
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 08 December 2022
Sec. Cardiovascular Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1047927
This article is part of the Research TopicAdvances in Research of the Cardiovascular Disease Continuum: Endocrine Aspects of Disease Pathophysiology, Risk Predictors, Therapeutics, and Management of Diabetes and Hypertension Volume IIView all 9 articles
 Shengnan Chen1,2,3†
Shengnan Chen1,2,3† Qiao Mei1,2,3†
Qiao Mei1,2,3† Li Guo4†
Li Guo4† Xiaoli Yang2,5
Xiaoli Yang2,5 Wenbin Luo2,5
Wenbin Luo2,5 Xuemei Qu6
Xuemei Qu6 Xiaoping Li2,5
Xiaoping Li2,5 Bingqing Zhou2,5
Bingqing Zhou2,5 Ken Chen3,6*
Ken Chen3,6* Chunyu Zeng1,2,3,5*
Chunyu Zeng1,2,3,5*Background: Insulin resistance is associated with atrial remodeling as well as atrial fibrillation (AF). However, there was limited evidence on the relationship of triglyceride-glucose index (TyG) index, a simple, valuable marker of insulin resistance, with AF. Thus, we aimed to investigate the association between TyG index and AF among hospitalized patients.
Methods: A retrospective observational study was conducted in Daping Hospital, which included 356 hospitalized patients from the Department of Cardiology. Clinical and biochemical parameters were collected from electronic medical records and AF was diagnosed from electrocardiogram (ECG) findings.
Results: We found that the TyG index was significantly higher in the AF group than in the group without AF. Multivariate logistic regression revealed that hypertension (OR = 1.756, 95%CI 1.135-2.717, P = 0.011) and TyG index (OR = 2.092, 95%CI 1.412-3.100, P<0.001) were positively associated with AF. The analysis of the area under the ROC curve was performed and revealed that area under curve (AUC) of TyG index was 0.600 (95%CI, 0.542-0.659, P = 0.001), the optimal critical value was 8.35, the sensitivity was 65.4%, and the specificity was 52.0%. Additional subgroup analyses of diabetic and non-diabetic subjects were also performed and found the TyG index was increased in non-diabetic subjects with AF. Furthermore, a logistic regression analysis showed TyG index was associated with AF (OR = 3.065, 95% CI, 1.819-5.166, P<0.001) in non-diabetic subjects. However, TyG index was not associated with AF in diabetic subjects.
Conclusion: Elevated TyG index is an independent risk factor for AF among non-diabetic hospitalized patients.
Atrial fibrillation (AF) is a cardiac arrhythmia associated with an elevated risk of stroke, heart failure, and mortality. Previous studies have indicated the fundamental electrophysiological and structural changes within the left atrium are the main pathophysiological basis of AF (1). And increased evidence has revealed the metabolism disorders, especially diabetes and insulin resistance (IR), could lead to the atrial electrical and structural remodeling (1–3), which contributes to the initiation of AF. However, current conclusions are conflicting. Various parameters of insulin sensitivity are used to evaluate the relationship between IR and the prevalence of AF. For example, fasting insulin has generally not been shown to increase the risk of incident AF (4). Other study showed AF development increased at the homeostatic model of insulin resistance (HOMA-IR) levels between 1 to 2.5, whereas no further increase occurred above HOMA-IR as 2.5, indicated a non-linear relationship between IR and AF in non-diabetics (5, 6). Since the calculation of HOMA-IR depends on atypical measurement of serum insulin levels, it has limited application in clinical practice (7). In this sense, triglyceride-glucose (TyG) index has been proposed as an alternative method and valuable marker to evaluate IR.
TyG index was first reported in 2008 by combining fasting triglyceride (TG) levels with fasting plasma glucose (FPG) levels (8). Previous studies showed TyG index has high sensitivity and specificity with a close relationship with HOMA-IR and the total glucose metabolism rates (8–10). Clinical studies have demonstrated that increased TyG index is significantly correlated to higher risk of coronary artery disease and carotid atherosclerosis and greater prevalence of major adverse cardiovascular events in patients with acute coronary syndrome (11–14). Since TyG index has been widely used as a marker of insulin resistance and a predictor of several metabolic and cardiovascular diseases, the purpose of this study was to evaluate the association between TyG index and AF.
This study was a retrospective, observational study and the clinical data was obtained from electronic medical records. The study was reviewed and approved by the Research Ethics Board in Daping Hospital (No. 181, 2022, Chongqing City, P.R. China).
A total of 179 AF hospitalized patients from the Department of Cardiology in Daping Hospital between November 2017 and December 2021 were enrolled retrospectively in this study, of which 36 patients were combined with diabetes mellitus. Figure 1 showed the results of the AF patient selection flow chart. AF was diagnosed based on electrocardiograph (ECG) findings (absence of consistent P waves, presence of rapid, irregular f waves with a frequency of 350-600 b.p.m. and an irregular ventricular response) (15). The inclusion criteria were as follows (1): an ECG-confirmed diagnosis of AF (2); aged >18 years (3); complete clinical data. The exclusion criteria are as follows (16–18) (1): structural heart diseases and ischemia induced AF, such as myocardial infarction, decompensated heart failure, severe valvular heart disease, rheumatic heart disease (2); diagnosed with AF due to a reversible cause, such as acute thyrotoxicosis, pulmonary embolism, or postoperative status, solitary atrial flutter without AF (3); previous diagnosis of severe kidney or liver disease, recent infection, active inflammatory disorder or rheumatologic diseases that might influence IR or the TyG index. In addition, 179 age- and gender-matched subjects without AF who were hospitalized in the department during the same time period were randomly sampled and enrolled as the control group. Moreover, the control group included 36 age- and sex-matched diabetic subjects for the subgroup analyses.
Clinical information including age, sex, body mass index, history of smoking and alcohol, history of hypertension, diabetes mellitus, coronary heart disease, and current medication history was collected. Coronary heart disease included acute coronary syndrome and chronic ischemic syndrome diagnosed by previous clinical manifestations, electrocardiogram evaluation and/or coronary angiography (19, 20). Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medications (21). Diabetes mellitus was defined as a fasting plasma glucose level ≥ 126 mg/dL, 2-h postload plasma glucose ≥ 200 mg/dL, HbA1c ≥ 6.5%, or history of medical treatment for diabetes (22).
In both groups, 5 mL of fasting venous blood was collected in the early morning of the day after admission, and the plasma was obtained by centrifugation at 3000 rpm for 5 min. Biochemical parameters, including fasting plasma glucose (FPG), total cholesterol (TC), triglycerides (TG), low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C) were measured using the AU5800 automatic biochemical analyzer (Beckman Coulter, United States). The TyG index was calculated using this formula: ln[fasting triglycerides (mg/dL) × fasting plasma glucose (mg/dL)/2] (23).
Statistical analysis was performed with SPSS version 25.0 (IBM). Quantitative variables if normally distributed were expressed as means ± standard deviation, and t-test was used for the intergroup comparison. Non-normally distributed continuous variables were expressed as median [P25, P75] and Mann–Whitney U test was employed. Categorical data were expressed as percentages, while the differences among groups were compared by Chi square test. The risk of AF was compared using logistic regression analysis. The receiver operating characteristic (ROC) curve was used to determine the optimal cut-off of TyG index for predicting AF and predictive accuracies were quantified and compared using the area under the ROC curve (AUC). All reported probability values were two-tailed, and P<0.05 was considered statistically significant.
The baseline characteristics of all participants are summarized in Table 1. The levels of BMI, FPG and TG were higher in the AF patients than the participants without AF, whereas the TC level was lower in the controls. And AF patients had a greater proportion of hypertension and history of medication taking. Moreover, the TyG index of the AF group was significantly higher compared to the control group (Figure 2).
Since previous studies have identified male sex, aging, hypertension, diabetes, obesity, smoking and excessive alcohol use are the as independent risk factors (24, 25), a bivariate logistic regression analysis was conducted to identify those candidates for multivariate logistic regression analysis. And the univariate logistic regression analysis indicated BMI, hypertension, TC, TG, HDL-C, LDL-C and TyG index were associated with AF (P<0.05 for all) (Table 2). All independent variables with a P-value of<0.05 in bivariate logistic regression analysis were included in the multivariable logistic regression analysis. This result showed hypertension (OR = 1.756, 95%CI 1.135-2.717, P = 0.011) and TyG index (OR = 2.092, 95%CI 1.412-3.100, P<0.001) were positively associated with AF (Table 3).
To assess the predictive value of TyG index for AF, the analysis of the area under the ROC curve was performed and revealed that area under curve (AUC) of TyG index was 0.600 (95%CI, 0.542-0.659, P = 0.001), the optimal critical value was 8.35, the sensitivity was 65.4%, and the specificity was 52.0% (Table 4 and Figure 3). A logistic regression model was established based on the results of the multivariate analysis: logistic (P) = -4.873 + 0.563×hypertension – 0.415×TC + 0.738×TyG index. The AUC of the model for predicting AF was 0.667 (95%CI, 0.611-0.723, P < 0.001), the cut-off value was 0.466, the sensitivity was 71.5% and the specificity was 58.1%.
Since previous studies show different result of the causal association between diabetes and AF in diabetic and non-diabetic subjects, we then analyzed the TyG index and AF in diabetic and non-diabetic subjects. The baseline characteristics of diabetic and non-diabetic subjects are summarized in Supplemental Tables S1, S2. And we found the TyG index is increased in non-diabetic subjects with AF, whereas there is no significantly difference between control and AF group in TyG index in diabetic subjects (Figure 4). Furthermore, a logistic regression analysis showed TyG index is associated with AF (OR = 3.065, 95% CI, 1.819-5.166, P<0.001) in non-diabetic subjects (Table 5). However, TyG index is not associated with AF in diabetic subjects (Supplemental Table S3). In non-diabetic subjects, the analysis of the area under the ROC curve showed the AUC was 0.625 (95%CI, 0.560-0.689, P<0.001) and the cut-off value was 8.34, the sensitivity was 61.5%, and the specificity was 60.1% (Supplemental Table S4). A logistic regression model was established based on the results of the multivariate analysis: logistic (P) = -7.558 + 0.758×hypertension –0.537×TC + 1.120×TyG index. The AUC of the model for predicting AF was 0.704 (95%CI, 0.644-0.764, P < 0.001), the cut-off value was 0.506, the sensitivity was 65.7% and the specificity was 70.6%.
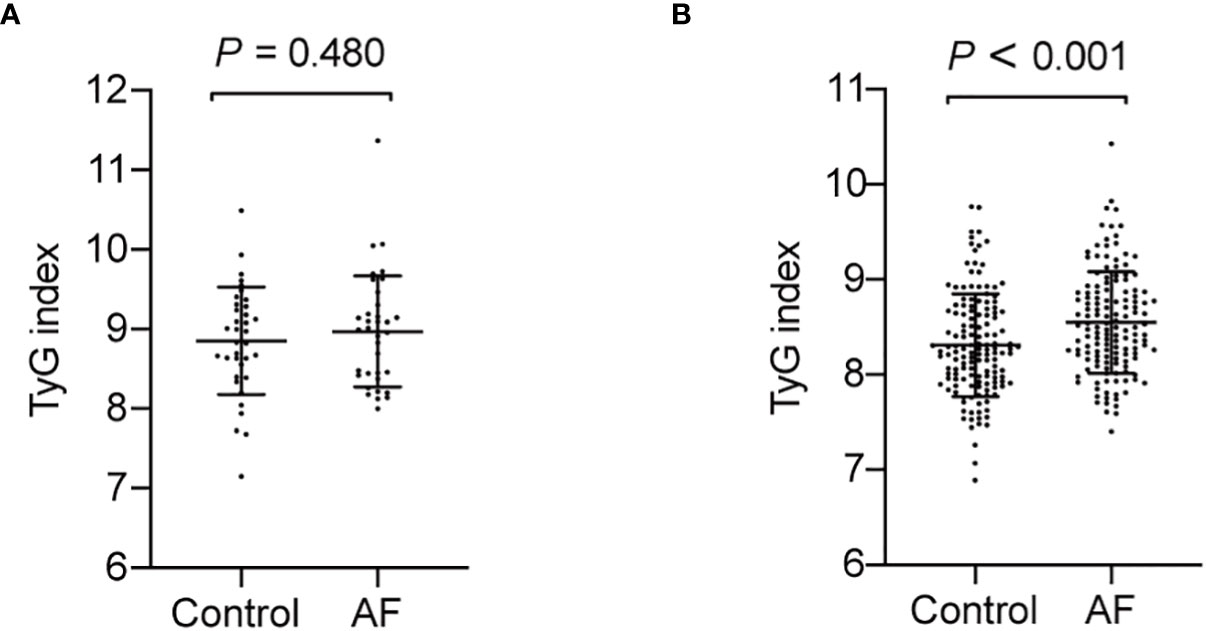
Figure 4 The TyG index in AF group and control in diabetic and non-diabetic subjects. (A) The TyG index is no significantly difference in diabetic subjects (P = 0.480). (B) The TyG index is increased in non-diabetic subjects with AF (P < 0.001).
Although numerous clinical and experimental studies show metabolism disorder is an important risk factor for the development of AF, whether diabetes and its related phenotypes (e.g., high FBG, insulin resistance) can contribute to AF development remains controversial. Our present study revealed that IR might be associated with AF. In this study, we found that the TyG index levels, a practical IR indicator, were higher in AF patients than the controls. Further logistic analysis indicated that the TyG index was an independent predictor for AF after adjusting for other factors. And the ROC curve confirmed the predictive value of the TyG index for AF.
Our present study suggested that IR is an independent predictor of AF, which has been demonstrated in previous cohort and experimental studies. Lee et al. found the baseline IR contributes to the long-term risk of incident AF in a community-based non-diabetes population (6). And a retrospective study of non-diabetic patients who have undergone radiofrequency catheter ablation (RFCA) showed the elevated TyG index was associated with an increased risk of late AF recurrence (26). Data from animal experiment also shows the IR mice were highly vulnerable to AF due to impaired glucose transport in the atria (27). And IR increases the levels of CaMKIIδ oxidizing and phospholamban/RyR-2 phosphorylation, which resulting in abnormal intracellular calcium homeostasis and the atrial structural remodeling (28). However, controversy has emerged regarding the association between IR and AF in various clinical studies. A study including a total of 60,620 individuals with atrial fibrillation with 970,216 control individuals did not find the causal association between diabetes and AF (29). The data from the Framingham Heart Study cannot indicate the association between IR and incident AF (30).
Some potential factors may explain those controversial outcomes. One possible explanation is that the correlation among known AF risk factor, such as hypertension, BMI and age, could obscure the individual contributions of specific risk factors to incident AF (30, 31). We also noticed that a variety of IR parameter in different studies has been proposed, which might lead to the bias of results. Fasting insulin level has been debated for a reliable IR parameter, for example, fasting insulin could not predict the risk of cardiovascular disease, such as coronary heart disease and stroke (31, 32). Thus, some indices are based on fasting plasma concentrations of glucose and insulin to substitute the fasting insulin level, such as the homeostasis model of insulin resistance (HOMA-IR), the quantitative insulin sensitivity check index (QUICKI) and the fasting insulin resistance index (FIRI) (33, 34). Those surrogate indices have been compared with hyperinsulinemic-euglycemic clamp, the gold standard of IR. In present study, we used TyG index, a simple and easy-to-calculate marker, which has been showed to be significantly correlated with IR measured by the hyperinsulinaemic-euglycaemic clamp test (35, 36). Our study found that hospitalized patients with elevated TyG index had a higher prevalence of AF and that after adjusting for traditional risk factors for AF, the TyG index remained an independent risk factor for AF. Moreover, since different cohorts may lead to the controversial results, an additional subgroup analysis of our study compared the difference between diabetic and non-diabetic population. Similar to what published researches reported (26, 29, 30), the present study showed the elevated TyG index was associated with AF in non-diabetic, whereas a role between TyG index with AF has not been observed in diabetic subjects. Lee et al. have identified the non-linear relationship between IR and the risk of AF, and the AF development increased at the HOMA-IR levels approximately between 1-2.5, and then plateaued afterwards (6). Therefore, the higher level of insulin resistance in patients with diabetes, as higher HOMA-IR or TyG index, might not associate with the risk of AF.
In our study, we showed the TC and LDL-C levels in AF group are significantly lower than the control. The lipid metabolism disorder might play a key role in the pathogenesis of AF (37). However, previous studies have identified higher levels of TC and LDL-C are associated with a lower incidence of AF (38, 39). The underlying mechanisms still remain largely unknown. Lower level of cholesterol could impair calcium handling, adrenergic signaling and the myofibril structure in cardiomyocytes (40), which might be involved in the development of AF.
Our study has several limitations. The current study is a cross-sectional clinical study. A causal correlation of TyG index with AF cannot be proposed due to the research design, which requires further prospective cohort researches for validation. Besides, we noticed that the AUC value in our study shows a mild-to-moderate significance. Although the range of AUC (0.6–0.7) is considered to be clinically useful (41–43), a study with a large sample size is needed to further verify the conclusion and to analyze the value of the TyG index in the evaluation of the progression and prognosis of AF.
In conclusion, our study found that TyG index was an independent risk factor of AF in non-diabetic subjects, which is expected to become a simple and practical biological indicator for predicting the occurrence of AF.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Research Ethics Board in Daping Hospital. The patients/participants provided their written informed consent to participate in this study.
SC, QM, XY and BZ enrolled the subjects and managed the case record form. SC, WL and XQ performed the statistical. XL, KC and CZ prepared the figures. LG, KC and CZ wrote the main manuscript text and prepared the revisions. All authors contributed to the article and approved the submitted version.
These studies were supported in part by grants from Senior Medical Talents Program of Chongqing for Young and Middle-aged (No. [2022]15).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1047927/full#supplementary-material
1. Gawalko M, Saljic A, Li N, Abu-Taha I, Jespersen T, Linz D, et al. Adiposity-associated atrial fibrillation: molecular determinants, mechanisms and clinical significance. Cardiovasc Res (2022) cvac093. doi: 10.1093/cvr/cvac093
2. Trieb M, Kornej J, Knuplez E, Hindricks G, Thiele H, Sommer P, et al. Atrial fibrillation is associated with alterations in HDL function, metabolism, and particle number. Basic Res Cardiol (2019) 114(4):27. doi: 10.1007/s00395-019-0735-0
3. Yang S, Pudasaini R, Zhi H, Wang L. The relationship between blood lipids and risk of atrial fibrillation: Univariable and multivariable mendelian randomization analysis. Nutrients14(1) (2021):181. doi: 10.3390/nu14010181
4. Chamberlain AM, Agarwal SK, Ambrose M, Folsom AR, Soliman EZ, Alonso A. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the atherosclerosis risk in communities (ARIC) study. Am Heart J (2010) 159(5):850–6. doi: 10.1016/j.ahj.2010.02.005
5. Polovina M, Krljanac G, Asanin M, Seferovic PM. Crouching tiger, hidden dragon: insulin resistance and the risk of atrial fibrillation. Eur J Prev Cardiol (2020) 27(18):1931–3. doi: 10.1177/2047487320912626
6. Lee Y, Cha SJ, Park JH, Shin JH, Lim YH, Park HC, et al. Association between insulin resistance and risk of atrial fibrillation in non-diabetics. Eur J Prev Cardiol (2020) 27(18):1934–41. doi: 10.1177/2047487320908706
7. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care (2004) 27(6):1487–95. doi: 10.2337/diacare.27.6.1487
8. Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord (2008) 6(4):299–304. doi: 10.1089/met.2008.0034
9. Aslam M, Mishra BK, Goyal S, Siddiqui AA, Madhu SV. Family history of diabetes determines the association of HOMA-IR with fasting and postprandial triglycerides in individuals with normal glucose tolerance. J Clin Lipidol (2021) 15(1):227–34. doi: 10.1016/j.jacl.2020.11.002
10. Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab (2010) 95(7):3347–51. doi: 10.1210/jc.2010-0288
11. Irace C, Carallo C, Scavelli FB, De Franceschi MS, Esposito T, Tripolino C, et al. Markers of insulin resistance and carotid atherosclerosis. a comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract (2013) 67(7):665–72. doi: 10.1111/ijcp.12124
12. Lambrinoudaki I, Kazani MV, Armeni E, Georgiopoulos G, Tampakis K, Rizos D, et al. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ (2018) 27(6):716–24. doi: 10.1016/j.hlc.2017.05.142
13. Li H, Zuo Y, Qian F, Chen S, Tian X, Wang P, et al. Triglyceride-glucose index variability and incident cardiovascular disease: a prospective cohort study. Cardiovasc Diabetol (2022) 21(1):105. doi: 10.1186/s12933-022-01541-5
14. Lu YW, Chang CC, Chou RH, Tsai YL, Liu LK, Chen LK, et al. Gender difference in the association between TyG index and subclinical atherosclerosis: results from the I-lan longitudinal aging study. Cardiovasc Diabetol (2021) 20(1):206. doi: 10.1186/s12933-021-01391-7
15. Tertulien T, Youmans QR, Essien UR. US Preventive services task force recommendation statement on screening for atrial fibrillation. JAMA (2022) 327(20):2021. doi: 10.1001/jama.2022.5204
16. Chen BX, Xie B, Zhou Y, Shi L, Wang Y, Zeng L, et al. Association of serum biomarkers and cardiac inflammation in patients with atrial fibrillation: Identification by positron emission tomography. Front Cardiovasc Med (2021) 8:735082. doi: 10.3389/fcvm.2021.735082
17. Inohara T, Shrader P, Pieper K, Blanco RG, Thomas L, Singer DE, et al. Association of of atrial fibrillation clinical phenotypes with treatment patterns and outcomes: A multicenter registry study. JAMA Cardiol (2018) 3(1):54–63. doi: 10.1001/jamacardio.2017.4665
18. Wei Z, Zhu E, Ren C, Dai J, Li J, Lai Y. Triglyceride-glucose index independently predicts new-onset atrial fibrillation after septal myectomy for hypertrophic obstructive cardiomyopathy beyond the traditional risk factors. Front Cardiovasc Med (2021) 8:692511. doi: 10.3389/fcvm.2021.692511
19. Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European society of cardiology. Eur Heart J (2013) 34(38):2949–3003. doi: 10.1093/eurheartj/eht296
20. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American college of Cardiology/American heart association task force on practice guidelines, and the American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Thorac Cardiovasc Surg (2015) 149(3):e5–23. doi: 10.1016/j.jtcvs.2014.11.002
21. Verhaar BJH, Collard D, Prodan A, Levels JHM, Zwinderman AH, Backhed F, et al. Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: the HELIUS study. Eur Heart J (2020) 41(44):4259–67. doi: 10.1093/eurheartj/ehaa704
22. Association American Diabetes. Diagnosis and classification of diabetes mellitus. Diabetes Care (2011) 34 Suppl:1, S62–9. doi: 10.2337/dc11-S062
23. Kang B, Yang Y, Lee EY, Yang HK, Kim HS, Lim SY, et al. Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int J Obes (Lond) (2017) 41(5):789–92. doi: 10.1038/ijo.2017.14
24. Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation (2017) 136(6):583–96. doi: 10.1161/CIRCULATIONAHA.116.023163
25. Middeldorp ME, Ariyaratnam J, Lau D, Sanders P. Lifestyle modifications for treatment of atrial fibrillation. Heart (2020) 106(5):325–32. doi: 10.1136/heartjnl-2019-315327
26. Tang Q, Guo XG, Sun Q, Ma J. The pre-ablation triglyceride-glucose index predicts late recurrence of atrial fibrillation after radiofrequency ablation in non-diabetic adults. BMC Cardiovasc Disord (2022) 22(1):219. doi: 10.1186/s12872-022-02657-y
27. Maria Z, Campolo AR, Scherlag BJ, Ritchey JW, Lacombe VA. Dysregulation of insulin-sensitive glucose transporters during insulin resistance-induced atrial fibrillation. Biochim Biophys Acta Mol Basis Dis (2018) 1864(4 Pt A):987–96. doi: 10.1016/j.bbadis.2017.12.038
28. Chan YH, Chang GJ, Lai YJ, Chen WJ, Chang SH, Hung LM, et al. Atrial fibrillation and its arrhythmogenesis associated with insulin resistance. Cardiovasc Diabetol (2019) 18(1):125. doi: 10.1186/s12933-019-0928-8
29. Harati H, Zanetti D, Rao A, Gustafsson S, Perez M, Ingelsson E, et al. No evidence of a causal association of type 2 diabetes and glucose metabolism with atrial fibrillation. Diabetologia (2019) 62(5):800–4. doi: 10.1007/s00125-019-4836-y
30. Fontes JD, Lyass A, Massaro JM, Rienstra M, Dallmeier D, Schnabel RB, et al. Insulin resistance and atrial fibrillation (from the framingham heart study). Am J Cardiol (2012) 109(1):87–90. doi: 10.1016/j.amjcard.2011.08.008
31. Garg PK, Biggs ML, Kaplan R, Kizer JR, Heckbert SR, Mukamal KJ. Fasting and post-glucose load measures of insulin resistance and risk of incident atrial fibrillation: The cardiovascular health study. Nutr Metab Cardiovasc Dis (2018) 28(7):716–21. doi: 10.1016/j.numecd.2018.02.016
32. Wiberg B, Sundstrom J, Zethelius B, Lind L. Insulin sensitivity measured by the euglycaemic insulin clamp and proinsulin levels as predictors of stroke in elderly men. Diabetologia (2009) 52(1):90–6. doi: 10.1007/s00125-008-1171-0
33. Otten J, Ahren B, Olsson T. Surrogate measures of insulin sensitivity vs the hyperinsulinaemic-euglycaemic clamp: a meta-analysis. Diabetologia (2014) 57(9):1781–8. doi: 10.1007/s00125-014-3285-x
34. Rudvik A, Mansson M. Evaluation of surrogate measures of insulin sensitivity - correlation with gold standard is not enough. BMC Med Res Methodol (2018) 18(1):64. doi: 10.1186/s12874-018-0521-y
35. Bastard JP, Lavoie ME, Messier V, Prud'homme D, Rabasa-Lhoret R. Evaluation of two new surrogate indices including parameters not using insulin to assess insulin sensitivity/resistance in non-diabetic postmenopausal women: a MONET group study. Diabetes Metab (2012) 38(3):258–63. doi: 10.1016/j.diabet.2012.01.004
36. Mirr M, Skrypnik D, Bogdanski P, Owecki M. Newly proposed insulin resistance indexes called TyG-NC and TyG-NHtR show efficacy in diagnosing the metabolic syndrome. J Endocrinol Invest (2021) 44(12):2831–43. doi: 10.1007/s40618-021-01608-2
37. Fang Y, Wu Y, Liu L, Wang H. The four key genes participated in and maintained atrial fibrillation process via reprogramming lipid metabolism in AF patients. Front Genet (2022) 13:821754. doi: 10.3389/fgene.2022.821754
38. Lopez FL, Agarwal SK, Maclehose RF, Soliman EZ, Sharrett AR, Huxley RR, et al. Blood lipid levels, lipid-lowering medications, and the incidence of atrial fibrillation: the atherosclerosis risk in communities study. Circ Arrhythm Electrophysiol (2012) 5(1):155–62. doi: 10.1161/CIRCEP.111.966804
39. Peters SAE, Carcel C, Millett ERC, Woodward M. Sex differences in the association between major risk factors and the risk of stroke in the UK biobank cohort study. Neurology (2020) 95(20):e2715–26. doi: 10.1212/WNL.0000000000010982
40. Hissa B, Oakes PW, Pontes B, Ramirez-San Juan G, Gardel ML. Cholesterol depletion impairs contractile machinery in neonatal rat cardiomyocytes. Sci Rep (2017) 7:43764. doi: 10.1038/srep43764
41. Xu X, Li X, Yang G, Li L, Hu W, Zhang L, et al. Circulating bone morphogenetic protein-9 in relation to metabolic syndrome and insulin resistance. Sci Rep (2017) 7(1):17529. doi: 10.1038/s41598-017-17807-y
42. Zhu Y, Liu K, Chen M, Liu Y, Gao A, Hu C, et al. Triglyceride-glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol (2021) 20(1):137. doi: 10.1186/s12933-021-01332-4
Keywords: atrial fibrillation, triglyceride-glucose index, insulin resistance, diabet mellitus type 2, arrhythmia
Citation: Chen S, Mei Q, Guo L, Yang X, Luo W, Qu X, Li X, Zhou B, Chen K and Zeng C (2022) Association between triglyceride-glucose index and atrial fibrillation: A retrospective observational study. Front. Endocrinol. 13:1047927. doi: 10.3389/fendo.2022.1047927
Received: 19 September 2022; Accepted: 11 November 2022;
Published: 08 December 2022.
Edited by:
Jyoti Rajan Sharma, South African Medical Research Council, South AfricaReviewed by:
Dongkai Shan, People’s Liberation Army General Hospital, ChinaCopyright © 2022 Chen, Mei, Guo, Yang, Luo, Qu, Li, Zhou, Chen and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunyu Zeng, Y2h1bnl1emVuZzAxQDE2My5jb20=; Ken Chen, Y2hlbmtlbkBjaWdpdC5hYy5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.