- 1Division of Endocrinology, Diabetes and Metabolism, University Hospital of Lausanne and University of Lausanne, Lausanne, Switzerland
- 2Endocrinology, Diabetes and Nutrition, Médical Center EndoDia-Centre, Biel-Bienne, Switzerland
- 3Division of Endocrinology, Metabolism and Molecular Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
Background: Iodine is essential for the biosynthesis of thyroid hormones, which are crucial for intrauterine growth and fetal neurocognitive development. Iodine requirements increase during pregnancy and lactation. The World Health Organization and the Swiss Confederation recommend a total daily iodine intake of 250 µg of iodine during preconception, pregnancy and lactation. To assure this goal, several professional organizations recommend complementing the nutritional iodine intake with supplements containing 150 μg of iodine daily.
Methods: Prenatal and adult multivitamins widely available in Switzerland were compiled to determine their iodine content. Obstetricians verified that the list includes the most frequently prescribed supplements in Switzerland.
Results: A total of 44 adult multivitamin supplements were identified, 23 of which are specifically intended for women planning pregnancy, pregnant, or breastfeeding. Seven out of 23 (30.4%) prenatal multivitamins products, and 12/21 (57.1%) adult multivitamins contained no iodine. Among all the products, only 18/44 (40.9%) contain 150 µg of iodine or more.
Conclusion: Several widely used products contain no or insufficient amounts (<150 ug) of iodine. Providers need to be informed about the variability in iodine content of supplements and established recommendations, and manufacturers of prenatal supplements should assure that their products contain iodine in adequate amounts.
Introduction
Iodine is an essential component for the production of the thyroid hormones thyroxine (T4) and triiodothyronine (T3). Normal levels of thyroid hormones are crucial for intrauterine growth and neurocognitive development of the fetus, and they are dependent on sufficient maternal dietary iodine intake. The consequences of iodine deficiency (ID) during fetal development and infancy are well established and can range from subtle changes in cognitive and/or neurologic function in the offspring to cretinism (1). Importantly, even mild and moderate degrees of ID may have adverse effects on the neurodevelopment of children (2), and ID is still considered the leading cause of preventable mental impairment worldwide (3). ID is easy to prevent, especially in vulnerable populations such as pregnant and lactating women, through the use of multivitamin supplements that contain sufficient amounts of potassium iodide or iodate. However, the content of multivitamin products is not regulated and among marketed products there may be differences in the iodine content declared on the product label and the measured concentration (4).
In 1922, Switzerland was the first country to fortify household salt with iodine to control endemic goiter and cretinism. In 1952, iodized salt was available across the whole country and, in response to decreasing salt consumption, the iodine amount in household salt was progressively increased from 3.75 mg/kg in 1952 to 25 mg/kg in 2014, resulting in the elimination of ID disorders in Switzerland (5). Swiss policy mandates iodization of salt on a voluntary basis, i.e. both iodized salt and non-iodized salt must be available. Salt samples from households were collected in the framework of multiple national studies between 1999 and 2015 (6–9). The results formally demonstrated that more than 80% of households use iodized salt. However, data on the current use of iodized and non-iodized salt by the Swiss food industry and canteens are limited; they suggest that coverage with iodized salt is incomplete and that many foods are prepared with non-iodized salt (data of Les Salines Suisses SA, the leading salt producer and supplier in the country (10)).
The urinary iodine concentration (UIC) is an excellent biomarker of the dietary iodine intake at the population level. The World Health Organization (WHO) defines sufficient iodine intake in a population as a median UIC ≥100 µg/l in adults and children, and ≥150 µg/l in pregnant and lactating women (3). Periodic national UIC surveys performed in Switzerland in 1999 and 2009 have shown borderline deficient iodine intake in pregnant women (8, 11), as well as in lactating women (11). In a cross-sectional national study performed in 2015-2016, the median UIC in women of reproductive age (n= 353) was 88 μg/L (bootstrapped 95% confidence interval (CI) 72, 103 μg/L), and in pregnant women (n = 363) it was 140 μg/L (bootstrapped 95% CI 124, 159 μg/L) (7). Among women of reproductive age, 92% reported using iodized salt, but only 1.4% used iodine-containing supplements. Among pregnant women, 37% used iodine-containing supplements, 62% did not, and 1.4% did not know. In pregnant women, the median thyroglobulin level (23.8 µg/l), which can be used as a biomarker for iodine status at the population level, was above the concentrations usually found in iodine-sufficient populations; it was elevated in 13% of the pregnant women, thus suggestive of increased thyroid activity. In aggregate, these findings suggest that the iodine intake is only borderline sufficient in pregnant and non-pregnant women in Switzerland (7).
In adults and adolescents, the recommended daily allowance of iodine is 150 µg (3). The Swiss Confederation and the World Health Organization recommend 250 µg of iodine during preconception, pregnancy and lactation (3, 12), the United States National Academy of Medicine (NAM) (formerly the Institute of Medicine (IoM)) recommends a daily iodine intake of 220 μg during pregnancy and 290 μg during lactation (13). The Swiss Society of Endocrinology and Diabetology (SSED) and The American Thyroid Association (ATA) recommend that women who are planning pregnancy, pregnant, or breastfeeding take a prenatal multivitamin containing 150 μg iodine per day to supplement the nutritional iodine intake (13). In these products, the recommended amount of 150 µg iodine is most commonly supplied by adding potassium iodide, which contains 76% iodide. The Swiss Society for Gynecology and Obstetrics does not specifically recommend iodine supplementation but suggests that women use iodized salt during pregnancy.
Materials and methods
Iodine content of multivitamin preparations
Using online resources, including the Swiss Compendium for drugs (https://compendium.ch/), we identified adult multivitamin supplements that are commonly available in Switzerland. The products were categorized into 1) products recommended for pregnancy and lactation (prenatal vitamins (PMV), and 2) other widely available multivitamin products for adults. Advice was sought from obstetricians to ensure the list includes the most frequently prescribed supplements for pregnant and lactating women in Switzerland. The contents and the amounts of the ingredients of every product were extracted and tabulated.
Results
Table 1 summarizes the iodine content, as well as the amount of selenium, a trace element that is essential for the normal function of selenoenzymes, including the deiodinases.
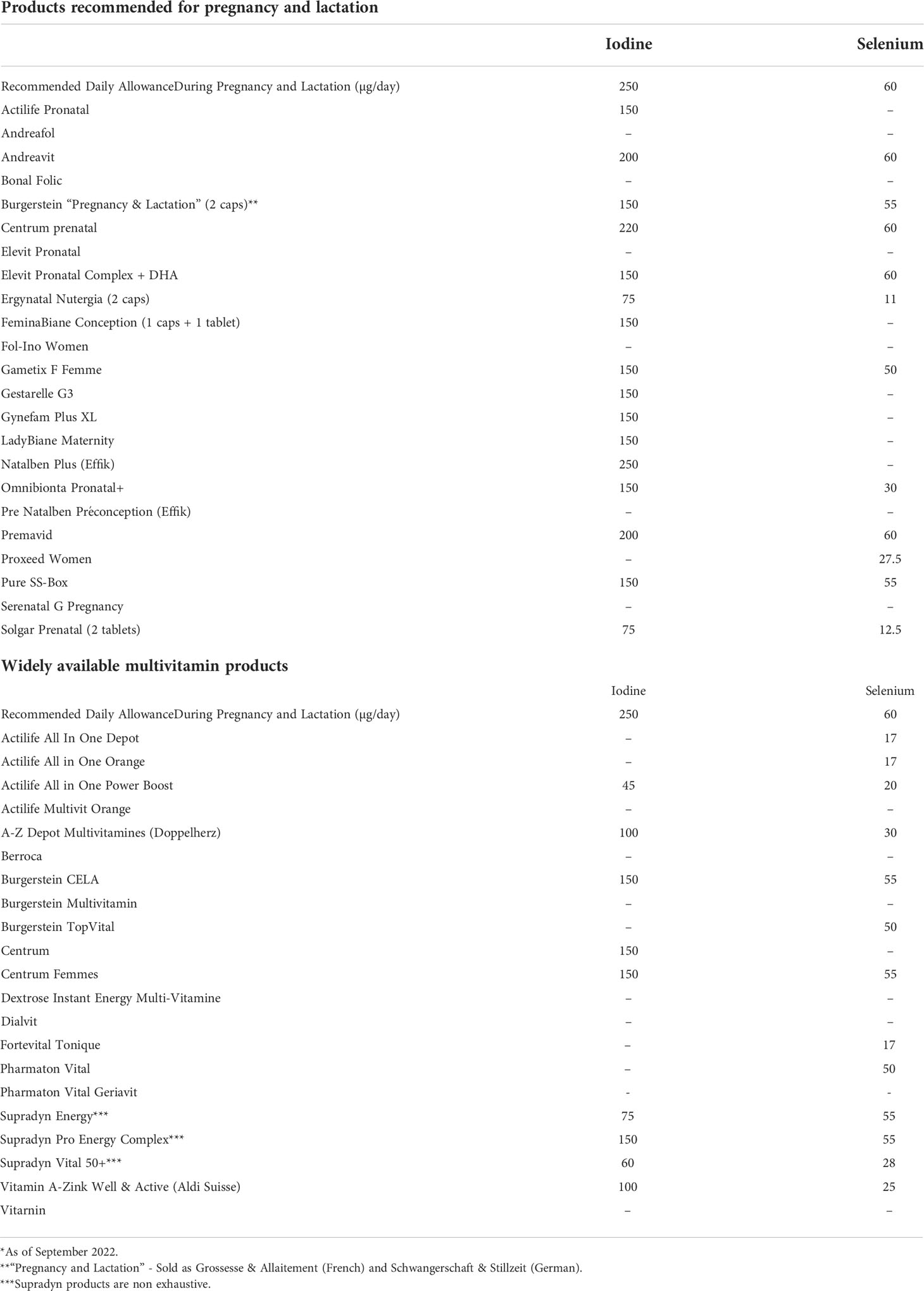
Table 1 Iodine and selenium content of the most frequently used multivitamin products in Switzerland*.
As illustrated in Figure 1, among prenatal multivitamins (PMV), 16/23 (69.6%) contain iodine according to their label. Fourteen out of 23 (60.9%) products contain 150 to 220 µg of iodine, matching or exceeding the recommendations of the SSED and the ATA. Two of 22 (8.7%) contains only 75 µg of iodine, and 7/23 (30.4%) products contain no iodine; surprisingly, the latter group includes the most widely recommended pregnancy supplement.
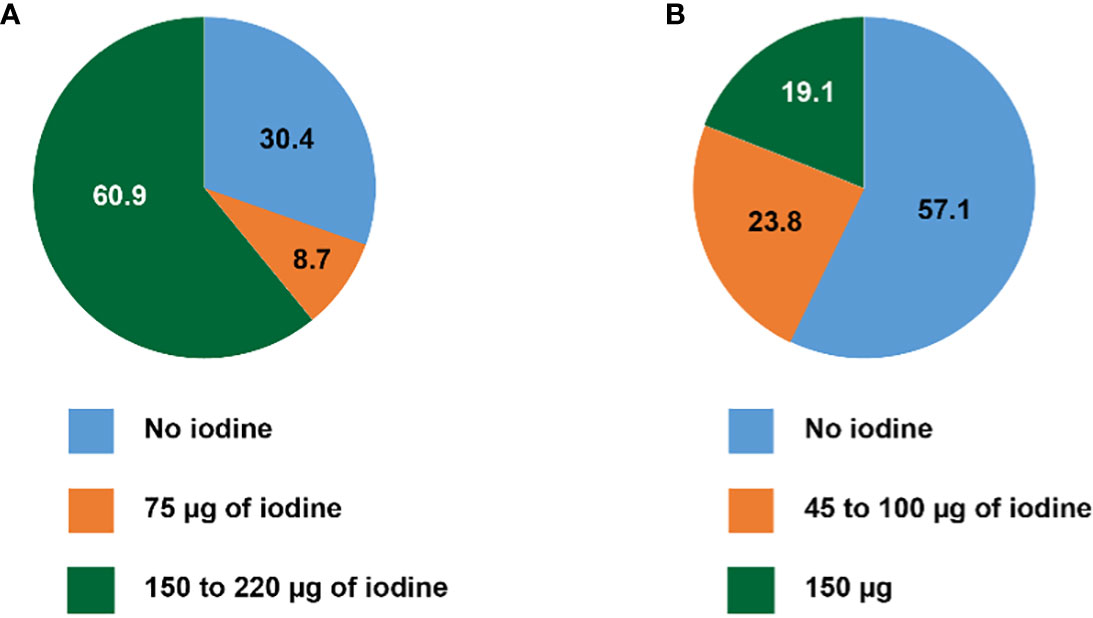
Figure 1 Iodine content of (A) multivitamin products recommended for pregnancy and lactation, and (B) widely available multivitamin products for adults (in percent).
Among adult multivitamins (AMV), 9/21 (42.9%) contain iodine. Twelve out of 21 (57.1%) contain no iodine, and 5/17 (23.8%) 100 µg of iodine or less (from 45 to 100 µg) (Figure 1). Four of 21 (19.1%) products contain 150 µg of iodine, reaching the recommended daily iodine intake for adults, and the supplementation recommended for pregnant and lactating women. Thus, in total, only 18/44 (40.9%) of all multivitamin supplements contain 150 µg of iodine or more.
Discussion
Despite the important consequences of ID during pregnancy and lactation, only 14/23 (60.9%) of vitamins supplements intended for women who are planning pregnancy, pregnant or lactating, contain at least 150 µg of iodine. Considering the products that are not labeled for specific use in pregnant and lactating women, only 9/21 (42.9%) contain iodine and among the iodine-containing multivitamins, the iodine content is variable with a range from 45 to 150 µg (Table 1).
Given that the iodine intake is currently only borderline sufficient in pregnant and non-pregnant women in Switzerland (7), despite the increase in iodine fortification of salt in 2014, the recommendation of the Swiss Society for Gynecology and Obstetrics to use iodized salt during pregnancy, yet not specifically recommending iodine supplementation during pregnancy, should be questioned.
Conclusions
In conclusion, physicians following pregnant and lactating women should be aware of the variability in iodine content of multivitamin products, and it is recommended to prescribe supplements that contain ≥150 µg of iodine. Manufacturers of prenatal multivitamins are encouraged to review their products and, if the iodine content is insufficient, appropriately enrich their products adhering to established recommendations.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization, GC, PK, MG; methodology, MG, PK; formal analysis, MG, PK, GC; writing—original draft preparation, MG, PK; writing—review and editing, PK, MG, GC. All authors contributed to the article and approved the submitted version.
Funding
The work has been partially supported by unrestricted funds by the Faculty of Biology and Medicine of the University of Lausanne to PK. Open access funding was provided by the University of Lausanne.
Acknowledgments
We thank our colleagues in obstetrics for information related to the use of multivitamin products in pregnant women.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zimmermann M. The effects of iodine deficiency in pregnancy and infancy. Paediatr Perinat Epidemiol (2012) 26(s1):108–17. doi: 10.1111/j.1365-3016.2012.01275.x
2. De Escobar G, Obregón M, Del Rey F. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab (2004) 18(2):225–48. doi: 10.1016/j.beem.2004.03.012
3. WHO. World health Organization/United nations children’s Fund/International council for the control of iodine deficiency disorders (WHO/UNICEF/ICCIDD). assessment of iodine deficiency disorders and monitoring their elimination. 3rd edition. Geneva, Switzerland: WHO Press, World Health Organization (2007).
4. Leung AM, Pearce EN, Braverman LE. Iodine content of prenatal multivitamins in the united states. N Engl J Med (2009) 360(9):939–40. doi: 10.1056/NEJMc0807851
5. Bürgi H, Andersson M. History and current epidemiology of iodine nutrition in Switzerland. 1-75: Federal Commission for Nutrition. Federal Office of Public Health (2013).
6. Andersson M, Aeberli I, Wust N, Piacenza A, Bucher T, Henschen I, et al. The Swiss iodized salt program provides adequate iodine for school children and pregnant women, but weaning infants not receiving iodine-containing complementary foods as well as their mothers are iodine deficient. J Clin Endocrinol Metab (2010) 95(12):5217–24. doi: 10.1210/jc.2010-0975
7. Andersson M, Hunziker S, Fingerhut R, Zimmermann MB, Herter-Aeberli I. Effectiveness of increased salt iodine concentration on iodine status: trend analysis of cross-sectional national studies in Switzerland. Eur J Nutr (2020) 59(2):581–93. doi: 10.1007/s00394-019-01927-4
8. Hess S, Zimmermann M, Torresani T, Bürgi H, Hurrell R. Monitoring the adequacy of salt iodization in Switzerland: A national study of school children and pregnant women. Eur J Clin Nutr (2001) 55:162–6. doi: 10.1038/sj.ejcn.1601140
9. Zimmermann M, Aeberli I, Torresani T, Bürgi H. Increasing the iodine concentration in the Swiss iodized salt program markedly improved iodine status in pregnant women and children: a 5-y prospective national study. Am J Clin Nutr (2005) 82(2):388–92. doi: 10.1093/ajcn/82.2.388
10. Andersson M, Herter-Aeberli I. Status en iode de la population suisse. In: Bulletin nutritionnel suisse. Bern, Switzerland: Office fédéral de la sécurité alimentaire et des affaires vétérinaires OSAV. (2019). p. 64–84. Available at: https://www.blv.admin.ch%20›%20blv%20›%20home%20›%20ernaehrung
11. Zimmermann M, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev (2012) 70(10):553–70. doi: 10.1111/j.1753-4887.2012.00528.x
12. Office fédéral de la sécurité alimentaire et des affaire vétérinaires OSAV. In: Denrées alimentaire et nutrition. recommandations concernant l'iode (2018). Bern, Switzerland: Swiss Confederation. Available at: https://www.blv.admin.ch/dam/blv/fr/dokumente/lebensmittel-und-ernaehrung/ernaehrung/empfehlungen-jod.pdf.download.pdf/Empfehlungen_Jod_FR.pdf.
Keywords: Iodine, iodine deficiency, pregnancy, thyroid function, multivitamins
Citation: Gfeller M, Colque G and Kopp PA (2022) Iodine content of frequently used prenatal and adult multivitamins in Switzerland. Front. Endocrinol. 13:1041232. doi: 10.3389/fendo.2022.1041232
Received: 10 September 2022; Accepted: 20 October 2022;
Published: 03 November 2022.
Edited by:
Christian Albert Koch, Fox Chase Cancer Center, United StatesReviewed by:
Simone Wajner, Federal University of Rio Grande do Sul, BrazilCopyright © 2022 Gfeller, Colque and Kopp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter A. Kopp, cGV0ZXIua29wcEBjaHV2LmNo
 Mandy Gfeller
Mandy Gfeller Gentiane Colque2
Gentiane Colque2 Peter A. Kopp
Peter A. Kopp