
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 10 November 2022
Sec. Systems Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1027047
This article is part of the Research TopicA year in review: Discussions in Systems EndocrinologyView all 5 articles
 Letizia Chiara Pezzaioli1
Letizia Chiara Pezzaioli1 Elisa Gatta1
Elisa Gatta1 Francesca Bambini1
Francesca Bambini1 Paolo Facondo1
Paolo Facondo1 Maria Gava1
Maria Gava1 Maria Cavadini1
Maria Cavadini1 Caterina Buoso1
Caterina Buoso1 Elena Di Lodovico1
Elena Di Lodovico1 Mario Rotondi2,3
Mario Rotondi2,3 Alberto Ferlin4
Alberto Ferlin4 Carlo Cappelli1*
Carlo Cappelli1*Purpose: The purpose of this study was to describe the current knowledge on the potential endocrine adverse effects post-COVID-19 vaccines.
Methods: A PubMed/MEDLINE, Web of Science, and Scopus research was performed. Case reports, case series, original studies, and reviews written in English and published online up to 31 July 2022 were selected and reviewed. The final reference list was defined based on the relevance of each paper to the scope of this review.
Results: The available data showed that endocrine side effects are generally rare and with favorable outcome, being thyroid disorders the most common. Conversely, data on type 1 diabetes mellitus are rare; adrenal and pituitary events are even anecdotal. Finally, the available clinical studies suggest no impact on female reproductive system and on male and couple fertility.
Conclusion: Overall, these data show that, after 2 years of COVID-19 vaccines, the endocrine system is not heavily threatened.
Since its appearance at the end of 2019, coronavirus disease 2019 (COVID-19) has globally affected over 580 million people, including over 6 million deaths reported to World Health Organization (WHO) (1).
While known to primarily affect the respiratory system, it has become evident that many relevant extrapulmonary manifestations contribute to the severity of COVID-19 (2), including cardiovascular, renal, gastrointestinal, urinary, and endocrine systems (3, 4). This marked that the variability of clinical manifestations has been attributed to the presence in all these tissues of angiotensin converting enzyme 2 (ACE2) receptor, which plays a pivotal role in COVID-19 pathogenesis (5).
Many vaccines have been developed to induce protection against severe COVID-19 manifestations, and, as of 9 August 2022, over 12 billion vaccine doses have been administered (1). WHO, as of 8 April 2022, confirmed the safety and effectiveness of the following vaccines: Moderna mRNA-1273, Pfzer/BioNTech BNT162b2, AstraZeneca/Oxford Vaxzevria ChAdOx1, Janssen Ad26.COV2, Sinopharm BIBP, Sinovac Coronavac, Covaxin BBV512, Convidecia AD5-nCOV, Covovax, and Nuvaxovid NVX-CoV2373 (6). Most of these vaccines contain messenger RNA (mRNA) or inactivate or vectorial virus that induces protective immune response. In detail, mRNA-based vaccines are encapsulated in lipid nanoparticles to transport nucleoside-modified mRNA encoding viral proteins to the host cell membrane; conversely, vaccines based on adenoviral or human vector are designed to invade cells without replication, whereas inactivated vaccines are chemically obtained and formulated with adjuvants (7).
The overall safety and efficacy of the available COVID-19 vaccines have been confirmed in multiple studies and metanalyses (8), even if the occurrence of a few cases of post-vaccination complications have been observed (9), including endocrine manifestations.
Besides the effects on hormonal structure (10) and long-term endocrine-metabolic complications (11), many different cases of endocrine adverse effects after COVID-19 vaccines have been reported, mainly as case reports/series. However, a comprehensive review is needed, especially because the endocrine system has been prone to many misconceptions regarding COVID-19 vaccines (3).
Therefore, we aimed to review the current findings on the effects of COVID-19 vaccines on endocrine system.
A PubMed/MEDLINE, Web of Science, and Scopus research was performed, for free-text words and terms related to “Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine”, “COVID-19 vaccine”, “SARS-CoV-2 vaccination”, “COVID-19 vaccination”, “SARS-CoV-2 immunization” variously combined with “endocrinopathies”, “endocrine system”, “thyroid”, “subacute thyroiditis”, “Graves’ disease”, “hypothyroidism”, “hyperthyroidism”, “adrenal”, “adrenal insufficiency”, “adrenal crisis”, “Addison disease”, “adrenal haemorrhage”, “pituitary gland”, “hypophysitis”, “hypopituitarism”, “pituitary apoplexy”, “type 1 diabetes”, “diabetic ketoacidosis”, “diabetes”, “ovary”, “amenorrhea”, “menstrual cycle”, “menstrual dysfunction”, “female fertility”, “male infertility”, “couple infertility”, “sperm”, “reproductive health”, “male hypogonadism”, and “sexual dysfunction”.
Case reports, case series, original studies, and reviews written in English and published online up to 31 July 2022 were selected and reviewed. The final reference list was defined based on the relevance of each paper to the scope of this review.
To date, 172 cases of thyroid involvement after COVID-19 vaccine have been reported as case reports/case series and are briefly summarized in Table 1. Subacute thyroiditis (SAT) is the most frequent finding, followed by new or recurrent Graves’ disease. Few clinical studies have been recently published, concerning the possible alterations of thyroid function and SAT characteristics, as shown in Supplementary Table 2.
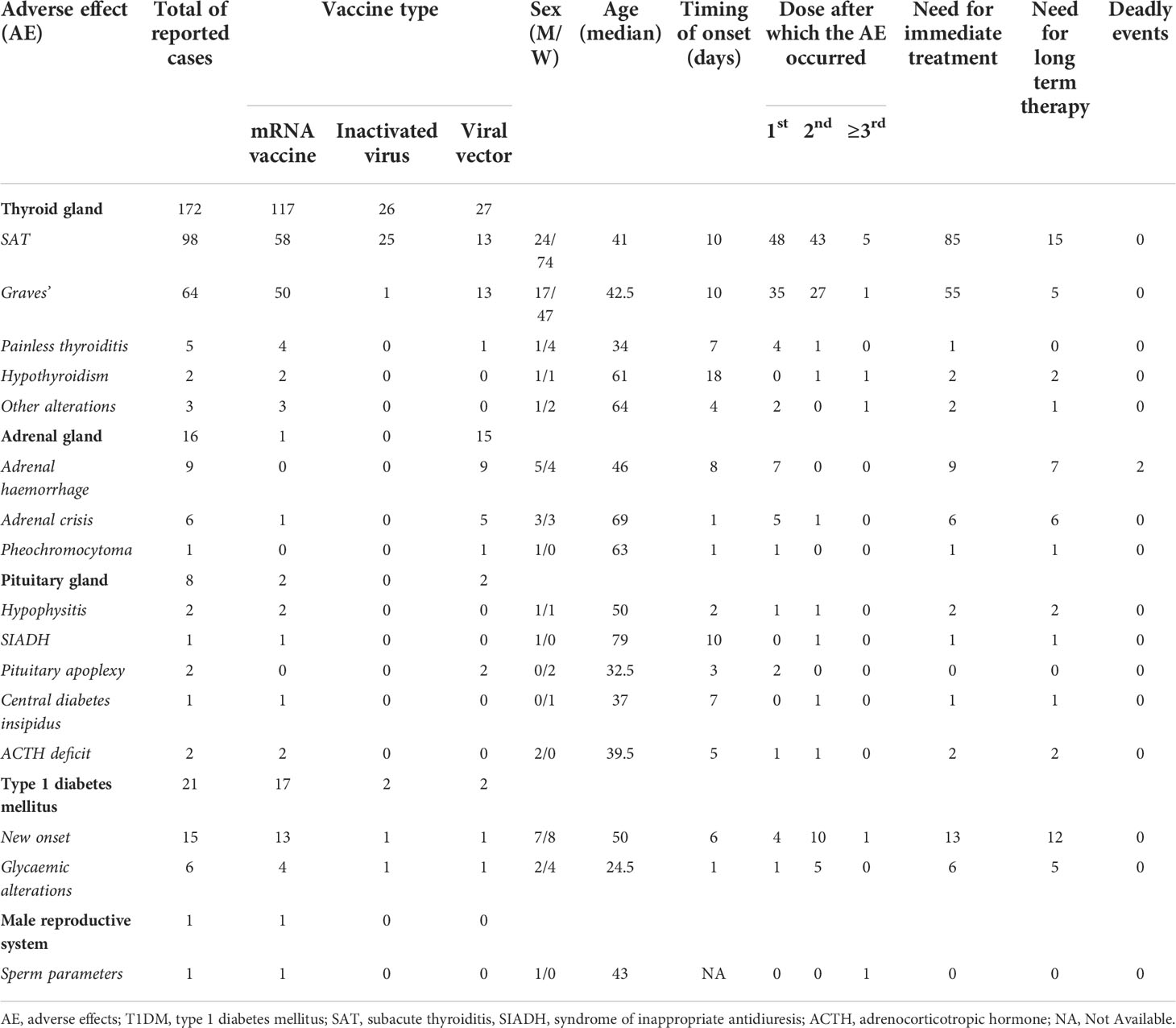
Table 1 Summarized data from case reports regarding thyroid gland, adrenal involvement, pituitary function alteration, type 1 diabetes mellitus (T1DM) occurrence/worsening, and male. reproductive function after COVID-19 vaccine.
A total of 98 cases of SAT after COVID-19 vaccine were retrieved, based on 46 articles, as summarized in Table 1. Complete data are available in Supplementary Table S1.
Median age of the 98 patients was 41 years (37–53), and 74 of them (75.5%) were women. In 14 patients (18.4%), a previous diagnosis of thyroid disease was reported, including primary hypothyroidism (18, 19), multinodular goiter (20), previous SAT (20–22) or Graves’ disease (21), multinodular goiter (20, 23), and papillary thyroid carcinoma (22).
Data concerning the type of vaccine were available for 96 patients. In detail, 58 patients (60.4%) received an mRNA vaccine (Pfizer/BioNTech BNT162b2 or Moderna mRNA-1273), 13 (13.5%) a viral vector vaccine (Vaxzevria ChAdOx1 or Convidecia AD5-nCOV), and 25 (26%) an inactivated virus (Sinovac Biotech CoronaVac or Bharat Biotech BBV152). In 48 cases (50%), SAT followed the first vaccine dose, in 43 cases (44.7%) the second one, and in five cases (5.2%) the third or subsequent doses. Patients developed SAT after a median of 10 days (4–11, 18–21) after the vaccine shot, independently from the type of vaccine administered. All the patients were symptomatic, being the most common symptoms anterior neck pain, fever, palpitations, fatigue, and weight loss. Concerning treatment, 39 patients (40.6%) were treated with corticosteroids (methylprednisolone, prednisone, or prednisolone), 31 (32.3%) with beta-blockers, 50 (52%) with nonsteroidal anti-inflammatory (NSAIDs), and only one (1%) with methimazole; nine patients (9.3%) did not receive any specific treatment.
Regarding follow-up, overt hypothyroidism was diagnosed in 14 cases and subclinical hypothyroidism was found in two patients; in nine cases, levothyroxine treatment was given. There was a recurrence of SAT after a subsequent vaccine dose in four cases (three after Pfizer/BioNtech BNT16b2 and one after Sinovac Biotech CoronaVac) (20, 24, 25).
Recently, four clinical studies have been published concerning SAT and COVID-19 vaccines, as shown in Supplementary Table 2.
In detail, Garcia et al. (12), in a large case/non-case study, analyzed 1,221,582 spontaneous cases of adverse reactions with mRNA and viral vector vaccines and found 162 cases of SAT, with a median time to onset of 10 days after any vaccine. They observed that SAT occurred more frequently after mRNA vaccines (reporting odds ratio of 3.58 and 3.44 for Pfizer/BioNtech BNT16b2 and Moderna mRNA-1273, respectively).
Bostan et al. (16) retrospectively examined 55 patients diagnosed with SAT, of whom 16 had previously undergone COVID-19 vaccine, with a median of 6.5 days before SAT development. They found no differences concerning the characteristics of vaccine.
Conversely, Topaloglu et al. (17) compared 23 cases of vaccine-related SAT with 62 cases of “classical” SAT; they found that SAT duration was longer in vaccinated patients compared with non-vaccinated ones.
Finally, Sendur et al. (26) performed a case-control study, including 14 patients with COVID-induced SAT, in order to assess the possible associations with specific haplotypes. With the limited sample size and the lack of many clinical information, they observed that Human Leukocyte Antigen (HLA)-B35 and HLA-C04 alleles were higher in patients with vaccine-induced SAT compared with healthy controls.
Concerning Graves’ disease, 29 articles were found and a total of 64 cases after COVID-19 vaccination were retrieved and are summarized in Table 1, whereas complete data are available in Supplementary Table S1.
The median age of the 64 patients was 42.5 years (35–53), and 47 of them (73.4%) were women.
First-onset Graves’ disease was diagnosed in 54 patients, including recently described cases (27, 28), whereas a relapse of a previously known and quiescent Graves’ disease was reported in 10 cases (14, 29–32), of whom five (14, 30, 31) developed an ophthalmopathy. Besides the patients with a previous diagnosis of Graves’ disease, further four patients had a known thyroid disease, including multinodular goiter (15), subclinical hypothyroidism under replacement therapy (33), Hashimoto’s thyroiditis (34), and an unspecified form of hyperthyroidism (31).
Of the 64 patients, 50 (78.1%) received an mRNA vaccine (Pfizer/BioNTech BNT162b2 or Moderna mRNA-1273), 13 (20.3%) received a viral vector vaccine (Vaxzevria ChAdOx1 or Convidecia AD5-nCOV or Jennsen), and only one (1.6%) received an inactivated virus vaccine (Sinovac Biotech CoronaVac). Median time between the vaccine shot (any type) and the symptoms’ development was 10 days (4–11, 18–23). Concerning the relationship between the dose of vaccine and Graves’ disease onset, 35 patients (54.7%) became symptomatic after the first dose, in 27 (42.2%) after the second dose, and in one case after the third dose. The most common symptoms and signs were anxiety, insomnia, irritability, diaphoresis, palpitation, tachycardia, hand tremors, and weight loss. Furthermore, five patients showed signs and symptoms of Graves’ ophthalmopathy (14, 30, 31).
Concerning treatment, Methimazole or Carbimazole, with or without beta blockers was prescribed to 51 patients (87.9%) and intravenous Teprotumumab infusions were given to four patients (6.9%) (14, 30, 31), whereas two patients (3.4%) (31, 35) did not receive any specific treatment.
Regarding follow-up, thyroid function returned to normal and symptoms improved in most cases; only four patients (23, 36–38) were still hyperthyroid and one (32) eventually required total thyroidectomy.
Regarding thyroid function alteration after COVID-19 vaccine, 10 cases were retrieved, as shown in Supplementary Table S1, and briefly summarized in Table 1.
In detail, five patients (four women and one man, aged 29–59 years) developed a painless thyroiditis after receiving an mRNA vaccine or viral vector vaccine, with a median time to onset of symptoms of 7 days. In almost all the cases, symptoms were developed after the first dose and none of the patients required specific treatment. Thyroid function was spontaneously normalized in all cases.
Moreover, two cases of overt hypothyroidism, one case of autoimmune thyroiditis with normal thyroid function and one case of thyrotoxicosis, were reported, all occurring after mRNA vaccine administration (first dose in two cases and third in one).
Recently, four clinical studies have been published concerning thyroid function alteration related to COVID-19 vaccine, as shown in Supplementary Table 2. In detail, three of them (39–41) concerned the possible alteration of thyroid hormones and thyroid antibodies in healthy people receiving mRNA or inactivated vaccines. They showed little (or no) modification of thyroid stimulating hormone (TSH) and free thyroid hormones, which remained within normal ranges. As for the autoantibodies titre, only Lui et al. (40) found a slight increase of thyroid peroxidase antibodies (TPOAb) and thyroglobulin antibodies (TgAb).
Finally, Xiong et al. (42) evaluated, in a large retrospective cohort study, the risk of vaccine related adverse events in hypothyroid patients under levothyroxine treatment. They observed that, after two doses, both Pfizer/BioNtechBNT162b2 and Sinovac Biotech Coronavac were not associated with alteration of thyroid status.
Adrenal adverse effects of COVID-19 vaccination were reported for 16 patients, as briefly summarized in Table 1. Complete data are available in Supplementary Table S1.
Of note, nine of the 16 cases (43–50) concerned episodes of adrenal insufficiency due to adrenal haemorrhage. In detail, median age of these patients was 46 years, five of nine were men, and four had predisposing factors of thromboembolism, including smoke (45, 49) and obesity (49, 50). In all cases, patients had received viral vector vaccines, Vaxzevria ChAdOx1 in eight patients (43, 44, 46, 47) and Johnson and Johnson in one (45). Clinical manifestations developed 8 days (median time) after the first dose of vaccine and included systemic thromboembolic and hemorrhagic complications, in addition to the aforementioned adrenal hemorrhage. In two patients, clinical presentation was fatal (46, 47). A diagnosis of vaccine-induced immune thrombotic thrombocytopenia (VITT) was made in seven cases (43–45, 48–50), and anti-platelet factor 4 (PF-4) antibodies were detected in eight patients (43, 44, 47–50).
Moreover, Maguire et al. (51) and Markovic et al. (52) described collectively six cases of adrenal crisis or incipient crisis, occurred after the administration of Vaxzevria ChAdOx1 vaccine (five patients) (51) or Pfizer/BioNtechBNT162b2 (one patient) (52). All the patients had a previous diagnosis of adrenal insufficiency, under proper replacement therapy. Adverse reactions developed within 24h after vaccination and caused adrenal crises, and hospitalization was needed in four cases. All the adrenal crises were promptly resolved by increasing the replacement therapy regimen.
Finally, Haji et al. (53) described a case of pheochromocytoma multisystem crisis occurred within 24h after the first dose of the Johnson and Johnson COVID-19 vaccine in a 63-years-old man. His clinical presentation was so severe to determine respiratory distress, acute kidney injury, cardiogenic shock, and cardiomyopathy. Once respiratory and hemodynamic stability were obtained, the patient was successfully treated with adrenal mass resection.
Pituitary gland disfunctions after COVID-19 vaccine, including hypophysitis, pituitary apoplexy (PA), adrenocorticotropic hormone (ACTH) deficiency, syndrome of inappropriate antidiuresis (SIADH), and central diabetes insipidus were reported in eight cases, as briefly shown in Table 1 (and extensively in Supplementary Table S1).
Regarding hypohphysitis, two cases were reported (54, 55), concerning a man and a woman; median age was 50 years; in both cases, symptoms of hypopituitarism occurred shortly after mRNA-based vaccines (one after first dose and one after second dose). Imaging findings and blood tests were consistent with hypophysitis, with central adrenal insufficiency and secondary hypothyroidism in one case (54) and central diabetes insipidus in the other one (55), both requiring long term hormonal replacement therapy.
Concerning PA, two cases (56, 57) were reported, both regarding young women (28–37 years), who showed intense headache 1–5 days after the first dose of Vaxzevria ChAdOx1 (56, 57). In both patients, haemorrhagic bleeding of the adenohypophysis was documented and in none of the cases any medical treatment was needed.
Isolated ACTH deficit was observed in two cases (58, 59), both occurring in young men (31–48 years) shortly after Pfizer/BioNtechBNT162b2 vaccine (first dose in one case and second dose in the other one). In one case (58), adrenal insufficiency due to ACTH deficit was the only clinical manifestation, whereas the patient described by Mizuno et al. (59) was diagnosed with concomitant neuroleptic malignant syndrome and ACTH deficit. Both patients were given adequate steroid replacement therapy with clinical improvement.
Finally, two more cases were reported, one concerning SIADH (60) occurred in a 79-year-old woman 10 days after the second dose of the Moderna mRNA-1273 and one regarding the onset of central diabetes insipidus (61) in a 37-year-old woman 7 days after the second dose of Pfizer/BioNtechBNT162b2 vaccine. In both cases, adequate treatment with desmopressin (61) or with intravenous crystalloid solutions, fluid restriction and oral urea (60) led to rapid improvement of the symptoms.
Case reports/series on type 1 diabetes mellitus (T1DM) after COVID-19 vaccine, regarding both new onset and disease worsening, are briefly shown in Table 1 (for complete data, see Supplementary Table S1).
Acute onset T1DM following COVID-19 vaccine was reported in 11 case reports/case series (62–72) concerning 15 patients. Median age was 50 years, and eight of 15 were women. T1DM occurred after mRNA-based vaccine (Pfizer/BioNtechBNT162b2 or Moderna mRNA-1273) in 13 of 15 cases, in one case after CoronaVac (65) and one after Vaxzevria ChAdOx1-S (70). Median time to onset of symptoms was 6 days, with a wide range from 3 days (63) to 2 months (71). Most common symptoms were fever, nausea, abdominal pain, fatigue, polydipsia, polyuria, and weight loss. Anti-glutamic acid decarboxylase antibodies and/or insulin autoantibodies were found in nine patients (62, 64, 67, 70, 71). Five patients (64–68) were found to have HLA haplotypes known to confer T1DM risk.
Diabetic ketoacidosis (DKA) in patients with known T1DM was reported in two case series (73, 74) and one case report (75), including five patients (four women and one man; median age was 25 years). In one case, DKA occurred less than 12h after the first dose of Pfizer/BioNTech BNT162b2 (73) in a patient who had consumed approximately 20 g of alcohol on the night before vaccination. The other four cases occurred, with a variable latency between 12h and 6 days, after the second dose of Moderna mRNA-1273 (75), Pfizer/BioNTech BNT162b2 (73), Vaxzevria ChAdOx1 (74) and Covaxin (BBV152-inactivated whole virion) (74). All patients were successfully treated with intravenous fluids and insulin. Furthermore, Infante et al. (76) reported the case of a young T1DM man, previously in optimal glycaemic control with only low basal insulin daily doses, who suffered from a transient deterioration of glucose control in the first days after both Pfizer/BioNTech BNT162b2.
Finally, some studies investigated the short-term effects of COVID-19 vaccination on blood glucose in individuals with T1DM, as shown in Supplementary Table 2.
Retrospective studies on patients with T1DM (77, 78) reported that COVID-19 vaccination might be associated with a temporary perturbation of blood glucose levels, especially in patients under oral hypoglycaemic drugs plus insulin and when HbA1c was lower, without differences between vaccine type (mRNA and viral vector).
Aberer and colleagues (79) prospectively investigated the impact of COVID-19 vaccination on time spent in different glycaemic ranges in 58 people with T1DM. No significant differences in time in range were observed, but a worsening of blood glucose was observed concurrently with general side effects after the vaccine.
Three further studies (80–82) retrospectively reported no significant differences in time in range between after and before any vaccination dose.
As regards the ovarian function in relation to COVID-19 vaccines, a few studies have been reportedwith different endpoints, as shown in Supplementary Table 2.
As far as follicular function is concerned, Mohr-Sasson and colleagues (83) analyzed anti- Müllerian hormone (AMH) levels as an ovarian reserve index in 132 fertile women (mean age was 29 years) before and after the mRNA vaccine. They found that interpersonal difference in plasma AMH levels was not significant between vaccinated and non-vaccinated women. Another study by Bentov et al. (84) assessed whether the immune response to the Pfizer/BioNTech BNT162b2 or virus infection affected ovarian follicles’ function in 32 women undergoing in vitro fertilization (IVF) (mean age was 33.7 years), and no effect was found.
Concerning possible ovarian cycle alterations, partially conflicting results were observed.
Indeed, Muhaidat et al. (85) performed a cross-sectional study, via online interviews, on 2,269 fertile young women (median age was 34.3 years) undergone to any type of COVID-19 vaccines. Some menstrual abnormalities were self-reported in 66.3% of cases, spontaneously resolving within 2 months. Mean menstrual duration and menstrual cycle length increased of 1 day after vaccine. Symptoms occurred after the first dose in 46.7% of cases and after the second dose in 32.4% of cases, without any differences between the types of vaccine. Of note, almost one-third of the patients had experienced menstrual abnormalities during the COVID-19 pandemic before vaccination.
Similarly, a cohort study performed by Edelman et al. (86) followed six consecutive menstrual cycles (three pre-vaccine and three post-vaccine) in 3,959 women, aged 18–45 years, in order to identify possible vaccine related changes in menses. The vaccinated group included women who had received both mRNA vaccines and viral vector vaccine. The vaccinated cohort experienced 0.64 days increase in the length of their menstrual cycles (compared with pre-vaccinal cycles). Considering patients who received both doses within the same menstrual cycle (358), 38 women (10.6%) experienced a change in cycle length of 8 days or more compared with the unvaccinated control group. These alterations, however, disappeared after two menstrual cycles post-vaccination.
Conversely, Laganà and colleagues (87) performed a cross-sectional study on 164 fertile young women via self-administered questionnaire. They found that the main cycle length alteration after COVID-19 vaccine (any type) was 1–5 days shortening of the time between menstruations, both after first and second dose, with a slightly higher prevalence after the second dose (60–70%). Menstrual irregularities were self-resolving within 2 months.
More recently, Lessans et al. (88), reported menstrual cycle alterations in almost 40% of 219 women aged 18-50 years who had undergone mRNA-based vaccine in the 3 months following the vaccine shots.
Finally, Lee et al. (89) retrospectively confirmed these menstrual alterations in a large observational study on 39,129 women (median age was 33 years).
Male fertility after COVID-19 vaccination has been assessed in one case report (90) (Supplementary Table S1) and 11 clinical studies (91, 93–102), as shown in Supplementary Table 2.
As for the case report (90), sperm parameters were analyzed after three doses of mRNA vaccine in a 43-year-old man with ankylosing spondylitis under therapy with anti-inflammatory drugs, and no detrimental impact was observed.
Concerning clinical studies, most papers (90, 93–102) evaluated the impact of different type of COVID-19 vaccines on sperm parameters, whereas one article (91) focused on risk of genital infections.
In detail, five studies retrospectively analyzed the potential impact of COVID-19 vaccine (both mRNA, inactivated and viral vector ones) on sperm parameters, three of them in men undergoing fertility treatments (94, 96, 100) and two in healthy semen donors (99, 101). Overall, sperm parameters showed no significant changes after vaccination, except a slight decrease in sperm volume observed by Safrai et al. (96) in patients undergoing IVF treatment, and a lower concentration and motility reported by Gat et al. (99), but the latter alterations spontaneously recovered at a subsequent control.
Moreover, five prospective studies were performed, four of which on healthy young volunteers/sperm donor men (93, 95, 97, 98), and one on men undergoing IVF (for female infertility factors) (102). Overall, no significant alterations of sperm parameters were observed, except in the study by Abd et al. (102), in which they observed a significant reduction of total and progressive sperm motility after the vaccine shot, albeit still in normal range.
Finally, a retrospective large cohort study (91) compared vaccinated and unvaccinated men to assess the risk of developing orchitis and/or epididymitis, and it showed that men who received any type of COVID-19 vaccine had a significantly reduced risk of infections compared with unvaccinated men.
To date, there are no data regarding a negative effect of COVID-19 vaccination on male hypogonadism, testosterone levels and sexual dysfunction.
The impact of COVID-19 vaccination on couple fertility was evaluated in five studies, four of which (13, 103–105) concerning couples undergoing IVF treatments, and one (106) regarding couples trying to conceive naturally (Supplementary Table 2). Overall, no differences were observed in fertility rates and in pregnancy outcomes in couple undergoing IVF. As far as spontaneous conceive is concerned (106), no effects were observed after COVID-19 vaccine, compared with SARS-CoV-2 infection, which, on the contrary, was associated with a transient reduction of fecundability in male subjects.
Vaccines are one of the most important advances in medical science and are the most effective method to prevent severe manifestations associated with various infections. However, since they are employed in healthy people, possible adverse effects are of great clinical interest (107). Vaccination campaigns against COVID-19 began more than 1 year ago, and the efficacy of COVID-19 vaccines at 5 months or longer after being fully vaccinated is now about 90% (95% CI, 87–92%) (8, 108, 109). Despite the increasing effectiveness evidence, the occurrence of adverse reactions after vaccines administration may elicit some concern, especially due to the spread misinformation about COVID-19 vaccination, such as infertility in men, which are not substantiated by the data available (3).
In the present review, we summarized the available data concerning COVID-19 vaccine and potential endocrine adverse effects in order to clarify and, possibly, quantify the risk for endocrinopathies after COVID-19 vaccination.
Most findings concern thyroid disorders, especially SAT and Graves’ disease after COVID-19 vaccine, as recently pointed out also by Jafarzadeh and colleagues (9).
With respect to SAT, the findings concerning its occurrence after COVID-19 vaccination are in substantial agreement with the demographic features of the cases previously reported in general population, such as a clear preference for female sex and age between 40 and 50 years (110).
Moreover, as for SAT following other causes (110), SAT occurring after COVID-19 vaccine was generally self-limited, leading to permanent hypothyroidism in about 5% of patients.
Concerning Graves’ disease, there was a clear prevalence of young female patients, in agreement with literature findings (111), being the female to male ratio about 3:1. Fortunately, as for SAT, a favorable outcome was observed in nearly all cases after proper treatment.
Regarding SAT pathogenesis, it is commonly accepted that a recent viral infection may be a triggering agent in SAT development in genetically susceptible individuals (112). Some infections have been associated with SAT occurrence, such as measles, mumps, coxsackie, rubella, adenovirus (112), and COVID-19 infection (113). Also, Graves’ disease onset has been associated with viral infections (111) and several cases of Graves’ disease have been reported after COVID-19 infection (114). Actually, it has been shown that thyroid cells express ACE2, which may facilitate COVID-19 infectivity and lead to a direct attack to the thyroid tissue, with subsequent gland dysfunction (115–117).
More interestingly, SAT has been reported also after vaccinations, such as influenza, human papillomavirus, and hepatitis B vaccines (9). Conversely, the risk of Graves’ disease occurrence after vaccinations has rarely been evaluated (33).
The mechanism by which COVID-19 vaccine may cause SAT and Graves’ disease is still unclear due to limited data.
Possible pathophysiological mechanisms include molecular mimicry, whereby antibodies directed against SARS-CoV-2 proteins cross-react with thyroid antigens and may trigger an autoimmune response in susceptible people (118). An alternative is represented by vaccine-induced higher viscosity status, which may cause an abnormal increase in thyroid hormone levels (119).
Furthermore, both SAT and Graves’ disease have been previously reported as part of the autoimmune/inflammatory syndrome in response to adjuvants (ASIA) (120). ASIA syndrome was firstly described in 2011 by Schoenfeld (121) to include clinical entities which shared similar signs and symptoms together with a previous exposure to adjuvants. Adjuvants exert an important role in vaccines and drugs, since their ability to increase the immunogenicity of the active ingredient is mandatory to achieve an adequate immune response (122). On the other side, adjuvants can induce the formation of autoantibodies. Even if the mRNA-based COVID-19 vaccines, which were more frequently used in patients who developed thyroid issues (both SAT and Graves’ disease), do not contain adjuvants, the mRNA itself could act as an adjuvant, being highly immunostimulatory (123); moreover, it is not clear if the lipid nanoparticles employed to preserve mRNA stability may act as adjuvant (122).
Furthermore, specific HLA haplotypes, such as HLA-B35, may have a role in the susceptibility to SAT in general population (124); interestingly, the association between vaccine-related SAT development and HLA-B35 was found in a recent study (26) even if further data are needed to confirm this relationship.
Taken all the above considerations into account, despite the increasing reports regarding SAT and Graves’ disease, vaccination against COVID-19 is still strongly encouraged, since their occurrence is rare and they are generally easily manageable and/or self-limited.
On the other side, when considering adrenal gland, the possible side effects after COVID-19 vaccine were extremely rare, but more severe than thyroid dysfunction. In fact, of the 16 case reports described so far, nine were about adrenal haemorrhage in the setting of immune thrombotic thrombocytopenia (ITT), and two patients eventually died due to the severity of clinical manifestations. Adrenal haemorrhage developed within 2 weeks and in all cases followed adenoviral vector vaccine administration.
As for the mechanisms by which an adenoviral vector vaccine may cause ITT, it has been hypothesized that there may be a reaction between the cationic PF4 and the anionic free DNA contained in the recombinant vaccine (45, 125). However, the underlying pathways by which COVID-19 vaccination may stimulate anti-PF4 antibody production need further examination.
Concerning the pituitary gland, very few cases of post-vaccination pituitary gland dysfunction have been reported since the beginning of COVID-19 vaccination campaign. So far, data are too sparse and heterogeneous to draw any conclusions about the possible pathogenesis of pituitary dysfunction after COVID-19 vaccination. However, several case reports about COVID-19–induced damage to hypothalamus and pituitary have been observed, such as panhypopituitarism (126), central diabetes insipidus (127) and SIADH resulting in hyponatremia (128, 129); significantly, the expression of ACE2 in the hypothalamus has been identified (92), and the SARS-CoV-2 genome has been detected in the cerebrospinal fluid of a patient with COVID-19, thereby confirming that SARS-CoV-2 can infiltrate into the brain including the hypothalamus and pituitary gland (130). In addition, an autoimmune mechanism triggered by vaccination could play a role in the pathogenetic pituitary damage (as autoimmune hypophysitis). In detail, anti-pituitary antibodies can primarily target corticotropic cells, with consequent isolated secondary hypoadrenalism after vaccination (58).
The possible link between vaccinations and the occurrence of T1DM has been investigated for many different vaccines to date, such as vaccines against influenza virus A/H1N1 (131), Rotavirus (132), human papilloma virus (HPV) (133), with the general agreement that vaccinations are not associated with increased risk of islet autoimmunity or T1DM (134, 135). To date, only few cases of post-COVID-19 vaccination acute diabetes onset have been reported, and in almost all patients a pre-existing genetic predisposition was confirmed (64–68). Therefore, these findings are too few to draw any conclusions about the possible pathogenetic mechanism.
Even when considering already diabetic patients, there are only few reports of DKA after COVID-19 vaccines (regardless of vaccine type), which proved to be easily manageable in all cases. Moreover, the available retrospective and prospective clinical studies showed that COVID-19 vaccines led to none or only temporary perturbations of blood glucose levels (7).
To date, clinical data support the general safety of COVID-19 vaccinations in T1DM patients, with the caveat that patients should be counselled and prepared for possible temporary glycaemic alterations following the vaccine (77). Encouraging vaccination is crucial, since it has been proven that COVID-19 infection may cause islet cells degeneration (7) and that both patients with T1DM and type 2 diabetes mellitus (T2DM), compared with healthy people, carry a worse prognosis in case of Sars-CoV-2 infection, especially in case of poor metabolic control (136).
Concerning female reproductive function related to COVID-19 vaccinations, the main worth mentioning findings are related to the possible and transient slight changes in menstrual cycle length (85–89), even if it is known that several factors may affect cycle length, such as infections, anxiety and hormonal changes, since stressors may activate the hypothalamic-pituitary-gonadal axis, disrupting of the regularity of hormone release (85).
Conflicting results regarding the impact of COVID-19 infection on semen quality have been reported (137). As ACE2 is expressed in spermatogonia and Sertoli and Leydig cells (138), there was concern about the fact that the virus could affect male fertility. Original studies included about 500 semen analyses from men infected with SARS-CoV-2, and some alterations were found in approximatively one third of cases, especially in patients with severe COVID-19 (138). Hence, these findings elicited some concern also about the possible role of COVID-19 vaccination on male fertility (137, 139). However, according to the published data, overall, no significant adverse effects of COVID-19 vaccines on male fertility have been reported so far, and no detrimental effect on both semen quality and fertility outcomes were observed in healthy donors (93, 95, 98, 99, 101), infertile men (94, 96) and couples undergoing assisted reproduction treatments (103, 104) or trying to conceive naturally (106). Of note, COVID-19 vaccination was shown to have a protective role on orchitis and epididymitis (91), which are known risk factor for male infertility (140).
Therefore, the concern regarding the impact of COVID-19 vaccination on male infertility appear as unfounded (141); instead, considering the potential impact of disease on male fertility, vaccination should be recommended (138).
Finally, as different types of COVID-19 vaccines might have different side effects, we tried to figure out a possible relationship between specific endocrine side effects and vaccine types, but we could not obtain clear evidence concerning these hypothetical associations, considering that most of the data came from case reports. Noteworthy, the only likely correlation was about vascular complications following adenoviral vector vaccine administration, but this issue was shown only for adrenal gland, and due to the scant data, we could not generalize this finding nor for adrenal gland or for other endocrine glands in case of adenoviral vector vaccine.
We summarized the available reports and studies regarding COVID-19 vaccines and potential endocrine adverse effects, showing that their occurrence is generally rare and with benign outcome, being thyroid alterations the most common—but generally easily manageable—findings.
Overall, these data do not call into question the safety and the efficacy of available COVID-19 vaccines, which are relevant and confirmed by multiple studies.
Data on endocrine adverse effects, albeit increasing, are not strong enough to draw any clear conclusion about their causal relationship with vaccine administration, since they are based mainly on case-reports; therefore, further clinical and preclinical studies are needed to clarify the possible mechanisms underlying these manifestations.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
CC conceived the project. LP and CC provided supervision and project administration. EG, FB, PF, MG, MC, CB and ED did the literature search. LP, EG, FB, PF, MG, MC, CB and ED wrote the original draft. EG, FB and PF cured the supplementary content. MR, AF and LP reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1027047/full#supplementary-material
1. World Health Organization Coronavirus (COVID-19) Dashboard. Available at: http://covid19.who.int.
2. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med (2020) 26(7):1017–32. doi: 10.1038/s41591-020-0968-3
3. Mirza SA, Sheikh AAE, Barbera M, Ijaz Z, Javaid MA, Shekhar R, et al. COVID-19 and the endocrine system: A review of the current information and misinformation. Infect Dis Rep (2022) 14(2):184–97. doi: 10.3390/idr14020023
4. Wang J, Zhu K, Xue Y, Wen G, Tao L. Research progress in the treatment of complications and sequelae of COVID-19. Front Med (Lausanne) (2021) 8:757605. doi: 10.3389/fmed.2021.757605
5. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell (2020) 181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052
6. World Health Organization. COVID-19 advice for the public: getting vaccinated. Available at: http://who.int.
7. Zhao Y, Wu X. Influence of COVID-19 vaccines on endocrine system. Endocrine (2022) 7:241–6. doi: 10.1007/s12020-022-03119-3
8. Asghar N, Mumtaz H, Syed AA, Eqbal F, Maharjan R, Bamboria A, et al. Safety, efficacy, and immunogenicity of COVID-19 vaccines; a systematic review. Immunol Med (2022) 1–13. doi: 10.1080/25785826.2022.2068331
9. Jafarzadeh A, Nemati M, Jafarzadeh S, Nozari P, Mortazavi SMJ. Thyroid dysfunction following vaccination with COVID-19 vaccines: a basic review of the preliminary evidence. J Endocrinol Invest (2022) 45:1835–63. doi: 10.1007/s40618-022-01786-7
10. La Vignera S, Cannarella R, Condorelli RA, Torre F, Aversa A, Calogero AE. SARS-CoV-2: the endocrinological protective clinical model derived from patients with prostate cancer. Ther Adv Endocrinol Metab (2020) 11:2042018820942385. doi: 10.1177/2042018820942385
11. Mongioì LM, Barbagallo F, Condorelli RA, Cannarella R, Aversa A, La Vignera S, et al. Possible long-term endocrine-metabolic complications in COVID-19: lesson from the SARS model. Endocrine (2020) 68(3):467–70. doi: 10.1007/s12020-020-02349-7
12. García M, Albizua-Madariaga I, Lertxundi U, Aguirre C. Subacute thyroiditis and COVID-19 vaccines: a case/non-case study. Endocrine (2022) 77:480–5. doi: 10.1007/s12020-022-03101-z
13. Avraham S, Kedem A, Zur H, Youngster M, Yaakov O, Yerushalmi GM, et al. Coronavirus disease 2019 vaccination and infertility treatment outcomes. Fertil Steril (2022) 117(6):1291–9. doi: 10.1016/j.fertnstert.2022.02.025
14. Rubinstein TJ. Thyroid eye disease following COVID-19 vaccine in a patient with a history graves' disease: A case report. Ophthal Plast Reconstr Surg (2021) 37(6):e221–3. doi: 10.1097/IOP.0000000000002059
15. Goblirsch TJ, Paulson AE, Tashko G, Mekonnen AJ. Graves' disease following administration of second dose of SARS-CoV-2 vaccine. BMJ Case Rep (2021) 14(12). doi: 10.1136/bcr-2021-246432
16. Bostan H, Kayihan S, Calapkulu M, Hepsen S, Gul U, Ozturk Unsal I, et al. Evaluation of the diagnostic features and clinical course of COVID-19 vaccine-associated subacute thyroiditis. Hormones (Athens) (2022) 21:447–55. doi: 10.1007/s42000-022-00380-z
17. Topaloğlu Ö., Tekin S, Topaloğlu SN, Bayraktaroglu T. Differences in clinical aspects between subacute thyroiditis associated with COVID-19 vaccines and classical subacute thyroiditis. Horm Metab Res (2022) 54(6):380–8. doi: 10.1055/a-1840-4374
18. Sözen M, Topaloğlu Ö., Çetinarslan B, Selek A, Cantürk Z, Gezer E, et al. COVID-19 mRNA vaccine may trigger subacute thyroiditis. Hum Vaccin Immunother (2021) 17(12):5120–5. doi: 10.1080/21645515.2021.2013083
19. Ippolito S, Gallo D, Rossini A, Patera B, Lanzo N, Fazzino GFM, et al. SARS-CoV-2 vaccine-associated subacute thyroiditis: insights from a systematic review. J Endocrinol Invest (2022) 45:1189–200. doi: 10.1007/s40618-022-01747-0
20. Oğuz SH, Şendur SN, İremli BG, Gürlek A, Erbas T, Ünlütürk U. SARS-CoV-2 vaccine-induced thyroiditis: Safety of revaccinations and clinical follow-up. J Clin Endocrinol Metab (2022) 107(5):e1823–34. doi: 10.1210/clinem/dgac049
21. Yorulmaz G, Sahin Tekin M. SARS-CoV-2 vaccine-associated subacute thyroiditis. J Endocrinol Invest (2022) 45:1341–7. doi: 10.1007/s40618-022-01767-w
22. Sigstad E, Grøholt KK, Westerheim O. Subacute thyroiditis after vaccination against SARS-CoV-2. Tidsskr Nor Laegeforen (2021) 141:(2021–14). doi: 10.4045/tidsskr.21.0554
23. Raven LM, McCormack AI, Greenfield JR. Letter to the Editor from raven et al: "Three cases of subacute thyroiditis following SARS-CoV-2 vaccine". J Clin Endocrinol Metab (2022) 107(4):e1767–8. doi: 10.1210/clinem/dgab822
24. Vasileiou V, Paschou SA, Tzamali X, Mitropoulou M, Kanouta F, Psaltopoulou T, et al. Recurring subacute thyroiditis after SARS-CoV-2 mRNA vaccine: A case report. Case Rep Womens Health (2022) 33:e00378. doi: 10.1016/j.crwh.2021.e00378
25. Topaloglu O, Tekin S, Topaloglu S, Bayraktaroglu T. Persistent subacute thyroiditis post SARS-CoV-2 vaccine in a Male patient with positive thyroid autoantibodies. Turkish J Of Endocrinol And Metab (2022) 54:380–8. doi: 10.25179/tjem.2021-86594
26. Şendur SN, Özmen F, Oğuz SH, İremli BG, Malkan Ü., Gürlek A, et al. Association of human leukocyte antigen genotypes with severe acute respiratory syndrome coronavirus 2 vaccine-induced subacute thyroiditis. Thyroid (2022) 32(6):640–7. doi: 10.1089/thy.2022.0010
27. Vera-Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sánchez Valadez TI, Jara LJ. Two cases of graves' disease following SARS-CoV-2 vaccination: An Autoimmune/Inflammatory syndrome induced by adjuvants. Thyroid (2021) 31(9):1436–9. doi: 10.1089/thy.2021.0142
28. Singh G, Howland T. Graves' disease following COVID-19 vaccination. Cureus (2022) 14(4):e24418. doi: 10.7759/cureus.24418
29. Sriphrapradang C. Aggravation of hyperthyroidism after heterologous prime-boost immunization with inactivated and adenovirus-vectored SARS-CoV-2 vaccine in a patient with graves' disease. Endocrine (2021) 74(2):226–7. doi: 10.1007/s12020-021-02879-8
30. Patrizio A, Ferrari SM, Antonelli A, Fallahi P. Worsening of graves' ophthalmopathy after SARS-CoV-2 mRNA vaccination. Autoimmun Rev (2022) 21:103096. doi: 10.1016/j.autrev.2022.103096
31. Park KS, Fung SE, Ting M, Ozzello DJ, Yoon JS, Liu CY, et al. Thyroid eye disease reactivation associated with COVID-19 vaccination. Taiwan J Ophthalmol (2022) 12(1):93–6. doi: 10.4103/tjo.tjo_61_21
32. Bostan H, Ucan B, Kizilgul M, Calapkulu M, Hepsen S, Gul U, et al. Relapsed and newly diagnosed graves' disease due to immunization against COVID-19: A case series and review of the literature. J Autoimmun (2022) 128:102809. doi: 10.1016/j.jaut.2022.102809
33. Lui DTW, Lee KK, Lee CH, Lee ACH, Hung IFN, Tan KCB. Development of graves' disease after SARS-CoV-2 mRNA vaccination: A case report and literature review. Front Public Health (2021) 9:778964. doi: 10.3389/fpubh.2021.778964
34. Bostan H, Unsal IO, Kizilgul M, Gul U, Sencar ME, Ucan B, et al. Two cases of subacute thyroiditis after different types of SARS-CoV-2 vaccination. Arch Endocrinol Metab (2022) 66(1):97–103. doi: 10.20945/2359-3997000000430
35. Weintraub MA, Ameer B, Sinha Gregory N. Graves disease following the SARS-CoV-2 vaccine: Case series. J Investig Med High Impact Case Rep (2021) 9:23247096211063356. doi: 10.1177/23247096211063356
36. Pla Peris B, Merchante Alfaro A, Maravall Royo FJ, Abellán Galiana P, Pérez Naranjo S, González Boillos M. Thyrotoxicosis following SARS-COV-2 vaccination: a case series and discussion. J Endocrinol Invest (2022) 45(5):1071–7. doi: 10.1007/s40618-022-01739-0
37. Chee YJ, Liew H, Hoi WH, Lee Y, Lim B, Chin HX, et al. SARS-CoV-2 mRNA vaccination and graves' disease: a report of 12 cases and review of the literature. J Clin Endocrinol Metab (2022) 107:e2324–30. doi: 10.1210/clinem/dgac119
38. Pierman G, Delgrange E, Jonas C. Recurrence of graves' disease (a Th1-type cytokine disease) following SARS-CoV-2 mRNA vaccine administration: A simple coincidence? Eur J Case Rep Intern Med (2021) 8(9):2807. doi: 10.12890/2021_002807
39. Paschou SA, Karalis V, Psaltopoulou T, Vasileiou V, Charitaki I, Bagratuni T, et al. Patients with autoimmune thyroiditis present similar immunological response to COVID-19 BNT162b2 mRNA vaccine with healthy subjects, while vaccination may affect thyroid function: A clinical study. Front Endocrinol (Lausanne) (2022) 13:840668. doi: 10.3389/fendo.2022.840668
40. Lui DTW, Lee CH, Cheung CYY, Cheung Mak JH, Fong CHY, Lui BWC, et al. Impact of COVID-19 vaccines on thyroid function and autoimmunity and impact of thyroid autoimmunity on antibody response. J Clin Endocrinol Metab (2022) 107:e3781–9. doi: 10.1210/clinem/dgac355
41. Li L, Chen X, Li B, Liu D, Liu YH, Mo R, et al. Effect of inactivated SARS-CoV-2 vaccine on thyroid function and autoimmunity within 28 days after the second dose. Thyroid (2022) 32:1051–8. doi: 10.1089/thy.2022.0101
42. Xiong X, Wong CKH, Au ICH, Lai FTT, Li X, Wan EYF, et al. Safety of inactivated and mRNA COVID-19 vaccination among patients treated for hypothyroidism: A population-based cohort study. Thyroid (2022) 32(5):505–14. doi: 10.1089/thy.2021.0684
43. Taylor P, Allen L, Shrikrishnapalasuriyar N, Stechman M, Rees A. Vaccine-induced thrombosis and thrombocytopenia with bilateral adrenal haemorrhage. Clin Endocrinol (Oxf) (2021) 97:26–7. doi: 10.1111/cen.14548
44. Varona JF, García-Isidro M, Moeinvaziri M, Ramos-López M, Fernández-Domínguez M. Primary adrenal insufficiency associated with Oxford-AstraZeneca ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia (VITT). Eur J Intern Med (2021) 91:90–2. doi: 10.1016/j.ejim.2021.06.025
45. Tews HC, Driendl SM, Kandulski M, Buechler C, Heiss P, Stöckert P, et al. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia with venous thrombosis, pulmonary embolism, and adrenal haemorrhage: A case report with literature review. Vaccines (Basel) (2022) 10(4). doi: 10.3390/vaccines10040595
46. D'Agostino V, Caranci F, Negro A, Piscitelli V, Tuccillo B, Fasano F, et al. A rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated to the COVID-19 vaccine administration. J Pers Med (2021) 11(4). doi: 10.3390/jpm11040285
47. Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas AM. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost (2021) 19(7):1771–5. doi: 10.1111/jth.15347
48. Al Rawahi B, BaTaher H, Jaffer Z, Al-Balushi A, Al-Mazrouqi A, Al-Balushi N. Vaccine-induced immune thrombotic thrombocytopenia following AstraZeneca (ChAdOx1 nCOV19) vaccine-a case report. Res Pract Thromb Haemost (2021) 5(6):e12578. doi: 10.1002/rth2.12578
49. Graf A, Armeni E, Dickinson L, Stubbs. M, Craven B, Srirangalingam ,U, et al. Adrenal haemorrhage and infarction in the setting of vaccine-induced immune thrombocytopenia and thrombosis after SARS-CoV-2 (Oxford–AstraZeneca) vaccination. Endocrinol Diabetes Metab Case Rep (2022). doi: 10.1530/EDM-21-0144
50. Efthymiadis A, Khan D, Pavord S, Pal A. A case of ChAdOx1 vaccine-induced thrombocytopenia and thrombosis syndrome leading to bilateral adrenal haemorrhage and adrenal insufficiency. Endocrinol Diabetes Metab Case Rep (2022). doi: 10.1530/EDM-22-0239
51. Maguire D, McLaren DS, Rasool I, Shah PM, Lynch J, Murray RD. ChAdOx1 SARS-CoV-2 vaccination: A putative precipitant of adrenal crises. Clin Endocrinol (Oxf) (2021). doi: 10.1111/cen.14566
52. Markovic N, Faizan A, Boradia C, Nambi S. Adrenal crisis secondary to COVID-19 vaccination in a patient with hypopituitarism. AACE Clin Case Rep (2022) 8(4):171–3. doi: 10.1016/j.aace.2022.04.004
53. Haji N, Ali S, Wahashi EA, Khalid M, Ramamurthi K. Johnson And Johnson COVID-19 vaccination triggering pheochromocytoma multisystem crisis. Cureus (2021) 13(9):e18196. doi: 10.7759/cureus.18196
54. Murvelashvili N, Tessnow A. A case of hypophysitis following immunization with the mRNA-1273 SARS-CoV-2 vaccine. J Investig Med High Impact Case Rep (2021) 9:23247096211043386. doi: 10.1177/23247096211043386
55. Ankiredypalli A, Chow LS, Radulescu A, Kawakami Y, Araki T. A case of hypophysitis associated with SARS-CoV2 vaccination. AACE Clin Case Rep (2022) 8:204–9. doi: 10.1016/j.aace.2022.06.001
56. Piñar-Gutiérrez A, Remón-Ruiz P, Soto-Moreno A. Case report: Pituitary apoplexy after COVID-19 vaccination. Med Clin (Barc) (2021) 158:498–9. doi: 10.1016/j.medcli.2021.09.028
57. Roncati L, Manenti A. Pituitary apoplexy following adenoviral vector-based COVID-19 vaccination. Brain Hemorrhages (2022). doi: 10.1016/j.hest.2022.04.002
58. Morita S, Tsuji T, Kishimoto S, Uraki S, Takeshima K, Iwakura H, et al. Isolated ACTH deficiency following immunization with the BNT162b2 SARS-CoV-2 vaccine: a case report. BMC Endocr Disord (2022) 22(1):185. doi: 10.1186/s12902-022-01095-3
59. Mizuno T, Takahashi R, Kamiyama T, Suzuki A, Suzuki M. Neuroleptic malignant syndrome with adrenal insufficiency after BNT162b2 COVID-19 vaccination in a man taking valproate: A case report. Am J Case Rep (2022) 23:e936217. doi: 10.12659/AJCR.936217
60. Lindner G, Ryser B. The syndrome of inappropriate antidiuresis after vaccination against COVID-19: case report. BMC Infect Dis (2021) 21(1):1000. doi: 10.1186/s12879-021-06690-8
61. Bouça B, Roldão M, Bogalho P, Cerqueira L, Silva-Nunes J. Central diabetes insipidus following immunization with BNT162b2 mRNA COVID-19 vaccine: A case report. Front Endocrinol (Lausanne) (2022) 13:889074. doi: 10.3389/fendo.2022.889074
62. Patrizio A, Ferrari SM, Antonelli A, Fallahi P. A case of graves' disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J Autoimmun (2021) 125:102738. doi: 10.1016/j.jaut.2021.102738
63. Ohuchi K, Amagai R, Tamabuchi E, Kambayashi Y, Fujimura T. Fulminant type 1 diabetes mellitus triggered by coronavirus disease 2019 vaccination in an advanced melanoma patient given adjuvant nivolumab therapy. J Dermatol (2022) 49(5):e167–8. doi: 10.1111/1346-8138.16304
64. Yano M, Morioka T, Natsuki Y, Sasaki K, Kakutani Y, Ochi A, et al. New-onset type 1 diabetes after COVID-19 mRNA vaccination. Intern Med (2022) 61(8):1197–200. doi: 10.2169/internalmedicine.9004-21
65. Tang X, He B, Liu Z, Zhou Z, Li X. Fulminant type 1 diabetes after COVID-19 vaccination. Diabetes Metab (2022) 48(2):101324. doi: 10.1016/j.diabet.2022.101324
66. Sasaki K, Morioka T, Okada N, Natsuki Y, Kakutani Y, Ochi A, et al. New-onset fulminant type 1 diabetes after severe acute respiratory syndrome coronavirus 2 vaccination: A case report. J Diabetes Investig (2022) 13:1286–9. doi: 10.1111/jdi.13771
67. Sasaki H, Itoh A, Watanabe Y, Nakajima Y, Saisho Y, Irie J, et al. Newly developed type 1 diabetes after coronavirus disease 2019 vaccination: A case report. J Diabetes Investig (2022) 13:1105–8. doi: 10.1111/jdi.13757
68. Sakurai K, Narita D, Saito N, Ueno T, Sato R, Niitsuma S, et al. Type 1 diabetes mellitus following COVID-19 RNA-based vaccine. J Diabetes Investig (2022) 13:1290–2. doi: 10.1111/jdi.13781
69. Makiguchi T, Fukushima T, Tanaka H, Taima K, Takayasu S, Tasaka S. Diabetic ketoacidosis shortly after COVID-19 vaccination in a non-small-cell lung cancer patient receiving combination of PD-1 and CTLA-4 inhibitors: A case report. Thorac Cancer (2022) 13(8):1220–3. doi: 10.1111/1759-7714.14352
70. Bleve E, Venditti V, Lenzi A, Morano S, Filardi T. COVID-19 vaccine and autoimmune diabetes in adults: report of two cases. J Endocrinol Invest (2022) 45(6):1269–70. doi: 10.1007/s40618-022-01796-5
71. Aydoğan B, Ünlütürk U, Cesur M. Type 1 diabetes mellitus following SARS-CoV-2 mRNA vaccination. Endocrine (2022) 78:42–6. doi: 10.1007/s12020-022-03130-8
72. Sato T, Kodama S, Kaneko K, Imai J, Katagiri H. Type 1 diabetes mellitus associated with nivolumab after second SARS-CoV-2 vaccination, Japan. Emerg Infect Dis (2022) 28(7):1518–20. doi: 10.3201/eid2807.220127
73. Yakou F, Saburi M, Hirose A, Akaoka H, Hirota Y, Kobayashi T, et al. A case series of ketoacidosis after coronavirus disease 2019 vaccination in patients with type 1 diabetes. Front Endocrinol (Lausanne) (2022) 13:840580. doi: 10.3389/fendo.2022.840580
74. Ganakumar V, Jethwani P, Roy A, Shukla R, Mittal M, Garg MK. Diabetic ketoacidosis (DKA) in type 1 diabetes mellitus (T1DM) temporally related to COVID-19 vaccination. Diabetes Metab Syndr (2022) 16(1):102371. doi: 10.1016/j.dsx.2021.102371
75. Zilbermint M, Demidowich AP. Severe diabetic ketoacidosis after the second dose of mRNA-1273 COVID-19 vaccine. J Diabetes Sci Technol (2022) 16(1):248–9. doi: 10.1177/19322968211043552
76. Infante M, Fabbri A, Padilla N, Pacifici F, Di Perna P, Vitiello L, et al. BNT162b2 mRNA COVID-19 vaccine does not impact the honeymoon phase in type 1 diabetes: A case report. Vaccines (Basel) (2022) 10(7). doi: 10.3390/vaccines10071096
77. Heald AH, Stedman M, Horne L, Rea R, Whyte M, Gibson JM, et al. The change in glycaemic control immediately after COVID-19 vaccination in people with type 1 diabetes. Diabetes Med (2022) 39(4):e14774. doi: 10.1111/dme.14774
78. Heald AH, Rea R, Horne L, Metters A, Steele T, Leivesley K, et al. Analysis of continuous glucose tracking data in people with type 1 diabetes after COVID-19 vaccination reveals unexpected link between immune and metabolic response, augmented by adjunctive oral medication. Int J Clin Pract (2021) 75(12):e14714. doi: 10.1111/ijcp.14714
79. Aberer F, Moser O, Aziz F, Sourij C, Ziko H, Lenz J, et al. Impact of COVID-19 vaccination on glycemia in individuals with type 1 and type 2 diabetes: Substudy of the COVAC-DM study. Diabetes Care (2022) 45(2):e24–6. doi: 10.2337/dc21-1563
80. D'Onofrio L, Coraggio L, Zurru A, Carlone A, Mignogna C, Moretti C, et al. Short-term safety profile of sars-Cov2 vaccination on glucose control: Continuous glucose monitoring data in people with autoimmune diabetes. Diabetes Res Clin Pract (2021) 179:109022. doi: 10.1016/j.diabres.2021.109022
81. Piccini B, Pessina B, Pezzoli F, Casalini E, Toni S. COVID-19 vaccination in adolescents and young adults with type 1 diabetes: Glycemic control and side effects. Pediatr Diabetes (2022) 23:469–72. doi: 10.1111/pedi.13326
82. Dicembrini I, Vitale V, Cosentino C, Cresci B, Pala L, Pieri M, et al. Interstitial glucose monitoring, type 1 diabetes and COVID-19 vaccine: the patient-reported outcomes and vaccine-associated changes in glucose and side effects (PRO-VACS). Acta Diabetol (2022) 59(3):435–8. doi: 10.1007/s00592-021-01837-0
83. Mohr-Sasson A, Haas J, Abuhasira S, Sivan M, Doitch Amdurski H, Dadon T, et al. The effect of covid-19 mRNA vaccine on serum anti-müllerian hormone levels. Hum Reprod (2022) 37(3):534–41. doi: 10.1093/humrep/deab282
84. Bentov Y, Beharier O, Moav-Zafrir A, Kabessa M, Godin M, Greenfield CS, et al. Ovarian follicular function is not altered by SARS-CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum Reprod (2021) 36(9):2506–13. doi: 10.1093/humrep/deab182
85. Muhaidat N, Alshrouf MA, Azzam MI, Karam AM, Al-Nazer MW, Al-Ani A. Menstrual symptoms after COVID-19 vaccine: A cross-sectional investigation in the MENA region. Int J Womens Health (2022) 14:395–404. doi: 10.2147/IJWH.S352167
86. Edelman A, Boniface ER, Benhar E, Han L, Matteson KA, Favaro C, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: A U.S. cohort. Obstet Gynecol (2022) 139:481–9. doi: 10.1097/AOG.0000000000004695
87. Laganà AS, Veronesi G, Ghezzi F, Ferrario MM, Cromi A, Bizzarri M, et al. Evaluation of menstrual irregularities after COVID-19 vaccination: Results of the MECOVAC survey. Open Med (Wars) (2022) 17(1):475–84. doi: 10.1515/med-2022-0452
88. Lessans N, Rottenstreich A, Stern S, Gilan A, Saar TD, Porat S, et al. The effect of BNT162b2 SARS-CoV-2 mRNA vaccine on menstrual cycle symptoms in healthy women. Int J Gynaecol Obstet (2022). doi: 10.1002/ijgo.14356
89. Lee KMN, Junkins EJ, Luo C, Fatima UA, Cox ML, Clancy KBH. Investigating trends in those who experience menstrual bleeding changes after SARS-CoV-2 vaccination. Sci Adv (2022) 8(28):eabm7201. doi: 10.1126/sciadv.abm7201
90. Chatzimeletiou K, Fleva A, Sioga A, Georgiou I, Nikolopoulos TT, Markopoulou M, et al. Effects of different drug therapies and COVID-19 mRNA vaccination on semen quality in a man with ankylosing spondylitis: A case report. Medicina (Kaunas) (2022) 58(2). doi: 10.3390/medicina58020173
91. Carto C, Nackeeran S, Ramasamy R. COVID-19 vaccination is associated with a decreased risk of orchitis and/or epididymitis in men. Andrologia (2022) 54(2):e14281. doi: 10.1111/and.14281
92. Chigr F, Merzouki M, Najimi M. Autonomic brain centers and pathophysiology of COVID-19. ACS Chem Neurosci (2020) 11(11):1520–2. doi: 10.1021/acschemneuro.0c00265
93. Gonzalez DC, Nassau DE, Khodamoradi K, Ibrahim E, Blachman-Braun R, Ory J, et al. Sperm parameters before and after COVID-19 mRNA vaccination. JAMA (2021) 326(3):273–4. doi: 10.1001/jama.2021.9976
94. Reschini M, Pagliardini L, Boeri L, Piazzini F, Bandini V, Fornelli G, et al. COVID-19 vaccination does not affect reproductive health parameters in men. Front Public Health (2022) 10:839967. doi: 10.3389/fpubh.2022.839967
95. Barda S, Laskov I, Grisaru D, Lehavi O, Kleiman S, Wenkert A, et al. The impact of COVID-19 vaccine on sperm quality. Int J Gynaecol Obstet (2022) 158:116–20. doi: 10.1002/ijgo.14135
96. Safrai M, Herzberg S, Imbar T, Reubinoff B, Dior U, Ben-Meir A. The BNT162b2 mRNA covid-19 vaccine does not impair sperm parameters. Reprod BioMed Online (2022) 44(4):685–8. doi: 10.1016/j.rbmo.2022.01.008
97. Lifshitz D, Haas J, Lebovitz O, Raviv G, Orvieto R, Aizer A. Does mRNA SARS-CoV-2 vaccine detrimentally affect male fertility, as reflected by semen analysis? Reprod BioMed Online (2022) 44(1):145–9. doi: 10.1016/j.rbmo.2021.09.021
98. Olana S, Mazzilli R, Salerno G, Zamponi V, Tarsitano MG, Simmaco M, et al. 4BNT162b2 mRNA COVID-19 vaccine and semen: What do we know? Andrology (2022) 10:1023–9. doi: 10.1111/andr.13199
99. Gat I, Kedem A, Dviri M, Umanski A, Levi M, Hourvitz A, et al. Covid-19 vaccination BNT162b2 temporarily impairs semen concentration and total motile count among semen donors. Andrology (2022) 10:1016–22. doi: 10.1111/andr.13209
100. Xia W, Zhao J, Hu Y, Fang L, Wu S. Investigate the effect of COVID-19 inactivated vaccine on sperm parameters and embryo quality in in vitro fertilization. Andrologia (2022) 54(6):e14483. doi: 10.1111/and.14483
101. Zhu H, Wang X, Zhang F, Zhu Y, Du MR, Tao ZW, et al. Evaluation of inactivated COVID-19 vaccine on semen parameters in reproductive-age males: a retrospective cohort study. Asian J Androl (2022) 24:441–4. doi: 10.4103/aja202225
102. Abd ZH, Muter SA, Saeed RAM, Ammar O. Effects of covid-19 vaccination on different semen parameters. Basic Clin Androl (2022) 32(1):13. doi: 10.1186/s12610-022-00163-x
103. Orvieto R, Noach-Hirsh M, Segev-Zahav A, Haas J, Nahum R, Aizer A. Does mRNA SARS-CoV-2 vaccine influence patients' performance during IVF-ET cycle? Reprod Biol Endocrinol (2021) 19(1):69. doi: 10.1186/s12958-021-00757-6
104. Odeh-Natour R, Shapira M, Estrada D, Freimann S, Tal Y, Atzmon Y, et al. Does mRNA SARS-CoV-2 vaccine in the follicular fluid impact follicle and oocyte performance in IVF treatments? Am J Reprod Immunol (2022) 87(5):e13530. doi: 10.1111/aji.13530
105. Wu Y, Cao M, Lin Y, Xu Z, Liang Z, Huang Q, et al. Inactivated COVID-19 vaccination does not affect in-vitro fertilization outcomes in women. Hum Reprod (2022) 37:2054–62. doi: 10.1093/humrep/deac160
106. Wesselink AK, Hatch EE, Rothman KJ, Wang TR, Willis MD, Yland J, et al. A prospective cohort study of COVID-19 vaccination, SARS-CoV-2 infection, and fertility. Am J Epidemiol (2022) 191(8):1383–95. doi: 10.1093/aje/kwac011
107. Jara LJ, Vera-Lastra O, Mahroum N, Pineda C, Shoenfeld Y. Autoimmune post-COVID vaccine syndromes: does the spectrum of autoimmune/inflammatory syndrome expand? Clin Rheumatol (2022) 41(5):1603–9. doi: 10.1007/s10067-022-06149-4
108. Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis (2022) 114:252–60. doi: 10.1016/j.ijid.2021.11.009
109. Ssentongo P, Ssentongo AE, Voleti N, Groff D, Sun A, Ba DM, et al. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis. BMC Infect Dis (2022) 22(1):439. doi: 10.1186/s12879-022-07418-y
110. Samuels MH. Subacute, silent, and postpartum thyroiditis. Med Clin North Am (2012) 96(2):223–33. doi: 10.1016/j.mcna.2012.01.003
111. Davies TF, Andersen S, Latif R, Nagayama Y, Barbesino G, Brito M, et al. Graves' disease. Nat Rev Dis Primers (2020) 6(1):52. doi: 10.1038/s41572-020-0184-y
112. Stasiak M, Lewiński A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev Endocr Metab Disord (2021) 22(4):1027–39. doi: 10.1007/s11154-021-09648-y
113. Aemaz Ur Rehman M, Farooq H, Ali MM, Ebaad Ur Rehman M, Dar QA, Hussain A. The association of subacute thyroiditis with COVID-19: a systematic review. SN Compr Clin Med (2021) 3:1–13. doi: 10.1007/s42399-021-00912-5
114. Mateu-Salat M, Urgell E, Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of graves' disease after COVID-19. J Endocrinol Invest (2020) 43(10):1527–8. doi: 10.1007/s40618-020-01366-7
115. Murugan AK, Alzahrani AS. SARS-CoV-2: Emerging role in the pathogenesis of various thyroid diseases. J Inflammation Res (2021) 14:6191–221. doi: 10.2147/JIR.S332705
116. Coperchini F, Ricci G, Croce L, Denegri M, Ruggiero R, Villani L, et al. Modulation of ACE-2 mRNA by inflammatory cytokines in human thyroid cells: a pilot study. Endocrine (2021) 74(3):638–45. doi: 10.1007/s12020-021-02807-w
117. Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, et al. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J Endocrinol Invest (2021) 44(5):1085–90. doi: 10.1007/s40618-020-01436-w
118. Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: Implications for autoimmune diseases. Front Immunol (2020) 11:617089. doi: 10.3389/fimmu.2020.617089
119. Popescu M, Ghemigian A, Vasile CM, Costache A, Carsote M, Ghenea AE. The new entity of subacute thyroiditis amid the COVID-19 pandemic: From infection to vaccine. Diagn (Basel) (2022) 12(4). doi: 10.3390/diagnostics12040960
120. Das L, Bhadada SK, Sood A. Post-COVID-vaccine autoimmune/inflammatory syndrome in response to adjuvants (ASIA syndrome) manifesting as subacute thyroiditis. J Endocrinol Invest (2022) 45(2):465–7. doi: 10.1007/s40618-021-01681-7
121. Shoenfeld Y, Agmon-Levin N. 'ASIA' - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun (2011) 36(1):4–8. doi: 10.1016/j.jaut.2010.07.003
122. Pujol A, Gómez LA, Gallegos C, Nicolau J, Sanchís P, González-Freire M, et al. Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA-based SARS-CoV2 vaccination: from graves' disease to silent thyroiditis. J Endocrinol Invest (2022) 45(4):875–82. doi: 10.1007/s40618-021-01707-0
123. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol (2021) 21(4):195–7. doi: 10.1038/s41577-021-00526-x
124. Caron P. Autoimmune and inflammatory thyroid diseases following vaccination with SARS-CoV-2 vaccines: from etiopathogenesis to clinical management. Endocrine (2022) 1–12. doi: 10.1007/s12020-022-03118-4
125. Dix C, McFadyen J, Huang A, Chunilal S, Chen V, Tran H. Understanding vaccine-induced thrombotic thrombocytopenia (VITT). Intern Med J (2022) 52(5):717–23. doi: 10.1111/imj.15783
126. Gülfem KM, Cem E, Muhammet G. Pituitary insufficiency diagnosed after coronavirus disease-19: A case report. Erciyes Med J (2022). doi: 10.14744/etd.2021.30676
127. Sheikh AB, Javed N, Sheikh AAE, Upadhyay S, Shekhar R. Diabetes insipidus and concomitant myocarditis: A late sequelae of COVID-19 infection. J Investig Med High Impact Case Rep (2021) 9:2324709621999954. doi: 10.1177/2324709621999954
128. Ho KS, Narasimhan B, Kumar A, Flynn E, Salonia J, El-Hachem K, et al. [Syndrome of inappropriate antidiuretic hormone as the initial presentation of COVID-19: A novel case report]. Nefrol (Engl Ed) (2021) 41(2):219–20. doi: 10.1016/j.nefro.2020.05.004
129. Yousaf Z, Al-Shokri SD, Al-Soub H, Mohamed MFH. COVID-19-associated SIADH: a clue in the times of pandemic! Am J Physiol Endocrinol Metab (2020) 318(6):E882–5. doi: 10.1152/ajpendo.00178.2020
130. Zhou L, Zhang M, Wang J, Gao J. Sars-Cov-2: Underestimated damage to nervous system. Travel Med Infect Dis (2020) 36:101642. doi: 10.1016/j.tmaid.2020.101642
131. Elding Larsson H, Lynch KF, Lönnrot M, Haller MJ, Lernmark Å., Hagopian WA, et al. Pandemrix® vaccination is not associated with increased risk of islet autoimmunity or type 1 diabetes in the TEDDY study children. Diabetologia (2018) 61(1):193–202. doi: 10.1007/s00125-017-4448-3
132. Hemming-Harlo M, Lähdeaho ML, Mäki M, Vesikari T. Rotavirus vaccination does not increase type 1 diabetes and may decrease celiac disease in children and adolescents. Pediatr Infect Dis J (2019) 38(5):539–41. doi: 10.1097/INF.0000000000002281
133. Klein NP, Goddard K, Lewis E, Ross P, Gee J, DeStefano F, et al. Long term risk of developing type 1 diabetes after HPV vaccination in males and females. Vaccine (2019) 37(14):1938–44. doi: 10.1016/j.vaccine.2019.02.051
134. Morgan E, Halliday SR, Campbell GR, Cardwell CR, Patterson CC. Vaccinations and childhood type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia (2016) 59(2):237–43. doi: 10.1007/s00125-015-3800-8
135. Hviid A, Stellfeld M, Wohlfahrt J, Melbye M. Childhood vaccination and type 1 diabetes. N Engl J Med (2004) 350(14):1398–404. doi: 10.1056/NEJMoa032665
136. Gregory JM, Slaughter JC, Duffus SH, Smith TJ, LeStourgeon LM, Jaser SS, et al. COVID-19 severity is tripled in the diabetes community: A prospective analysis of the pandemic's impact in type 1 and type 2 diabetes. Diabetes Care (2021) 44(2):526–32. doi: 10.2337/dc20-2260
137. Lo SP, Hsieh TC, Pastuszak AW, Hotaling JM, Patel DP. Effects of SARS CoV-2, COVID-19, and its vaccines on male sexual health and reproduction: where do we stand? Int J Impot Res (2022) 34(2):138–44. doi: 10.1038/s41443-021-00483-y
138. Braun AS, Feil K, Reiser E, Weiss G, von Steuben T, Pinggera GM, et al. Corona and reproduction, or why the corona vaccination does not result in infertility. Geburtshilfe Frauenheilkd (2022) 82(5):490–500. doi: 10.1055/a-1750-9284
139. Diaz P, Zizzo J, Balaji NC, Reddy R, Khodamoradi K, Ory J, et al. Fear about adverse effect on fertility is a major cause of COVID-19 vaccine hesitancy in the united states. Andrologia (2022) 54(4):e14361. doi: 10.1111/and.14361
140. Schuppe HC, Pilatz A, Hossain H, Diemer T, Wagenlehner F, Weidner W. Urogenital infection as a risk factor for Male infertility. Dtsch Arztebl Int (2017) 114(19):339–46. doi: 10.3238/arztebl.2017.0339
Keywords: COVID-19, COVID-19 vaccines, endocrine system, SARS-CoV-2 vaccine, endocrine adverse effects
Citation: Pezzaioli LC, Gatta E, Bambini F, Facondo P, Gava M, Cavadini M, Buoso C, Di Lodovico E, Rotondi M, Ferlin A and Cappelli C (2022) Endocrine system after 2 years of COVID-19 vaccines: A narrative review of the literature. Front. Endocrinol. 13:1027047. doi: 10.3389/fendo.2022.1027047
Received: 24 August 2022; Accepted: 20 October 2022;
Published: 10 November 2022.
Edited by:
Andrea Sansone, University of Rome Tor Vergata, ItalyReviewed by:
Giuseppe Bellastella, Second University of Naples, ItalyCopyright © 2022 Pezzaioli, Gatta, Bambini, Facondo, Gava, Cavadini, Buoso, Di Lodovico, Rotondi, Ferlin and Cappelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlo Cappelli, Y2FybG8uY2FwcGVsbGlAdW5pYnMuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.