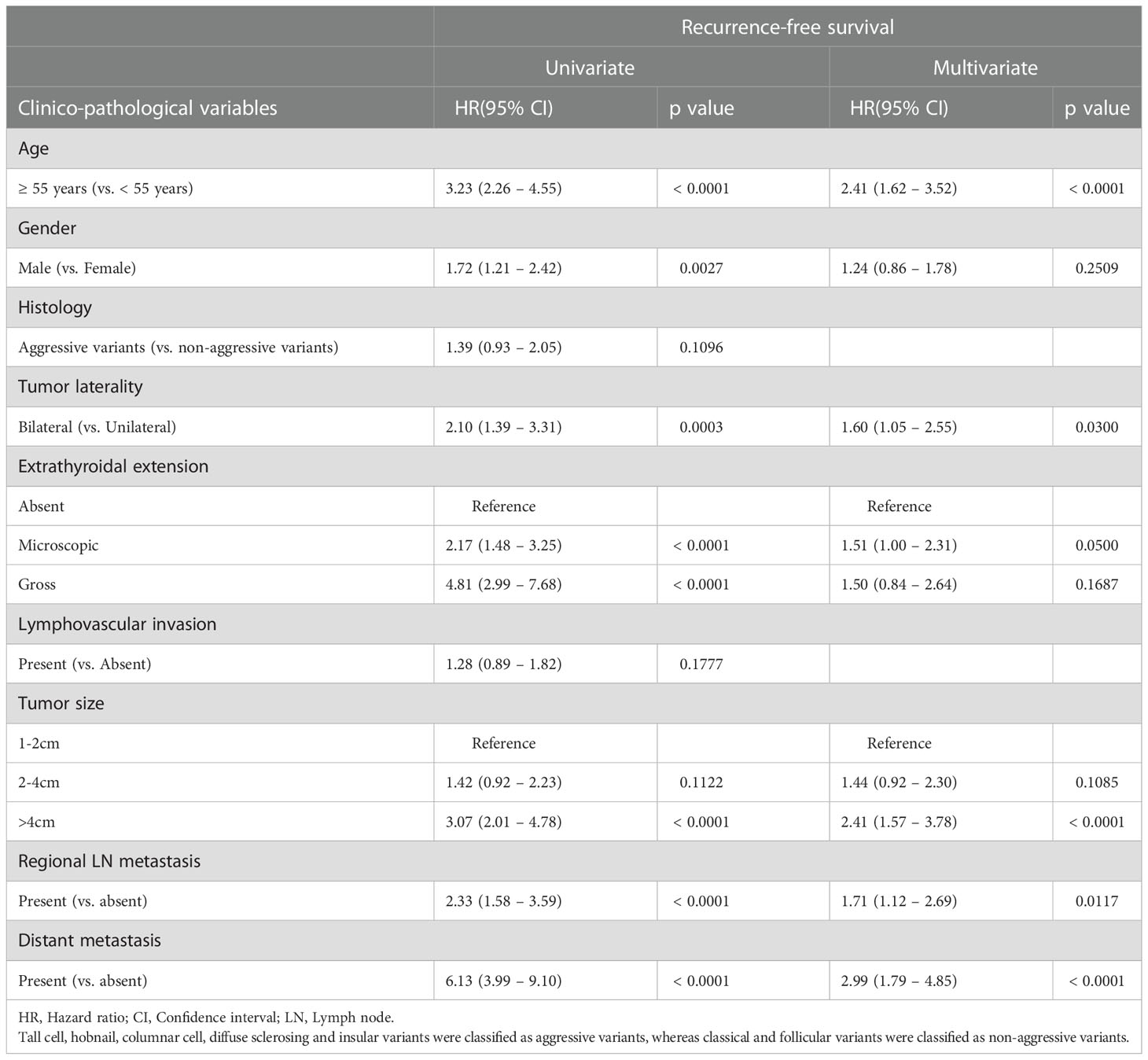
Table 3 Cox proportional hazards model for predictors of recurrence-free survival (RFS) in multifocal PTC.
Next, we sought to determine the clinico-pathological features that could predict bilateral multifocality in PTCs. On univariate analysis using logistic regression model, we found older at diagnosis, male gender, gross extrathyroidal extension, larger tumor size (>4cm), regional lymph node metastasis and distant metastasis at diagnosis to be significantly associated with bilateral multifocality. We included these significant clinico-pathological parameters in a multivariate logistic regression model and found that only tumor size >4cm (Odds ratio = 1.86; 95% Confidence Interval = 1.13 – 3.07; p = 0.0155) was an independent predictor for bilateral multifocality in PTCs (Table 4).
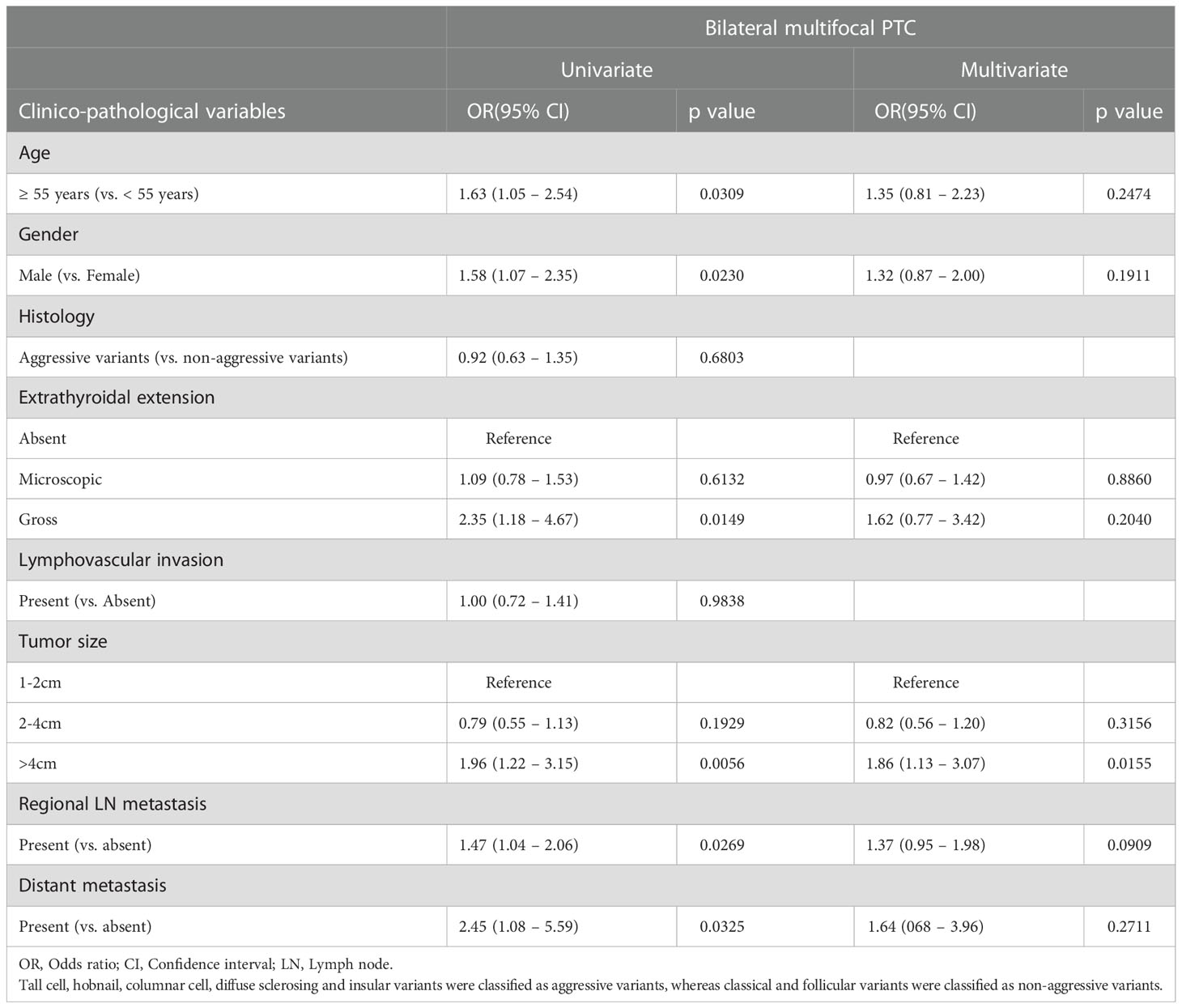
Table 4 Multivariate analysis using logistic regression model for predictors of bilateral multifocality in PTC.
The optimal clinical management of PTC depends on appropriate clinico-pathological risk stratification. Therefore, accurately evaluating the clinico-pathological risk factors is important in achieving the balance between higher therapy benefit and lower adverse complications. Multifocality as prognostic marker remains a highly controversial subject (15, 20–23, 29–33). These conflicting results in the literature could be attributed to the clinical behavior difference between unilateral and bilateral tumors. Even among the few studies that compared unilateral and bilateral multifocal PTC, the prognostic impact of multifocality remains inconsistent. While Sefika et al. (24) could not find association between bilateral involvement in multifocal PTC and prognosis, Jiu et al. (25) found both bilaterality and multifocality to be associated with aggressive tumor but multifocality only was associated with increased risk of recurrence. Another study by Woo et al. (34) found no significant difference between unifocal and multifocal PTC in terms of prognosis, but reported that multifocality was an independent risk factor for recurrence in a cohort of more than 2000 PTCs. In this study, we evaluated for the first time, the incidence of multifocality and clinical impact of unilateral and bilateral subgroups in a large cohort of PTCs from Middle Eastern ethnicity.
In our cohort, multifocality was observed in 54.3% of patients, which is comparable with what has been reported in literature (15, 22, 23, 35). The rate of bilateral multifocal PTC was 69.9% (487/697). The incidence reported in literature ranges from 13 – 71% (23, 24, 36–38). Results of our study show that bilaterality in multifocal PTC is associated with aggressive clinico-pathological markers such as older age, male gender, gross extrathyroidal extension, larger tumor size, locoregional and distant metastasis. Previous studies have also reported an association between multifocality and aggressive clinico-pathological characteristics (23, 24, 39–41).
Interestingly, bilateral multifocality was significantly associated with unfavorable clinical outcomes, being an independent predictor of RFS. The prognostic value of multifocality was significant in bilateral multifocality subgroup compared to unilateral multifocal PTC subgroup, showing an approximately two-fold increased risk of recurrence. Therefore, this study has identified that bilateral multifocality is a risk factor for recurrence after total thyroidectomy in Middle Eastern PTC patients. This is consistent with a previous report which showed that bilateral multifocality was an independent risk factor for neck recurrence as well as distant metastasis (42).
Given the strong prognostic impact of bilateral multifocality, we further evaluated if there are any predictive markers of bilateral multifocal PTCs. We found that tumor diameter of >4cm was the only independent predictor for bilaterality in multifocal PTCs. Previous studies have identified number of tumor foci, TSH level, and BRAF mutation as predictors of bilateral multifocal disease (24, 43). However, number of tumor foci and preoperative TSH level data were not used as variables in this cohort due to lack of documented data on these variables. BRAF mutation was not associated with bilateral multifocal disease in this study.
The present study supported the finding of previous studies that multifocality is associated with aggressive disease (22, 42, 43). Bilateral multifocality has a strong association with advanced disease and more metastatic potential as well as increased risk of recurrence. In addition, bilateral multifocality plays an important role in patient prognosis and in predicting RFS in PTC patients.
Our study has a few limitations. Firstly, this is a retrospective single institute study which includes only PTC patients from Saudi Arabia and hence selection bias is inevitable. The results of this study should be interpreted with caution and generalizability of our results to other ethnicities needs to be validated in large studies from different ethnicities. Secondly, data on the number of tumor foci are not routinely recorded in our histopathological reports and hence we were unable to incorporate this interesting data in our current study.
In conclusion, bilateral multifocality is high in Middle Eastern PTC and is associated with aggressive clinico-pathological markers. Tumor diameter >4cm was predictive of bilateral multifocality in PTC. Bilateral multifocality appears to be an important prognostic factor for PTC and an independent predictor of RFS. Therefore, patient with bilateral multifocal PTC may benefit from more frequent follow-up to identify recurrences earlier.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Research Advisory Council, King Faisal Specialist Hospital and Research Centre. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Study concept and design: KA-K, SP, AS. Executed the study: SP, AS, PA, NS, SA-S, FA-D. Statistical analysis: SP. Drafting the article: KA-K, AS, SP. Critical revision of the article for important intellectual content, writing of the article, and approval of the final version: KA-K, SP, AS, PA, NS, SA-S, FA-D. All authors contributed to the article and approved the submitted version.
The authors would like to thank Felisa DeVera for her technical assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer (2015) 136(9):2187–95. doi: 10.1002/ijc.29251
3. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol (2013) 2013:965212. doi: 10.1155/2013/965212
4. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol (2016) 12(11):646–53. doi: 10.1038/nrendo.2016.110
5. Alrawaji, Alshahrani, Alzahrani, Alomran, Almadouj, Alshehri, et al. Cancer incidence report Saudi Arabia 2015. Council SH, editor. Riyadh: Saudi Cancer Registry (2018).
6. Ritter A, Mizrachi A, Bachar G, Vainer I, Shimon I, Hirsch D, et al. Detecting recurrence following lobectomy for thyroid cancer: Role of thyroglobulin and thyroglobulin antibodies. J Clin Endocrinol Metab (2020) 105(6):dgaa152. doi: 10.1210/clinem/dgaa152
7. Póvoa AA, Teixeira E, Bella-Cueto MR, Melo M, Oliveira MJ, Sobrinho-Simões M, et al. Clinicopathological features as prognostic predictors of poor outcome in papillary thyroid carcinoma. Cancers (2020) 12(11):3186. doi: 10.3390/cancers12113186
8. Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol (2015) 33(1):42. doi: 10.1200/JCO.2014.56.8253
9. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
10. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid (2009) 19(11):1167–214. doi: 10.1089/thy.2009.0110
11. Maciel J, Donato S, Simões H, Leite V. Clinical outcomes of a conservative approach in cervical lymph node metastases of thyroid cancer. Clin Endocrinol (2021) 94(3):460–5. doi: 10.1111/cen.14306
12. Sawant R, FitzGerald A, Hey S, Hulse K, Hay A, Adamson R, et al. Oncological outcomes in differentiated thyroid cancer in south East Scotland. Surg (2021) 19(6):e372–e8. doi: 10.1016/j.surge.2020.11.003
13. van Gerwen M, Sinclair C, Rahman M, Genden E, Taioli E. The impact of surgery refusal on thyroid cancer survival: a SEER-based analysis. Endocr (2020) 70(2):356–63. doi: 10.1007/s12020-020-02301-9
14. Schlumberger M, Leboulleux S, Catargi B, Deandreis D, Zerdoud S, Bardet S, et al. Outcome after ablation in patients with low-risk thyroid cancer (ESTIMABL1): 5-year follow-up results of a randomised, phase 3, equivalence trial. Lancet Diabetes Endocrinol (2018) 6(8):618–26. doi: 10.1016/S2213-8587(18)30113-X
15. Iacobone M, Jansson S, Barczyński M, Goretzki P. Multifocal papillary thyroid carcinoma–a consensus report of the European society of endocrine surgeons (ESES). Langenbeck's Arch Surg (2014) 399(2):141–54. doi: 10.1007/s00423-013-1145-7
16. Filetti S, Durante C, Hartl D, Leboulleux S, Locati L, Newbold K, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2019) 30(12):1856–83. doi: 10.1093/annonc/mdz400
17. Woo J, Kim H, Kwon H. Impact of multifocality on the recurrence of papillary thyroid carcinoma. J Clin Med (2021) 10(21):5144. doi: 10.3390/jcm10215144
18. Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. European Consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol (2006) 154(6):787–803. doi: 10.1530/eje.1.02158
19. Momesso DP, Tuttle RM. Update on differentiated thyroid cancer staging. Endocrinol Metab Clinics (2014) 43(2):401–21. doi: 10.1016/j.ecl.2014.02.010
20. Geron Y, Benbassat C, Shteinshneider M, Or K, Markus E, Hirsch D, et al. Multifocality is not an independent prognostic factor in papillary thyroid cancer: a propensity score–matching analysis. Thyroid (2019) 29(4):513–22. doi: 10.1089/thy.2018.0547
21. Joseph KR, Edirimanne S, Eslick GD. Multifocality as a prognostic factor in thyroid cancer: a meta-analysis. Int J Surg (2018) 50:121–5. doi: 10.1016/j.ijsu.2017.12.035
22. Wang F, Yu X, Shen X, Zhu G, Huang Y, Liu R, et al. The prognostic value of tumor multifocality in clinical outcomes of papillary thyroid cancer. J Clin Endocrinol Metab (2017) 102(9):3241–50. doi: 10.1210/jc.2017-00277
23. Kim HJ, Sohn SY, Jang HW, Kim SW, Chung JH. Multifocality, but not bilaterality, is a predictor of disease recurrence/persistence of papillary thyroid carcinoma. World J Surg (2013) 37(2):376–84. doi: 10.1007/s00268-012-1835-2
24. Polat SB, Cakir B, Evranos B, Baser H, Cuhaci N, Aydin C, et al. Preoperative predictors and prognosis of bilateral multifocal papillary thyroid carcinomas. Surg Oncol (2019) 28:145–9. doi: 10.1016/j.suronc.2018.12.004
25. Feng J-W, Qu Z, Qin A-C, Pan H, Ye J, Jiang Y. Significance of multifocality in papillary thyroid carcinoma. Eur J Surg Oncol (2020) 46(10):1820–8. doi: 10.1016/j.ejso.2020.06.015
26. Tuttle RM, Haugen B, Perrier ND. Updated American joint committee on cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer: what changed and why? 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA: Mary Ann Liebert, Inc (2017) p. 751–6.
27. Pearce EN, Farwell AP, Braverman LE. Thyroiditis. New Engl J Med (2003) 348(26):2646–55. doi: 10.1056/NEJMra021194
28. Siraj AK, Parvathareddy SK, Pratheeshkumar P, Divya SP, Al-Sobhi SS, Al-Dayel F, et al. PD-L1 is an independent prognostic marker in middle Eastern PTC and its expression is upregulated by BRAFV600E mutation. Cancers (2021) 13(3):555. doi: 10.3390/cancers13030555
29. Kim K-J, Kim S-M, Lee YS, Chung WY, Chang H-S, Park CS. Prognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patients. Ann Surg Oncol (2015) 22(1):125–31. doi: 10.1245/s10434-014-3899-8
30. Ardito G, Revelli L, Giustozzi E, Salvatori M, Fadda G, Ardito F, et al. Aggressive papillary thyroid microcarcinoma: prognostic factors and therapeutic strategy. Clin Nucl Med (2013) 38(1):25–8. doi: 10.1097/RLU.0b013e318279bc65
31. Kiriakopoulos A, Petralias A, Linos D. Multifocal versus solitary papillary thyroid carcinoma. World J Surg (2016) 40(9):2139–43. doi: 10.1007/s00268-016-3628-5
32. Tam AA, Ozdemir D, Ogmen BE, Fakı S, Dumlu EG, Yazgan AK, et al. Should multifocal papillary thyroid carcinomas classified as T1A with a tumor diameter sum of 1 to 2 centimeters be reclassified as T1B? Endocr Pract (2017) 23(5):526–35. doi: 10.4158/EP161488.OR
33. Kaliszewski K, Diakowska D, Wojtczak B, Migoń J, Kasprzyk A, Rudnicki J. The occurrence of and predictive factors for multifocality and bilaterality in patients with papillary thyroid microcarcinoma. Medicine (2019) 98(19):e15609. doi: 10.1097/MD.0000000000015609
34. Choi WR, Roh J-L, Gong G, Cho K-J, Choi S-H, Nam SY, et al. Multifocality of papillary thyroid carcinoma as a risk factor for disease recurrence. Oral Oncol (2019) 94:106–10. doi: 10.1016/j.oraloncology.2019.05.023
35. Shi X, Liu R, Basolo F, Giannini R, Shen X, Teng D, et al. Differential clinicopathological risk and prognosis of major papillary thyroid cancer variants. J Clin Endocrinol (2016) 101(1):264–74. doi: 10.1210/jc.2015-2917
36. Pitt SC, Sippel RS, Chen H. Contralateral papillary thyroid cancer: does size matter? Am J Surg (2009) 197(3):342–7. doi: 10.1016/j.amjsurg.2008.09.011
37. Huang G, Tian X, Li Y, Ji F. Clinical characteristics and surgical resection of multifocal papillary thyroid carcinoma: 168 cases. Int J Clin Exp Med (2014) 7(12):5802.
38. Mazeh H, Samet Y, Hochstein D, Mizrahi I, Ariel I, Eid A, et al. Multifocality in well-differentiated thyroid carcinomas calls for total thyroidectomy. Am J Surg (2011) 201(6):770–5. doi: 10.1016/j.amjsurg.2010.03.004
39. Del Rio P, Loderer T, Giuffrida M, Cozzani F, Rossini M, Bonfili D, et al. Multifocality in patients treated for papillary thyroid carcinoma: a preliminary analysis of related risk factors. Acta Bio Med: Atenei Parmensis (2021) 92(5):e2021017. doi: 10.23750/abm.v92i5.11897
40. Han JR, Jung JH, Kim WW, Lee J, Park HY, Kim HJ, et al. Bilateral papillary thyroid cancer increases the risk of lymph node metastasis compared with unilateral multifocal papillary thyroid cancer. J Endocr Surg (2017) 17(2):63–72. doi: 10.16956/jes.2017.17.2.63
41. Suh YJ, Kwon H, Kim S-j, Choi JY, Lee KE, Park YJ, et al. Factors affecting the locoregional recurrence of conventional papillary thyroid carcinoma after surgery: a retrospective analysis of 3381 patients. Ann Surg Oncol (2015) 22(11):3543–9. doi: 10.1245/s10434-015-4448-9
42. Qu N, Zhang L, Wu W-l, Ji Q-h, Lu Z-w, Zhu Y-x, et al. Bilaterality weighs more than unilateral multifocality in predicting prognosis in papillary thyroid cancer. Tumor Biol (2016) 37(7):8783–9. doi: 10.1007/s13277-015-4533-5
Keywords: papillary thyroid carcinoma, multifocality, bilateral, recurrence-free survival, prognosis
Citation: Parvathareddy SK, Siraj AK, Annaiyappanaidu P, Siraj N, Al-Sobhi SS, Al-Dayel F and Al-Kuraya KS (2023) Bilateral multifocality is an independent predictor of patients’ outcome in Middle Eastern papillary thyroid carcinoma. Front. Endocrinol. 13:1060301. doi: 10.3389/fendo.2022.1060301
Received: 03 October 2022; Accepted: 12 December 2022;
Published: 04 January 2023.
Edited by:
Marco Raffaelli, Fondazione Policlinico Universitario Agostino Gemelli IRCCS - Università Cattolica del Sacro Cuore, ItalyReviewed by:
Gian Luigi Canu, University of Cagliari, ItalyCopyright © 2023 Parvathareddy, Siraj, Annaiyappanaidu, Siraj, Al-Sobhi, Al-Dayel and Al-Kuraya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khawla S. Al-Kuraya, a2t1cmF5YUBrZnNocmMuZWR1LnNh
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.