- 1Department of Oncology, Suzhou Ninth People’s Hospital, Suzhou, China
- 2Department of Gastrointestinal Surgery, Xuzhou Central Hospital, The Affiliated Xuzhou Hospital of Medical College of Southeast University, Xuzhou, China
- 3Department of Pathology, Xuzhou Central Hospital, The Affiliated Xuzhou Hospital of Medical College of Southeast University, Xuzhou, China
- 4Department of Ultrasound, Xuzhou Central Hospital, The Affiliated Xuzhou Hospital of Medical College of Southeast University, Xuzhou, China
- 5Department of Stomatology, Xuzhou Central Hospital, The Affiliated Xuzhou Hospital of Medical College of Southeast University, Xuzhou, China
Purpose: Clinical guidelines presently recommend total thyroidectomy for the treatment of medullary thyroid cancer (MTC). This study was aimed to investigate whether lobectomy could be the initial treatment for stage I MTC patients.
Methods: The retrospective study was based on data from the Surveillance, Epidemiology, and End Results (SEER) database between 2004 and 2015. The risk factors of survival were estimated by the univariate and multivariate Cox proportional-hazards model. The effect of age on death risk was estimated using restricted cubic splines. Survival curves were constructed according to the Kaplan–Meier method.
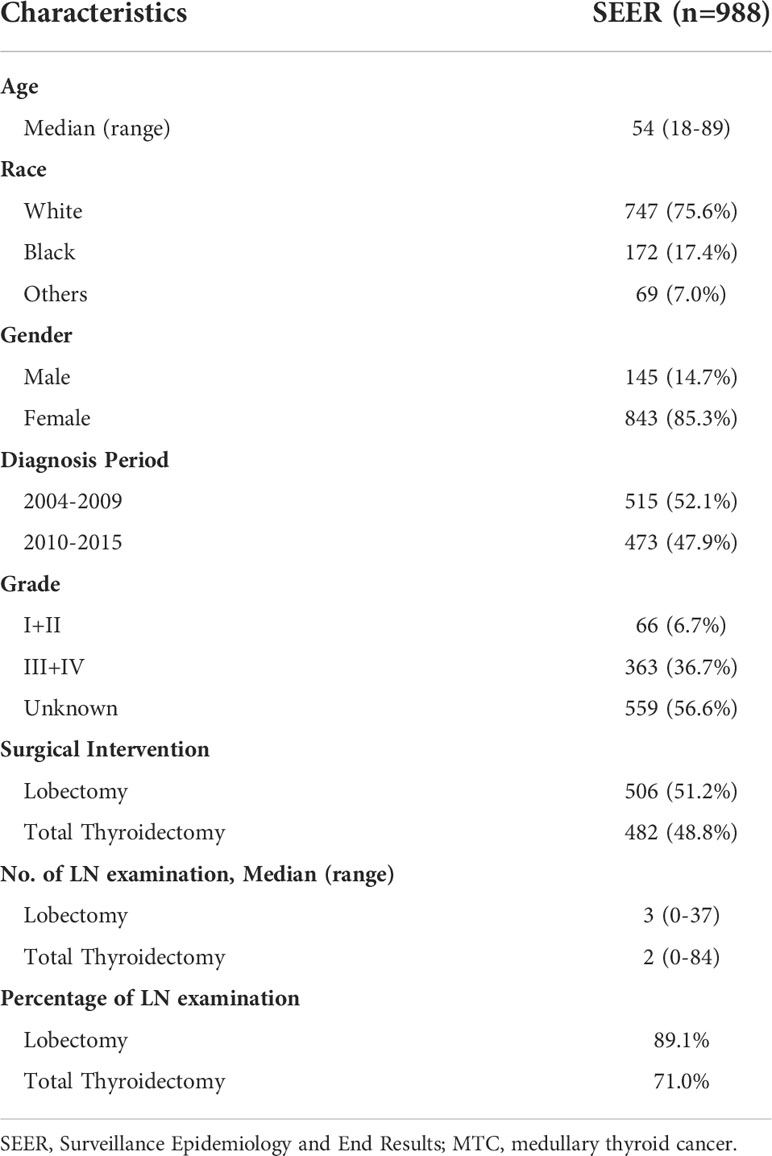
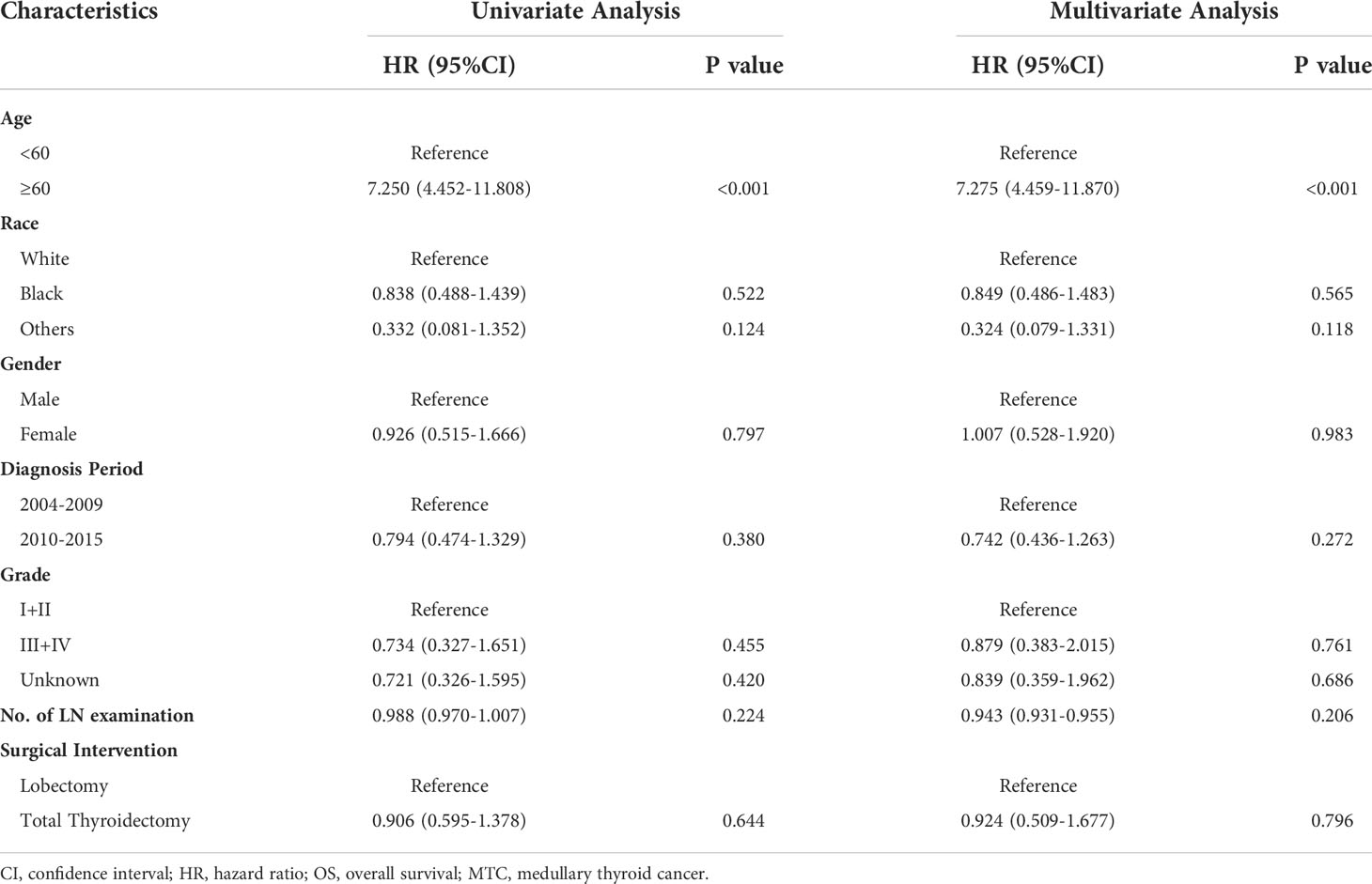
Results: A total of 988 stage I MTC patients was included in the study. Among them, 506 (51.2%) MTC patients received lobectomy and 482 (48.8%) received total thyroidectomy. The only independent prognostic factor for overall survival (OS) and disease-specific survival (DSS) was age, according to univariate and multivariate Cox regression analysis. The hazard ratio (HR) increased relatively slowly with age growing under the age of approximately 60 years. However, the death risk of MTC patients began to rise sharply with increasing age above 60 years. For patients under the age of 60, a significant survival difference for OS and DSS was observed between the lobectomy group and total thyroidectomy group (p < 0.05). However, for patients aged above 60, no significant survival difference was observed for OS or DSS (p > 0.05).
Conclusion: Total thyroidectomy was an appropriate treatment for stage I MTC patients under the age of 60, which was consistent with the recommendation of the clinical guidelines. However, for those over the age of 60, lobectomy may be explored as a better surgical option. The findings may provide the evidence base for improving the clinical management of stage I MTC patients. Further prospective multicenter clinical studies are needed including information regarding RET status as well as calcitonin and CEA levels.
Introduction
Thyroid cancer (TC) is the most frequent endocrine neoplasm (1). There are four major pathological types of TC: papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), medullary thyroid cancer (MTC), and anaplastic thyroid cancer (ATC). Unlike the other three types, MTC originates from parafollicular calcitonin secreting cells of the thyroid gland (2), which can produce calcitonin, an important diagnostic and prognostic marker for MTC (3). It accounts for 5%-10% of all TC cases with an annual incidence rate of 0.19/100,000 (4, 5). According to the etiology, MTC could be classified into sporadic MTC and hereditary MTC, accounting for 75% and 25%, respectively (6). RET mutations occur in almost all hereditary MTC and a considerable fraction of apparently sporadic MTC cases (7).
Although the 5-year survival rate of early-stage MTC patients is >90%, the overall survival prognosis of MTC patients with all stages is still poor with a 5-year survival rate of less than 40% (8, 9). Therefore, early detection and treatment of MTC are critical. Due to heredity and the lack of effective and safe pharmacotherapy, total thyroidectomy is recommended for treatment of MTC by both the American Thyroid Association (ATA) and British Thyroid Association (BTA) guidelines to prevent cancer recurrence and improve the prognosis (10). However, as compared to lobectomy, total thyroidectomy may increase the risk of postoperative complications such as chronic hypoparathyroidism and laryngeal nerve injury (11). Hence, there have been various attempts to identify a patient subpopulation that would benefit most from minimally invasive surgery, thus reducing the risk of complications (12–14). According to the 2015 ATA guidelines, the initial surgical procedure for patients with solitary intrathyroidal PTC (1-4 cm in size) could be a near-total or total thyroidectomy or lobectomy (12). Mingzhao Xing et al. (15) found that BRAF gene status could provide guidance for further optimization of surgical modalities. However, the pros and cons of surgical modalities of MTC patients have been less investigated. Miyauchi et al. (16) analyzed 48 sporadic MTC patients without germline RET mutations and found that lobectomy might be an appropriate surgical modality. A single-center, retrospective study from Japan including 118 MTC patients between 1975 and 2005 showed that lobectomy could be considered in sporadic MTC cases with primary tumors located only in one lobe (17). Some scholars suggested that age was an independent prognostic predictor and a less extensive surgical technique might be considered for hereditary MTC patients of younger age (18, 19). These findings remain highly controversial due to small sample sizes or the relatively short follow up period of the referred studies. In the present study, we used the Surveillance, Epidemiology, and End Results (SEER) database to investigate whether lobectomy could be the initial treatment for stage I MTC patients.
Materials and methods
Patients
Patient data were extracted from the SEER database between 2004 and 2015. The SEER database is the largest publicly available clinical database covering 18 registries in the United States (20). Patients with MTC were identified using ICD-O-3 codes 8345/3 and 8510/3. Baseline characteristics were collected, including age, race, gender, diagnosis period, grade, and surgical modalities. Inclusion criteria were as follows: (1) patients over 18 years; (2) MTC diagnosis confirmed by histopathological examination; (3) MTC as the only malignancy or the first primary malignancy; (4) stage I (T1N0M0) classification according to the 8th edition of American Joint Committee on Cancer (AJCC) staging system for MTC; and (5) patients underwent lobectomy (surgery codes 20: lobectomy and/or isthmectomy; surgery codes 21: lobectomy only; surgery codes 22: isthmectomy only; surgery codes 23: lobectomy with isthmus) or total thyroidectomy (surgery codes 50: total thyroidectomy). The exclusion criteria were: (1) MTC diagnosis based on radiographic or cytological evidence; (2) no surgical treatment or unclear surgical modalities; and (3) lack of follow-up information. The primary endpoints for survival analysis were overall survival (OS) and disease-specific survival (DSS). OS refers to the time between initial MTC diagnosis and death time from any cause or last follow-up time. DSS refers to the time between initial MTC diagnosis and death time from MTC or last follow-up time. The median follow-up time was 100 months (ranging from 0 to 179 months). The study had been reported in line with the STROCSS criteria (21), and approved by the Medical Scientific Research Ethics Committee of Suzhou Ninth People’s Hospital.
Statistical analysis
The risk factors of survival were estimated by the univariate and multivariate Cox proportional-hazards model. Hazard ratio (HR) and 95% confidence interval (95% CI) were calculated. Survival curves were constructed according to the Kaplan–Meier method. The effect of age on death risk was estimated using restricted cubic splines. Two-tailed P values of <0.05 indicated a significant difference.
Results
A total of 988 stage I MTC patients was included in the study (Table 1). Age ranged from 18 to 89 years (median: 54). In terms of race, 75.6% of the entire cohort was White, 17.4% was Black and 7.0% was other races. The majority was female, accounting for 85.3%. The percentage of MTC patients was slightly higher during the period 2004-2009 than during 2010-2015. 66 (6.7%) patients had well or moderately differentiated tumors (grade I+II) and 363 (36.7%) had poorly differentiated or undifferentiated tumors (grade III+IV). More than half of the patients (56.6%) had no information about grade. 506 (51.2%) MTC patients underwent lobectomy and 482 (48.8%) underwent total thyroidectomy. The median number of lymph node (LN) examination was 2 in the lobectomy group while that was 3 in the total thyroidectomy group. Patients without LN examination were further excluded and 793 cases were finally included for the following survival analysis.
The cohort was categorized into two groups to investigate the effect of different surgical modalities on stage I MTC patients: lobectomy and total thyroidectomy. Kaplan–Meier survival analysis showed that no significant survival difference for OS was observed between the two groups (Figure 1A, p = 0.199). DSS in patients who underwent total thyroidectomy seemed to be better than those undergone lobectomy, although not reaching statistical significance (Figure 1B, p = 0.075).
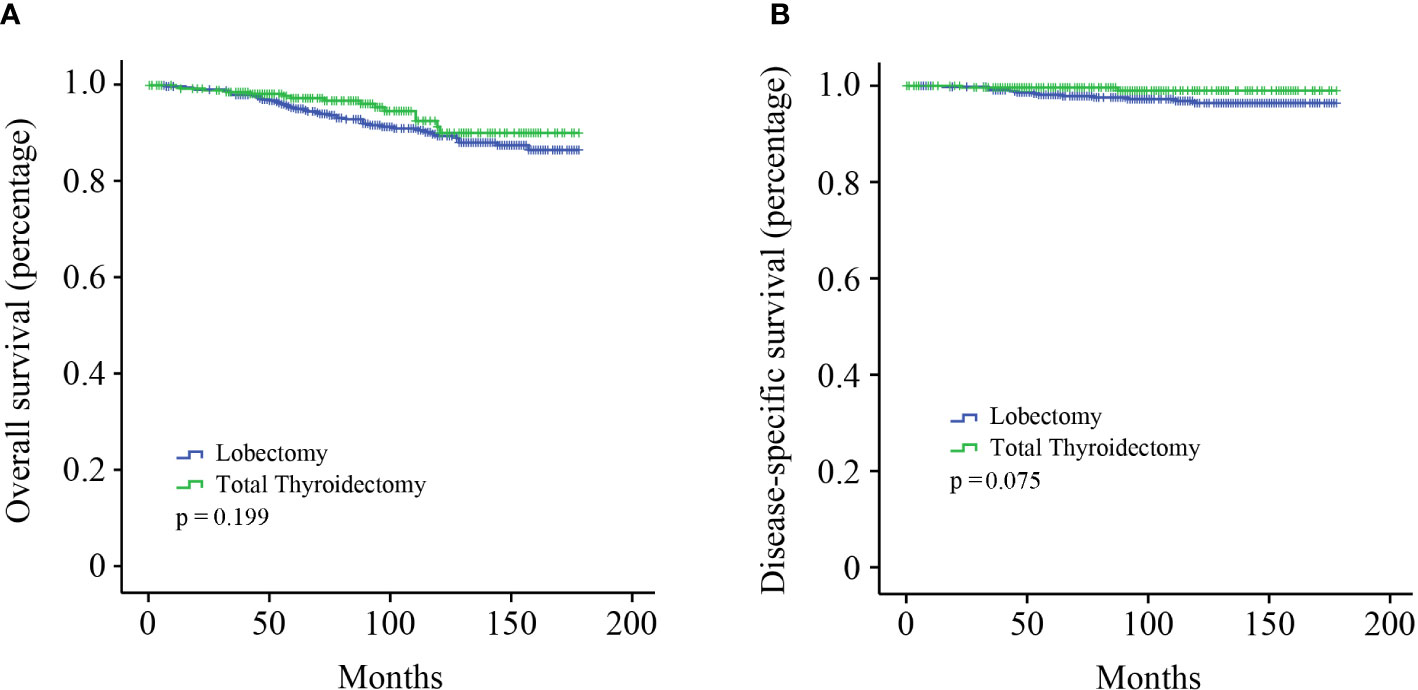
Figure 1 Kaplan–Meier survival analysis. (A) OS curve of stage I MTC patients; (B) DSS curve of stage I MTC patients.
All the variables were included to perform univariate and multivariate Cox regression analysis (Table 2). Age is a continuous variable, and we investigated the optimal age cutoff value to transform age into a categorical variable. As shown in Figure 2A, the distribution of age followed a normal distribution, and the age group 50-60 years had the highest percentage (29.8%). The HR increased relatively slowly with age growing under the age of approximately 60 years (Figure 2B). However, the risk of mortality in MTC patients began to rise sharply with increasing age above 60 years. Thus, the age of 60 years was considered as the optimal cutoff value. Both univariate and multivariate analyses showed that age was the only independent prognostic factor. Given this, the two groups (lobectomy and total thyroidectomy) were further separated into age-related subgroups. There were 505 cases under the age of 60 and 288 cases over 60. The median follow-up time of patients under the age of 60 was 105 months while that of patients over 60 was 90 months. For patients under the age of 60, a significant survival difference for OS and DSS was observed between the lobectomy group and total thyroidectomy group (Figures 3A, B, p < 0.05). For patients aged above 60, however, no significant survival difference was observed for OS or DSS (Figures 3C, D).
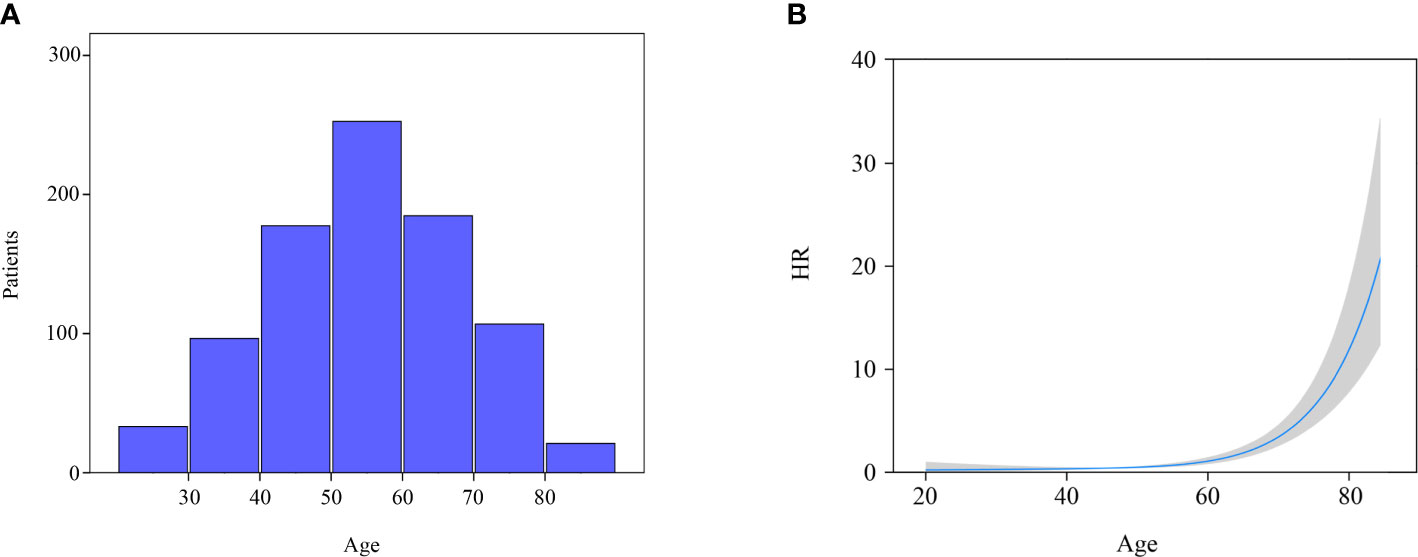
Figure 2 Distribution and effect of age. (A) Age distribution of entire cohort; (B) Influence of age on the death risk of stage I MTC patients by means of restricted cubic splines.
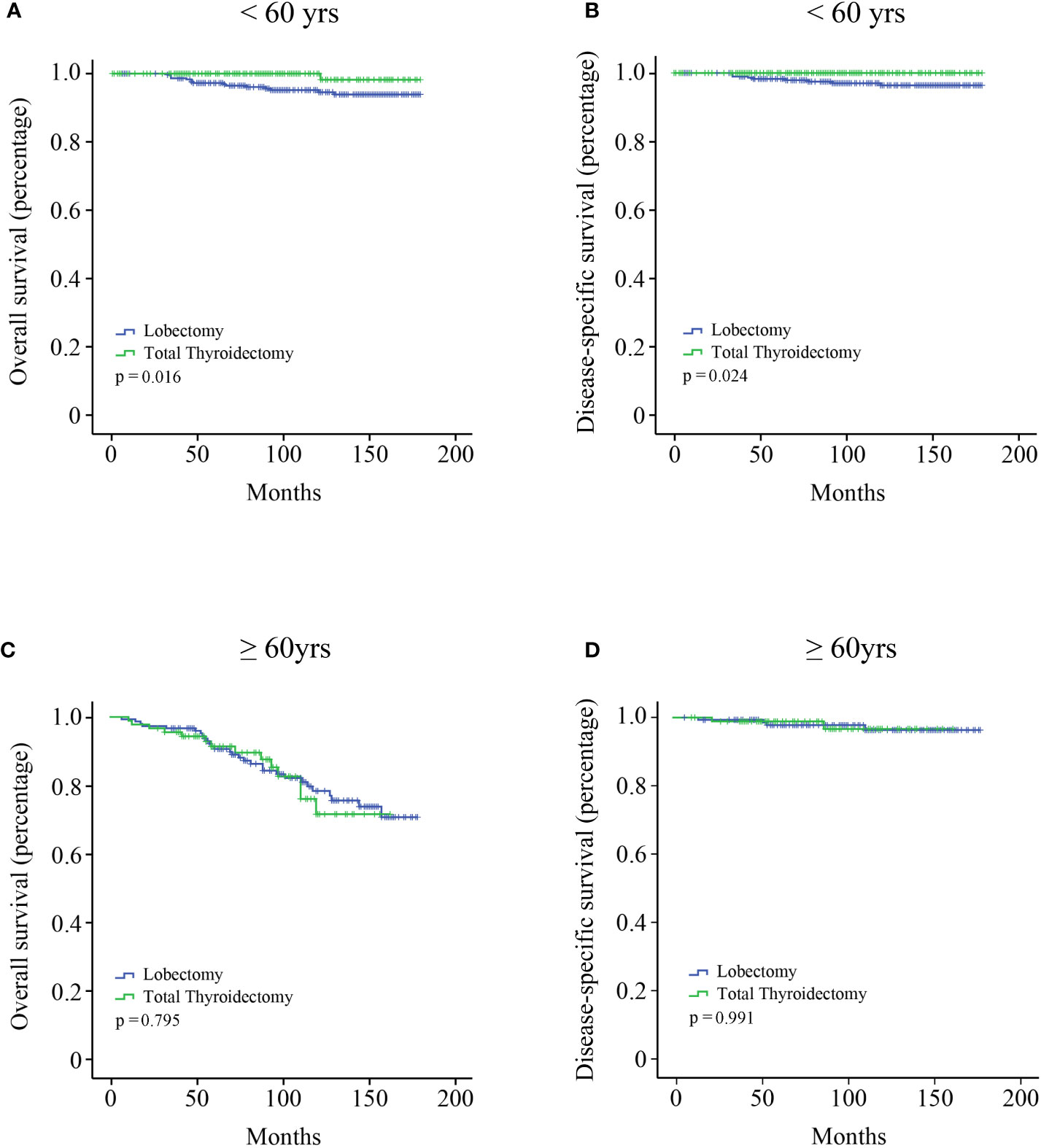
Figure 3 Kaplan–Meier survival analysis according to the age subgroups. (A) OS curve of stage I MTC patients under the age of 60 years; (B) DSS curve of stage I MTC patients under the age of 60 years; (C) OS curve of stage I MTC patients above the age of 60 years; (D) DSS curve of stage I MTC patients above the age of 60 years.
Discussion
Early-stage MTC patients usually have a favorable prognosis. It is still unknown whether there is a subset of early MTC patients that are overtreated based on the commonly used clinical guidelines. Using the population-based database, it was found that total thyroidectomy was an appropriate treatment for stage I MTC patients under the age of 60. However, for those older than 60 years of age, lobectomy may be considered as a better surgical procedure since total thyroidectomy could not provide an additional survival benefit. The less extensive operation would reduce the incidence of complications and improve the quality of life. These findings may provide some guidance for clinical decisions.
Age was reported to be an independent prognostic factor for MTC patients in various large sample studies (18, 22, 23). A study by Sahli et al. (18) classified MTC patients into three groups (18-64 years, 65-79 years, and ≥ 80 years) based on the previous studies of PTC and found that elderly patients (≥ 65 years) had a greater risk of mortality than young patients (with age group 18-64 years as reference, HR: 1.787). MTC patients were also classified into three groups (18-49 years, 50-69 years, and ≥ 70 years) in the study by Qu et al. (24) With age group 18-49 years as reference, the HR of age group 50-69 years and ≥ 70 years were 2.853 and 5.804, respectively. In the present study, we investigated the effect of age on the risk of mortality using restricted cubic splines and found that the survival curve was anti-L-shaped. The HR was virtually unchanged under the age of 60 years and began to rise sharply above 60 years, indicating it was justified to classify these MTC patients into two groups rather than three groups. Our results also demonstrated that the HR of elderly patients (≥ 60 years) was significantly higher compared to elderly group in the research by Sahli et al. and Qu et al. (with age group <60 years as reference, HR: 7.275).
MTC tumors in elderly patients (≥ 60 years) tend to present with more aggressive biological behavior and higher lethality. Generally, more extensive and radical surgery is needed for tumors with more aggressive histology. However, our findings revealed that elderly patients (≥ 60 years) did not benefit from more extensive surgery (total thyroidectomy) while young patients did. This might be explained by the fact that the age factor played a much more important role in elderly patients than surgery. Whatever surgical procedures were used, the clinical outcomes of elderly patients could not be improved. The findings provided a foundation for improving the ATA recommendations. For young patients (< 60 years) whose prognosis was hardly affected by the age factor, total thyroidectomy could significantly improve the clinical outcomes. These findings were consistent with the recommendation of the ATA guidelines. Sahli et al. (18) also found that young patients underwent total thyroidectomy at a higher proportion compared to elderly patients by analyzing MTC patients with all stages in the SEER database.
LN metastasis of MTC is common (25), and the ATA guidelines recommended that central LN dissection should be performed regardless of LN status (10). There is currently a disagreement over lateral LN dissection (26–28). However, Hamy et al. (29) showed that early MTC tumors (T1a) had almost no LN metastatic potential, suggesting LN dissection may not be suitable for early-stage MTC. Niederle et al. (30) indicated that MTC desmoplastic stromal reaction negative (DSR-negative) tumors generally had a small diameter (T1a, <1cm) and no lateral LN metastasis. Lateral LN dissection was not necessary for individuals with DSR-negative tumors. Our study also demonstrated that the number of LN examination was not an independent prognostic factor for stage I MTC. Nonetheless, the SEER database did not distinguish the extent and location of LN examination (central or lateral), and definitive and reliable conclusions could not be reached.
There are several limitations in the present study. First, it is the well-known limitation of the retrospective nature of the SEER database. Further prospective, multicenter clinical trials should be performed to validate our results. Second, the RET status, CEA or calcitonin levels were not recorded in the SEER database. A more comprehensive multivariate Cox regression analysis could not be carried out. Third, stage I MTC patients have a comparatively favorable outcome and statistically significant survival differences are difficult to be recorded. There might be differences in OS between the lobectomy and total thyroidectomy groups for patients under the age of 60 regarding longer follow-up time and larger cohort. Fourth, the decision about the appropriate surgical approaches could not only be dependent on OS and DSS but also the postoperative complications and the quality of life. Due to a lack of information about postoperative complications and recurrence, a more accurate and comprehensive assessment could not be conducted. Fifth, in 56.6% of stage I MTC patients information about the grade in the SEER database were not available. Thus, it was not able to identify whether grade was an independent prognostic factor for stage I MTC patients. Studies indicated that grade was significantly associated with survival outcomes of MTC with all stages (31, 32).
In summary, total thyroidectomy was an appropriate treatment for stage I MTC patients under the age of 60, which was consistent with the recommendation of the clinical guidelines. However, for those over the age of 60, lobectomy may be considered as a better surgical option. The findings may provide the evidence base for improving the clinical management of stage I MTC patients. Further prospective multicenter clinical studies are needed including information regarding RET status as well as calcitonin and CEA levels.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Scientific Research Ethics Committee of Suzhou Ninth People’s Hospital. The patients/participants provided their written informed consent in the SEER database.
Author contributions
BY and SH made substantial contributions to the design of the study, carried out the analysis, interpreted the data. GN and XL contributed to the review of previous literature. FM and YM contributed substantially to the data discussion and critically commented on the manuscript for scientific content. All authors made substantial contributions to the conception and design of the study, data interpretation and drafting of the manuscript, were responsible for the quality of the overall manuscript. All authors approved the final version of the manuscript.
Acknowledgments
The authors are thankful to the statistician Aidibai Simayi from Department of Epidemiology and Health Statistics, School of Public Health, Southeast University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lindquist D, Alsina FC, Herdenberg C, Larsson C, Hoppener J, Wang N, et al. LRIG1 negatively regulates RET mutants and is downregulated in thyroid cancer. Int J Oncol (2018) 52(4):1189–97. doi: 10.3892/ijo.2018.4273
2. Valenciaga A, Grubbs EG, Porter K, Wakely PE Jr., Williams MD, Cote GJ, et al. Reduced retinoblastoma protein expression is associated with decreased patient survival in medullary thyroid cancer. Thyroid (2017) 27(12):1523–33. doi: 10.1089/thy.2017.0113
3. Hu MI, Ying AK, Jimenez C. Update on medullary thyroid cancer. Endocrinol Metab Clin North Am (2014) 43(2):423–42. doi: 10.1016/j.ecl.2014.02.004
4. Mathiesen JS, Kroustrup JP, Vestergaard P, Poulsen PL, Rasmussen AK, Feldt-Rasmussen U, et al. Replication of newly proposed TNM staging system for medullary thyroid carcinoma: A nationwide study. Endocr Connect (2019) 8(1):1–7. doi: 10.1530/EC-18-0494
5. Zamperini P, Gibelli B, Gilardi D, Tradati N, Chiesa F. Pregnancy and thyroid cancer: ultrasound study of foetal thyroid. Acta Otorhinolaryngol Ital (2009) 29(6):339–44.
6. Gogna S, Goldberg M, Samson D, Gachabayov M, Felsenreich DM, Azim A, et al. Medullary thyroid cancer in patients older than 45-epidemiologic trends and predictors of survival. Cancers (Basel) (2020) 12(11):3124. doi: 10.3390/cancers12113124
7. Starenki D, Sosonkina N, Hong SK, Lloyd RV, Park JI. Mortalin (GRP75/HSPA9) promotes survival and proliferation of thyroid carcinoma cells. Int J Mol Sci (2019) 20(9):2069. doi: 10.3390/ijms20092069
8. Bhoj VG, Li L, Parvathaneni K, Zhang Z, Kacir S, Arhontoulis D, et al. Adoptive T cell immunotherapy for medullary thyroid carcinoma targeting GDNF family receptor alpha 4. Mol Ther Oncolytics (2021) 20:387–98. doi: 10.1016/j.omto.2021.01.012
9. Adam MA, Thomas S, Roman SA, Hyslop T, Sosa JA. Rethinking the current American joint committee on cancer TNM staging system for medullary thyroid cancer. JAMA Surg (2017) 152(9):869–76. doi: 10.1001/jamasurg.2017.1665
10. Wells SA Jr., Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid (2015) 25(6):567–610. doi: 10.1089/thy.2014.0335
11. Jorgensen CU, Homoe P, Dahl M, Hitz MF. Postoperative chronic hypoparathyroidism and quality of life after total thyroidectomy. JBMR Plus (2021) 5(4):e10479. doi: 10.1002/jbm4.10479
12. Pitoia F, Miyauchi A. 2015 American Thyroid association guidelines for thyroid nodules and differentiated thyroid cancer and their implementation in various care settings. Thyroid (2016) 26(2):319–21. doi: 10.1089/thy.2015.0530
13. Kluijfhout WP, Pasternak JD, Lim J, Kwon JS, Vriens MR, Clark OH, et al. Frequency of high-risk characteristics requiring total thyroidectomy for 1-4 cm well-differentiated thyroid cancer. Thyroid (2016) 26(6):820–4. doi: 10.1089/thy.2015.0495
14. Kim BW, Yousman W, Wong WX, Cheng C, McAninch EA. Less is more: Comparing the 2015 and 2009 American thyroid association guidelines for thyroid nodules and cancer. Thyroid (2016) 26(6):759–64. doi: 10.1089/thy.2016.0068
15. Huang Y, Qu S, Zhu G, Wang F, Liu R, Shen X, et al. BRAF V600E mutation-assisted risk stratification of solitary intrathyroidal papillary thyroid cancer for precision treatment. J Natl Cancer Inst (2018) 110(4):362–70. doi: 10.1093/jnci/djx227
16. Miyauchi A, Matsuzuka F, Hirai K, Yokozawa T, Kobayashi K, Ito Y, et al. Prospective trial of unilateral surgery for nonhereditary medullary thyroid carcinoma in patients without germline RET mutations. World J Surg (2002) 26(8):1023–8. doi: 10.1007/s00268-002-6665-1
17. Ito Y, Miyauchi A, Yabuta T, Fukushima M, Inoue H, Tomoda C, et al. Alternative surgical strategies and favorable outcomes in patients with medullary thyroid carcinoma in Japan: Experience of a single institution. World J Surg (2009) 33(1):58–66. doi: 10.1007/s00268-008-9795-2
18. Sahli ZT, Canner JK, Zeiger MA, Mathur A. Association between age and disease specific mortality in medullary thyroid cancer. Am J Surg (2021) 221(2):478–84. doi: 10.1016/j.amjsurg.2020.09.025
19. Gimm O, Sutter T, Dralle H. Diagnosis and therapy of sporadic and familial medullary thyroid carcinoma. J Cancer Res Clin Oncol (2001) 127(3):156–65. doi: 10.1007/s004320000173
20. Zhu C, Zheng T, Kilfoy BA, Han X, Ma S, Ba Y, et al. A birth cohort analysis of the incidence of papillary thyroid cancer in the united states, 1973-2004. Thyroid (2009) 19(10):1061–6. doi: 10.1089/thy.2008.0342
21. Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G, et al. STROCSS 2019 guideline: Strengthening the reporting of cohort studies in surgery. Int J Surg (2019) 72:156–65. doi: 10.1016/j.ijsu.2019.11.002
22. Ho AS, Wang L, Palmer FL, Yu C, Toset A, Patel S, et al. Postoperative nomogram for predicting cancer-specific mortality in medullary thyroid cancer. Ann Surg Oncol (2015) 22(8):2700–6. doi: 10.1245/s10434-014-4208-2
23. Esfandiari NH, Hughes DT, Yin H, Banerjee M, Haymart MR. The effect of extent of surgery and number of lymph node metastases on overall survival in patients with medullary thyroid cancer. J Clin Endocrinol Metab (2014) 99(2):448–54. doi: 10.1210/jc.2013-2942
24. Qu N, Shi RL, Luo TX, Wang YL, Li DS, Wang Y, et al. Prognostic significance and optimal cutoff of age in medullary thyroid cancer. Oncotarget (2016) 7(13):15937–47. doi: 10.18632/oncotarget.7556
25. Wu X, Li B, Zheng C, Liu W, Hong T, He X. Risk factors for lateral lymph node metastases in patients with sporadic medullary thyroid carcinoma. Technol Cancer Res Treat (2020) 19:1533033820962089. doi: 10.1177/1533033820962089
26. Bowen A, Mani N, Penney S, Loughran S, Yap B. Surgical management of medullary thyroid cancer: which guidelines should we follow? J Laryngol Otol (2015) 129(5):478–83. doi: 10.1017/S0022215115000237
27. Stamatakos M, Paraskeva P, Katsaronis P, Tasiopoulou G, Kontzoglou K. Surgical approach to the management of medullary thyroid cancer: when is lymph node dissection needed? Oncology (2013) 84(6):350–5. doi: 10.1159/000351148
28. Ahn HS, Kim DW, Lee YJ, Lee CY, Kim JH, Choi YJ, et al. Postoperative neck ultrasonography surveillance after thyroidectomy in patients with medullary thyroid carcinoma: A multicenter study. Front Endocrinol (Lausanne) (2018) 9:102. doi: 10.3389/fendo.2018.00102
29. Hamy A, Pessaux P, Mirallie E, Mucci-Hennekinne S, Gibelin H, Mor-Martinez C, et al. Central neck dissection in the management of sporadic medullary thyroid microcarcinoma. Eur J Surg Oncol (2005) 31(7):774–7. doi: 10.1016/j.ejso.2005.03.007
30. Niederle MB, Riss P, Selberherr A, Koperek O, Kaserer K, Niederle B, et al. Omission of lateral lymph node dissection in medullary thyroid cancer without a desmoplastic stromal reaction. Br J Surg (2021) 108(2):174–81. doi: 10.1093/bjs/znaa047
31. Alzumaili B, Xu B, Spanheimer PM, Tuttle RM, Sherman E, Katabi N, et al. Grading of medullary thyroid carcinoma on the basis of tumor necrosis and high mitotic rate is an independent predictor of poor outcome. Mod Pathol (2020) 33(9):1690–701. doi: 10.1038/s41379-020-0532-1
Keywords: medullary thyroid cancer, AJCC, age, total thyroidectomy, lobectomy
Citation: Yang B, Niu G, Li X, Ma F, Ma Y and Hu S (2022) Lobectomy may be more appropriate for patients with early-stage medullary thyroid cancer older than 60 years old. Front. Endocrinol. 13:1015319. doi: 10.3389/fendo.2022.1015319
Received: 10 August 2022; Accepted: 10 October 2022;
Published: 21 October 2022.
Edited by:
David Viola, University of Pisa, ItalyReviewed by:
George Simeakis, 401 General Military Hospital of Athens, GreeceBarbara Maria Jarzab, Maria Skłodowska-Curie National Research Institute of Oncology, Poland
Copyright © 2022 Yang, Niu, Li, Ma, Ma and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaojun Hu, aHNqcmFpbkAxNjMuY29t; Yanhong Ma, bWF5YW5ob25nODdAMTYzLmNvbQ==; Fenfen Ma, NTk1NTA3OTIzQHFxLmNvbQ==
†These authors have contributed equally to this work
 Binfeng Yang1†
Binfeng Yang1† Shaojun Hu
Shaojun Hu