- 1Department of Health Services Administration, China Medical University, Taichung, Taiwan
- 2School of Nursing, Chung Shan Medical University, Taichung, Taiwan
- 3Department of Nursing, Chung Shan Medical University Hospital, Taichung, Taiwan
- 4School of Pharmacy, China Medical University, Taichung, Taiwan
- 5Department of Pharmacy, China Medical University Hospital, Taichung, Taiwan
- 6School of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 7Department of Pharmacology, Chung Shan Medical University, Taichung, Taiwan
- 8Department of Pharmacy, Chung Shan Medical University Hospital, Taichung, Taiwan
Background and aims: Studies have demonstrated that the short-term use of metformin benefits liver function among patients with type 2 diabetes mellitus (T2DM). However, few studies have reported on the effects of long-term metformin treatment on liver function or liver histology. This study investigated the correlation between metformin use and the incidence of nonalcoholic fatty liver disease (NAFLD) among patients with T2DM.
Methods: This population-based study investigated the risk of NAFLD among patients with T2DM who received metformin treatment between 2001-2018. Metformin users and metformin nonusers were enrolled and matched to compare the risk of NAFLD.
Results: After 3 years, the patients who received <300 cDDD of metformin and those with metformin use intensity of <10 and 10–25 DDD/month had odds ratios (ORs) of 1.11 (95% confidence interval [CI] = 1.06–1.16), 1.08 (95% CI = 1.02–1.13), and 1.18 (95% CI = 1.11–1.26) for NAFLD, respectively. Moreover, metformin users who scored high on the Diabetes Complications and Severity Index (DCSI) were at high risk of NAFLD. Patients with comorbid hyperlipidemia, hyperuricemia, obesity, and hepatitis C were also at high risk of NAFLD.
Conclusion: Patients with T2DM who received metformin of <300 cDDD or used metformin at an intensity of <10 and 10–25 DDD/month were at a high risk of developing NAFLD. The results of this study also indicated that patients with T2DM receiving metformin and with high scores on the DCSI were at a high risk of developing NAFLD.
Highlights
1. According to results from 3-year follow-up, metformin users with type 2 diabetes had an increased risk for NAFLD, with odds ratio of 1.11 (95% confidence interval [CI] = 1.06–1.16).
2. Metformin users who scored high on the Diabetes Complications and Severity Index (DCSI) were at high risk of NAFLD.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a major public health concern worldwide because of its high prevalence. NAFLD is characterized by increased hepatic triglycerides in patients who do not consume alcohol excessively (1). NAFLD is typically classified into nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH); NASH is characterized by liver inflammation and hepatocyte damage due to the development of NAFLD (2). The accumulation of triglycerides within the cytoplasm of hepatocytes is a distinguishing characteristic of NAFLD (1).
The correlation between NAFLD and type 2 diabetes mellitus (T2DM) is indicated by insulin resistance (IR) and the progression of compensatory hyperinsulinemia leading to defective lipid metabolism and hepatic triglyceride accumulation (3). NAFLD is highly prevalent among patients with T2DM, accompanied by frequent incidences of obesity and IR (4). Hepatic fat accumulation among patients with T2DM is more likely to progress to NASH and fibrosis than among patients without T2DM (5). Patients with T2DM exhibit more than a twofold increase in the prevalence of NAFLD, regardless of the diagnostic method used (6).
Recent studies have reported that metformin can improve IR and hyperinsulinemia and may aid in the treatment of NAFLD (7). Evidence from animal and human studies has indicated that metformin may attenuate the onset and progression of NAFLD (8–11). Several studies have attributed the alleviating effects of metformin on NAFLD to the anti-inflammatory effects of metformin (12, 13),. However, metformin is not used for treating NAFLD because of a lack of evidence that metformin significantly improves liver histology (2, 14).
Few epidemiological studies have reported the effects of long-term metformin use on the risk of NAFLD among patients with T2DM. Therefore, we investigated whether long-term metformin use is associated with the risk of NAFLD by using the patient population in Taiwan’s National Health Insurance Research Database (NHIRD).
Material and methods
Data source
Secondary data analysis was performed in this study by using the Longitudinal Health Insurance Database (LHID; a subset of the NHIRD) from 2001 to 2018 released by the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW). The LHID is prepared from claims from Taiwan’s National Health Insurance (NHI) program that enrolls up to 99% of Taiwanese citizens. Hence, the database is a nationally representative health database for Taiwan. The data in the LHID, including detailed clinical data of outpatient visits, hospitalizations, diagnostic results, and prescriptions, have demonstrated high concordance between NHI claims records and patient self-reports (15). Therefore, the LHID was used to analyze the risk of NAFLD among patients with DM receiving metformin. The data in the LHID are anonymized, and the HWDC assigns scrambled random identification numbers to insured patients to protect their privacy. The requirement of informed consent was waived.
Ethics approval
This study was conducted in compliance with the Declaration of Helsinki. Data used in the analysis were anonymized and released by the HWDC, MOHW, Taiwan. The HWDC assigns scrambled random identification numbers to insured patients to protect their privacy. The study was approved by the Central Regional Research Ethics Committee of China Medical University, Taiwan, as meeting all ethical criteria (No. CRREC-109-011).
Study participants
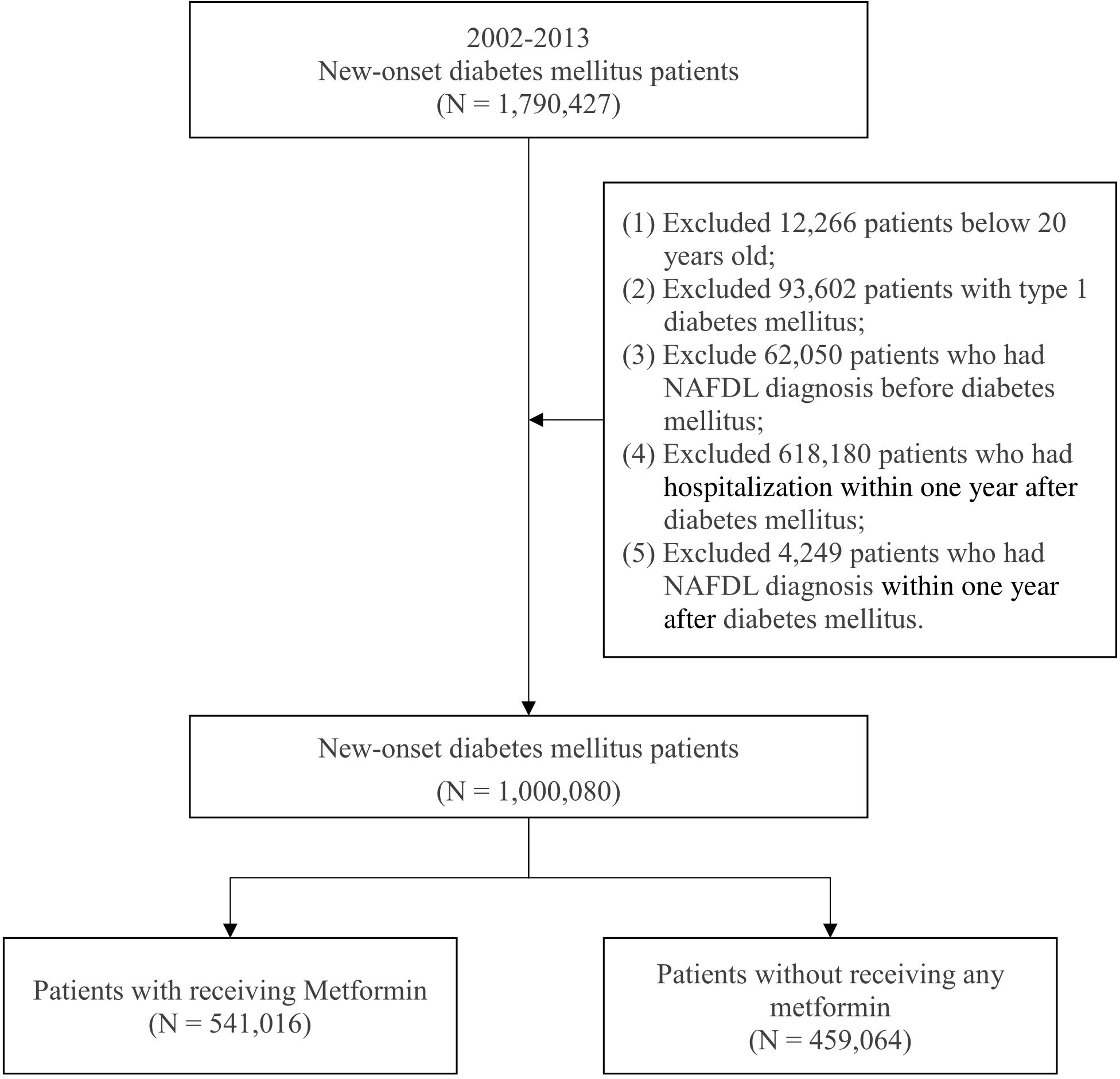
Patients with new-onset DM who were aged above 20 years were enrolled in this study to investigate the effects of metformin on incident NAFLD from 2002 to 2013. The criterion for DM was three diagnoses in a year according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM; code 250). The criterion for metformin use was based on Anatomical Therapeutic Chemical (ATC) code A10BA02. To reduce study bias, we excluded patients with type 1 DM diagnosed with NAFLD before the onset of DM, those diagnosed with NAFLD in the first year after the onset of DM, and those hospitalized within one year after the onset of DM. The patients were divided into two groups: a case group and a comparison group. The case group included patients who had received metformin in the first year after the onset of DM, and the comparison group included patients who had not received any metformin. A total of 1,000,080 patients with new-onset DM were included from 2002 to 2013; of them, 459,064 patients had not received any metformin, and 541,016 patients had received metformin in the first year after the onset of DM. Figure 1 illustrates the process of selecting study participants.
Study design
This study had a cross-sectional design and investigated the risk of NAFLD among patients with DM who received metformin for 3 or 5 years. The defined daily dose (DDD) is used as a standard unit for measuring drug utilization and drug exposure in a population. The World Health Organization defines DDD as the estimated average maintenance dose per day of a drug used to treat a condition in adults. The DDD does not necessarily reflect the recommended or prescribed daily dose (16). Each patient was observed for one year after the diagnosis of DM to assess the use of metformin. The DDD of metformin used to evaluate the medication was 2 g (17). The cumulative DDD (cDDD) of metformin use in the first year was calculated and categorized into five groups for dose–response analysis: nonusers, <300 cDDD, 300–500 cDDD, and above 500 cDDD. Furthermore, we calculated and categorized the average monthly DDD into four groups to investigate the association of metformin use intensity with NAFLD incidence: nonusers, <10 DDD, 10–25 DDD, and above 25 DDD. All patients were observed for 3 and 5 years to analyze the association between metformin use and NAFLD incidence. The criterion for NAFLD in this study was three or more diagnoses within one year, according to ICD-9-CM code 571.8 and ICD-10-CM codes K75.81 and K76.0. The control variables included diabetes severity and related comorbidities. We used the Diabetes Complications Severity Index (DCSI) to adjust the diabetes severity. The DCSI was used to assess the DM patients’ risks of adverse outcomes calculated by the information from the seven diabetes complication categories (retinopathy, nephropathy, neuropathy, cerebrovascular, cardiovascular, peripheral vascular disease, and metabolic) (18, 19). The assessed comorbidities included hypertension (ICD-9-CM 401–405), hyperlipidemia (ICD-9-CM 272.0–272.4), hyperuricemia (ICD-9-CM 790.6), chronic kidney disease (CKD; ICD-9-CM 585), obesity (ICD-9-CM 278.00), Helicobacter pylori infection (ICD-9-CM 041.86), psoriasis (ICD-9-CM 696.1), rheumatoid arthritis (RA ICD-9-CM 714), hypothyroidism (ICD-9-CM 244.9), polycystic ovary syndrome (ICD-9-CM 256.4), and hepatitis C virus (HCV; ICD-9-CM 070.4, 070.5, 070.70).
Statistical analysis
All analyses in the study were performed using SAS version 9.4. The chi-square test was used to evaluate the distribution of the baseline characteristics between metformin users and nonusers. Differences in the incidence of NAFLD between metformin users and nonusers were estimated through multiple logistic regression with the adjustment of the relevant variables, and the results are presented as odds ratios (ORs) with 95% confidence intervals (CI). Two adjusted models were developed to estimate the incidence of NAFLD among metformin users; the estimation involved calculating the cDDD and intensity of metformin use (DDD/month). Statistical significance in this study was indicated by p-values <0.05.
Results
Characteristic distribution of study participants
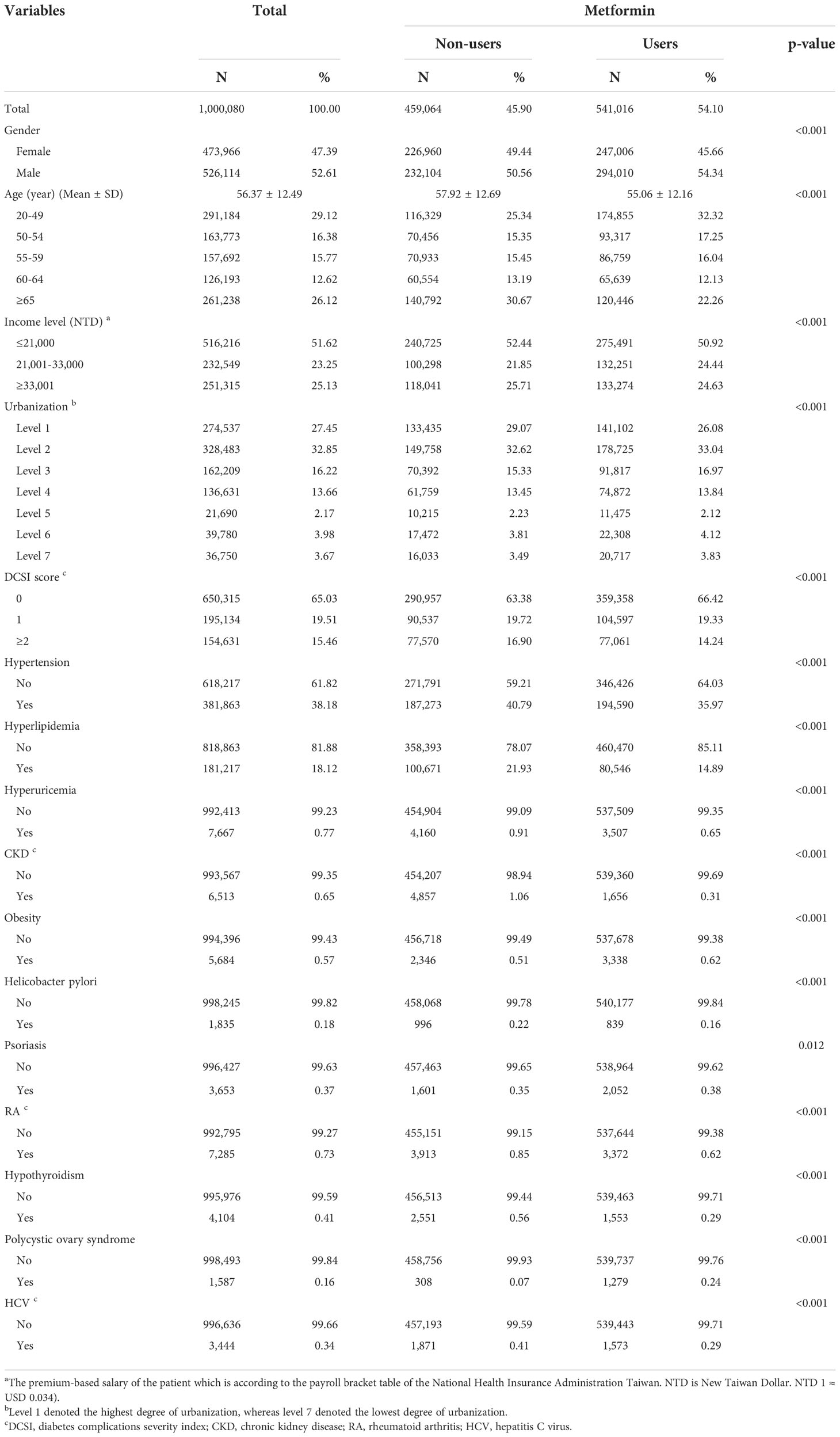
Table 1 displays the baseline characteristics of the patients. The average age of all the patients was 56.37 ± 12.49 years. Among the selected patients, 47.39% were female and 52.61% were male. Further, 29.12% of the patients were aged 20–49 years, 16.38% were aged 50–54 years, 15.77% were aged 55–59 years, 12.62% were aged 60–64 years, and 26.12% were aged ≥65 years.
Among metformin users, the average age was 55.06 ± 12.16 years. Among the selected patients, 194,590 patients (35.97%) had hypertension, 80,546 patients (14.89%) had hyperlipidemia, 3,507 patients (0.65%) had hyperuricemia, 1,656 patients (0.31%) had CKD, 3,338 (0.62%) patients had obesity, 839 patients (0.16%) had H. pylori infection, 2,052 patients (0.38%) had psoriasis, 3,372 patients (0.62%) had RA, 1,553 patients (0.29%) had hypothyroidism, 1,279 patients (0.24%) had polycystic ovary syndrome, and 1,573 patients (0.29%) had HCV. Furthermore, the difference in the distribution of each comorbid disease between metformin users and nonusers was statistically significant.
Incident NAFLD in patients with new-onset dm receiving metformin medication
Table S1 displays the distribution of incident NAFLD among patients with T2DM. Table 2 displays the data on NAFLD incidence obtained through 3-year follow-up; 7,451 patients (0.75%) developed NAFLD within 3 years after the diagnosis of DM. The incidence rate of NAFLD among metformin nonusers was 0.70%, and those among the metformin users were 0.79% for cDDD <300, 0.81% for cDDD 300–500, and 0.88% for cDDD ≥500. In terms of metformin use intensity, the incidence rate of NAFLD was 0.76% for <10 DDD/month, 0.85% for 10–25 DDD/month, and 0.81% for ≥25 DDD/month. After 3-year follow-up, the ORs for the incidence of NAFLD among patients with DM receiving cDDD <300, 300–500, and >500 were 1.11 (95% CI = 1.06–1.16), 1.08 (95% CI = 0.85–1.37), and 1.19 (95% CI = 0.39–3.70), respectively. In terms of metformin use intensity, the ORs for the incidence of NAFLD among patients with DM receiving <10, 10–25, and ≥25 DDD/month were 1.08 (95% CI = 1.02–1.13), 1.18 (95% CI = 1.11–1.26), and 1.09 (95% CI = 0.86–1.37), respectively. In terms of risk factors, the ORs for dementia among patients with DM scoring 1 and ≥2 on the DCSI were 1.07 (95% CI = 1.01–1.14) and 1.16 (95% CI = 1.09–1.24), respectively. Furthermore, the patients with DM comorbid with hyperlipidemia (OR = 1.30, 95% CI = 1.22–1.38) and obesity (OR = 1.78, 95% CI = 1.44–2.21) were at high risk of developing NAFLD. Patients with DM comorbid with hypertension (OR = 0.94, 95% CI = 0.89–0.99) and CKD (OR = 0.66, 95% CI = 0.47–0.94) were at low risk of developing NAFLD. By contrast, patients with comorbid hyperuricemia, H. pylori infection, psoriasis, RA, hypothyroidism, polycystic ovary syndrome, and HCV were not at risk of developing dementia.
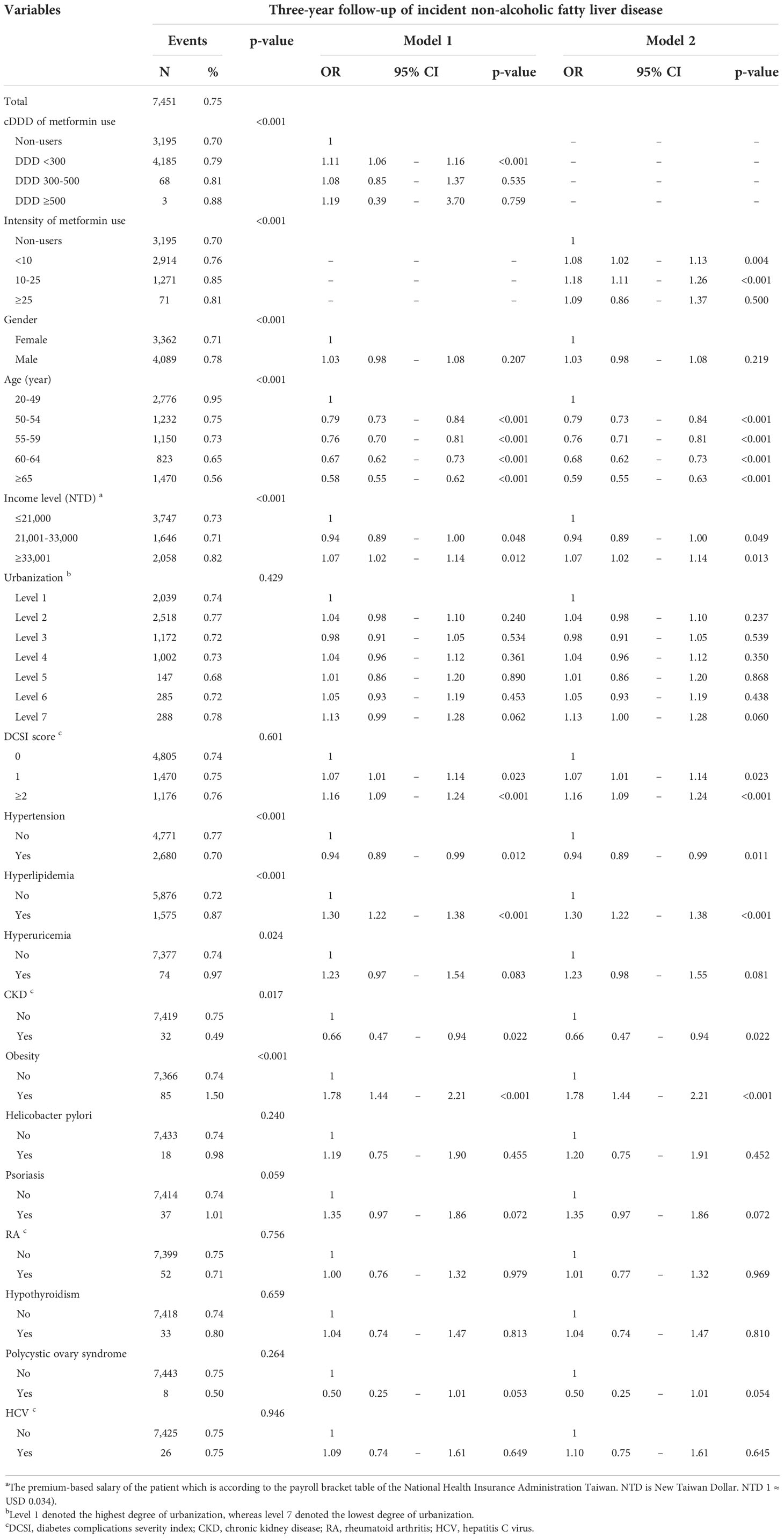
Table 2 Three-year follow-up of incident non-alcoholic fatty liver disease in new-onset diabetes mellitus patients with metformin medication.
Table 3 displays the 5-year follow-up data on NAFLD incidence. After adjusting the related variables, we discovered that the ORs for NAFLD incidence among patients with DM receiving cDDD <300, 300–500, and ≥500 were 1.06 (95% CI = 1.02–1.09), 0.96 (95% CI = 0.80–1.15), and 1.02 (95% CI = 0.43–2.46), respectively. In terms of the intensity of metformin use, the ORs for NAFLD incidence among patients receiving <10, 10–25, and >25 DDD/month were 1.04 (95% CI = 1.00–1.08), 1.11 (95% CI = 1.06–1.16), and 0.96 (95% CI = 0.80–1.15). Adjusted model 1 also indicated that the ORs for NAFLD incidence among patients with DM who scored 1 and ≥2 on the DCSI were 1.08 (95% CI = 1.04–1.13) and 1.14 (95% CI = 1.09–1.20), respectively. In terms of risk factors, patients with DM having hyperlipidemia (OR = 1.33, 95% CI = 1.28–1.39), hyperuricemia (OR = 1.23, 95% CI = 1.04–1.45), obesity (OR = 1.62, 95% CI = 1.38–1.91), and HCV (OR = 1.46, 95% CI = 1.15–1.86) were at high risk of developing NAFLD. Patients with comorbid hypertension (OR = 0.93, 95% CI = 0.90–0.97) and CKD (OR = 0.72, 95% CI = 0.57–0.92) were at low risk of developing NAFLD.
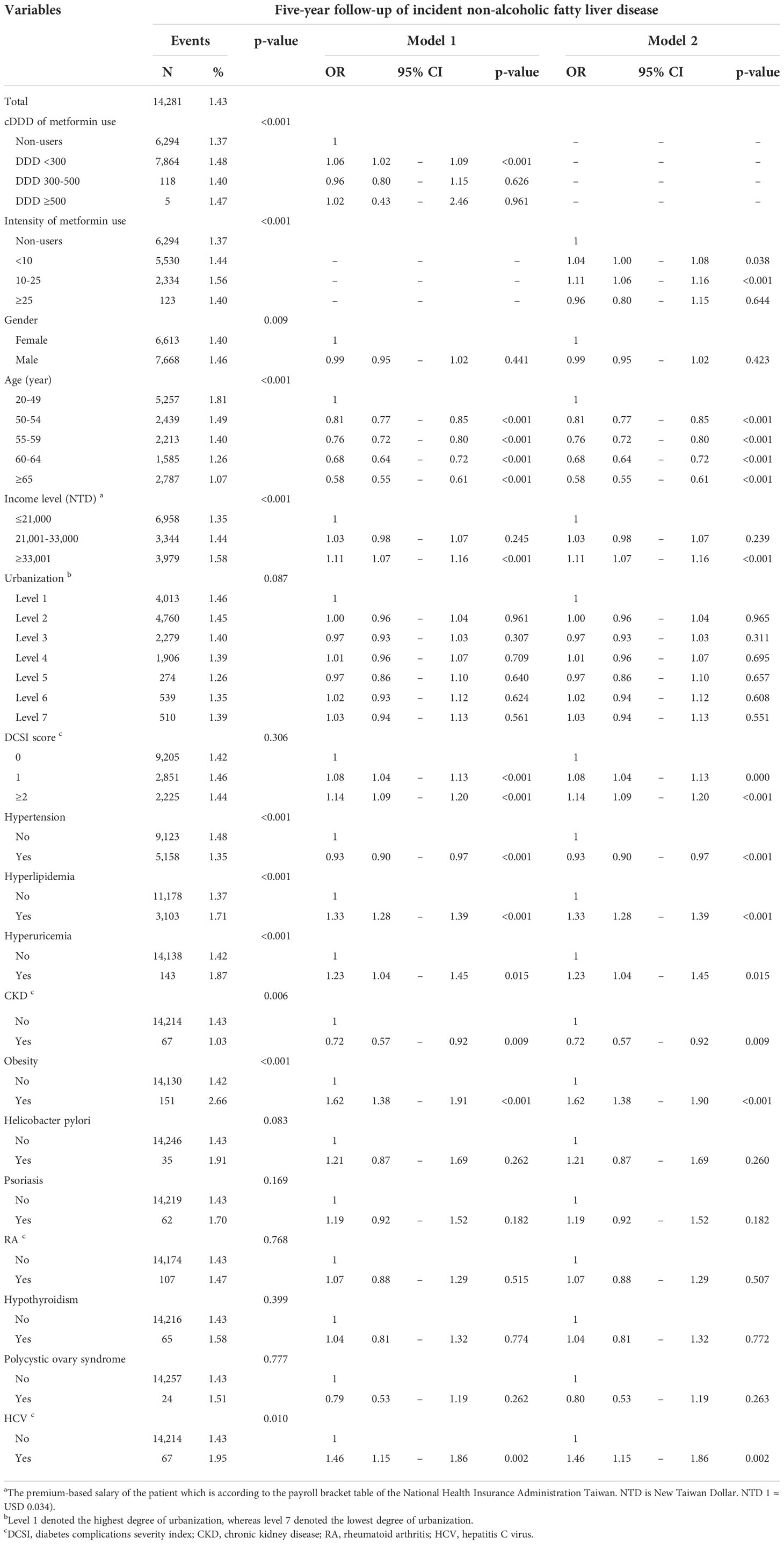
Table 3 Five-year follow-up of incident non-alcoholic fatty liver disease in new-onset diabetes mellitus patients with metformin medication.
Discussion
To the best of our knowledge, few large-scale epidemiological studies have evaluated the risk of NAFLD incidence among patients with T2DM receiving metformin. The results obtained after 3-year and 5-year follow-up indicated that patients with T2DM receiving metformin in cDDD <300 or at intensities of <10 and 10–25 DDD/month were at high risk for developing NAFLD. However, in patients with T2DM receiving metformin in cDDD of 300–500 and >500 or at intensities of >25 DDD/month, metformin exhibited no protective effects against NAFLD. In addition, metformin users who scored high on the DCSI had high ORs for NAFLD incidence among patients with T2DM; patients with comorbid hyperlipidemia, hyperuricemia, obesity and HCV were also at high risk of NAFLD.
Several studies have reported that patients with T2DM and fatty liver disease exhibited improved aminotransferase levels and IR after metformin therapy (20–23). Therefore, metformin may aid the treatment of NAFLD (8, 20, 24). Animal and physiological studies have proposed various possible mechanisms to explain the relationship between metformin use and the risk of NAFLD incidence. Metformin is considered an activator of AMP-activated protein kinase (AMPK), which is a major cellular regulator of glucose and lipid metabolism. This serves as a key mechanism through which metformin treatment aids glucose metabolism and alleviates diabetes-related complications (25). Metformin decreases triglyceride accumulation in hepatocytes due to high-fat diets in vivo and in vitro (26). Moreover, metformin can activate intracellular AMPK and stimulate NO synthesis in human aortic endothelial cells (27). The beneficial effects of metformin extend beyond glycemic control and include the improvement of hepatocyte lipid metabolism and the suppression of hepatocyte and macrophage inflammatory responses (13).
Our results indicated that in patients with T2DM receiving metformin in cDDD of 300–500 and >500 or at an intensity of >25 DDD/month, metformin exhibited no protective effects against NAFLD after 3-year and 5-year follow-up periods. Several studies have investigated the effects of metformin therapy on liver aminotransferase levels and liver histology of patients with NASH or NAFLD (10, 22, 23, 28–31). Several small open-label studies have demonstrated decreases in IR and liver aminotransferase levels with metformin use (10, 29, 31), but liver histology was not considerably improved (10, 29). Although histological necroinflammation improved among the metformin treatment group, the improvement was not statistically significant and no difference in liver fibrosis was observed between the metformin user and nonuser groups (29). Other studies have failed to demonstrate significant improvements in insulin sensitivity, aminotransferase level, or liver histology due to metformin treatment (22, 23). A meta-analysis study that included a subanalysis of the effects of metformin on biochemical and histological outcomes among NASH patients demonstrated that metformin did not improve NASH-related outcomes (28). Another meta-analysis study also demonstrated that metformin therapy did not improve liver histology among patients with NASH or NAFLD (28, 32). Therefore, the clinical administration of metformin among patients with NAFLD is limited because of mixed study results, the heterogeneous effects of treatment, and the small number of patients involved in the studies. Preclinical studies on rodents have suggested that metformin may be a useful therapeutic medication for reducing intrahepatic triacylglycerol (IHTAG) content; however, the effectiveness of metformin therapy in reducing IHTAG levels among patients has yet to be confirmed (33). Therefore, owing to a lack of evidence for significant histological improvement of the liver, metformin is not recommended for treating NASH or NAFLD in adult patients (2, 14).
Our results indicated that patients with T2DM receiving metformin in cDDD of <300 or at intensity of <10 and 10–25 DDD/month were at high risk of developing NAFLD after 3-year and 5-year follow-up periods. The effectiveness of short-term metformin treatment in reducing lipid levels and preventing lipid accumulation in hepatocytes has been frequently reported (34, 35). Metformin treatment has been reported to cause only transient improvement in liver chemistry. The reduction in insulin sensitivity due to metformin therapy was not sustainable (10). Animal and physiological studies on the effects of long-term metformin treatment have been inconclusive. Furthermore, data regarding the long-term effects of metformin therapy on liver function among patients with NAFLD are controversial. An animal study demonstrated that long-term treatment with metformin had no preventive effects against NAFLD in Zucker diabetic fatty rats (36). Studies on long-term metformin therapy have not demonstrated any histological protective effects in the liver (20–23). Moreover, metformin-induced hepatotoxic effects, including acute hepatitis, liver transaminitis, and intrahepatic cholestasis, have rarely been reported (37–40). Vitamin deficiency has been reported in many causes of chronic liver disease, and has been associated with the development of NAFLD (41). Furthermore, low vitamin B12 serum levels were revealed to be significantly correlated with NAFLD, especially in grade 2 to grade 3 hepato-steatosis (42). Another study also demonstrated that low level of vitamin B12 has been related to NAFLD patients, and the histological severity of NASH (43). Low levels of vitamin B12 have been linked to high levels of homocysteine characterizing hyper-homocysteinemia as an indicator for oxidative stress (44). Subjects with chronic liver disease can benefit from vitamin B, since its antioxidant effect has possessed hepatoprotective activity to ameliorate chronic liver injury (41). A low vitamin B12 serum level is an independent predictor of NASH histological severity and fibrosis grade (43). Serum vitamin B12 levels were significantly lower among patients with NAFLD than in controls, indicating a correlation with a higher grade of steatohepatitis (43). The prevalence of B12 deficiency was higher in metformin users than non-metformin users (45). Metformin induces vitamin B12 malabsorption may be dose-related which may increase the risk of vitamin B12 deficiency in T2DM patients (46). Several studies demonstrated that vitamin B12 deficiency occurred when patients taken metformin for more than 2-4 years (45, 47).
Metformin use is associated with vitamin B12 deficiency, which is dependent upon the cumulative dose of metformin (48). Due to the clinical benefits of metformin use, its associated side effects such as vitamin B12 deficiency is often overlooked in T2DM patients. However, the diagnosis of metformin-induced vitamin B12 deficiency may be difficult (46). Vitamin B12 deficiency play a pivotal role in the risk of NAFLD development in T2DM patients receiving cumulative dose of metformin treatment over the long term.
In summary, short-term metformin use is effective in treating NAFLD, whereas long-term cumulative dose of metformin use may not alleviate NAFLD but may instead have harmful effects. Vitamin B12 deficiency may increase the risk of NAFLD among patients with T2DM receiving cumulative dose of metformin use over the long term. However, the actual mechanism of the effects of metformin dosage on the risk of NAFLD remains unclear and should be investigated in the future. Randomized-controlled studies are warranted to verify these effects.
Our study revealed that patients with DM receiving metformin and having higher scores on the DCSI were at high risk of developing NAFLD. The prevalence of NAFLD among young adults was significantly higher than among older adults, likely because of the higher prevalence among women and metabolic syndrome among young adults (49). The DCSI is an effective tool for predicting the risk of hospitalization and mortality among patients with T2DM (18). DCSI may also be used as an indicator for estimating the risk of developing NAFLD.
The results of this study indicated that patients with DM receiving metformin with comorbid hyperlipidemia, obesity, hyperuricemia, and HCV were at high risk of developing NAFLD. Studies have demonstrated that NAFLD is a multisystem disease. Evidence indicated a strong correlation between NAFLD and increased risk of hyperlipidemia (50). Obesity is strongly correlated with the development of NAFLD (51). T2DM, IR, and obesity are key factors influencing the development of NAFLD and NASH (52). The risk of NAFLD among patients with hyperuricemia was significantly higher than among patients with normal uric acid levels (53). NAFLD is a predominant outcome of chronic HCV infection (54), which causes impairment of lipid and glucose metabolism (55).
We included data approximately covering the entire Taiwanese population in this study; thus, the sample size was large and highly representative of patients with T2DM at risk of developing NAFLD, and the data obtained were of high quality.
The follow-up period of metformin use in this study was divided into 3 years and 5 years. The cDDD of metformin use was divided into three levels: ≤300, 300–500, and >500. Similarly, the intensity of metformin use was divided into three levels, namely ≤10, 10–25, and >25 DDD/month, to investigate the correlation between T2DM and the risk of developing NAFLD.
We investigated the correlation between the risk factors of comorbidities and the risk of NAFLD incidence among patients with T2DM.
This study has several limitations that should be addressed by future studies. First, the algorithm used to categorize the severity of liver disease could not be validated because of the limitation of the NHIRD (the Child–Pugh–Turcotte score used for the prognosis of chronic liver disease was not available in the NHIRD).
Second, the ICD codes from the NHIRD data did not include detailed computed tomography findings. Third, a few factors, including alcohol consumption behavior, laboratory parameters, and abdominal ultrasonography findings, that influence NAFLD development could not be determined from the LHID, thereby affecting the findings of this study. Fourth, physical activity and eating habit are the leading causes for developing NAFDL in T2DM patients. However, we could not get information of physical activity and eating habit from these patients. Finally, although the LHID includes a large amount of data, it does not include personal information of patients, such as self-pay medical information, which could influence the development of NAFLD.
Conclusions
Patients with T2DM who received metformin of <300 cDDD or used metformin at an intensity of <10 and 10–25 DDD/month were at a high risk of developing NAFLD. Moreover, patients receiving 300–500 and >500 cDDD of metformin or using metformin at an intensity of >25 DDD/month did not exhibit any protective effects against NAFLD.
Data availability statement
The National Health Insurance Database used to support the findings of this study were provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW) under license and so cannot be made freely available. Requests to access these datasets should be directed to https://dep.mohw.gov.tw/dos/np-2497-113.html.
Ethics statement
The studies involving human participants were reviewed and approved by Central Regional Research Ethics Committee of China Medical University, Taiwan (No. CRREC-109-011). The ethics committee waived the requirement of written informed consent for participation.
Author contributions
All the authors involved in drafting or revising the article and approved of the submitted version. Study conception and design: K-HH, C-HL, Y-DC, S-YG, T-HT, N-JC and C-YL. Data acquisition: K-HH and C-YL. Data analysis and demonstration: K-HH, T-HT and C-YL. Original draft preparation: K-HH, C-HL, Y-DC, S-YG, T-HT, N-JC and C-YL.
Funding
This research was supported by the Chung Shan Medical University Hospital, Taiwan (CSH-2022-C-046), China Medical University Taiwan (CMU110-MF-113), and the Ministry of Science and Technology Taiwan (MOST 109-2410-H-039-004-MY2).
Acknowledgments
Our special thanks to Chung Shan Medical University, Chung Shan Medical University Hospital, and China Medical University, which has contributed to the completion of this study. This study is based in part on data from the NHIRD. The interpretation and conclusions contained herein do not represent those of National Health Insurance Administration, Ministry of Health and Welfare or National Health Research Institutes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1027484/full#supplementary-material
References
1. Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol (2013) 48(4):434–41. doi: 10.1007/s00535-013-0758-5
2. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American association for the study of liver diseases. Hepatology (2018) 67(1):328–57. doi: 10.1002/hep.29367
3. Forlani G, Giorda C, Manti R, Mazzella N, De Cosmo S, Rossi MC, et al. The burden of NAFLD and its characteristics in a nationwide population with type 2 diabetes. J Diabetes Res 2016 (2016) p:2931985. doi: 10.1155/2016/2931985
4. Bhatt HB, Smith RJ. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg Nutr (2015) 4(2):101–8. doi: 10.3978/j.issn.2304-3881.2015.01.03
5. Leite NC, Villela-Nogueira CA, Pannain VL, Bottino AC, Rezende GF, Cardoso CR, et al. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: prevalences and correlated factors. Liver Int (2011) 31(5):700–6. doi: 10.1111/j.1478-3231.2011.02482.x
6. Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW, et al. Non-alcoholic fatty liver disease and diabetes. Metabolism (2016) 65(8):1096–108. doi: 10.1016/j.metabol.2016.01.001
7. Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. BioMed Rep (2013) 1(1):57–64. doi: 10.3892/br.2012.18
8. Feng W, Gao C, Bi Y, Wu M, Li P, Shen S, et al. Randomized trial comparing the effects of gliclazide, liraglutide, and metformin on diabetes with non-alcoholic fatty liver disease. J Diabetes (2017) 9(8):800–9. doi: 10.1111/1753-0407.12555
9. Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med (2000) 6(9):998–1003. doi: 10.1038/79697
10. Nair S, Diehl AM, Wiseman M, Farr GH Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther (2004) 20(1):23–8. doi: 10.1111/j.1365-2036.2004.02025.x
11. Spruss A, Kanuri G, Stahl C, Bischoff SC, Bergheim I. Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab Invest (2012) 92(7):1020–32. doi: 10.1038/labinvest.2012.75
12. Kita Y, Takamura T, Misu H, Ota T, Kurita S, Takeshita Y, et al. Metformin prevents and reverses inflammation in a non-diabetic mouse model of nonalcoholic steatohepatitis. PloS One (2012) 7(9):e43056. doi: 10.1371/journal.pone.0043056
13. Woo SL, Xu H, Li H, Zhao Y, Hu X, Zhao J, et al. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PloS One (2014) 9(3):e91111. doi: 10.1371/journal.pone.0091111
14. European Association for the Study of the, L, D. European Association for the Study, O. European Association for the Study. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol (2016) 64(6):1388–402. doi: 10.1007/s00125-016-3902-y
15. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan's national health insurance research database: past and future. Clin Epidemiol (2019) 11:349–58. doi: 10.2147/CLEP.S196293
16. Grimmsmann T, Himmel W. Discrepancies between prescribed and defined daily doses: a matter of patients or drug classes? Eur J Clin Pharmacol (2011) 67(8):847–54. doi: 10.1007/s00228-011-1014-7
17. Wellington K. Rosiglitazone/Metformin. Drugs (2005) 65(11):1581–92; discussion 1593-4. doi: 10.2165/00003495-200565110-00013
18. Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care (2008) 14(1):15–23.
19. Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted diabetes complications severity index in claims data. Am J Manag Care (2012) 18(11):721–6.
20. Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther (2009) 29(2):172–82. doi: 10.1111/j.1365-2036.2008.03869.x
21. Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A randomized controlled trial of metformin versus vitamin e or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol (2005) 100(5):1082–90. doi: 10.1111/j.1572-0241.2005.41583.x
22. Idilman R, Mizrak D, Corapcioglu D, Bektas M, Doganay B, Sayki M, et al. Clinical trial: insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther (2008) 28(2):200–8. doi: 10.1111/j.1365-2036.2008.03723.x
23. Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The effect of metformin and standard therapy versus standard therapy alone in nondiabetic patients with insulin resistance and nonalcoholic steatohepatitis (NASH): A pilot trial. Therap Adv Gastroenterol (2009) 2(3):157–63. doi: 10.1177/1756283X09105462
24. Haukeland JW, Konopski Z, Eggesbo HB, von Volkmann HL, Raschpichler G, Bjoro K, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol (2009) 44(7):853–60. doi: 10.1080/00365520902845268
25. Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, et al. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem (2004) 279(46):47898–905. doi: 10.1074/jbc.M408149200
26. Doycheva I, Loomba R. Effect of metformin on ballooning degeneration in nonalcoholic steatohepatitis (NASH): when to use metformin in nonalcoholic fatty liver disease (NAFLD). Adv Ther (2014) 31(1):30–43. doi: 10.1007/s12325-013-0084-6
27. Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem (2003) 278(34):31629–39. doi: 10.1074/jbc.M212831200
28. Rakoski MO, Singal AG, Rogers MA, Conjeevaram H. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther (2010) 32(10):1211–21. doi: 10.1111/j.1365-2036.2010.04467.x
29. Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther (2004) 19(5):537–44. doi: 10.1111/j.1365-2036.2004.01888.x
30. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther (2011) 34(3):274–85. doi: 10.1111/j.1365-2036.2011.04724.x
31. Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet (2001) 358(9285):893–4. doi: 10.1016/S0140-6736(01)06042-1
32. Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology (2010) 52(1):79–104. doi: 10.1002/hep.23623
33. Green CJ, Marjot T, Tomlinson JW, Hodson L. Of mice and men: Is there a future for metformin in the treatment of hepatic steatosis? Diabetes Obes Metab (2019) 21(4):749–60. doi: 10.1111/dom.13592
34. Wang N, Zhang J, Wu Y, Liu J, Liu L, Guo X. Metformin improves lipid metabolism disorders through reducing the expression of microsomal triglyceride transfer protein in OLETF rats. Diabetes Res Clin Pract (2016) 122:170–8. doi: 10.1016/j.diabres.2016.10.006
35. Madsen A, Bozickovic O, Bjune JI, Mellgren G, Sagen JV. Metformin inhibits hepatocellular glucose, lipid and cholesterol biosynthetic pathways by transcriptionally suppressing steroid receptor coactivator 2 (SRC-2). Sci Rep (2015) 5:16430. doi: 10.1038/srep16430
36. Sui Y, Kong X, Fan R, Ye Y, Mai H, Zhuo S, et al. Long-term treatment with metformin in the prevention of fatty liver in zucker diabetic fatty rats. Diabetol Metab Syndr (2019) 11:94. doi: 10.1186/s13098-019-0491-1
37. Cone CJ, Bachyrycz AM, Murata GH. Hepatotoxicity associated with metformin therapy in treatment of type 2 diabetes mellitus with nonalcoholic fatty liver disease. Ann Pharmacother (2010) 44(10):1655–9. doi: 10.1345/aph.1P099
38. Miralles-Linares F, Puerta-Fernandez S, Bernal-Lopez MR, Tinahones FJ, Andrade RJ, Gomez-Huelgas R. Metformin-induced hepatotoxicity. Diabetes Care (2012) 35(3):e21. doi: 10.2337/dc11-2306
39. Kutoh E. Possible metformin-induced hepatotoxicity. Am J Geriatr Pharmacother (2005) 3(4):270–3. doi: 10.1016/j.amjopharm.2005.12.002
40. Desilets DJ, Shorr AF, Moran KA, Holtzmuller KC. Cholestatic jaundice associated with the use of metformin. Am J Gastroenterol (2001) 96(7):2257–8. doi: 10.1111/j.1572-0241.2001.03972.x
41. Licata A, Zerbo M, Como S, Cammilleri M, Soresi M, Montalto G, et al. The role of vitamin deficiency in liver disease: To supplement or not supplement? Nutrients (2021) 13(11):4014. doi: 10.3390/nu13114014
42. Koplay M, Gulcan E, Ozkan F. Association between serum vitamin B12 levels and the degree of steatosis in patients with nonalcoholic fatty liver disease. J Investig Med (2011) 59(7):1137–40. doi: 10.2310/JIM.0b013e31822a29f5
43. Mahamid M, Mahroum N, Bragazzi NL, Shalaata K, Yavne Y, Adawi M, et al. Folate and B12 levels correlate with histological severity in NASH patients. Nutrients (2018) 10(4):440. doi: 10.3390/nu10040440
44. Li L, Huang Q, Yang L, Zhang R, Gao L, Han X, et al. The association between non-alcoholic fatty liver disease (NAFLD) and advanced fibrosis with serological vitamin B12 markers: Results from the NHANES 1999-2004. Nutrients (2022) 14(6):1224. doi: 10.3390/nu14061224
45. Alharbi TJ, Tourkmani AM, Abdelhay O, Alkhashan HI, Al-Asmari AK, Bin Rsheed AM, et al. The association of metformin use with vitamin B12 deficiency and peripheral neuropathy in Saudi individuals with type 2 diabetes mellitus. PloS One (2018) 13(10):e0204420. doi: 10.1371/journal.pone.0204420
46. Al-Hamdi A, Al-Gahhafi M, Al-Roshdi S, Jaju S, Al-Mamari A, Al Mahrezi AM. Vitamin B12 deficiency in diabetic patients on metformin therapy: A cross-sectional study from oman. Sultan Qaboos Univ Med J (2020) 20(1):e90–4. doi: 10.18295/squmj.2020.20.01.013
47. Wong CW, Leung CS, Leung CP, Cheng JN. Association of metformin use with vitamin B12 deficiency in the institutionalized elderly. Arch Gerontol Geriatr (2018) 79:57–62. doi: 10.1016/j.archger.2018.07.019
48. Farooq MD, Tak FA, Ara F, Rashid S, Mir IA. Vitamin B12 deficiency and clinical neuropathy with metformin use in type 2 diabetes. J Xenobiot (2022) 12(2):122–30. doi: 10.3390/jox12020011
49. Dai W, Ye L, Liu A, Wen SW, Deng J, Wu X, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A meta-analysis. Med (Baltimore) (2017) 96(39):e8179. doi: 10.1097/MD.0000000000008179
50. Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism (2016) 65(8):1109–23. doi: 10.1016/j.metabol.2016.05.003
51. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology (2010) 51(2):679–89. doi: 10.1002/hep.23280
52. Zhou J, Massey S, Story D, Li L. Metformin: An old drug with new applications. Int J Mol Sci (2018) 19(10):2863. doi: 10.3390/ijms19102863
53. Wijarnpreecha K, Panjawatanan P, Lekuthai N, Thongprayoon C, Cheungpasitporn W, Ungprasert P. Hyperuricaemia and risk of nonalcoholic fatty liver disease: A meta-analysis. Liver Int (2017) 37(6):906–18. doi: 10.1111/liv.13329
54. Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis c patients and correlates with specific HCV genotype and visceral obesity. Hepatology (2001) 33(6):1358–64. doi: 10.1053/jhep.2001.24432
Keywords: nonalcoholic fatty liver disease, metformin, type 2 diabetes mellitus, cumulative defined daily dose, NHIRD
Citation: Huang K-H, Lee C-H, Cheng Y-D, Gau S-Y, Tsai T-H, Chung N-J and Lee C-Y (2022) Correlation between long-term use of metformin and incidence of NAFLD among patients with type 2 diabetes mellitus: A real-world cohort study. Front. Endocrinol. 13:1027484. doi: 10.3389/fendo.2022.1027484
Received: 25 August 2022; Accepted: 09 November 2022;
Published: 30 November 2022.
Edited by:
Nick Giannoukakis, Allegheny Health Network, United StatesReviewed by:
Anu Grover, Ipca Laboratories, IndiaSolaleh Emamgholipour, Tehran University of Medical Sciences, Iran
Copyright © 2022 Huang, Lee, Cheng, Gau, Tsai, Chung and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chien-Ying Lee, Y3NoZDAxNUBjc211LmVkdS50dw==
†These authors have contributed equally to this work
 Kuang-Hua Huang1†
Kuang-Hua Huang1† Shuo-Yan Gau
Shuo-Yan Gau Chien-Ying Lee
Chien-Ying Lee