- 1Department of Internal Medicine, Chung-Ang University Gwangmyeong Hospital, Chung-Ang University College of Medicine, Gwangmyeong, South Korea
- 2Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea
- 3Asan Diabetes Center, Asan Medical Center, Seoul, South Korea
Introduction: Type 2 diabetes mellitus (T2DM) is a chronic, progressive disease requiring lifelong treatment, and durable medication is essential for maintaining stable glycemic control. This study aimed to evaluate the long-term efficacy of dulaglutide in participants who have continued the drug for more than one year.
Methods: We conducted a retrospective study on 605 participants, who used dulaglutide for over one year between 2016 and 2020. Changes in glycosylated hemoglobin (HbA1c), fasting plasma glucose, and bodyweight from baseline to last prescription day were assessed. Adherence was evaluated by the proportion of days covered (PDC), and a PDC value ≥ 0.80 was considered adherent.
Results: The mean age was 54.0 ± 11.1 years, and 46.1% were female. The mean baseline HbA1c, bodyweight, and duration of diabetes were 8.8% (72.7 mmol/mol), 75.6 kg, and 12.2 years, respectively. During the mean follow-up of 33.1 months, HbA1c and bodyweight decreased by 1.28% (14 mmol/mol, P < 0.001) and by 3.19 kg (P < 0.001), respectively. The participants were highly adherent with PDC ≥ 0.80 in 92.4% of the participants.
Conclusion: In T2DM patients, long-term dulaglutide treatment was effective in maintaining HbA1c and weight reduction. Dulaglutide could be a favorable option of long-term treatment in real-world clinical practice.
Introduction
Type 2 Diabetes Mellitus (T2DM) is a chronic progressive disease affecting nearly 10% of the adult population worldwide (1). The extent of glycemic control is strongly associated with diabetic complications and mortality (2). Therefore, maintenance of the glycemic goal is critical for patients with T2DM. Antidiabetic drugs show variable durability. Thiazolidinedione has been associated with the most durable glycemic response, followed by sulfonylurea and dipeptidyl-peptidase-4 inhibitor (3). Also, sodium-glucose co-transporter 2 inhibitor show greater durability than dipeptidyl-peptidase-4 inhibitor (4).
The control rate of diabetes is low with only about half of the US diabetic population reaching the target glycosylated hemoglobin (HbA1c) of < 7.0% (5, 6). For patients who do not reach the goal, intensification of medical treatment is often necessary. For those who achieve the target, sustenance of the glycemic control is essential. Although it should be supported by diet and exercise, medical therapy is of great importance. In the last decade, the novel drug class glucagon-like peptide-1 receptor agonist (GLP-1RA) has been introduced. Due to its additional effects on weight loss and low risk of hypoglycemia, GLP-1RA use has been increasing continuously (7). Among the various currently available GLP-1RAs, dulaglutide or semaglutide is preferred because of the convenience of once-weekly administration (8–10).
Dulaglutide has been associated with significant reductions in HbA1c and bodyweight as well as cardiovascular benefits in the Assessment of Weekly Administration of Dulaglutide in Diabetes (AWARD) and Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND) trials (11, 12). Following the actual clinical use of dulaglutide, retrospective real-world studies were also published globally, confirming the efficacy of dulaglutide. However, in most studies, the duration of drug usage was quite short (13–15). Since diabetes is a chronic and progressive disease, it is important to maintain glycemic control for a long period of time (16). Although a few studies did confirm the long-term efficacy of dulaglutide, there has been no report, to our knowledge, on the long-term efficacy of dulaglutide in Asian patients. Therefore, we conducted this real-world study to investigate the durability of dulaglutide treatment in Korean patients with T2DM.
Material and methods
Participants
We initially identified 1398 T2DM patients who were prescribed with dulaglutide at least once from June 2016 to November 2020 (Figure 1). After thorough review of medical records, patients with missing laboratory data (n = 46), steroid use (n = 11), active cancer (n = 29), and previous GLP-1RA use (n = 17) were excluded. We further excluded patients who had been using dulaglutide for less than a year by the time of analysis (n = 91) and those who were not present for follow-up or were referred to regional hospitals (n = 185). Of the 1019 patients eligible for analysis, 605 patients continued dulaglutide treatment for over one year and were included in the final analysis. Minimum 12-month use was suggested for an anti-diabetic drug to be considered durable (17). This study followed the principles of the Declaration of Helsinki and Korean Good Clinical Practice and was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2020-1914).
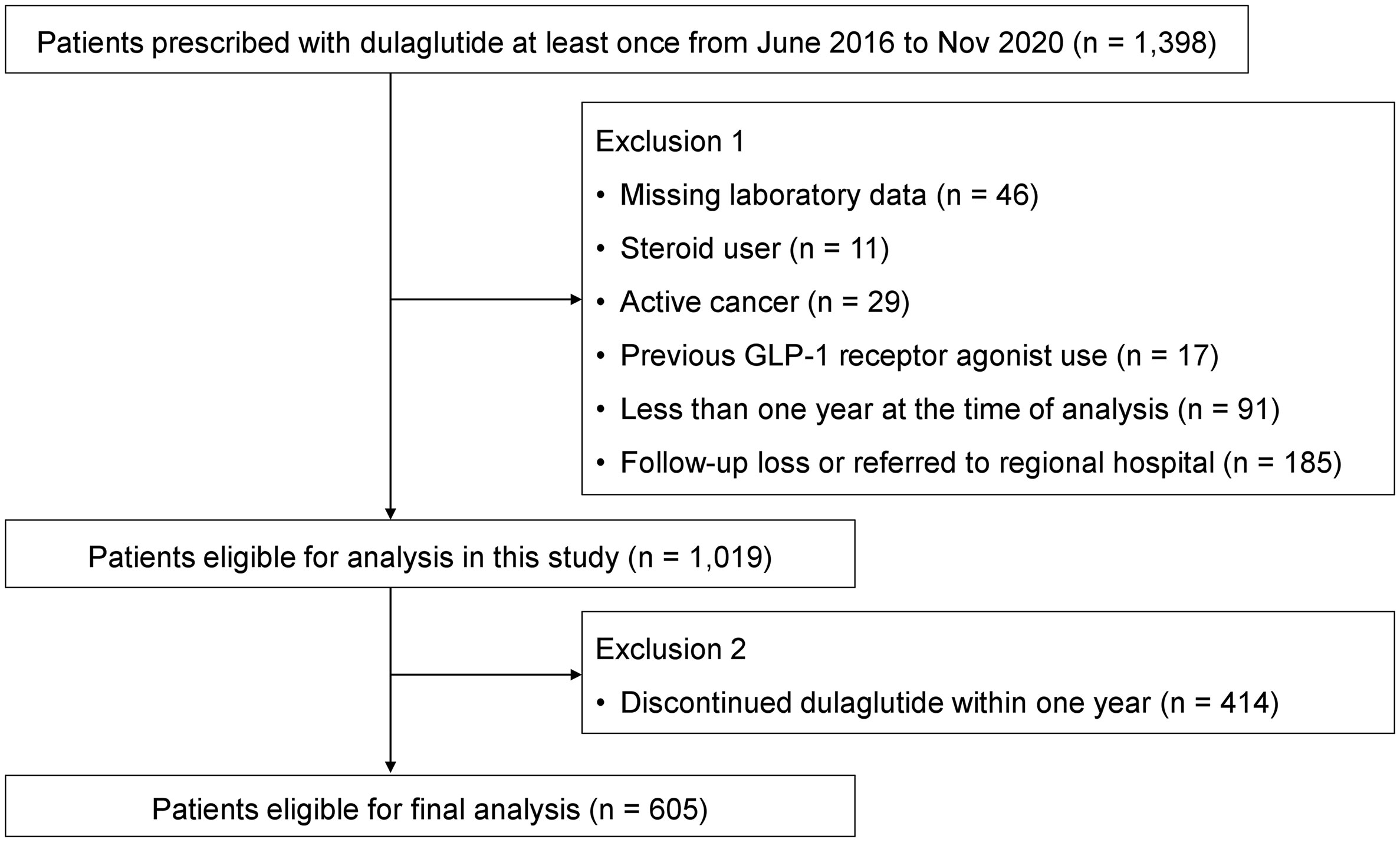
Figure 1 Flow diagram showing the selection process of the study population for durability of dulaglutide.
Clinical and laboratory measurements
Clinical data including age, sex, bodyweight, height, body mass index (BMI), blood pressure (BP), duration of diabetes, co-existing hypertension, dyslipidemia, diabetic complications, and use of other medications were collected. For participants who had not continued dulaglutide treatment for a year, the reasons for discontinuation were documented. Laboratory measurements consisted of glycosylated hemoglobin (HbA1c), fasting plasma glucose (FPG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, estimated glomerular filtration rate (eGFR), and fasting c-peptide. Data on concomitant anti-diabetic drugs, HbA1c, FPG, and weight on the last prescription day were assembled.
Outcomes
The primary outcome measure was the evaluation of glycemic control in participants who initiated dulaglutide treatment and continued for at least one year. For this purpose, change of HbA1c from baseline to last prescription day was measured. Additionally, HbA1c, fasting plasma glucose (FPG), and bodyweight were also assessed every six months. The secondary outcome measure was adherence, which was measured by the proportion of days covered (PDC), evaluated by chart review. PDC was calculated as the total number of days covered by prescribed dulaglutide divided by the total number of days in the post-initiation follow-up period (13). Participants with a PDC of 0.80 or higher were considered adherent (13). Subgroup analyses of HbA1c reduction according to age, baseline HbA1c, BMI, duration of diabetes, starting dulaglutide dose, and final dulaglutide dose were performed. Subgroup analyses of HbA1c and weight reduction according to baseline antidiabetic drug use and change in other antidiabetic drugs were also conducted.
Statistical analysis
Continuous and categorical variables were each shown as mean ± standard deviation and number (percentages), respectively. Changes in HbA1c, FPG, and bodyweight over time were analyzed using a repeated measures model. Paired t-tests were performed for subgroup analyses. To evaluate the parameters affecting the glycemic response, univariate and multivariate linear regression analyses were performed. All statistical analyses were performed using SPSS version 23.0 for Windows (IBM Co., Armonk, NY, USA).
Results
Selected population and baseline characteristics
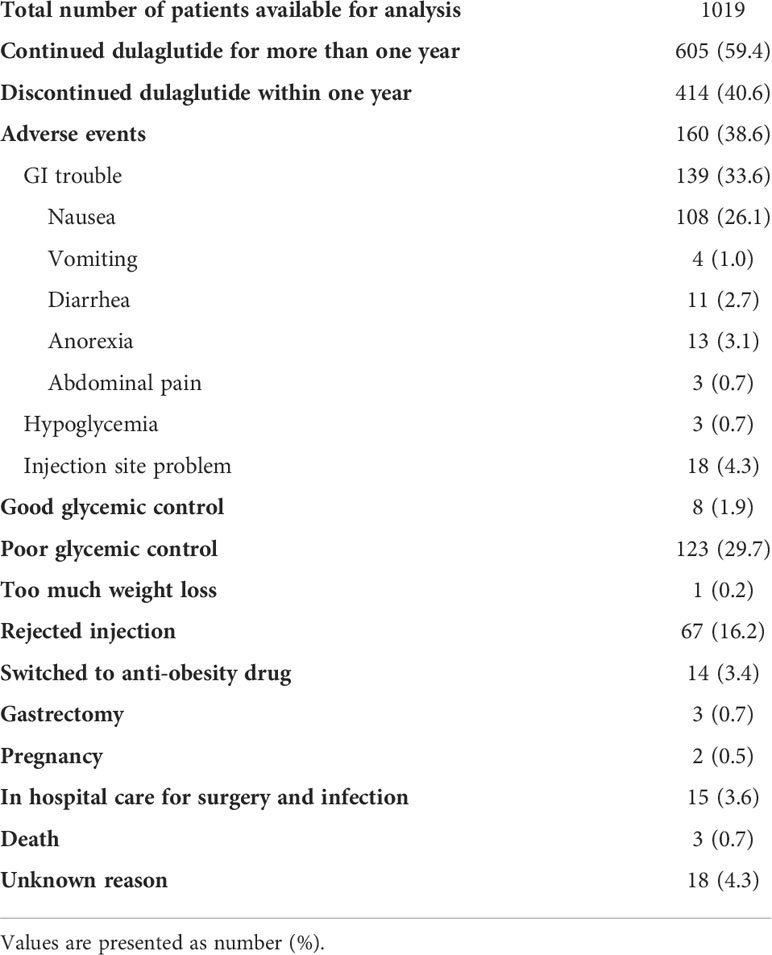
A total of 1019 participants were eligible for analysis after applying the exclusion criteria (Figure 1). Of these, 414 participants were further excluded because of discontinuation of dulaglutide within a year after initiation. As a result, 605 participants who continued dulaglutide for more than one year (mean follow-up 33.1 months) were included in the final analyses. The reasons for discontinued dulaglutide use are presented in Table 1. The most common reason for stopping dulaglutide was adverse events (160 participants; 38.6%). Among the 160 participants who discontinued the drug due to adverse events, 139 experienced gastrointestinal trouble, of which nausea was by far the most experienced by 108 participants. Eighteen and three participants stopped the drug due to injection site problem and hypoglycemia, respectively. Other reasons for discontinuing dulaglutide were the use of injection as the mode of administration (67 participants; 16.2%), poor glycemic control (123 participants; 29.7%), and good glycemic control (8 participants; 1.9%). Decision to discontinue dulaglutide for poor or good glycemic control was based on the clinician’s judgment. All patients who stopped dulaglutide due to poor glycemic control were switched to insulin. Patients who reached the individualized HbA1c target and de-escalated treatment by discontinuing dulaglutide were considered good glycemic control. Three deaths were identified, and the causes of death were stroke and pneumonia for two and unknown for one.
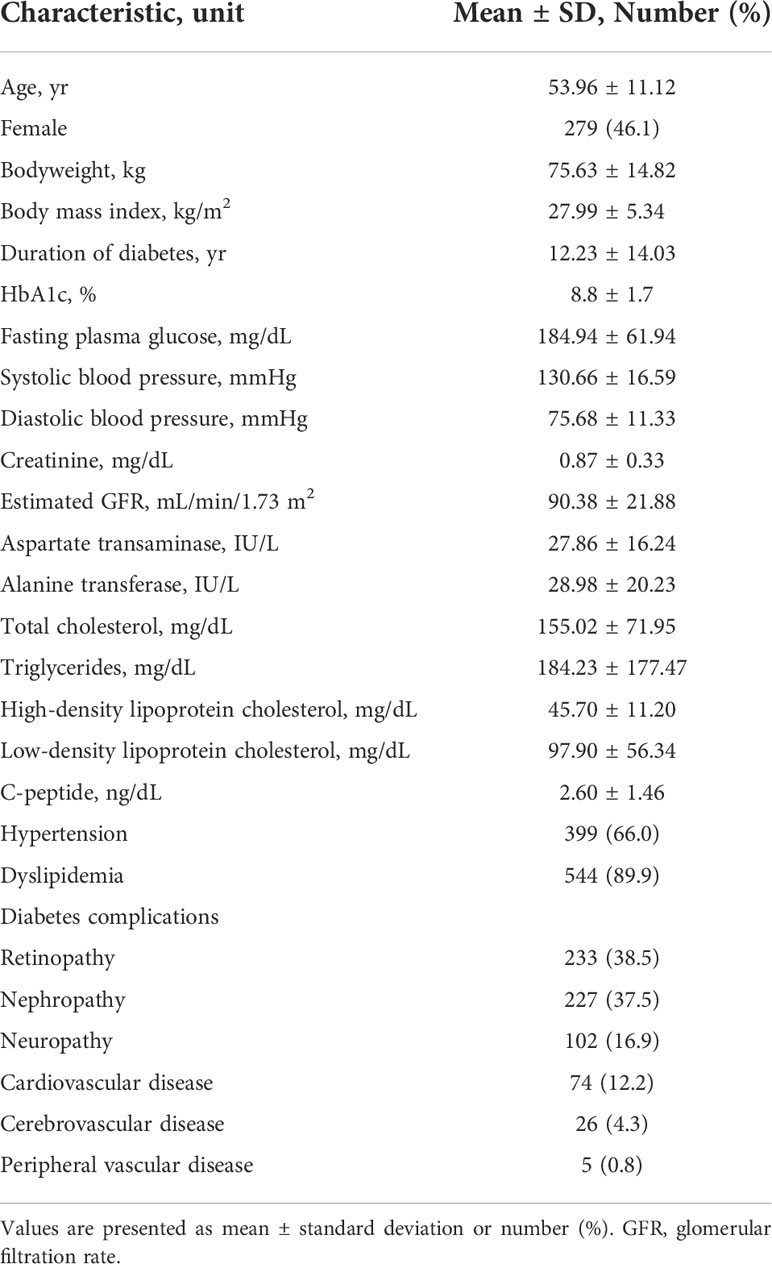
Baseline characteristics of the 605 participants who continued dulaglutide for over one year are shown in Table 2. The mean age was 53.96 ± 11.12 years, and 46.2% of the participants were female. The mean bodyweight was 75.63 kg, and the mean BMI was 27.99 kg/m2. The mean duration of diabetes was 12.23 years. HbA1c and FPG levels were 8.8% and 184.94 mg/dL, respectively. The mean fasting c-peptide concentration was 2.60 ng/dL. Hypertension and dyslipidemia were present in 66.0% and 89.9% of the study population, respectively.
Treatment patterns
Majority of the participants (555 of 605; 91.7%) started the treatment with 0.75 mg of dulaglutide, and 83.4% of these participants switched to 1.5 mg of dulaglutide during follow-up. Of the 50 (8.3%) participants who initiated dulaglutide use at 1.5 mg, only one patient decreased the dose to 0.75 mg during follow-up.
All the participants were on at least one oral antihyperglycemic drug at baseline (Table 3). Metformin was the most common oral antihyperglycemic drug used by the participants at baseline (592 participants; 97.9%), followed by sulfonylurea (487 participants; 80.5%). Insulin was used by 138 participants (22.8%). At the last follow-up visit, the number of participants using metformin and sulfonylurea decreased to 579 (95.7%) and 426 (70.4%), respectively. Conversely, the use of thiazolidinedione, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, and insulin increased. Number of participants using SGLT2 inhibitor showed the greatest increase from 63 (10.4%) at baseline to 141 (23.3%) at last follow-up.
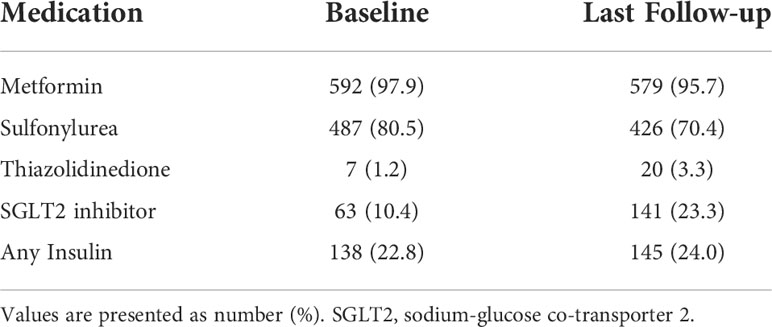
Table 3 Use of concomitant anti-diabetic medication with dulaglutide at baseline and last follow-up.
Efficacy of long-term dulaglutide
HbA1c levels reduced significantly after using dulaglutide for six months and were maintained through follow-up (P < 0.001) (Figure 2A). Mean reduction in HbA1c was 1.2 ± 1.6% from baseline to last follow-up. FPG levels also significantly decreased after six months of dulaglutide use and were maintained until last follow-up (P < 0.001) (Figure 2B). Mean FPG decrease was 46.1 ± 59.5 mg/dL. Bodyweight was significantly reduced by 3.3 ± 5.4 kg from baseline to last follow-up (P < 0.001) (Figure 2C). The ratio of participants with PDC ≥ 0.80 was 92.4%, and the mean PDC was 0.98.
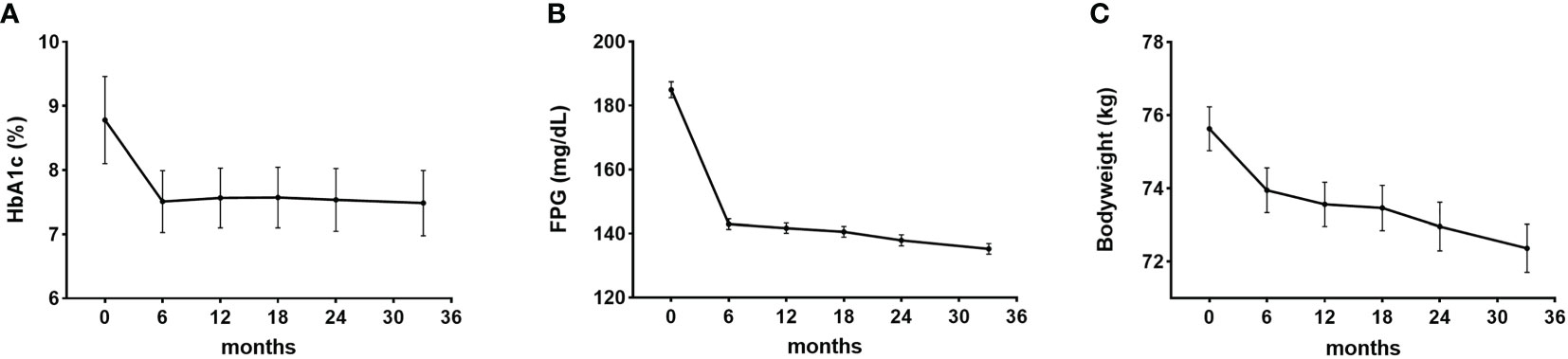
Figure 2 Efficacy of dulaglutide. Changes in (A) glycosylated hemoglobin (HbA1c), (B) fasting plasma glucose (FPG), and (C) bodyweight after dulaglutide use. Data are presented as mean ± standard error. All values for 6–36 months in each of the panels (A–C) were significantly different (P < 0.05) compared with the corresponding baseline values.
Clinical parameters affecting the glucose-lowering effect of long-term dulaglutide
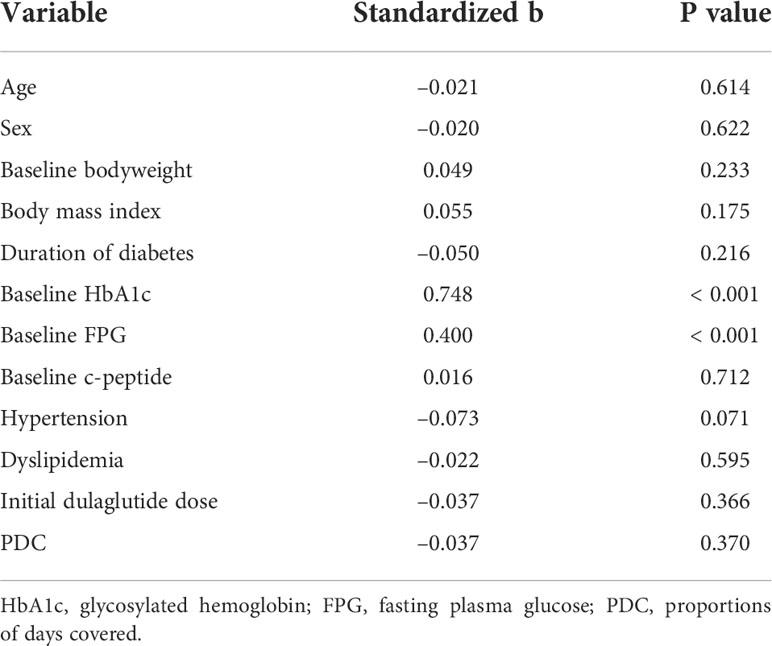
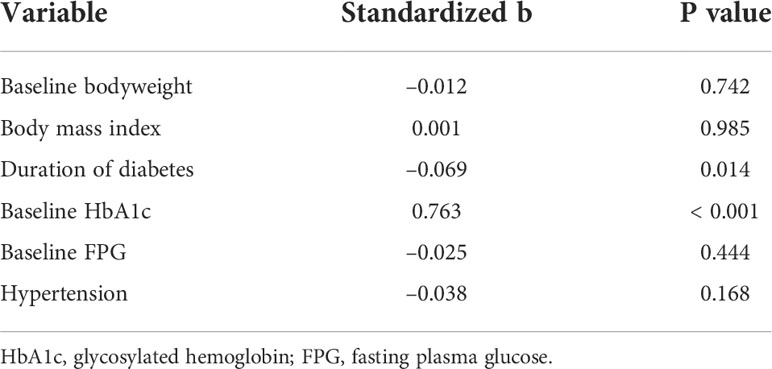
According to univariate linear regression analysis, factors predicting the glucose-lowering efficacy of long-term dulaglutide treatment were baseline HbA1c and baseline FPG levels (Table 4). Multiple linear regression analysis showed that duration of diabetes and baseline HbA1c levels significantly affected HbA1c reduction (Table 5). Subgroups divided by baseline HbA1c levels (< 9.0% versus ≥ 9.0%) and diabetes duration (< 10 years versus ≥ 10 years) showed significant differences in the levels of HbA1c reduction; participants with higher baseline HbA1c levels and shorter diabetes durations responded better to dulaglutide. However, no significant difference in HbA1c reduction was observed between the subgroups defined according to age, BMI, or initial/final dulaglutide dose (Figure 3). In all subgroups regarding other antidiabetic drug use at baseline, significant decreases in HbA1c and bodyweight were observed (Supplemental Table 1). When the subjects were divided according to change in other antidiabetic drugs, HbA1c and bodyweight also showed significant reduction (Supplemental Table 2).
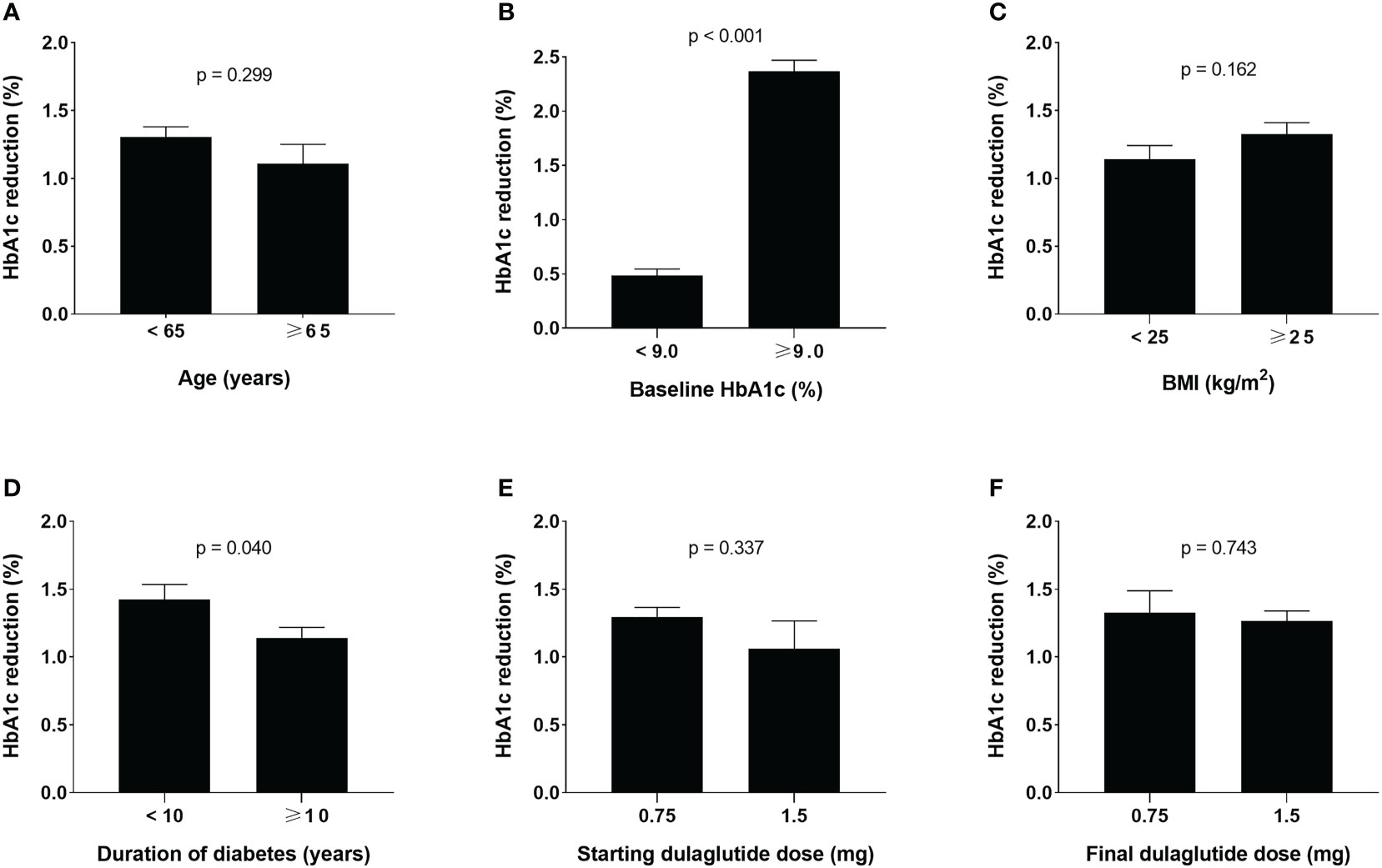
Figure 3 Glucose-lowering effects of dulaglutide among subgroups. Subgroup analyses for changes in HbA1c levels according to (A) age, (B) baseline HbA1c, (C) BMI, (D) duration of diabetes, (E) initial dulaglutide dose, and (F) final dulaglutide dose. Data are presented as mean ± standard error. HbA1c, glycosylated hemoglobin; BMI, body mass index.
Discussion
Our results showed that long-term dulaglutide treatment was effective and safe in patients with T2DM. In real-world clinical practice, dulaglutide significantly improved glycemic control in the first six months, and this effect was maintained throughout the period of dulaglutide treatment. In parallel, FPG and bodyweight also showed similar patterns of sustainable improvement. Higher baseline HbA1c levels and shorter duration of diabetes were associated with greater reductions in HbA1c levels.
While most studies on dulaglutide evaluated its effectiveness over a period of 6–12 months, two previous retrospective studies evaluated its effects after 2 years of usage. Moreno Obregón et al. analyzed 163 Spanish patients and found 1.4% (15 mmol/mol) reduction in HbA1c (P < 0.001) and 30 mg/dL reduction in FPG levels (P < 0.001) after 6 months of drug use, both of which were maintained until the follow-up at 2 years (18). Bodyweight, evaluated every 6 months, showed a continues decrease, resulting in a reduction of 7.27 kg after 2 years (P < 0.001). Compared with our results, the study on Spanish patients reported a greater reduction in HbA1c, a smaller decrease in FPG, and almost 4 kg greater reduction in bodyweight. This might be explained by the difference in the baseline bodyweight (75.63 kg in our study population versus 99.57 kg in the Spanish study population). Another study recently conducted in the United States (US) evaluated the efficacy of dulaglutide over a period of 2 years in 872 patients (19). The baseline HbA1c level was 8.7%, similar to that 8.8% in our study. The extent of HbA1c reduction was 1.3% and 1.2% in the US study and the current study, respectively. Unfortunately, the US study did not report data on weight reduction or percentage of Asian ethnic group included in the study. So, we cannot make any comparisons in these aspects. However, subgroup analyses of both studies showed that HbA1c levels reduced regardless of sex, age, and index dulaglutide dose, which is also accordant with the results of post-hoc analysis of the AWARD trials (20). The patterns of other antihyperglycemic medication use at baseline and at 24-month follow-up in the US study were similar to those in our study with increased use of SGLT2 inhibitor, thiazolidinedione, and insulin and decreased use of sulfonylurea. Previous reports of GLP-1RA and SGLT2 inhibitor combination therapy suggest significant weight reduction benefits and possible additional cardiovascular benefits, which could explain their increased use (21–23).
Results of our analysis comparing BMI subgroups did not differ from those of the post-hoc analysis of the AWARD trials, showing no significant differences in HbA1c reduction levels among the subgroups (24). However, the mean baseline BMI levels in the AWARD trials, ranging between 31.2 and 33.3 kg/m2, were greater compared with the corresponding levels of 27.99 kg/m2 in our study. In addition to the disparity in the mean BMI values, the BMI categories were also defined by different intervals (<30 kg/m2; ≥30 and <35 kg/m2; ≥35 kg/m2 versus <25 kg/m2; ≥25 kg/m2). The use of different criteria for BMI categories seems reasonable, as the diagnostic criteria for obesity are different in East Asian (25). Another real-world study by Morieri et al. showed that improvements in HbA1c and weight indices were significant and consistent regardless of age, obesity, and chronic kidney disease, while these effects were greater in patients with shorter durations of diabetes and in those who had not used GLP-1RAs previously (26). Since the patients in our study were all GLP-1RA-naïve, we could not analyze the data to examine the effects of previous GLP-1RA exposure. Nevertheless, our study adds further evidence that shorter duration of diabetes is associated with a more pronounced HbA1c reduction.
The AWARD-5 trial evaluated the effects of long-term dulaglutide use (over 24 months) (27). HbA1c and bodyweight indices were significantly reduced by 1.0% and 2.9 kg, respectively, after 2 years (P < 0.001 for both) (27). The lower baseline HbA1c levels (8.1% vs 8.8% in our study) could have influenced the extent of reductions in HbA1c and bodyweight, which were smaller than those in our study (1.2% and 3.3 kg). Also, all patients in the AWARD-5 trial were on metformin as the only background hypoglycemic medication. Additionally, since AWARD-5 trial was a controlled randomized clinical trial, the patients who discontinued dulaglutide during the 2-year follow-up period were also included in the final analysis of the results.
Adherence is frequently suboptimal in T2DM patients, which can worsen glycemic control, increase hospitalization, and lead to diabetic complications (28). Mody et al. previously reported an adherence of 61% over a dulaglutide treatment period of 6 months with a mean PDC of 0.76 (13), which are substantially less than the 92% adherence and mean PDC of 0.98 in our study. The high adherence in our study might have ensued from selection bias because our analysis only included patients who continued dulaglutide treatment for more than one year. Also, since a total of 320 patients showed a PDC greater than 1.0, PDC might not completely reflect the actual administration of the drug. Additionally, Durden et al. showed that early response to GLP-1RA (defined as at least 1% reduction in HbA1c and at least 3% reduction in bodyweight within 3–6 months) is associated with higher adherence (29). The present study population showed mean reductions of 1.2% in HbA1c and 4.4% in bodyweight from baseline during the first 6 months of dulaglutide initiation, which might have contributed to the high adherence rate.
This study has some limitations. First, it is a retrospective study based on data acquired from chart review, so the adverse events and hypoglycemic episodes reported by patients could be under-estimated or over-estimated. Additionally, the retrospective nature of the study could have caused selection bias, and the patients excluded could have influenced the results. Second, the results might not be generalized, because the analyses were conducted on data obtained from a single institution. Third, the current study was not controlled, so other medications taken in parallel could have affected the results. Finally, while GLP-1RA is in the limelight for its pleiotropic benefits in addition to its glucose-lowering and weight-reducing effects, we have not assessed its cardiovascular or renal outcomes, which we plan to investigate in the near future.
While the retrospective, observational aspect of the current study might be a limitation, our results may provide valuable insights on the real-world clinical practice of T2DM management. As clinical trials follow a tightly defined criteria for patient enrollment, study populations in clinical trials may not be truly representative of general patient populations. In contrast, real-world evidence provides objective data about treatment trends and patient outcomes in general clinical practice. To the best of our knowledge, this is the first study to evaluate long-term efficacy of dulaglutide in Asian population in real world. In addition, relatively large number of participants were analyzed, and data on adherence and sustainability of dulaglutide were presented.
In summary, our study revealed that dulaglutide shows sustainable efficacy and safety in real-world clinical practice. HbA1c, FPG, and bodyweight indices were significantly improved after 6 months of dulaglutide use, and the improvements were maintained throughout the period of dulaglutide treatment. As the first real-world study of long-term dulaglutide treatment in Asia, this study contributes valuable data to the literature on glycemic effectiveness and sustainability of dulaglutide.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Asan Medical Center (IRB No. 2020-1914). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
WL designed the research; HK and MK conducted research; YC, CJ, WL, and J-YP provided essential materials; HK, MK, YC analyzed data and wrote paper; HK, YC, CJ, and WL had primary responsibility of the final content; All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1032793/full#supplementary-material
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj. (2000) 321(7258):405–12. doi: 10.1136/bmj.321.7258.405
3. Mamza J, Mehta R, Donnelly R, Idris I. Important differences in the durability of glycaemic response among second-line treatment options when added to metformin in type 2 diabetes: a retrospective cohort study. Ann Med (2016) 48(4):224–34. doi: 10.3109/07853890.2016.1157263
4. Bailey CJ, Del Prato S, Wei C, Reyner D, Saraiva G. Durability of glycaemic control with dapagliflozin, an SGLT2 inhibitor, compared with saxagliptin, a DPP4 inhibitor, in patients with inadequately controlled type 2 diabetes. Diabetes Obes Metab (2019) 21(11):2564–9. doi: 10.1111/dom.13841
5. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med (2013) 368(17):1613–24. doi: 10.1056/NEJMsa1213829
6. Carls G, Huynh J, Tuttle E, Yee J, Edelman SV. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther (2017) 8(4):863–73. doi: 10.1007/s13300-017-0280-5
7. Dave CV, Schneeweiss S, Wexler DJ, Brill G, Patorno E. Trends in clinical characteristics and prescribing preferences for SGLT2 inhibitors and GLP-1 receptor agonists, 2013-2018. Diabetes Care (2020) 43(4):921–4. doi: 10.2337/dc19-1943
8. Alatorre C, Fernández Landó L, Yu M, Brown K, Montejano L, Juneau P, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: Higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab (2017) 19(7):953–61. doi: 10.1111/dom.12902
9. Mody R, Huang Q, Yu M, Zhao R, Patel H, Grabner M, et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12-month follow-up in a real-world setting in the united states. Diabetes Obes Metab (2019) 21(4):920–9. doi: 10.1111/dom.13603
10. Uzoigwe C, Liang Y, Whitmire S, Paprocki Y. Semaglutide once-weekly persistence and adherence versus other GLP-1 RAs in patients with type 2 diabetes in a US real-world setting. Diabetes Ther (2021) 12(5):1475–89. doi: 10.1007/s13300-021-01053-7
11. Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care (2014) 37(8):2168–76. doi: 10.2337/dc13-2759
12. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. (2019) 394(10193):121–30. doi: 10.1016/S0140-6736(19)31149-3
13. Mody R, Grabner M, Yu M, Turner R, Kwan AYM, York W, et al. Real-world effectiveness, adherence and persistence among patients with type 2 diabetes mellitus initiating dulaglutide treatment. Curr Med Res Opin (2018) 34(6):995–1003. doi: 10.1080/03007995.2017.1421146
14. Yoo JH, Cho YK, Lee J, Kim HS, Kang YM, Jung CH, et al. Clinical efficacy and parameters affecting the response to dulaglutide treatment in patients with type 2 diabetes: A retrospective, real-world data study. Diabetes Ther (2019) 10(4):1453–63. doi: 10.1007/s13300-019-0658-7
15. Robinson S, Boye KS, Mody R, Strizek AA, Konig M, Malik RE, et al. Real-world effectiveness of dulaglutide in patients with type 2 diabetes mellitus: A literature review. Diabetes Ther (2020) 11(7):1437–66. doi: 10.1007/s13300-020-00839-5
16. Kim KJ, Choi JH, Kim KJ, An JH, Kim HY, Kim SG, et al. Determinants of long-term durable glycemic control in new-onset type 2 diabetes mellitus. Diabetes Metab J (2017) 41(4):284–95. doi: 10.4093/dmj.2017.41.4.284
17. Kalra S, Kamaruddin NA, Visvanathan J, Santani R. Defining disease progression and drug durability in type 2 diabetes mellitus. Eur Endocrinol (2019) 15(2):67–9. doi: 10.17925/EE.2019.15.2.67
18. Moreno Obregón F, Miramontes-González JP, Romo Guajardo-Fajardo C, Nieto-Sánchez Á, López-Suárez JM, Martín-Vallejo J, et al. Real-life experience with dulaglutide: Analysis of clinical effectiveness to 24 months. Diabetes Res Clin Pract (2019) 158:107916. doi: 10.1016/j.diabres.2019.107916
19. Mody R, Yu M, Grabner M, Boye K, Teng CC, Kwan AYM. Dulaglutide shows sustained reduction in glycosylated hemoglobin values: 2-year US real-world study results. Clin Ther (2020) 42(11):2184–95. doi: 10.1016/j.clinthera.2020.09.011
20. Boustani MA, It P, Yu M, Thieu VT, Varnado OJ, Juneja R. Similar efficacy and safety of once-weekly dulaglutide in patients with type 2 diabetes aged ≥65 and <65 years. Diabetes Obes Metab (2016) 18(8):820–8. doi: 10.1111/dom.12687
21. Ludvik B, Frías JP, Tinahones FJ, Wainstein J, Jiang H, Robertson KE, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol (2018) 6(5):370–81. doi: 10.1016/S2213-8587(18)30023-8
22. Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. Bmj (2021) 372:m4573. doi: 10.1136/bmj.m4573
23. Kim HS, Yoon T, Jung CH, Park JY, Lee WJ. Clinical efficacy of sodium-glucose cotransporter 2 inhibitor and glucagon-like peptide-1 receptor agonist combination therapy in type 2 diabetes mellitus: Real-world study. Diabetes Metab J (2022) 46(4):658–62. doi: 10.4093/dmj.2021.0232
24. Gentilella R, Sesti G, Vazquez L, Sapin H, Reed V, Romera I, et al. Dulaglutide is an effective treatment for lowering HbA1c in patients with type 2 diabetes regardless of body mass index. Diabetes Obes Metab (2019) 21(12):2660–6. doi: 10.1111/dom.13853
25. Seo MH, Lee WY, Kim SS, Kang JH, Kang JH, Kim KK, et al. 2018 Korean Society for the study of obesity guideline for the management of obesity in Korea. J Obes Metab Syndr (2019) 28(1):40–5. doi: 10.7570/jomes.2019.28.1.40
26. Morieri ML, Frison V, Rigato M, D'Ambrosio M, Tadiotto F, Paccagnella A, et al. Effectiveness of dulaglutide in the real world and in special populations of type 2 diabetic patients. J Clin Endocrinol Metab (2020) 105(7):e2617–25. doi: 10.1210/clinem/dgaa204
27. Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab (2015) 17(9):849–58. doi: 10.1111/dom.12479
28. Giorgino F, Penfornis A, Pechtner V, Gentilella R, Corcos A. Adherence to antihyperglycemic medications and glucagon-like peptide 1-receptor agonists in type 2 diabetes: clinical consequences and strategies for improvement. Patient Prefer Adherence. (2018) 12:707–19. doi: 10.2147/PPA.S151736
Keywords: type 2 diabetes mellitus, adherence, dulaglutide, real-world evidence, GLP-1 receptor agonist
Citation: Kim HS, Cho YK, Kim MJ, Jung CH, Park J-Y and Lee WJ (2022) Durability of glucose-lowering effect of dulaglutide in patients with type 2 diabetes mellitus: A real-world data study. Front. Endocrinol. 13:1032793. doi: 10.3389/fendo.2022.1032793
Received: 31 August 2022; Accepted: 18 October 2022;
Published: 31 October 2022.
Edited by:
Habib Yaribeygi, Semnan University of Medical Sciences, IranReviewed by:
Abdurezak Ahmed Abdela, Addis Ababa University, EthiopiaAbilash Nair, Government Medical College, Thiruvananthapuram, India
Copyright © 2022 Kim, Cho, Kim, Jung, Park and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Woo Je Lee, bHdqYXRsYXNAZ21haWwuY29t
 Hwi Seung Kim
Hwi Seung Kim Yun Kyung Cho
Yun Kyung Cho Myung Jin Kim2,3
Myung Jin Kim2,3 Chang Hee Jung
Chang Hee Jung Woo Je Lee
Woo Je Lee