- State Key Laboratory of Cardiovascular Disease, Cardiometabolic Medicine Center, Fuwai Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
Objective: The aim of the present study was to examine the value of high-density lipoprotein (HDL) subfractions for predicting cardiovascular events (CVEs) in untreated type 2 diabetes mellitus (T2DM) patients with stable coronary artery disease (SCAD) using an age- and gender-matched case-control study.
Methods: In total, 185 SCAD patients and 185 T2DM patients with SCAD were enrolled and subjected to a clinical follow-up of CVEs. HDL subfractions were analyzed using the Quantimetrix Lipoprint System. The relationship between HDL subfractions and CVEs in T2DM patients with SCAD was evaluated by Kaplan–Meier analysis and Cox proportional hazard models.
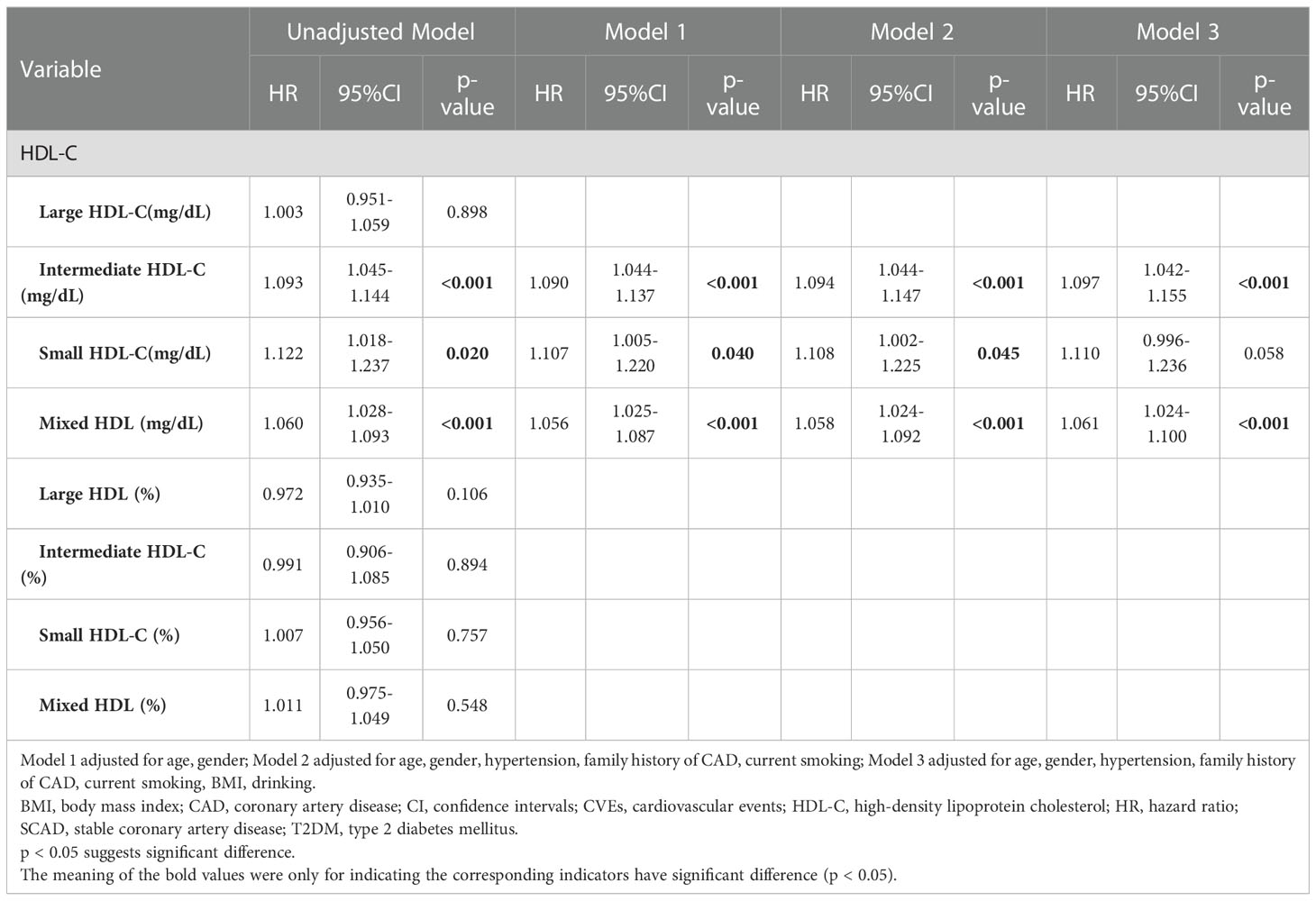
Results: During a median 37.7-month follow-up, T2DM patients with SCAD had a higher percentage of CVEs compared to SCAD patients (p=0.039). The concentration of the combined intermediate and small HDL-C subfraction (defined as the mixed HDL subfraction) was related to the event incidence in T2DM patients with SCAD (p=0.004), and it was positively associated with increased CVEs even after adjustment in three models. Kaplan-Meier curve analysis indicated that T2DM patients with SCAD in the high mixed HDL subfraction group (>28 mg/dL) had lower event-free survival rates (p=0.008).
Conclusions: Elevated concentration of the mixed HDL subfraction predicts events in T2DM patients with SCAD.
Introduction
Cardiovascular disease (CVD) is one of major causes of death in the world with atherosclerotic cardiovascular disease (ASCVD) accounting for half of these deaths. Patients with type 2 diabetes mellitus (T2DM) are at a 2- to 4-fold increased risk of ASCVD, which accounts for the leading cause of mortality (1). Dyslipidemia in T2DM is characterized by hypertriglyceridemia, normal to mildly elevated levels of low-density lipoprotein-cholesterol (LDL-C; with increased levels of small dense LDL particles) and low high-density lipoprotein-cholesterol (HDL-C) levels (with variable changes in HDL composition) (2). Previous study has indicated that atherosclerotic lesions of the vascular system in T2DM patients is not only dependent on glycemic control but also the presence of coexisting risk factors, including dyslipidemia (3).
HDL is highly heterogenous in its size and composition, and HDL subclasses are generally classified as HDL2 (larger and less dense) and HDL3 (smaller and denser) (4). The conformational and functional properties of HDL particles may be altered by several factors. The findings of lipid-lowering drugs that increase HDL-C but do not reduce cardiovascular events (CVEs) or atherosclerosis have attracted an interest in alternative indexes of HDL quantity [i.e., HDL particle or apolipoprotein A1 (ApoA1)] or HDL quality, such as particle size, subclass distribution or various measures of HDL functionality (5, 6). Moreover, a previous study has shown that the transformation of HDL particles into pathogenic ones occurs in T2DM (7). Garvey et al. (8) demonstrated that lipoprotein subclass alterations in T2DM are moderately exacerbated as evidenced by a decrease in HDL size as a result of depletion of large HDL particles and a modest increase in small HDL. Furthermore, our group previously identified significant associations between HDL-C subfractions and T2DM or stable coronary artery disease (SCAD) (9). Thus, it is important to understand these features to help improve the prevention of macrovascular complications.
Although previous studies have shown the prognostic role of dyslipidemia in T2DM patients with SCAD, the effects of plasma HDL-C subfractions on cardiovascular outcomes in T2DM patients with SCAD are unclear. Thus, the aim of the present study was to investigate the relationship between HDL-C subfractions and the risk of CVEs in SCAD patients or T2DM patients with SCAD.
Materials and methods
Study design and population
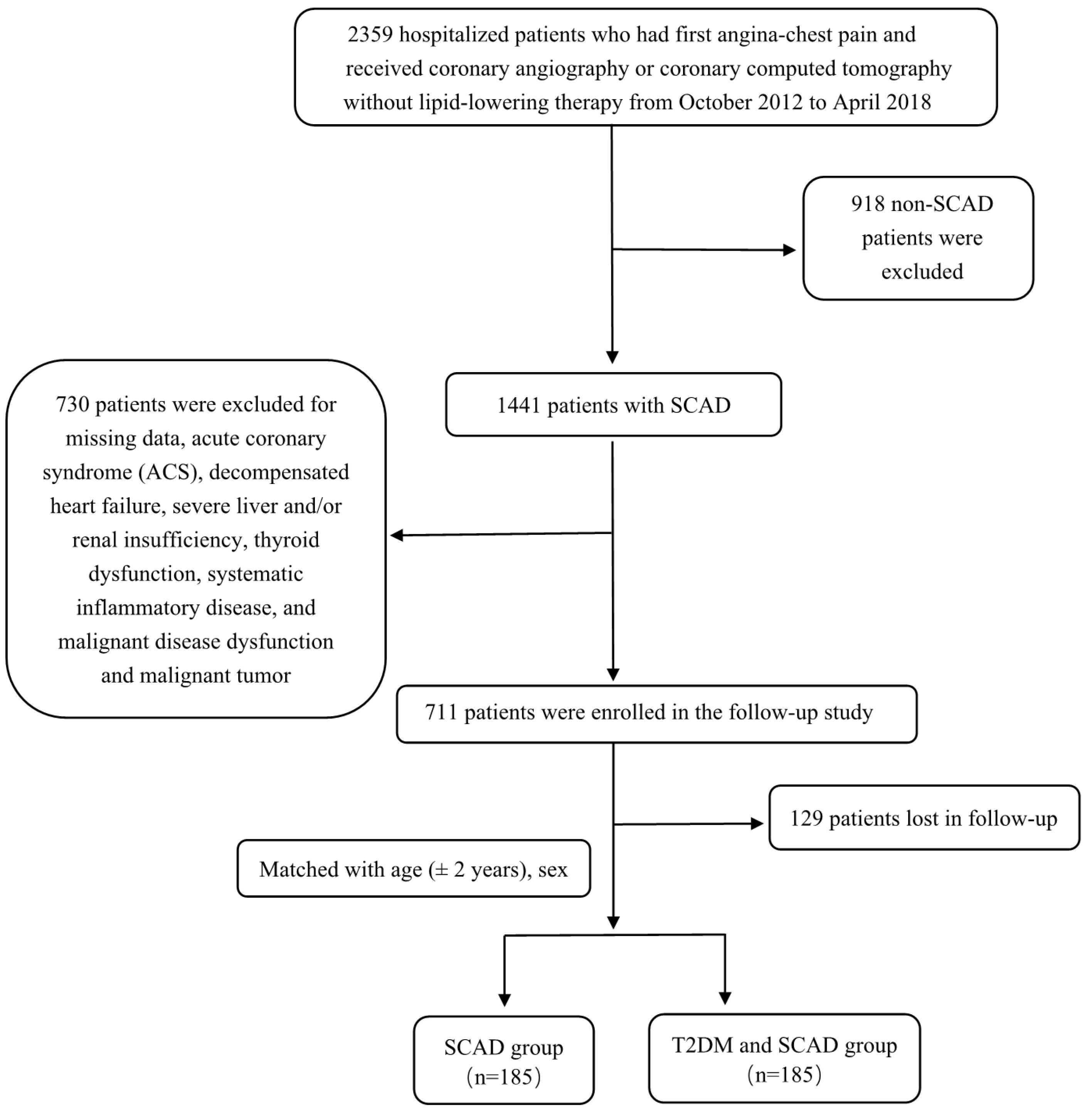
A total of 2359 participants with angina-like chest pain and/or positive treadmill exercise test or clinically suspected CVD from Fuwai Hospital were consecutively enrolled between October 2012 and April 2018. The exclusion criteria of patients were as follows: 1) aged <18 years old; 2) treatment history of lipid-lowering drugs and antidiabetic drugs prior to entering the study; 3) severe end-stage diseases, such as renal and/or liver dysfunction, heart failure and malignant carcinoma; 4) systematic inflammatory disease or severe infection; 5) thyroid disorder; and 6) pregnancy. In total, 918 patients were excluded due to the lack of angiography-proven SCAD. SCAD was defined as typical angina-like chest pain brought on by exertion and relieved by rest or sublingual nitrates or both, a positive treadmill exercise test >1-mm ST-segment depression) and stable obstructive lesion >50% in at least 1 of the 3 major coronary arteries or major branches assessed by at least 2 independent senior interventional cardiologists. Another 730 patients were excluded due to the following reasons: acute coronary syndrome (ACS); decompensated heart failure; severe liver and/or renal insufficiency; thyroid dysfunction; systematic inflammatory disease; malignant disease dysfunction; and malignant tumor. All patients in the present study were followed up every year by clinic revisit or by phone interview with trained nurses or doctors blinded to the clinical data until the first CVE occurred or up to the last day of the follow-up period. In total, 711 patients were enrolled for follow-up, but 129 patients were lost to follow-up, resulting in 582 patients with intact follow-up information. Among these patients, there were 185 T2DM patients with SCAD according to the definition. From the remaining pool of patients (n=397), we randomly selected control subjects at a 1:1 ratio matched with the same sex, and the age difference was ± 2 years old. Thus, there were 185 T2DM patients with SCAD and 185 SCAD patients in the final analysis. CVEs were defined as follows: hospitalized unstable angina; non-fatal myocardial infarction (MI); stroke; unplanned percutaneous coronary intervention or coronary artery bypass grafting; and cardiovascular death. Non-fatal MI was diagnosed as positive cardiac troponins along with typical chest pain or typical electrocardiogram serial changes. Cardiovascular death was diagnosed based on death primarily caused by acute myocardial infarction (AMI), congestive heart failure, malignant arrhythmia and other structural or functional cardiac diseases. Follow-up time was calculated as the number of months from the enrollment till the last traceable hospital outpatient record, hospital inpatient record or telephone interview before April 2019. A study population enrollment flowchart is presented in Figure 1.
T2DM was diagnosed by fasting plasma glucose (FPG) 7.0 ≥ mmol/L, 2-h plasma glucose of oral glucose tolerance test ≥ 11.1 mmol/L, hemoglobin A1c (HbA1c) level ≥ 6.5%, currently using antidiabetic drugs or currently using insulin. Hypertension was diagnosed as medical history of hypertension, currently receiving antihypertensive drugs or hospital recorded systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg for three or more consecutive times. The body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Patients who had a smoking habit of at least one cigarette per day on admission were classified as current smokers. Patients who reported alcohol intake at least once a week were classified as a current drinker. Family history of CAD was defined when myocardial ischemia or infarction was documented in at least one first-degree relative. Baseline medications (medications before admission) were collected by interviewing or from hospital-recorded medical history. Patients who received statin therapy at the time of discharging from hospital were also recorded.
The present study fully complied with the Declaration of Helsinki and received approval from the Ethics Committee of Fu Wai Hospital and Cardiovascular Institute (Beijing, China). All included patients provided prior written consent.
Blood sample measurement
Blood samples were collected into EDTA-containing tubes. After centrifugation at 3000 rpm for 10 min at 4°C, plasma was collected and stored at −80°C. Plasma concentrations of total cholesterol (TC), triglyceride (TG), LDL-C and HDL-C were measured by an automatic biochemistry analyzer (Hitachi 7150, Tokyo, Japan) using an enzymatic assay. HbA1c was measured using the Tosoh Automated Glycohemoglobin Analyzer (HLC-723G8, Tokyo, Japan). Plasma concentrations of ApoA1, apolipoprotein B (ApoB) and lipoprotein (a) [Lp(a)] were measured using a turbidimetric immunoassay with the automatic biochemistry analyzer mentioned above. Lipoprotein subfraction analysis was performed using the Lipoprint System (Quantimetrix Corporation, Redondo Beach, CA, USA) according to the manufacturer’s instructions. The HDLs were divided into 10 subfractions as follows: subfractions 1–3 indicated large HDL particles; subfractions 4–7 represented intermediate HDL particles; and subfractions 8–10 represented small HDL particles. Subsequently, the cholesterol concentration (mg/dL) and lipoprotein subfraction proportion (%) were determined for each lipoprotein subfraction.
Statistical analysis
All statistical analyses were performed using statistical package for social science (SPSS) version 25 (IBM Corporation, Armonk, New York, USA). Continuous variables with normal distribution are presented as mean ± standard deviation (SD), and variables with abnormal distribution are expressed as the median (interquartile range). Categorical variables are presented as a number (frequency). The independent sample Student’s t-test or Mann–Whitney U test was performed to analyze the differences in variables among groups. χ2 analysis and Fisher’s test were performed to determine the statistical difference in categorical variables between two groups. The logistic regression analysis was performed to determine the relationship between HDL subfractions and T2DM prevalence. The hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated by univariate and multivariate Cox regression analyses. The cumulative event-free survival rates of the high mixed HDL subfractions (>28 mg/dL) and the low mixed HDL subfractions (≤ 28 mg/dL) were estimated by the Kaplan–Meier curve with the log-rank test. Moreover, p<0.05 was considered statistically significant.
Results
Baseline characteristics
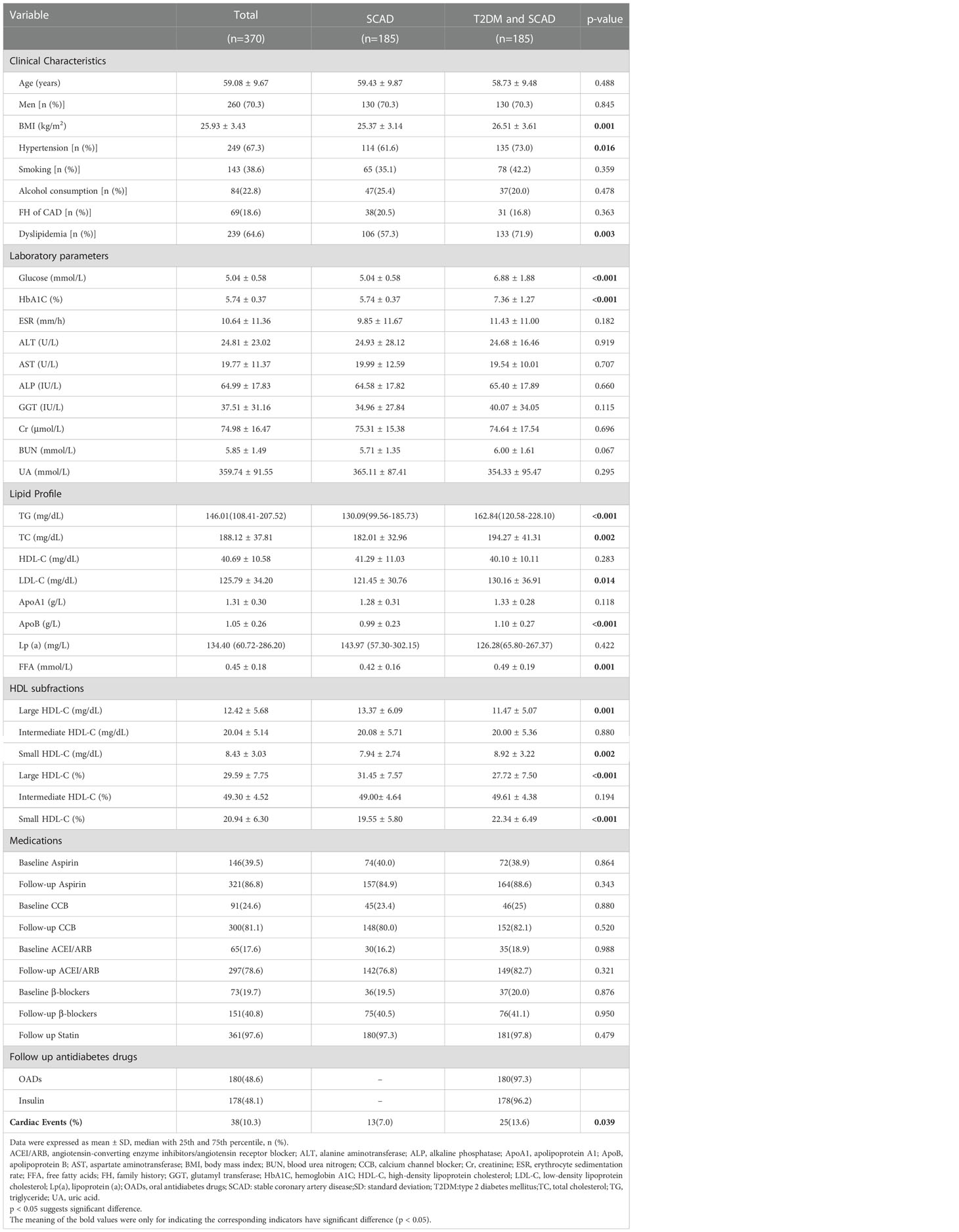
The demographics and clinical characteristics of T2DM patients with SCAD and SCAD patients are summarized in Table 1. The average age was 59.08 ± 9.67 years, and males accounted for 70.3% (n = 260) of the enrolled patients. Compared to the SCAD group, the T2DM and SCAD group had significantly higher levels of glucose, HbA1C, TG, TC, LDL-C, ApoB, free fatty acids (FFAs) and BMI (p<0.001, p<0.001, p<0.001, p=0.002, p=0.014, p<0.001, p=0.001 and p=0.001, respectively). Moreover, the T2DM and SCAD group had a higher morbidity of hypertension and dyslipidemia compared to the SCAD group. The levels of HDL-C and Lp(a) in the T2DM and SCAD group displayed a descending tendency, and the ApoA1 level showed an increasing tendency; however, the differences were not statistically significant (p=0.283, p=0.422 and p=0.118, respectively). Moreover, the T2DM and SCAD group had a higher concentration and percentage of the small HDL-C subfraction (p=0.002 and p<0.001, respectively) compared to the SCAD group. Nevertheless, the concentration and percentage of the large HDL-C subfraction was significantly reduced in the T2DM and SCAD group (p=0.001 and p<0.001, respectively) compared to the SCAD group. In addition, no significant difference was observed in the concentration and percentage of the intermediate HDL-C subfraction between the two groups.
Association of HDL subfractions in T2DM patients with SCAD
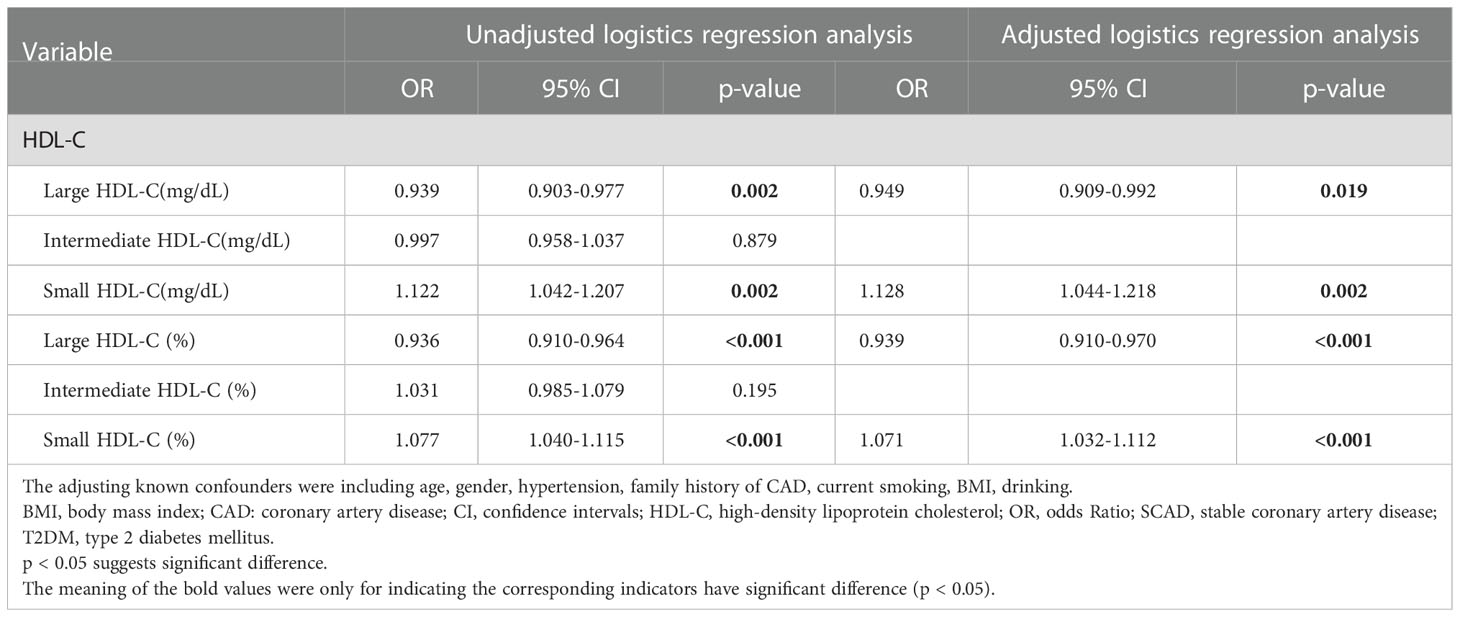
The median follow-up time was 37.7 months (interquartile range: 13.2–47.1 months). During the follow-up period, 13 (7.0%) patients in the SCAD group developed CVEs, and 25 (13.6%) patients in the T2DM and SCAD group developed CVEs; there was a significant difference between two groups (p=0.039). A logistic regression analysis was performed to further assess the association of HDL subfractions with the coexistence of T2DM and SCAD (Table 2). After adjusted logistic regression analysis, the concentration and percentage of the small HDL subfraction were significantly positively associated with the coexistence of T2DM and SCAD [unadjusted OR (95% CI): 1.122 (1.042–1.207), p=0.002; adjusted OR (95% CI): 1.128 (1.044–1.218), p=0.002; unadjusted OR (95% CI): 1.077 (1.040–1.115), p<0.001; adjusted OR (95% CI): 1.071 (1.032–1.112), p<0.001; respectively], while the concentration and percentage of the large HDL-C subfraction were inversely related to the coexistence of T2DM and SCAD [unadjusted OR (95% CI): 0.939 (0.903–0.977), p=0.002; adjusted OR (95% CI): 0.949 (0.909–0.992), p=0.019; unadjusted OR (95% CI): 0.936 (0.910–0.964), p<0.001; adjusted OR (95% CI): 0.939 (0.910–0.970), p<0.001; respectively].
Clinical and biochemical characteristics of patients in the T2DM and SCAD group with or without CVEs
The demographics and clinical characteristics of patients in the T2DM and SCAD group with or without CVEs are shown in Table 3. Patients with CVEs had significantly higher levels of ALT, AST and glutamyl transferase (GGT) (p=0.007, p=0.015 and p=0.033, respectively), while the ages of patients with CVEs were lower (p=0.038). Furthermore, patients with CVEs had higher intermediate HDL-C concentrations, small HDL-C concentrations and combined intermediate HDL-C and small HDL-C (defined as mixed HDL subfraction) concentrations (p<0.001, p=0.028 and p<0.001, respectively).
Association of HDL-C subfractions with CVEs in T2DM patients with SCAD
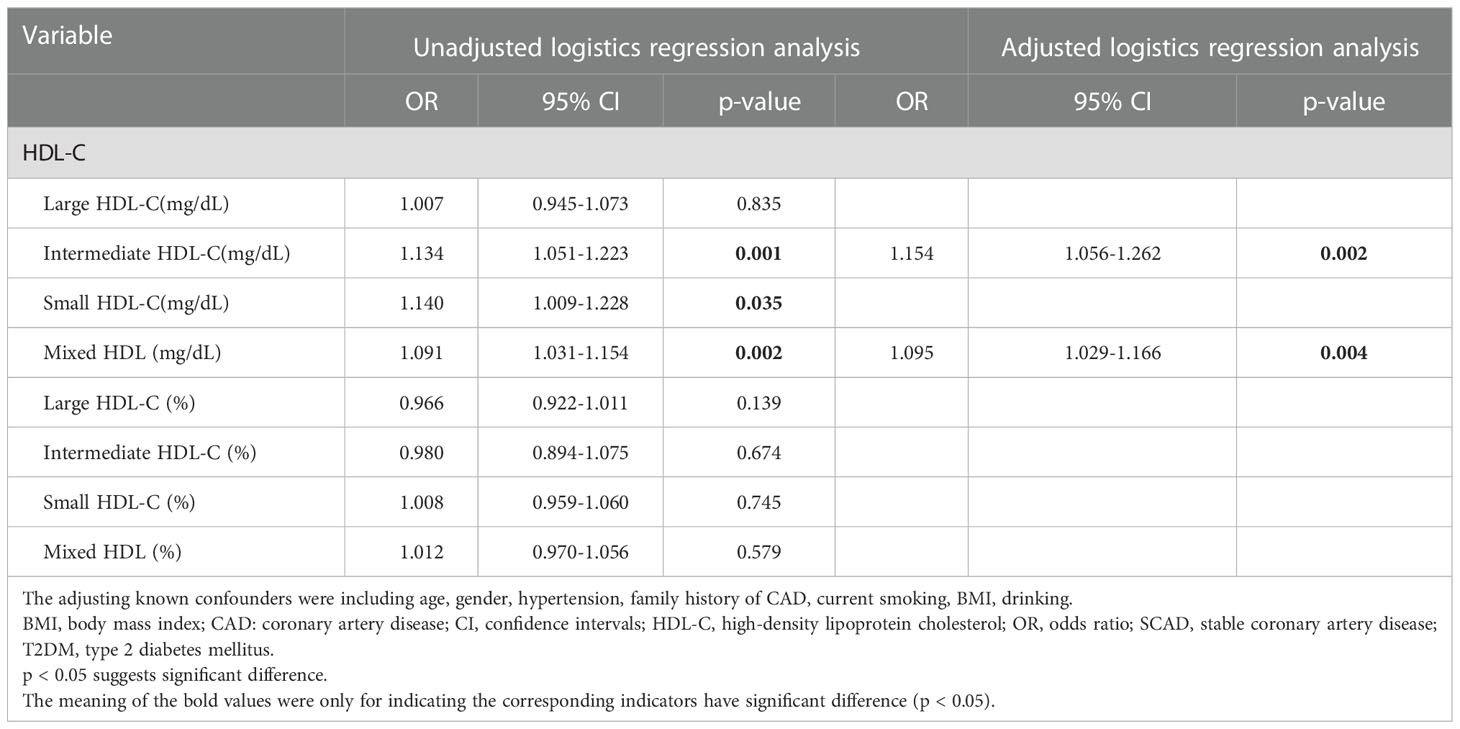
A logistic regression analysis was performed to further assess the association of HDL subfractions with CVEs in T2DM patients with SCAD (Table 4). In the present study, adjusted logistic regression analysis indicated that intermediate HDL-C concentrations and mixed HDL subfraction concentrations were positively related to the existence of CVEs in T2DM patients with SCAD [adjusted OR (95% CI): 1.154 (1.056–1.262), p=0.002; adjusted OR (95% CI): 1.095 (1.029–1.166), p=0.004; respectively].
Relationship between HDL-C subfractions and outcomes of T2DM patients with SCAD
As shown in Table 5, univariate Cox regression and multivariate Cox regression analyses revealed that the association of CVEs with intermediate HDL-C concentrations and mixed HDL subfraction concentrations remained significant after adjusting in three models [adjusted HR (95% CI): 1.097 (1.042–1.155); p<0.001; adjusted HR (95% CI): 1.061 (1.024–1.100); p<0.001; respectively]. Kaplan–Meier curve analysis of the probability of CVEs event-free survival during the follow-up period according to the low mixed HDL subfractions (≤ 28 mg/dL) and high mixed HDL subfractions (> 28 mg/dL) indicated that patients with higher levels of mixed HDL subfractions had lower event-free survival rates (p=0.008) (Figure 2).
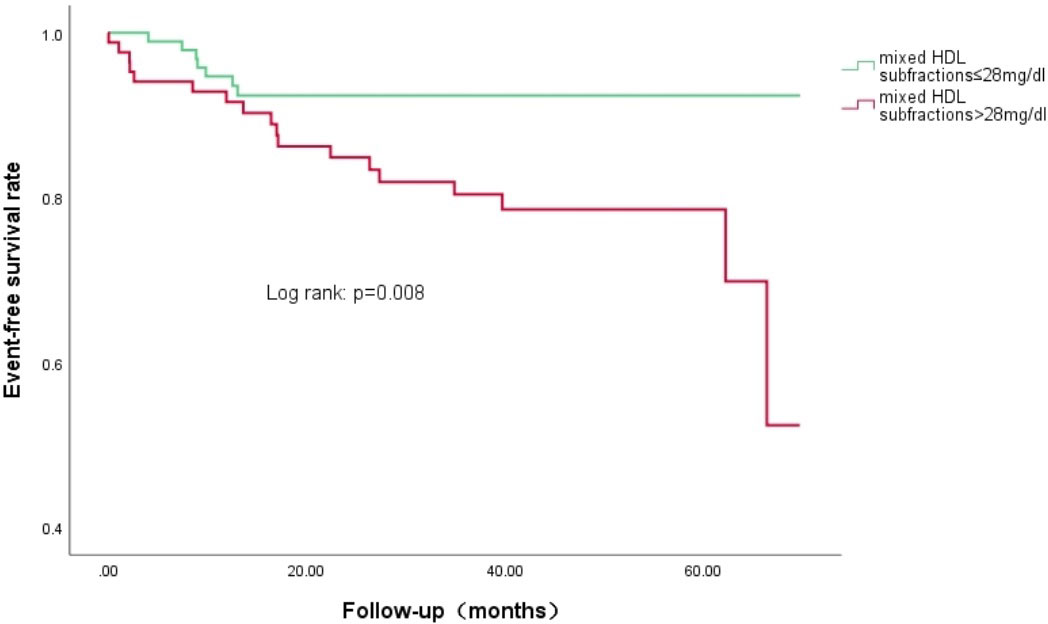
Figure 2 Kaplan-Meier curves of the cumulative event-free survival analyses according to mixed HDL subfractions levels in T2DM and SCAD patients at baseline.
Discussion
Many studies have investigated the changes in HDL-C subfractions or particles resulting from diabetes. We previously reported that the concentration of large HDL-C subfractions in SCAD patients is lower compared to healthy individuals but that the concentration of small HDL-C subfractions is higher in SCAD patients compared to healthy individuals (10). In the present study, we found that the concentration of large HDL-C subfractions was decreased and that the concentration of small HDL-C subfractions was increased in T2DM patients with SCAD compared to patients with SCAD alone. Moreover, the present data indicated that the concentrations of large HDL-C subfractions and small HDL-C subfractions were negatively and positively correlated, respectively, with the coexistence of T2DM and SCAD even after adjusting for traditional cardiovascular risk factors. More importantly, the present study is the first to report that the mixed HDL subfraction concentration predicts the risk of CVEs in T2DM patients with SCAD even after adjusting for traditional cardiovascular risk factors. Thus, the present findings suggested that measurement of HDL-C subfractions may be a novel useful tool for predicting clinical outcomes for T2DM patients with SCAD.
Data from numerous epidemiological and clinical studies have indicated that the mortality of SCAD is much higher in patients with T2DM, and the potential risk has been reported to be three times higher than that in patients without T2DM (11–13). The characteristics of dyslipidemia in T2DM patients consist of elevated concentrations of TG and low levels of HDL-C, which are significantly associated with the incidence of ASCVD (14). Previous study has also indicated that HDL has antiatherogenic and cardioprotective functions (15), while the role of HDL particles or subfractions in SCAD patients or T2DM patients with SCAD is not completely understood. HDL particles vary in size and function, which may be strongly associated with incident T2DM (16). Interestingly, we have previously reported that there is a significant increase in small HDL-C subfraction concentrations and a decrease in large HDL subfraction percentages in Chinese Han T2DM patients (9, 17). A previous study performed in 51 healthy subjects and 98 patients with T2DM or coronary heart disease using nuclear magnetic resonance (NMR) spectroscopy revealed a shift of HDL particle composition with a loss of large and very large particles as well as a gain of small triglyceride-rich particles in T2DM patients (18). Another study has reported that the HDL-C subfraction in T2DM patients with SCAD consists mostly of small triglyceride-rich HDL particles with a loss of large and very large HDL particles (19). Furthermore, Syvänne et al. (20) found that HDL particles in T2DM patients with SCAD are small in size and have lower free cholesterol content. Similarly, the present study found that the concentration and percentage of large HDL-C subfractions were significantly lower in T2DM patients with SCAD compared to SCAD patients, whereas the concentration and percentage of small HDL-C subfractions were higher in T2DM patients with SCAD compared to SCAD patients. Finally, the concentration and percentage of HDL-C large subfractions were negatively correlated with the coexistence of T2DM and SCAD, while the concentration and percentage of small HDL-C was positively correlated with the coexistence of T2DM and SCAD.
Although previous studies have shown a significant association between HDL lipoprotein subfractions and the coexistence of T2DM and SCAD, the prognostic value of different HDL-C subfractions in the outcome of T2DM patients with SCAD has been investigated less. The LUdwigshafen RIsk and Cardiovascular Health (LURIC) study reported that high concentrations of HDL-C particles are inversely related to cardiovascular mortality in SCAD patients, which is primarily driven by the smallest HDL-C particles (21). The Insulin Resistance Atherosclerosis Study (IRAS) showed a relationship between small HDL and incident diabetes in 830 individuals who had no diabetes at baseline and subsequently developed diabetes after a mean follow-up of 5.2 years (22). In a prospective study of 26,836 initially healthy women with 13 years follow-up for incident T2DM, the data showed that the small HDL particles were positively associated with diabetes (23). Moreover, higher levels of small HDL particles at baseline were associated with a higher risk of future T2DM during a median 7.3-year follow-up among 4828 subjects of the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study without T2DM at baseline (24). The present study focused on the association of HDL-C subfraction concentrations with CVEs in SCAD patients with or without T2DM, and our data indicated that the concentrations of the mixed HDL subfractions were positively correlated with CVEs after adjustment for established cardiometabolic factors, which suggested that high levels of mixed HDL subfractions indicate higher risk of CVEs. These findings provide novel information regarding the relation of HDL-C subfraction and prognosis in T2DM patients with SCAD.
Recent studies reported that the changes of HDL particles or subfractions can predict the severity and outcomes of T2DM patients with or without SCAD, but the exact mechanisms underlying the HDL changes are not fully understood. It has been demonstrated that HDL exerts various antiatherogenic properties, including enhancing reverse cholesterol transport, antioxidative capacities and anti-inflammatory capacities in physiological conditions (25, 26). HDL may become dysfunctional in the pathological state which consists of a change in the composition of lipids and proteins in HDL, such as paraoxonase-1 and lipoprotein-associated phospholipase 2 (27–29). The size of HDLs in T2DM patients is altered with a loss of large and very large HDL2 (subclass of large and more buoyant HDL) as well as a shift toward small HDL3 (subclass of small and dense HDL) (30). This change may be a potential factor contributing to the proinflammatory properties of these particles in T2DM patients. It has been suggested that the predominant small HDLs are primary carriers of ceramides, which are recognized as potent activators of the NF-κB transcription factor involved in inflammation (31). The proteins in HDLs are significantly modified in T2DM patients with increased levels of serum amyloid A, fibrinogen, ApoC2 and ApoC3 levels as well as reduced levels of ApoA1, ApoA2, ApoE, ApoM and PON1 (32, 33). A previous study has shown that the function of large HDL2 protecting against LDL oxidation in individuals with T2DM is decreased compared to that of healthy controls, which is associated with decreased free cholesterol and increased TGs (34). A previous study using a small cohort of patients in whom insulin sensitivity was decreased has suggested another potential mechanism, in which insulin resistance may drive HDL subclass distribution towards smaller particles in T2DM patients (8). In a recent study of 8365 individuals aged 52 ± 13 years who have not taken lipid-lowering drugs, HDL2-C was reported to be inversely associated with exacerbation of insulin resistance but that HDL3-C showed the opposite results. Furthermore, after 5 years of follow-up, the HDL2-C level was negatively associated with a risk of T2DM incidence, clarifying the difference in prognostic significance of different HDL subclasses for exacerbation of insulin resistance and incidence of T2DM in the general population (35). Further investigation is needed to better understand the pathological mechanisms of HDL dysfunction, especially in T2DM patients with SCAD, in which there is an interaction between abnormal glucose metabolism and dyslipidemia.
Despite providing more information concerning changes of HDL subfractions in T2DM and SCAD, there were several limitations in the present study. First, the data was obtained from a single center, and there was a small sample size, indicating that the present findings should be confirmed by large-scale multicenter study in the future. Second, the short duration of follow-up, which was related to the small number of patients with CVEs, was another limitation. Third, because there were different measurements of lipoprotein subfractions, the present findings need to be confirmed using different methods. Additionally, the present study did not contain a group of T2DM patients without SCAD as a negative control, and there were no data for the plasma insulin and C-peptide levels as well as the mean duration of diabetes to evaluate the severity of T2DM. Finally, we did not assess the impact of lipoprotein subfraction on microvascular complications in T2DM patients with SCAD, suggesting that further study may be needed in the future.
Conclusion
T2DM patients with SCAD have higher levels of mixed HDL subfractions, resulting in a higher risk of CVEs. Thus, an elevated concentration of the mixed HDL subfraction may be a novel prognostic marker for T2DM patients with SCAD.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Fu Wai Hospital and Cardiovascular Institute, Beijing, China. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WZ analyzed the data and wrote the manuscript. R-XX and J-JL designed the study, interpreted the data, and contributed to critically revising the manuscript. J-LJ and H-WZ contributed to analyzing the data. YXZ, QD and JS contributed to data collection and procedure of laboratory examination. Y-LG and K-FD contributed to recruitment of patients. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Peking Union Medical College Youth Fund (3332016018 and 3332018200) and Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016-I2M-1-011).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: Cardiovascular disease in diabetes mellitus: Atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus - mechanisms, management, and clinical considerations. Circulation (2016) 133(24):2459–502. doi: 10.1161/circulationaha.116.022194
2. Taskinen MR, Borén J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis (2015) 239(2):483–95. doi: 10.1016/j.atherosclerosis.2015.01.039
3. Eckel RH, Bornfeldt KE, Goldberg IJ. Cardiovascular disease in diabetes, beyond glucose. Cell Metab (2021) 33(8):1519–45. doi: 10.1016/j.cmet.2021.07.001
4. Femlak M, Gluba-Brzózka A, Ciałkowska-Rysz A, Rysz J. The role and function of hdl in patients with diabetes mellitus and the related cardiovascular risk. Lipids Health Dis (2017) 16(1):207. doi: 10.1186/s12944-017-0594-3
5. Kingwell BA, Chapman MJ, Kontush A, Miller NE. Hdl-targeted therapies: Progress, failures and future. Nat Rev Drug Discovery (2014) 13(6):445–64. doi: 10.1038/nrd4279
6. Superko HR, Pendyala L, Williams PT, Momary KM, King SB 3rd, Garrett BC. High-density lipoprotein subclasses and their relationship to cardiovascular disease. J Clin lipidology (2012) 6(6):496–523. doi: 10.1016/j.jacl.2012.03.001
7. Fanni G, Rosato R, Gentile L, Anselmino M, Frea S, Ponzo V, et al. Is hdl cholesterol protective in patients with type 2 diabetes? a retrospective population-based cohort study. J Trans Med (2020) 18(1):189. doi: 10.1186/s12967-020-02357-1
8. Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes (2003) 52(2):453–62. doi: 10.2337/diabetes.52.2.453
9. Zhao X, Zhang HW, Zhang Y, Li S, Xu RX, Sun J, et al. Analysis of lipoprotein subfractions in 920 patients with and without type 2 diabetes. Heart Lung Circ (2017) 26(3):211–8. doi: 10.1016/j.hlc.2016.10.020
10. Xu RX, Li S, Li XL, Zhang Y, Guo YL, Zhu CG, et al. High-density lipoprotein subfractions in relation with the severity of coronary artery disease: A gensini score assessment. J Clin lipidology (2015) 9(1):26–34. doi: 10.1016/j.jacl.2014.11.003
11. Preis SR, Hwang SJ, Coady S, Pencina MJ, D'Agostino RB Sr., Savage PJ, et al. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the framingham heart study, 1950 to 2005. Circulation (2009) 119(13):1728–35. doi: 10.1161/circulationaha.108.829176
12. Niccoli G, Giubilato S, Di Vito L, Leo A, Cosentino N, Pitocco D, et al. Severity of coronary atherosclerosis in patients with a first acute coronary event: A diabetes paradox. Eur Heart J (2013) 34(10):729–41. doi: 10.1093/eurheartj/ehs393
13. Jin JL, Cao YX, Zhang HW, Sun D, Hua Q, Li YF, et al. Lipoprotein(a) and cardiovascular outcomes in patients with coronary artery disease and prediabetes or diabetes. Diabetes Care (2019) 42(7):1312–8. doi: 10.2337/dc19-0274
14. Hirano T. Pathophysiology of diabetic dyslipidemia. J Atheroscl Thromb (2018) 25(9):771–82. doi: 10.5551/jat.RV17023
15. Cao P, Pan H, Xiao T, Zhou T, Guo J, Su Z. Advances in the study of the antiatherogenic function and novel therapies for hdl. Int J Mol Sci (2015) 16(8):17245–72. doi: 10.3390/ijms160817245
16. Rosenson RS, Brewer HB Jr., Chapman MJ, Fazio S, Hussain MM, Kontush A, et al. Hdl measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem (2011) 57(3):392–410. doi: 10.1373/clinchem.2010.155333
17. Xu RX, Zhang Y, Ye P, Chen H, Li YF, Hua Q, et al. Analysis of lipoprotein subfractions in Chinese han patients with stable coronary artery disease. Heart Lung Circ (2015) 24(12):1203–10. doi: 10.1016/j.hlc.2015.05.002
18. Cardner M, Yalcinkaya M, Goetze S, Luca E, Balaz M, Hunjadi M, et al. Structure-function relationships of hdl in diabetes and coronary heart disease. JCI Insight (2020) 5(1):e131491. doi: 10.1172/jci.insight.131491
19. El Khoudary SR, Chen X, Nasr AN, Billheimer J, Brooks MM, McConnell D, et al. Hdl (High-density lipoprotein) subclasses, lipid content, and function trajectories across the menopause transition: Swan-hdl study. Arterioscler Thromb Vasc Biol (2021) 41(2):951–61. doi: 10.1161/atvbaha.120.315355
20. Syvänne M, Ahola M, Lahdenperä S, Kahri J, Kuusi T, Virtanen KS, et al. High density lipoprotein subfractions in non-Insulin-Dependent diabetes mellitus and coronary artery disease. J Lipid Res (1995) 36(3):573–82. doi: 10.1016/S0022-2275(20)39891-6
21. Winkelmann BR, März W, Boehm BO, Zotz R, Hager J, Hellstern P, et al. Rationale and design of the luric study–a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics (2001) 2(1 Suppl 1):S1–73. doi: 10.1517/14622416.2.1.S1
22. Festa A, Williams K, Hanley AJ, Otvos JD, Goff DC, Wagenknecht LE, et al. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the insulin resistance atherosclerosis study. Circulation (2005) 111(25):3465–72. doi: 10.1161/circulationaha.104.512079
23. Mora S, Otvos JD, Rosenson RS, Pradhan A, Buring JE, Ridker PM. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes (2010) 59(5):1153–60. doi: 10.2337/db09-1114
24. Sokooti S, Flores-Guerrero JL, Kieneker LM, Heerspink HJL, Connelly MA, Bakker SJL, et al. Hdl particle subspecies and their association with incident type 2 diabetes: The prevend study. J Clin Endocrinol Metab (2021) 106(6):1761–72. doi: 10.1210/clinem/dgab075
25. Pownall HJ, Rosales C, Gillard BK, Gotto AM Jr. High-density lipoproteins, reverse cholesterol transport and atherogenesis. Nat Rev Cardiol (2021) 18(10):712–23. doi: 10.1038/s41569-021-00538-z
26. Rohatgi A, Westerterp M, von Eckardstein A, Remaley A, Rye KA. Hdl in the 21st century: A multifunctional roadmap for future hdl research. Circulation (2021) 143(23):2293–309. doi: 10.1161/circulationaha.120.044221
27. Rosenson RS, Brewer HB Jr., Ansell BJ, Barter P, Chapman MJ, Heinecke JW, et al. Dysfunctional hdl and atherosclerotic cardiovascular disease. Nat Rev Cardiol (2016) 13(1):48–60. doi: 10.1038/nrcardio.2015.124
28. Khalil A, Fulop T, Berrougui H. Role of Paraoxonase1 in the regulation of high-density lipoprotein functionality and in cardiovascular protection. Antioxid Redox Signal (2021) 34(3):191–200. doi: 10.1089/ars.2019.7998
29. Baziar N, Nasli-Esfahani E, Djafarian K, Qorbani M, Hedayati M, Mishani MA, et al. The beneficial effects of alpha lipoic acid supplementation on lp-Pla2 mass and its distribution between hdl and apob-containing lipoproteins in type 2 diabetic patients: A randomized, double-blind, placebo-controlled trial. Oxid Med Cell Longev (2020) 2020:5850865. doi: 10.1155/2020/5850865
30. Bonilha I, Zimetti F, Zanotti I, Papotti B, Sposito AC. Dysfunctional high-density lipoproteins in type 2 diabetes mellitus: Molecular mechanisms and therapeutic implications. J Clin Med (2021) 10(11):2233. doi: 10.3390/jcm10112233
31. Ståhlman M, Fagerberg B, Adiels M, Ekroos K, Chapman JM, Kontush A, et al. Dyslipidemia, but not hyperglycemia and insulin resistance, is associated with marked alterations in the hdl lipidome in type 2 diabetic subjects in the diwa cohort: Impact on small hdl particles. Biochim Biophys Acta (2013) 1831(11):1609–17. doi: 10.1016/j.bbalip.2013.07.009
32. Hedrick CC, Thorpe SR, Fu MX, Harper CM, Yoo J, Kim SM, et al. Glycation impairs high-density lipoprotein function. Diabetologia (2000) 43(3):312–20. doi: 10.1007/s001250050049
33. Xepapadaki E, Nikdima I, Sagiadinou EC, Zvintzou E, Kypreos KE. Hdl and type 2 diabetes: The chicken or the egg? Diabetologia (2021) 64(9):1917–26. doi: 10.1007/s00125-021-05509-0
34. Gowri MS, van der Westhuyzen DR, Bridges SR, Anderson JW. Decreased protection by hdl from poorly controlled type 2 diabetic subjects against ldl oxidation may be due to the abnormal composition of hdl. Arterioscler Thromb Vasc Biol (1999) 19(9):2226–33. doi: 10.1161/01.atv.19.9.2226
35. Tabara Y, Arai H, Hirao Y, Takahashi Y, Setoh K, Kawaguchi T, et al. Different inverse association of Large high-density lipoprotein subclasses with exacerbation of insulin resistance and incidence of type 2 diabetes: The nagahama study. Diabetes Res Clin Pract (2017) 127:123–31. doi: 10.1016/j.diabres.2017.03.018
Keywords: cardiovascular events, coronary artery disease, diabetes, high-density lipoprotein subfractions, predicting value
Citation: Zhang W, Jin J-L, Zhang H-W, Zhu Y-X, Dong Q, Sun J, Guo Y-L, Dou K-F, Xu R-X and Li J-J (2023) The value of HDL subfractions in predicting cardiovascular outcomes in untreated, diabetic patients with stable coronary artery disease: An age- and gender-matched case-control study. Front. Endocrinol. 13:1041555. doi: 10.3389/fendo.2022.1041555
Received: 11 September 2022; Accepted: 28 December 2022;
Published: 12 January 2023.
Edited by:
Konstantinos Tziomalos, Aristotle University of Thessaloniki, GreeceCopyright © 2023 Zhang, Jin, Zhang, Zhu, Dong, Sun, Guo, Dou, Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui-Xia Xu, cnVpeGlheHVAc2luYS5jb20=; Jian-Jun Li, bGlqaWFuanVuOTM4QDEyNi5jb20=
 Wei Zhang
Wei Zhang Ke-Fei Dou
Ke-Fei Dou Rui-Xia Xu
Rui-Xia Xu Jian-Jun Li
Jian-Jun Li