
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 07 December 2022
Sec. Systems Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1037098
This article is part of the Research Topic Environmental Exposomics and Metabolic Disorders View all 5 articles
 Qiming Yuan1†
Qiming Yuan1† Kun Jin1†
Kun Jin1† Xianghong Zhou1†
Xianghong Zhou1† Zhimei Qiu2
Zhimei Qiu2 Jiakun Li1
Jiakun Li1 Di Jin1
Di Jin1 Zilong Zhang1
Zilong Zhang1 Chichen Zhang1
Chichen Zhang1 Lu Yang1
Lu Yang1 Yu Zhan2
Yu Zhan2 Shi Qiu1*
Shi Qiu1* Qiang Wei1*
Qiang Wei1*Background: It has been reported for several years that polycyclic aromatic hydrocarbons (PAHs) could disturb human endocrine function. However, there is still a short of consistent conclusion about the relationship between PAH exposure and levels of sexual hormones. The aim of our study is to explore whether exposure to PAHs and how PAHs affect the levels of serum testosterone (T) and estradiol (E2) in adults, hoping to fulfill the knowledge gap.
Materials and methods: This study included adults aged 20 and above who participated in the National Health and Nutrition Examination Survey (NHANES) from 2011 to 2016. We included 10 PAH metabolites in this study. The levels of urinary PAH metabolites were log-transformed and divided into quartiles. The associations between PAH metabolites and both serum T levels of males and E2 levels of females were investigated using multivariate regression models. We furtherly calculated PAHs scores by sum of ranks across 10 PAHs metabolites, which represented the exposure levels of PAHs mixtures, and the association between PAHs scores and serum T and E2 levels were analyzed.
Results: A total of 4,654 subjects were included in this study, including 2,460 males and 2,194 females. After adjusting for confounders, 2-hydroxynapthalene and 3-hydroxyfluorene were positively associated with serum T levels of males (p-value for trend=0.047, and p-value for trend=0.006, respectively), while 1-hydroxyphenanthrene was positively associated with serum E2 levels of females (p-value for trend=0.013). In the adjusted models, no significant association was found between PAHs scores and either T levels of males or E2 levels of females (p-value for trend=0.615, and p-value for trend=0.241, respectively).
Conclusions: This study showed urinary 2-hydroxynapthalene and 3-hydroxyfluorene were associated with increased T levels of males, and urinary 1-hydroxyphenanthrene was associated with increased E2 levels of females. The observed association indicated disrupting effects of PAH exposure on reproductive health.
Polycyclic aromatic hydrocarbons (PAHs) are a family of environment toxin which are ubiquitous in urban atmospheres. PAHs are produced mainly by incomplete combustion of organic compounds, including diesel, gasoline, coal, oil, wood, et al. (1), and people can expose to PAHs through smoking, vehicle exhaust, industrial production, and food (2). To evaluate the exposure level of people to PAHs, previous studies mainly applied levels of urinary PAH metabolites (3–6). Studies have reported environment toxins could disturb the levels of reproductive hormone and increase the risk of relative diseases such as breast cancer (7–10). As an important environment toxin, the relationship between PAHs and people’s reproductive system is under intense investigation.
Testosterone (T) as the major male sex hormone can promote the development of male reproductive tissue and spermatogenesis. The secretion of T is regulated by luteinizing hormone (LH), which is secreted at anterior pituitary responded to gonadotropin-releasing hormone (GnRH). A previous study found PAHs could disturb the balance of hypothalamic-pituitary-gonadal axis, and in this study a positive association between secretion of GnRH and exposure to low-dosage PAHs was detected, which led to an increase of serum T level (11). However, another study reported that PAHs may disturb the function of Leydig cells, which are a group of cells in testis secreting T, resulting in a decrease of T in serum (12).
Estradiol (E2), as the most abundant and most active estrogen, is secreted by granulosa cells of follicles in the ovary. E2 plays an important role in regulating women’s somatic and psychological health. For example, women will show increased susceptibility to nausea and motion sickness during the early (low serum E2 level) versus late follicular (high serum E2 level) phase (13, 14). One previous study found exposure to PAHs could enhance the production of reactive quinone species of endogenous estrogen, and there was a positive correlation between PAHs exposure and activation of estrogen, including E2 (15). However, another study found PAHs could decrease E2 secretion by disturbing the function of granulosa cells via ESR1 and GPER1 receptors (16).
Although the association between PAH exposure and people’s reproductive health has aroused more and more attention, there is still a short of consistent conclusion about the relationship between PAH exposure and levels of sexual hormones. This may attribute to geographical differences, racial differences, selected PAHs biomarkers and exposure levels. In addition, as present studies mainly performed on animals or in vitro (17–21), studies investigating the association directly among human are still lacking. Thus, the aim of our study is to explore whether exposure to PAHs and which PAH could affect the levels of serum T and E2 in adults, hoping to fulfill the knowledge gap.
The study subjects were selected from participants of the National Health and Nutrition Examination Survey (NHANES) from 2011 to 2016. The NHANES is a cross-sectional survey of non-institutionalized citizens conducted by NCHS of the Centers for Disease Control and Prevention. The data of participants such as race, income status, dietary condition and medical history was collected through household interviews. Meanwhile, participants would undergo physical examination, and blood and urine would be collected at specific examination centers. All participants were given informed consents, and the NHANES protocol was approved by the National Center for Health Statistics Research Ethics Review Board. We included subjects with complete urinary PAHs metabolites levels and complete serum T and E2 levels. As there was a significant difference in sexual hormone levels between minors and adults, we only included adults aged 20 and above. Subjects using endocrine disrupting drugs were furtherly excluded. A total of 4,654 subjects were included in our study, including 2,460 males and 2,194 females. Details of inclusion and exclusion criteria were shown in Figure 1.
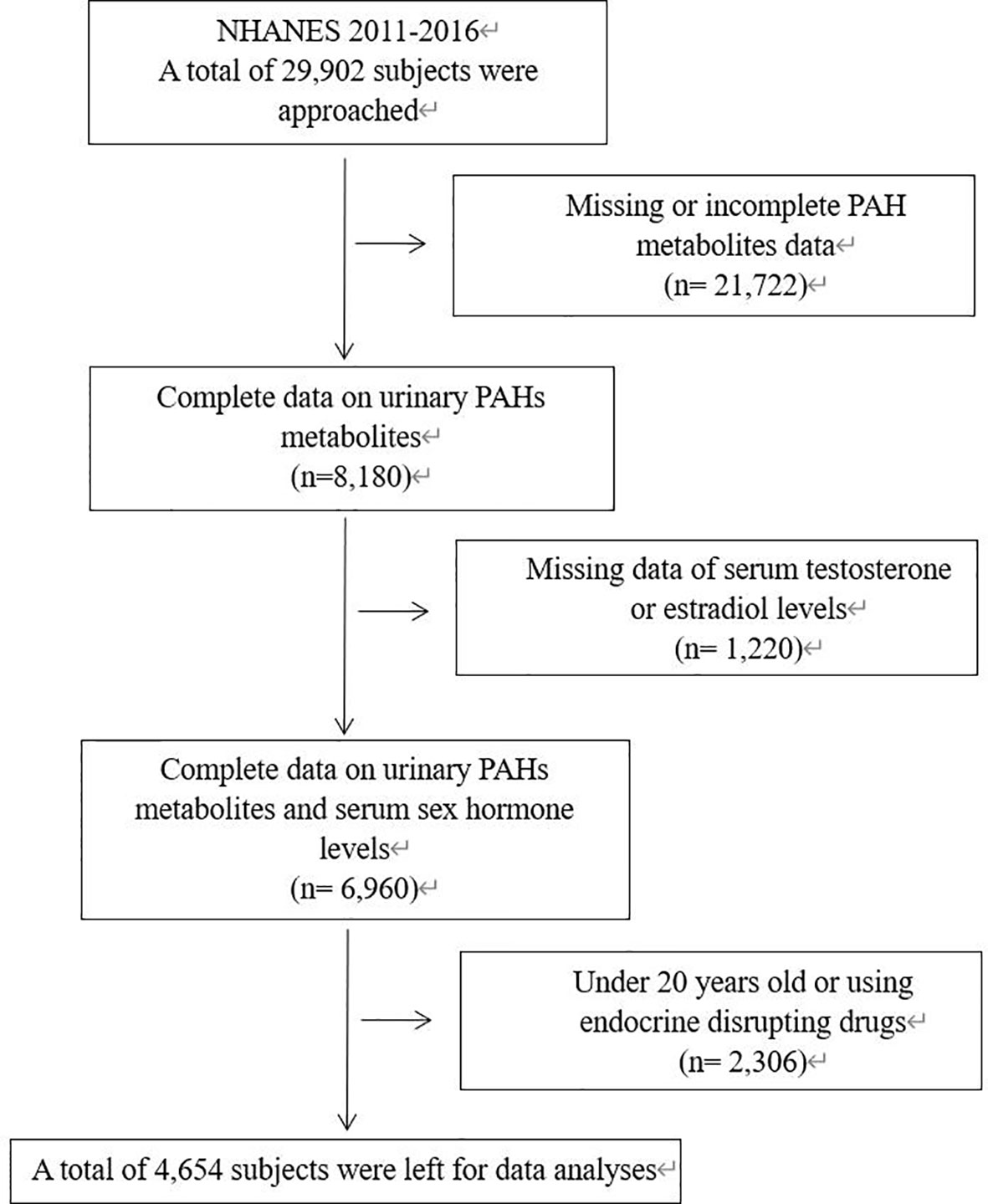
Figure 1 Flowchart describing the selection of patients. NHANES, National Health and Nutrition Examination Survey; PAH, Polycyclic aromatic hydrocarbons.
The urine samples were collected at mobile examination center by trained professionals. After the collection, urine samples were stored at a temperature of -20°C or lower and transported to National Center for Environmental Health for analysis. The glucuronidated or sulfated OH-PAH metabolites in urine experienced enzymatic hydrolysis, extraction, and derivatization. Then the values of metabolites were measured using isotope dilution capillary gas chromatography tandem mass spectrometry (GC-MS/MS). The detailed information about detection and measurement of urine PAH metabolites was provided in the NHANES laboratory method (http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/PAH_G_met.pdf). We included 10 PAH metabolites in this study: 1-hydroxynapthalene (1-naphthol), 2-hydroxynapthalene (2-naphthol), 3-hydroxyfluorene, 2-hydroxyfluorene, 3-hydroxyphenanthrene, 1-hydroxyphenanthrene, 2-hydroxyphenanthrene, 1-hydroxypyrene, 9-hydroxyfluorene, and 4-hydroxyphenanthrene. These 10 PAHs metabolites are major monohydroxylated metabolites of four important PAHs, including naphthalene, fluorene, phenanthrene, and pyrene. In the NHANES database from 2011 to 2016, only these 10 metabolites were available.
The collected serum samples were stored at a temperature of -20°C or lower until they were transported to the Division of Environmental Health Laboratory Sciences for analysis. Before the quantitative analysis, serum T and E2 were dissociated from binding proteins, extracted from sample matrix, and potentially interfering compounds were also removed. Then, serum T levels and E2 levels were measured using isotope dilution liquid chromatography tandem mass spectrometry (ID-LC-MS/MS) by the CDC (https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/TST_H.htm).
To perform the analyses, we used EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) and statistical software packages R (http://www.R-project.org, The R Foundation). A p value of <0.05 was considered statistically significant. The continuous variables were presented as mean ± standard deviation, and the categorical variables were presented as the frequency and its proportion. When the values of PAH metabolites were below the limit of detection, the values were reserved as the detection limit divided by the square root of two. The following categorical variables were included: race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), ratio of family income to poverty (<1.3, 1.3-3.5, >3.5), education level (Less than high school, High school or General educational development, Above high school), marital (Married or living with partner, Living alone), BMI (<=25, >25 and <=30, >30), comorbidity index (0, 1, 2–5), smoking (never, former, current), alcohol intake (none, moderate, heavy). The levels of 10 urinary PAH metabolites were log-transformed to acquire a normal distribution, and the geometric mean levels of creatinine-corrected urinary PAH metabolites were divided into quartiles. The first quartile (lowest level) was set as the reference group, and the β-coefficient and 95% confidence interval (CI) were analyzed using linear regression model to determine whether there was a dose-response relationship between PAH exposure and serum T levels of males and E2 levels of females. P-value for trends were calculated to evaluate the statistical significance of trends. Extended models were performed to adjust covariates, including age, race, BMI, time of venipuncture, ratio of family income to poverty, education level, marital, comorbidity index, smoking, and alcohol intake. As the levels of T and E2 vary physiologically in relation to age, we furtherly performed age-stratified analysis. Subjects were divided into three groups: <40, >=40 and <60, >=60 groups. Associations between PAHs metabolites and sex hormones were tested in the three age groups separately. The association between PAHs metabolites and sex hormone binding globulin (SHBG) levels of males was also analyzed. Additionally, to investigate the association between exposure to PAHs mixtures and serum sexual hormone levels, we sorted the 10 urinary PAH metabolites from lowest to highest to create ranks separately, and PAHs scores were established by sum of ranks across 10 urinary PAH metabolites for each participant (22). High value of PAHs score signified heavy burden of exposure to PAHs mixtures. Association between PAHs scores and serum T and E2 levels were further investigated. Age-stratified analysis was also performed between PAHs scores and sex hormones.
Demographic characteristics, serum T and E2 levels of 4,654 subjects from NHANES 2011-2016 were shown in Table 1. The mean age of male subjects was 48.88 years old, while females were 46.29. The BMI of 33.58% male subjects were over 30 kg/m2, and the BMI of 41.75% female subjects were over 30 kg/m2. For males, smokers, including former and current smokers, were up to 52.56% of subjects, while the percentage of females was 32.68%. The mean T and SHBG levels of male subjects were 409.29 ng/dl and 44.41 nmol/l, while the mean E2 level of female subjects was 82.91 pg/ml.
Table 2 showed the geometric mean and creatine-corrected mean concentration of 10 urinary PAH metabolites. Urinary 1-hydroxynapthalene and 2-hydroxynapthalene metabolites accounted for almost 90% of total urinary PAH metabolites. Besides, urinary 2-hydroxyfluorene and 9-hydroxyfluorene metabolites were higher than the rest PAH metabolites.
The associations between 10 types of urinary PAH metabolites and serum T levels of males are shown in Table 3. In the non-adjusted model, 1-hydroxynapthalene, 2-hydroxynapthalene, 3-hydroxyfluorene, 2-hydroxyfluorene, 3-hydroxyphenanthrene, and 1-hydroxypyrene were associated with increased serum T levels of males (p-value for trends were all <0.05). After adjusting for confounders, 2-hydroxynapthalene and 3-hydroxyfluorene remained positively correlated with serum T level [βs for increasing quartiles: 0, -2.74 (95%CI -21.18 to 15.70), 0.49 (95%CI -18.65 to 19.63), 18.08 (95%CI -3.23 to 39.39), p-value for trend=0.047; and βs for increasing quartiles: 0, 1.43 (95%CI -16.92 to 19.78), -2.71 (95%CI -21.24 to 15.83), 29.13 (95%CI 5.14 to 53.12), p-value for trend=0.006, respectively]. No significant association between PAHs metabolites and SHBG was found (Supplementary Table 1). In the age-stratified analysis, 2-hydroxynapthalene was positively associated with serum T levels among males aged 40-60 (p-value for trend=0.007), while the positive association was found between 3-hydroxyfluorene and serum T among males aged 20-40 (p-value for trend=0.002) (Supplementary Table 2). The correlation between PAHs scores and serum T levels of males were shown in Table 4. In non-adjusted model, serum T levels were positively associated with PAHs score (p-value for trend=0.031). However, after adjusting for confounders, the association did not remain significant (p-value for trend=0.615). In addition, no significant association was found between PAHs scores and serum T levels of males in the three different age groups (Supplementary Table 4).
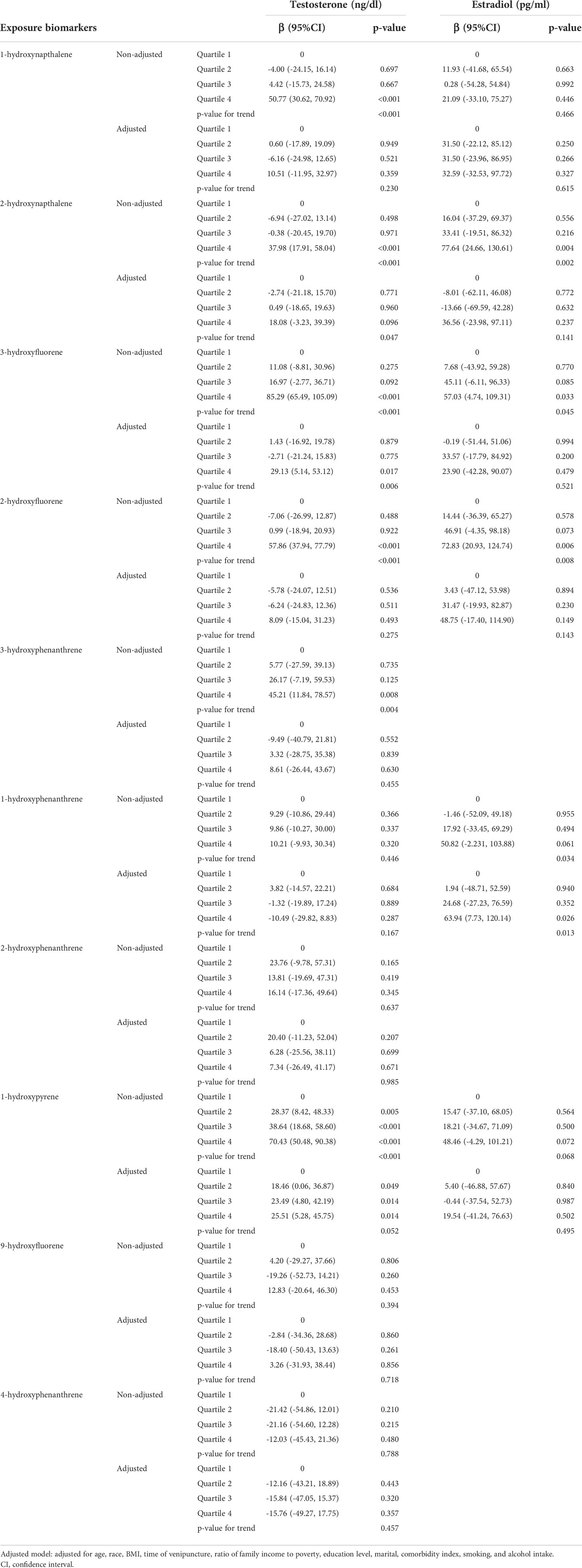
Table 3 Relationships between urinary PAH metabolites and sex hormones in the non-adjusted and adjusted model.
6 out of 10 urinary PAH metabolites showed liner relationships with serum E2 levels of females, including 1-hydroxynapthalene, 2-hydroxynapthalene, 3-hydroxyfluorene, 2-hydroxyfluorene, 1-hydroxyphenanthrene, and 1-hydroxypyrene (Table 3). In the non-adjusted model, 2-hydroxynapthalene, 2-hydroxyfluorene, 3-hydroxyfluorene, and 1-hydroxyphenanthrene were positively associated with serum E2 levels (p=0.002, p=0.045, p=0.008, and p=0.034, respectively). After adjusting for confounders, 1-hydroxyphenanthrene remained positively associated with serum E2 levels [βs for increasing quartiles: 0, 1.94 (95%CI -48.71 to 52.59), 24.68 (95%CI -27.23 to 76.59), 63.94 (95%CI 7.73 to 120.14), p-value for trend=0.013]. In the age-stratified analysis, 1-hydroxyphenanthrene was positively associated with serum E2 levels among females aged 20-40 (p-value for trend=0.002) (Supplementary Table 3). The correlation between PAHs scores and serum E2 levels were shown in Table 4. In non-adjusted model, serum E2 levels were positively associated with PAHs scores (p-value for trend=0.005). Similarly, after adjusting for confounders, the association did not remain significant (p-value for trend=0.241), and no significant association was found between PAHs scores and serum E2 levels of females in three different age groups (Supplementary Table 4).
We examined the association between urinary PAH metabolites and serum T levels of males and serum E2 levels of females separately. The results of this study showed urinary 2-hydroxynapthalene and 3-hydroxyfluorene were positively associated with serum T levels of males, and urinary 1-hydroxyphenanthrene was positively associated with serum E2 levels of females. 2-hydroxynapthalene, 3-hydroxyfluorene, and 1-hydroxyphenanthrene were major metabolites of naphthalene, fluorene, and phenanthrene respectively. This indicated exposure to naphthalene and fluorene might result in an increased level of T of males, and phenanthrene might cause the increasing of E2 level of females.
In the stratified analysis, urinary 2-hydroxynapthalene and 3-hydroxyfluorene were positively associated with serum T levels of males aged 40-60 and 20-40 respectively, and urinary 1-hydroxyphenanthrene was positively associated with serum E2 levels of females aged 20-40. This indicated that young and middle-aged people were more sensitive to the exposure of PAHs than the elderly. PAHs scores was not significantly associated with sex hormones in the three different age groups, which was consistent with the results of total population.
Previous studies focusing on the relationship between PAH exposure and serum sexual hormone levels did not reach a consistent result. In addition, as PAHs consist of hundreds of compounds, the types of PAHs or their metabolites included in different studies were not the same, which made it more difficult to collate and compare the results of different studies. A cross-sectional study which included 371 men in an infertility clinic in Wuhan, China observed 2-hydroxynaphthalene was associated with decreased serum free T (p-value for trend=0.01) (23). However, in another study including 642 males which analyzed the associations between PAH metabolites and several different reproductive hormones, no significant correlation was found between 2-hydroxynapthalene metabolites and serum T levels (24). Interestingly, we observed a positive association between urinary 2-hydroxynapthalene and serum T levels of males in our study. Similarly, results of present studies investigating the association between PAH exposure and serum E2 levels showed a divergence. One study including 120 pregnant Chinese women detected the exposure to both low-molecular-weight and high-molecular weight PAHs could negatively affect the E2 level in the umbilical cord serum (25). There were studies showed a positive association between PAHs exposure and serum E2 level. In one study including 122 non-smoker males, there was a positive association between hydroxypyrene and serum E2 levels (26). Benefiting from NHANES database, we included more kinds of PAH metabolites, and the sample size of our study was larger compared with previous studies, which made our results more systematic and reliable.
The positive association between PAHs exposure and serum T and E2 levels may be explained by several mechanisms. One study explored the association between GnRH expression and short-term benzo(a)pyrene (one type of PAH) exposure, which showed benzo(a)pyrene was positively associated with GnRH by increased the mRNA expression of GnRH2 and GnRH3 (27). This indicated some kinds of PAHs could promote the secretion of GnRH, and furtherly promote the secretion of LT and T. Another study explored the alteration of sex hormone by PAH in rats and a significant increase of LH was observed after exposing to PAHs. In this study, serum T levels were decreased during PAH exposure, while T levels were gradually increased within 48 hours after the exposure. At 72h, the T levels were higher than control group (28). This could be explained by that remaining vital Leydig cells conducted a compensatory synthesis and release of T, and this likely be another reason why PAH metabolites were positively associated with serum T in our study. The positive association between PAHs exposure and serum E2 levels can also be explained by the hypothalamic-pituitary-gonadal axis. Previous studies reported exposure to PAHs was related to increased levels of FSH and LH (24), which could furtherly lead to an increase of serum E2 levels. The monohydroxy derivatives of some PAHs have similar structures with 17β-estradiol, and some of the PAH metabolites can interact with estrogen receptors, demonstrating anti-estrogenic activity (29). This can lead to an increased secretion of E2 to maintain the normal functions of estrogenic hormones.
Our study still has some limitations. First, given the characteristics of cross-sectional design, we cannot make any conclusion about causal link between PAH exposure and serum T and E2 levels. Second, as an observational study, although a number of covariates have been adjusted, unmeasured confounding factors still cannot be excluded. Third, as the half-life of PAHs are short, the urinary PAH metabolites show a within-subject variability over time, which may result in a slight difference from the actual exposure levels (30).
In conclusion, association between PAHs exposure and sex hormones varied by specific PAHs. This study showed urinary 2-hydroxynapthalene and 3-hydroxyfluorene were associated with increased T levels of males, and urinary 1-hydroxyphenanthrene was associated with increased E2 levels of females. The observed association indicated disrupting effects of PAH exposure on reproductive health.
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/default.aspx.
The studies involving human participants were reviewed and approved by the National Center for Health Statistics Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
QY: Data analysis, Manuscript writing. KJ: Data analysis, Manuscript writing. XZ: Data management, Data analysis. ZQ: Data collection and management. JL: Data collection and management. DJ: Data curation, Validation. ZZ Data collection. CZ: Data collection. LY: Manuscript editing. YZ: Manuscript editing. SQ: Project development, Manuscript editing. QW: Project development, Manuscript editing. All authors contributed to the article and approved the submitted version.
This work was supported by the National key research and development program of China (Grant No. 2017YFC0908003), National Natural Science Foundation of China (Grant No.81902578, 81974098), China Postdoctoral Science Foundation (2017M612971), Post-doctoral Science Research Foundation of Sichuan University (2020SCU12041), Post-Doctor Research Project, West China Hospital, Sichuan University (2018HXBH085), National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2018C01).
The authors gratefully thank Dr. Changzhong Chen, Chi Chen, and Xin-Lin Chen (EmpowerStats X&Y Solutions, Inc., Boston, MA) for providing statistical methodology consultation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1037098/full#supplementary-material
1. Larsen RK 3rd, Baker JE. Source apportionment of polycyclic aromatic hydrocarbons in the urban atmosphere: a comparison of three methods. Environ Sci Technol (2003) 37(9):1873–81. doi: 10.1021/es0206184
2. Ramesh A, Walker SA, Hood DB, Guillén MD, Schneider K, Weyand EH. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int J Toxicol (2004) 23(5):301–33. doi: 10.1080/10915810490517063
3. Best EA, Juarez-Colunga E, James K, LeBlanc WG, Serdar B. Biomarkers of exposure to polycyclic aromatic hydrocarbons and cognitive function among elderly in the united states (National health and nutrition examination survey: 2001-2002). PloS One (2016) 11(2):e0147632. doi: 10.1371/journal.pone.0147632
4. Chou CW, Chen YY, Wang CC, Kao TW, Wu CJ, Chen YJ, et al. Urinary biomarkers of polycyclic aromatic hydrocarbons and the association with hearing threshold shifts in the united states adults. Environ Sci pollut Res Int (2020) 27(1):562–70. doi: 10.1007/s11356-019-06883-4
5. Xu C, Liu Q, Liang J, Weng Z, Xu J, Jiang Z, et al. Urinary biomarkers of polycyclic aromatic hydrocarbons and their associations with liver function in adolescents. Environ pollut (2021) 278:116842. doi: 10.1016/j.envpol.2021.116842
6. Lee TW, Kim DH, Ryu JY. Association between urinary polycyclic aromatic hydrocarbons and hypertension in the Korean population: data from the second Korean national environmental health survey (2012-2014). Sci Rep (2020) 10(1):17142. doi: 10.1038/s41598-020-74353-w
7. Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update (2017) 23(6):646–59. doi: 10.1093/humupd/dmx022
8. Mima M, Greenwald D, Ohlander S. Environmental toxins and Male fertility. Curr Urol Rep (2018) 19(7):50. doi: 10.1007/s11934-018-0804-1
9. Krzastek SC, Farhi J, Gray M, Smith RP. Impact of environmental toxin exposure on male fertility potential. Transl Androl Urol (2020) 9(6):2797–813. doi: 10.21037/tau-20-685
10. Bonner MR, Han D, Nie J, Rogerson P, Vena JE, Muti P, et al. Breast cancer risk and exposure in early life to polycyclic aromatic hydrocarbons using total suspended particulates as a proxy measure. Cancer Epidemiol Biomarkers Prev (2005) 14(1):53–60. doi: 10.1158/1055-9965.53.14.1
11. Evanson M, Van Der Kraak GJ. Stimulatory effects of selected PAHs on testosterone production in goldfish and rainbow trout and possible mechanisms of action. Comp Biochem Physiol C Toxicol Pharmacol (2001) 130(2):249–58. doi: 10.1016/s1532-0456(01)00246-0
12. Chung JY, Kim YJ, Kim JY, Lee SG, Park JE, Kim WR, et al. Benzo[a]pyrene reduces testosterone production in rat leydig cells via a direct disturbance of testicular steroidogenic machinery. Environ Health Perspect (2011) 119(11):1569–74. doi: 10.1289/ehp.1003391
13. Motta-Mena NV, Puts DA. Endocrinology of human female sexuality, mating, and reproductive behavior. Horm Behav (2017) 91:19–35. doi: 10.1016/j.yhbeh.2016.11.012
14. Rieder JK, Darabos K, Weierich MR. Estradiol and women's health: Considering the role of estradiol as a marker in behavioral medicine. Int J Behav Med (2020) 27(3):294–304. doi: 10.1007/s12529-019-09820-4
15. Lin C, Chen DR, Wang SL, Hsieh WC, Yu WF, Wang TW, et al. Cumulative body burdens of polycyclic aromatic hydrocarbons associated with estrogen bioactivation in pregnant women: Protein adducts as biomarkers of exposure. J Environ Sci Health A Tox Hazard Subst Environ Eng (2014) 49(6):634–40. doi: 10.1080/10934529.2014.865416
16. Zajda K, Gregoraszczuk EL. Environmental polycyclic aromatic hydrocarbons mixture, in human blood levels, decreased oestradiol secretion by granulosa cells via ESR1 and GPER1 but not ESR2 receptor. Hum Exp Toxicol (2020) 39(3):276–89. doi: 10.1177/0960327119886027
17. Madeen EP, Williams DE. Environmental PAH exposure and male idiopathic infertility: A review on early life exposures and adult diagnosis. Rev Environ Health (2017) 32(1-2):73–81. doi: 10.1515/reveh-2016-0045
18. Xia Y, Zhu P, Han Y, Lu C, Wang S, Gu A, et al. Urinary metabolites of polycyclic aromatic hydrocarbons in relation to idiopathic male infertility. Hum Reprod (2009) 24(5):1067–74. doi: 10.1093/humrep/dep006
19. Izawa H, Kohara M, Watanabe G, Taya K, Sagai M. Diesel exhaust particle toxicity on spermatogenesis in the mouse is aryl hydrocarbon receptor dependent. J Reprod Dev (2007) 53(5):1069–78. doi: 10.1262/jrd.19025
20. de Campos MF, Lo Nostro FL, Da Cuña RH, Moreira RG. Endocrine disruption of phenanthrene in the protogynous dusky grouper epinephelus marginatus (Serranidae: Perciformes). Gen Comp Endocrinol (2018) 257:255–63. doi: 10.1016/j.ygcen.2017.06.020
21. Kennedy CJ, Smyth KR. Disruption of the rainbow trout reproductive endocrine axis by the polycyclic aromatic hydrocarbon benzo[a]pyrene. Gen Comp Endocrinol (2015) 219:102–11. doi: 10.1016/j.ygcen.2015.03.013
22. Guo J, Huang Y, Bian S, Zhao C, Jin Y, Yu D, et al. Associations of urinary polycyclic aromatic hydrocarbons with bone mass density and osteoporosis in U.S. adults, NHANES 2005-2010. Environ pollut (2018) 240:209–18. doi: 10.1016/j.envpol.2018.04.108
23. Yang P, Sun H, Gong YJ, Wang YX, Liu C, Chen YJ, et al. Repeated measures of urinary polycyclic aromatic hydrocarbon metabolites in relation to altered reproductive hormones: A cross-sectional study in China. Int J Hyg Environ Health (2017) 220(8):1340–6. doi: 10.1016/j.ijheh.2017.09.004
24. Han Y, Xia Y, Zhu P, Qiao S, Zhao R, Jin N, et al. Reproductive hormones in relation to polycyclic aromatic hydrocarbon (PAH) metabolites among non-occupational exposure of males. Sci Total Environ (2010) 408(4):768–73. doi: 10.1016/j.scitotenv.2009.11.021
25. Yin S, Tang M, Chen F, Li T, Liu W. Environmental exposure to polycyclic aromatic hydrocarbons (PAHs): The correlation with and impact on reproductive hormones in umbilical cord serum. Environ pollut (2017) 220(Pt B):1429–37. doi: 10.1016/j.envpol.2016.10.090
26. Sancini A, Montuori L, Chighine A, Caciari T, Giubilati R, Sacco C, et al. Urinary hydroxypyrene and estradiol in an occupationally exposed "outdoor" population. Ann Ig (2014) 26(4):311–20. doi: 10.7416/ai.2014.1991
27. Wang W, Chen J, Fang Y, Wang B, Zou Q, Wang L, et al. Identification of gnrh2 and gnrh3 and their expression during brood pouch growth and short-term benzo(a)pyrene exposure in lined seahorse (Hippocampus erectus). Comp Biochem Physiol C Toxicol Pharmacol (2019) 225:108579. doi: 10.1016/j.cbpc.2019.108579
28. Jeng HA, Yu L. Alteration of sperm quality and hormone levels by polycyclic aromatic hydrocarbons on airborne particulate particles. J Environ Sci Health A Tox Hazard Subst Environ Eng (2008) 43(7):675–81. doi: 10.1080/10934520801959815
29. Arcaro KF, O'Keefe PW, Yang Y, Clayton W, Gierthy JF. Antiestrogenicity of environmental polycyclic aromatic hydrocarbons in human breast cancer cells. Toxicology (1999) 133(2-3):115–27. doi: 10.1016/s0300-483x(99)00018-9
30. Li Z, Romanoff LC, Lewin MD, Porter EN, Trinidad DA, Needham LL, et al. Variability of urinary concentrations of polycyclic aromatic hydrocarbon metabolite in general population and comparison of spot, first-morning, and 24-h void sampling. J Expo Sci Environ Epidemiol (2010) 20(6):526–35. doi: 10.1038/jes.2009.41
Keywords: polycyclic aromatic hydrocarbon, testosterone, estradiol, reproductive health, national health and nutrition examination survey
Citation: Yuan Q, Jin K, Zhou X, Qiu Z, Li J, Jin D, Zhang Z, Zhang C, Yang L, Zhan Y, Qiu S and Wei Q (2022) Urinary polycyclic aromatic hydrocarbon metabolites are positively related to serum testosterone levels of males and serum estradiol levels of females among U.S. adults. Front. Endocrinol. 13:1037098. doi: 10.3389/fendo.2022.1037098
Received: 05 September 2022; Accepted: 31 October 2022;
Published: 07 December 2022.
Edited by:
Cristina De Angelis, University of Naples Federico II, ItalyReviewed by:
Marco Ghezzi, Independent Researcher, Padova, ItalyCopyright © 2022 Yuan, Jin, Zhou, Qiu, Li, Jin, Zhang, Zhang, Yang, Zhan, Qiu and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi Qiu, cWl1c2hpQHNjdS5lZHUuY24=; Qiang Wei, d2VpcWlhbmc5MzNAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.