
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 14 November 2022
Sec. Cardiovascular Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1033354
This article is part of the Research TopicThe Role of Metabolic Syndrome and Disorders in Cardiovascular DiseaseView all 17 articles
 Chi Liu1,2
Chi Liu1,2 Qi Zhao1,2
Qi Zhao1,2 Ziwei Zhao1,2
Ziwei Zhao1,2 Xiaoteng Ma1,2
Xiaoteng Ma1,2 Yihua Xia1,2
Yihua Xia1,2 Yan Sun1,2
Yan Sun1,2 Dai Zhang1,2
Dai Zhang1,2 Xiaoli Liu1,2*
Xiaoli Liu1,2* Yujie Zhou1,2*
Yujie Zhou1,2*Background: Insulin resistance (IR) is closely associated with in-stent restenosis (ISR) following percutaneous coronary intervention (PCI). Nevertheless, the predictive power of the newly developed simple assessment method for IR, estimated glucose disposal rate (eGDR), for ISR after PCI in individuals with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) remains unclear.
Methods: NSTE-ACS cases administered PCI in Beijing Anzhen Hospital between January and December 2015 were enrolled. The included individuals were submitted to at least one coronary angiography within 48 months after discharge. Patients were assigned to 2 groups according to ISR occurrence or absence. eGDR was derived as 21.16 - (0.09 * waist circumference [cm]) - (3.41 * hypertension) - (0.55 * glycated hemoglobin [%]). Multivariate logistic regression analysis and receiver operating characteristic (ROC) curve analysis were performed for evaluating eGDR’s association with ISR.
Results: Based on eligibility criteria, 1218 patients were included. In multivariate logistic analysis, the odds ratios (ORs) of eGDR as a nominal variate and a continuous variate were 3.393 (confidence interval [CI] 2.099 - 5.488, P < 0.001) and 1.210 (CI 1.063 - 1.378, P = 0.004), respectively. The incremental effect of eGDR on ISR prediction based on traditional cardiovascular risk factors was reflected by ROC curve analysis (AUC: baseline model + eGDR 0.644 vs. baseline model 0.609, P for comparison=0.013), continuous net reclassification improvement (continuous-NRI) of -0.264 (p < 0.001) and integrated discrimination improvement (IDI) of 0.071 (p = 0.065).
Conclusion: In NSTE-ACS cases administered PCI, eGDR levels show an independent negative association with increased ISR risk.
Although the popularization of second-generation drug-eluting stents (DESs) has largely decreased in-stent hyperproliferation, the incidence of in-stent restenosis (ISR) remains high, between 3% and 20%, which confirms coronary anatomical characteristics, patient indexes and surgical factors are highly correlated (1–3). The mechanism of ISR development is complex: besides vascular factors such as endothelial dysfunction, smooth muscle hyperplasia and inflammation (4–6), age, gender, hypertension, hyperlipidemia, diabetes and smoking are also considered risk factors for ISR (4, 7–10). Because of such complexity, accurate prediction and prevention of ISR has important clinical significance in improving prognosis in atherosclerotic cardiovascular disease (ASCVD) treated with stents.
Type 2 diabetes mellitus (T2DM) represents a major risk factor for ASCVD, which includes coronary heart disease, cerebrovascular disease and peripheral arterial disease (PAD), and also plays a key role in ISR (11). As an important pathogenetic mechanism of T2DM, insulin resistance (IR) has been shown to be correlated with the occurrence of ISR (12–14). IR measurement and assessment have attracted extensive attention recently. Hyperinsulinemic-euglycemic clamp is presently considered the gold standard for IR evaluation, but its wide clinical application is hampered by its high cost, time-consuming, complex and invasive characteristics. Using hyperinsulinemic-euglycemic clamp as a validation criterion, investigators established an estimated glucose disposal rate (eGDR) to enable the evaluation of insulin sensitivity in type 1 diabetes mellitus (T1DM) (15, 16). In the original study, waist-to-hip ratio (WHR), hypertension, and glycated hemoglobin (HbA1c) were included in the formula of eGDR. However, further studies have shown utilizing waist circumference (WC) in lieu of WHR for eGDR determination yields comparable results (15, 17). Patients with high eGDR have higher insulin sensitivity; conversely, low eGDR is associated with enhanced IR (18).
It was demonstrated that low eGDR independently predicts all-cause mortality in T2DM cases administered coronary artery bypass grafting (CABG) (19). Nevertheless, no studies have explored the relationship between eGDR and ISR. Therefore, we conducted the current work to investigate eGDR’s predictive value in ISR for individuals with non-ST-elevation acute coronary syndrome (NSTE-ACS) administered percutaneous coronary intervention (PCI).
This single-center observational trial enrolled individuals diagnosed with coronary artery disease (CAD) in Beijing Anzhen Hospital between January and December 2015. Inclusion criteria were: (1) diagnosis of NSTE-ACS (including on-ST-segment elevation myocardial infarction [NSTEMI] and unstable angina [UA]); (2) successful elective PCI; (3) coronary angiography performed at least once within 48 months after discharge. Relevant diagnostic criteria were based on the latest guidelines (20, 21). Exclusion criteria were: (1) missing baseline and/or follow-up data; (2) T1DM diagnosis; (3) history of CABG, cardiogenic shock, acute decompensated heat failure, chronic infectious disease, or cancer; (4) impaired kidney function, with estimated glomerular filtration rate (eGFR) below 30 mL/(min × 1.73 m2) or kidney replacement treatment; (5) serious liver dysfunction, with alanine transaminase and/or aspartate transaminase ≥ 5 times the respective upper reference limits. A total of 1218 individuals were finally included (Figure 1). The study was approved by the Hospital Clinical Research Ethics Committee and was conducted in accordance with the Helsinki Declaration.
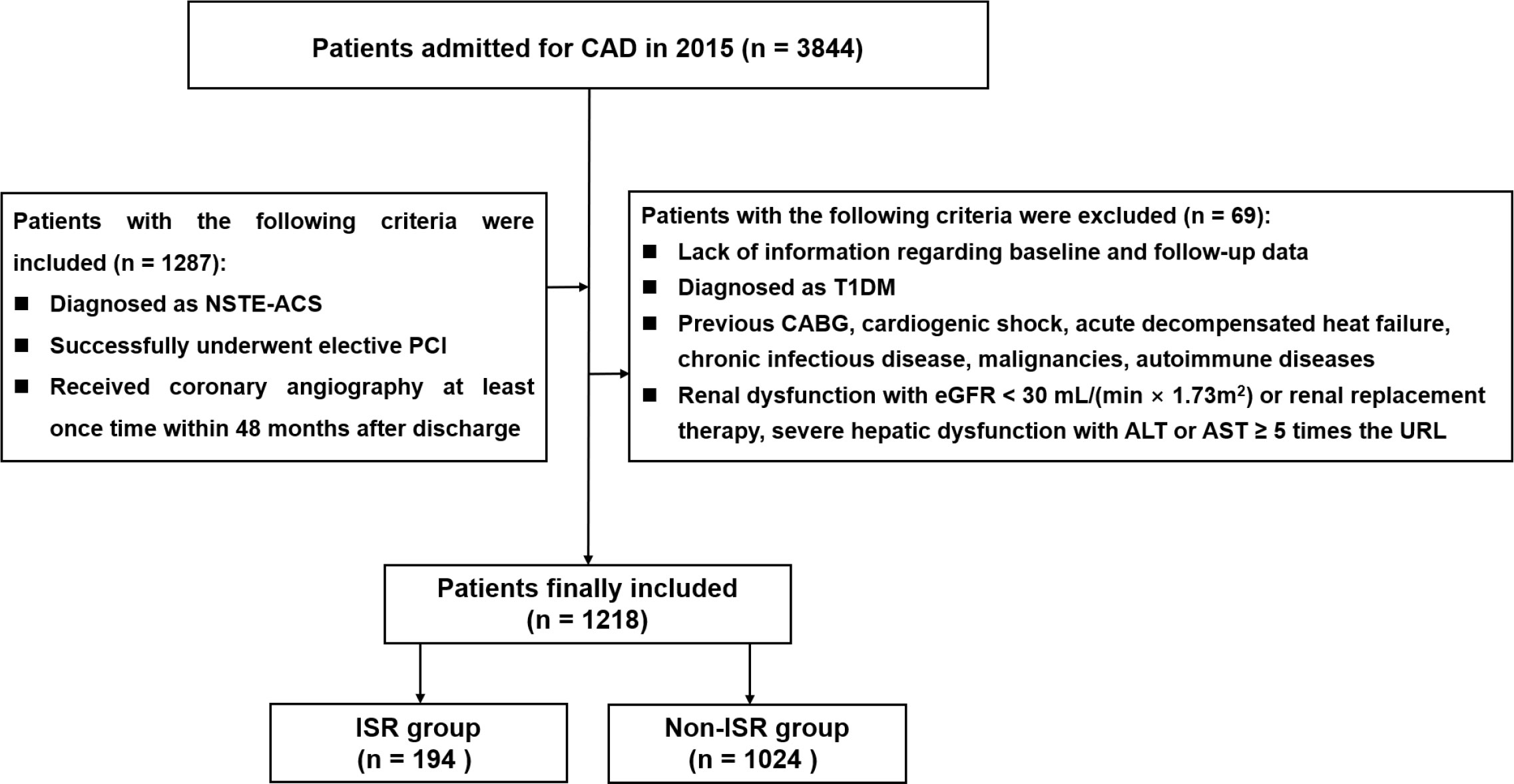
Figure 1 Flow diagram for the enrollment of study population. CAD coronary artery disease, NSTE-ACS non-ST-segment elevation acute coronary syndrome, PCI percutaneous coronary intervention, T1DM Type 1 Diabetes mellitus, CABG coronary artery bypass grafting, eGFR estimated glomerular filtration rate, ALT alanine transaminase, AST aspartate transaminase, URL upper reference limit, ISR in-stent restenosis.
Coronary angiography, coronary stent implantation, and perioperative management were all performed by two experienced interventional cardiologists, with the implementation path and management process based on current guidelines (21). Cases underwent antiplatelet treatment, with loading doses of 300, 300 and 180 mg for aspirin, clopidogrel and ticagrelor, respectively, prior to interventional therapy. During the procedure, 100 IU/kg unfractionated heparin was also administered for anticoagulation to maintain an activated clotting time >300 seconds. Successful stent placement was considered with residual stenosis <20% in the target lesion, as assessed by visual inspection or quantitative coronary angiography, and grade-III anterior thrombolysis in myocardial infarction (TIMI) flow.
Demographic and clinical characteristics were recorded by hospital information center professionals. NSTE-ACS includes non-ST segment elevation myocardial infarction (NSTEMI) and unstable angina pectoris (UA), whose definitions refer to recognized guidelines (22). Diagnostic criteria for related diseases (T2DM, hypertension, dyslipidemia, stroke, and PAD) followed current guidelines (23–27). WC was measured by taking the distance of the midpoint line between the rib’s lowest point and the iliac crest’s upper border. Echocardiography-based diagnostic reports were evaluated and reviewed by two sonographers. Blood samples were collected after fasting for 8-12 hours and transported to the hospital’s testing center for testing of hematological and biochemical parameters. A variety of biochemical and hematological indicators were collected. The standard enzymatic method was used to determine triglyceride (TG), total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C). The homogeneous direct method was performed to determine low-density lipoprotein cholesterol (LDL-C). The enzymatic hexokinase technique was performed to detect fasting blood glucose (FBG). Other parameters and indicators were determined by the standard laboratory method in the central laboratory of the hospital. The synergy between PCI and taxus and cardiac surgery (SYNTAX) score was determined using a standard formula (http://www.syntaxscore.com).
The formula for calculating eGDR was as follows (15, 17, 28): eGDR = 21.16 - (0.09 * WC [cm]) - (3.41 * Hypertension [yes or no]) - (0.55 * HbA1c [%]).
All the 1218 patients included in this study completed a 48-month follow-up period and underwent at least one coronary angiography in our hospital within 48 months of discharge. ISR was considered with a stenosis ≥ 50% in diameter within the stent or involving 5 mm proximal and distal to the stent (29). Quantitative coronary angiography was used to assess coronary stenosis. Similarly, angiographic findings and the presence of ISR were examined by two independent experienced cardiologists. Participants were assigned to the ISR and non-ISR groups, based on ISR status at 48 months.
Participants’ baseline data were described by the following methods. Continuous data with normal and skewed distributions were described as mean ± standard deviation (SD) and median with 25th and 75th percentiles, respectively, and compared by the two-sample t-test and the Mann-Whitney U test, respectively. Nominal variables were described as number and percentage, and comparison used the chi-square, continuity-adjusted chi-square, or Fisher’s exact test.
Univariate logistic regression analysis was used to identify parameters associated with ISR. Baseline variables with significant associations in univariate analysis and those clinically significant for ISR development were further assessed by multivariable logistic regression analysis, excluding variates that may have collinearity. eGDR was evaluated as both nominal and continuous. Nominal variables were analyzed for the low and high eGDR groups, categorized based on median eGDR (lower eGDR [eGDR ≤ 6.92]; higher eGDR [eGDR > 6.92]). Odds ratio (OR) and 95% confidence interval (CI) were determined for each association. Four multivariable logistic regression models were built for assessing eGDR’s association with ISR. In Model 1, adjustment was made for age, sex and body mass index (BMI). Model 2 was adjusted for Model 1’s variables besides a history of smoking, previously diagnosed myocardial infarction (MI), a history of PCI and previously detected stroke. In Model 3, adjustment was made for Model 2’s variables in addition to TG, LDL-C, high-sensitivity C-reactive protein (hs-CRP), eGFR and left ventricular ejection fraction (LVEF). Model 4 was adjusted for Model 3’s variables as well as angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB) at discharge, left main artery (LM) lesion, bifurcation, multi-vessel lesion, chronic total occlusion (CTO) lesion, SYNTAX score, complete revascularization and DES amount.
Subgroup analysis was performed after stratification by T2DM, adjusted for model 4 variates. The area under the receiver operating characteristic (ROC) curve (AUC) was obtained to assess eGDR’s predictive value in ISR. Net reclassification improvement (NRI) and integrated discrimination improvement (IDI) illustrated the incremental impact of introducing eGDR on the predictive ability of currently accepted risk models. The baseline model used for comparison included the following cardiovascular risk factors: age, sex, BMI, smoking history, family history of CAD, previously diagnosed MI, previously diagnosed PCI, previously detected stroke, hyperlipidemia, LVEF and SYNTAX score.
SPSS 26.0 and R 3.6.3 were utilized for data analysis, with two-sided P < 0.05 indicating statistical significance.
Totally 1218 participants averaging 59.93 ± 8.90 years old were included, with a male proportion of 70.4% (n=858). Details of demographics, past medical history, laboratory tests, drug status and interventions for the non-ISR and ISR groups are presented in Table 1. In comparison with non-ISR cases, the ISR group showed elevated WC and higher rates of smoking history, drinking history, diabetes, hypertension, previous MI, and previous PCI. Regarding laboratory tests, ISR cases showed elevated FBG and HbA1c amounts, but reduced TC and LDL-C levels. For admission medication, patients with ISR had higher rates of dual antiplatelet therapy (DAPT), aspirin, P2Y12 inhibitors, β-blockers, statins, oral hypoglycemic agents (OHA) and insulin. For discharge medication, the rates of ACEI/ARB, OHA and insulin used were elevated in ISR cases. Regarding coronary angiography and PCI, ISR cases displayed elevated rates of bifurcation and SYNTAX score, but lower rates of complete revascularization. The mean length of stent was higher in the ISR group, but there was no difference in minimal stent diameter between the two groups. Baseline data grouped by median eGDR are presented in Supplementary Table S1
Univariate analysis was performed for initially identifying factors associated with ISR (Supplementary Table S2). Based on univariate logistic regression analysis and clinically relevant risk factors, we screened variates and built four multivariate logistic regression models to measure eGDR’s predictive value in ISR. Whether defined as a nominal variate (with higher median eGDR as reference) or a continuous variate (per 1-unit decrease), eGDR had an independent predictive value across all 4 models. After fully adjusting for potential confounders in Model 4, ORs for eGDR as a nominal variate and a continuous variate were 3.393 (2.099-5.488) and 1.210 (1.063 - 1.378), respectively (Table 2).
Subgroup analysis of the independent association between eGDR and ISR based on T2DM status was carried out (Figure 2). The results revealed eGDR’s predictive potential in ISR was higher in non-T2DM cases [OR (95%CI): T2DM no 1.216 (1.025-1.442) vs. T2DM yes 0.978 (0.826–1.157), P for interaction = 0.010].
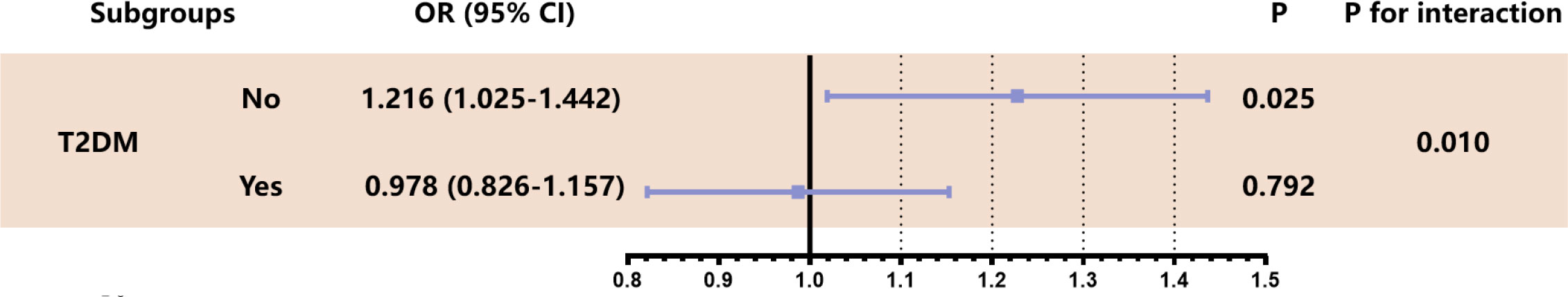
Figure 2 Stratified analysis of eGDR predicting ISR in T2DM subgroup. The analysis was adjusted for Model 4 except for variates applied for grouping. OR was evaluated by per 1-unit decrease of eGDR. eGDR estimated glucose disposal rate calculated, ISR in-stent restenosis, OR odds ratio, T2DM type 2 diabetes mellitus.
We established baseline models based on currently recognized cardiovascular risk factors as mentioned in Methods. Based on this model, addition of eGDR significantly enhanced its predictive power for ISR (AUCs of 0.644 and 0.609 for baseline model + eGDR and baseline model, respectively; P = 0.013) (Table 3, Figure 3). Estimation of continuous-NRI (-0.264, p < 0.001) also showed similar results, although IDI values (0.071, p = 0.065) were not significantly different (Table 3).
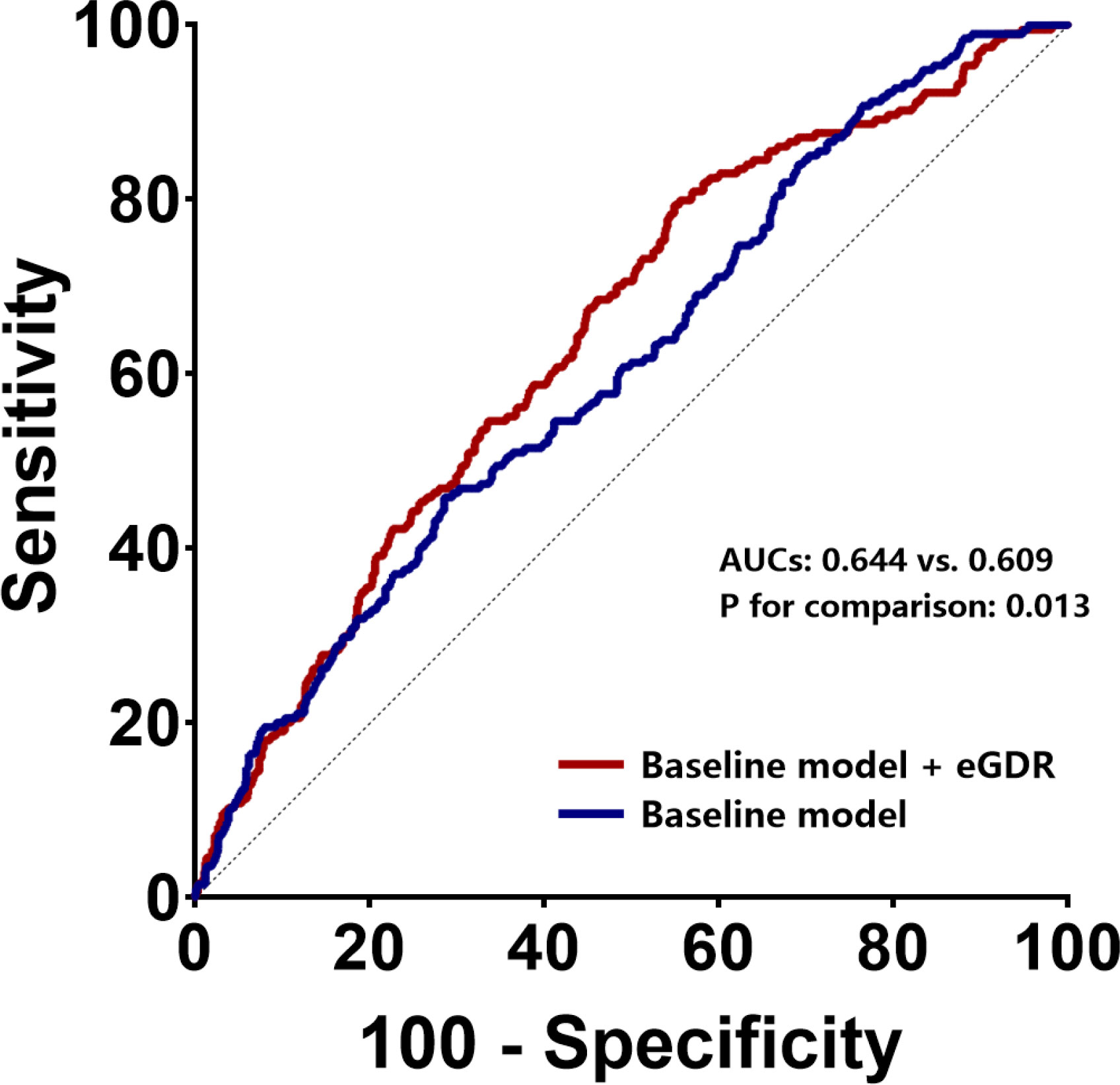
Figure 3 ROC curves to assess the predictive value of eGDR for ISR in general population. ROC receiver-operating characteristic, eGFR estimated glomerular filtration rate, ISR in-stent restenosis, AUC area under curve.
In non-diabetic cases, eGDR showed an incremental effect similar to that of the general population, with AUCs of 0.671 and 0.636 for baseline model + eGDR and baseline model, respectively (P = 0.043); continuous-NRI was 0.091 (P < 0.001) and IDI was 0.081 (P = 0.103) (Table 4, Figure 4B). In contrast, in the diabetic population, addition of eGDR did not increase the predictive potential of the baseline model in ROC curve analysis (AUCs of 0.655 and 0.658 for baseline model + eGDR and baseline model, respectively; P = 0.503), and continuous-NRI (-0.021, P = 0.107) and IDI (-0.021, P = 0.394) differences were not statistically significant (Table 4, Figure 4A).
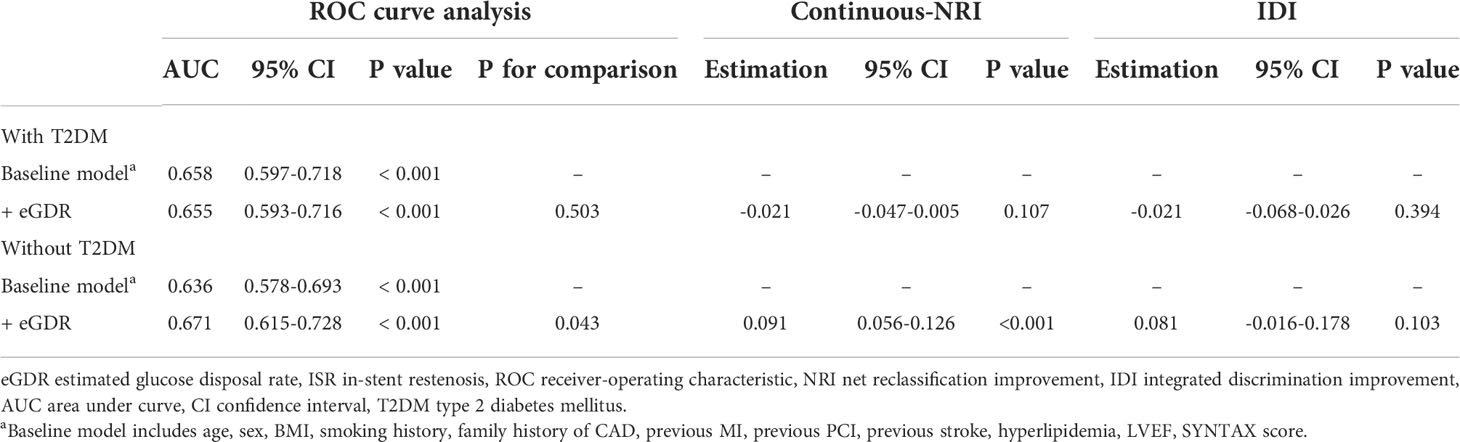
Table 4 Incremental effect of eGDR on ISR prediction by existing risk model in populations with and without T2DM.
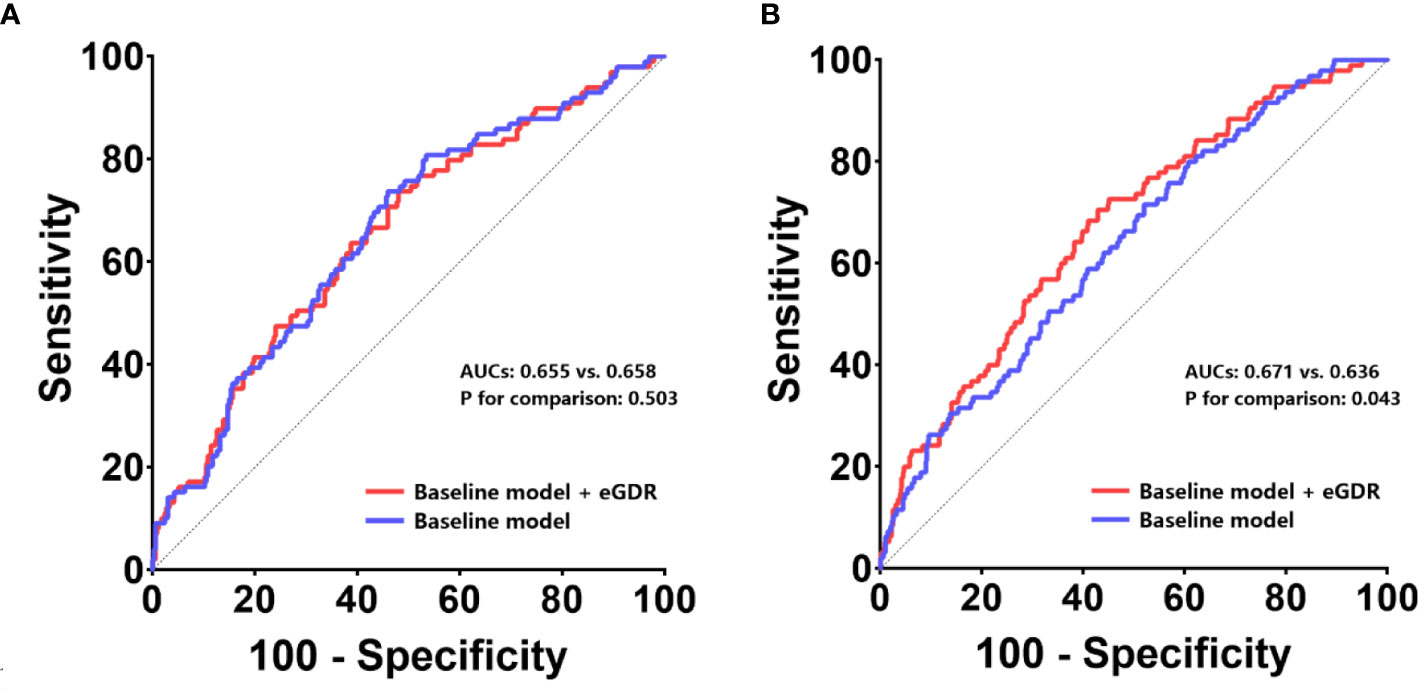
Figure 4 ROC curves to assess the predictive value of eGDR for ISR in populations with and without T2DM. The predictive values of the eGDR and baseline models were assessed in populations with T2DM (A) and without T2DM (B). ROC receiver-operating characteristic, eGFR estimated glomerular filtration rate, ISR in-stent restenosis, AUC area under curve, T2DM type 2 diabetes mellitus.
The present work firstly assessed eGDR’s association with ISR following PCI in CAD. The results revealed eGDR was independently and negatively associated with increased risk of ISR following PCI in NSTE-ACS; furthermore, eGDR improved the predictive ability of routine cardiovascular risk factors for ISR; moreover, the predictive value of eGDR for ISR was mainly reflected in patients without T2DM.
IR is the most important pathogenetic mechanism of diabetes and metabolic syndrome, with the main features including the following two aspects: decreased ability of insulin to induce glucose uptake and use; body compensation by enhanced insulin secretion for inducing hyperinsulinemia to stabilize blood sugar. Insulin resistance causes endothelial dysfunction, oxidative stress, and the activation of inflammatory responses, ultimately leading to the formation of atherosclerotic plaques (30). Currently, assessment techniques for insulin resistance mostly encompass two categories: direct assessment methods and simple surrogate assessment indicators. The hyperinsulinemic-euglycemic clamp and the insulin suppression test are both direct assessment methods for insulin resistance. By applying the hyperinsulinemic-euglycemic clamp, researchers confirmed that IR is tightly associated with coronary atherosclerotic heart disease, with a predictive role independent of other risk factors (31–33). For simple surrogate assessment indicators of IR, many clinical studies have used homoeostasis model assessment of insulin resistance (HOMA-IR) as an assessment method to explore the relationship between IR and cardiovascular disease (CVD), with consistent results. Indeed, IR is highly associated with atherosclerosis (34) and predicts CVD onset and poor prognosis in non-diabetic individuals (35–37). However, in clinical practice, fasting insulin levels are not routinely measured even in diabetics, let alone in individuals without diabetes. In addition, insulin measurement methods do not yield consistent data across laboratories, especially in case of low insulin levels. Therefore, researchers have proposed a variety of simpler alternative assessment indicators of insulin resistance, including triglyceride-glucose (TyG) index, triglyceride/high-density lipoprotein cholesterol (TG/HDL-C), visceral adiposity index (VAI) and lipid accumulation product (LAP), which are highly correlated with the incidence and prognosis of ASCVD (38–41). eGDR is also a simple surrogate measure of this type of IR.
It has long been admitted that diabetes could predict the occurrence of ISR (42, 43), and a study suggested that diabetes is the most effective predictor of ISR (44). In addition, a meta-analysis showed ISR incidence is markedly elevated in diabetic patients in comparison with non-diabetics (45). Therefore, diabetes can almost be considered the clearest risk factor for ISR. Previously, it was shown that IR is a common feature of CVD patients undergoing stent surgery, and an important marker of restenosis after PCI, with a deterioration process related to endothelial dysfunction, nitric oxide production disorders and activity defects (13). In recent years, studies applying HOMA-IR have confirmed that insulin resistance is highly correlated with ISR occurrence after PCI, representing an independent predictor of ISR (12, 14). In addition, a study using TyG as an evaluation index of IR found that TyG is independently and positively correlated with ISR risk following DES implantation in ACS patients (46).
As for eGDR, its associations with stroke incidence and mortality in T2DM patients have been demonstrated (47). In addition, eGDR was also shown to be closely related to elevated risk of all-cause mortality after CABG in T2DM patients, independent of other cardiovascular and metabolic risk factors (19). The above findings suggest that eGDR has great potential in predicting ASCVD prognosis and ISR events after PCI. This study clarified the predictive potential of eGDR for ISR occurrence post-PCI in NSTE-ACS cases, which is consistent with previous findings. The present work not only confirmed IR could predict ISR occurrence upon PCI in NSTE-ACS cases, but also revealed a new and effective indicator applicable for ISR prediction. The population of this study was mainly UA patients. On the one hand, we excluded patients who underwent primary PCI due to severe and complex disease as well as confounding factors that were difficult to adjust for. On the other hand, the patient data available to our research team came from general wards with relatively few NSTEMI patients. In data analysis, we attempted to include diabetes and related variates in the multivariate analysis, but after final adjustment, eGDR lost statistical significance in ISR prediction. Therefore, a subgroup analysis was carried out based on the diabetes status. The results revealed eGDR only had a predictive value in ISR for the non-diabetic subgroup. Furthermore, incremental effect analysis in the diabetes and non-diabetes groups was also consistent with the above subgroup analysis. This could explain the lack of significance for eGDR in models incorporating diabetes and associated variates. As mentioned above, IR assessed by various methods has important predictive value for CVD development in patients without diabetes. Although such finding is novel, we believe that eGDR can predict the adverse prognosis of CVD in non-diabetic patients. However, the results of the subgroup analysis in this study need further research to verify. It is certain that eGDR has the potential as a routine evaluation index of CVD cases, which requires further investigation in large prospective trials. In the era of widespread PCI treatment, there is a lack of simple and effective evaluation methods for long-term prognosis of patients. eGDR is expected to become an effective index to evaluate the ISR risk of patients after PCI and guide follow-up treatment. Finally, whether eGDR can really be used clinically as a powerful predictor of ISR after PCI needs to be assessed via comparison with other IR evaluation indicators.
There were some limitations in the present study that need to be further confirmed by more rationally designed studies. First, this was a single-center observational study of Chinese individuals, with unavoidable selection bias. Therefore, multi-center trials or even randomized controlled studies with larger samples and greater racial diversity are warranted to further clarify the current results. Additionally, because of the exclusion of patients undergoing emergency PCI, UA cases in this study cohort constituted the greatest part of all cases, and the current findings might not reflect the prognostic value of eGDR for ISR in NSTEMI. Furthermore, regarding repeat coronary angiography after discharge, ISR detection was not based on intracoronary imaging, and its accuracy was insufficient. Moreover, this work did not clarify the specific time when ISR occurred within 48 months after discharge and lacked short-term and long-term ISR analyses. In addition, this study did not compare the predictive value of eGDR and other IR evaluation methods on ISR.
eGDR independently predicts ISR after PCI in NSTE-ACS cases and improves the predictive power of routine cardiovascular risk factors in ISR. Finally, eGDR’s predictive potential in ISR was mainly demonstrated in non-T2DM patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CL made substantial contributions to data collection, data analysis and manuscript writing. YZ and XL made substantial contributions to study design and intellectual direction. QZ, ZZ, XM, YX, YS, and DZ made contributions to data collection and analysis. All authors read and approved the final manuscript.
The study was funded by Beijing Municipal Administration of Hospitals “Mission plan” (SML20180601) and the National Key Research and Development Program of China (2017YFC0908800).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1033354/full#supplementary-material
DES, drug-eluting stent; ISR, in-stent restenosis; ASCVD, atherosclerotic cardiovascular disease; T2DM, type 2 diabetes mellitus; PAD, peripheral arterial disease; IR, insulin resistance; eGDR, estimated glucose disposal rate; T1DM, type 1 diabetes mellitus; WHR, waist-to-hip ratio; HbA1c, glycosylated hemoglobin; WC, waist circumference; CABG, coronary artery bypass grafting; NSTE-ACS, non-ST-segment elevation acute coronary syndrome; PCI, percutaneous coronary intervention; CAD, coronary artery disease; NSTEMI, non-ST-segment elevation myocardial infarction; UA, unstable angina; eGFR, estimated glomerular filtration rate; TIMI, thrombolysis in myocardial infarction; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FBG, fasting blood glucose; SYNTAX, the synergy between PCI with taxus and cardiac surgery; SD, standard deviation; OR, odds ratio; CI, confidence interval; BMI, body mass index; MI, myocardial infarction; hs-CRP, high-sensitivity C-reactive protein; LVEF, left ventricular ejection fraction; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; LM, left main artery; CTO, chronic total occlusion; ROC, receiver operating characteristic; AUC, area under curve; NRI, net reclassification improvement; IDI, integrated discrimination improvement; OHA, oral hypoglycemic agents; HOMA-IR, homoeostasis model assessment of insulin resistance; CVD, cardiovascular disease; TyG, triglyceride-glucose; HDL-C, high-density lipoprotein cholesterol; VAI, visceral adiposity index; LAP, lipid accumulation product

1. Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol (2010) 56(23):1897–907. doi: 10.1016/j.jacc.2010.07.028
2. Stolker JM, Cohen DJ, Kennedy KF, Pencina MJ, Lindsey JB, Mauri L, et al. Repeat revascularization after contemporary percutaneous coronary intervention: an evaluation of staged, target lesion, and other unplanned revascularization procedures during the first year. Circ Cardiovasc Interv (2012) 5(6):772–82. doi: 10.1161/CIRCINTERVENTIONS.111.967802
3. Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, et al. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart (2014) 100(2):153–9. doi: 10.1136/heartjnl-2013-304933
4. Bauters C, Hubert E, Prat A, Bougrimi K, Van Belle E, McFadden EP, et al. Predictors of restenosis after coronary stent implantation. J Am Coll Cardiol (1998) 31(6):1291–8. doi: 10.1016/S0735-1097(98)00076-X
5. Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell (2001) 104(4):503–16. doi: 10.1016/S0092-8674(01)00238-0
6. Schillinger M, Exner M, Mlekusch W, Haumer M, Ahmadi R, Rumpold H, et al. Balloon angioplasty and stent implantation induce a vascular inflammatory reaction. J Endovasc Ther (2002) 9(1):59–66. doi: 10.1177/152660280200900111
7. Arora RR, Konrad K, Badhwar K, Hollman J. Restenosis after transluminal coronary angioplasty: a risk factor analysis. Cathet Cardiovasc Diagn (1990) 19(1):17–22. doi: 10.1002/ccd.1810190106
8. Bernat R, Szavits-Nossan J, Trbović A, Kapov-Svilicić K, Sesto I, Sipić T. Relationship of genetic markers for atherosclerosis and long-term outcome after percutaneous coronary intervention with stenting. Coll Antropol (2012) 36(4):1385–90.
9. Dzavik V, Kharbanda R, Ivanov J, Ing DJ, Bui S, Mackie K, et al. Predictors of long-term outcome after crush stenting of coronary bifurcation lesions: importance of the bifurcation angle. Am Heart J (2006) 152(4):762–9. doi: 10.1016/j.ahj.2006.04.033
10. Sajadian M, Alizadeh L, Ganjifard M, Mardani A, Ansari MA, Falsoleiman H. Factors affecting in-stent restenosis in patients undergoing percutaneous coronary angioplasty. Galen Med J (2018) 7:e961. doi: 10.31661/gmj.v7i0.961
11. Jakubiak GK, Pawlas N, Cieślar G, Stanek A. Pathogenesis and clinical significance of in-stent restenosis in patients with diabetes. Int J Environ Res Public Health (2021) 18(22):11970. doi: 10.3390/ijerph182211970
12. Piatti P, Di Mario C, Monti LD, Fragasso G, Sgura F, Caumo A, et al. Association of insulin resistance, hyperleptinemia, and impaired nitric oxide release with in-stent restenosis in patients undergoing coronary stenting. Circulation (2003) 108(17):2074–81. doi: 10.1161/01.CIR.0000095272.67948.17
13. Piatti P, Monti LD. Insulin resistance, hyperleptinemia and endothelial dysfunction in coronary restenosis. Curr Opin Pharmacol (2005) 5(2):160–4. doi: 10.1016/j.coph.2004.10.004
14. Zhao LP, Xu WT, Wang L, Li H, Shao CL, Gu HB, et al. Influence of insulin resistance on in-stent restenosis in patients undergoing coronary drug-eluting stent implantation after long-term angiographic follow-up. Coron Artery Dis (2015) 26(1):5–10. doi: 10.1097/MCA.0000000000000170
15. Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes (2000) 49(4):626–32. doi: 10.2337/diabetes.49.4.626
16. Chillarón JJ, Goday A, Flores-Le-Roux JA, Benaiges D, Carrera MJ, Puig J, et al. Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J Clin Endocrinol Metab (2009) 94(9):3530–4. doi: 10.1210/jc.2009-0960
17. Kietsiriroje N, Pearson S, Campbell M, Ariëns R, Ajjan RA. Double diabetes: A distinct high-risk group? Diabetes Obes Metab (2019) 21(12):2609–18. doi: 10.1111/dom.13848
18. Chillarón JJ, Flores LJ, Benaiges D, Pedro-Botet J. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism (2014) 63(2):181–7. doi: 10.1016/j.metabol.2013.10.002
19. Nyström T, Holzmann MJ, Eliasson B, Svensson AM, Kuhl J, Sartipy U. Estimated glucose disposal rate and long-term survival in type 2 diabetes after coronary artery bypass grafting. Heart Vessels (2017) 32(3):269–78. doi: 10.1007/s00380-016-0875-1
20. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J (2021) 42(14):1289–367. doi: 10.1093/eurheartj/ehaa575
21. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: A report of the American college of Cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol (2022) 79(2):e21–e129. doi: 10.1161/CIR.0000000000001038
22. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European society of cardiology (ESC). Eur Heart J (2016) 37(3):267–315. doi: 10.1093/eurheartj/ehv320
23. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension (2020) 75(6):1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026
24. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2020. Diabetes Care (2020) 43(Suppl 1):S14–31. doi: 10.2337/dc20-S002
25. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J (2020) 41(1):111–88. doi: 10.1093/eurheartj/ehz455
26. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American heart Association/American stroke association. Stroke (2018) 49(3):e46–110. doi: 10.1161/STR.0000000000000158
27. Creager MA, Belkin M, Bluth EI, Casey DJ, Chaturvedi S, Dake MD, et al. 2012 ACCF/AHA/ACR/SCAI/SIR/STS/SVM/SVN/SVS key data elements and definitions for peripheral atherosclerotic vascular disease: a report of the American college of cardiology Foundation/American heart association task force on clinical data standards (Writing committee to develop clinical data standards for peripheral atherosclerotic vascular disease). J Am Coll Cardiol (2012) 59(3):294–357. doi: 10.1016/j.jacc.2011.10.860
28. Epstein EJ, Osman JL, Cohen HW, Rajpathak SN, Lewis O, Crandall JP. Use of the estimated glucose disposal rate as a measure of insulin resistance in an urban multiethnic population with type 1 diabetes. Diabetes Care (2013) 36(8):2280–5. doi: 10.2337/dc12-1693
29. Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol (2014) 63(24):2659–73. doi: 10.1016/j.jacc.2014.02.545
30. Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol (2018) 17(1):122. doi: 10.1186/s12933-018-0762-4
31. Laakso M, Sarlund H, Salonen R, Suhonen M, Pyörälä K, Salonen JT, et al. Asymptomatic atherosclerosis and insulin resistance. Arterioscler Thromb (1991) 11(4):1068–76. doi: 10.1161/01.ATV.11.4.1068
32. Bressler P, Bailey SR, Matsuda M, DeFronzo RA. Insulin resistance and coronary artery disease. Diabetologia (1996) 39(11):1345–50. doi: 10.1007/s001250050581
33. Zethelius B, Lithell H, Hales CN, Berne C. Insulin sensitivity, proinsulin and insulin as predictors of coronary heart disease. a population-based 10-year, follow-up study in 70-year old men using the euglycaemic insulin clamp. Diabetologia (2005) 48(5):862–7. doi: 10.1007/s00125-005-1711-9
34. Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. results from a cross-sectional study in malmö, Sweden. Diabetes Med (2000) 17(4):299–307. doi: 10.1046/j.1464-5491.2000.00280.x
35. Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care (2001) 24(4):683–9. doi: 10.2337/diacare.24.4.683
36. Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio heart study. Diabetes Care (2002) 25(7):1177–84. doi: 10.2337/diacare.25.7.1177
37. Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLos One (2012) 7(12):e52036. doi: 10.1371/journal.pone.0052036
38. Cho YR, Ann SH, Won KB, Park GM, Kim YG, Yang DH, et al. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci Rep (2019) 9(1):6129. doi: 10.1038/s41598-019-42700-1
39. Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest (2016) 46(2):189–97. doi: 10.1111/eci.12583
40. Kouli GM, Panagiotakos DB, Kyrou I, Georgousopoulou EN, Chrysohoou C, Tsigos C, et al. Visceral adiposity index and 10-year cardiovascular disease incidence: The ATTICA study. Nutr Metab Cardiovasc Dis (2017) 27(10):881–9. doi: 10.1016/j.numecd.2017.06.015
41. Kyrou I, Panagiotakos DB, Kouli GM, Georgousopoulou E, Chrysohoou C, Tsigos C, et al. Lipid accumulation product in relation to 10-year cardiovascular disease incidence in Caucasian adults: The ATTICA study. Atherosclerosis (2018) 279:10–6. doi: 10.1016/j.atherosclerosis.2018.10.015
42. Wong SC, Baim DS, Schatz RA, Teirstein PS, King SR, Curry RJ, et al. Immediate results and late outcomes after stent implantation in saphenous vein graft lesions: the multicenter U.S. palmaz-schatz stent experience. the palmaz-schatz stent study group. J Am Coll Cardiol (1995) 26(3):704–12. doi: 10.1016/0735-1097(95)00217-r
43. Van Belle E, Abolmaali K, Bauters C, McFadden EP, Lablanche JM, Bertrand ME. Restenosis, late vessel occlusion and left ventricular function six months after balloon angioplasty in diabetic patients. J Am Coll Cardiol (1999) 34(2):476–85. doi: 10.1016/S0735-1097(99)00202-8
44. Lau KW, Ding ZP, Sigwart U, Lam L. Percutaneous interventional strategies in the treatment of chronic total coronary occlusions. Singapore Med J (2000) 41(9):468–70.
45. Gürlek A, Dağalp Z, Oral D, Omürlü K, Erol C, Akyol T, et al. Restenosis after transluminal coronary angioplasty: a risk factor analysis. J Cardiovasc Risk (1995) 2(1):51–5. doi: 10.1097/00043798-199502000-00008
46. Zhu Y, Liu K, Chen M, Liu Y, Gao A, Hu C, et al. Triglyceride-glucose index is associated with in-stent restenosis in patients with acute coronary syndrome after percutaneous coronary intervention with drug-eluting stents. Cardiovasc Diabetol (2021) 20(1):137. doi: 10.1186/s12933-021-01332-4
Keywords: estimated glucose disposal rate, non-ST-segment elevation acute coronary syndrome, percutaneous coronary intervention, insulin resistance, in-stent restenosis
Citation: Liu C, Zhao Q, Zhao Z, Ma X, Xia Y, Sun Y, Zhang D, Liu X and Zhou Y (2022) Correlation between estimated glucose disposal rate and in-stent restenosis following percutaneous coronary intervention in individuals with non-ST-segment elevation acute coronary syndrome. Front. Endocrinol. 13:1033354. doi: 10.3389/fendo.2022.1033354
Received: 31 August 2022; Accepted: 26 October 2022;
Published: 14 November 2022.
Edited by:
Paola Di Pietro, University of Salerno, ItalyReviewed by:
Leila Warszawski, Instituto Estadual de Diabetes e Endocrinologia Luiz Capriglione, BrazilCopyright © 2022 Liu, Zhao, Zhao, Ma, Xia, Sun, Zhang, Liu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoli Liu, bGl1eGw5ODgxQDE2My5jb20=; Yujie Zhou, YXp6eWoxMkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.