- 1Department of Health and Pediatric Sciences, University of Turin, Turin, Italy
- 2Department of Pediatric Endocrinology, Regina Margherita Children’s Hospital, Turin, Italy
- 3Department of Public Health and Pediatrics, University of Turin, Turin, Italy
- 4Department of General Surgery, "Maria Vittoria" Hospital Azienda Sanitaria Locale (ASL) Città di Torino, Turin, Italy
Background: Pediatric thyroid nodules have a lower prevalence but a higher rate of malignancy (ROM) than those in adults. Ultrasound features suspected of malignancy lead to fine needle aspiration biopsy (FNAB) and subsequent cytological determination, upon which management is decided. Based on the characteristics of ultrasound, to standardize clinician decisions and avoid unnecessary FNAB, the European Thyroid Association and the American Radiology College have established guidelines for Thyroid Imaging, Reporting and Data System (EU-TIRADS and ACR-TIRADS) for ROM stratification of thyroid nodules. The aim of this study is to evaluate the diagnostic performance of ACR-TIRADS and EU-TIRADS in pediatric age.
Materials and methods: Subjects younger than 18 years of age with thyroid nodules greater than 0.5 cm observed in the 2000-2020 period were included.
Results: Data from 200 subjects were collected. The overall ROM was 13%, rising to 26% if nodules with a diameter >1 cm were considered. Patients with a malignant nodule were more likely to have a higher EU-TIRADS score (p=0.03). Missed cancer diagnoses were 26.9%. Using the EU-TIRADS system, 40% of FNABs could have been avoided, while this scoring system would have resulted in FNAB being performed in 12% of cases where the assessment of ultrasound features would not recommend FNAB. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were 73.1%, 57.1%, 73.1%, and 50%, respectively. Even considering the ACR-TIRADS, a higher score correlated with a higher ROM (p<0.001). This system missed 6 diagnoses of cancer (23.1%). Using the ACR-TIRADS system, 45.3% of FNABs could have been avoided, while FNAB should have been performed in 12% of cases where it was not recommended by ultrasound characteristics. Sensitivity, specificity, PPV and NPV were 76.9%, 50%, 76.9%, and 42.9%, respectively.
Conclusion: The present study confirms the correspondence of the EU-TIRADS and ACR-TIRADS categories with respect to malignancy but indicates not entirely satisfactory performance compared to FNAB alone. However, the use of the two TIRADS systems should be encouraged in multicentre studies to increase their performance and establish paediatric-specific points in the scoring criteria.
Introduction
Nodular thyroid disease in paediatric age has a lower prevalence (0.2-5.1%) than in adulthood (1-10%) (1–3), but the main difference between paediatric and adult age lies in the rate of malignancy (ROM) (16-26% vs 5-10%) (4–6). The most important risk factors for the development of thyroid cancer include underlying thyroid disease, radiation exposure, previous malignancy, family history, young age, male gender, and genetic predisposition (1–21).
Nodule size >1 cm, hypoechoic pattern, intranodal vascularization or microcalcifications, irregular edges and neck lymph nodes are the main ultrasound features that indicate malignancy (5, 22–29). Once suspicious ultrasound features are present, fine needle aspiration biopsy (FNAB) is required to determine the cytological category, identified based on the most widely used cytological classifications, namely the Bethesda System for Reporting Thyroid Cytopathology (BSRTC), the British Thyroid Association (BTA) and, in Italy, the Guidance of the Italian Society of Anatomic Pathology and Cytology (SIAPEC) (30–32). The cytological category assignment leads to different clinical management that includes clinical-radiological follow-up or surgery, with some differences between these classifications. Despite the differences, all agree on a higher ROM in paediatric age for all categories, especially for indeterminate nodules (6, 33, 34).
Based on the ultrasound features, the European Thyroid Association and the American Radiology College have established guidelines for Thyroid Imaging, Reporting and Data System (EU-TIRADS and ACR-TIRADS respectively) for risk stratification of malignancy of thyroid nodules. Once the TIRADS category is assigned, both guidelines determine whether to perform FNAB or adopt an active surveillance strategy, depending primarily on the size of the nodule (35, 36). The main reasons that led to the definition of these guidelines were the need to standardize the ultrasound description of thyroid nodules as much as possible, provide selection criteria to perform FNAB, avoid unnecessary procedures, and provide clinicians with an additional tool for the management of thyroid nodules, especially in the category of the indeterminate cytology.
Most existing studies evaluating ACR-TIRADS in adulthood have established that the score is useful for managing thyroid nodules and reducing the number of unnecessary FNAB procedures, while there are some concerns about its reliability for the evaluation of nodules with indeterminate cytology (37–51). Among the different TIRADS classification systems, ACR-TIRADS has been indicated as the most accurate classification system for identifying high-risk nodules and preventing most unnecessary FNABs (35, 36, 52–60), although in 10.2- 96 20% of cases a failure to diagnose malignancy has been reported (46, 55, 61).
To improve the diagnostic performance of ACR-TIRADS, some Authors have indicated additional nodules features or PET activity as risk factors (61, 62). Many efforts have been made to evaluate the diagnostic performance of ACR-TIRADS also in paediatric age (63). Its performance has been mostly defined as suboptimal, with a higher rate of cancers missed than in adulthood, up to 25% of cases (63, 64), suggesting that FNAB should be performed in all the 4 and 5 ACR-TIRADS categories (65–71). EU-TIRADS has also been considered a useful tool for physicians managing adults with thyroid nodules, although its performance should be improved and a FNAB should be performed in all nodules assessed as EU-TIRADS ≥4 (50, 72–75). Considering the EU-TIRADS, the rate of missed diagnosis of cancer is higher than that found in ACR-TIRADS, reaching up to 37.7% of cases (55, 72–79).
The purpose of this retrospective study is to evaluate the diagnostic performance of ACR-TIRADS and EU-TIRADS in risk stratification of paediatric thyroid nodules and determine whether extensive use of these tools can help the paediatric endocrinologist better manage thyroid nodules in pediatric age.
Materials and methods
The study included all subjects under the age of 18 with thyroid nodules greater than 0.5 cm followed at the Tertiary Center of Paediatric Endocrinology of the Regina Margherita Children’s Hospital in Turin in the period 2000-2020. Patients with nodules less than 0.5 cm in diameter and with suspicious characteristics were also initially considered. However, none of these were then included in the study as no malignant features were found in any of these nodules. After approval by the Institute’s Ethical Committee, clinical, laboratory and radiographic data were collected from electronic medical records. All patients underwent thyroid ultrasound evaluation, which assessed the diameter of the nodule and the ultrasound pattern; they were therefore classified as anechoic, hypoechoic, isoechoic, hyperechoic, or mixed nodules. All lymph node changes were then recorded, such as rounded swollen shape, irregular margins, increased size, absence of echogenic hilum, heterogeneous echo pattern, presence of calcifications or cystic areas, and irregular vascularization. Patients undergoing multiple ultrasound monitoring were considered as a single case. In patients with multiple nodules, the largest nodule was considered.
All ultrasound evaluations were performed in the same institution and the images were retrospectively evaluated by two independent radiologists blinded for the outcome. The TIRADS category was indicated according to both EU-TIRADS and ACR-TIRADS. Patients with inadequate ultrasound images to correctly assess the TIRADS category were excluded. In case of nodules >1 cm or suspicious features of malignancy on ultrasound evaluation, a cytological sample was obtained by fine needle aspiration biopsy (FNAB) within one month of the ultrasound finding. Histological specimens were also obtained from subjects undergoing lobectomy or total thyroidectomy. All specimens were evaluated by a single pathologist.
Statistical analysis and graphs construction were performed using Graphpad 7 (GraphPad Software, La Jolla, CA, USA). Sensitivity (number of true positives divided by the sum of true positives and false negatives), specificity (number of true negatives divided by the sum of true negatives and false positives), positive predictive value (number of true positive divided by the sum of true positive and false positive), negative predictive value (number of true negative divided by the sum of true negative and false negative) and diagnostic accuracy (sum of true positives and true negatives divided by the samples’ number) were calculated based on the results of patients undergoing both FNAB and surgery. Differences between groups were established by t test to compare mean values of continuous variables. The calculations were considered statistically significant when the P-value was <0.05. Cohen’s kappa coefficient was calculated to measure the inter-rater reliability among the radiologists assigning the TIRADS score.
Results
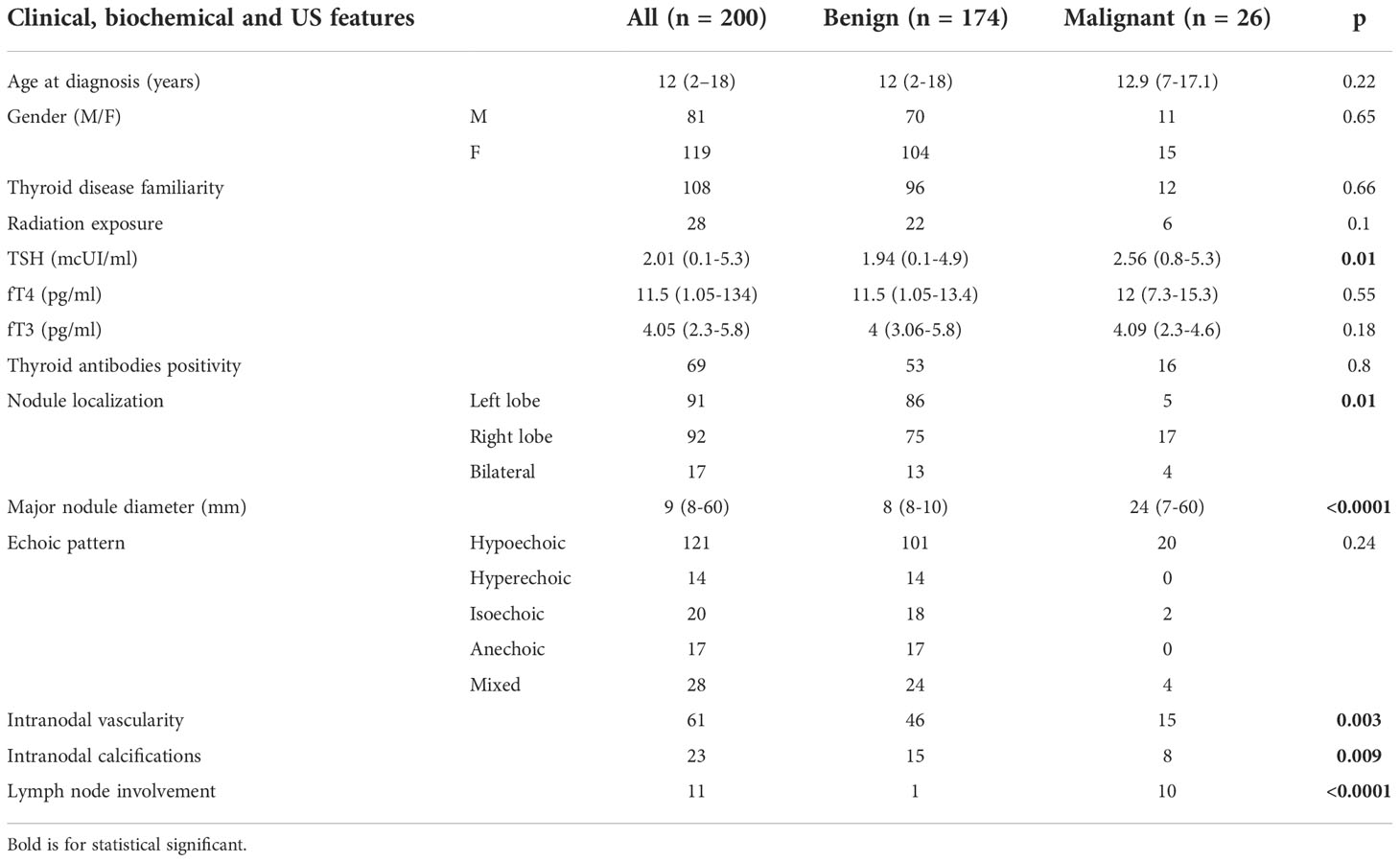
We collected clinical, laboratory and ultrasound retrospective data from 200 subjects (119 females and 81 males) aged less than 18 years with thyroid nodules (Table 1). The observed overall rate of malignancy (ROM) was 13% (26/200 malignant nodules), which rose to 26% if nodules with a diameter >1 cm were considered. The mean age at diagnosis was 11.6 years (range 2-18), with a mean follow-up of 8.6 years. The ratio of female to male was 1.47 and dropped to 1.27 considering the malignant nodules.
Regarding risk factors such as age, gender, family history of thyroid diseases, positive thyroid antibodies and radiation exposure for cancer previously treated with radiotherapy, no difference was observed between benign and malignant nodules.
All subjects had normal levels of fT4 and fT3, but the TSH level was significantly lower in subjects with a benign nodule than in subjects with a malignant nodule (p=0.01).
Bilateral and right lobe involvement was associated with a higher malignancy rate than left lobe localization (23.6% vs 18.5% vs 5.5% malignancy rate, respectively, p=0.01), as also observed for intranodal vascularization and calcification (p=0.003 and p=0.009, respectively), and lymph node involvement (p<0.0001). A larger nodule diameter was significantly more present in the malignant nodule than in the benign nodule group (mean diameter 24 mm vs 8 mm, respectively, p=<0.001). The echogenic pattern was not related to ROM.
FNAB was performed based on nodule size and ultrasound features in 75/200 (37.5%) of subjects, including 7 TIR1 (9.3%), 4 TIR1c (5.3%), 22 TIR2 (29.3%), 14 TIR3a (18.7%), 9 TIR3b (12%), 3 TIR4 (4%) and 16 TIR5 (21.4%).
Surgery was performed in 40/200 (20%), with a total malignancy rate of 65% (0% for the TIR1-TIR3a, 77.8% for the TIR3b and 100 % for the TIR4-TIR5 categories) as shown in Table 2.
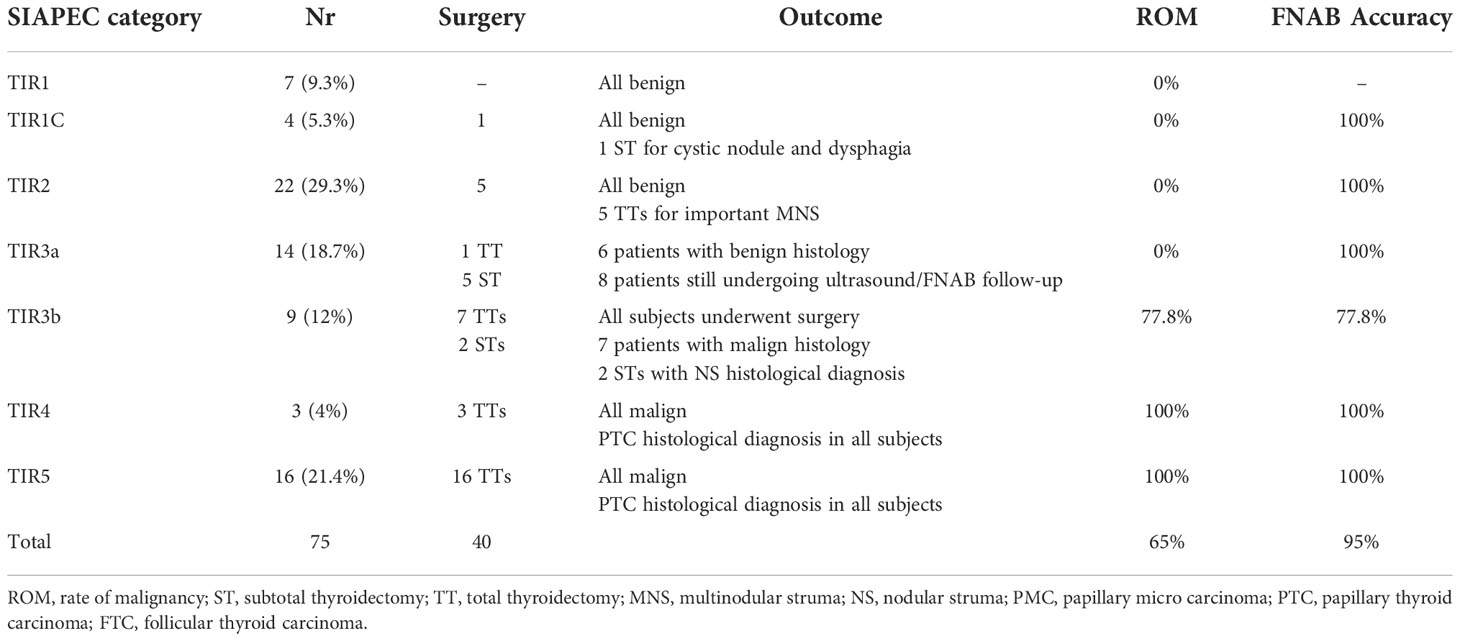
Table 2 Cytological, histological data, malignancy rate and FNAB accuracy for each cytological category.
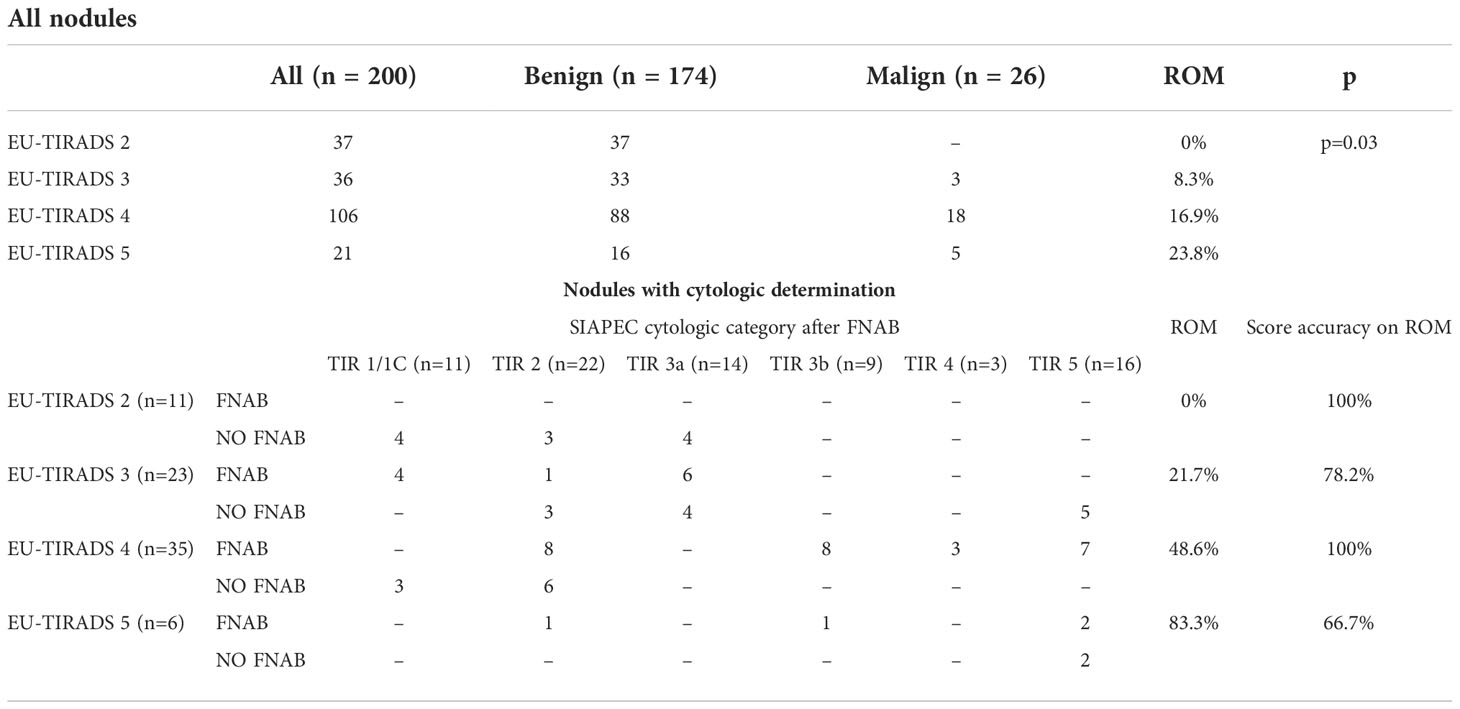
Cohen’s kappa coefficient among the radiologists assigning the TIRADS score was 0.85. The correlation between cytological categories after FNAB and the EU-TIRADS score is represented in Table 3. Patients with a malignant nodule were more likely to have a higher EU-TIRADS score (p=0.03). If all nodules are considered, the most frequently assigned category was EU-TIRADS 4 (53%, ROM 16.9%), followed by EU-TIRADS 2 (18.5%, ROM 0%), EU-TIRADS 3 (18%, ROM 8.3%) and EU-TIRADS 5 (10.5%, ROM 23.8%). Nodules with cytological determination were mainly assigned to EU-TIRADS 4 (46.7%), with ROM up to 48.6%, followed by EU-TIRADS 3 (30.1%, ROM 21.7%), EU-TIRADS 2 (14.7%, ROM 0%) and EU-TIRADS 5 (8%, ROM 83.3%).
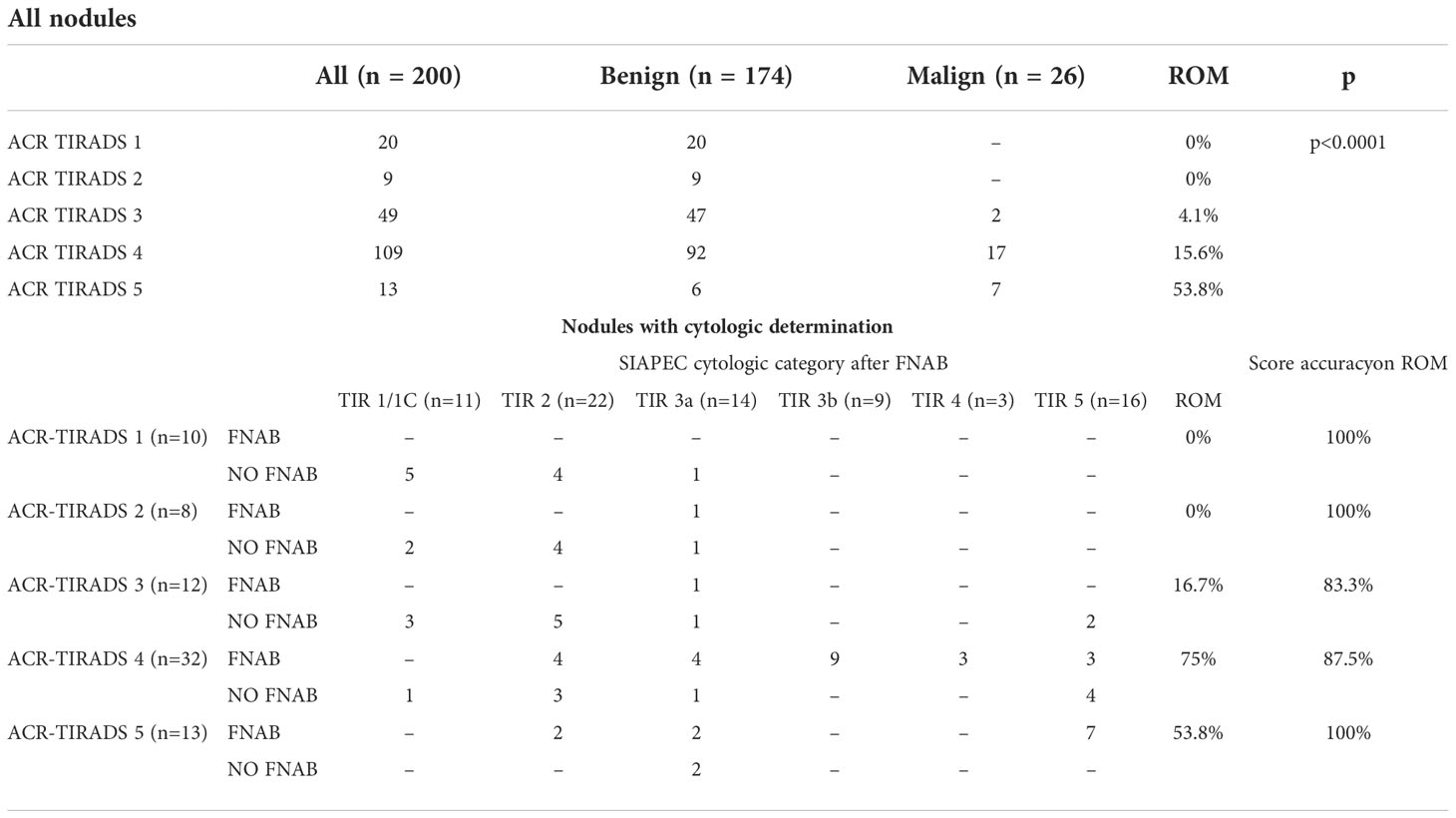
The correlation between the FNAB categories and the ACR-TIRADS system score is represented in Table 4. Higher scores correlated with higher ROMs (p<0.001). Most nodules were classified as ACR-TIRADS 4 (54.5%, ROM 15.6%), followed by ACR-TIRADS 3 (24.5%, ROM 4.1 %), ACR-TIRADS 1 (10%, ROM 0%), ACR-TIRADS 5 (6.5%, ROM 53.8%) and ACR-TIRADS 2 (4.5%, ROM 0%). When only nodules with cytological determination were considered, the category assigned in most cases was ACR-TIRADS 4 (42.7%, ROM 75%), followed by ACR-TIRADS 5 (17.3%, ROM 53.8 %), ACR-TIRADS 3 (16%, ROM 16.7%), ACR-TIRADS 1 (13.3%, ROM 0%) and ACR-TIRADS 2 (10.7%, ROM 0%).
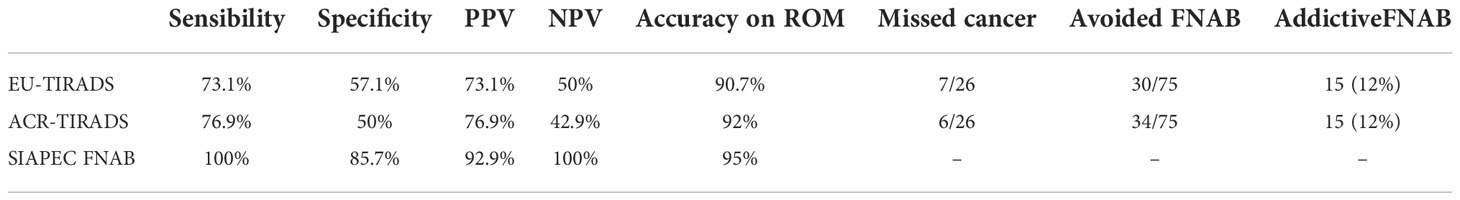
Based on the EU-TIRADS score, missed cancer diagnoses would have occurred in 7 cases (26.9%), with 5 nodules classified in category 3 and 2 nodules in category 5 (Table 5). All nodules in category 3 were < 20 mm and in category 5 < 10 mm. All missed diagnoses were assigned to the TIR5 cytological category. Using the EU-TIRADS system, 40% (30/75) of the FNABs performed could have been avoided, while this scoring system would have led to perform a FNAB in 12% (15/125) of the cases in which the assessment of the ultrasound features would not have recommended FNAB. Sensitivity, specificity, positive predictive value, and negative predictive value based on histological outcome were 73.1%, 57.1%, 73.1%, and 50% respectively.
With the ACR-TIRADS system, cancer diagnosis would have been lost in 6 cases (23.1%), with 2 nodules assigned to ACR-TIRADS category 3 and 4 nodules to ACR-TIRADS category 4. All missed diagnoses were classified cytologically as TIR5. Using the ACR-TIRADS system, 45.3% (34/75) of the FNABs performed could have been avoided; on the other hand, a FNAB was indicated in 12% (15/125) of cases in which it was not recommended by the evaluation of the ultrasound features. Sensitivity, specificity, positive predictive value, and negative predictive value based on histological outcome were 76.9%, 50%, 76.9%, and 42.9%, respectively. The sensitivity and specificity of FNAB based on histological outcome for all categories were 100% and 85.7%, respectively, while PPV and NPV were 92.9% and 100%, respectively. Considering the high ROM of the nodules within the TIR3b category, all nodules classified in TIR3b were considered cytologically malignant. Compared to FNAB, ROM accuracy was lower for both EU-TIRADS and ACR-TIRADS (95% vs 90.7% and 92% respectively).
Discussion
Thyroid nodules in paediatric age have a lower prevalence than in adulthood, but greater ROM (1–7). Considering only nodules >1 cm, the ROM rate of our cohort was 26%, in line with previous published studies. The overall ROM rate was 13%, probably underestimated as most patients did not have suspicious ultrasound features leading to FNAB.
The behaviour of pediatric thyroid cancer is different from that of adults, with higher rates of extrathyroid extension and disease recurrence, but much better prognosis and survival rates; to date their management therefore remains challenging. Giving the invasiveness of FNAB, to avoid unnecessary procedures and anxiety for children and their parents, the best follow-up strategy should include this procedure only when strictly necessary, in presence of certain clinical and ultrasound features. The most important, reported by the current guidelines for adults, is the size of the nodule greater than 1 cm. Other features include intranodal calcification or vascularization, lymph node involvement, marked hypoechoic pattern, bilateral or right lobe localization of the nodule, poorly defined nodule margins and some clinical risk factors, particularly radiation exposure for cancer treatment, increased TSH values, young age and male gender. In our cohort, TSH levels were correlated to malignancy, as previously reported (6, 26). Considering the child’s body size and the presence of microcarcinomas, in presence of multiple risk factors FNAB should be performed even if the nodule size is smaller than 1 cm (1–7).
For both the paediatric and adult populations, numerous efforts have been made to improve the selection criteria that lead clinicians to perform FNAB. To standardize the ultrasound description of thyroid nodules as much as possible and better select candidates for FNAB, the European Thyroid Association and the American Radiology College have established guidelines for Thyroid Imaging, Reports and Data System. Despite several limitations of both scoring system, previous studies in paediatric and adult cohorts have encouraged their use to increase the available data that can improve their performance. EU-TIRADS categories have been observed to be related to thyroid nodules malignancy, although the performance of such system should be improved and therefore a FNAB is currently recommended in all EU-TIRADS ≥4 nodules (50, 72–75), as cancer underdiagnosis rate rises to 37.7% (55, 72–80). The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of EU-TIRADS in adulthood are between 70.6-83.5%, 51.2-94.1%, 11.8-76.1% and 85.4-94.9%, respectively (62, 73–75, 77, 78). The performance of EU-TIRADS in paediatric age has been evaluated in a few studies that showed lower efficacy than in adults, with sensitivity, specificity, PPV and NPV ranging between 41.7-100%, 25-75.9%, 41.7-44%, 75.9-100% respectively (63, 78, 79). The data from our study confirm the significant correlation of the EU-TIRADS category with malignancy. Sensitivity, specificity, PPV and NPV were 73.1%, 57.1%, 73.1% and 50 %, respectively, showing an underestimation of malignant lesions and a low ability to detect histologically determined benign nodules, which do not require FNAB. Lost cancer diagnoses in our cohort were 26.9%, while 40% of FNABs could have been avoided and 12% of patients who were not selected for needle-biopsy should have undergone FNAB.
Most existing studies evaluating ACR-TIRADS in adulthood have determined that the system score is useful for managing thyroid nodules and reducing the number of unnecessary FNAB, while some concerns remain about its reliability in evaluating nodules of indeterminate cytology (37–51). Among the different TIRADS, ACR-TIRADS was ranked as the best performing classification for identifying high-risk nodules and unnecessary FNABs (35, 36, 52–60), although a missed malignancy diagnosis occurred in 10.2-20% of cases in which ACR-TIRADS have not indicated the need for FNAB (46, 55, 61). The combined sensitivity and specificity of ACR-TIRADS in adults were 89% and 70%, respectively (61). Many efforts have been made to evaluate the diagnostic performance of ACR-TIRADS also in paediatric age (63), mostly defined as suboptimal, with a rate of up to 25% of undiagnosed cancers, higher than that of adulthood (63, 64), which suggests performing FNAB in all ACR-TIRADS categories 4 and 5 (65–71). Sensitivity, specificity, PPV and NPV in the paediatric age group vary between 70-75%, 64-92.3%, 21.8-83.3% and 64-97.2%, respectively (65, 67–71). In our study, the performance of ACR-TIRADS was similar to that indicated by the literature for sensitivity, but the specificity was lower, and the cancer underdiagnosis rate higher (23.1%). Using ACR-TIRADS, 45.3% of FNAB could have been avoided, while 12% of unselected patients would have had to undergo FNAB.
Considering the two scoring systems, ACR-TIRADS performed better than EU-TIRADS as also observed in previous studies (52–60). The number of potentially avoidable FNABs was substantial, although malignant nodules were underestimated and the performance of both scores to avoid FNAB in definitive benign nodules was not satisfactory. The interpretation of FNAB results according to the SIAPEC classification has a significantly greater risk stratification capacity, with a sensitivity of 100%. This is mainly due to the interpretation of the indeterminate category TIR3b as cytologically malignant, with consistently high ROM (77.8%), while the ROM observed in the indeterminate category TIR3a was 0%. This result differs from the BSRTC system which assigns similar ROMs in the indeterminate grouped categories Bethesda III and IV.
Despite the limitations of TIRADS scores, we confirm that their use should be encouraged to improve their performance and have an additional tool in the management of paediatric thyroid nodules. The main limitation of TIRADS in children is the criterion of the size as a determinant for the execution of FNAB. We must be aware that the current guidelines have been established for adults and are not at all suitable for children, especially considering their body size. The EU-TIRADS score does not include lymph node involvement in the score but indicates the need for FNAB in case of suspicious ultrasound features; intranodal vascularization, described as a risk factor for malignancy, is also not included in the EU or in the ACR-TIRADS. Bilateral and right lobe localization should also be considered in the final score. To improve the diagnostic performance of ACR-TIRADS, some authors have indicated additional characteristics as risk profiles or PET activity (61, 62). The association of ultrasound data with clinical data could be an additional aid to performance improvement. The final score could also include an age <10 years, male gender, previous radiation exposure for cancer treatment, a higher TSH level, as well as a familial history or genetic predisposition to thyroid cancer (1–5).
The present study has several limitations. The retrospective nature of the study limits the statistical power of the data analysis. The number of histologically and cytologically determined malignant nodules is limited due to the low prevalence of pediatric thyroid nodules and restrictive criteria for FNAB, which can lead to underestimation, despite the case series being recruited in a tertiary centre of Paediatric Endocrinology over a 20-years period.
In conclusion, in the present study the correlation of the EU-TIRADS and ACR-TIRADS categories with malignancy was confirmed, even if their performance was not entirely satisfactory compared to FNAB alone. However, their use should be encouraged within multicentre studies, to increase the performance of both TIRADS systems and to allow for an update of the scoring criteria, including pediatric-specific points.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the City of Health and Science University Hospital of Turin. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
GT and JM contributed to the study concept, the statistical analysis and to the first draft of manuscript. MS contributed to the data collection and literature research. FQ and LS contributed to the study concept and the revision con the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bauer AJ. Thyroid nodules in children and adolescents. Curr Opin Endocrinol Diabetes Obes (2019) 26:266–74. doi: 10.1097/MED.0000000000000495
2. Bauer AJ. Pediatric thyroid cancer: genetics, therapy and outcome. Endocrinol Metab Clin North Am (2020) 49:589–611. doi: 10.1016/j.ecl.2020.08.001
3. Niedziela M. Pathogenesis, diagnosis and management of thyroid nodules in children. Endocr Relat Cancer (2006) 13:427–53. doi: 10.1677/erc.1.00882
4. Corrias A, Mussa A, Baronio F, Arrigo T, Salerno M, Segni M, et al. Diagnostic features of thyroid nodules in pediatrics. Arch Pediatr Adolesc Med (2010) 164:714–9. doi: 10.1001/archpediatrics.2010.114
5. Niedziela M. Thyroid nodules. Best Pract Res Clin Endocrinol Metab (2014) 28:245–77. doi: 10.1016/j.beem.2013.08.007
6. Tuli G, Munarin J, Agosto E, Matarazzo P, Quaglino F, Mormile A, et al. Predictive factors of malignancy in pediatric patients with thyroid nodules and performance of the Italian classification (SIAPEC 2014) in the outcome of the cytological FNA categories. Endocrine (2021) 74:365–74. doi: 10.1007/s12020-021-02784-0
7. Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric thyroid carcinoma: Incidence and outcomes in 1753 patients. J Surg Res (2009) 156:167–72. doi: 10.1016/j.jss.2009.03.098
8. Parisi MT, Mankoff D. Differentiated pediatric thyroid cancer: Correlates with adult disease, controversies in treatment. Semin Nucl Med (2007) 37:340–56. doi: 10.1053/j.semnuclmed.2007.05.001
9. Pritchard-Jones K, Kaatschb P, Steliarova-Foucher E, Stiller CA, Coebergh JW. Cancer in children and adolescents in Europe: Developments over 20 years and future challenges. Eur J Cancer (2006) 42:2183–90. doi: 10.1016/j.ejca.2006.06.006
10. Wells A, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF. American Thyroid association guidelines task force on medullary thyroid carcinoma . revised American thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid (2015) 25:567–610. doi: 10.1089/thy.2014.0335
11. Vuong HG, Kondo T, Oishi N, Nakazawa T, Mochizuki K, Miyauchi A, et al. Paediatric follicular thyroid carcinoma - indolent cancer with low prevalence of RAS mutations and absence of PAX8-PPARG fusion in a Japanese population. Histopathology (2017) 71:760–8. doi: 10.1111/his.13285
12. Corrias A, Cassio A, Weber G, Mussa A, Wasniewska M, Rapa A, et al. Thyroid nodules and cancer in children and adolescents affected by autoimmune thyroiditis. Arch Pediatr Adolesc Med (2008) 162:526–31. doi: 10.1001/archpedi.162.6.526
13. Alvarez AL, Mulder M, Handelsman RS, Lew JI, Farra JC. High rates of underlying thyroid cancer in patients undergoing thyroidectomy for hyperthyroidism. J Surg Res (2020) 245:523–8. doi: 10.1016/j.jss.2019.07.048
14. Waguespack SG. Thyroid sequelae of pediatric cancer. Horm Res Paediatr (2019) 91:104–17. doi: 10.1159/000495040
15. Sigurdson AJ, Ronckers SM, Mertens AC, Stovall M, Smith SA, Liu Y, et al. Primary thyroid cancer after a first tumour in childhood (the childhood cancer survivor study): A nested case-control study. Lancet (2005) 365:2014–23. doi: 10.1016/S0140-6736(05)66695-0
16. Pekova B, Dvorakova S, Sykorova V, Vacinova G, Vaclavikova E, Moravcova J, et al. Somatic genetic alterations in a large cohort of pediatric thyroid nodules. Endocr Connect (2019) 8:796–805. doi: 10.1530/EC-19-0069
17. Mostoufi-Moab S, Labourier E, Sullivan L, LiVolsi V, Li Y, Xiao R, et al. Molecular testing for oncogenic gene alterations in pediatric thyroid lesions. Thyroid (2018) 28:60–7. doi: 10.1089/thy.2017.0059
18. Zdravkovic V. Update on the management of pediatric thyroid cancer. Adv Pediatrics Adol Med (2018) 1:1–5.
19. Wells SA Jr., Santoro M. Targeting the RET pathway in thyroid cancer. Clin Cancer Res (2009) 15:7119–23. doi: 10.1158/1078-0432.CCR-08-2742
20. Waguespack SG, Rich TA, Perrier ND, Jimenez C, Cote GJ. Management of medullary thyroid carcinoma and MEN2 syndromes in childhood. Nat Rev Endocrinol (2011) 7:596–607. doi: 10.1038/nrendo.2011.139
21. Corrias A, Mussa A. Thyroid nodules in pediatrics: Which ones can be left alone, which ones must be investigated, when and how. J Clin Res Pediatr Endocrinol (2013) 5:57–69. doi: 10.4274/jcrpe.853
22. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer, the American thyroid association guidelines task force on pediatric thyroid cancer. Thyroid (2015) 25:716–59. doi: 10.1089/thy.2014.0460
23. Moon WJ, Jung S, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation–multicenter retrospective study. Radiology (2008) 247:762–70. doi: 10.1148/radiol.2473070944
24. Saavedra J, Deladoëy J, Saint-Vil D, Boivin Y, Alos N, Deal C, et al. Is ultrasonography useful in predicting thyroid cancer in children with thyroid nodules and apparently benign cytopathologic features? Horm Res Paediatr (2011) 75:269–75. doi: 10.1159/000322877
25. Roy R, Kouniavsky G, Schneider E, Allendorf JD, Chabot JA, Logerfo P, et al. Predictive factors of malignancy in pediatric thyroid nodules. Surgery (2011) 150:1228–33. doi: 10.1016/j.surg.2011.09.023
26. Mussa A, De Andrea M, Motta M, Mormile A, Palestini N, Corrias A. Predictors of malignancy in children with thyroid nodules. J Pediatr (2015) 167:886–92. doi: 10.1016/j.jpeds.2015.06.026
27. Singh Ospina N, Sebo TJ, Morris JC, Castro MR. The value of repeat thyroid fine-needle aspiration biopsy in patients with a previously benign result: How often does it alter management? Thyroid (2015) 25:1121–6. doi: 10.1089/thy.2015.0146
28. Hess J, Schafernak K, Newbern D, Vern-Gross T, Foote J, Van Tassel D, et al. Ultrasound is superior to palpation for thyroid cancer detection in high-risk childhood cancer and BMT survivors. Support Care Cancer (2020) 28:5117–24. doi: 10.1007/s00520-020-05340-0
29. Corrias A A, Einaudi S S, Chiorboli E, Weber G, Crinò A, Andreo M, et al. Accuracy of fine needle aspiration biopsy of thyroid nodules in detecting malignancy in childhood: Comparison with conventional clinical, laboratory, and imaging approaches. J Clin Endocrinol Metab (2001) 86:4644–8. doi: 10.1210/jcem.86.10.7950
30. Nardi F, Basolo F, Crescenzi A, Fadda G, Frasoldati A, Orlandi F, et al. Italian Consensus for the classification and reporting of thyroid cytology. J Endocrinol Invest (2014) 37:593–9. doi: 10.1007/s40618-014-0062-0
31. Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid (2017) 27:1341–6. doi: 10.1089/thy.2017.0500
32. Cross C, Chandra A, Giles T, Johnson S, Kocjan G, Poller D, et al. Guidance on the reporting of thyroid cytology specimens (2016). Available at: http://ukeps.com/docs/thyroidfna.pdf.
33. Trimboli P, Crescenzi A, Castellana M, Giorgino F, Giovanella L, Bongiovanni M. Italian Consensus for the classification and reporting of thyroid cytology: The risk of malignancy between indeterminate lesions at low or high risk. a systematic review and meta-analysis. Endocrine (2019) 63:430–8. doi: 10.1007/s12020-018-1825-8
34. Vuong HGG, Chung DGB, Ngo LM, Bui TQ, Hassell L, Jung CK, et al. The use of the Bethesda system for reporting thyroid cytopathology in pediatric thyroid nodules - a meta-analysis. Thyroid (2021) (8):1203–11. doi: 10.1089/thy.2020.0702
35. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS committee. J Am Coll Radiol (2017) 14:587–95. doi: 10.1016/j.jacr.2017.01.046
36. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: The EU-TIRADS. Eur Thyroid J (2017) 6:225–37. doi: 10.1159/000478927
37. Barbosa TLM, Junior COM, Graf H, Cavalvanti T, Trippia MA, da Silveira Ugino RT, et al. ACR TI-RADS and ATA US scores are helpful for the management of thyroid nodules with indeterminate cytology. BMC Endocr Disord (2019) 19:112. doi: 10.1186/s12902-019-0429-5
38. Chaigneau E, Russ G, Royer B, Bigorgne C, Bienvenu-Perrard M, Rouxel A, et al. TIRADS score is of limited clinical value for risk stratification of indeterminate cytological results. Eur J Endocrinol (2018) 179:13–20. doi: 10.1530/EJE-18-0078
39. Shayganfar A, Hashemi P, Esfahani MM, Ghanei AM, Moghadam NA, Ebrahimian S. Prediction of thyroid nodule malignancy using thyroid imaging reporting and data system (TIRADS) and nodule size. Clin Imaging (2020) 60:222–7. doi: 10.1016/j.clinimag.2019.10.004
40. Mauri G, Gitto S, Cantisani V, Vallone G, Schiavone C, Papini, et al. Use of the thyroid imaging reporting and data system (TIRADS) in clinical practice: an Italian survey. Endocrine (2020) 68:329–35. doi: 10.1007/s12020-020-02199-3
41. Modi L, Sun W, Shafizadeh N, Negron R, Yee-Chang M, Zhou F, et al. Does a higher American college of radiology thyroid imaging reporting and data system (ACR TI-RADS) score forecast an increased risk of malignancy? a correlation study of ACR TI-RADS with FNA cytology in the evaluation of thyroid nodules. Cancer Cytopathol (2020) 128:470–81. doi: 10.1002/cncy.22254
42. Leni D, Seminati D, Fior D, Vacirca F, Capitoli G, Cazzaniga L, et al. Diagnostic performances of the ACR-TIRADS system in thyroid nodules triage: A prospective single center study. Cancers (Basel) (2021) 13:2230. doi: 10.3390/cancers13092230
43. Håskjold OI, Foshaug HS, Iversen TB, Kjøren HC, Brun VH. Prediction of thyroid nodule histopathology by expert ultrasound evaluation. Endocr Connect (2021) 10:776–81. doi: 10.1530/EC-21-0192
44. Malhi HS, Grant EG. Ultrasound of thyroid nodules and the thyroid imaging reporting and data system. Neuroimaging Clin N Am (2021) 31:285–300. doi: 10.1016/j.nic.2021.04.001
45. Solymosi T, Hegedüs L, Bonnema SJ, Frasoldati A, Jambor L, Kovacs GL, et al. Ultrasound-based indications for thyroid fine-needle aspiration: Outcome of a TIRADS-based approach versus operators' expertise. Eur Thyroid J (2021) 10:416–24. doi: 10.1159/000511183
46. Araruna Bezerra de Melo R, Menis F, Calsavara VF, Stefanini FS, Novaes T, Saieg M. The impact of the use of the ACR-TIRADS as a screening tool for thyroid nodules in a cancer center. Diagn Cytopathol (2022) 50:18–23. doi: 10.1002/dc.24904
47. Aksoy SH, Uygun O, Yurdaisik I, Ates L, Aydin S. The relationship between ultrasound-based TIRADS and BETHESDA categories in patients undergoing thyroid biopsy. Clin Exp Med (2022) (4):661–6. doi: 10.1007/s10238-021-00779-9
48. Rago T, Vitti P. Risk stratification of thyroid nodules: From ultrasound features to TIRADS. Cancers (Basel) (2022) 14:717. doi: 10.3390/cancers14030717
49. Li W, Sun Y, Xu H, Shang W, Dong A. Systematic review and meta-analysis of American college of radiology TI-RADS inter-reader reliability for risk stratification of thyroid nodules. Front Oncol (2022) 49. doi: 10.3389/fonc.2022.840516
50. Eidt LB, Nunes de Oliveira C, Lagos YBB, Solera GLM, Izquierdo R, Meyer ELS, et al. A prospective comparison of ACR-TIRADS and EU-TIRADS in thyroid nodule assessment for FNA-US. Clin Endocrinol (Oxf) (2022). doi: 10.1111/cen.14799
51. Zhong M, Zhang Z, Xiao Y, He Y, Chen Y, Huang W, et al. The predictive value of ACR TI-RADS classification for central lymph node metastasis of papillary thyroid carcinoma: A retrospective study. Int J Endocrinol (2022). doi: 10.1155/2022/4412725
52. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Korean Society of thyroid radiology (KSThR) and Korean society of radiology. ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol (2016) 17:370–95. doi: 10.3348/kjr.2016.17.3.370
53. Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo B, et al. Chinese Guidelines for ultrasound malignancy risk stratification of thyroid nodules: the c-TIRADS. Endocrine (2020) 70:256–79. doi: 10.1007/s12020-020-02441-y
54. Lauria Pantano A, Maddaloni E, Briganti SI, Beretta Anguissola G, Perrella E, Taffon C, et al. Differences between ATA, AACE/ACE/AME and ACR TI-RADS ultrasound classifications performance in identifying cytological high-risk thyroid nodules. Eur J Endocrinol (2018) 178:595–603. doi: 10.1530/EJE-18-0083
55. Grani G, Lamartina L, Ascoli V, Bosco D, Biffoni M, Giacomelli L, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: Toward the "Right" TIRADS. J Clin Endocrinol Metab (2019) 104:95–102. doi: 10.1210/jc.2018-01674
56. Castellana M, Castellana C, Treglia G, Giorgino F, Giovanella L, Russ G, et al. Performance of five ultrasound risk stratification systems in selecting thyroid nodules for FNA. J Clin Endocrinol Metab (2020) 105:dgz170. doi: 10.1210/clinem/dgz170
57. Pandya A, Caoili EM, Jawad-Makki F, Wasnik AP, Shankar PR, Bude R, et al. Retrospective cohort study of 1947 thyroid nodules: A comparison of the 2017 American college of radiology TI-RADS and the 2015 American thyroid association classifications. AJR Am J Roentgenol (2020) 214:900–6. doi: 10.2214/AJR.19.21904
58. Li W, Wang Y, Wen J, Zhang L, Sun Y. Diagnostic performance of American college of radiology TI-RADS: A systematic review and meta-analysis. AJR Am J Roentgenol (2021) 216:38–47. doi: 10.2214/AJR.19.22691
59. Kim PH, Suh CH, Baek JH, Chung SR, Choi YJ, Lee JH. Unnecessary thyroid nodule biopsy rates under four ultrasound risk stratification systems: a systematic review and meta-analysis. Eur Radiol (2021) 31:2877–85. doi: 10.1007/s00330-020-07384-6
60. Hoang JK, Middleton WD, Langer JE, Schmidt K, Gillis LB, Nair SS, et al. Comparison of thyroid risk categorization systems and fine-needle aspiration recommendations in a multi-institutional thyroid ultrasound registry. J Am Coll Radiol (2021) 18:1605–13. doi: 10.1016/j.jacr.2021.07.019
61. Tappouni RR, Itri JN, McQueen TS, Lalwani N, Ou JJ. ACR TI-RADS: Pitfalls, solutions, and future directions. Radiographics (2019) 39:2040–52. doi: 10.1148/rg.2019190026
62. Russ G, Trimboli P, Buffet C. The new era of TIRADSs to stratify the risk of malignancy of thyroid nodules: Strengths, weaknesses and pitfalls. Cancers (Basel) (2021) 13:4316. doi: 10.3390/cancers13174316
63. Scappaticcio L, Maiorino MI, Iorio S, Docimo G, Longo M, Grandone A, et al. Exploring the performance of ultrasound risk stratification systems in thyroid nodules of pediatric patients. Cancers (Basel) (2021) 13:5304. doi: 10.3390/cancers13215304
64. Kim PH, Yoon HM, Hwang J, Lee JS, Jung AY, Cho YA, et al. Diagnostic performance of adult-based ATA and ACR-TIRADS ultrasound risk stratification systems in pediatric thyroid nodules: a systematic review and meta-analysis. Eur Radiol (2021) 31:7450–63. doi: 10.1007/s00330-021-07908-8
65. Uner C, Aydin S, Ucan B. Thyroid image reporting and data system categorization: Effectiveness in pediatric thyroid nodule assessment. Ultrasound Q (2020) 36:15–9. doi: 10.1097/RUQ.0000000000000476
66. Lim-Dunham JE, Toslak IE, Reiter MP, Martin B. Assessment of the American college of radiology thyroid imaging reporting and data system for thyroid nodule malignancy risk stratification in a pediatric population. AJR Am J Roentgenol (2019) 212:188–94. doi: 10.2214/AJR.18.20099
67. Richman DM, Cherella CE, Smith JR, Modi BP, Zendejas B, Frates MC, et al. Clinical utility of sonographic features in indeterminate pediatric thyroid nodules. Eur J Endocrinol (2021) 184:657–65. doi: 10.1530/EJE-20-1480
68. Martinez-Rios C, Daneman A, Bajno L, van der Kaay DCM, Moineddin R, Wasserman JD. Utility of adult-based ultrasound malignancy risk stratifications in pediatric thyroid nodules. Pediatr Radiol (2018) 48:74–84. doi: 10.1007/s00247-017-3974-y
69. Arora S, Khoury J, Trout AT, Chuang J. Improving malignancy prediction in AUS/FLUS pediatric thyroid nodules with the aid of ultrasound. Horm Res Paediatr (2020) 93:239–44. doi: 10.1159/000509118
70. Ahmad H, Al-Hadidi A, Bobbey A, Shah S, Stanek J, Nicol K, et al. Pediatric adaptions are needed to improve the diagnostic accuracy of thyroid ultrasound using TI-RADS. J Pediatr Surg (2021) 56:1120–5. doi: 10.1016/j.jpedsurg.2021.02.034
71. Piccardo A, Fiz F, Bottoni G, De Luca C, Massollo M, Catrambone U, et al. Facing thyroid nodules in paediatric patients previously treated with radiotherapy for non-thyroidal cancers: Are adult ultrasound risk stratification systems reliable? Cancers (Basel) (2021) 213:4692. doi: 10.3390/cancers13184692
72. Hekimsoy İ, Öztürk E, Ertan Y, Orman MN, Kavukçu G, Özgen AG, et al. Diagnostic performance rates of the ACR-TIRADS and EU-TIRADS based on histopathological evidence. Diagn Interv Radiol (2021) 27:511–8. doi: 10.5152/dir.2021.20813
73. Skowrońska A, Milczarek-Banach J, Wiechno W, Chudziński W, Żach M, Mazurkiewicz M, et al. Accuracy of the European thyroid imaging reporting and data system (EU-TIRADS) in the valuation of thyroid nodule malignancy in reference to the post-surgery histological results. Pol J Radiol (2018) 83:e579–86. doi: 10.5114/pjr.2018.81556
74. Castellana M, Grani G, Radzina M, Guerra V, Giovanella L, Deandrea M, et al. Performance of EU-TIRADS in malignancy risk stratification of thyroid nodules: a meta-analysis. Eur J Endocrinol (2020) 183:255–64. doi: 10.1530/EJE-20-0204
75. Kovatcheva RD, Shinkov AD, Dimitrova ID, Ivanova RB, Vidinov KN, Ivanova RS. Evaluation of the diagnostic performance of EU-TIRADS in discriminating benign from malignant thyroid nodules: A prospective study in one referral center. Eur Thyroid J (2021) 9:304–12. doi: 10.1159/000507575
76. Magri F, Chytiris S, Croce L, Molteni M, Bendotti G, Gruosso G, et al. Performance of the ACR TI-RADS and EU TI-RADS scoring systems in the diagnostic work-up of thyroid nodules in a real-life series using histology as reference standard. Eur J Endocrinol (2020) 183:521–8. doi: 10.1530/EJE-20-0682
77. Seminati D, Capitoli G, Leni D, Fior D, Vacirca F, Di Bella C, et al. Use of diagnostic criteria from ACR and EU-TIRADS systems to improve the performance of cytology in thyroid nodule triage. Cancers (Basel) (2021) 13:5439. doi: 10.3390/cancers13215439
78. Dobruch-Sobczak K, Adamczewski Z, Dedecjus M, Lewiński A, Migda B, Ruchała M, et al. Summary of meta-analyses of studies involving TIRADS classifications (EU-TIRADS, ACR-TIRADS, and K-TIRADS) in evaluating the malignant potential of focal lesions of the thyroid gland. J Ultrason (2022) 22:121–9. doi: 10.15557/JoU.2022.0020
79. Yeste Fernández D, Vega Amenabar E, Coma Muñoz A, Arciniegas Vallejo L, Clemente León M, Planes-Conangla M, et al. Ultrasound criteria (EU-TIRADS) to identify thyroid nodule malignancy risk in adolescents. correlation with cyto-histological findings. Endocrinol Diabetes Nutr (Engl Ed) (2021) 68:728–34. doi: 10.1016/j.endien.2020.11.006
Keywords: thyroid nodule, pediatric age, ACR-TIRADS, EU-TIRADS, diagnostic performance, thyroid nodules outcome
Citation: Tuli G, Munarin J, Scollo M, Quaglino F and De Sanctis L (2022) Evaluation of the efficacy of EU-TIRADS and ACR-TIRADS in risk stratification of pediatric patients with thyroid nodules. Front. Endocrinol. 13:1041464. doi: 10.3389/fendo.2022.1041464
Received: 10 September 2022; Accepted: 07 November 2022;
Published: 22 November 2022.
Edited by:
Hanneke M. Van Santen, Wilhelmina Children's Hospital, NetherlandsReviewed by:
Barbara Maria Jarzab, Maria Skłodowska-Curie National Research Institute of Oncology, PolandChantal Lebbink, Wilhelmina Children's Hospital, Netherlands
Copyright © 2022 Tuli, Munarin, Scollo, Quaglino and De Sanctis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerdi Tuli, Z2VyZGkudHVsaUB1bml0by5pdA==
 Gerdi Tuli
Gerdi Tuli Jessica Munarin2,3
Jessica Munarin2,3 Luisa De Sanctis
Luisa De Sanctis