
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 16 January 2023
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1019390
This article is part of the Research TopicInnovation in Diabetes Self‐Care Management and InterventionsView all 14 articles
 Takaaki Matsui1
Takaaki Matsui1 Hiroshi Okada1,2*
Hiroshi Okada1,2* Masahide Hamaguchi1
Masahide Hamaguchi1 Kazushiro Kurogi3
Kazushiro Kurogi3 Hiroaki Murata4
Hiroaki Murata4 Masato Ito3
Masato Ito3 Michiaki Fukui1
Michiaki Fukui1Aim: This study aimed to investigate the association between change in body weight (BW) and type 2 diabetes remission in Japanese men with new-onset type 2 diabetes.
Methods: This study enrolled 1,903 patients with new-onset type 2 diabetes between 2008 and 2013 from a medical health checkup program conducted by the Panasonic Corporation, Osaka, Japan. The baseline was defined as the year of new-onset diabetes. We assessed the type 2 diabetes remission five years after baseline and the association between the change in BW and type 2 diabetes remission using logistic regression analyses. To evaluate the predictive performance of the change in BW, we employed the receiver operating characteristic curves and the area under the receiver operating characteristic (ROC) curve (AUC).
Results: The BW loss was associated with type 2 diabetes remission in the participants with a BMI ≥25 kg/m2 but not in the participants with a BMI <25 kg/m2. The odds ratios were 1.96 (95% CI: 1.19–3.29) and 3.72 (95% CI: 2.14–6.59) in the participants with a loss of 5–9.9% and loss of ≥10% for five years, respectively, in the participants with a BMI ≥25 kg/m2 (reference; stable group [0.9% gain to 0.9% loss]). The AUC and cut-off values for the rate of change in BW for type 2 diabetes remission were 0.59 and 5.0%.
Discussion: Body weight loss of ≥5% effectively achieved diabetes remission in Japanese men with a BMI ≥25 kg/m2 and new-onset type 2 diabetes.
There are several well-known risk factors for the onset of diabetes in the Japanese population, such as hyperlipidemia, hypertension, aging, weight gain, smoking history, impaired glucose tolerance (IGT), and family history (1–8). One of the goals in clinical care is the prevention of diabetes by focusing on these risk factors. However, in recent decades, the number of patients with diabetes and medical costs of diabetes have been increasing worldwide. Therefore, diabetes remission is as important as diabetes prevention.
Several Japanese studies have reported the association between body weight loss and diabetes prevention in patients with IGT. Kosaka et al. (9) conducted an intervention trial on whether body weight reduction by diet and exercise could prevent progression to diabetes among male patients with IGT in an outpatient clinic. Their study showed that the reduction in risk of diabetes was 67.4% lower in the intervention group than in the control group, and body weight loss was higher in the intervention group than in the control group (loss of 2.18 kg versus 0.39 kg) for 4 years. Kawahara et al. (10) conducted an intervention trial on whether a short-term hospital program of diabetes education could prevent progression to diabetes in patients with IGT. They reported that the incidence of diabetes was 42% lower in the intervention group than in the control group for 3 years. They also observed that body weight loss was higher in the intervention group than in the control group (loss of 2.1 kg versus gain of 0.4 kg). Furthermore, Saito et al. (11) conducted an intervention trial involving patients with IGT in an outpatient clinic. They reported that the risk of incident diabetes was 59% lower in the intervention group than that in the control group and that body weight loss was higher in the intervention group than in the control group (loss of 2.5 kg versus 1.1 kg). Thus, the association between body weight loss and prevention of type 2 diabetes in patients with IGT might be obvious. However, to our knowledge, there are no studies that assessed the association between change in body weight and diabetes remission in Japanese patients with type 2 diabetes. The findings of such studies may help in setting strategies to achieve type 2 diabetes remission. This is the first study to investigate the association between change in body weight and type 2 diabetes remission in Japanese men with new-onset type 2 diabetes.
This retrospective cohort study included participants of a physical examination program at Panasonic Corporation, Osaka, Japan. This study was named Panasonic cohort study and used 2008–2018 data from Panasonic Corporation’s database. All the participants partook in the physical examination program yearly from 2008 to 2018. The baseline was defined as the year of new-onset diabetes. The participants’ baseline characteristics were evaluated using a self-administered questionnaire. The participants were classified into current smokers, past smokers, and non-smokers based on smoking habits. The participants who regularly practiced any sport twice a week for more than one year were classified as regular exercisers.
The study was approved by the local ethics committee of Panasonic Health Insurance Organization (Approval number: 2021-001) and was conducted in accordance with the principles of the Declaration of Helsinki.
Body weight and height of all participants were recorded using an automatic machine yearly. We collected body weight five years after baseline to evaluate the change in this variable. Change in body weight was calculated as follows: body weight five years after baseline – body weight at baseline. The rate of change in body weight (%) was calculated as follows: (body weight five years after baseline – body weight at baseline) × 100/body weight at baseline.
Participants with a fasting plasma glucose concentration ≥126 mg/dL and/or who were on antihyperglycemic medication were considered as having type 2 diabetes. Participants with a fasting plasma glucose concentration <126 mg/dL and who were not taking antihyperglycemic medication were considered as having type 2 diabetes remission. We calculated the incidence of new-onset type 2 diabetes between 2008 and 2013 among the participants who did not have diabetes in 2008 and type 2 diabetes remission five years after baseline to evaluate the association between the change in body weight and type 2 diabetes remission in the participants with new-onset type 2 diabetes.
Overall, 84,997 men partook in the physical examination program in 2008. We excluded the participants with diabetes in 2008 (n = 4943). Of 80,054 men without diabetes in 2008, 3,264 men developed type 2 diabetes between 2008 and 2013. Of 3,264 men with new-onset type 2 diabetes, we excluded 1361 men who did not partake in the physical examination program five years after baseline. The final analysis involved the data of 1,903 men with new-onset diabetes between 2008 and 2013.
The differences in the general characteristics at baseline (the year of new-onset diabetes) according to the type 2 diabetes remission were evaluated using Student’s t-test and chi-square test, as appropriate. The association between the change in body weight and type 2 diabetes remission was assessed by logistic regression analyses. The multivariate analysis was adjusted for the factors related to type 2 diabetes, such as body mass index (BMI), age, systolic blood pressure (SBP), serum high-density lipoprotein (HDL) cholesterol concentrations, serum low-density lipoprotein (LDL) cholesterol concentrations, serum triglycerides concentrations, serum fasting plasma glucose concentrations, serum uric acid concentrations, smoking status, physical exercise habits, and change in body weight. The categorical values of the change in body weight and the rate of change in body weight were added into multivariate models to assess the association between categorical values and type 2 diabetes remission. The five groups according to change in body weight (gain [≥ 1 kg gain], stable [0.9 kg gain to 0.9 kg loss], loss of 1–4.9 kg, loss of 5–9.9 kg, and loss of ≥10 kg) and the rate of change in body weight (gain [≥ 1% gain], stable [0.9% gain to 0.9% loss], loss of 1–4.9%, loss of 5–9.9%, and loss of ≥10%) were added to the multivariate analyses of data of all the participants and participants with obesity (BMI ≥25 kg/m2) at baseline, respectively. The four groups according to change in body weight (gain [≥ 1 kg gain], stable [0.9 kg gain to 0.9 kg loss], loss of 1–4.9 kg, and loss of ≥5 kg) and the rate of change in body weight (gain [≥ 1% gain], stable [0.9% gain to 0.9% loss], loss of 1–4.9%, and loss of ≥5%) were added to the multivariate analyses of data of non-obese participants (BMI <25 kg/m2) at baseline, respectively. A receiver operating characteristic curve analysis was performed for change in body weight to assess the ability to identify patients with type 2 diabetes remission. We used JMP software (SAS Institute, NC, USA) to performe all statistical analyses. Continuous variables are expressed as mean ± standard deviation or absolute numbers. P values <0.05 were considered statistically significant. The associations are presented as hazard ratios with 95% confidence intervals (CIs).
The baseline characteristics of all the participants with new-onset type 2 diabetes are shown in Table 1. In total, 619 participants (32.5%) had type 2 diabetes remission five years after baseline. In the participants with a BMI ≥25 kg/m2 and BMI < 25 kg/m2, 298 (27.1%) and 321 (40.0%) participants had type 2 diabetes remission five years after baseline. The average change in body weight was -2.3 ± 4.9 kg, -3.3 ± 5.5 kg, and -0.9 ± 3.8 kg in the overall participants, participants with BMI ≥25 kg/m2, and participants with BMI <25 kg/m2. The proportions of participants with type 2 diabetes remission in the overall study population are shown in Figures 1 and 2. The proportion of type 2 diabetes remission varied with the degree of change in body weight loss in the overall participants and the participants with a BMI ≥25 kg/m2.
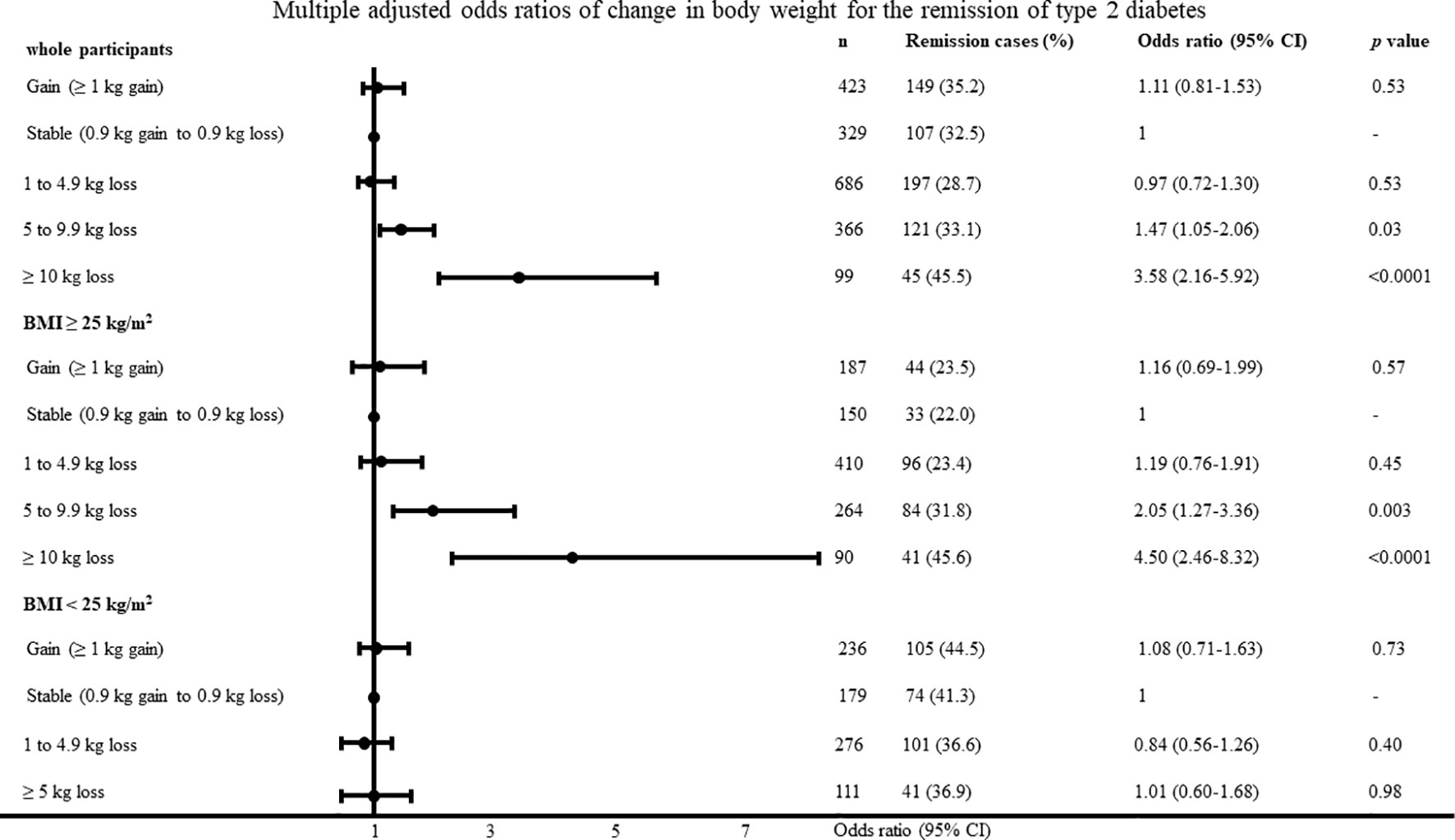
Figure 1 Multiple adjusted odds ratios of change in body weight for the remission of type 2 diabetes adjusted for age, body mass index, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, fasting plasma glucose, uric acid, smoking habit and physical activity.
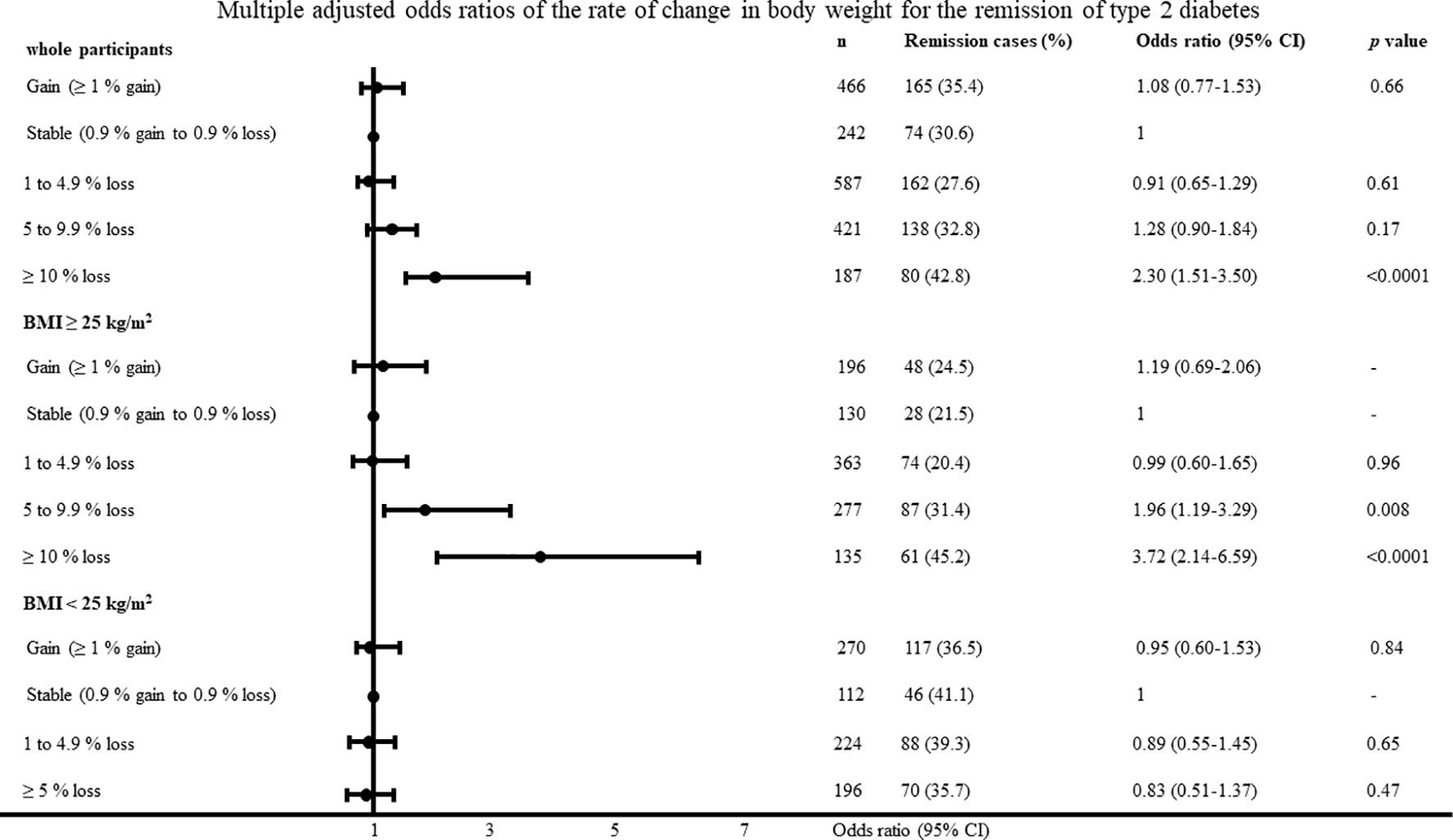
Figure 2 Multiple adjusted odds ratios of change in body weight for the remission of type 2 diabetes adjusted for age, body mass index, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride, fasting plasma glucose, uric acid, smoking habit and physical activity.
The unadjusted and adjusted odds ratios in the multivariate models for type 2 diabetes remission are shown in Table 2. For every 1 kg reduction in body weight for 5 years, the odds ratio of type 2 diabetes remission increased by 6% in the overall participants. The adjusted odds ratios in the multivariate models for type 2 diabetes remission according to BMI category are shown in Table 3. For every 1 kg reduction in body weight for 5 years, the odds ratio of type 2 diabetes remission increased by 9% in the participants with a BMI ≥25 kg/m2. Conversely, the degree of reduction in body weight was not associated with type 2 diabetes remission in the participants with a BMI <25 kg/m2. The BMI, LDL cholesterol, fasting plasma glucose, and smoking habit were associated with type 2 diabetes remission in the participants with a BMI <25 kg/m2.
The multiple adjusted odds ratios of change in body weight and rate of change in body weight according to the categorical values for type 2 diabetes remission with the body weight stable group as reference are shown in Figures 1 and 2. In the participants with a BMI ≥25 kg/m2, the odds ratios were 2.05 (95% CI, 1.27–3.36; P = 0.003) and 4.50 (95% CI, 2.46–8.32; P <0.0001) in the participants with a loss of 5–9.9 kg and ≥10 kg, respectively. In the participants with a BMI <25 kg/m2, change in body weight, and rate of change in body weight was not associated with type 2 diabetes remission. The results were almost identified with the change in body weight when we evaluated odds ratios regarding the rate of change in body weight. When diabetes remission was defined as < 110mg/dl, the results was almost same as definition of diabetes remission < 126 mg/dl. In the participants with a BMI ≥25 kg/m2, the odds ratios were 7.96 (95% CI, 3.72–17.69; P < 0.0001) in the participants with a loss of ≥10 kg. When diabetes remission was defined as < 110mg/dl, change in body weight was not associated with type 2 diabetes remission in the participants with a BMI <25 kg/m2.
The area under the curve and cut-off values of the change in body weight and rate of change in body weight for type 2 diabetes remission were 0.58 and 3.9 kg loss, and 0.59 and 5.0% loss in the participants with a BMI ≥25 kg/m2, respectively.
This study assessed the association between the change in body weight and type 2 diabetes remission in participants with new-onset diabetes. The major findings of our study were as follows: (1) body weight loss was associated with new-onset type 2 diabetes remission in the participants with a BMI ≥25 kg/m2 (obese) but not in the participants with a BMI <25 kg/m2 (non-obese); and (2) in patients with a BMI ≥25 kg/m2 (obese), a body weight loss of ≥3.9 kg or ≥5.0% might be effective for new-onset type 2 diabetes remission. Our findings are largely consistent with the guidelines of the Japan Society for the Study of Obesity, which recommends a body weight loss of ≥3.0% in the participants with a 25 ≤ BMI < 35 kg/m2 and a body weight loss of ≥5.0% in the participants with a BMI ≥35 kg/m2.
Body weight is strongly associated with the development of diabetes in Western people and Japanese people (12). Several studies have reported the association between body weight loss and diabetes remission. The Diabetes Remission Clinical Trial (DiRECT) was conducted to assess effective body weight management for diabetes remission (13). DiRECT showed that almost half of the participants achieved diabetes remission at 12 months in the intervention group, whose average body weight loss was –10.0 ± 8.0 kg (13). However, because eligible participants in DiRECT had a BMI of ≥27 kg/m2, it is unclear whether those findings were similar to persons with a BMI <27 kg/m2. An observational study in Scotland reported that more than 5 kg of body weight loss was associated with diabetes remission (14). The strengths of their study were that it involved the Western population in general and used data from their register that included information of 99% of the patients with diabetes in their country. We should consider ethnic differences in diabetes etiology. It has been reported that the BMI cut-off value for incident type 2 diabetes was lower in the Asian population than in the Western population (15). The mean BMI was 35.1 ± 4.5 kg/m2 in DiRECT, and the median BMI was 30.9 (27.4-35.3) kg/m2 in the Scottish study; their findings are not applicable to Asian people. Moreover, it might be difficult for participants to achieve a body weight loss of 10 kg in 1 year in a clinical care setting. Therefore, our findings can address the target body weight loss in Japanese in a clinical care setting.
Interestingly, 619 (32.5%) participants achieved type 2 diabetes remission in this study. This rate of remission was higher than that in a Western population (the Scottish study). This might be due to the difference in BMI from the Western population and whether the participants had no new-onset or new-onset diabetes, which could be more prone to remission. A proportion of 42.1% of the participants in our study had a BMI <25.0 kg/m2 (non-obese). As expected, in these participants, body weight loss was not associated with new-onset type 2 diabetes remission. The findings in participants with a BMI <25.0 kg/m2 might be due to diminished insulin secretion, which is characteristic of patients with diabetes in Asian countries (16). In the participants with a BMI <25.0 kg/m2, lipid disorder, increased plasma glucose concentration, and smoking habits might be more important than the change in body weight in new-onset type 2 diabetes remission. A body weight loss in participants with a BMI <25.0 kg/m2 might be associated with the comorbidity including malignancy, resulting in no improvement of diabetes remission. We found ten patients who have malignancy both in participants with BMI <25 kg/m2 and with BMI ≥25 kg/m2. Our results were almost the same when the participants with malignancy were excluded.
It has been proposed that the development of diabetes is triggered by insulin resistance, which eventually leads to the exhaustion of pancreatic β cells (17). Obesity reportedly induces chronic inflammation (18) and insulin resistance (19, 20), which is partly attributed to the dysregulation of adipocytokines such as tumor necrosis factor-α, adiponectin, leptin, and plasminogen activator inhibitor-1 (21, 22). The increased visceral adipose tissue, causing the adipocytes to produce more tumor necrosis factor-α and less adiponectin is associated with weight gain. It has been reported that body weight loss beneficially affects adipocytokines (23). Hence, participants with a BMI ≥25 kg/m2 and new-onset diabetes needed to lose body weight in the early disease stage to achieve type 2 diabetes remission.
The strengths of our study include its long follow-up period, real-world nature, and consecutive enrolment. However, this study had several limitations. First, in general, the diagnosis is mainly judged by plasma glucose and HbA1c. The diabetes remission is mainly defined as HbA1c below the level of 6.5% and remaining at that level for at least 3 months without continuation of the usual antihyperglycemic medication (24). However, diagnosis and remission of diabetes were judged only by fasting plasma glucose level and use of antihyperglycemic medication, but not HbA1c. Second, change in body composition rather than body weight may be more important for remission (25). However, we have no data about body composition. Thirdly, education on diabetes could have affected the rate of diabetes remission; however, we had no such data. Lastly, our study population was made up of relatively young Japanese men. We have no data in elder participants because our data was derived from cohort of a physical examination program at Panasonic Corporation. Therefore, it is unclear whether our findings are generalizable to women, other ethnic groups and age groups.
In conclusion, our study found that a body weight loss of ≥3.9 kg or ≥5.0% effectively achieved diabetes remission in Japanese men with a BMI ≥25 kg/m2 and new-onset type 2 diabetes. Therefore, it is important to focus on early body weight loss in participants with a BMI ≥25 kg/m2 and new-onset diabetes in clinical settings to achieve diabetes remission.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
TM wrote this manuscript. KK, MH, and HM contributed to the discussion. HO analyzed the data. HO and MI collected the data and contributed to the design and discussion. HO and MF edited and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by Osaka foundation for the prevention of cancer and cardiovascular disease.
We thank Kenya Kasahara, Moe Horiuchi, Shinta Yamamoto, Taro Hirozane, Akifumi Shiota, Nozomi Yoshioka, Tomoyuki Matsuyama, Momoko Habu and Yoshitaka Hashimoto for their assistance in this study.
HO received personal fees from Kowa Pharma Co. Ltd; Novo Nordisk Pharma Ltd.; Sumitomo Dainippon Pharma Co. Ltd and Eli Lilly, Japan, outside work. MH received grants from Yamada Bee Farm, AstraZeneca K.K. and Oishi Kenko Inc.; and received personal fees from Eli Lilly, Japan; AstraZeneca K.K.; Daiichi Sankyo Co. Ltd.; Ono Pharma Co. Ltd., Kowa Pharma Co. Ltd.; Mitsubishi Tanabe Pharma Corp., Sanofi K.K., and Sumitomo Dainippon Pharma Co. Ltd., outside the submitted work. MF received grants from Terumo Corp.; Yamada Bee Farm; Kowa Pharma Co. Ltd.; Taiyo Kagaku Co., Ltd; Nippon Boehringer Ingelheim Co. Ltd.; Novo Nordisk Pharma Ltd.; Sanofi K.K.; Kissei Pharma Co. Ltd.; MSD K.K.; Taisho Pharma Co. Ltd.; Abbott Japan Co. Ltd.; Daiichi Sankyo Co. Ltd.; Kyowa Kirin Co. Ltd.; Oishi Kenko Inc.; Sumitomo Dainippon Pharma Co. Ltd.; Eli Lilly, Japan, K.K.; Nippon Chemiphar Co. Ltd.; Astellas Pharma Inc.; Sanwa Kagagu Kenkyusho Co. Ltd.; Tejin Pharma Ltd.; Mitsubishi Tanabe Pharma Corp.; and Johnson & Johnson K.K. Medical Co.; and received personal fees from Kissei Pharma Co., Ltd.; Mitsubishi Tanabe Pharma Corp.; Ono Pharma Co. Ltd.; AstraZeneca K.K.; Novo Nordisk Pharma Ltd.; Kyowa Kirin Co. Ltd.; Astellas Pharma Inc.; Daiichi Sankyo Co. Ltd.; Mochida Pharma Co. Ltd.; Eli Lilly, Japan K.K.; Taisho Pharma Co. Ltd.; Sanwa Kagaku Kenkyusho Co. Ltd.; Medtronic Japan Co. Ltd.; Bayer Yakuhin Ltd.; Teijin Pharma Ltd.; Nipro Corp.; Sanofi K.K.; Nippon Boehringer Ingelheim Co. Ltd.; Arkray Inc.; MSD K.K.; Kowa Pharma Co. Ltd.; Sumitomo Dainippon Pharma Co. Ltd.; and Abbott Japan Co. Ltd., outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Doi Y, Ninomiya T, Hata J, Hirakawa Y, Mukai N, Iwase M, et al. Two risk score models for predicting incident type 2 diabetes in Japan. Diabetes Med (2012) 29:107–14. doi: 10.1111/j.1464-5491.2011.03376.x
2. Noda M, Kato M, Takahashi Y, Matsushita Y, Mizoue T, Inoue M, et al. Fasting plasma glucose and 5-year incidence of diabetes in the JPHC diabetes study - suggestion for the threshold for impaired fasting glucose among Japanese. Endocr J (2010) 57:629–37. doi: 10.1507/endocrj.k10e-010
3. Heianza Y, Arase Y, Hsieh SD, Saito K, Tsuji H, Kodama S, et al. Development of a new scoring system for predicting the 5 year incidence of type 2 diabetes in Japan: the toranomon hospital health management center study 6 (TOPICS 6). Diabetologia (2012) 55:3213–23. doi: 10.1007/s00125-012-2712-0
4. Mukai N, Doi Y, Ninomiya T, Hata J, Hirakawa Y, Fukuhara M, et al. Cut-off values of fasting and post-load plasma glucose and HbA1c for predicting type 2 diabetes in community-dwelling Japanese subjects: the hisayama study. Diabetes Med (2012) 29:99–106. doi: 10.1111/j.1464-5491.2011.03378.x
5. Heianza Y, Arase Y, Fujihara K, Hsieh SD, Saito K, Tsuji H, et al. Longitudinal trajectories of HbA1c and fasting plasma glucose levels during the development of type 2 diabetes: the toranomon hospital health management center study 7 (TOPICS 7). Diabetes Care (2012) 35:1050–2. doi: 10.2337/dc11-1793
6. Nanri A, Nakagawa T, Kuwahara K, Yamamoto S, Honda T, Okazaki H, et al. Development of risk score for predicting 3-year incidence of type 2 diabetes: Japan epidemiology collaboration on occupational health study. PloS One (2015) 10:e0142779. doi: 10.1371/journal.pone.0142779
7. Heianza Y, Hara S, Arase Y, Saito K, Fujiwara K, Tsuji H, et al. HbA1c 5·7-6·4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet (2011) 378:147–55. doi: 10.1016/S0140-6736(11)60472-8
8. Saijo Y, Okada H, Hamaguchi M, Habu M, Kurogi K, Murata H, et al. The risk factors for development of type 2 diabetes: Panasonic cohort study 4. Int J Environ Res Public Health (2022) 19:571. doi: 10.3390/ijerph19010571
9. Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract (2005) 67:152–62. doi: 10.1016/j.diabres.2004.06.010
10. Kawahara T, Takahashi K, Inazu T, Arao T, Kawahara C, Tabata T, et al. Reduced progression to type 2 diabetes from impaired glucose tolerance after a 2-day in-hospital diabetes educational program: the joetsu diabetes prevention trial. Diabetes Care (2008) 31:1949–54. doi: 10.2337/dc07-2272
11. Saito T, Watanabe M, Nishida J, Izumi T, Omura M, Takagi T, et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med (2011) 171:1352–60. doi: 10.1001/archinternmed.2011.275
12. Maskarinec G, Erber E, Grandinetti A, Verheus M, Oum R, Hopping BN, et al. Diabetes incidence based on linkages with health plans: the multiethnic cohort. Diabetes (2009) 58:1732–8. doi: 10.2337/db08-1685
13. Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet (2018) 391:541–51. doi: 10.1016/S0140-6736(17)33102-1
14. Captieux M, Fleetwood K, Kennon B, Sattar N, Lindsay R, Guthrie B, et al. Epidemiology of type 2 diabetes remission in Scotland in 2019: A cross-sectional population-based study. PloS Med (2021) 18:e1003828. doi: 10.1371/journal.pmed.1003828
15. Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care (2011) 34:1741–8. doi: 10.2337/dc10-2300
16. Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA (2007) 298:2654–64. doi: 10.1001/jama.298.22.2654
17. Ogihara T, Mirmira RG. An islet in distress: β cell failure in type 2 diabetes. J Diabetes Investig (2010) 1:123–33. doi: 10.1111/j.2040-1124.2010.00021.x
18. Tokunaga K, Matsuzawa Y, Kotani K, Keno Y, Kobatake T, Fujioka S, et al. Ideal body weight estimated from the body mass index with the lowest morbidity. Int J Obes (1991) 15:1–5.
19. Campbell PJ, Carlson MG. Impact of obesity on insulin action in NIDDM. Diabetes (1993) 42:405–10. doi: 10.2337/diab.42.3.405
20. Bogardus C, Lillioja S, Mott DM, Hollenbeck C, Reaven G. Relationship between degree of obesity and in vivoinsulin action in man. Am J Physiol (1985) 248:E286–91. doi: 10.1152/ajpendo.1985.248.3.E286
21. Esteve E, Ricart W, Fernández-Real JM. Adipocytokines and insulin resistance: the possible role of lipocalin-2, retinol binding protein-4, and adiponectin. Diabetes Care (2009) 32(Suppl 2):S362–7. doi: 10.2337/dc09-S340
22. Lorenzo M, Fernández-Veledo S, Vila-Bedmar R, Garcia-Guerra L, De Alvaro C, Nieto-Vazquez I. Insulin resistance induced by tumor necrosis factor-alpha in myocytes and brown adipocytes. J Anim Sci (2008) 86(14):E94–E104. doi: 10.2527/jas.2007-0462
23. Salehi-Abargouei A, Izadi V, Azadbakht L. The effect of low calorie diet on adiponectin concentration: a systematic review and meta-analysis. Horm Metab Res (2015) 47:549–55. doi: 10.1055/s-0035-1549878
24. Riddle MC, Cefalu WT, Evans PH, Gerstein HC, Nauck MA, Oh WK, et al. Consensus report: Definition and interpretation of remission in type 2 diabetes. Diabetes Care (2021) 44:2438–44. doi: 10.2337/dci21-0034
Keywords: type 2 diabetes, body weight loss, diabetes remission, medical health checkup, obesity
Citation: Matsui T, Okada H, Hamaguchi M, Kurogi K, Murata H, Ito M and Fukui M (2023) The association between the reduction of body weight and new-onset type 2 diabetes remission in middle-aged Japanese men: Population-based Panasonic cohort study 8. Front. Endocrinol. 13:1019390. doi: 10.3389/fendo.2022.1019390
Received: 15 August 2022; Accepted: 28 December 2022;
Published: 16 January 2023.
Edited by:
Lingling Xu, Peking Union Medical College Hospital (CAMS), ChinaCopyright © 2023 Matsui, Okada, Hamaguchi, Kurogi, Murata, Ito and Fukui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroshi Okada, Y29udGlAa290by5rcHUtbS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.