- 1Department of Medicine, School of Clinical Medicine, University of Hong Kong, Queen Mary Hospital, Hong Kong, Hong Kong SAR, China
- 2State Key Laboratory of Pharmaceutical Biotechnology, University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 3Department of Obstetrics and Gynaecology, University of Hong Kong, Queen Mary Hospital, Hong Kong, Hong Kong SAR, China
- 4The School of Public Health, University of Hong Kong, Queen Mary Hospital, Hong Kong, Hong Kong SAR, China
- 5Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 6State Key Laboratory of Liver Research, University of Hong Kong, Hong Kong, Hong Kong SAR, China
Background: Non-diabetic overweight/obese metabolic dysfunction-associated fatty liver disease (MAFLD) represents the largest subgroup with heterogeneous liver fibrosis risk. Metabolic dysfunction promotes liver fibrosis. Here, we investigated whether incorporating additional metabolic risk factors into clinical evaluation improved liver fibrosis risk stratification among individuals with non-diabetic overweight/obese MAFLD.
Materials and methods: Comprehensive metabolic evaluation including 75-gram oral glucose tolerance test was performed in over 1000 participants from the New Hong Kong Cardiovascular Risk Factor Prevalence Study (HK-NCRISPS), a contemporary population-based study of HK Chinese. Hepatic steatosis and fibrosis were evaluated based on controlled attenuation parameter and liver stiffness (LS) measured using vibration-controlled transient elastography, respectively. Clinically significant liver fibrosis was defined as LS ≥8.0 kPa. Our findings were validated in an independent pooled cohort comprising individuals with obesity and/or polycystic ovarian syndrome.
Results: Of the 1020 recruited community-dwelling individuals, 312 (30.6%) had non-diabetic overweight/obese MAFLD. Among them, 6.4% had LS ≥8.0 kPa. In multivariable stepwise logistic regression analysis, abnormal serum aspartate aminotransferase (AST) (OR 7.95, p<0.001) and homeostasis model assessment of insulin resistance (HOMA-IR) ≥2.5 (OR 5.01, p=0.008) were independently associated with LS ≥8.0 kPa, in a model also consisting of other metabolic risk factors including central adiposity, hypertension, dyslipidaemia and prediabetes. A sequential screening algorithm using abnormal AST, followed by elevated HOMA-IR, was developed to identify individuals with LS ≥8.0 kPa, and externally validated with satisfactory sensitivity (>80%) and negative predictive value (>90%).
Conclusion: A sequential algorithm incorporating AST and HOMA-IR levels improves fibrosis risk stratification among non-diabetic overweight/obese MAFLD individuals.
Introduction
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a new nomenclature defining fatty liver disease with metabolic dysfunction proposed by an international expert panel in 2020, and affects one-third of the global adult population (1–3). MAFLD can be classified into three subtypes, based on the presence of hepatic steatosis co-existing with any one of the following including (1) type 2 diabetes (T2D-MAFLD); (2) overweight or obesity (overweight/obese MAFLD), defined as body mass index (BMI) ≥23kg/m2 in Asians and 25kg/m2 in Caucasians; and (3) in lean/normal weight individuals, the presence of any two of the other evidence of metabolic dysfunction (Lean MAFLD), including central obesity, elevated blood pressure (BP), dyslipidaemia, prediabetes, increased homeostasis model assessment of insulin resistance (HOMA-IR), and elevated circulating high-sensitivity C-reactive protein (hsCRP) levels (1).
In fatty liver disease, liver fibrosis is the most important determinant of adverse long-term outcomes. Higher stage of liver fibrosis is associated with all-cause, as well as liver-related, mortality and morbidity (4, 5). Early identification of individuals with clinically significant liver fibrosis, who are at risk of compensated advanced chronic liver disease (cACLD) (6, 7), is important to facilitate more targeted follow-up and surveillance, especially among overweight and obese individuals in whom the prevalence of MAFLD is over 50% (8). Several recent studies have suggested that the prevalence and risk of liver fibrosis differ across the three MAFLD subtypes (9–11). However, while individuals with overweight/obese MAFLD constitute the largest subgroup within the MAFLD population, no report thus far has evaluated the optimal strategy for stratifying liver fibrosis risk in these individuals. Previous studies in non-alcoholic fatty liver disease (NAFLD) have shown that the presence of metabolic dysfunction was closely associated with the development of liver fibrosis (12). Hence, we investigated whether incorporating additional metabolic risk factors into clinical evaluation would improve liver fibrosis risk stratification among individuals with overweight/obese MAFLD, using a contemporary population-based study of Hong Kong (HK) Chinese with comprehensive metabolic assessment.
Materials and methods
Study participants
All participants were recruited from the New HK Cardiovascular Risk Factor Prevalence Study (NCRISPS), an ongoing population-based, cross-sectional study established since December 2019, to determine the updated sex and age-stratified prevalence of cardiovascular risk factors, cardiovascular diseases (CVD) and related disorders in HK Chinese. The protocol was similar to the previously published HK Cardiovascular Risk Factor Prevalence Study (CRISPS) (13). Individuals aged 25 – 74 years were recruited from the community through systematic sampling of representative replicates of living quarters in HK, obtained from the Census and Statistics Department of the HK Special Administrative Region. Pregnant women, individuals with physical or mental illness which precluded them from travelling to the study centre for health assessment, or from providing informed consent, were excluded. The protocol of NCRISPS was approved by the Ethics Committee of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (IRB Ref: UW-18-610). Written informed consent was obtained from all participants before any study-related procedures.
Clinical assessments
All NCRISPS participants attended the study visit after an overnight fast of at least 8 hours. Each participant completed a detailed questionnaire which included demographics (age, sex, occupation, income, education level, smoking and alcohol intake), family history, medical (personal history of diabetes, hypertension, hyperlipidaemia, CVD, cancers, chronic liver diseases in particular viral hepatitis, Wilson’s disease, alpha-1 antitrypsin deficiency, autoimmune hepatitis and primary biliary cholangitis), and drug histories (anti-diabetic, lipid lowering, anti-hypertensive, and steatogenic medications such as amiodarone, tamoxifen, methotrexate etc.). Bloods were drawn for complete blood count and serum creatinine levels. Estimated glomerular filtration rate (eGFR) of the participants was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation as described previously (14).
Metabolic assessments
Anthropometric parameters including body weight (BW), height (BH), BMI, waist and hip circumferences (WC and HC, respectively) and BP were measured as previously described (13). Fasting bloods were drawn for glycated haemoglobin (HbA1c) and lipid profile. All participants, except those on anti-diabetic medications, underwent a 75-gram oral glucose tolerance test (OGTT). Moreover, except for those receiving insulin therapy, fasting insulin level was measured to determine the HOMA-IR level (15).
Hepatic assessments
Liver biochemistry including serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured at the Pathology Department of Queen Mary Hospital, Hong Kong, a laboratory accredited by the College of American Pathologists. The definitions of abnormal ALT and AST levels in the laboratory were based on age- and sex-specific reference ranges established using data collected from a cohort of local healthy Chinese (13). Hepatitis B surface antigen (HBsAg) were measured in all participants, whereas antibody against hepatitis C virus (Anti-HCV) was measured in those with elevated ALT or AST levels above the upper normal range (ALT: 58U/L for men, and 36 U/L for women aged ≤50 years and 45 U/L for women aged >50 years; AST: 38 U/L for men, and 30 U/L for women aged ≤50 years and 37 U/L for women aged >50 years), and/or HBsAg-positivity. Two commonly used non-invasive conventional fibrosis scores including Fibrosis-4 index (FIB-4) and NAFLD fibrosis score (NFS) were determined using published formulae (16).
During the study visit, all participants underwent vibration controlled transient elastography (VCTE) assessments, performed by trained operators using Fibroscan (Echosens, Paris, France) as per protocol described previously (17). In all participants, M probe was used during assessments, unless prompted by the VCTE machine. Controlled attenuation parameter (CAP) and liver stiffness (LS), which assessed the severity of liver steatosis and fibrosis, respectively, were measured with values represented by the median of 10 reliable measurements, defined when the interquartile range was <30% and the success rate was >60%. Only CAP values with an inter-quartile range of 40 dB/m were used to ensure data validity.
Definition of outcomes and clinical variables
Hepatic steatosis was defined as CAP ≥248 dB/m. Liver fibrosis was graded by LS cut-offs: <8.0 kPa (low risk), 8.0 – 9.5 kPa (intermediate to high risk), ≥9.6 kPa (advanced fibrosis and cirrhosis) (18). In this study, clinically significant liver fibrosis was defined as LS ≥8.0 kPa (7).
In this study consisting of exclusively HK Chinese, overweight and obesity were defined as BMI ≥23kg/m2 and ≥27.5kg/m2, respectively (1, 19). Central obesity was defined as WC ≥90cm in men and ≥80cm in women (1, 20). Hypertension was defined as BP ≥140/90mmHg or on anti-hypertensive medications. Dyslipidaemia was defined as fasting triglycerides (TG) ≥1.7 mmol/L, high density lipoprotein-cholesterol (HDL-C) <1.3 mmol/L in women and <1.0 mmol/L in men, low density lipoprotein-cholesterol (LDL-C) ≥3.4 mmol/L, or on lipid-lowering medications. Normal glucose tolerance (NGT) was defined as FG <5.6 mmol/L and 2-hour blood glucose (2hG) <7.8 mmol/L. IFG was defined as FG ≥5.6 mmol/L and <7.0 mmol/L, whereas IGT was defined as 2hG ≥7.8 mmol/l and <11.1 mmol/L. Prediabetes included IFG, IGT or elevated HbA1c ≥5.7% and <6.5%. Type 2 diabetes was defined as the presence of any two of the following biochemical abnormalities: FG ≥7.0 mmol/L, or 2hG ≥11.1 mmol/L on OGTT, or HbA1c ≥6.5%, or on anti-diabetic medications (21). CKD was defined as eGFR <60ml/min/1.73m2. CVD was defined as any self-reported or medical history of cardiovascular event, including myocardial infarction, stroke, transient ischaemic attack, peripheral vascular disease, heart failure, recorded based on diagnostic codes (402, 404, 410-414,425-447, and 518.4) from the HK Hospital Authority database. Excessive alcohol intake was defined as daily alcohol consumption of >3 drinks in men and >2 drinks in women (1).
Independent cohorts for external validation
Two independent cohorts consisting of individuals without type 2 diabetes and fulfilled the diagnostic criteria of overweight/obese MAFLD, based on reliable M probe measurements with VCTE, were used to form a pooled external cohort for validating our findings. The first cohort comprised individuals from the Obesity Clinic of Queen Mary Hospital, HK (N=40), whereas the second cohort involved participants from a longitudinal follow-up study of polycystic ovarian syndrome (PCOS) at Queen Mary Hospital, HK (N=31) (22).
Statistical analysis
All data were analysed with IBM SPSS Statistics 26.0 (http://www.IBM.com/SPSS). Data normality was determined by the Kolmogorov-Smirnov test. Values were reported as mean ± standard deviation (SD), medians with interquartile range (IQR), or percentages, as appropriate. Continuous variables between two groups were compared using independent t-test or Mann-Whitney U test, whereas one-way analysis of variance (ANOVA) or Kruskal-Wallis test were used to compare among multiple groups. Categorical variables were compared using Chi-square or Fisher Exact test, as appropriate. Bonferroni correction was applied for multiple comparisons. Cochran-Armitage test was applied for evaluating trend for binary variables, whereas ANOVA linear test or Jonckheere-Terpstra test was used for evaluating continuous variables. Multiple quantile regression analysis was conducted to investigate the associations of clinical variables with LS, accounting for the potential heterogeneity in the association of differently explanatory variables across the different quantiles of LS. Multivariable stepwise logistic regression analysis was performed to evaluate the independent determinants of the presence of LS ≥8.0 kPa and develop a screening algorithm for identifying individuals with overweight/obese MAFLD who had LS ≥8.0 kPa. Sensitivity, specificity, positive and negative predictive values (PPV and NPV) and the area under the receiver operating characteristic curve (AUROC) of the screening algorithm were evaluated to determine its performance. In all statistical tests, a two-sided p-value of <0.05 was considered significant.
Results
Overweight/obese MAFLD constituted the largest MAFLD subgroup
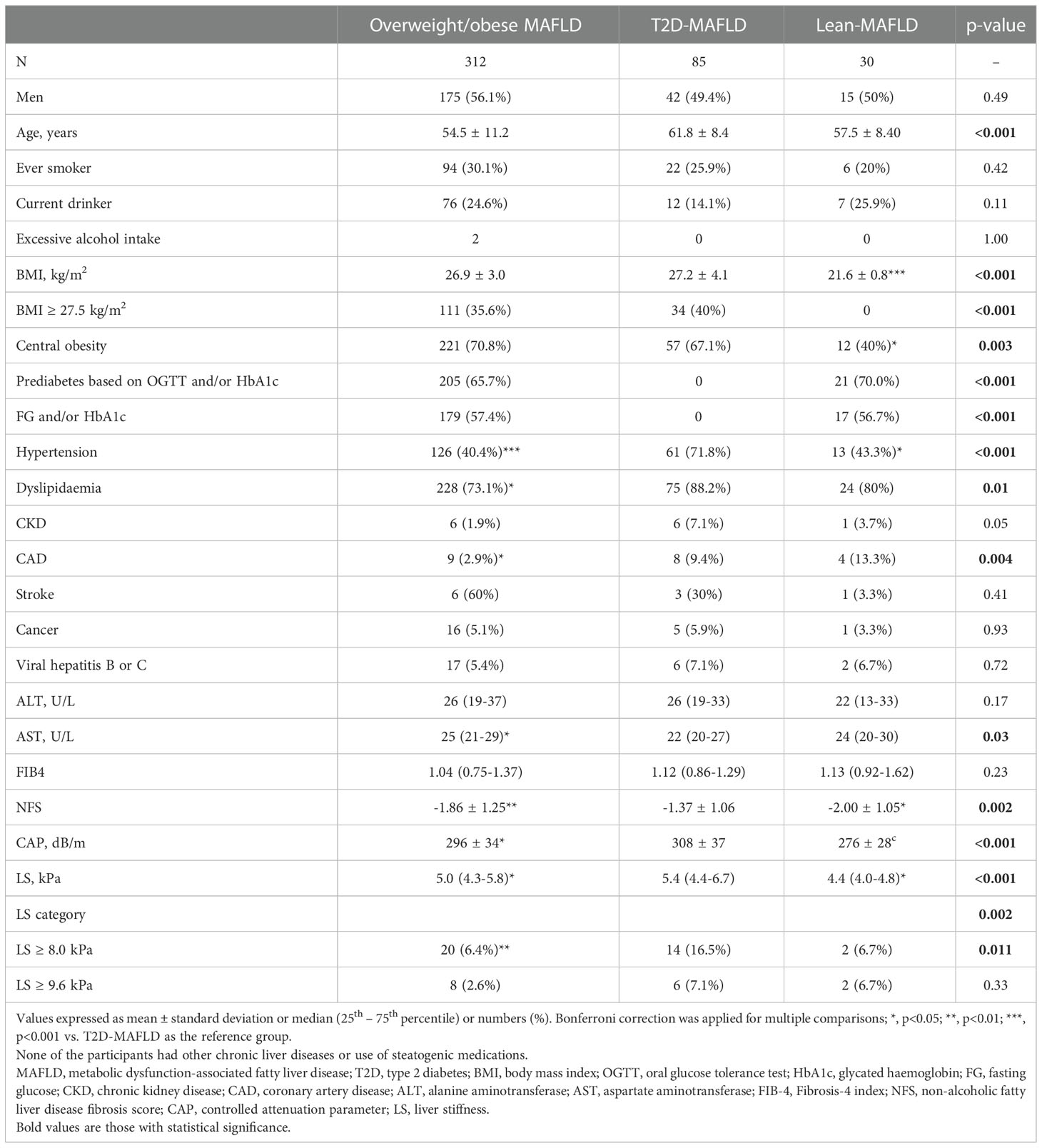
Of 1020 NCRISPS participants, 445 (43.6%) of them had fatty liver disease as defined by CAP ≥248 dB/m. All participants had valid CAP and LS measurements on VCTE using M probe. Participants who had fatty liver disease were significantly older (56.1 vs. 54.1 years) (p=0.004), being men (53.7% vs 40.9%) (p<0.001) and ever-smoker (28.1% vs. 20.7%) (p=0.006), with higher BMI (26.4 kg/m2 vs. 22.3 kg/m2), HOMA-IR (2.31 vs. 1.27) and prevalence of hypertension (43.4% vs. 25.7%), diabetes (19.1% vs 7.0%), dyslipidaemia (74.4% vs. 47.3%) (all p<0.001) and CKD (2.9% vs. 0.9%) (p=0.014) than those who did not. Among these 445 participants, 427 fulfilled the diagnostic criteria of MAFLD, and the majority (73%) had overweight/obese MAFLD, followed by T2D-MAFLD (20%) and lean-MAFLD (7%). Notably, participants with T2D-MAFLD, as compared to the other two subgroups, had significantly higher NFS (p=0.002), CAP (p<0.001) and LS measurements (p<0.001) despite similar serum ALT levels (Table 1).
Associations of metabolic risk factors with liver fibrosis risk in overweight/obese MAFLD
Among the 312 participants who did not have diabetes and had overweight/obese MAFLD, the majority (93.6%) were at low risk of liver fibrosis with LS <8.0 kPa. (Table 2) Higher stages of liver fibrosis were significantly associated with higher serum ALT (p for trend <0.001) and AST levels (p for trend <0.001), CAP (p for trend = 0.002) and NFS (p for trend =0.002). Moreover, with regard to the metabolic risk factors considered in the MAFLD definition (1), participants with higher stages of liver fibrosis had significantly higher prevalence of prediabetes based on OGTT and/or HbA1c (p for trend = 0.01), and were more insulin resistant as indicated by HOMA-IR ≥2.5 (p for trend <0.001). An increasing number of these metabolic risk factors was significantly associated with higher LS values (p for trend <0.001) (Table 2).
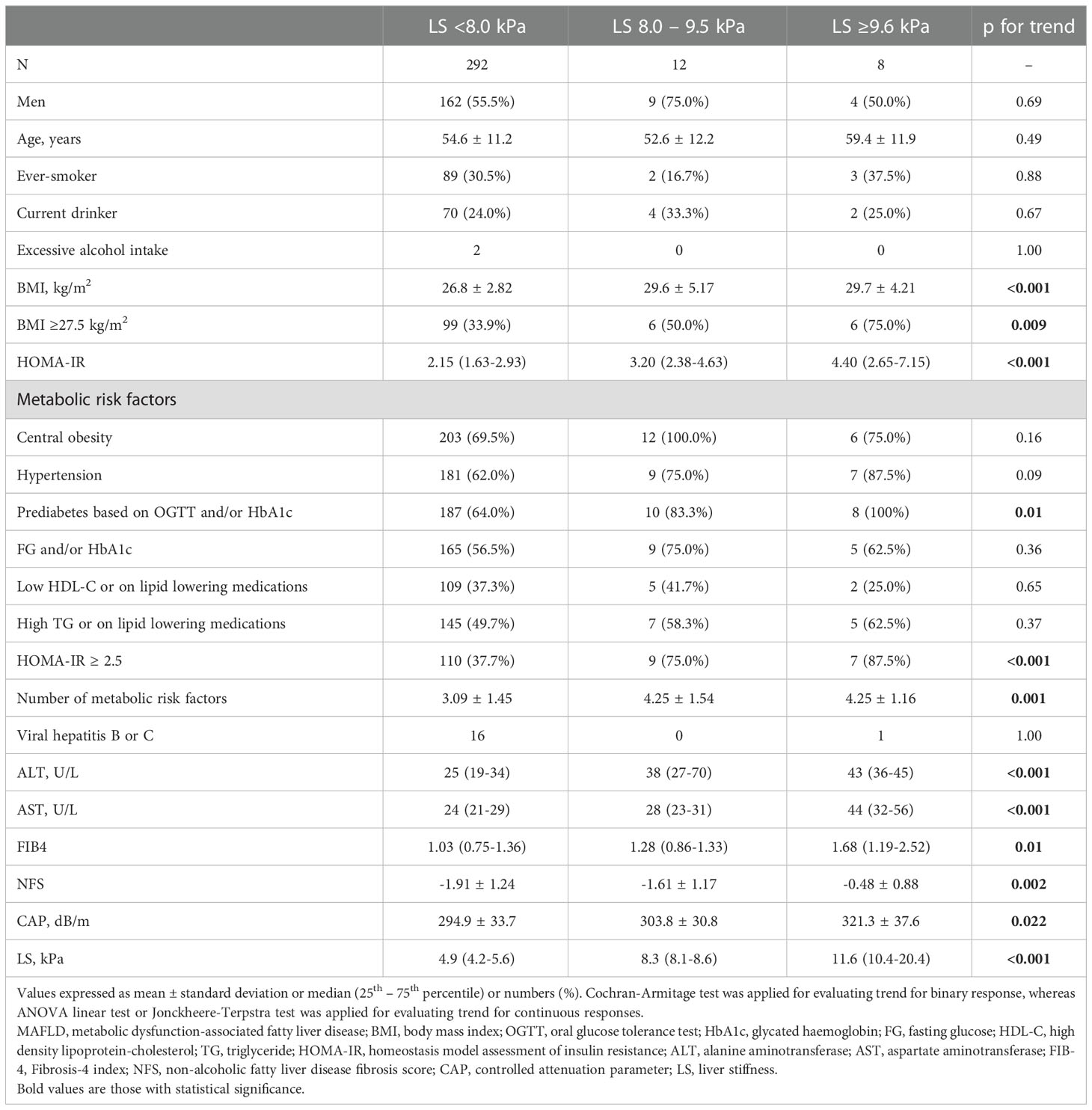
Table 2 Associations of clinical characteristics with the severity of liver fibrosis among participants with overweight/obese MAFLD (N=312).
In multiple quantile regression analysis, in the first quantile, only BMI ≥27.5 kg/m2 (p=0.017) and central obesity (p=0.004) were significantly associated with LS, whereas in the second quantile, abnormal AST level became the only significant determinant (p <0.001). In the third quantile and the 90th percentile, both abnormal AST level (p<0.001) and HOMA-IR ≥2.5 (p<0.001) were significant independent determinants of LS, in a model also consisting of hypertension, abnormal serum ALT level, prediabetes based on OGTT and/or HbA1c, as well as high TG or on lipid lowering medications. Moreover, the effects of abnormal AST and HOMA-IR ≥2.5 on LS increased with higher LS quantiles (Table 3).
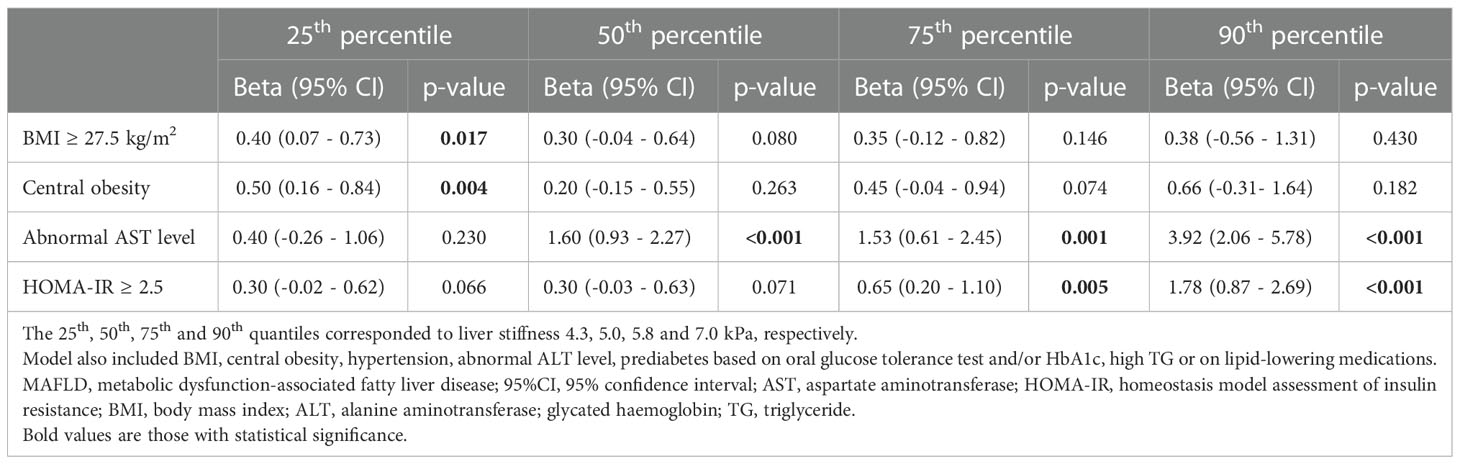
Table 3 Multiple quantile regression analysis showing the independent determinants of higher liver stiffness in participants with overweight/obese MAFLD (N=312).
Sequential screening algorithm for identifying individuals with non-diabetic overweight/obese MAFLD who had clinically significant liver fibrosis (LS ≥8.0 kPa)
Among these 312 participants with overweight/obese MAFLD and without diabetes, 20 (6.4%) of them had LS ≥8.0 kPa. Participants with LS ≥8.0 kPa had significantly higher BMI (p<0.001), abnormal ALT (p=0.038) and AST levels (p<0.001), NFS ≥-1.5 (p=0.003), prevalence of prediabetes based on OGTT and/or HbA1c levels (p=0.031) and HOMA-IR ≥2.5 (p=0.001) than those without (Supplementary Table S1).
To derive a screening algorithm for identifying individuals with overweight/obese MAFLD who had LS ≥8.0 kPa, multivariable stepwise logistic regression analysis was conducted in a stepwise fashion, based on the availability of parameters during the routine clinical care for patients with MAFLD. In the first step which consisted of BMI ≥27.5kg/m2, a cut-off used to define obesity among Asian individuals (19), as well as abnormal transaminase levels in the model, only abnormal AST level was independently associated with the presence of significant liver fibrosis (OR 8.96, 95%CI 3.3 – 24.3, p<0.001). In the next step when metabolic risk factors including prediabetes (based on OGTT and/or HbA1c) and HOMA-IR ≥2.5 were also included in the model, both abnormal AST level (OR 7.95, 95%CI 2.83 – 22.4, p<0.001) and HOMA-IR ≥2.5 (OR 5.01, 95%CI 1.54 – 16.3, p=0.008) remained independently associated with LS ≥8.0 kPa. (Table 4) The results were similar when abnormal serum transaminase levels were replaced by elevated NFS (Supplementary Table S2), or when BMI ≥27.5kg/m2 was replaced by a more generally used obesity cut-off of ≥30kg/m2. Notably, among the metabolic risk factors (Supplementary Table S1), HOMA-IR ≥2.5 was the only independent determinant of significant liver fibrosis (OR 4.08, 95%CI 1.54 – 16.0) in multivariable logistic regression analysis.
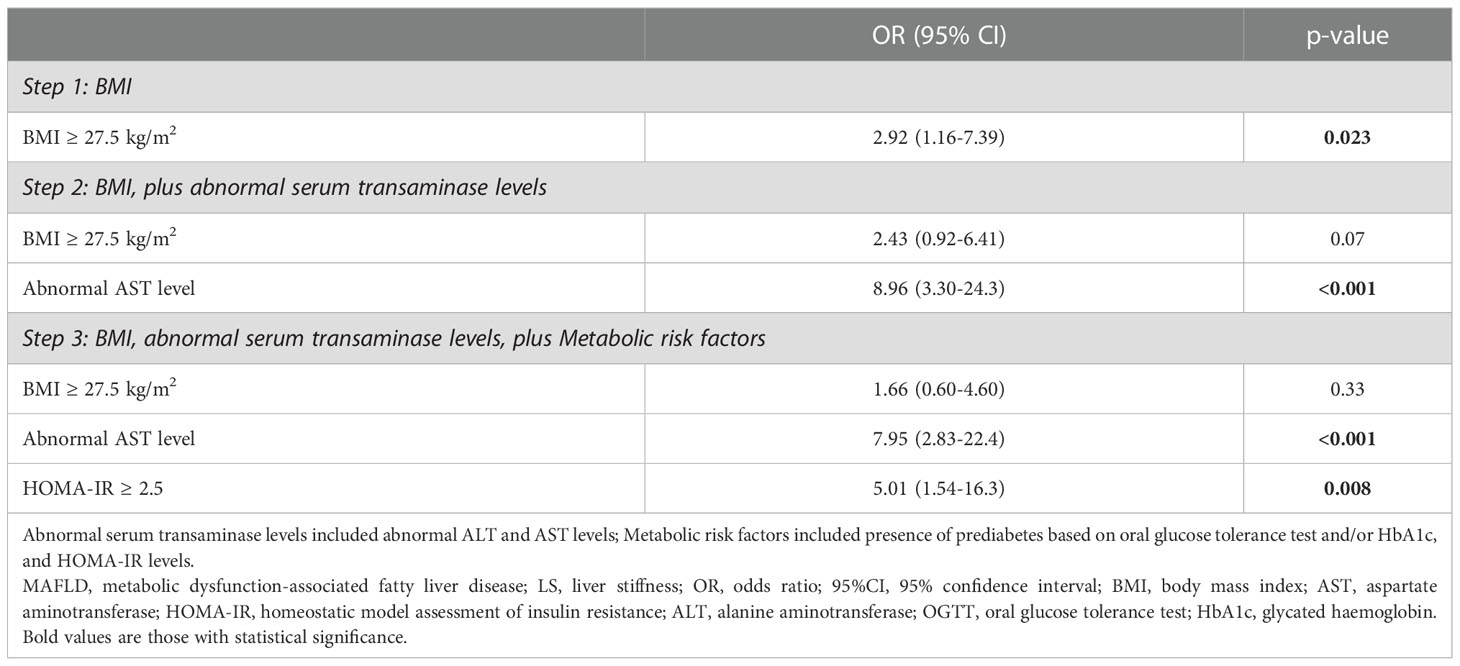
Table 4 Multivariable stepwise logistic regression showing the associations of clinical variables with LS ≥8.0 kPa in participants with overweight/obese MAFLD (N=312).
Performance of sequential screening algorithm in derivation and validation cohorts
In NCRISPS, the sensitivity and specificity of this sequential screening algorithm (Figure 1) to identify individuals with non-diabetic overweight/obese MAFLD who were at risk of clinically significant liver fibrosis was 90% and 58.6%, respectively. Importantly, the NPV was 98.8% with a PPV of 12.9%. (Table 5) The AUROC was 0.80 (95%CI 0.71 – 0.90). The performance was similar between men and women (Supplementary Table S3). In the pooled validation cohort, with baseline characteristics of the participants shown in Supplementary Table S3, the AUROC was 0.68 (95%CI 0.50 – 0.87). The sensitivity was 81.8% with an NPV of 91.3% (Table 5).
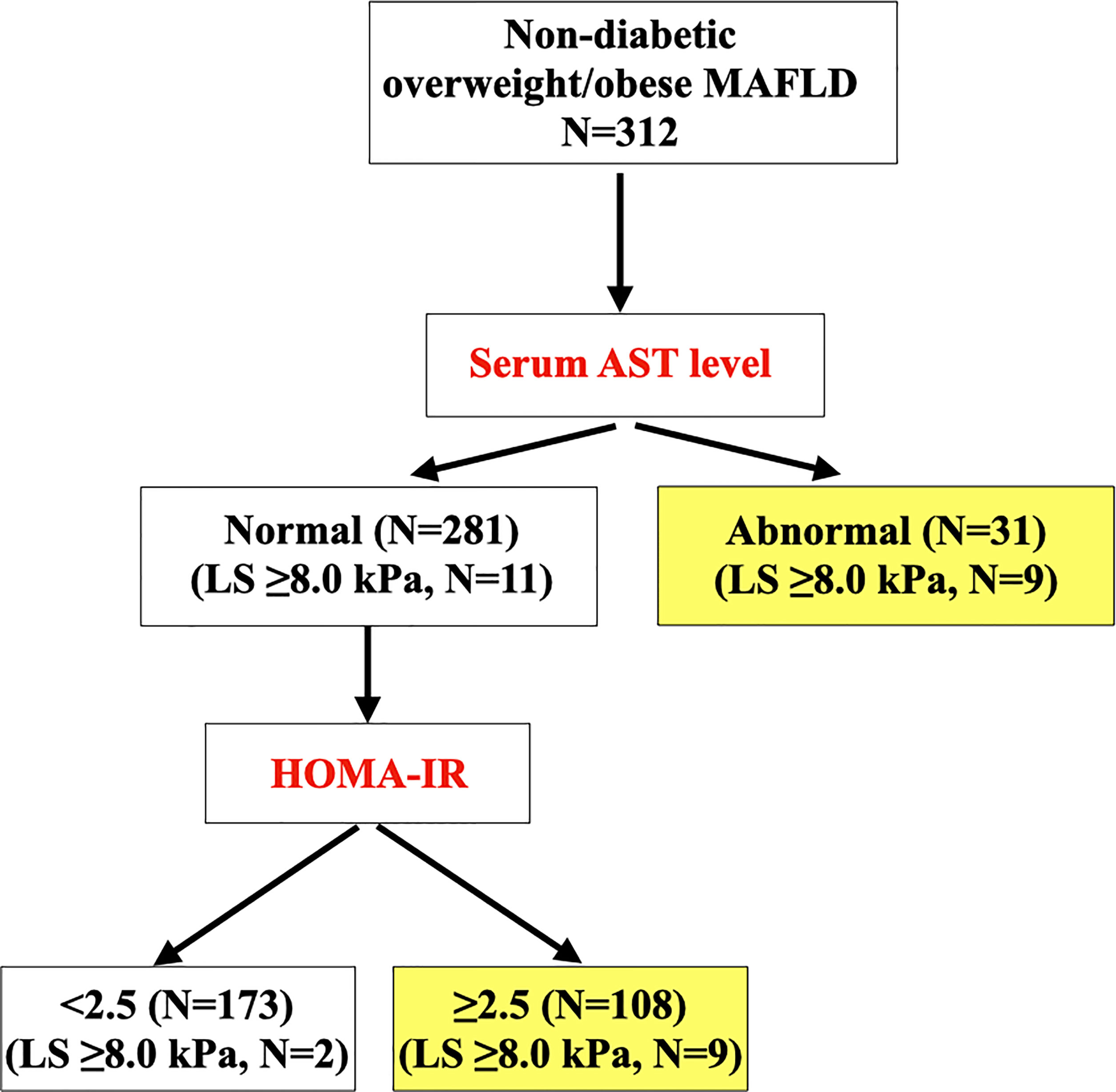
Figure 1 Sequential clinical algorithm for identifying overweight/obese participants with MAFLD at risk of clinically significant liver fibrosis (i.e. LS ≥8.0 kPa) (N=312). MAFLD, metabolic dysfunction-associated fatty liver disease; cACLD, compensated advanced chronic liver disease; LS, liver stiffness; AST, aspartate aminotransferase; HOMA-IR, homeostasis model assessment of insulin resistance.
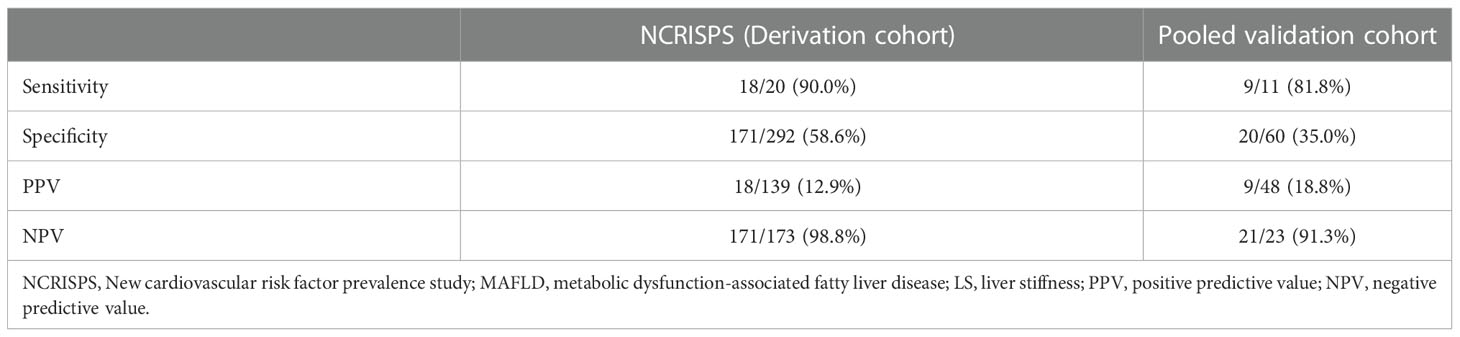
Table 5 Performance of the sequential screening algorithm in NCRISPS and the pooled validation cohort for identifying non-diabetic overweight/obese participants with MAFLD at risk of at risk of clinically significant liver fibrosis (i.e. LS ≥8.0 kPa).
Discussion
In this contemporary population-based study of HK Chinese with comprehensive metabolic evaluation, we demonstrated that 1 in 3 of our local community-dwelling individuals had overweight/obese MAFLD. However, despite this high prevalence, only <3% and 6.4% of them had advanced and clinically significant liver fibrosis, respectively. Therefore, we have developed a simple, sequential screening algorithm based on abnormal AST, followed by elevated HOMA-IR levels. We demonstrated, with external validation, that the clinical performance of this algorithm was satisfactory with sensitivity over 80% and NPV over 90%. Although the specificity and PPV were relatively low, the high sensitivity and NPV were particularly important for a screening algorithm, which allowed us to optimally identify, among this large group of individuals with non-diabetic overweight/obese MAFLD, those who were at risk of cACLD and would require referral to hepatologists for VCTE and/or further hepatic evaluation.
Since the proposal of the new diagnostic entity of MAFLD, only a few studies have directly compared the three different MAFLD subgroups (9–11). The Rotterdam Study showed that the prevalence of hepatic fibrosis, defined as LS ≥8.0 kPa, increased significantly when individuals fulfilled all three diagnostic criteria of MAFLD which included type 2 diabetes, overweight/obesity or having two or more metabolic abnormalities, as compared to those satisfying only one or two inclusion criteria (23). It is also well established that type 2 diabetes is an important risk factor of fibrosis progression in fatty liver disease (24). A recent meta-analysis reported that 1 in 5 patients with type 2 diabetes had elevated LS (25). Consistently, in our study, individuals with T2D-MAFLD had significantly higher LS and prevalence of clinically significant liver fibrosis than the other two MAFLD subgroups. Indeed, several non-invasive fibrosis scores and novel biomarkers have been investigated over the years for their performance to stratify liver fibrosis risk specifically among individuals with T2D-MAFLD (26–28). (29) On the other hand, as shown by us and others, non-diabetic overweight/obese MAFLD constitutes the largest MAFLD population within the community (9–11). Although the studies were not directly comparable, our 73% prevalence of non-diabetic overweight/obese MAFLD was overall similar to the 77.5% reported in a community-based survey in Beijing, and lower than the 95.2% in a Korean study (9, 10). However, we found that their overall risk of significant liver fibrosis was much lower than that of T2D-MAFLD and correlated significantly with the presence of additional metabolic comorbidities.
Hence, in this study, we evaluated whether taking into consideration the presence of additional metabolic risk factors would improve the identification of clinically significant liver fibrosis specifically among individuals with overweight/obese MAFLD. The contemporary study population, together with the comprehensive metabolic assessments, which included OGTT in all our participants (except for those taking anti-diabetic medications), are two major strengths of our study. Indeed, we found that 41.7% of our study participants had MAFLD, a prevalence rate that was considerably higher than the 25.9% reported in a local population study using the HK census database performed over a decade ago (30). Although the two studies differed in the imaging modality employed for the detection of hepatic steatosis, our updated local MAFLD prevalence was overall in keeping with that reported globally in a recent meta-analysis (2). This probably reflected the soaring prevalence of obesity and related metabolic diseases such as prediabetes both locally and globally (31, 32), and the additional use of OGTT for evaluating glycaemic status in our study.
We found that, of all the metabolic risk factors including obesity, central adiposity, hypertension, dyslipidaemia and prediabetes diagnosed based on OGTT and/or HbA1c, elevated HOMA-IR was the only independent determinant of LS >8.0 kPa. These findings, which were derived from non-diabetic overweight/obese MAFLD participants, concurred with those reported in a previous study of obese individuals with NAFLD that HOMA-IR was an independent predictor of worsening histological fibrosis (33). Indeed, amongst the multiple hits in the pathogenesis of fatty liver disease, insulin resistance is a key driver of its progression (34). With increased lipolysis in the adipocytes and de novo lipogenesis in the liver, free fatty acid accumulates and lipo-toxicity ensues. Hepatocyte injury causes inflammation with increased cytokines production by the Kupffer cells, vascular remodeling, and activation of regenerative processes. Repetitive unsuccessful regenerative responses lead to progressive scarring, advanced fibrosis and cirrhosis (35). Furthermore, hyperinsulinaemia, which occurs secondary to insulin resistance, also promotes hepatic fibrosis through stimulating the proliferation of hepatic stellate cells, collagen synthesis, and up-regulation of the hepatic expression of connective tissue growth factors (36, 37).
In this study, with the inclusion of OGTT for metabolic evaluation in our study, we found that both HOMA-IR≥2.5 and prediabetes based on either OGTT or HbA1c were important metabolic risk factors of liver fibrosis among individuals with non-diabetic overweight/obese MAFLD. However, our findings in multivariable analyses showed that elevated HOMR-IR outperformed prediabetes, which included also individuals with normal HbA1c but abnormal OGTT, a cumbersome test to perform. This led to the development of the current screening algorithm as a simpler strategy to use clinically, based on parameters that can be conveniently measured during the routine care for patients with MAFLD. On the other hand, we found that FIB-4, a commonly used non-invasive fibrosis score, was not significantly associated with LS ≥8.0 kPa, which was likely due to the low prevalence of liver fibrosis in this community-based cohort. Moreover, although we demonstrated that replacing abnormal AST level with NFS resulted in similar conclusions, it is noteworthy that NFS is a composite score that requires several other parameters including platelet count, serum albumin and ALT levels. Certainly, the relatively small sample size of the two external cohorts to validate our findings was a major limitation of the study. Nonetheless, we found that the clinical performance of this screening algorithm remained satisfactory in the pooled validation cohort with reasonable sensitivity and high NPV of over 90%, indicating that this algorithm should be also applicable to individuals who are relatively insulin resistant either due to PCOS or more severe obesity.
Our study had several other limitations. First, the cross-sectional study design precluded the evaluation of a causal relationship between metabolic dysfunction and the development of liver fibrosis, or cACLD, in patients with overweight/obese MAFLD. Secondly, the sample size of both derivation and validation cohorts were relatively small, and all our participants were HK Chinese. Further studies in other populations with a larger sample size are required to confirm these findings and validate our proposed clinical algorithm. Moreover, serum hsCRP level was not measured and liver biopsy was not performed in our study participants. However, it was not feasible and ethically not justified to perform liver biopsy in these asymptomatic community-dwelling individuals. Nonetheless, the prevalence of 6.4% with LS ≥8.0 kPa on VCTE in our study, which involved community-dwelling individuals aged ≥25 years, was overall in line with those reported in two recent Korean studies based on magnetic resonance elastography (9, 10). In these two studies conducted among individuals attending health check-up aged ≥18 years and ≥40 years, the prevalence of significant liver fibrosis was found to be 4.2% and 9.9%, respectively (9, 10). Lastly, although the recruitment process from the community was through random sampling, participation in this population-based study was entirely voluntary. Therefore, it is possible that the participants were overall relatively more health conscious, which could have also explained the small number of individuals with excessive alcohol intake that is associated with increased liver fibrosis development in MAFLD (38). Moreover, the relatively small sample size of individuals with viral hepatitis or the significant alcohol intake also rendered it difficult for further subgroup analysis based on MAFLD with single or dual etiologies.
Conclusion
It is conceivable that a MAFLD pandemic, in particular overweight/obese MAFLD, will soon follow alongside the rising global prevalence of obesity (2, 39). From a clinical perspective, our findings suggest the recommendations of measuring serum AST level in all individuals with non-diabetic overweight/obese MAFLD detected on imaging techniques such as ultrasound or blood biomarkers, and have serum fasting insulin level measured to determine the HOMA-IR if their serum AST levels are normal. Individuals who have elevated serum AST level and/or HOMR-IR ≥2.5 should be referred for VCTE and/or hepatologist assessment for the presence of clinically significant liver fibrosis. Since MAFLD research has only started since 2020, future prospective studies should focus on the role of metabolic dysfunction in stratifying the long-term risks of incident adverse hepatic outcomes including liver-related mortality in this largest subgroup within the MAFLD population.
Data availability statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (IRB Ref: UW-18-610). The patients/participants provided their written informed consent to participate in this study.
Author contributions
C-HL researched the data and wrote the manuscript. DL, MY, RL, LM and Y-CW researched the data. CF, AL and JC performed statistical analyses. T-HL, JW, AX, H-FT, KT, BC, M-FY and KL critically reviewed and edited the manuscript. KL initiated and supervised the study, had full access to all the data and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Commissioned Research to Support Local Cohorts and Follow-up Studies 2019 (Ref: CFS-HKU5) and the Health and Medical Research Fund (Ref: 08192856).
Acknowledgments
We thank Mr John Yuen and Ms Rachel Wong for their technical assistance in the measurements of serum levels of HBsAg and anti-HCV antibody of the participants.
Conflict of interest
C-HL received speaker’s fees from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly and Sanofi Aventis. M-FY reports grant/research support from AbbVie, Assembly Biosciences, Arrowhead Pharmaceuticals, Bristol Myers Squibb, Fujirebio Incorporation, Gilead Sciences, Immuncore, Merck Sharp and Dohme, Springbank Pharmaceuticals, Sysmex Corporation and Roche, consultancy for AbbVie, Aligos Therapeutics, AiCuris, Antios Therapeutics, Arbutus Biopharma, Arrowhead Pharmaceuticals, Assembly Biosciences, Bristol Myers Squibb, Clear B Therapeutics, Dicerna Pharmaceuticals, Finch Therapeutics, Fujirebio Incorporation, GSK, Gilead Sciences, Immunocore, Janssen, Merck Sharp and Dohme, Roche, Springbank Pharmaceuticals, Silverback Therapeutics, Sysmex Corporation and Vir Biotechnology, and lecture fees from AbbVie, Dicerna Pharmaceuticals, Fujirebio Incorporation, Gilead Sciences, Merck Sharp and Dohme, Roche and Sysmex Corporation. KL is an advisory board member of Merck Sharp and Dohme.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1056562/full#supplementary-material
References
1. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
2. Hui Lim GE, Tang A, Ng CH, Chin YH, Lim WH, Hao Tan DJ, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol (2021) S1542–3565(21)01276–3. doi: 10.1016/j.cgh.2021.11.038
3. Ayada I, van Kleef LA, Alferink LJM, Li P, de Knegt RJ, Pan Q. Systematically comparing epidemiological and clinical features of MAFLD and NAFLD by meta-analysis: Focusing on the non-overlap groups. Liver Int (2022) 42:277–87. doi: 10.1111/liv.15139
4. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology (2017) 65:1557–65. doi: 10.1002/hep.29085
5. Taylor RS, Taylor RJ, Bayliss S, Hagstrom H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterology (2020) 158:1611–25.e12. doi: 10.1053/j.gastro.2020.01.043
6. Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med (2021) 385:1559–69. doi: 10.1056/NEJMoa2029349
7. Papatheodoridi M, Hiriart JB, Lupsor-Platon M, Bronte F, Boursier J, Elshaarawy O, et al. Refining the baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J Hepatol (2021) 74:1109–16. doi: 10.1016/j.jhep.2020.11.050
8. Liu J, Ayada I, Zhang X, Wang L, Li Y, Wen T, et al. Estimating global prevalence of metabolic dysfunction-associated fatty liver disease in overweight or obese adults. Clin Gastroenterol Hepatol (2021) 20(3):e573–e582. doi: 10.1016/j.cgh.2021.02.030
9. Sohn W, Kwon HJ, Chang Y, Ryu S, Cho YK. Liver fibrosis in asians with metabolic dysfunction-associated fatty liver disease. Clin Gastroenterol Hepatol (2022) 20:e1135–48. doi: 10.1016/j.cgh.2021.06.042
10. Kim M, Yoon EL, Cho S, Lee CM, Kang BK, Park H, et al. Prevalence of advanced hepatic fibrosis and comorbidity in metabolic dysfunction-associated fatty liver disease in Korea. Liver Int (2022) 42(7):1536–1544. doi: 10.21203/rs.3.rs-799868/v1
11. Yuan Q, Wang H, Gao P, Chen W, Lv M, Bai S, et al. Prevalence and risk factors of metabolic-associated fatty liver disease among 73,566 individuals in Beijing, China. Int J Environ Res Public Health (2022) 19(4):2096. doi: 10.3390/ijerph19042096
12. Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. PloS Med (2020) 17:e1003100. doi: 10.1371/journal.pmed.1003100
13. Lui DTW, Lee CH, Woo YC, Fong CHY, Tso AWK, Cheung BMY, et al. Cohort profile: The Hong Kong cardiovascular risk factor prevalence study (CRISPS) and the follow-up studies. Int J Epidemiol (2021) 50:1069–1069h. doi: 10.1093/ije/dyaa240
14. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman 3HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
15. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia (1985) 28:412–9. doi: 10.1007/BF00280883
16. Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J Hepatol (2018) 68:305–15. doi: 10.1016/j.jhep.2017.11.013
17. Lee CH, Seto WK, Lui DT, Fong CH, Wan HY, Cheung CY, et al. Circulating thrombospondin-2 as a novel fibrosis biomarker of nonalcoholic fatty liver disease in type 2 diabetes. Diabetes Care (2021) 44(9):2089–2097. doi: 10.2337/figshare.14673267.v1
18. e.e.e. European Association for the Study of the Liver. P. clinical practice guideline, chair, E.G.B. representative, and m. panel, EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol (2021) 75:659–89. doi: 10.1016/j.jhep.2021.05.025
19. Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (2004) 363:157–63. doi: 10.1016/S0140-6736(03)15268-3
20. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. International association for the study of, harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
21. American Diabetes Association Professional Practice C. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care (2022) 45:S17–38. doi: 10.2337/dc22-S002
22. Wong HYQ, Li HWR, Lam KSL, Tam S, Shek CC, Lee CYV, et al. Independent association of serum vitamin d with anti-mullerian hormone levels in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) (2018) 89:634–41. doi: 10.1111/cen.13816
23. van Kleef LA, Ayada I, Alferink LJM, Pan Q, de Knegt RJ. Metabolic dysfunction-associated fatty liver disease improves detection of high liver stiffness: The Rotterdam study. Hepatology (2022) 75:419–29. doi: 10.1002/hep.32131
24. Lee CH, Lui DT, Lam KS. Non-alcoholic fatty liver disease and type 2 diabetes: An update. J Diabetes Investig (2022) 13(6):930–940. doi: 10.1111/jdi.13756
25. Ciardullo S, Perseghin G. Prevalence of elevated liver stiffness in patients with type 1 and type 2 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract (2022) 190:109981. doi: 10.1016/j.diabres.2022.109981
26. Bril F, McPhaul MJ, Caulfield MP, Clark VC, Soldevilla-Pico C, Firpi-Morell RJ, et al. Performance of plasma biomarkers and diagnostic panels for nonalcoholic steatohepatitis and advanced fibrosis in patients with type 2 diabetes. Diabetes Care (2020) 43:290–7. doi: 10.2337/dc19-1071
27. Singh A, Garg R, Lopez R, Alkhouri N. Diabetes liver fibrosis score to detect advanced fibrosis in diabetics with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol (2021) 20(3):e624–e626. doi: 10.1016/j.cgh.2021.01.010
28. Castera L. Non-invasive tests for liver fibrosis in NAFLD: Creating pathways between primary healthcare and liver clinics. Liver Int (2020) 40 Suppl 1:77–81. doi: 10.1111/liv.14347
29. Lee CH, Seto WK, Ieong K, Lui DTW, Fong CHY, Wan HY, et al. Development of a non-invasive liver fibrosis score based on transient elastography for risk stratification in patients with type 2 diabetes. Endocrinol Metab (Seoul) (2021) 36:134–45. doi: 10.3803/EnM.2020.887
30. Wong VW, Wong GL, Woo J, Abrigo JM, Chan CK, Shu SS, et al. Impact of the new definition of metabolic associated fatty liver disease on the epidemiology of the disease. Clin Gastroenterol Hepatol (2021) 19:2161–71.e5. doi: 10.1016/j.cgh.2020.10.046
31. Collaboration N.C.D.R.F. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19. 2 million participants. Lancet (2016) 387:1377–96. doi: 10.1016/S0140-6736(16)30054-X
32. Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
33. Sorrentino P, Terracciano L, D'Angelo S, Ferbo U, Bracigliano A, Vecchione R. Predicting fibrosis worsening in obese patients with NASH through parenchymal fibronectin, HOMA-IR, and hypertension. Am J Gastroenterol (2010) 105:336–44. doi: 10.1038/ajg.2009.587
34. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism (2016) 65:1038–48. doi: 10.1016/j.metabol.2015.12.012
35. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med (2017) 377:2063–72. doi: 10.1056/NEJMra1503519
36. Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology (1999) 29:1743–51. doi: 10.1002/hep.510290632
37. Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: A potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology (2001) 34:738–44. doi: 10.1053/jhep.2001.28055
38. Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int (2020) 40:2082–9. doi: 10.1111/liv.14548
Keywords: obesity, MAFLD (metabolic associated fatty liver disease), overweight, fatty liver disease, population based study
Citation: Lee C-H, Lui DT-W, Li RH-W, Yuen MM-A, Fong CH-Y, Leung AP-W, Chu JC-M, Mak LL-Y, Lam T-H, Woo J, Woo Y-C, Xu A, Tse H-F, Tan KC-B, Cheung BM-Y, Yuen M-F and Lam KS-L (2023) Sequential algorithm to stratify liver fibrosis risk in overweight/obese metabolic dysfunction-associated fatty liver disease. Front. Endocrinol. 13:1056562. doi: 10.3389/fendo.2022.1056562
Received: 29 September 2022; Accepted: 16 December 2022;
Published: 06 January 2023.
Edited by:
Gabriel Rufino Estrela, National Institute of Diabetes and Digestive and Kidney Diseases (NIH), United StatesReviewed by:
Angelo Armandi, University of Turin, ItalyStefano Ciardullo, University of Milano Bicocca, Italy
Copyright © 2023 Lee, Lui, Li, Yuen, Fong, Leung, Chu, Mak, Lam, Woo, Woo, Xu, Tse, Tan, Cheung, Yuen and Lam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karen Siu-Ling Lam, a3NsbGFtQGhrdS5oaw==
 Chi-Ho Lee
Chi-Ho Lee David Tak-Wai Lui
David Tak-Wai Lui Raymond Hang-Wun Li
Raymond Hang-Wun Li Michele Mae-Ann Yuen1
Michele Mae-Ann Yuen1 Carol Ho-Yi Fong
Carol Ho-Yi Fong Loey Lung-Yi Mak
Loey Lung-Yi Mak Tai-Hing Lam
Tai-Hing Lam Jean Woo
Jean Woo Aimin Xu
Aimin Xu Kathryn Choon-Beng Tan
Kathryn Choon-Beng Tan Bernard Man-Yung Cheung
Bernard Man-Yung Cheung Karen Siu-Ling Lam
Karen Siu-Ling Lam