
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 01 December 2022
Sec. Obesity
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1035418
This article is part of the Research Topic Metabolic Associated Fatty Liver Disease: Clinical Perspectives From Pathogenesis to Diagnosis and Treatment View all 8 articles
Objective: Metabolic dysfunction-associated fatty liver disease (MAFLD) affects 25% of the population without approved drug therapy. According to the latest consensus, MAFLD is divided into three subgroups based on different diagnostic modalities, including Obesity, Lean, and Type 2 diabetes mellitus (T2DM) MAFLD subgroups. This study aimed to find out the optimum non-invasive metabolism-related indicators to respectively predict MAFLD and its subgroups.
Design: 1058 Chinese participants were enrolled in this study. Anthropometric measurements, laboratory data, and ultrasonography features were collected. 22 metabolism-related indexes were calculated, including fatty liver index (FLI), lipid accumulation product (LAP), waist circumference-triglyceride index (WTI), etc. Logistic regression analyzed the correlation between indexes and MAFLD. Receiver operating characteristics were conducted to compare predictive values among 22 indicators for screening the best indicators to predict MAFLD in different subgroups.
Results: FLI was the best predictor with the maximum odds ratio (OR) values of overall MAFLD (OR: 6.712, 95%CI: 4.766-9.452, area under the curve (AUC): 0.879, P < 0.05) and T2DM MAFLD subgroup (OR: 14.725, 95%CI: 3.712-58.420, AUC: 0.958, P < 0.05). LAP was the best predictor with the maximum OR value of Obesity MAFLD subgroup (OR: 2.689, 95%CI: 2.182-3.313, AUC: 0.796, P < 0.05). WTI was the best predictor with the maximum OR values of Lean MAFLD subgroup (OR: 3.512, 95%CI: 2.286-5.395, AUC: 0.920, P < 0.05).
Conclusion: The best predictors of overall MAFLD, Obesity, Lean, and T2DM MAFLD subgroups were respectively FLI, LAP, WTI, and FLI.
Metabolic dysfunction-associated fatty liver disease (MAFLD) has become one of the most common chronic liver diseases and affected more than 25% of the population (1). The etiology of MAFLD is multifactorial and not understood completely, including genetic make-up, accumulation of intrahepatic lipids, insulin resistance (IR), and inflammatory responses (2). Furthermore, most patients with MAFLD do not have symptoms at an early stage, which limits the early detection and prevention of MAFLD. However, MAFLD is not a benign static liver disease. On one hand, it could progress silently to cirrhosis and liver-related death. On the other hand, as the hepatic manifestation of metabolic disorder, it affects the metabolic state of the body, which acts on the heart and cerebral vessels, increasing the risk of cardiovascular death (3).
Unfortunately, there are no approved therapies for MAFLD treatment (4). The early stages of MAFLD can be reversible with a healthy lifestyle. Hence, it is necessary to develop early identification, diagnosis, and lifestyle intervention. As the gold standard for diagnosis of MAFLD, the application of liver biopsy is limited because it is expensive and invasive with up to 1% risk of serious complications (5). Until now, abdominal ultrasound has been recommended as the preferred diagnostic method for fatty liver disease (FLD), which is still inconvenient for large populations (6).
Some anthropometric measures and lipid parameters are proven to be simple and useful tools for predicting metabolic syndrome (MS) in clinical practice (7). Previous studies suggested some indicators could predict hepatic steatosis, such as fatty liver index (FLI), etc. Nima Motamed et al. found that the FLI, as a noninvasive and relatively inexpensive index, had an acceptable ability to predict the occurrence of new cases of NAFLD in the study population from northern Iran (8). Besides, atherogenic index of plasma (AIP) was considered as a regular monitoring index of NAFLD for obese men due to its strong correlation with NAFLD in obese participants (9). However, most researchers only aimed at one or two parameters without comparison among more indexes (10, 11). The latest consensus suggests that there are three subgroups in the diagnosis of MAFLD, including Obesity, Lean, and T2DM MAFLD subgroups (12). Given the high complexity of the pathogenesis of MAFLD, we need specific diagnostic markers for MAFLD and its different subgroups, but they have been unclear yet. Hence, in this study, we compared the predictive values of 22 metabolic indicators and screened out specific indexes to predict MAFLD, especially discriminating different subgroups.
This is a retrospective case-control study of 1,058 Chinese participants with or without MAFLD who were recruited consecutively at the China-Japan Friendship Hospital from January 2021 to February 2022. There were 621 patients with MAFLD and 437 participants with non-MAFLD. MAFLD and non-MAFLD control groups were respectively divided into Obesity, Lean and T2DM subgroups. In the MAFLD population, there were 464, 36, and 121 patients in the Obesity, Lean, and T2DM subgroups. In the non-MAFLD group, there were 244, 152, and 41 participants in the Obesity, Lean, and T2DM non-MAFLD subgroups.
Inclusion criteria were as follows: 1) Age ≥18 years and ≤80 years; 2) Diagnosis of MAFLD. The standards of diagnosis were as follows:
(1) Diagnosed with hepatic steatosis by ultrasound (13). At least two of the three conditions: ① hypoechogenicity in the far field of the liver; ② hyperechogenicity in the near field of the liver or bright liver, as well as signs of it being stronger than the kidney cortex; and ③ a blurry intrahepatic tubular structure.
(2) At least two of the three conditions: ① Obesity: BMI ≥23 kg/m2; ② Lean: BMI<23 kg/m2 and with at least two of the following metabolic risk abnormalities: a. Waist circumference ≥90/80 cm in Asian men and women; b. Blood pressure ≥130/85 mmHg or specific drug treatment; c. Plasma triglycerides ≥150 mg/dL (≥1.70 mmol/L) or specific drug treatment; d. Plasma HDL-C<40 mg/dL (<1.0 mmol/L) for men and<50 mg/dL (<1.3 mmol/L) for women or specific drug treatment; e. Prediabetes (i.e., fasting glucose levels 100-125 mg/dL (5.6-6.9 mmol/L), or 2-hour post-load glucose levels 140-199 mg/dL (7.8-11.0 mmol/L) or HbA1c 5.7%-6.4% (39-47 mmol/L); f. Homeostasis model assessment of insulin resistance score ≥2.5; g. Plasma high-sensitivity C-reactive protein level >2 mg/L. ③ T2DM: diagnosed with type 2 diabetes mellitus according to widely accepted international criteria (14).
Exclusion criteria: 1) Missing important information (such as age, gender or ultrasonography). 2) Cushing’s syndrome, total parenteral nutrition, drugs (amiodarone, ammonium valproate, glucocorticoids, methotrexate), etc., which can lead to special conditions of fatty liver. 3) Suffering from serious cardiovascular and cerebrovascular diseases, lung diseases, kidney diseases and so on. 4) Malignant tumors of the liver and other systems. 5) Pregnancy and lactation.
The flow chart was shown in Figure 1.
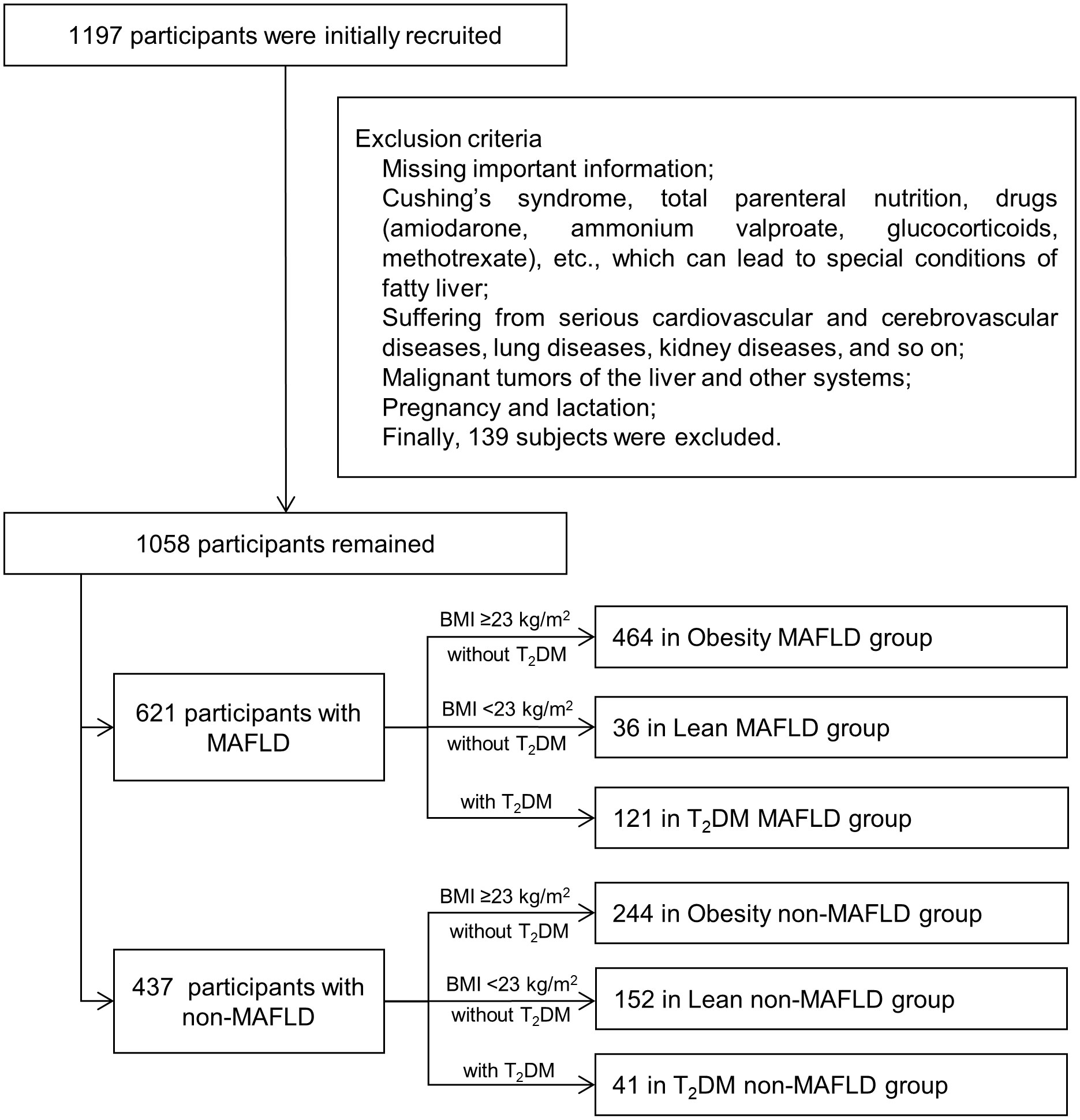
Figure 1 Flow diagram of research procedure. MAFLD, metabolic dysfunction-associated fatty liver disease; T2DM, type 2 diabetes mellitus.
The definition of non-MAFLD controls was the individuals without fatty liver diagnosed by ultrasound. Obesity non-MAFLD control included obese individuals (BMI ≥23 kg/m2) without T2DM and fatty liver diagnosed by ultrasound. Lean non-MAFLD control included lean individuals (BMI <23 kg/m2) without T2DM and didn’t conform to the diagnostic criteria of MAFLD. T2DM non-MAFLD control included T2DM patients without fatty liver diagnosed by ultrasound.
The study was approved by China-Japan Friendship Hospital Clinical Research Ethics Committee (ID: 2018-110-K79-1).
The researchers administered a structured questionnaire to document specified data on demographic, health-related behaviors, previous history, and medication history. Anthropometric indices were measured by an eligible physician, including weight, height, waist circumference (WC), hip circumference (HC), and blood pressure (BP). Weight and height were measured in light indoor clothing without shoes and heavy clothes, using a calibrated measuring apparatus. WC was measured using an inelastic measuring tape at midline between the lowest rib and the iliac crest. HC was measured at the maximum extension of the buttocks. The BP was measured using automatic electronic sphygmomanometer with the arm supported at the level of the heart. The mean readings of three replicate measurements were recorded.
Health examinations were performed in the morning after the examinees fasted overnight. Laboratory evaluation included aspartate aminotransferase (AST), Alanine transaminase (ALT), Gamma-glutamyl transferase (GGT), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), triglyceride (TG), total bilirubin (TBil), direct bilirubin (DBil), blood glucose (GLU). We performed the blood tests once and obtained all the data. There was no interval.
The calculation methods of the indicators were shown in Table 1.
Data analysis was conducted using SPSS 26.0 and Medcalc 20.022 statistical software. Measurement data were expressed as mean ± standard deviation () and analyzed by student’s t-test. Counting data expressed as a percentage (%) were analyzed by chi-square test. P<0.05 was considered statistically significant.
Logistic regression was used to assess the relationship between indexes (including quartiles) and MAFLD, expressed by odds ratios (OR) with their 95% confidence intervals (CI). The values of OR were normalized. Cramer’s V was used to evaluate the strength of the relationship. It means a weak association when 0.1< Cramer’s V< 0.3, a medium association when 0.3 ≤ Cramer’s V< 0.5, a strong association when Cramer’s V ≥ 0.5. The area under the curves (AUCs) with a 95% CI of receiver operating characteristic (ROC) were calculated to compare the predictive value among indicators for screening the best indicators to predict MAFLD in different subgroups and determine optimal cutoff points and Youden index with maximum concomitant sensitivity and specificity. The case/control quartile trends of indexes were analyzed, and the indexes levels were stratified into 4 quartiles (quartile 1: ≤1st quartile; quartile 2: >1st quartile and ≤median; quartile 3: >median and ≤3rd quartile, and quartile 4: >3rd quartile). The lowest quartile (quartile 1) was defined as the reference category and compared with quartile 2, 3, and 4.
In the design phase, we selected on-set FLD patients as cases as possible. When data were collected, repeated measurements were applied. In the analysis phase, a multivariate logistic regression analysis was used to control confounding factors such as age and gender. And the outcome assessors were blinded to the exposure status of participants and the study aims.
A total of 1058 subjects were included in this study, including 621 (58.69%) MAFLD patients and 437 (41.31%) non-MAFLD patients. Compared with non-MAFLD, MAFLD patients had higher TG, Glu, GGT, WC, HC, ALT, AST, and BP (Table 2), and the values of composite indexes, including BMI, AI2, AIP, LCI, BLCI2, TG/HDL-C, BAI, BRI, LAP, VAI, WTI, WWI, CVAI, FLI, ZJU index, TyG index, FLDI, and CMI (Table 3). In the Obesity, Lean, and T2DM subgroups, MAFLD patients had higher values of most of the composite indexes than respective non-MAFLD control (P all<0.05).
As shown in Figure 2, univariate logistic regression results indicated that FLI had the strongest association with the overall MAFLD (unadjusted OR: 6.654, 95%CI: 4.762-9.297, P<0.05). After adjusting for age and gender, multivariate logistic regression analyses indicated that FLI was still the indicator with the strongest association (adjusted OR: 6.712, 95%CI: 4.766-9.452, P<0.05). ROC analyses found that the ability to predict substantially increased fatty liver burden was the strongest for FLI with the highest AUC value (AUC: 0.879, P<0.05). The ROC curves were plotted (see Figure 2), and the values of cutoff points, sensitivity, specificity, and Youden index of FLI were determined (see Table 4). In analyses of quartiles, as shown in Table 5, there were statistically significant differences in the prevalence of MAFLD in different FLI quartile groups. And the FLI level was strongly correlated to MAFLD, (Cramer’s V: 0.642). Taking the F1 group as a reference, after adjusting for gender and age, the risks of MAFLD in the F2, F3, and F4 groups were higher than that in the F1 group (P all<0.05). These results suggested that FLI was the best predictor of overall MAFLD and the risk of overall MAFLD enhanced with the increasing level of FLI.
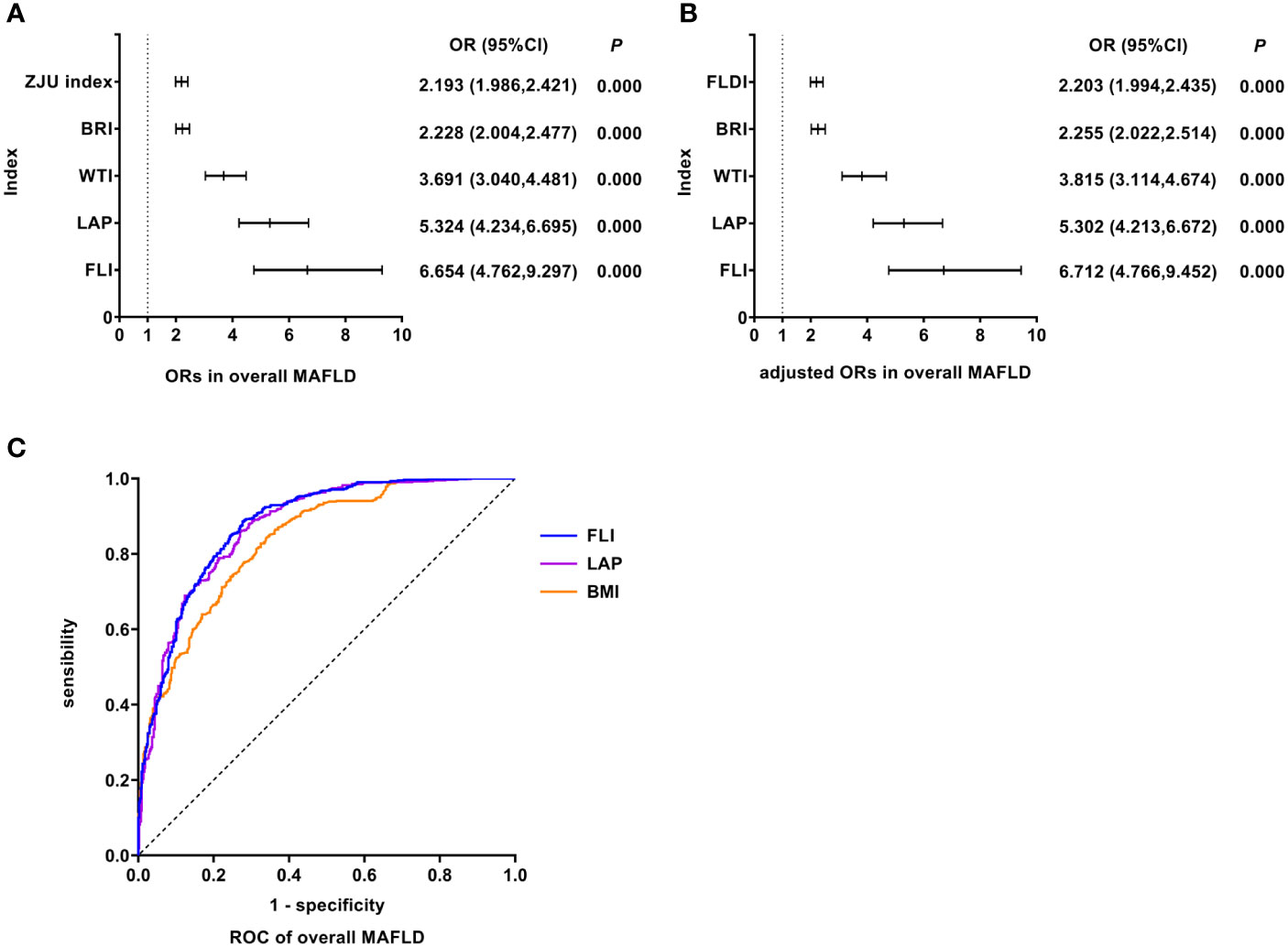
Figure 2 Logistic regression and ROC analysis of indicators in overall MAFLD. (A) ORs in overall MAFLD. (B) adjusted ORs in overall MAFLD. (C) ROC of overall MAFLD. MAFLD, metabolic dysfunction-associated fatty liver disease; OR, odds ratio; adjusted ORs: the values of ORs adjusted for gender and age (for age of LAP); ROC, receiver operating characteristic; ZJU index, Zhejiang University index; BRI, body roundness index; WTI, waist triglyceride index; LAP, lipid accumulation product; FLI, fatty liver index; FLDI, fatty liver disease index; BMI, body mass index.
As shown in Figure 3, univariate logistic regression results indicated that LAP had the strongest association with the Obesity MAFLD (unadjusted OR: 2.724, 95%CI: 2.210-3.358, P<0.05). After adjusting for age, multivariate logistic regression analyses indicated that LAP still was the indicator with the strongest association (adjusted OR: 2.689, 95%CI: 2.182-3.313, P<0.05). In Obesity MAFLD, ROC analyses found that the AUC of LAP was smaller than that of ZJU index, but the difference was not statistically significant (AUC: LAP vs. ZJU index: 0.796 vs. 0.816, P >0.05). The ROC curves were plotted (see Figure 3), and the values of cutoff points, sensitivity, specificity and Youden index of LAP were determined (see Table 4). In analyses of quartiles, as shown in Table 5, there were statistically significant differences in the prevalence of Obesity MAFLD in different LAP quartile groups. And the LAP level was moderately correlated to Obesity MAFLD (Cramer’s V: 0.498). Taking the L1 group as a reference, after adjusting for age, the risks of Obesity MAFLD in the L2, L3, and L4 groups were higher than that in the L1 group (P all<0.05). These results suggested that LAP was the best predictor of Obesity MAFLD and the risk of Obesity MAFLD enhanced with the increasing level of LAP.
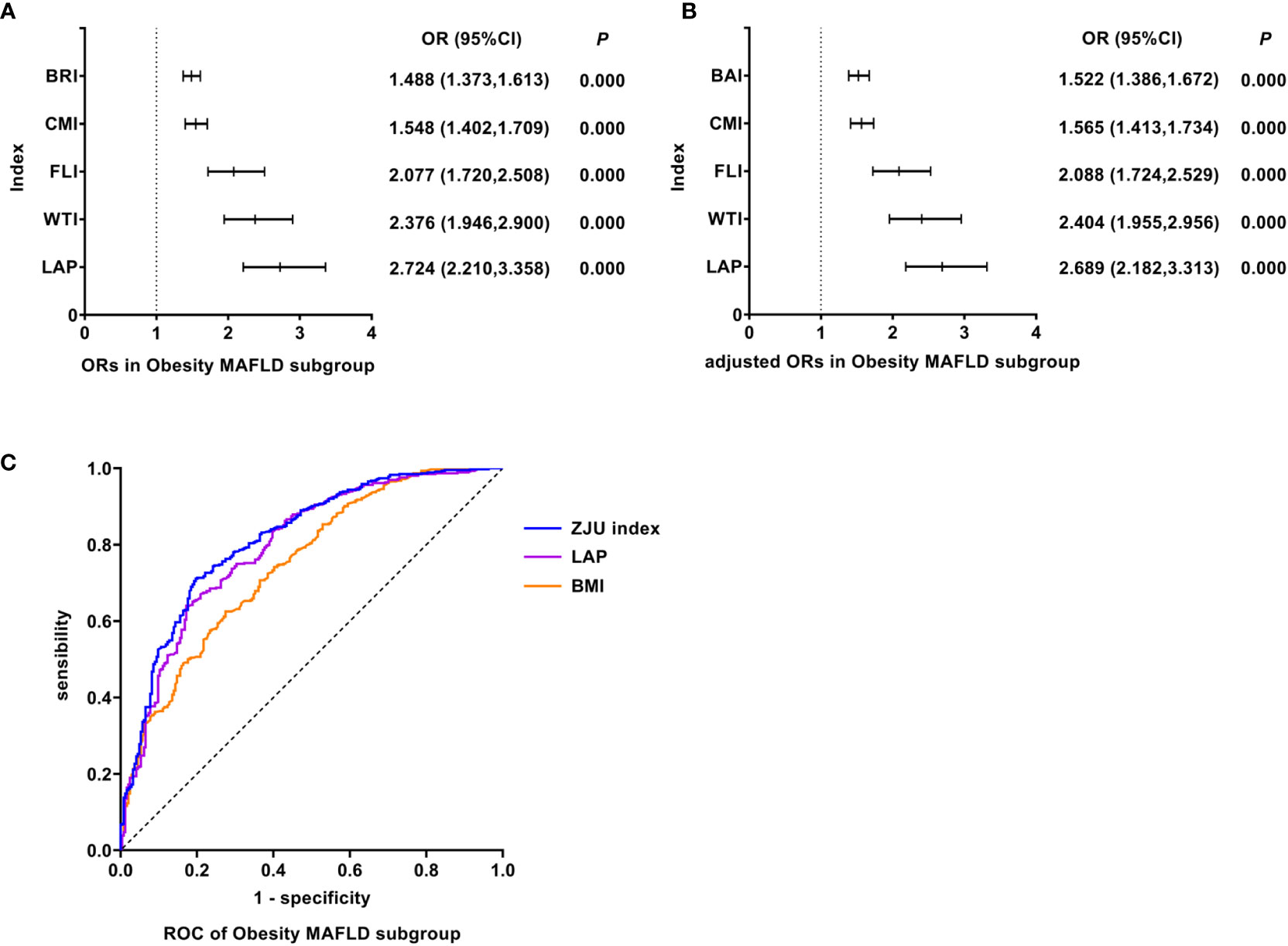
Figure 3 Logistic regression and ROC analysis of indicators in Obesity MAFLD subgroup. (A) ORs in Obesity MAFLD subgroup. (B) adjusted ORs in Obesity MAFLD subgroup. (C) ROC of Obesity MAFLD subgroup. MAFLD, metabolic dysfunction-associated fatty liver disease; OR, odds ratio; adjusted ORs: the values of ORs adjusted for gender and age (for age of LAP); ROC, receiver operating characteristic; BRI, body roundness index; CMI, cardiometabolic index; FLI, fatty liver index; WTI, waist triglyceride index; LAP, lipid accumulation product; BAI, body adiposity index; ZJU index, Zhejiang University index; LAP, lipid accumulation product; BMI, body mass index.
As shown in Figure 4, univariate logistic regression results indicated that WTI had the strongest association with the Lean MAFLD (unadjusted OR: 3.255, 95%CI: 2.236-4.740, P<0.05). After adjusting for age and gender, multivariate logistic regression analyses indicated that WTI still was the indicator with the strongest association (adjusted OR: 3.512, 95%CI: 2.286-5.395, P<0.05). In Lean MAFLD, ROC analyses found that the AUC of WTI was smaller than that of ZJU index, but the difference was not statistically significant (AUC: WTI vs. ZJU index: 0.920 vs. 0.952, P > 0.05). The ROC curves were plotted (see Figure 4), and the values of cutoff points, sensitivity, specificity, and Youden index of WTI were determined (see Table 4). In analyses of quartiles, as shown in Table 5, there were statistically significant differences in the prevalence of Lean MAFLD in different WTI quartile groups. And the WTI level was moderately correlated to Lean MAFLD (Cramer’s V: 0.645). Taking the W1 group as a reference, after adjusting for age and gender, the risks of Lean MAFLD in the W2, W3, W4 groups were higher than that in the W1 group (P all<0.05). These results suggested that WTI was the best predictor of Lean MAFLD and the risk of Lean MAFLD enhanced with the increasing level of WTI.
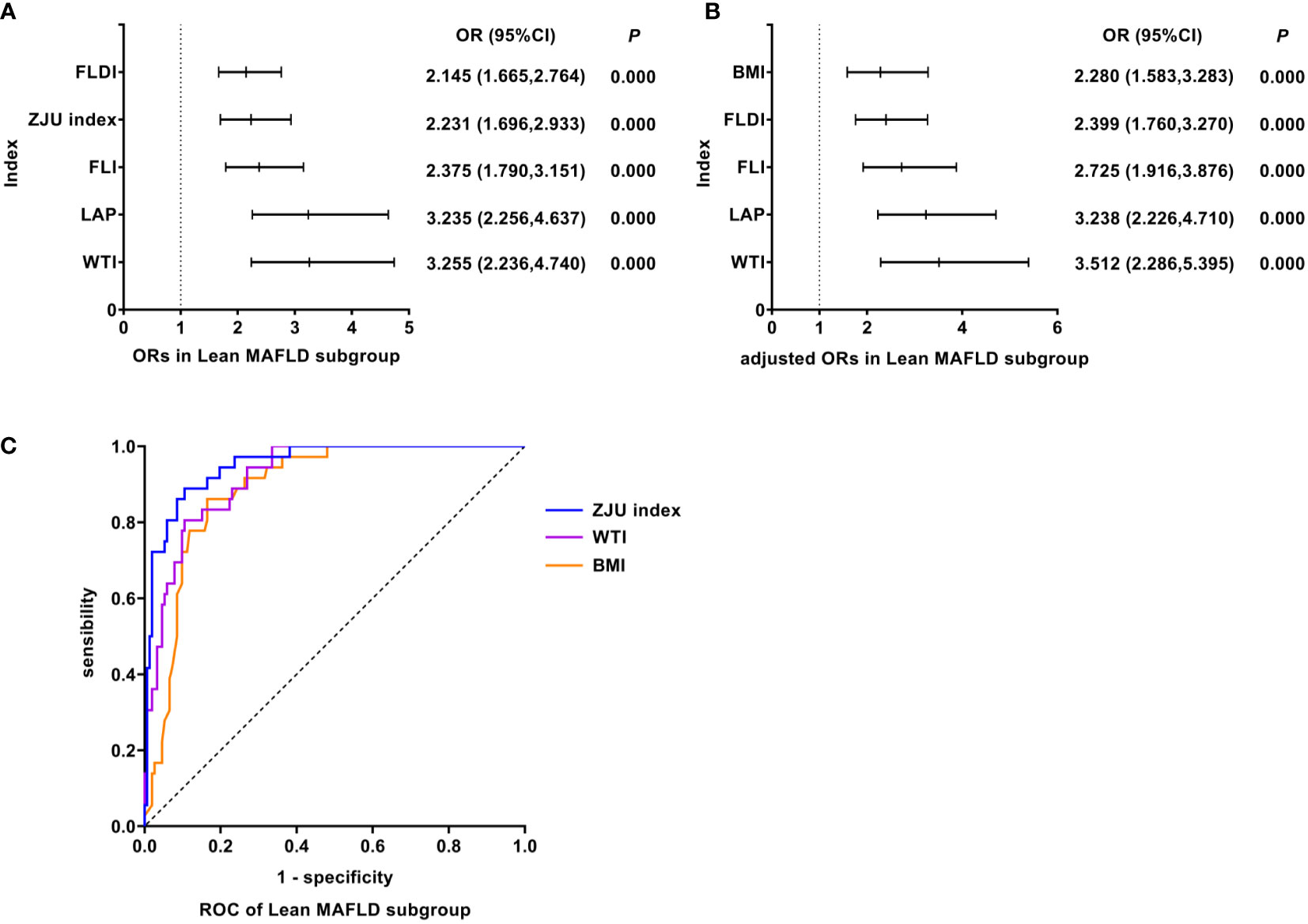
Figure 4 Logistic regression and ROC analysis of indicators in Lean MAFLD subgroup. (A) ORs in Lean MAFLD subgroup. (B) adjusted ORs in Lean MAFLD subgroup. (C) ROC of Lean MAFLD subgroup. MAFLD, metabolic dysfunction-associated fatty liver disease; OR, odds ratio; adjusted ORs: the value of ORs adjusted for gender and age (for age of LAP); ROC, receiver operating characteristic; FLDI, fatty liver disease index; ZJU index, Zhejiang University index; FLI, fatty liver index; LAP, lipid accumulation product; WTI, waist triglyceride index; BMI, body mass index; OR, odds ratio; adjusted ORs: The value of ORs after being adjusted for gender and age; ROC, receiver operating characteristic; MAFLD, metabolic dysfunction-associated fatty liver disease.
As shown in Figure 5, univariate logistic regression results indicated that FLI had the strongest association with the T2DM MAFLD (unadjusted OR: 19.138, 95%CI: 5.321-68.835, P<0.05). After adjusting for age and gender, multivariate logistic regression analyses indicated that FLI still was the indicator with the strongest association (adjusted OR: 14.725, 95%CI: 3.712-58.420, P<0.05). In T2DM MAFLD, ROC analyses found that the AUC of FLI was smaller than that of BRI, but the difference was not statistically significant (AUC: FLI vs. BRI: 0.958 vs. 0.963, P >0.05). The ROC curves were plotted (see Figure 5), and the values of cutoff points, sensitivity, specificity, and Youden index of FLI were determined (see Table 4). In analyses of quartiles, as shown in Table 5, there were statistically significant differences in the prevalence of T2DM MAFLD in different FLI quartile groups. And the FLI level was strongly correlated to T2DM MAFLD, (Cramer’s V: 0.778). Taking the F1 group as a reference, after adjusting for age and gender, the risks of T2DM MAFLD in the F3 and F4 groups were higher than that in the F1 group (P all< 0.05). These results suggested that FLI was the best predictor of T2DM MAFLD, and the risk of T2DM MAFLD enhanced with the increasing level of FLI.
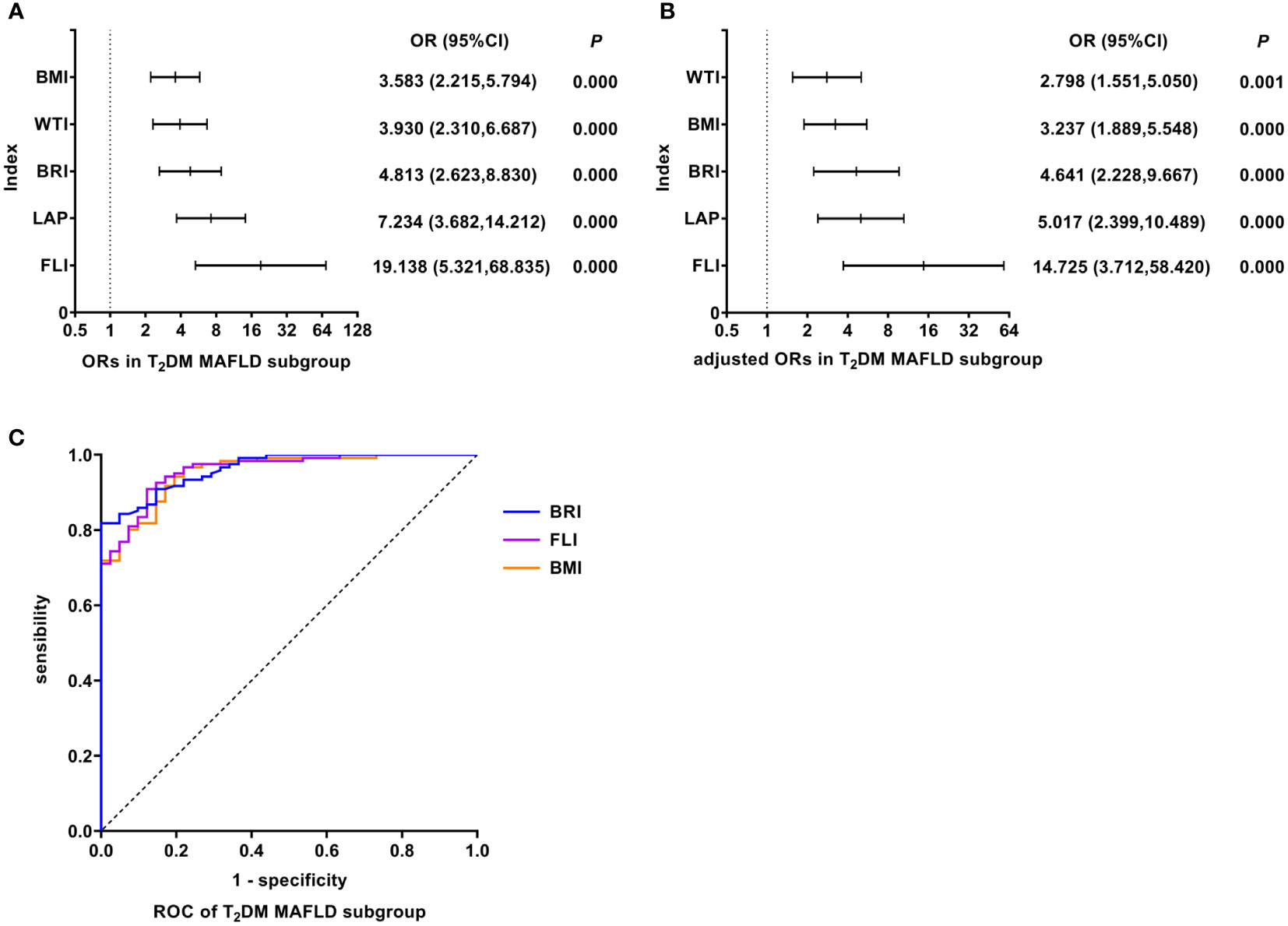
Figure 5 Logistic regression and ROC analysis of indicators in T2DM MAFLD subgroup. (A) ORs in T2DM MAFLD subgroup. (B) adjusted ORs in T2DM MAFLD subgroup. (C) ROC of T2DM MAFLD subgroup. MAFLD, metabolic dysfunction-associated fatty liver disease; T2DM, type 2 diabetes mellitus; OR, odds ratio; adjusted ORs: the value of ORs adjusted for gender and age (for age of LAP); ROC, receiver operating characteristic; BMI, body mass index; WTI, waist triglyceride index; BRI, body roundness index; LAP, lipid accumulation product; FLI, fatty liver index.
The results of other indicators were shown in Supplementary Material.
It is the first step to recognizing fatty liver in screening people at high risk for steatohepatitis. Due to differences in the distribution of metabolic risk factors in population with discriminating characteristics, the performances of diagnostic models are also distinguished. We analyzed and compared multiple composite measurements to determine the best predictive tools for different subgroups of MAFLD.
FLI is an algorithm combining BMI, WC, GGT, and TG levels, originally developed in a population residing in northern Italy and proposed by Bedogni to predict fatty liver (15). GGT is an early and sensitive marker of oxidative stress associated well with the levels of prooxidants, which can serve as an indirect marker of the risk of future oxidative damage (16). The high FLI level is related to increased C-reactive protein (CRP), blood pressure, IR, and fibrinogen levels (17).
Research conducted by Otgonsuren suggested FLI provided good diagnostic accuracy for fatty liver since it showed high concordance with the imaging and histological criteria for fatty liver (18). Kabisch et al. found that FLI could predict most cases of NAFLD with an AUC of 0.84 in ROC analysis (10). Nima’s study in northern Iran showed the ability of FLI to predict new cases of NAFLD was superior to other indexes such as BMI, WC, WHR, WHtR, and LAP. In their 7 years of follow-up, a one-unit increase in the FLI enhanced the chance of the occurrence of NAFLD by 3.8% in men and 3.2% in women (8). A meta-analysis of ten studies was conducted by Marco, evaluating the performance of FLI among 8273 subjects diagnosed with and 18,948 subjects without NAFLD, in which FLI showed an adequate performance in stratifying the risk of NAFLD (19).
Our study obtained supportive results that FLI was the best predictor of overall MAFLD and T2DM MAFLD, which suggested that the key mechanism of MAFLD may be strongly associated with IR. In a meta-analysis of 70,198 participants including 27 cohort studies, individuals with higher FLI levels had a significantly higher risk of diabetes development (20). Gerhard found patients with T2DM had a higher risk of progressive NAFLD with abnormal lipid metabolism and IR. Due to IR, adipose tissue becomes resistant to the anti-adipose effects of insulin to promote the synthesis of free fatty acids and TG in liver cells, which contributes to the accumulation of fat in liver cells, ultimately leading to FLD (21).
In America, the leading cause of mortality in patients with NAFLD was cardiovascular disease (22). FLD has been considered an early mediator of atherosclerosis (23, 24). Coexisting T2DM with FLD could aggravate liver diseases and atherosclerosis. Risks of cardiovascular events and kidney disease were enhanced in T2DM MAFLD patients (25). Lerchbaum et al. observed that a high level of FLI could predict all-cause, cardiovascular as well as hepatic mortality (17). FLI can confirm the diagnosis of MAFLD and evaluate the risks of related complications. Therefore, we contend that FLI is the most appropriate diagnostic index for overall MAFLD and T2DM MAFLD subgroup.
The most common explanation for the high prevalence of FLD is the obesity epidemic and weight loss is related to improvement in histology features of FLD (26). IR and excess adiposity increased lipid influx into the liver and de novo hepatic lipogenesis to promote hepatic triglyceride accumulation (27). It is generally recognized that serious complications of FLD (i.e., cirrhosis and liver cancer) are common in middle age and older. Whereas obesity and its related metabolic disorders could accelerate the process of disease (28). Obesity MAFLD will expose adolescents and young adults to the long-term risk of MAFLD, progressive hepatic lipotoxicity, and future end-stage liver disease (29, 30). Hence, it is significant to use the LAP index to screen MAFLD for obese people.
In a cross-sectional study involving 40,459 participants from southern China, LAP showed a strong association with the diagnosis and severity of NAFLD (31), in line with findings from northern China (11). Besides, LAP is one of the few indicators that can reflect sex differences in FLD, which is calculated for males and females respectively. Sex hormones and regional fat distribution take part in regulating metabolic disorders and FLD (32). In Liu’s study, LAP exhibited a stronger correlation with FLD in females than in males. The risk of FLD tended to increase in females who were about 55 years old (11). During this period, most women underwent menopause, during which estrogen levels sharply fell and body fat distribution shifted to the abdominal region leading to the increase of WC. Estrogen could regulate lipid metabolism and inhibit inflammation and plaque advancement in premenopausal women. Menopausal triggered a cascade of biological and physiological alterations, including fat redistribution (i.e., accumulation of visceral fat), dyslipidemia, and glucose intolerance, which are correlated with enhanced IR, cardiovascular disease, and NAFLD (32).
Lean MAFLD refers to FLD with normal BMI and metabolic disorders, involving WC, BP, TG, HDL-C, prediabetes, IR scores, and high-sensitivity CRP level. We found the ability to predict Lean MAFLD was strongest for WTI, which was based on WC and TG. As reported by Lee, WC and TG levels elevated significantly with the severity of hepatic steatosis. Furthermore, abdominal obesity with increased WC is a major manifestation of Lean MAFLD and appears to be associated with poorer metabolic status than general obesity and increased hepatic fibrosis (33). Excess VAT and SAT serve as two remarkable characteristics of abdominal obesity. Compared with SAT, the lipolytic activity is stronger in VAT, which plays a leading role in the development of Lean MAFLD (34). VAT induces the synthesis of cytokines like IL-6 and TNF-α, promoting macrophage infiltration and chronic inflammation (35). The signal transduction pathway of surrounding cells like T cells, eosinophils, B-regulatory cells, and macrophages was activated, leading to IR, liver steatosis, and eventually induced and deteriorated NAFLD (36). What’s more, Chinese individuals have a greater amount of VAT than Europeans at the same level of BMI or WC (37).
According to the NHANES III survey in the US, Kahn et al. found that people with high WC and TG had higher levels of fasting insulin and fasting plasma glucose than those with normal WC and TG. Excessive lipid accumulation reflected by WC and TG caused metabolic abnormalities (38). A Chinese study showed WTI was a better predictor of MetS in both men and women (39). In addition, WTI was also a predictor for the development of coronary artery disease (40). A mild to moderate increase in TG can reflect an increase in chylomicrons and very low density lipoprotein cholesterol remnants. Meanwhile, the particles of the remnant lipoprotein become smaller, they may directly cause atherosclerosis. Previous reports have noted that simultaneously increased WC and TG were associated with each metabolic index in CHD patients and the severity of lesions in the coronary artery (41, 42).
Individuals with Lean MAFLD may have worse outcomes, incorporating enhanced incidence of hepatitis, cardiometabolic complications, and even mortality, than those with metabolically healthy obese (43). Unfortunately, due to deceptive BMI and obscure metabolic abnormalities, patients with Lean MAFLD often go undetected and missed diagnosis at an early stage, to subsequently suffering from more severe diseases. Hence, as an easily available and the most accurate measurement of Lean MAFLD, WTI is demanding in the Chinese population, especially those whose BMI is normal.
Previous studies compared the predictive ability of AIP, AVI, BAI, BMI, BRI, and TyG index, usually the ROAUC of BMI was the maximum (44). On the contrary, our study found that BMI was not in the top 3 for maximum OR in all the MAFLD groups. Additionally, the diagnosis ability of BMI was poorer than other indexes in all groups with lower AUCs. Although BMI used to be an anthropometric indicator of obesity and FLD, increasing evidence suggests that BMI is criticized for its single estimate of the degree of body adipose tissue, and Asians are more likely to have central fat deposition even with a lower BMI. It does neither reflect the status of metabolic disorders nor specifically distinguish among different subtypes of MAFLD. Hence, it is conceivable that BMI is not suitable for diagnosing metabolic disorders, such as MAFLD, especially in Asians.
MAFLD is considered as a hepatic manifestation of metabolic disorders. In this study, almost all commonly used anthropometric and metabolic indicators related to metabolic disorders were compared as far as possible. What’s more, we found specific diagnostic indicators for various subtypes of MAFLD, which were rarely seen in other studies, providing more accurate information and treatment measures for the performance of personalized protocols in clinical practice and public health consultation. In addition, simple alternative markers are significant for screening MAFLD at an early stage, while CT and MRI are not readily available due to the cost of money and time, especially in rural and grassroots areas of China with large population bases and relatively inadequate health resources. Our findings provide simple and inexpensive ways to identify and distinguish subtypes of MAFLD and monitor the risks of MAFLD and associated complications.
Firstly, we could not obtain results of the biopsy considered the gold standard of FLD, because in Chinese and other countries’ guidelines, liver biopsy is not recommended and applicable to all participants. As hepatic steatosis was evaluated using hepatic ultrasonography, the accuracy of the reference test might be reduced. Hence, the index test results can also be affected. Secondly, due to the observational study design, causality cannot be confirmed nor negated. Most included noninvasive indexes and scores used parameters that do not directly reflect the processes involved in hepatic steatosis and liver fibrosis, which might mean that these noninvasive indexes and scores do not directly indicate hepatic steatosis or liver fibrosis changes. In further studies, we would perform the prospective study to explore the proper noninvasive index to distinguish NASH or liver fibrosis and clarify the causality. Thirdly, the increased values of several hepatic steatosis scores and indexes that contain age in the formula, can partially be attributed to the increased age in different subgroups. And the severity of MAFLD was not classified, which prevented us from quantitatively evaluating steatosis. Besides, the data of other confounders including drinking status and exercise were not analyzed because relative information was not available. Finally, given that our results were predominantly applicable to Chinese adults, generalizability to other racial or ethnic populations is uncertain.
The best predictors of overall MAFLD, Obesity, Lean, and T2DM MAFLD subgroups were respectively FLI, LAP, WTI, and FLI.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by China-Japan Friendship Hospital Clinical Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
JL designed the study, analyzed the data, and wrote the manuscript. SD analyzed the data and revised the manuscript. CW, YW, HP, and ZN collected the data. SY designed the study and revised the manuscript. All authors read and approved the final manuscript.
Science and Technology Project Task Book of Beijing, No. Z171100001717008.
The authors thank the China-Japan Friendship Hospital for support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1035418/full#supplementary-material
BMI, Body mass index; AI1, Atherosclerosis index1; AI2, Atherosclerosis index2; non-HDL-C, Non-high-density lipoprotein cholesterol; R-CHR, Coronary heart index; AIP, Atherogenic index of plasma; LCI, Lipid comprehensive index; BLCI1, Bilirubin lipid composite index1; BLCI2, Bilirubin lipid composite index2; TG/HDL-C, Triglyceride/(high-density lipoprotein cholesterol); BAI, Body adiposity index; BRI, Body roundness index; LAP, Lipid accumulation product; VAI, Visceral adiposity index; WTI, Waist circumference-triglyceride index; WWI, Weight-adjusted waist index; CVAI, Chinese visceral adiposity index; FLI, Fatty liver index; ZJU index, Zhejiang University index; TyG index, Triglyceride-glucose index; FLDI, Fatty liver disease index.
1. Perez-Diaz-Del-Campo N, Riezu-Boj JI, Marin-Alejandre BA, Monreal JI, Elorz M, Herrero JI, et al. Three different genetic risk scores based on fatty liver index, magnetic resonance imaging and lipidomic for a nutrigenetic personalized management of NAFLD: The fatty liver in obesity study. Diagnostics (Basel) (2021) 11(6):1083. doi: 10.3390/diagnostics11061083
2. Trepo E, Valenti L. Update on NAFLD genetics: From new variants to the clinic. J Hepatol (2020) 72(6):1196–209. doi: 10.1016/j.jhep.2020.02.020
3. Jiang ZY, Xu CY, Chang XX, Li WW, Sun LY, Yang XB, et al. Fatty liver index correlates with non-alcoholic fatty liver disease, but not with newly diagnosed coronary artery atherosclerotic disease in Chinese patients. BMC Gastroenterol (2013) 13:110. doi: 10.1186/1471-230X-13-110
4. Hou W, Gupta S, Beauchamp MC, Yuan L, Jerome-Majewska LA. Non-alcoholic fatty liver disease in mice with heterozygous mutation in TMED2. PloS One (2017) 12(8):e0182995. doi: 10.1371/journal.pone.0182995
5. Fu CP, Ali H, Rachakonda VP, Oczypok EA, DeLany JP, Kershaw EE. The ZJU index is a powerful surrogate marker for NAFLD in severely obese north American women. PloS One (2019) 14(11):e0224942. doi: 10.1371/journal.pone.0224942
6. Unger LW, Forstner B, Muckenhuber M, Scheuba K, Eigenbauer E, Scheiner B, et al. Hepatic steatosis in lean patients: Risk factors and impact on mortality. Dig Dis Sci (2020) 65(9):2712–8. doi: 10.1007/s10620-019-06000-y
7. Ching YK, Chin YS, Appukutty M, Gan WY, Chan YM. Comparisons of conventional and novel anthropometric obesity indices to predict metabolic syndrome among vegetarians in Malaysia. Sci Rep (2020) 10(1):20861. doi: 10.1038/s41598-020-78035-5
8. Motamed N, Faraji AH, Khonsari MR, Maadi M, Tameshkel FS, Keyvani H, et al. Fatty liver index (FLI) and prediction of new cases of non-alcoholic fatty liver disease: A population-based study of northern Iran. Clin Nutr (2020) 39(2):468–74. doi: 10.1016/j.clnu.2019.02.024
9. Wang Q, Zheng D, Liu J, Fang L, Li Q. Atherogenic index of plasma is a novel predictor of non-alcoholic fatty liver disease in obese participants: A cross-sectional study. Lipids Health Dis (2018) 17(1):284. doi: 10.1186/s12944-018-0932-0
10. Kabisch S, Bather S, Dambeck U, Kemper M, Gerbracht C, Honsek C, et al. Liver fat scores moderately reflect interventional changes in liver fat content by a low-fat diet but not by a low-carb diet. Nutrients (2018) 10(2):157. doi: 10.3390/nu10020157
11. Liu Y, Wang W. Sex-specific contribution of lipid accumulation product and cardiometabolic index in the identification of nonalcoholic fatty liver disease among Chinese adults. Lipids Health Dis (2022) 21(1):8. doi: 10.1186/s12944-021-01617-3
12. Eslam M, Sanyal AJ, George J, International Consensus P. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology (2020) 158(7):1999–2014 e1. doi: 10.1053/j.gastro.2019.11.312
13. Gao X, Fan JG, Study Group of L, Metabolism CSoE. Diagnosis and management of non-alcoholic fatty liver disease and related metabolic disorders: consensus statement from the study group of liver and metabolism, Chinese society of endocrinology. J Diabetes. (2013) 5(4):406–15. doi: 10.1111/1753-0407.12056
14. Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group, Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Society, Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Health Care Society, Geriatric Professional Committee of Beijing Medical Award Foundation, National Clinical Medical Research Center for Geriatric Diseases (PLA General Hospital). Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition). Zhonghua Nei Ke Za Zhi. (2022) 61(1):12–50. doi: 10.3760/cma.j.cn112138-20211027-00751
15. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol (2006) 6:33. doi: 10.1186/1471-230X-6-33
16. Lee DH, Blomhoff R, Jacobs DR Jr. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic Res (2004) 38(6):535–9. doi: 10.1080/10715760410001694026
17. Lerchbaum E, Pilz S, Grammer TB, Boehm BO, Stojakovic T, Obermayer-Pietsch B, et al. The fatty liver index is associated with increased mortality in subjects referred to coronary angiography. Nutr Metab Cardiovasc Dis (2013) 23(12):1231–8. doi: 10.1016/j.numecd.2013.02.004
18. Otgonsuren M, Estep MJ, Hossain N, Younossi E, Frost S, Henry L, et al. Single non-invasive model to diagnose non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). J Gastroenterol Hepatol (2014) 29(12):2006–13. doi: 10.1111/jgh.12665
19. Castellana M, Donghia R, Guerra V, Procino F, Lampignano L, Castellana F, et al. Performance of fatty liver index in identifying non-alcoholic fatty liver disease in population studies. a meta-analysis. J Clin Med (2021) 10(9):1877. doi: 10.3390/jcm10091877
20. Movahedian M, Rahmani J, Hashemi Nazari SS, Mohamadi S, Naik G, Hekmatdoost A. Fatty liver index and risk of diabetes incidence: A systematic review and dose-response meta-analysis of cohort studies. Prim Care Diabetes. (2020) 14(6):577–83. doi: 10.1016/j.pcd.2020.02.011
21. Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J, et al. A role for fibroblast growth factor 19 and bile acids in diabetes remission after roux-en-Y gastric bypass. Diabetes Care (2013) 36(7):1859–64. doi: 10.2337/dc12-2255
22. Lonardo A, Targher G. NAFLD: Is there anything new under the sun? Int J Mol Sci (2017) 18(9):1955. doi: 10.3390/ijms18091955
23. Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis (2007) 191(2):235–40. doi: 10.1016/j.atherosclerosis.2006.08.021
24. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med (2010) 363(14):1341–50. doi: 10.1056/NEJMra0912063
25. Xie F, Pei Y, Zhou Q, Cao D, Wang Y. Comparison of obesity-related indices for identifying nonalcoholic fatty liver disease: a population-based cross-sectional study in China. Lipids Health Dis (2021) 20(1):132. doi: 10.1186/s12944-021-01560-3
26. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology (2015) 149(2):367–78 e5; quiz e14-5. doi: 10.1053/j.gastro.2015.04.005
27. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology (2010) 51(2):679–89. doi: 10.1002/hep.23280
28. Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in obesity prevalence among children and adolescents in the united states, 1988-1994 through 2013-2014. JAMA (2016) 315(21):2292–9. doi: 10.1001/jama.2016.6361
29. Nobili V, Alisi A, Valenti L, Miele L, Feldstein AE, Alkhouri N. NAFLD in children: new genes, new diagnostic modalities and new drugs. Nat Rev Gastroenterol Hepatol (2019) 16(9):517–30. doi: 10.1038/s41575-019-0169-z
30. Doycheva I, Watt KD, Alkhouri N. Nonalcoholic fatty liver disease in adolescents and young adults: The next frontier in the epidemic. Hepatology (2017) 65(6):2100–9. doi: 10.1002/hep.29068
31. Dai H, Wang W, Chen R, Chen Z, Lu Y, Yuan H. Lipid accumulation product is a powerful tool to predict non-alcoholic fatty liver disease in Chinese adults. Nutr Metab (Lond). (2017) 14:49. doi: 10.1186/s12986-017-0206-2
32. Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: State of the art and identification of research gaps. Hepatology (2019) 70(4):1457–69. doi: 10.1002/hep.30626
33. Lee JH, Jeong SJ. What is the appropriate strategy for diagnosing NAFLD using ultrasonography in obese children? World J Pediatr (2017) 13(3):248–54. doi: 10.1007/s12519-017-0008-7
34. Lima-Martinez MM, Blandenier C, Iacobellis G. [Epicardial adipose tissue: more than a simple fat deposit]? Endocrinol Nutr (2013) 60(6):320–8. doi: 10.1016/j.endonu.2012.08.001
35. Tantanavipas S, Vallibhakara O, Sobhonslidsuk A, Phongkitkarun S, Vallibhakara SA, Promson K, et al. Abdominal obesity as a predictive factor of nonalcoholic fatty liver disease assessed by ultrasonography and transient elastography in polycystic ovary syndrome and healthy women. BioMed Res Int (2019) 2019:9047324. doi: 10.1155/2019/9047324
36. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol (2006) 6(10):772–83. doi: 10.1038/nri1937
37. Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the international study of prediction of intra-abdominal adiposity and its relationship with cardiometabolic Risk/Intra-abdominal adiposity. Am J Clin Nutr (2012) 96(4):714–26. doi: 10.3945/ajcn.112.035758
38. Kahn HS, Valdez R. Metabolic risks identified by the combination of enlarged waist and elevated triacylglycerol concentration. Am J Clin Nutr (2003) 78(5):928–34. doi: 10.1093/ajcn/78.5.928
39. Ma CM, Lu N, Wang R, Liu XL, Lu Q, Yin FZ. Three novel obese indicators perform better in monitoring management of metabolic syndrome in type 2 diabetes. Sci Rep (2017) 7(1):9843. doi: 10.1038/s41598-017-10446-3
40. Yang RF, Liu XY, Lin Z, Zhang G. Correlation study on waist circumference-triglyceride (WT) index and coronary artery scores in patients with coronary heart disease. Eur Rev Med Pharmacol Sci (2015) 19(1):113–8.
41. Blackburn P, Lamarche B, Couillard C, Pascot A, Bergeron N, Prud’homme D, et al. Postprandial hyperlipidemia: another correlate of the “hypertriglyceridemic waist” phenotype in men. Atherosclerosis (2003) 171(2):327–36. doi: 10.1016/j.atherosclerosis.2003.06.001
42. Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Almeras N, et al. Hypertriglyceridemic waist: A marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein b; small, dense LDL) in men? Circulation (2000) 102(2):179–84. doi: 10.1161/01.cir.102.2.179
43. Cruz ACD, Bugianesi E, George J, Day CP, Liaquat H, Charatcharoenwitthaya P, et al. 379 Characteristics and long-term prognosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology (2014) 146(5):S-909. doi: 10.1016/s0016-5085(14)63307-2
Keywords: metabolic dysfunction-associated fatty liver disease, non-invasive predictive indicators, metabolic disorders, fatty liver index, lipid accumulation product, waist circumference-triglyceride index
Citation: Liu J, Duan S, Wang C, Wang Y, Peng H, Niu Z and Yao S (2022) Optimum non-invasive predictive indicators for metabolic dysfunction-associated fatty liver disease and its subgroups in the Chinese population: A retrospective case-control study. Front. Endocrinol. 13:1035418. doi: 10.3389/fendo.2022.1035418
Received: 02 September 2022; Accepted: 15 November 2022;
Published: 01 December 2022.
Edited by:
Dewei Ye, Guangdong Pharmaceutical University, ChinaReviewed by:
Abdulrahman Ismaiel, Iuliu Hațieganu University of Medicine and Pharmacy, RomaniaCopyright © 2022 Liu, Duan, Wang, Wang, Peng, Niu and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shukun Yao, c2h1a3VueWFvQDEyNi5jb20=
†These authors share first authorship
‡ORCID: Yao Shukun, orcid.org/0000-0002-8512-2589
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.