
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 24 October 2022
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1035929
This article is part of the Research Topic PCOS: from Infertility to Pregnancy View all 12 articles
Polycystic ovary syndrome (PCOS) is a common endocrine and metabolic disorder among women of reproductive age. Current standard treatment includes lifestyle change, oral pharmacological agents, and surgical modalities. However, the efficacy of current therapies is less than satisfactory. Clinical evidence has shown that acupuncture is effective for regulating hormone levels, promoting ovulation, and attenuating insulin resistance in patients with PCOS. Acupuncture may affect the production of β‐endorphin, which may lead to gonadotropin-releasing hormone secretion and then affect ovulation, menstrual cycle, and fertility. The mechanism of acupuncture for patients with PCOS has not been comprehensively reviewed so far. Better understanding of the mechanisms of acupuncture would help popularize the use of acupuncture therapy for patients with PCOS. In this narrative review, we aimed to overview the potential mechanisms and evidence-based data of acupuncture on PCOS, and analyze the most frequently used acupoints based on animal and clinical studies. The results of this study will contribute to a better understanding of the current situation in this field.
Polycystic ovary syndrome (PCOS) is a common endocrine-metabolic disorder in reproductive-aged women, affecting up to 15% of reproductive age women (1), and has become the leading cause of menstrual disorders and anovulatory infertility in women (2, 3). The major clinical manifestations of PCOS consist of ovulatory dysfunction, hyperandrogenism, and polycystic ovaries, along with insulin resistance, obesity, and metabolic dysfunction (4, 5). In addition, PCOS is also linked with other complications, such as type 2 diabetes, endometrial dysfunction and cancer, cardiovascular disease, depression, and pregnancy complications (6–10). The current standard treatment for PCOS includes lifestyle change, pharmacological therapy, and surgical modalities, but the effect is less than satisfactory (11, 12). Lifestyle change is the first-line therapy for PCOS patients, especially for overweight and obese women. However, this is a very difficult task for many people (13). The main pharmacological therapy for PCOS patients is the oral selective estrogen receptor modulator like clomiphene citrate (CC). CC is ineffective in 40% of PCOS patients and is associated with significant side effects, such as headaches, bloating, mood swings, and breast tenderness (14, 15). Letrozole is considered the first-line treatment to induce ovulation, with the ability to improve clinical pregnancy rates and reduce time-to-pregnancy in women with PCOS (15). However, it is associated with higher risks of hot flashes, arthralgias, fatigue, and myalgias (16). Metformin, an insulin sensitizer, has been widely used for the treatment of PCOS patients (17). It increases insulin sensitivity, but associated with unsatisfactory weight loss and increased risk of hypoglycemia (18, 19). Gonadotropin has been proven to be effective in ovulation induction. But it may lead to the development of multiple follicles and increase the risk of ovarian hyperstimulation syndrome (20). Therefore, there is a need for a new therapy, which should be inexpensive, easily administered, and free of serious side effects.
Acupuncture has been used as a medical means in China for thousands of years (21). The use of acupuncture in the reproductive endocrinology and infertility of PCOS has recently gained increased popularity worldwide. Several clinical trials have shown that acupuncture may have beneficial effects on ovulatory dysfunction and infertility in patients with PCOS. Acupuncture has also been reported to improve insulin sensitivity and decrease testosterone in patients and animals with PCOS (22). However, there is an insufficient amount of research evidence to support the clinical efficacy of acupuncture treatment for PCOS women, and the mechanisms of acupuncture are unclear, previous clinical and experimental studies indicate that acupuncture influences PCOS-like symptoms via various mechanisms. Recent reviews have demonstrated that acupuncture adjusts hormone levels by regulating hypothalamic-pituitary-ovarian (HPO) axis or regulating levels of anti-Müllerian hormone (AMH) and P450arom. A recent systematic review on effect of acupuncture on PCOS in animal models summarized that acupuncture could improve insulin resistance by upregulating the insulin receptor substrate-1 (IRS-1)/PI3K/glucose transporter 4 (GLUT4) pathway, or inhibiting the PI3K/AKT/mTOR pathway, or activating the adenosine 5’-monophosphate activated protein kinase (AMPK) pathway in PCOS animals (23).
Until now, no review has been published to summarize the effects of acupuncture both in PCOS patients and animal models. Therefore, the present study aims to review the potential mechanism and evidence-based data of acupuncture on PCOS, and analyze the most commonly used acupoints. The results of this narrative review may provide directions for future research in this area.
A comprehensive literature search was performed by two independent investigators in PubMed, Web of Science, and Scopus databases from establishment to July 2022 to identify related publications. The advanced search option was used to select relevant keywords and identify Medical Subject Headings (MeSH) terms [i.e., (Acupuncture* OR Electroacupuncture*) AND (Polycystic Ovary Syndrome* OR Polycystic Ovarian Syndrome* OR PCOS*)].
All human and animal studies concerning the effect of acupuncture on PCOS treatment were included in this review. There was no country restriction, but the language was limited to English. Moreover, no restrictions were imposed involving publishing date, type of subjects or type of reported outcomes. Researches were excluded if the type of intervention was moxibustion or transcutaneous electrical acupoint stimulation or acupressure.
The following assignments were independently accomplished by two investigators. We identified 150 articles on PubMed, 246 articles on Web of Science, and 158 articles on Scopus. After duplicates were removed, 263 articles conformed to our search criteria. The titles and abstracts of the identified articles were reviewed, and irrelevant search results were deleted. After that step, the full text of the remaining literature was assessed. A total of 62 publications were finally included in this review (Table 1). Among them, 28 publications were animal studies, and 34 publications were clinical studies. The flow chart of the screening process is shown in Figure 1.
The exact etiology of PCOS is still unclear because of the complicated pathophysiological processes. Mounting evidence suggests that PCOS may be related to hyperandrogenism, insulin resistance, genetic factors, and negative emotions. Hyperandrogenemia plays a vital role in the pathogenesis of PCOS (70). Androgen stimulation may increase the release of gonadotropin-releasing hormone (GnRH), which may lead to an increase in the frequency and amplitude of luteinizing hormone (LH) pulses. Excessive LH release, in turn, may cause excessive production of androgens (71). Moreover, the low levels of follicle-stimulating hormone (FSH) and inadequate conversion of androgen to estradiol prevent the recruitment of dominant follicles, leading to anovulation (71–73). Insulin resistance is another important factor in the pathogenesis of PCOS (74). An abnormal insulin signaling pathway was found in the ovarian tissues of PCOS patients. This may be because prolonged hyperinsulinemia activates the mTOR/S6 kinase pathway, which enhances the serine phosphorylation of IRS-1 and eventually induces insulin resistance in the hypothalamus (75, 76). In addition, insulin levels are also positively correlated with androgen levels in PCOS patients (77). The secretion of androgens increased in PCOS patients under insulin stimulation, which enhanced the activity of cytochrome P450c17α hydroxylase and then increased androgen production. Adiponectin, an adipocyte-specific protein that regulates insulin sensitivity and glucose catabolism, have been found decreased in patients with PCOS (78, 79). Furthermore, high insulin levels in PCOS patients may also accelerate the pulse of LH secretion and stimulate the synthesis of androgens by follicular membrane cells, resulting in hyperandrogenemia and anovulation (80). The incidence of PCOS is often clustered in families, and first-degree relatives are at higher risk (81). Genes such as the CYP17 gene, androgen receptor gene, and SHBG gene have been confirmed to be involved in androgen metabolism (82–84). Recent studies have shown that insulin receptor genes (IRS1 and IRS2) are associated with the incidence of PCOS (85). Chronic negative emotions such as depression and low self-esteem could make the body in a state of stress. Such emotions can directly inhibit the hypothalamic-pituitary-adrenal axis, leading to obstacles in the HPO axis regulation mechanism and ovarian dysfunction, which induce PCOS (86).
Acupuncture, a representative of traditional Chinese medicine, has been widely used for treating diseases in China for at least 2,000 years. Currently, acupuncture is increasingly accepted as a complementary therapy for many disorders worldwide (87). The effectiveness of acupuncture for diseases such as chronic prostatitis, chronic musculoskeletal pain, and chronic severe functional constipation, etc. have been confirmed by many high-quality randomized controlled trials (88–90). Electroacupuncture (EA) is a new form of acupuncture treatment in which acupuncture is combined with electrical stimulation. Multiple clinical trials have shown that manual acupuncture and EA are both effective for treating PCOS (48, 57, 64, 65, 68). The effects of acupuncture for PCOS involved improvement in ovulation rate, pregnancy rate, insulin resistance, negative emotion, sexual hormone disturbance, and lipid metabolism dysfunction (Figure 2) (91).
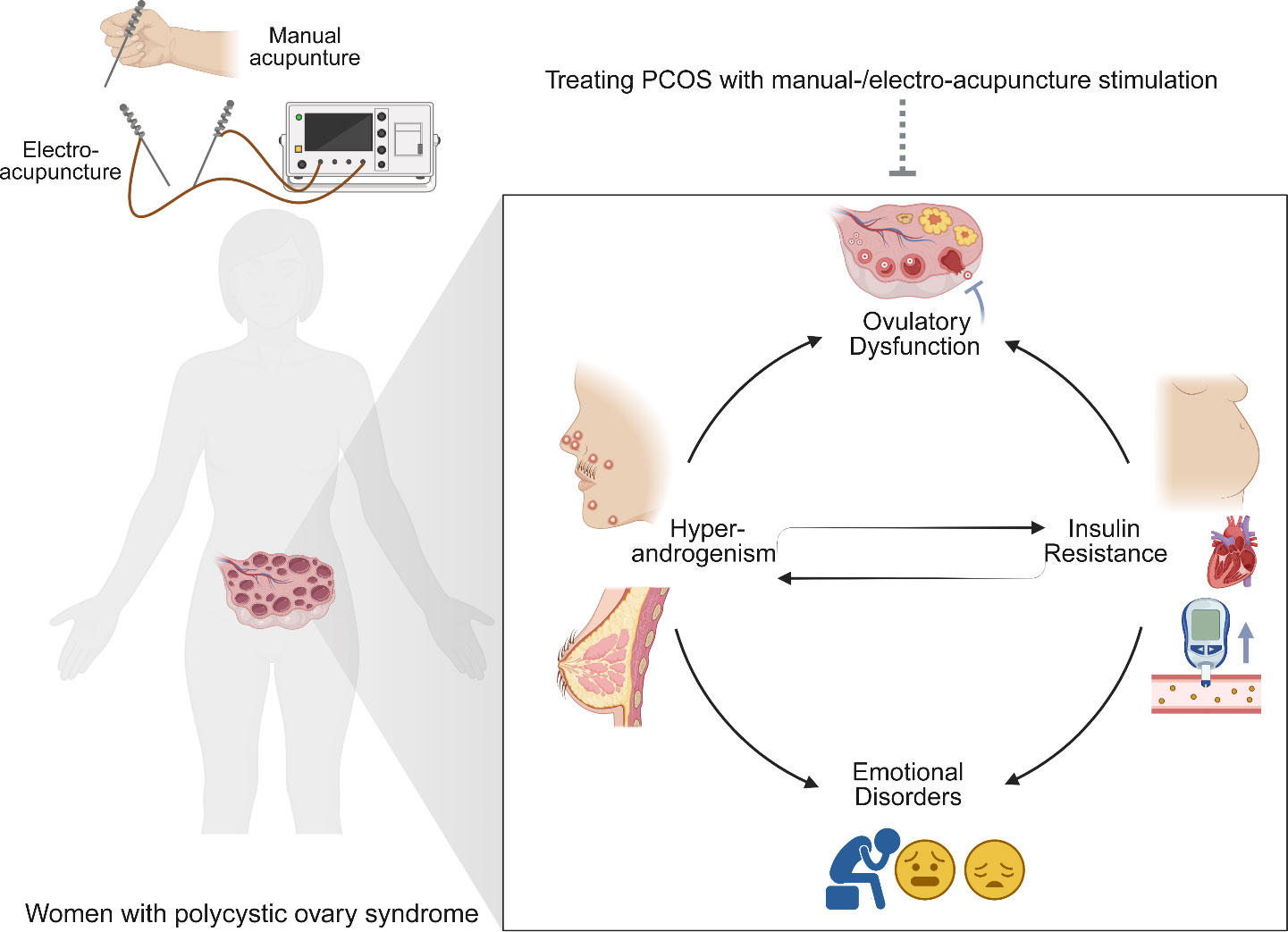
Figure 2 Acupuncture treatment attenuates the major clinical manifestations of polycystic ovary syndrome (PCOS). PCOS is featured by hyperandrogenism (hirsutism, acne, alopecia, etc.), ovulatory dysfunction, polycystic ovaries, obesity, insulin resistance, metabolic dysfunction, and emotional disorders. Manual-/electro-acupuncture improves the PCOS-related symptoms through different biological mechanisms.
Acupuncture therapy has been used as a complementary and alternative treatment for oligo/anovulatory women with PCOS (91). Studies have revealed that acupuncture might reduce cortisol concentrations and regulate central and peripheral β‐endorphin production and secretion (92). Considering that acupuncture has a potential effect on β-endorphin, which can impact GnRH secretion and levels, it is postulated that acupuncture may play an important role in improving ovulation induction and fertility. In 2016, a systematic review including five randomized controlled trials (RCTs) with 413 women reported insufficient evidence to support the use of acupuncture for the treatment of ovulation disorders in women with PCOS (93). Further in 2019, the updated review added three other new RCTs with a total of 1546 women covering the uncertainty of the effect of acupuncture on the live birth rate, multiple pregnancy rate and ovulation rate compared to sham acupuncture (91). However, acupuncture may ameliorate the restoration of regular menstrual periods. In recent years, accumulating scientific studies have investigated the acupuncture meridians and the neuroendocrinological aspects of the meridians, considering that acupuncture may have a role in normalizing the HPO axis, which in turn influences the menstruation cycle pattern (91, 94). In addition, the evolving omics techniques and emerging analysis tools of biological information may facilitate acupuncture research and help to reveal the mechanisms of acupuncture action on PCOS.
A lot of systematic reviews and meta-analyses have been performed to provide evidence-based information in this field. A Cochrane systematic review have been conducted by Lim et al. in 2011, and updated in 2016 and 2019 (91, 93, 95). These studies assessed the effectiveness of acupuncture treatment for oligo/anovulatory women with PCOS for both fertility and symptom control. They concluded that the efficacy of acupuncture on pregnancy outcomes in PCOS patients was uncertain due to the limited number of RCTs and the low quality of evidence (91). A systematic review in 2017 revealed that acupuncture is likely to improve ovulation rate and menstruation rate, but the level of evidence was low (96). In 2020, Wu et al. believed that there was no sufficient evidence supporting the effectiveness of acupuncture to promote live birth, pregnancy, and ovulation in PCOS patients (22). Interestingly, this systematic review suggested that acupuncture could promote the recovery of menstrual cycles as well as downregulate the levels of LH and testosterone in PCOS patients. A recent systematic review showed that acupuncture combined with metformin improved pregnancy rate, ovulation rate, and insulin resistance in PCOS patients compared to using metformin alone (97). Another study found that acupuncture combined with moxibustion improved pregnancy, ovulation, and miscarriage rates, as well as the levels of some sex hormones and metabolic indicators (98). The effectiveness of acupuncture on PCOS patients undergoing in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) was also evaluated by a systematic review (99). The results showed that acupuncture may increase the clinical pregnancy rate and ongoing pregnancy rate and decrease the risk of ovarian hyperstimulation syndrome in patients with PCOS undergoing IVF or ICSI. A systematic review by Zheng et al. found that acupuncture was relatively effective in improving glucose metabolism and insulin sensitivity in patients with PCOS (100). Furthermore, a systematic review assessed the efficacy of acupuncture on animal models with PCOS (23). They found that a definite conclusion was difficult to draw because the methodology was weak and heterogeneity was high. The methodological and reporting quality of systematic reviews on acupuncture treatment for patients with PCOS was also evaluated by a systematic review. This study demonstrated poor methodological and reporting quality of systematic reviews assessing acupuncture in patients with PCOS (101). Information of these systematic reviews and meta-analyses is shown in Table 2.
Ovulatory dysfunction is one of the most sovereign characteristics of PCOS (102). Oocyte quality has been proved important for reproductive potential in women with PCOS (103). EA was proved to be effective in improving oocyte quality and embryonic development potential in infertile patients with PCOS (64). Upregulation of the IRS-1/PI3K/GLUT4 signaling pathway appears to be involved in the effect of EA. A similar study demonstrated that EA improved abnormal follicular development in PCOS patients by inhibiting the overexpression of AMH and increasing the expression of P450arom (66). The protective effect of EA on follicle growth in patients with PCOS was further confirmed by another clinical study (53, 60). In addition, acupuncture at an early stage of oocyte recruitment improved embryo quality in PCOS patients undergoing in vitro fertilization (39). A recent animal study revealed that acupuncture improved ovulation disorder by downregulating LncMEG3 expression, inhibiting the PI3K/AKT/mTOR pathway, and reducing granulosa cell autophagy (61). EA was also reported to improve follicular arrest in PCOS rats by decreasing the overexpression of AMH to normalize FSH and AMH imbalance in granulosa cells (51). Follicular maturation may be affected by endogenous ovarian angiogenesis, which may be another mechanism underlying EA in the treatment of PCOS (49). Interestingly, another animal study demonstrated that EA upregulates the numbers of preovulatory follicles and corpora lutea by increasing innervation of blood vessels near the hilum (58).
PCOS is a multi-symptom disorder linked with a range of reproductive hormonal disturbances (104). A recent clinical study showed that acupuncture improved the pregnancy rate and ovulation rate in infertile women with PCOS, and the effect may be related to the modulation of acupuncture on sex hormones disturbance (68). In another study, acupuncture induced a higher ovulation frequency in lean/overweight PCOS women (41). Meanwhile, acupuncture also reduced the serum levels of ovarian and adrenal sex steroid. In an animal study, EA improved the disturbed estrous cycles and upregulated the number of corpora lutea and area of the ovary in a pubertal rat model of PCOS (63). The increased LH and decreased estradiol and GnRH were all normalized by EA in this study. Furthermore, EA attenuated the upregulation of kisspeptin protein level in the arcuate nucleus, which might explain the efficacy of EA (63).
Evidence suggests that hyperandrogenism is an important clinical feature and mechanism of PCOS (105). Many clinical studies have shown that acupuncture can lower the serum level of testosterone in PCOS women (33, 41, 68, 106). In a study, the circulating and adipose tissue androgen levels in PCOS patients were decreased by EA (44). The effect of EA may be associated with decreased level of hemoglobin A1C. Another study showed that EA improved hyperandrogenism in PCOS patients, and regulation of AMH and P450arom may be involved in the potential mechanism of EA (66). In an animal study, acupuncture inhibited excessive androgen secretion in a rat model of PCOS. The efficacy of acupuncture may be related to the inhibitory effect on overexpression of androgen receptor and connexin 43 (59). Another animal study revealed that EA improved the local ovarian hyperandrogenic environment, probably through increasing P450arom level and decreasing P450C17a level (42). In addition, research showed that EA decreased the overexpression of AMH and regulated FSH and AMH imbalance in granulosa cells, improving hyperandrogenism in a rat model of PCOS (51). Low-frequency EA also decreased serum testosterone in rats with PCOS, and the efficacy may be mediated by central opioid receptors such as Oprk1 and Oprm1 in the hypothalamic arcuate nucleus (35).
There is general agreement that PCOS patients are insulin resistant, especially obese PCOS patients (107). Insulin resistance and related hyperinsulinemia may induce both the endocrine and reproductive traits of PCOS (108). The efficiency of acupuncture on insulin resistance in PCOS patients has been confirmed by many clinical studies (43, 57, 64). A recent study demonstrated that EA improved the insulin resistance score compared with the control group in PCOS patients, and the protective effect of EA might be through an upregulation of the IRS-1/PI3K/GLUT4 signaling pathway (64). Abdominal acupuncture also improved insulin resistance in patients with obesity-type PCOS, which may be related to the efficacy of acupuncture treatment on body–mass index, waist-to-hip ratio (WHR), and lipid metabolism dysfunctions (43). Consistent with this study, EA was found to be effective in improving insulin resistance, as well as decreasing WHR and the levels of total cholesterol and low-density lipoprotein (LDL) cholesterol (54, 62). EA was also reported to attenuate insulin resistance by inactivating the mTOR/4E-BP1 signaling pathway in a rat model of PCOS (12). Simultaneously, EA ameliorated mitochondrial dysfunction and endoplasmic reticulum stress by enhancing autophagy. EA improved insulin sensitivity in PCOS models, and this efficacy may be associated with increased plasma insulin-like growth factor-I, increased expression of leptin and interleukin-6 (IL-6) and decreased expression of uncoupling protein 2 in visceral adipose tissue (28). Sterol regulatory element-binding protein-1 (SREBP-1) is an important transcription factor that regulates the expression of genes involved in lipogenesis and glycolysis (109). A study found that EA induced the activation of the AMPK pathway to suppress SREBP-1 expression and finally inhibited insulin resistance, mitochondrial dysfunction and oxidative stress in a PCOS rat model (56). A study investigated whether EA and manual acupuncture have different effects on insulin sensitivity in PCOS rats. They found that EA improved insulin sensitivity in soleus muscle and mesenteric adipose tissue, while manual had a greater effect on glucose tolerance (Figure 3) (38).
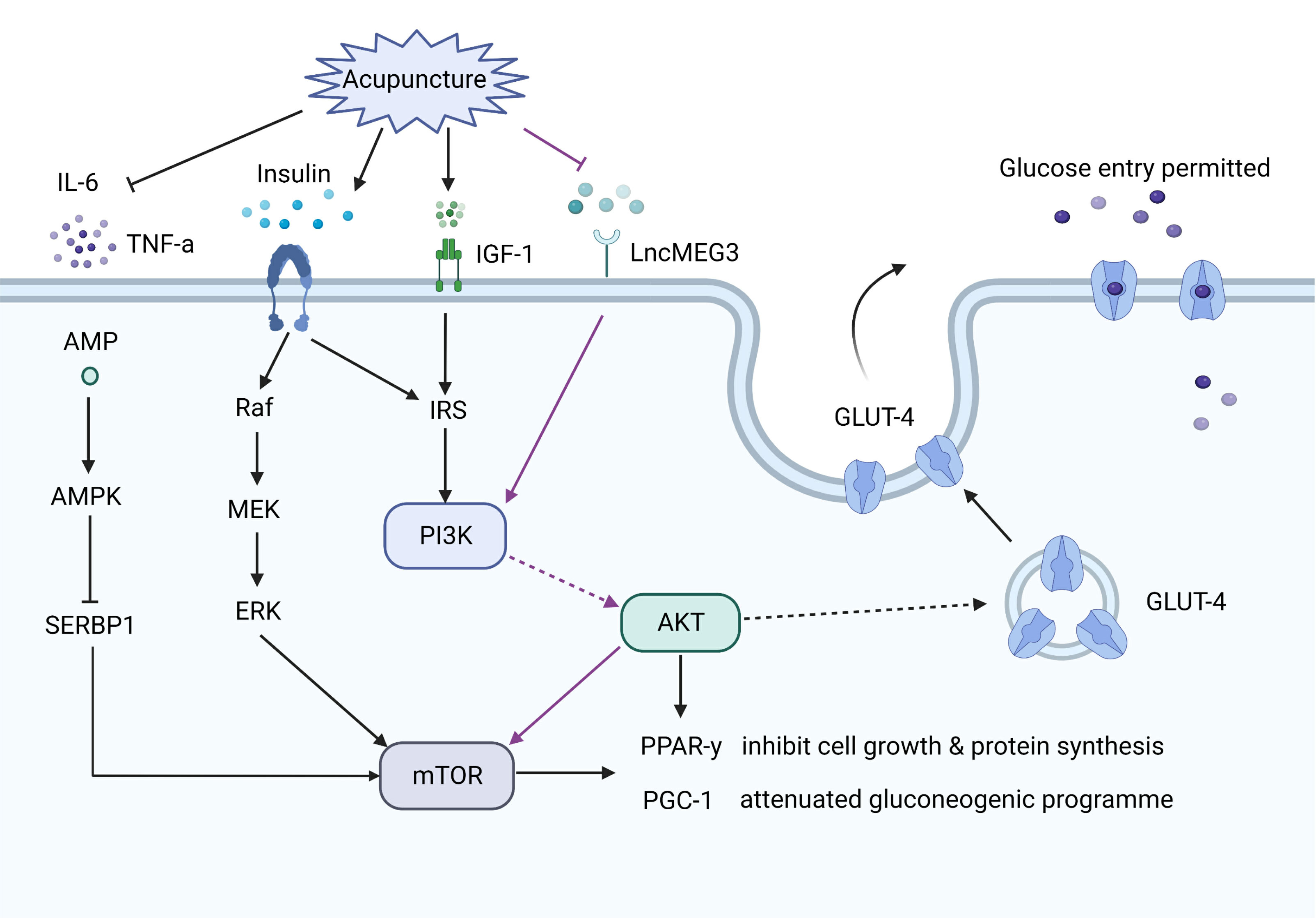
Figure 3 The effect of acupuncture on the insulin pathways. Acupuncture increases glucose transporter 4 (GLUT4) expression via upregulating the insulin receptor substrates-1/PI3K/GLUT4 pathway, or inhibiting the PI3K/AKT/mTOR pathway, or activating the adenosine monophosphate activated protein kinase (AMPK) pathway. Acupuncture also decreases the levels of linterleukin-6 and tumor necrosis factor-α, which are involved in insulin resistance.
Glucose and lipid metabolism dysfunctions are found in most obese PCOS patients (110). In a clinical study, acupuncture treatment decreased miR-32-3p levels and increased the expression of PLA2G4A, leading to improvement in PCOS patients with diabetes (65). Gene expression and methylation were analyzed to reveal the mechanism of EA on glucose metabolism dysfunctions in PCOS patients. The results showed that EA regulated gene expression (such as MSX1 and SRNX1) in skeletal muscle in insulin-resistant overweight/obese PCOS women (55). Interestingly, EA increased LDL cholesterol without affecting insulin sensitivity or adipose tissue function in a rat model of PCOS, which might suggest that a balance of sex hormones is necessary to restore metabolic function (47). The same research team also found that EA improved insulin sensitivity and decreased total high-density lipoprotein and LDL cholesterol in the same PCOS models (32). The protein expression of GLUT4 was found to be increased in skeletal muscle, which may be involved in the mechanism of EA on insulin sensitivity. In addition, the gut microbiota is known to be causal in the development of obesity/insulin resistance (111). A recent study showed that EA intervention decreased body weight, probably through regulating gut microbiota in PCOS rats (112). This study also demonstrated that EA can normalize visceral and subcutaneous fat content, brown adipose tissue weight, and glucose tolerance in the PCOS model.
An increased risk of depression and anxiety has been found in patients with PCOS (113, 114). It has been reported that acupuncture can improve depression and anxiety scores in women with PCOS (37). Interestingly, a recent study revealed that EA appears to improve symptoms of anxiety and depression and regulate the serum levels of norepinephrine (NE) and serotonin (5-HT) in unmarried PCOS patients (52). Anxiety and depression are associated with an autonomic nervous system imbalance (115). Hyperactivation of the sympathetic nervous system is involved in many psychological disorders, such as anxiety and depression (116). Chronic sympathetic overactivity also plays a critical role in the pathogenesis of PCOS (117). A previous clinical study showed that low-frequency EA decreased high muscle sympathetic nerve activity in PCOS patients (31). In addition, another study showed that the efficiency of EA was associated with activation of the sympathetic nervous system (48). In a PCOS rat model, low-frequency EA and physical exercise restored the ovarian expression of markers of sympathetic nervous system activity (30). EA intervention inhibited hyperactivity of the sympathetic nervous system in PCOS rats, which may be related to the inhibitory effects of EA on nerve growth factor (NGF) concentrations in ovaries (24). In addition, the p75 neurotrophin receptor (p75NTR) plays a vital role in patterning the sympathetic nervous system during development (118). EA prevented the increase in p75NTR expression, probably by normalizing the sympathetic ovarian response to NGF action (27). Interestingly, the effect of EA on NGF abundance was only found in the ovaries of PCOS rats, but not in the brain (Figure 4) (26).
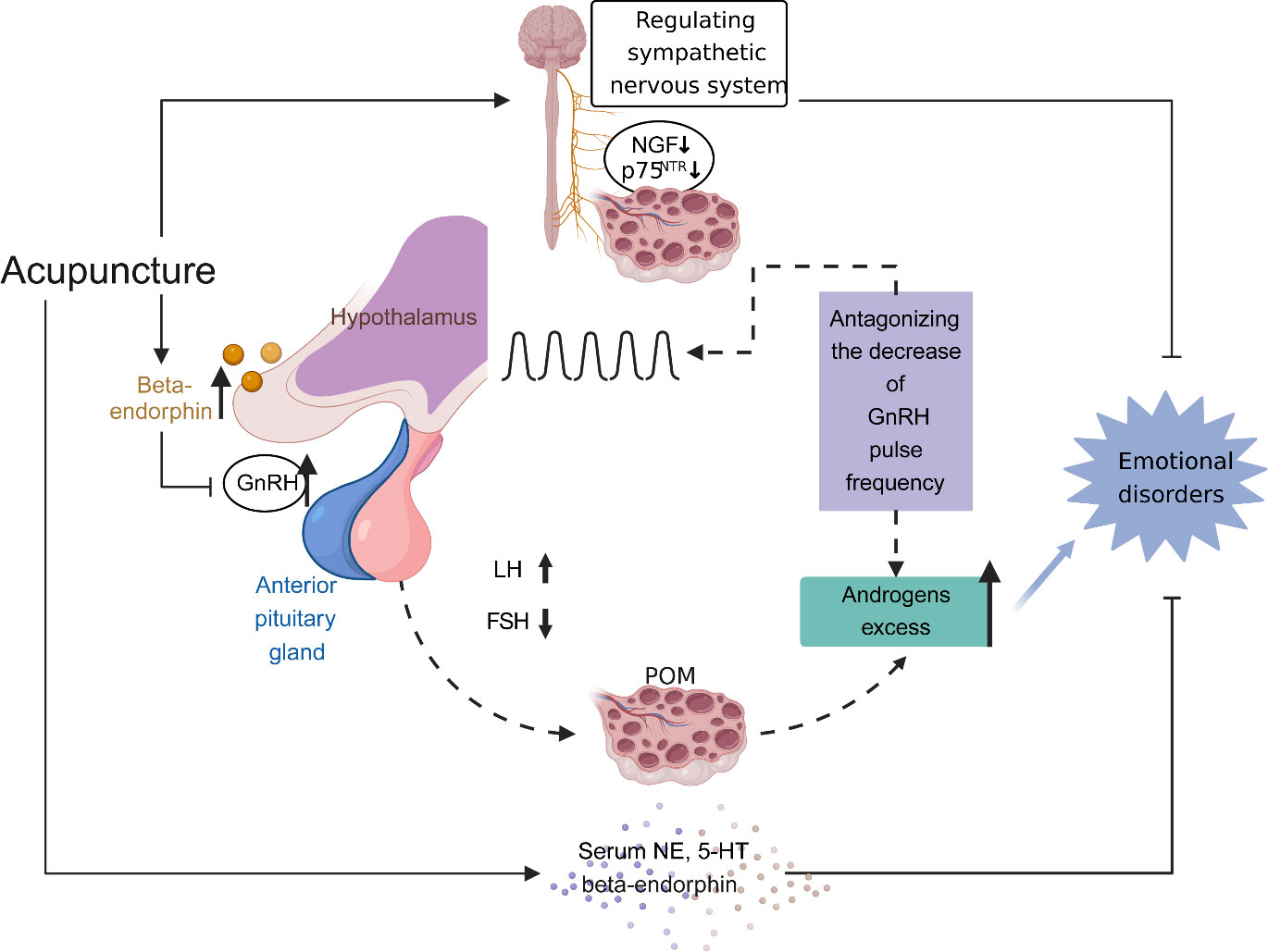
Figure 4 The effect of acupuncture on restoration of the hypothalamic-pituitary-ovarian (HPO) axis and amelioration of emotional disorders. Increased activity and secretion of gonadotropin-releasing hormone (GnRH) with persistently high pulse frequency causes elevated levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), contributing to the polycystic ovarian pathology (including impaired follicular development and excess androgen production). Elevated ovarian androgen antagonizes the ability of the progesterone to descend GnRH pulse frequency, leading to a proposed vicious cycle in the HPO axis in polycystic ovary syndrome (PCOS). Meanwhile, androgen excess has an effect on emotion disorders of women with PCOS. Acupuncture modulates central and peripheral β‐endorphin production and secretion, influencing the release of GnRH, then normalising the ratio of LH and FSH, and eventually normalising the HPO axis. Moreover, acupuncture ameliorates emotion disorders of women with PCOS through regulating serum norepinephrine, 5-hydroxytryptamine and β‐endorphin levels, balancing autonomic nervous system, and inhibiting the concentrations of nerve growth factor and the expression of p75 neurotrophin receptor in ovaries.
The conception of the acupoint is introduced in Traditional Chinese Medicine (TCM) as the matter that acupuncture acts on the body physiology and relieves symptoms. Increasing evidence has suggested that acupoints are mostly collagen fiber-rich regions, such as intermuscular connective tissue, peri-neurovascular connective tissue, and organ portal and peri-neural connective tissue (26). Moreover, acupoints on different meridians have different effects.
Acupoints SP6, ST29, CV6, LI4, CV3, ST36, SP9, and CV4 have been frequently used in these scientific studies (Figures 5, 6). In clinical applications, the most widely used acupoint was SP6, as it had been selected in sixteen researches. According to the theory of TCM, SP6 (Sanyinjiao) is mainly characterized by the ability to nourish organs, activate blood, soothe the liver and regulate Qi, which can contribute to addressing gynecological problems. The second most involved acupoint was ST29 (Guilai), which was mentioned in fifteen studies. It promotes circulation to remove stasis, regulate menstruation and relieve pain. In addition, CV6, LI4, CV3, ST36, SP9, and CV4 were used in at least eight studies for treating women with PCOS.
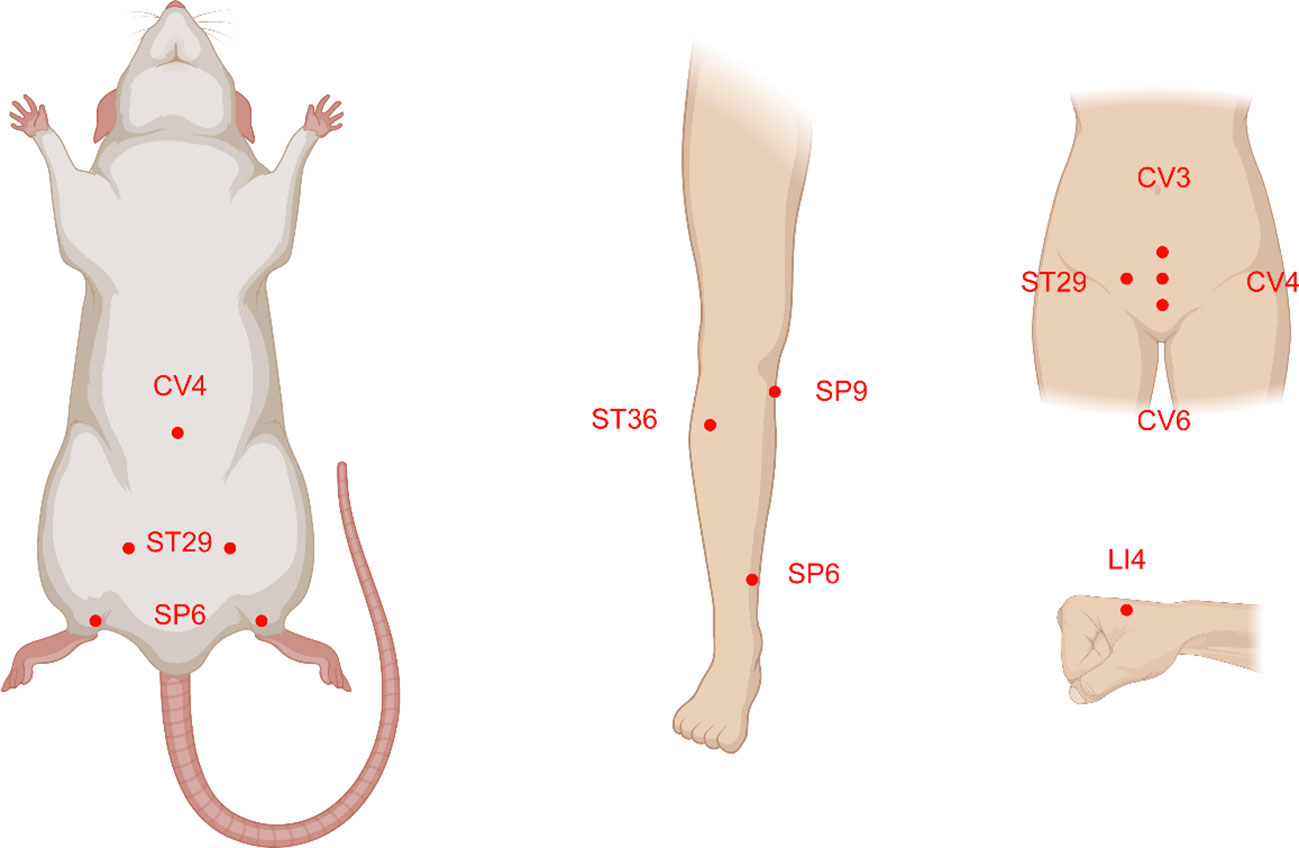
Figure 5 The acupoints common selected and their location distributions in animals and humans with polycystic ovary syndrome (PCOS). The majority of the acupoints are located on the abdomen, upper and lower extremities.
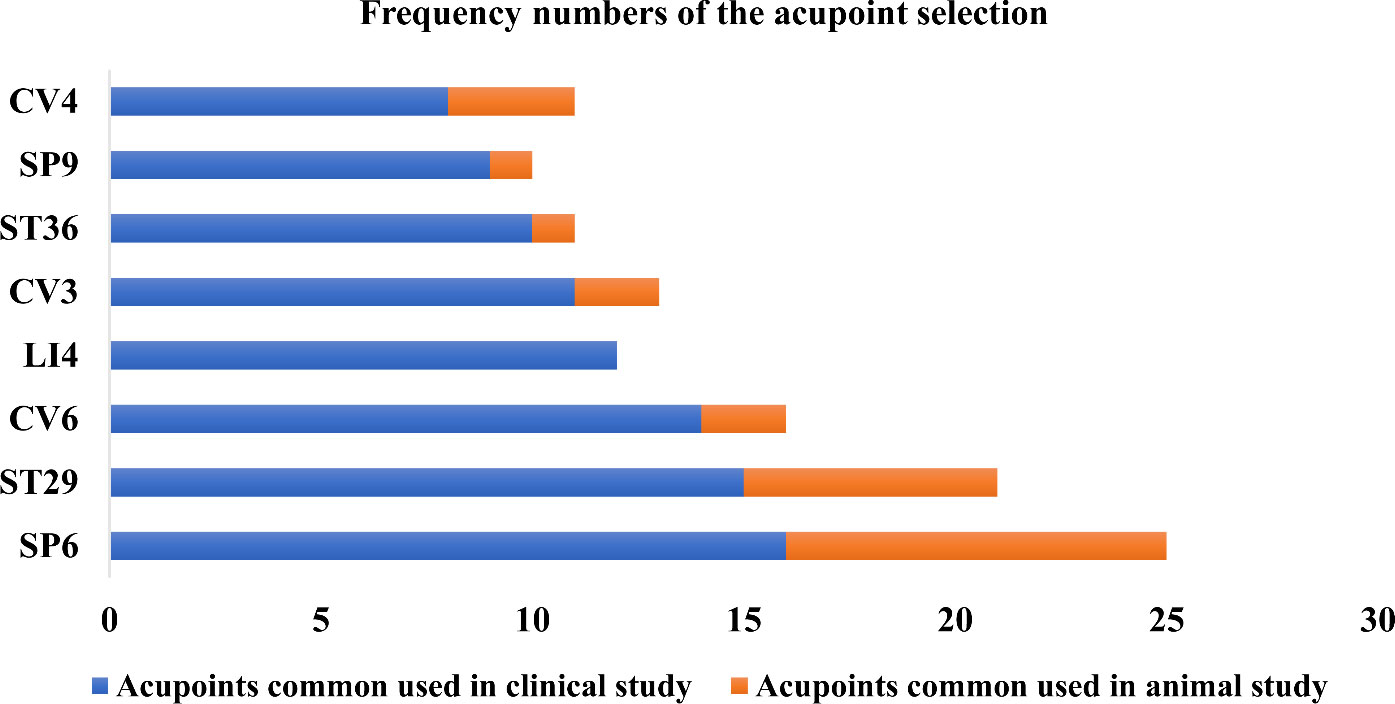
Figure 6 The use frequency of acupoints. SP6 (Sanyinjiao) and ST29 (Guilai) are the most frequently stimulated two acupoints both in animals and humans with polycystic ovary syndrome.
Compared with clinical trials, fewer acupoints were stimulated in animal researches. EA was often applied for androgen excess-induced PCOS rat/mouse models. Among the acupoints selected for treatment, SP6 acupoint was the most commonly chosen, either alone or in association with other acupoints, which was consistent with the findings of clinical studies. Additionally, ST29 was more frequently used to mitigate hyperandrogenism and ovulatory dysfunction and to modulate the menstrual cycle. CV4 and ST36 were also frequently used to treat animals with PCOS. Acupoint ST36, in particular, has the ability to tonify Qi and circulation. It was demonstrated that acupuncture at ST36 could lower the levels of IL-6 and tumor necrosis factor (TNF-α) in PCOS animal serum, which might be associated with the ability of acupuncture to inhibit inflammation and oxidative stress (119).
PCOS is a common but heterogeneous disease with symptoms that vary from age to age in patients, typically featuring chronic oligo-anovulation, hyperandrogenism, and/or metabolic disturbance (120). The routine administration after recommending lifestyle modification and some adscititious tips is symptomatic therapy with various agents (121, 122). Patients with PCOS often suffer from a high symptom burden but low tolerance and compliance to pharmacotherapy. Additional regimens need to be explored. With the building availability of acupuncture all over the world, patients with PCOS are increasingly seeking and accepting acupuncture to maintain reproductive health (91). Our previous research manifested that acupuncture can effectively relieve anxiety and depression in patients with PCOS, and its mechanism may be related to the regulation of the levels of serum β-endorphin and androgen (123). In addition, our multinational study protocol on acupuncture or metformin to improve insulin resistance in women with PCOS has been published (124). In recent years, there have been an increasing number of systematic reviews and/or meta-analyses on the effect of acupuncture on PCOS in both patients and animal models (22, 23, 96, 100). This study reviewed the feasibility and efficacy of acupuncture for managing PCOS and summarized the potential mechanisms of acupuncture on treating PCOS. To the best of our knowledge, this is the first detailed review to address acupuncture-specific action on PCOS-related symptoms, including ovulatory dysfunction, hyperandrogenism, insulin resistance, obesity, and emotional disorders.
The disordered hypothalamic–pituitary–ovarian/adrenal axis is one of the most important pathological and physiological states of PCOS (125). As the main metabolic feature of PCOS, insulin resistance is considered to be a crucial pathophysiological basis for the pathogenesis of PCOS (107). Inhibiting phosphoinositide-3 kinase and phosphorylation of IRS-1 impairs insulin signaling by affecting GLUT-4 expression and glucose uptake (126, 127). Another vital pro-inflammatory agent relevant to the pathogenesis of PCOS is adipose tissue (128). It has been proven that adipose tissue-resident macrophages lead to the release of TNF-α and IL-6, which are implicated in the induction of insulin resistance (129). Hyperandrogenism causes the aberration of adipose tissue functions in PCOS. Insulin resistance, hyperandrogenism, chronic low-grade inflammation, and adipose tissue hypertrophy and dysfunction may affect a vicious cycle in the pathophysiology of PCOS (130, 131). Evidence has shown that acupuncture elevates the level of β-endorphin not only in the central endocrine system but also in the peripheral circulation (94), which is associated with both direct and indirect tonic inhibitions of GnRH and subsequent LH release (132). Aberrant sympathetic neurogenic regulation of the ovary is involved in the pathogenesis of PCOS (23), and acupuncture can also inhibit the overexpression of NGF to decrease sympathetic activity, resulting in a restoration to the normal level of the ovarian steroid response to gonadotropins (25). Moreover, acupuncture regulates the phosphorylation of insulin substrates and receptors and inhibits the abnormal expression of signaling pathways, thereby improving metabolic dysfunction such as insulin resistance (32, 56, 133). Acupuncture may also ameliorate cholesterol metabolism, affecting lipid metabolism enzyme activity, inhibiting the synthesis of fatty acids, and then promoting fat decomposition and energy metabolism (100, 134).
Many review articles concerning PCOS and acupuncture have been published during the last 10 years (23, 132, 135–137). However, our present paper is different from those published papers. Firstly, this review summarized current available information from both clinical studies and animal studies. Secondly, the mechanisms of acupuncture on PCOS-related main symptoms (ovulatory dysfunction, hyperandrogenism, insulin resistance, obesity, and negative emotion) were all overviewed. Thirdly, the acupoints that commonly used in PCOS patients and animals were also overviewed in this study. To our knowledge, this is the most comprehensive review that summarized current progress on acupuncture treatment for PCOS.
Some points should be noted. At present, most studies on the effect of acupuncture on PCOS are statistical comparisons, with insufficient depth and breadth of its mechanism of action. System biology and omics techniques have become a new trend, and transcriptomics technology will better analyze the specific expression factors and biological mechanisms of acupuncture treatment. Additionally, there is considerable heterogeneity in terms of animal models (dihydrotestosterone, dehydroepiandrosterone, and testosterone propionate), research intervention (acupoint selection, frequency, electrical current range, pulse width and length of stimulation) and major endpoints (live birth, multiple pregnancy rate, ovulation rate, clinical pregnancy rate, and miscarriage rate), lessening the generalizability of the results from those studies. Moreover, the majority of these studies were conducted in various phenotypes of patients and animal models with PCOS. Although the pathophysiology of the symptoms is similar between several phenotypes and models, there could be differences, making data from one not entirely applicable to the other. As a recent review reported, clinical practice and health policy underuse beneficial acupuncture therapies.
At present, a large number of clinical studies have confirmed that acupuncture could improve many symptoms in patients with PCOS. These symptoms include chronic and continuous anovulation, hyperandrogenemia, insulin resistance, negative emotion, glucose and lipid metabolism dysfunction, etc. The effect of acupuncture may be induced by stimulating muscle conduction and chemical signals to induce the central release of key factors through sympathetic nerve conduction and then regulating the female reproductive axis. The selection of acupoints and EA frequency may also impact the therapeutic effect, which needs to be verified by more studies.
With recent advances in technology, the effect of acupuncture could be observed from a more microscopic point of view. Whether the clinical effect of acupuncture is associated with the traditional Chinese meridian theory is still unclear. This needs to be verified and discussed in the next few decades. Clarifying the mechanism of acupuncture in the treatment of PCOS will help to make acupuncture therapy accepted by more people.
YY and C-CZ contributed to writing the original draft, literature search, and data collection; H-QH contributed to figure presentation and manuscript editing; IF contributed to writing, corrections, and editing; H-LZ contributed to conceiving, designing, editing, and supervising. All authors contributed to this article and approved this submitted version.
The work was supported by the Special Grant for Capital Health Research and Development (Grant No. 2022-2-4098), National Natural Science Foundation of China (Grant No. 82174151), Peking University Third Hospital “Key Young Talents” Training Program (Grant No. BYSYFY2021032). The funders have had no role in study design, and will not have any role in data collection and analysis, decision to publish, or preparation of the manuscripts.
We sincerely thank everyone in the department of traditional Chinese medicine, Peking University Third Hospital for discussion and constructive criticism. we would like to thank the BioRender for figure making.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Shen H, Xu X, Fu Z, Xu C, Wang Y. The interactions of CAP and LYN with the insulin signaling transducer CBL play an important role in polycystic ovary syndrome. Metabolism (2022) 131:155164. doi: 10.1016/j.metabol.2022.155164
2. Szeliga A, Rudnicka E, Maciejewska-Jeske M, Kucharski M, Kostrzak A, Hajbos M, et al. Neuroendocrine determinants of polycystic ovary syndrome. Int J Environ Res Public Health (2022) 19:3089. doi: 10.3390/ijerph19053089
3. Palomba S. Is fertility reduced in ovulatory women with polycystic ovary syndrome? an opinion paper. Hum Reprod (2021) 36:2421–8. doi: 10.1093/humrep/deab181
4. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol (2018) 14:270–84. doi: 10.1038/nrendo.2018.24
5. Zhang J, Xin X, Zhang H, Zhu Y, Ye Y, Li D. The efficacy of Chinese herbal medicine in animal models of polycystic ovary syndrome: A systematic review and meta-analysis. Evid Based Complement Alternat Med (2022) 2022:4892215. doi: 10.1155/2022/4892215
6. Chen Z, Liu L, Xi X, Burn M, Karakaya C, Kallen AN. Aberrant H19 expression disrupts ovarian Cyp17 and testosterone production and is associated with polycystic ovary syndrome in women. Reprod Sci (2022) 29:1357–67. doi: 10.1007/s43032-021-00700-5
7. Schoretsanitis G, Gastaldon C, Kalaitzopoulos DR, Ochsenbein-Koelble N, Barbui C, Seifritz E. Polycystic ovary syndrome and postpartum depression: A systematic review and meta-analysis of observational studies. J Affect Disord (2022) 299:463–9. doi: 10.1016/j.jad.2021.12.044
8. Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc Med (2020) 30:399–404. doi: 10.1016/j.tcm.2019.08.010
9. Palomba S, De Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update (2015) 21:575–92. doi: 10.1093/humupd/dmv029
10. Palomba S, Piltonen TT, Giudice LC. Endometrial function in women with polycystic ovary syndrome: a comprehensive review. Hum Reprod Update (2021) 27:584–618. doi: 10.1093/humupd/dmaa051
11. Calcaterra V, Verduci E, Cena H, Magenes VC, Todisco CF, Tenuta E, et al. Polycystic ovary syndrome in insulin-resistant adolescents with obesity: The role of nutrition therapy and food supplements as a strategy to protect fertility. Nutrients (2021) 13:1848. doi: 10.3390/nu13061848
12. Peng Y, Guo L, Gu A, Shi B, Ren Y, Cong J, et al. Electroacupuncture alleviates polycystic ovary syndrome-like symptoms through improving insulin resistance, mitochondrial dysfunction, and endoplasmic reticulum stress via enhancing autophagy in rats. Mol Med (2020) 26:73. doi: 10.1186/s10020-020-00198-8
13. Billhult A, Stener-Victorin E. Acupuncture with manual and low frequency electrical stimulation as experienced by women with polycystic ovary syndrome: A qualitative study. BMC Complement Altern Med (2012) 12:32. doi: 10.1186/1472-6882-12-32
14. Ersahin AA, Caliskan E. Clomiphene citrate changes metabolite content of follicular fluid of PCOS women. Eur Rev Med Pharmacol Sci (2018) 22:4359–62. doi: 10.26355/eurrev_201807_15434
15. Wang R, Li W, Bordewijk EM, Legro RS, Zhang H, Wu X, et al. First-line ovulation induction for polycystic ovary syndrome: An individual participant data meta-analysis. Hum Reprod Update (2019) 25:717–32. doi: 10.1093/humupd/dmz029
16. Vaklavas C, Roberts BS, Varley KE, Lin NU, Liu MC, Rugo HS, et al. TBCRC 002: a phase II, randomized, open-label trial of preoperative letrozole with or without bevacizumab in postmenopausal women with newly diagnosed stage 2/3 hormone receptor-positive and HER2-negative breast cancer. Breast Cancer Res (2020) 22:22. doi: 10.1186/s13058-020-01258-x
17. Palomba S, Falbo A, Zullo F, Orio F Jr. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: A comprehensive review. Endocr Rev (2009) 30:1–50. doi: 10.1210/er.2008-0030
18. Ma RL, Deng Y, Wang YF, Zhu SY, Ding XS, Sun AJ. Short-term combined treatment with exenatide and metformin for overweight/obese women with polycystic ovary syndrome. Chin Med J (Engl) (2021) 134:2882–9. doi: 10.1097/CM9.0000000000001712
19. Glintborg D, Mumm H, Holst JJ, Andersen M. Effect of oral contraceptives and/or metformin on GLP-1 secretion and reactive hypoglycaemia in polycystic ovary syndrome. Endocr Connect (2017) 6:267–77. doi: 10.1530/EC-17-0034
20. Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update (2016) 22:687–708. doi: 10.1093/humupd/dmw025
21. Lu L, Zhang Y, Tang X, Ge S, Wen H, Zeng J, et al. Evidence on acupuncture therapies is underused in clinical practice and health policy. BMJ (2022) 376:e067475. doi: 10.1136/bmj-2021-067475
22. Wu J, Chen D, Liu N. Effectiveness of acupuncture in polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Med (Baltimore) (2020) 99:e20441. doi: 10.1097/MD.0000000000020441
23. Li Y, Zhang L, Gao J, Yan J, Feng X, He X, et al. Effect of acupuncture on polycystic ovary syndrome in animal models: A systematic review. Evid Based Complement Alternat Med (2021) 2021:5595478. doi: 10.1155/2021/5595478
24. Stener-Victorin E, Lundeberg T, Waldenstrom U, Manni L, Aloe L, Gunnarsson S, et al. Effects of electro-acupuncture on nerve growth factor and ovarian morphology in rats with experimentally induced polycystic ovaries. Biol Reprod (2000) 63:1497–503. doi: 10.1095/biolreprod63.5.1497
25. Stener-Victorin E, Lundeberg T, Cajander S, Aloe L, Manni L, Waldenstrom U, et al. Steroid-induced polycystic ovaries in rats: effect of electro-acupuncture on concentrations of endothelin-1 and nerve growth factor (NGF), and expression of NGF mRNA in the ovaries, the adrenal glands, and the central nervous system. Reprod Biol Endocrinol (2003) 1:33. doi: 10.1186/1477-7827-1-33
26. Bai YH, Lim SC, Song CH, Bae CS, Jin CS, Choi BC, et al. Electro-acupuncture reverses nerve growth factor abundance in experimental polycystic ovaries in the rat. Gynecol Obstet Invest (2004) 57:80–5. doi: 10.1159/000075382
27. Manni L, Lundeberg T, Holmang A, Aloe L, Stener-Victorin E. Effect of electro-acupuncture on ovarian expression of alpha (1)- and beta (2)-adrenoceptors, and p75 neurotrophin receptors in rats with steroid-induced polycystic ovaries. Reprod Biol Endocrinol (2005) 3:21. doi: 10.1186/1477-7827-3-21
28. Manneras L, Jonsdottir IH, Holmang A, Lonn M, Stener-Victorin E. Low-frequency electro-acupuncture and physical exercise improve metabolic disturbances and modulate gene expression in adipose tissue in rats with dihydrotestosterone-induced polycystic ovary syndrome. Endocrinology (2008) 149:3559–68. doi: 10.1210/en.2008-0053
29. Feng Y, Johansson J, Shao R, Manneras L, Fernandez-Rodriguez J, Billig H, et al. Hypothalamic neuroendocrine functions in rats with dihydrotestosterone-induced polycystic ovary syndrome: effects of low-frequency electro-acupuncture. PloS One (2009) 4:e6638. doi: 10.1371/journal.pone.0006638
30. Manneras L, Cajander S, Lonn M, Stener-Victorin E. Acupuncture and exercise restore adipose tissue expression of sympathetic markers and improve ovarian morphology in rats with dihydrotestosterone-induced PCOS. Am J Physiol Regul Integr Comp Physiol (2009) 296:R1124–31. doi: 10.1152/ajpregu.90947.2008
31. Stener-Victorin E, Jedel E, Janson PO, Sverrisdottir YB. Low-frequency electroacupuncture and physical exercise decrease high muscle sympathetic nerve activity in polycystic ovary syndrome. Am J Physiol Regul Integr Comp Physiol (2009) 297:R387–95. doi: 10.1152/ajpregu.00197.2009
32. Johansson J, Feng Y, Shao R, Lonn M, Billig H, Stener-Victorin E. Intense electroacupuncture normalizes insulin sensitivity, increases muscle GLUT4 content, and improves lipid profile in a rat model of polycystic ovary syndrome. Am J Physiol Endocrinol Metab (2010) 299:E551–9. doi: 10.1152/ajpendo.00323.2010
33. Jedel E, Labrie F, Oden A, Holm G, Nilsson L, Janson PO, et al. Impact of electro-acupuncture and physical exercise on hyperandrogenism and oligo/amenorrhea in women with polycystic ovary syndrome: A randomized controlled trial. Am J Physiol Endocrinol Metab (2011) 300:E37–45. doi: 10.1152/ajpendo.00495.2010
34. Pastore LM, Williams CD, Jenkins J, Patrie JT. True and sham acupuncture produced similar frequency of ovulation and improved LH to FSH ratios in women with polycystic ovary syndrome. J Clin Endocrinol Metab (2011) 96:3143–50. doi: 10.1210/jc.2011-1126
35. Feng Y, Johansson J, Shao R, Manneras-Holm L, Billig H, Stener-Victorin E. Electrical and manual acupuncture stimulation affect oestrous cyclicity and neuroendocrine function in an 5alpha-dihydrotestosterone-induced rat polycystic ovary syndrome model. Exp Physiol (2012) 97:651–62. doi: 10.1113/expphysiol.2011.063131
36. Franasiak J, Young SL, Williams CD, Pastore LM. Longitudinal anti-mullerian hormone in women with polycystic ovary syndrome: an acupuncture randomized clinical trial. Evid Based Complement Alternat Med (2012) 2012:973712. doi: 10.1155/2012/973712
37. Stener-Victorin E, Holm G, Janson PO, Gustafson D, Waern M. Acupuncture and physical exercise for affective symptoms and health-related quality of life in polycystic ovary syndrome: secondary analysis from a randomized controlled trial. BMC Complement Altern Med (2013) 13:131. doi: 10.1186/1472-6882-13-131
38. Johansson J, Manneras-Holm L, Shao R, Olsson A, Lonn M, Billig H, et al. Electrical vs manual acupuncture stimulation in a rat model of polycystic ovary syndrome: Different effects on muscle and fat tissue insulin signaling. PloS One (2013) 8:e54357. doi: 10.1371/journal.pone.0054357
39. Rashidi BH, Tehrani ES, Hamedani NA, Pirzadeh L. Effects of acupuncture on the outcome of in vitro fertilisation and intracytoplasmic sperm injection in women with polycystic ovarian syndrome. Acupunct Med (2013) 31:151–6. doi: 10.1136/acupmed-2012-010198
40. Yu L, Liao Y, Wu H, Zhao J, Wu L, Shi Y, et al. Effects of electroacupuncture and Chinese kidney-nourishing medicine on polycystic ovary syndrome in obese patients. J Tradit Chin Med (2013) 33:287–93. doi: 10.1016/s0254-6272(13)60166-1
41. Johansson J, Redman L, Veldhuis PP, Sazonova A, Labrie F, Holm G, et al. Acupuncture for ovulation induction in polycystic ovary syndrome: a randomized controlled trial. Am J Physiol Endocrinol Metab (2013) 304:E934–43. doi: 10.1152/ajpendo.00039.2013
42. Sun J, Jin C, Wu H, Zhao J, Cui Y, Liu H, et al. Effects of electro-acupuncture on ovarian P450arom, P450c17alpha and mRNA expression induced by letrozole in PCOS rats. PloS One (2013) 8:e79382. doi: 10.1371/journal.pone.0079382
43. Zheng YH, Wang XH, Lai MH, Yao H, Liu H, Ma HX. Effectiveness of abdominal acupuncture for patients with obesity-type polycystic ovary syndrome: A randomized controlled trial. J Altern Complement Med (2013) 19:740–5. doi: 10.1089/acm.2012.0429
44. Stener-Victorin E, Maliqueo M, Soligo M, Protto V, Manni L, Jerlhag E, et al. Changes in HbA1c and circulating and adipose tissue androgen levels in overweight-obese women with polycystic ovary syndrome in response to electroacupuncture. Obes Sci Pract (2016) 2:426–35. doi: 10.1002/osp4.78
45. Ramadoss M, Ramanathan G, Subbiah AJ, Natrajan C. Heart rate changes in electroacupuncture treated polycystic ovary in rats. J Clin Diagn Res (2016) 10:CF01–3. doi: 10.7860/JCDR/2016/18303.7395
46. Benrick A, Kokosar M, Hu M, Larsson M, Maliqueo M, Marcondes RR, et al. Autonomic nervous system activation mediates the increase in whole-body glucose uptake in response to electroacupuncture. FASEB J (2017) 31:3288–97. doi: 10.1096/fj.201601381R
47. Maliqueo M, Benrick A, Marcondes RR, Johansson J, Sun M, Stener-Victorin E. Acupuncture does not ameliorate metabolic disturbances in the P450 aromatase inhibitor-induced rat model of polycystic ovary syndrome. Exp Physiol (2017) 102:113–27. doi: 10.1113/EP085983
48. Kokosar M, Benrick A, Perfilyev A, Nilsson E, Kallman T, Ohlsson C, et al. A single bout of electroacupuncture remodels epigenetic and transcriptional changes in adipose tissue in polycystic ovary syndrome. Sci Rep (2018) 8:1878. doi: 10.1038/s41598-017-17919-5
49. Ma T, Cui P, Tong X, Hu W, Shao LR, Zhang F, et al. Endogenous ovarian angiogenesis in polycystic ovary syndrome-like rats induced by low-frequency electro-acupuncture: The CLARITY three-dimensional approach. Int J Mol Sci (2018) 19:3500. doi: 10.3390/ijms19113500
50. Cui P, Ma T, Tamadon A, Han S, Li B, Chen Z, et al. Hypothalamic DNA methylation in rats with dihydrotestosterone-induced polycystic ovary syndrome: effects of low-frequency electro-acupuncture. Exp Physiol (2018) 103:1618–32. doi: 10.1113/EP087163
51. Shi Y, Li L, Zhou J, Sun J, Chen L, Zhao J, et al. Efficacy of electroacupuncture in regulating the imbalance of AMH and FSH to improve follicle development and hyperandrogenism in PCOS rats. BioMed Pharmacother (2019) 113:108687. doi: 10.1016/j.biopha.2019.108687
52. Wang Z, Dong H, Wang Q, Zhang L, Wu X, Zhou Z, et al. Effects of electroacupuncture on anxiety and depression in unmarried patients with polycystic ovarian syndrome: secondary analysis of a pilot randomised controlled trial. Acupunct Med (2019) 37:40–6. doi: 10.1136/acupmed-2017-011615
53. Budihastuti UR, Melinawati E, Sulistyowati S, Nurwati I. Electroacupuncture effect on polycystic ovary syndrome to improve oocytes’ growth. Med Acupunct (2019) 31:379–83. doi: 10.1089/acu.2019.1354
54. Rouhani M, Motavasselian M, Taghipoor A, Layegh P, Asili J, Hamedi SS, et al. Efficacy of a Persian herbal remedy and electroacupuncture on metabolic profiles and anthropometric parameters in women with polycystic ovary syndrome: A randomized controlled trial. Galen Med J (2019) 8:e1389. doi: 10.31661/gmj.v8i0.1389
55. Benrick A, Pillon NJ, Nilsson E, Lindgren E, Krook A, Ling C, et al. Electroacupuncture mimics exercise-induced changes in skeletal muscle gene expression in women with polycystic ovary syndrome. J Clin Endocrinol Metab (2020) 105:2027–41. doi: 10.1210/clinem/dgaa165
56. Peng Y, Yang X, Luo X, Liu C, Cao X, Wang H, et al. Novel mechanisms underlying anti-polycystic ovary like syndrome effects of electroacupuncture in rats: Suppressing SREBP1 to mitigate insulin resistance, mitochondrial dysfunction and oxidative stress. Biol Res (2020) 53:50. doi: 10.1186/s40659-020-00317-z
57. Li J, Wu W, Stener-Victorin E, Ng EHY, Li RHW, Li M, et al. A prospective pilot study of the effect of acupuncture on insulin sensitivity in women with polycystic ovary syndrome and insulin resistance. Acupunct Med (2020) 38:310–8. doi: 10.1177/0964528420902144
58. Tong X, Liu Y, Xu X, Shi J, Hu W, Ma T, et al. Ovarian innervation coupling with vascularity: The role of electro-acupuncture in follicular maturation in a rat model of polycystic ovary syndrome. Front Physiol (2020) 11:474. doi: 10.3389/fphys.2020.00474
59. Xu G, Zhang A, Liu J, Wang X, Feng J, Chen Y. Effects of electroacupuncture on ovarian expression of the androgen receptor and connexin 43 in rats with letrozole-induced polycystic ovaries. Evid Based Complement Alternat Med (2020) 2020:3608062. doi: 10.1155/2020/3608062
60. Budihastuti UR, Melinawati E, Anggraini NWP, Anggraeni A, Yuliantara EE, Sulistyowati S, et al. Electroacupuncture to improve endometrial receptivity and folliculogenesis in polycystic ovary syndrome. Med Acupunct (2021) 33:428–34. doi: 10.1089/acu.2020.1503
61. Chen X, Tang H, Liang Y, Wu P, Xie L, Ding Y, et al. Acupuncture regulates the autophagy of ovarian granulosa cells in polycystic ovarian syndrome ovulation disorder by inhibiting the PI3K/AKT/mTOR pathway through LncMEG3. BioMed Pharmacother (2021) 144:112288. doi: 10.1016/j.biopha.2021.112288
62. Dong HX, Wang Q, Wang Z, Wu XK, Cheng L, Zhou ZM, et al. Impact of low frequency electro-acupuncture on glucose and lipid metabolism in unmarried PCOS women: A randomized controlled trial. Chin J Integr Med (2021) 27:737–43. doi: 10.1007/s11655-021-3482-z
63. Wang Z, Yang L, Dong H, Dong H, Cheng L, Yi P, et al. Effect of electroacupuncture on the kisspeptin system in a pubertal rat model of polycystic ovary syndrome. Acupunct Med (2021) 39:491–500. doi: 10.1177/0964528420971299
64. Xiang S, Xia MF, Song JY, Liu DQ, Lian F. Effect of electro-acupuncture on expression of IRS-1/PI3K/GLUT4 pathway in ovarian granulosa cells of infertile patients with polycystic ovary syndrome-insulin resistance of phlegm-dampness syndrome. Chin J Integr Med (2021) 27:330–5. doi: 10.1007/s11655-020-3219-z
65. Wu J, Chen X. Acupuncture therapy protects PCOS patients with diabetes by regulating miR-32-3p/PLA2G4A pathway. Am J Transl Res (2021) 13:8819–32.
66. Zhao QY, Sun Y, Zhou J, Gao YL, Ma GZ, Hu ZH, et al. Effectiveness of herb-partitioned moxibustion combined with electroacupuncture on polycystic ovary syndrome in patients with symptom pattern of kidney deficiency and phlegm-dampne. J Tradit Chin Med (2021) 41:985–93. doi: 10.19852/j.cnki.jtcm.2021.06.017
67. Dong H, Wang Q, Cheng L, Wang Z, Wu X, Zhou Z, et al. Effect of low-frequency electro-acupuncture in unmarried women with polycystic ovary syndrome: A randomized controlled study. Altern Ther Health Med (2022) 28:24–33.
68. Pan W, Li FX, Wang Q, Huang ZQ, Yan YM, Zhao L, et al. A randomized sham-controlled trial of manual acupuncture for infertile women with polycystic ovary syndrome. Integr Med Res (2022) 11:100830. doi: 10.1016/j.imr.2021.100830
69. Zhang F, Ma T, Tong X, Liu Y, Cui P, Xu X, et al. Electroacupuncture improves metabolic and ovarian function in a rat model of polycystic ovary syndrome by decreasing white adipose tissue, increasing brown adipose tissue, and modulating the gut microbiota. Acupunct Med (2022) 40:347–59. doi: 10.1177/09645284211056663
70. Liao B, Qi X, Yun C, Qiao J, Pang Y. Effects of androgen excess-related metabolic disturbances on granulosa cell function and follicular development. Front Endocrinol (Lausanne) (2022) 13:815968. doi: 10.3389/fendo.2022.815968
71. Rodriguez Paris V, Bertoldo MJ. The mechanism of androgen actions in PCOS etiology. Med Sci (Basel) (2019) 7:89. doi: 10.3390/medsci7090089
72. Ryu Y, Kim SW, Kim YY, Ku SY. Animal models for human polycystic ovary syndrome (PCOS) focused on the use of indirect hormonal perturbations: A review of the literature. Int J Mol Sci (2019) 20:2720. doi: 10.3390/ijms20112720
73. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): The hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev (2016) 37:467–520. doi: 10.1210/er.2015-1104
74. Yi Y, Liu J, Xu W. Naringenin and morin reduces insulin resistance and endometrial hyperplasia in the rat model of polycystic ovarian syndrome through enhancement of inflammation and autophagic apoptosis. Acta Biochim Pol (2022) 69:91–100. doi: 10.18388/abp.2020_5722
75. Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: Possible implications in prenatal androgenization. Hum Reprod (2002) 17:2573–9. doi: 10.1093/humrep/17.10.2573
76. Pani A, Gironi I, Di Vieste G, Mion E, Bertuzzi F, Pintaudi B. From prediabetes to type 2 diabetes mellitus in women with polycystic ovary syndrome: Lifestyle and pharmacological management. Int J Endocrinol (2020) 2020:6276187. doi: 10.1155/2020/6276187
77. Xu Y, Qiao J. Association of insulin resistance and elevated androgen levels with polycystic ovarian syndrome (PCOS): A review of literature. J Healthc Eng (2022) 2022:9240569. doi: 10.1155/2022/9240569
78. Singh A, Choubey M, Bora P, Krishna A. Adiponectin and chemerin: Contrary adipokines in regulating reproduction and metabolic disorders. Reprod Sci (2018) 25:1462–73. doi: 10.1177/1933719118770547
79. Behboudi-Gandevani S, Ramezani Tehrani F, Bidhendi Yarandi R, Noroozzadeh M, Hedayati M, Azizi F. The association between polycystic ovary syndrome, obesity, and the serum concentration of adipokines. J Endocrinol Invest (2017) 40:859–66. doi: 10.1007/s40618-017-0650-x
80. Moran LJ, Teede HJ, Noakes M, Clifton PM, Norman RJ, Wittert GA. Sex hormone binding globulin, but not testosterone, is associated with the metabolic syndrome in overweight and obese women with polycystic ovary syndrome. J Endocrinol Invest (2013) 36:1004–10. doi: 10.3275/9023
81. Dapas M, Sisk R, Legro RS, Urbanek M, Dunaif A, Hayes MG. Family-based quantitative trait meta-analysis implicates rare noncoding variants in DENND1A in polycystic ovary syndrome. J Clin Endocrinol Metab (2019) 104:3835–50. doi: 10.1210/jc.2018-02496
82. Rahimi Z, Mohammadi MSE. The CYP17 MSP AI (T-34C) and CYP19A1 (Trp39Arg) variants in polycystic ovary syndrome: A case-control study. Int J Reprod BioMed (2019) 17:201–8. doi: 10.18502/ijrm.v17i3.4519
83. Abu-Hijleh TM, Gammoh E, Al-Busaidi AS, Malalla ZH, Madan S, Mahmood N, et al. Common variants in the sex hormone-binding globulin (SHBG) gene influence SHBG levels in women with polycystic ovary syndrome. Ann Nutr Metab (2016) 68:66–74. doi: 10.1159/000441570
84. Nikanfar S, Hamdi K, Haiaty S, Samadi N, Shahnazi V, Fattahi A, et al. Oncostatin m and its receptor in women with polycystic ovary syndrome and association with assisted reproductive technology outcomes. Reprod Biol (2022) 22:100633. doi: 10.1016/j.repbio.2022.100633
85. Jamshidi M, Mohammadi Pour S, Bahadoram M, Mahmoudian-Sani MR, Saeedi Boroujeni A. Genetic polymorphisms associated with polycystic ovary syndrome among Iranian women. Int J Gynaecol Obstet (2021) 153:33–44. doi: 10.1002/ijgo.13534
86. Eggers S, Kirchengast S. The polycystic ovary syndrome–a medical condition but also an important psychosocial problem. Coll Antropol (2001) 25:673–85.
87. Zhang B, Shi H, Cao S, Xie L, Ren P, Wang J, et al. Revealing the magic of acupuncture based on biological mechanisms: A literature review. Biosci Trends (2022) 16:73–90. doi: 10.5582/bst.2022.01039
88. Sun Y, Liu Y, Liu B, Zhou K, Yue Z, Zhang W, et al. Efficacy of acupuncture for chronic Prostatitis/Chronic pelvic pain syndrome: A randomized trial. Ann Intern Med (2021) 174:1357–66. doi: 10.7326/M21-1814
89. Mao JJ, Liou KT, Baser RE, Bao T, Panageas KS, Romero SAD, et al. Effectiveness of electroacupuncture or auricular acupuncture vs usual care for chronic musculoskeletal pain among cancer survivors: The PEACE randomized clinical trial. JAMA Oncol (2021) 7:720–7. doi: 10.1001/jamaoncol.2021.0310
90. Liu Z, Yan S, Wu J, He L, Li N, Dong G, et al. Acupuncture for chronic severe functional constipation: A randomized trial. Ann Intern Med (2016) 165:761–9. doi: 10.7326/M15-3118
91. Lim CED, Ng RWC, Cheng NCL, Zhang GS, Chen H. Acupuncture for polycystic ovarian syndrome. Cochrane Database Syst Rev (2019) 7:CD007689. doi: 10.1002/14651858.CD007689.pub4
92. Stener-Victorin E, Jedel E, Manneras L. Acupuncture in polycystic ovary syndrome: Current experimental and clinical evidence. J Neuroendocrinol (2008) 20:290–8. doi: 10.1111/j.1365-2826.2007.01634.x
93. Lim CE, Ng RW, Xu K, Cheng NC, Xue CC, Liu JP, et al. Acupuncture for polycystic ovarian syndrome. Cochrane Database Syst Rev (2016) CD007689. doi: 10.1002/14651858.CD007689.pub3
94. Cui J, Song W, Jin Y, Xu H, Fan K, Lin D, et al. Research progress on the mechanism of the acupuncture regulating neuro-Endocrine-Immune network system. Vet Sci (2021) 8:149. doi: 10.3390/vetsci8080149
95. Lim DC, Chen W, Cheng LN, Xue CC, Wong FW, O’sullivan AJ, et al. Acupuncture for polycystic ovarian syndrome. Cochrane Database Syst Rev (2011) CD007689. doi: 10.1002/14651858.CD007689.pub2
96. Jo J, Lee YJ, Lee H. Acupuncture for polycystic ovarian syndrome: A systematic review and meta-analysis. Med (Baltimore) (2017) 96:e7066. doi: 10.1097/MD.0000000000007066
97. Chen X, Lan Y, Yang L, Liu Y, Li H, Zhu X, et al. Acupuncture combined with metformin versus metformin alone to improve pregnancy rate in polycystic ovary syndrome: A systematic review and meta-analysis. Front Endocrinol (Lausanne) (2022) 13:978280. doi: 10.3389/fendo.2022.978280
98. Li P, Peng J, Ding Z, Zhou X, Liang R. Effects of acupuncture combined with moxibustion on reproductive and metabolic outcomes in patients with polycystic ovary syndrome: A systematic review and meta-analysis. Evid Based Complement Alternat Med (2022) 2022:3616036. doi: 10.1155/2022/3616036
99. Jo J, Lee YJ. Effectiveness of acupuncture in women with polycystic ovarian syndrome undergoing in vitro fertilisation or intracytoplasmic sperm injection: a systematic review and meta-analysis. Acupunct Med (2017) 35:162–70. doi: 10.1136/acupmed-2016-011163
100. Zheng R, Qing P, Han M, Song J, Hu M, Ma H, et al. The effect of acupuncture on glucose metabolism and lipid profiles in patients with PCOS: A systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med (2021) 2021:5555028. doi: 10.1155/2021/5555028
101. Luo YN, Zheng QH, Liu ZB, Zhang FR, Chen Y, Li Y. Methodological and reporting quality evaluation of systematic reviews on acupuncture in women with polycystic ovarian syndrome: A systematic review. Complement Ther Clin Pract (2018) 33:197–203. doi: 10.1016/j.ctcp.2018.10.002
102. Dumesic DA, Hoyos LR, Chazenbalk GD, Naik R, Padmanabhan V, Abbott DH. Mechanisms of intergenerational transmission of polycystic ovary syndrome. Reproduction (2020) 159:R1–R13. doi: 10.1530/REP-19-0197
103. Palomba S, Daolio J, La Sala GB. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol Metab (2017) 28:186–98. doi: 10.1016/j.tem.2016.11.008
104. Krishnan A, Muthusami S. Hormonal alterations in PCOS and its influence on bone metabolism. J Endocrinol (2017) 232:R99–R113. doi: 10.1530/JOE-16-0405
105. Stener-Victorin E, Deng Q. Epigenetic inheritance of polycystic ovary syndrome - challenges and opportunities for treatment. Nat Rev Endocrinol (2021) 17:521–33. doi: 10.1038/s41574-021-00517-x
106. Dong H, Wang Q, Cheng L, Wang Z, Wu X, Zhou Z, et al. Effect of low-frequency electro-acupuncture in unmarried women with polycystic ovary syndrome: A randomized controlled study. Altern Ther Health Med (2021) 28:24–33.
107. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev (2012) 33:981–1030. doi: 10.1210/er.2011-1034
108. Moghetti P, Tosi F. Insulin resistance and PCOS: chicken or egg? J Endocrinol Invest (2021) 44:233–44. doi: 10.1007/s40618-020-01351-0
109. Ruiz R, Jideonwo V, Ahn M, Surendran S, Tagliabracci VS, Hou Y, et al. Sterol regulatory element-binding protein-1 (SREBP-1) is required to regulate glycogen synthesis and gluconeogenic gene expression in mouse liver. J Biol Chem (2014) 289:5510–7. doi: 10.1074/jbc.M113.541110
110. Bozdag G, Yildiz BO. Interventions for the metabolic dysfunction in polycystic ovary syndrome. Steroids (2013) 78:777–81. doi: 10.1016/j.steroids.2013.04.008
111. Giron M, Thomas M, Dardevet D, Chassard C, Savary-Auzeloux I. Gut microbes and muscle function: Can probiotics make our muscles stronger? J Cachexia Sarcopenia Muscle (2022) 13:1460–76. doi: 10.1002/jcsm.12964
112. Zhang F, Ma T, Tong X, Liu Y, Cui P, Xu X, et al. Electroacupuncture improves metabolic and ovarian function in a rat model of polycystic ovary syndrome by decreasing white adipose tissue, increasing brown adipose tissue, and modulating the gut microbiota. Acupunct Med (2021) 40:347–59. doi: 10.1177/09645284211056663
113. Yin X, Ji Y, Chan CLW, Chan CHY. The mental health of women with polycystic ovary syndrome: a systematic review and meta-analysis. Arch Womens Ment Health (2021) 24:11–27. doi: 10.1007/s00737-020-01043-x
114. Rodriguez-Paris D, Remlinger-Molenda A, Kurzawa R, Glowinska A, Spaczynski R, Rybakowski F, et al. Psychiatric disorders in women with polycystic ovary syndrome. Psychiatr Pol (2019) 53:955–66. doi: 10.12740/PP/OnlineFirst/93105
115. Kiani AK, Maltese PE, Dautaj A, Paolacci S, Kurti D, Picotti PM, et al. Neurobiological basis of chiropractic manipulative treatment of the spine in the care of major depression. Acta BioMed (2020) 91:e2020006. doi: 10.23750/abm.v91i13-S.10536
116. Ito K, Hirooka Y, Matsukawa R, Nakano M, Sunagawa K. Decreased brain sigma-1 receptor contributes to the relationship between heart failure and depression. Cardiovasc Res (2012) 93:33–40. doi: 10.1093/cvr/cvr255
117. Lansdown A, Rees DA. The sympathetic nervous system in polycystic ovary syndrome: a novel therapeutic target? Clin Endocrinol (Oxf) (2012) 77:791–801. doi: 10.1111/cen.12003
118. Hickman FE, Stanley EM, Carter BD. Neurotrophin responsiveness of sympathetic neurons is regulated by rapid mobilization of the p75 receptor to the cell surface through TrkA activation of Arf6. J Neurosci (2018) 38:5606–19. doi: 10.1523/JNEUROSCI.0788-16.2018
119. Oh JE, Kim SN. Anti-inflammatory effects of acupuncture at ST36 point: A literature review in animal studies. Front Immunol (2021) 12:813748. doi: 10.3389/fimmu.2021.813748
120. Rotterdam Ea-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril (2004) 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004
121. Moran LJ, Tassone EC, Boyle J, Brennan L, Harrison CL, Hirschberg AL, et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: Lifestyle management. Obes Rev (2020) 21:e13046. doi: 10.1111/obr.13046
122. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril (2018) 110:364–79. doi: 10.1016/j.fertnstert.2018.05.004
123. Zhang HL, Huo ZJ, Wang HN, Wang W, Chang CQ, Shi L, et al. Acupuncture ameliorates negative emotion in PCOS patients: a randomized controlled trial. Zhongguo Zhen Jiu (2020) 40:385–90. doi: 10.13703/j.0255-2930.20191231-k0005
124. Stener-Victorin E, Zhang H, Li R, Friden C, Li D, Wang W, et al. Acupuncture or metformin to improve insulin resistance in women with polycystic ovary syndrome: Study protocol of a combined multinational cross sectional case-control study and a randomised controlled trial. BMJ Open (2019) 9:e024733. doi: 10.1136/bmjopen-2018-024733
125. Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol (2016) 37:80–97. doi: 10.1016/j.bpobgyn.2016.03.005
126. Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med (2006) 12:324–32. doi: 10.1016/j.molmed.2006.05.006
127. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev (2018) 98:2133–223. doi: 10.1152/physrev.00063.2017
128. Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflammation (2013) 2013:139239. doi: 10.1155/2013/139239
129. Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med (2008) 14:222–31. doi: 10.2119/2007-00119.Tilg
130. Sadeghi HM, Adeli I, Calina D, Docea AO, Mousavi T, Daniali M, et al. Polycystic ovary syndrome: A comprehensive review of pathogenesis, management, and drug repurposing. Int J Mol Sci (2022) 23:583. doi: 10.3390/ijms23020583
131. Armanini D, Boscaro M, Bordin L, Sabbadin C. Controversies in the pathogenesis, diagnosis and treatment of PCOS: Focus on insulin resistance, inflammation, and hyperandrogenism. Int J Mol Sci (2022) 23:4110. doi: 10.3390/ijms23084110
132. Johansson J, Stener-Victorin E. Polycystic ovary syndrome: effect and mechanisms of acupuncture for ovulation induction. Evid Based Complement Alternat Med (2013) 2013:762615. doi: 10.1155/2013/762615
133. Yin J, Kuang J, Chandalia M, Tuvdendorj D, Tumurbaatar B, Abate N, et al. Hypoglycemic effects and mechanisms of electroacupuncture on insulin resistance. Am J Physiol Regul Integr Comp Physiol (2014) 307:R332–9. doi: 10.1152/ajpregu.00465.2013
134. Bi Y, Yin B, Fan G, Xia Y, Huang J, Li A, et al. Effects of acupoint therapy on nonalcoholic fatty liver disease: A systematic review and meta-analysis. Complement Ther Clin Pract (2021) 43:101376. doi: 10.1016/j.ctcp.2021.101376
135. Stener-Victorin E. Hypothetical physiological and molecular basis for the effect of acupuncture in the treatment of polycystic ovary syndrome. Mol Cell Endocrinol (2013) 373:83–90. doi: 10.1016/j.mce.2013.01.006
136. Wu Y, Robinson N, Hardiman PJ, Taw MB, Zhou J, Wang FF, et al. Acupuncture for treating polycystic ovary syndrome: guidance for future randomized controlled trials. J Zhejiang Univ Sci B (2016) 17:169–80. doi: 10.1631/jzus.B1500301
Keywords: acupuncture, animal studies, clinical studies, mechanism, polycystic ovary syndrome, review
Citation: Ye Y, Zhou C-C, Hu H-Q, Fukuzawa I and Zhang H-L (2022) Underlying mechanisms of acupuncture therapy on polycystic ovary syndrome: Evidences from animal and clinical studies. Front. Endocrinol. 13:1035929. doi: 10.3389/fendo.2022.1035929
Received: 03 September 2022; Accepted: 11 October 2022;
Published: 24 October 2022.
Edited by:
Stefano Palomba, Magna Græcia University, ItalyReviewed by:
Mayank Choubey, New York University, United StatesCopyright © 2022 Ye, Zhou, Hu, Fukuzawa and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao-Lin Zhang, em9lQGJqbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.