- 1Human Cancer Genomic Research, Research Center, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 2Department of Surgery, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 3Department of Pathology, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
Background: X-linked inhibitor of apoptosis (XIAP) is the most potent caspase inhibitory IAP family member and its over-expression is implicated in aggressive behavior of various solid tumors, including papillary thyroid carcinoma (PTC). BRAFV600E mutation is the most common oncogenic event in PTC and is also known to be associated with aggressive clinico-pathological characteristics. In this study, we investigated the prevalence of XIAP expression in more than 1600 PTCs from Middle Eastern ethnicity and its prognostic value to predict disease-free survival (DFS), in combination with the BRAFV600E mutation.
Methods: Clinical data, XIAP expression by immunohistochemistry and BRAF mutation status were analyzed in 1640 Saudi PTC patients seen at our institute between 1988 - 2020.
Results: BRAFV600E mutation was found in 910 of 1640 patients (55.5%) and was significantly correlated with older age, extrathyroidal extension, bilaterality, multifocality and lymph node metastasis, but was not an independent predictor of DFS. XIAP was over-expressed in 758 of 1640 (46.2%) and was associated with aggressive clinico-pathological features. It was also found to be an independent prognostic marker for DFS (HR = 1.28, 95% CI = 1.02 – 1.60, P = 0.0342). XIAP overexpression was correlated with presence of BRAFV600E mutation in PTC patients. Interestingly, we found the ability to predict shorter DFS was 2.7-fold higher in PTCs with over-expression of XIAP and BRAFV600E mutation compared to patients with high XIAP and wild-type BRAFV600E status (HR = 2.74, 95% CI = 2.19 – 3.44, p < 0.0001).
Conclusion: XIAP expression is an independent predictor of prognosis in Middle Eastern PTC patients. Combination of XIAP expression and BRAFV600E mutation can synergistically improve the DFS prediction in PTC patients, which may help clinicians to establish the most appropriate initial care and long-term surveillance strategies.
Introduction
Thyroid carcinoma is the most common endocrine malignancy (1) and papillary thyroid carcinoma (PTC) accounts for the majority (~90%) of all thyroid cancers (2, 3). In Saudi Arabia, PTC is the second commonest cancer affecting women, after breast cancer (4). Despite the indolent course and excellent prognosis of PTC, there are some patients who present with aggressive disease and poor prognosis, which remains a major concern for clinicians (5, 6). Identifying molecular markers that can predict poor prognosis is of paramount importance in helping healthcare providers to tailor therapy and follow-up of PTC patients.
The role of genetic alteration in MAPK signaling pathway in PTC initiation and progression has been previously well documented (7, 8). A BRAFV600E point mutation in exon 15 is commonly detected in PTC and accounts for more than 90% of all BRAF mutations (9). BRAF mutation is present in more than 50% of PTCs (10, 11). Numerous studies have documented the oncogenic molecular mechanisms of BRAFV600E in driving aggressiveness of PTC (11–14). Despite the strong association between BRAF mutation and aggressive clinico-pathological features, its ability as a sole marker to predict prognosis is still a subject of debate. While some studies have clearly demonstrated that BRAFV600E significantly affected prognosis (12, 15–19), others have failed to establish BRAF mutation as an independent prognostic factor in PTC patients (14, 20–22). Therefore, synergistic interaction between BRAF mutations and other oncogenic markers may better predict prognosis of PTC.
The known marker that has been confirmed by us previously to play an important functional and prognostic role in PTC is the X-linked inhibitor of apoptosis protein (XIAP) (23). The inhibitor of apoptosis is a family of endogenous caspase inhibitors that share a common Baculoviral IAP repeat domain (24, 25). XIAP is the best characterized member of the IAP family in terms of the caspase inhibitory mechanism (26). XIAP dysregulation has been shown to be associated with aggressive clinical behavior and worse outcome in several cancers, including PTC (27–31).
Therefore, we hypothesized that synergistic association between BRAFV600E mutation and XIAP alteration may better predict the prognosis of PTC. The aim of the present study was to investigate whether BRAFV600E mutational status is associated with XIAP alteration and whether these associations can synergistically improve the ability to predict prognosis in a large cohort of Middle Eastern PTC patients.
Materials and methods
Patient selection and clinico-pathological data
One thousand seven-hundred and sixteen consecutive unselected PTC patients diagnosed between 1988 and 2020 at King Faisal Specialist Hospital and Research Centre (Riyadh, Saudi Arabia) were available to be included in the study. However, complete data for XIAP expression and BRAF mutation were available for 1640 PTC patients and hence only these cases were included for further analysis. Cases were identified based on clinical history followed by fine needle aspiration cytology for confirmation. The Institutional Review Board of the hospital approved this study. Since only retrospective patient data was utilized, the Research Advisory Council (RAC) provided waiver of consent under project RAC # 221 1168 and # 2110 031.
Baseline clinico-pathological data were collected from case records and have been summarized in Table 1. Staging of PTC was performed using the eighth edition of American Joint Committee on Cancer (AJCC) staging system (32).
BRAFV600E mutation analysis
BRAFV600E mutation data was assessed in our laboratory by Sanger sequencing and has been published by us previously (33).
Tissue microarray construction and immunohistochemistry analysis
Tissue microarray (TMA) format was utilized for immunohistochemical analysis of the PTC samples. TMA was constructed as previously described (34). Briefly, modified semiautomatic robotic precision instrument (Beecher Instruments, Woodland, WI) was used to punch tissue cylinders with a diameter of 0.6 mm from representative tumor area of the donor tissue block and brought into the recipient paraffin block. Two 0.6-mm cores of PTC were arrayed from each case.
XIAP immunohistochemical staining was performed on 1022 PTC samples previously by us (23). We expanded the staining to 1640 cases using the same protocol. Scoring (H-score) and cutoff for analysis was also performed as described previously (23). Briefly, X-tile plots were constructed for assessment of biomarker and optimization of cutoff points based on outcome as has been described earlier (35). PTCs were categorised into two groups based on X-tile plots: one with complete absence or reduced staining of XIAP (H score = 0–40) and the other group showed overexpression of XIAP (H score > 40).
Follow-up and study endpoint
Patients were regularly followed by both physical examinations and imaging studies to identify tumor recurrence. The median follow-up was 7.5 years (range 1.0 – 30.2 years). The study end-point was disease-free survival (DFS). Patients were grouped according to disease status, with patients considered to be disease-free in the absence of clinical, biochemical (unstimulated serum thyroglobulin (Tg) levels of < 0.2 µg/L or stimulated Tg levels of < 1 µg/L in the absence of interfering thyroglobulin antibodies (TgAb)) or radiological evidence of disease persistence or recurrence. In contrast, active disease was defined by the presence of unstimulated serum Tg levels ≥ 0.2 µg/L or stimulated Tg levels ≥1 µg/L; a rising or denovo appearance of TgAb; or abnormal findings on radio-imaging.
Statistical analysis
The associations between clinico-pathological variables and XIAP expression/BRAFV600E mutation was performed using contingency table analysis and Chi square tests. Mantel-Cox log-rank test was used to evaluate DFS. Survival curves were generated using the Kaplan-Meier method. Cox proportional hazards model was used for univariate and multivariate analysis. Two-sided tests were used for statistical analyses with a limit of significance defined as p value < 0.05. Data analyses were performed using the JMP14.0 (SAS Institute, Inc., Cary, NC) software package.
Results
Patient and tumor characteristics
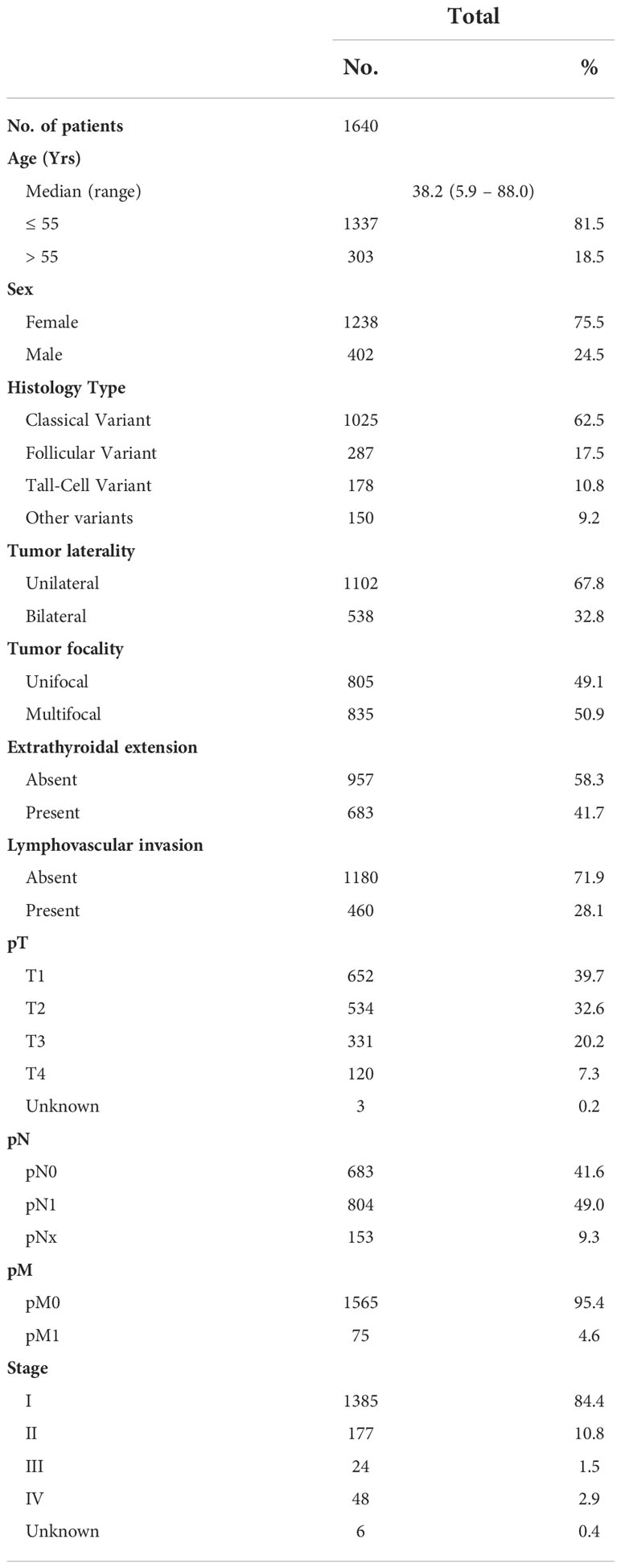
Median age of the study population was 38.2 years (range: 5.9 – 88 years), with a male to female ratio of 1:3. The majority of tumors were classical variant of PTC (62.5%; 1025/1640). 32.8% (538/1640) of tumors were bilateral and 50.9% (835/1640) were multifocal. 41.7% (683/1640) of tumors exhibited extrathyroidal extension and 28.1% (460/1640) showed lymphovascular invasion. Lymph node metastasis was noted in 49.0% (804/1640) and distant metastasis in 4.6% (75/1640) of PTCs (Table 1).
Frequency of BRAFV600E mutation and its clinico-pathological associations
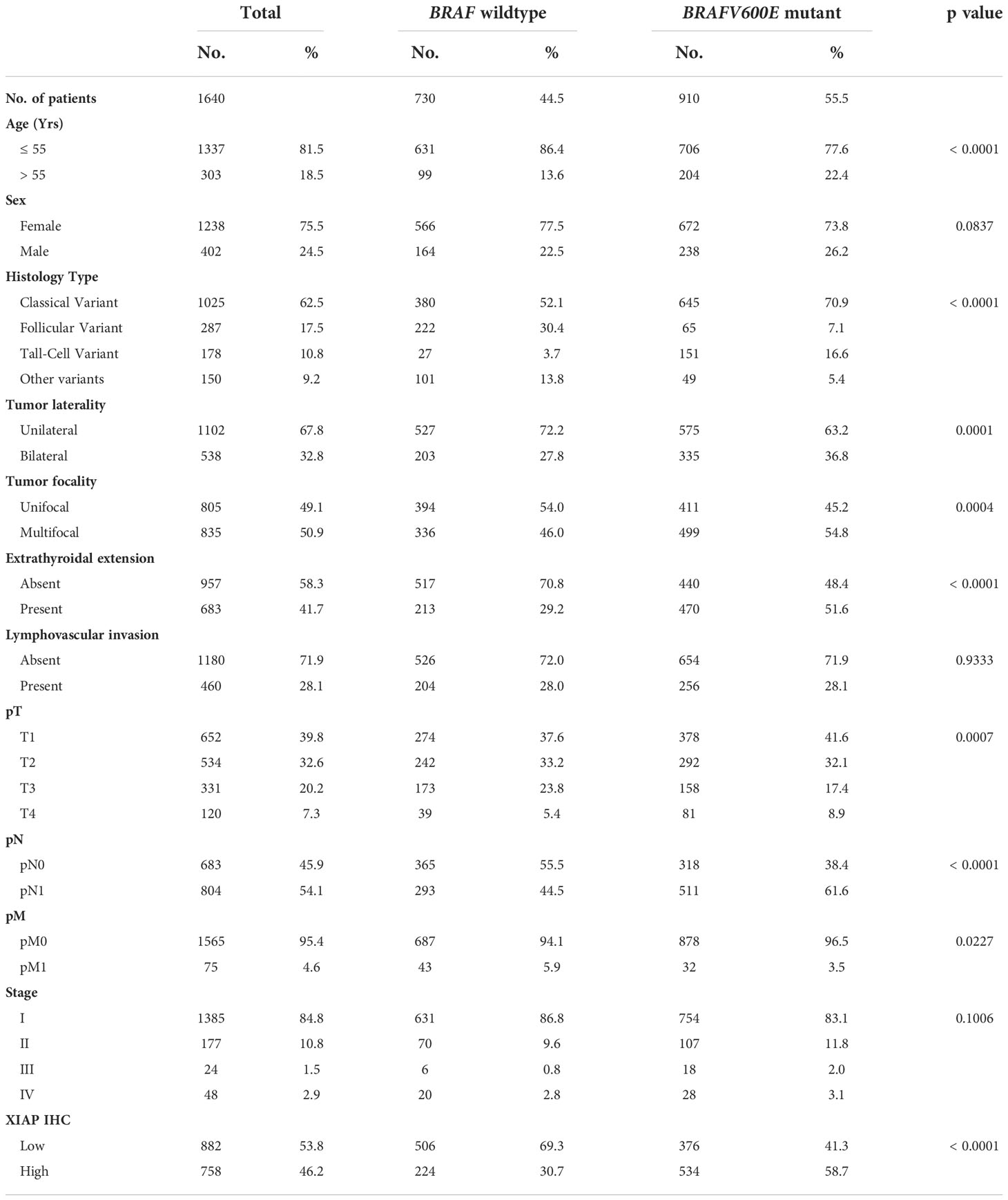
BRAFV600E mutation was noted in 55.5% (910/1640) of PTCs in our cohort and was significantly associated with adverse clinico-pathological characteristics such as older age at diagnosis (p < 0.0001), tall cell variant of PTC (p < 0.0001), bilateral tumors (p = 0.0001), multifocality (p = 0.0004), extrathyroidal extension (p < 0.0001) and lymph node metastasis (p < 0.0001). Interestingly, we also found a significant association between BRAFV600E mutation and XIAP over-expression (p < 0.0001) (Table 2).
XIAP expression and its clinico-pathological associations
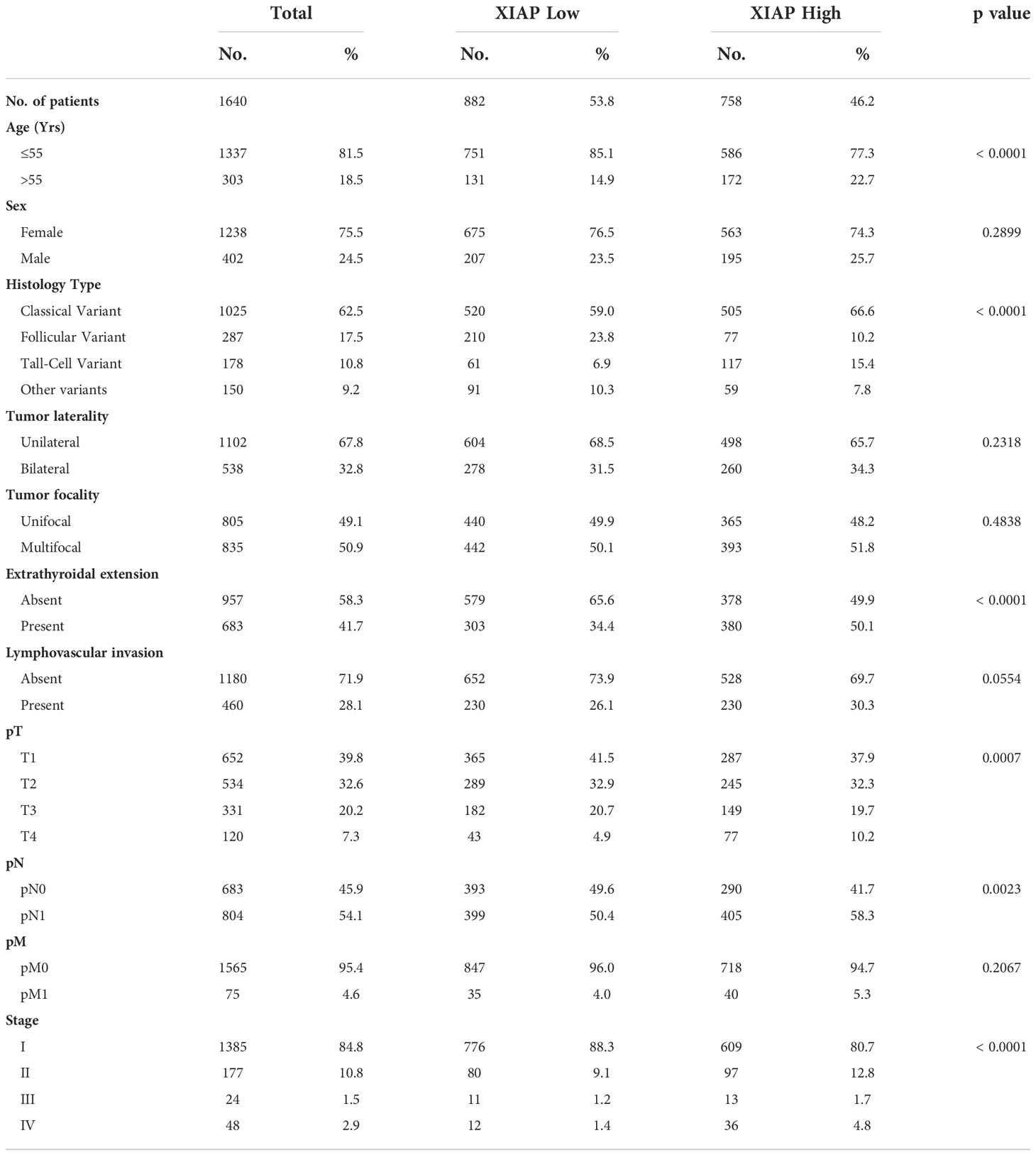
XIAP over-expression was seen in 46.2% (758/1640) of PTCs. Over-expression of XIAP was found to be associated with older age at diagnosis (p < 0.0001), tall cell variant of PTC (p < 0.0001), extrathyroidal extension (p < 0.0001), larger tumor size (p = 0.0007), lymph node metastasis (p = 0.0023) and advanced stage (p < 0.0001) (Table 3).
Prediction of disease-free survival by XIAP expression and BRAFV600E mutation status
We next analyzed the ability of XIAP and BRAFV600E to predict outcome in PTC patients in our cohort. Both XIAP and BRAFV600E could predict DFS on univariate analysis (XIAP: Hazard ratio (HR) = 1.74, 95% confidence interval (CI) = 1.41 – 2.15, p value < 0.0001; BRAFV600E: HR = 1.64, 95% CI = 1.32 – 2.05, P < 0.0001) (Table 4). However, on multivariate analysis (after adjusting for age, sex, laterality, tumor size, focality, extrathyroidal extension, lymph node metastasis and distant metastasis), XIAP was found to be an independent predictor of DFS (HR = 1.28, 95% CI = 1.02 – 1.60, P = 0.0342) but BRAFV600E was not (HR = 1.22, 95% CI = 0.96 – 1.56, P = 0.1041) (Table 4).
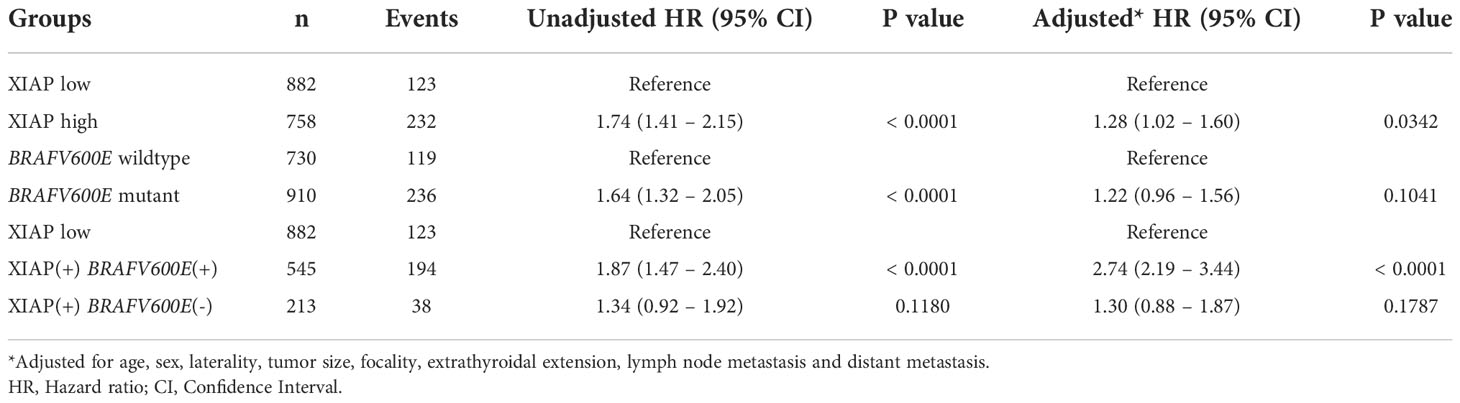
Table 4 Comparison of multivariate analysis to predict DFS using the XIAP status alone or in combination with BRAFV600E mutation.
To determine whether the combination of XIAP and BRAFV600E could be a better predictor of prognosis in our cohort, we classified the patients into three groups: XIAP low expression, XIAP over-expression and BRAFV600E mutant, XIAP over-expression and BRAFV600E wildtype. Indeed, the combination of XIAP over-expression and BRAFV600E mutation was found to be a better independent predictor of DFS (HR = 2.74, 95% CI = 2.19 – 3.44, p < 0.0001) in our cohort (Table 4; Figure 1).
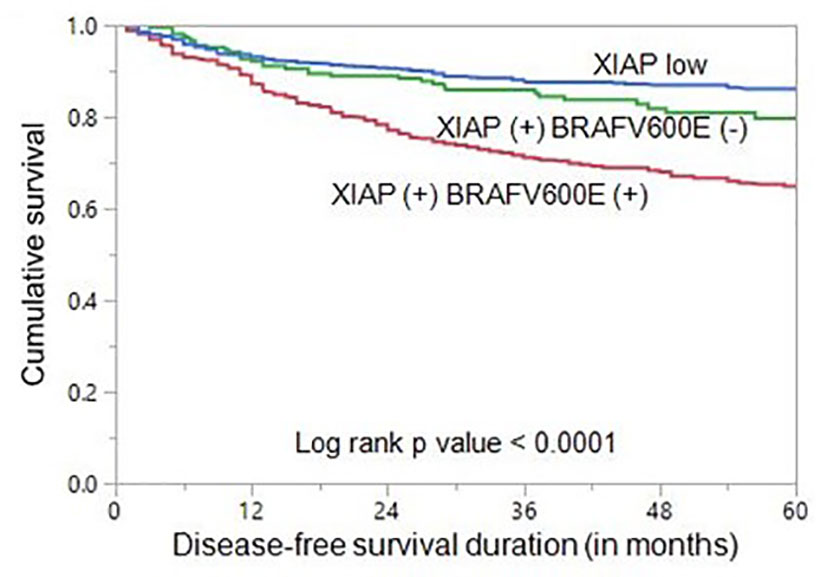
Figure 1 Disease-free survival (DFS) in papillary thyroid carcinoma (PTC). Kaplan-Meier survival curve showing shorter DFS in patients with co-existing XIAP over-expression and BRAF mutation compared to those with co-existing XIAP over-expression and BRAF wildtype or XIAP low expression groups (p < 0.0001).
Discussion
Our study offers further evidence that the BRAFV600E mutation is associated with aggressive form of PTC in Middle Eastern patients. The BRAFV600E mutation is the most common mutation in PTC and occurs in 40-80% of PTCs (36). The prevalence of BRAFV600E in this cohort was 55.5%. Despite the association with aggressive clinico-pathological characteristics in our cohort such as older age, bilateral tumors, multifocality, extrathyroidal extension and lymphnode metastasis, BRAFV600E mutation alone was not found to be an independent predictor of disease-free survival in Middle Eastern patients with PTC. Previous reports have been controversial regarding the prognostic significance of BRAFV600E mutation, with some studies identifying BRAFV600E mutation as an independent prognostic marker in PTC (15–19), whereas other studies have failed to show the prognostic value of BRAFV600E mutation (14, 20–22). The prevalence of BRAFV600E mutation, cohort size, patients’ selection criteria and ethnicity might contribute to these differences.
Given the high survival among thyroid cancer, there is a need to identify molecular marker to predict prognosis for continued surveillance and help in clinical decision making for PTC patients. We have looked for association of other markers that could improve the limited prognostic value of BRAFV600E mutation. In this study, we investigated whether BRAFV600E mutation may better predict PTC recurrence in the presence of XIAP alteration. In a previous study (23), we found XIAP to be an oncogenic marker in PTC cells. We also showed that XIAP expression was high in PTC clinical samples and was associated with aggressive clinico-pathological markers.
In this current study, we found XIAP overexpression in 46.2% of PTC cases and it was associated with older age, advanced tumor stage and lymph node metastasis among other clinico-pathological parameters. Interestingly, XIAP overexpression was strongly associated with BRAFV600E mutations in PTC patients. More importantly, XIAP was an independent molecular marker for shorter disease-free survival in multivariate analysis. The ability of XIAP to predict prognosis in PTC patient and its strong association with BRAFV600E mutation led us to hypothesize that BRAFV600E, if used in combination with XIAP, may improve the ability for prediction of prognosis in PTC patients. Interestingly, upon classifying patients based on XIAP expression in tumors, we found that the ability to predict shorter disease-free survival was 2.7-fold higher in PTCs with high expression of XIAP and BRAFV600E mutation compared to patients with high XIAP and wild-type BRAFV600E status. Therefore, XIAP could be a potential prognostic biomarker that may be used in combination with BRAFV600E mutations in the clinical setting to predict prognosis in Middle Eastern PTC. Previous studies have demonstrated that XIAP is a NF-kappaB (NFκB)-dependent member of the IAP gene family (37, 38). MAPK signalling can also lead to the activation of the transcription factor NFκB, causing upregulation of survival genes such as XIAP (39, 40). This could be a possible explanation for the association between XIAP and BRAF, as well as their ability to better predict prognosis in PTC when used in combination.
A previous study has reported the association between BRAFV600E mutation and molecular marker XIAP, in predicting patients’ outcome (41). They conducted a study on 164 PTC patients from South Korea and found that PTCs positive for BRAFV600E mutation and negative for XIAP expression had significantly higher rate of recurrence. This is inconsistent with our study, where we found that BRAFV600E mutations in XIAP positive PTC was more useful in improving prediction of disease-free survival. Whether differences in cohort size and ethnicity played a role in this contradictory results are not clear. In addition, the younger median age of our study cohort could also be a contributing factor to the differences in results of our study compared to other studies. The younger age of our cohort most likely represents the inherent aggressive nature of PTC in the Middle Eastern ethnicity, as shown by us and other studies from this region (42–46). Another study analyzed 123 PTCs and found that presence of BRAFV600E mutation was unrelated to XIAP expression (47). Hence, more studies are required to establish the prognostic role of BRAFV600E mutation and XIAP expression in PTCs.
In summary, the XIAP expression in PTC better predicts aggressive disease and prognosis based on stratification by BRAFV600E mutation status. The synergistic interaction between XIAP overexpression and BRAFV600E mutations could potentially be helpful to clinicians in establishing optimal decision making regarding PTC patients’ therapy, follow-up and surveillance.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by King Faisal Specialist Hospital and Research Centre. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
Study concept and design: KA-K, SP, AS, RB. Executed the study: SP, AS, RB, KI, MA-R, WA-H, PA, NS, SA, SA-S, FA-D. Statistical analysis: SP. Drafting the article: KA-K, AS, SP. Critical revision of the article for important intellectual content, writing of the article, and approval of the final version: KA-K, SP, AS, RB, KI, MA-R, WA-H, PA, NS, SA, SA-S, FA-D. All authors contributed to the article and approved the submitted version.
Acknowledgements
The authors would like to thank Felisa DeVera for her technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA: Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Pereira M, Williams VL, Hallanger Johnson J, Valderrabano P. Thyroid cancer incidence trends in the united states: association with changes in professional guideline recommendations. Thyroid (2020) 30(8):1132–40. doi: 10.1089/thy.2019.0415
4. Alrawaji, Alshahrani, Alzahrani, Alomran, Almadouj, Alshehri, et al. Cancer incidence report Saudi Arabia 2015. In: Council SH, editor. Saudi Cancer registry. Riyadh, Saudi Arabia: Saudi Cancer Registry (2018).
5. Póvoa AA, Teixeira E, Bella-Cueto MR, Melo M, Oliveira MJ, Sobrinho-Simões M, et al. Clinicopathological features as prognostic predictors of poor outcome in papillary thyroid carcinoma. Cancers (2020) 12(11):3186. doi: 10.3390/cancers12113186
6. Park S, Kim WG, Song E, Oh H-S, Kim M, Kwon H, et al. Dynamic risk stratification for predicting recurrence in patients with differentiated thyroid cancer treated without radioactive iodine remnant ablation therapy. Thyroid (2017) 27(4):524–30. doi: 10.1089/thy.2016.0477
7. Zaballos MA, Santisteban P. Key signaling pathways in thyroid cancer. J Endocrinol (2017) 235(2):R43–61. doi: 10.1530/JOE-17-0266
8. Braicu C, Buse M, Busuioc C, Drula R, Gulei D, Raduly L, et al. A comprehensive review on MAPK: a promising therapeutic target in cancer. Cancers (2019) 11(10):1618. doi: 10.3390/cancers11101618
9. Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocrine Rev (2007) 28(7):742–62. doi: 10.1210/er.2007-0007
10. Ge J, Wang J, Wang H, Jiang X, Liao Q, Gong Q, et al. The BRAF V600E mutation is a predictor of the effect of radioiodine therapy in papillary thyroid cancer. J Cancer (2020) 11(4):932. doi: 10.7150/jca.33105
11. Yan C, Huang M, Li X, Wang T, Ling R. Relationship between BRAF V600E and clinical features in papillary thyroid carcinoma. Endocrine Connections (2019) 8(7):988–96. doi: 10.1530/EC-19-0246
12. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer (2013) 13(3):184–99. doi: 10.1038/nrc3431
13. Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK, Lee YJ, et al. The association of the BRAFV600E mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: A meta-analysis. Cancer (2012) 118(7):1764–73. doi: 10.1002/cncr.26500
14. Gan X, Shen F, Deng X, Feng J, Lu J, Cai W, et al. Prognostic implications of the BRAF−V600E mutation in papillary thyroid carcinoma based on a new cut−off age stratification. Oncol Letters (2020) 19(1):631–40. doi: 10.3892/ol.2019.11132
15. Fraser S, Go C, Aniss A, Sidhu S, Delbridge L, Learoyd D, et al. BRAFV600E mutation is associated with decreased disease-free survival in papillary thyroid cancer. World J Surgery (2016) 40(7):1618–24. doi: 10.1007/s00268-016-3534-x
16. Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, et al. BRAFV600E mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab (2008) 93(10):3943–9. doi: 10.1210/jc.2008-0607
17. Kebebew E, Weng J, Bauer J, Ranvier G, Clark OH, Duh Q-Y, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surgery (2007) 246(3):466. doi: 10.1097/SLA.0b013e318148563d
18. Enumah S, Fingeret A, Parangi S, Dias-Santagata D, Sadow PM, Lubitz CC. BRAFV600E mutation is associated with an increased risk of papillary thyroid cancer recurrence. World J Surgery (2020) 44(8):2685–91. doi: 10.1007/s00268-020-05521-2
19. Chen Y, Sadow PM, Suh H, Lee KE, Choi JY, Suh YJ, et al. BRAFV600E is correlated with recurrence of papillary thyroid microcarcinoma: a systematic review, multi-institutional primary data analysis, and meta-analysis. Thyroid (2016) 26(2):248–55. doi: 10.1089/thy.2015.0391
20. Takacsova E, Kralik R, Waczulikova I, Zavodna K, Kausitz J. A different prognostic value of BRAFV600E mutation positivity in various age groups of patients with papillary thyroid cancer. Neoplasma (2017) 64(1):156–64. doi: 10.4149/neo_2017_120
21. Henke LE, Pfeifer JD, Ma C, Perkins SM, DeWees T, El-Mofty S, et al. BRAF mutation is not predictive of long-term outcome in papillary thyroid carcinoma. Cancer Med (2015) 4(6):791–9. doi: 10.1002/cam4.417
22. Niederer-Wüst SM, Jochum W, Förbs D, Brändle M, Bilz S, Clerici T, et al. Impact of clinical risk scores and BRAF V600E mutation status on outcome in papillary thyroid cancer. Surgery (2015) 157(1):119–25. doi: 10.1016/j.surg.2014.07.015
23. Hussain AR, Bu R, Ahmed M, Jehan Z, Beg S, Al-Sobhi S, et al. Role of X-linked inhibitor of apoptosis as a prognostic marker and therapeutic target in papillary thyroid carcinoma. J Clin Endocrinol Metab (2015) 100(7):E974–E85. doi: 10.1210/jc.2014-4356
24. Verhagen AM, Coulson EJ, Vaux DL. Inhibitor of apoptosis proteins and their relatives: IAPs and other BIRPs. Genome Biol (2001) 2(7):1–10. doi: 10.1186/gb-2001-2-7-reviews3009
25. Silke J, Meier P. Inhibitor of apoptosis (IAP) proteins–modulators of cell death and inflammation. Cold Spring Harbor Perspect Biol (2013) 5(2):a008730. doi: 10.1101/cshperspect.a008730
26. Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep (2006) 7(10):988–94. doi: 10.1038/sj.embor.7400795
27. Tu H, Costa M. XIAP’s profile in human cancer. Biomolecules (2020) 10(11):1493. doi: 10.3390/biom10111493
28. Gao X, Zhang L, Wei Y, Yang Y, Li J, Wu H, et al. Prognostic value of XIAP level in patients with various cancers: a systematic review and meta-analysis. J Cancer (2019) 10(6):1528. doi: 10.7150/jca.28229
29. Devi GR, Finetti P, Morse MA, Lee S, de Nonneville A, Van Laere S, et al. Expression of x-linked inhibitor of apoptosis protein (Xiap) in breast cancer is associated with shorter survival and resistance to chemotherapy. Cancers (2021) 13(11):2807. doi: 10.3390/cancers13112807
30. Xiao G-Q, Unger PD, Burstein DE. Immunohistochemical detection of X-linked inhibitor of apoptosis (XIAP) in neoplastic and other thyroid disorders. Ann Diagn Pathol (2007) 11(4):235–40. doi: 10.1016/j.anndiagpath.2006.06.010
31. Gu L-Q, Li F-Y, Zhao L, Liu Y, Chu Q, Zang X-X, et al. Association of XIAP and P2X7 receptor expression with lymph node metastasis in papillary thyroid carcinoma. Endocrine (2010) 38(2):276–82. doi: 10.1007/s12020-010-9384-7
32. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA: Cancer J Clin (2017) 67(2):93–9. doi: 10.3322/caac.21388
33. Siraj AK, Parvathareddy SK, Pratheeshkumar P, Divya SP, Al-Sobhi SS, Al-Dayel F, et al. PD-L1 is an independent prognostic marker in middle Eastern PTC and its expression is upregulated by BRAFV600E mutation. Cancers (2021) 13(3):555. doi: 10.3390/cancers13030555
34. Siraj A, Bavi P, Abubaker J, Jehan Z, Sultana M, Al-Dayel F, et al. Genome-wide expression analysis of middle Eastern papillary thyroid cancer reveals c-MET as a novel target for cancer therapy. J Pathol (2007) 213(2):190–9. doi: 10.1002/path.2215
35. Camp RL, Dolled-Filhart M, Rimm DL. X-Tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res (2004) 10(21):7252–9. doi: 10.1158/1078-0432.CCR-04-0713
36. Li X, Kwon H. The impact of BRAF mutation on the recurrence of papillary thyroid carcinoma: A meta-analysis. Cancers (2020) 12(8):2056. doi: 10.3390/cancers12082056
37. Hofer-Warbinek R, Schmid JA, Stehlik C, Binder BR, Lipp J, De Martin R. Activation of NF-κB by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1. J Biol Chem (2000) 275(29):22064–8. doi: 10.1074/jbc.M910346199
38. Lu M, Lin S-C, Huang Y, Kang YJ, Rich R, Lo Y-C, et al. XIAP induces NF-κB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol Cell (2007) 26(5):689–702. doi: 10.1016/j.molcel.2007.05.006
39. Evans MK, Brown MC, Geradts J, Bao X, Robinson TJ, Jolly MK, et al. XIAP regulation by MNK links MAPK and NFκB signaling to determine an aggressive breast cancer phenotype. Cancer Res (2018) 78(7):1726–38. doi: 10.1158/0008-5472.CAN-17-1667
40. Shao Y, Le K, Cheng H, Aplin AE. NF-κB regulation of c-FLIP promotes TNFα-mediated RAF inhibitor resistance in melanoma. J Invest Dermatol (2015) 135(7):1839–48. doi: 10.1038/jid.2015.91
41. Yim JH, Kim WG, Jeon MJ, Han JM, Kim TY, Yoon JH, et al. Association between expression of X-linked inhibitor of apoptosis protein and the clinical outcome in a BRAFV600E-prevalent papillary thyroid cancer population. Thyroid (2014) 24(4):689–94. doi: 10.1089/thy.2012.0585
42. Siraj AK, Kumar Parvathareddy SK, Annaiyappanaidu P, Siraj N, Al-Sobhi SS, Al-Dayel F, et al. Male Sex is an independent predictor of recurrence-free survival in middle Eastern papillary thyroid carcinoma. Front Endocrinol (2022) 292. doi: 10.3389/fendo.2022.777345
43. Doubi A, Al-Qannass A, Al-Angari SS, Al-Qahtani KH, Alessa M, Al-Dhahri S. Trends in thyroid carcinoma among thyroidectomy patients: a 12-year multicenter study. Ann Saudi Med (2019) 39(5):345–9. doi: 10.5144/0256-4947.2019.345
44. Samargandy S, Qari R, Aljadani A, Assaqaf S, Etaiwi A, Alghamdi D, et al. Clinicopathological characteristics of thyroid cancer in a Saudi academic hospital. Cureus (2020) 12(5). doi: 10.7759/cureus.8044
45. Al-Zaher N, Al-Salam S, El Teraifi H. Thyroid carcinoma in the united Arab Emirates: perspectives and experience of a tertiary care hospital. Hematol/Oncol Stem Cell Ther (2008) 1(1):14–21. doi: 10.1016/S1658-3876(08)50055-0
46. Keinan-Boker L, Silverman BG. Trends of thyroid cancer in Israel: 1980–2012. Rambam Maimonides Med J (2016) 7(1):e0001. doi: 10.5041/RMMJ.10228
Keywords: papillary thyroid carcinoma, XIAP, BRAFV600E, disease-free survival, prognosis
Citation: Parvathareddy SK, Siraj AK, Bu R, Iqbal K, Al-Rasheed M, Al-Haqawi W, Annaiyappanaidu P, Siraj N, Ahmed SO, Al-Sobhi SS, Al-Dayel F and Al-Kuraya KS (2022) X-linked inhibitor of apoptosis protein (XIAP) predicts disease-free survival in BRAFV600E mutant papillary thyroid carcinoma in middle eastern patients. Front. Endocrinol. 13:1054882. doi: 10.3389/fendo.2022.1054882
Received: 27 September 2022; Accepted: 23 November 2022;
Published: 12 December 2022.
Edited by:
Sadegh Rajabi, Shahid Beheshti University of Medical Sciences, IranReviewed by:
Barbara Maria Jarzab, Maria Skłodowska-Curie National Research Institute of Oncology, PolandGuido Sauter, University Medical Center Hamburg-Eppendorf, Germany
Copyright © 2022 Parvathareddy, Siraj, Bu, Iqbal, Al-Rasheed, Al-Haqawi, Annaiyappanaidu, Siraj, Ahmed, Al-Sobhi, Al-Dayel and Al-Kuraya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khawla S. Al-Kuraya, a2t1cmF5YUBrZnNocmMuZWR1LnNh
†These authors have contributed equally to this work
 Sandeep Kumar Parvathareddy1†
Sandeep Kumar Parvathareddy1† Abdul K. Siraj
Abdul K. Siraj Rong Bu
Rong Bu Kaleem Iqbal
Kaleem Iqbal Khawla S. Al-Kuraya
Khawla S. Al-Kuraya