- 1Department of Pharmacy, Second Xiangya Hospital of Central South University, Changsha, China
- 2Phase I Clinical Trial Center, The Second Xiangya Hospital of Central South University, Changsha, China
- 3Xiangxing College, Hunan University of Chinese Medicine, Changsha, China
- 4Department of Pharmacy, Medical College, Yueyang Vocational Technical College, YueYang, China
- 5Hunan Provincial Engineering Research Central of Translational Medical and Innovative Drug, The Second Xiangya Hospital of Central South University, Changsha, China
Epidemic obesity is contributing to increases in the prevalence of obesity-related metabolic diseases and has, therefore, become an important public health problem. Adipose tissue is a vital energy storage organ that regulates whole-body energy metabolism. Triglyceride degradation in adipocytes is called lipolysis. It is closely tied to obesity and the metabolic disorders associated with it. Various natural products such as flavonoids, alkaloids, and terpenoids regulate lipolysis and can promote weight loss or improve obesity-related metabolic conditions. It is important to identify the specific secondary metabolites that are most effective at reducing weight and the health risks associated with obesity and lipolysis regulation. The aims of this review were to identify, categorize, and clarify the modes of action of a wide diversity of plant secondary metabolites that have demonstrated prophylactic and therapeutic efficacy against obesity by regulating lipolysis. The present review explores the regulatory mechanisms of lipolysis and summarizes the effects and modes of action of various natural products on this process. We propose that the discovery and development of natural product-based lipolysis regulators could diminish the risks associated with obesity and certain metabolic conditions.
1 Introduction
Obesity is excessive lipid accumulation in adipose tissue. It is caused by an imbalance between energy intake and energy consumption. According to the World Health Organization (WHO), more than 650 million adults over 18 years of age were obese as of 2016 (1). Obesity is a risk factor for cardiovascular disease (CVD), insulin resistance (IR), type 2 diabetes mellitus (T2DM), hypertension, dyslipidemia, and certain cancers (2). Increased adipocyte number (hyperplasia) and size (hypertrophy) are morphological manifestations of obesity (3). Adipose tissue is classified into three distinct types: white (WAT), brown (BAT), and beige (4). WAT stores excess energy in the form of triglycerides (TG), whereas BAT and beige adipose tissues catabolize TG into heat (5). Adipose tissue also functions as an endocrine organ and filler tissue and cushions, supports, and insulates the body (6).
WAT is generally considered a ‘troublesome and excessive tissue’. Body weight may be lost via intermittent fasting, medication, exercise, or surgery (7). However, it is uncertain whether these approaches maintain weight loss or have unacceptable side effects in the long term. Exercise appears to be an effective weight loss method, although its efficacy depends largely on its duration, frequency, and intensity (8). The administration of certain drugs is promising for obesity prevention and treatment. Intermittent fasting, drugs, and exercise decompose TG faster than they are synthesized in the adipocytes. Hence, pharmacological and nutritional enhancements of this process are potential strategies for weight loss and the prevention of obesity-related metabolic syndrome.
The reservoir effect of WAT protects other tissues against the toxic effects of glycolipids associated with excess energy storage. Adipocytes have limited lipid storage capacity and can hold no additional TG when their volume expands beyond a critical point. At this stage, the adipose tissue becomes dysfunctional. This condition is observed in patients with insulin resistance, T2DM, and obesity and is manifested by decreased TG synthesis and excess free fatty acid (FFA) release (9). In cases of adipose tissue dysfunction, certain compounds improve whole-body energy metabolism by inhibiting lipolysis.
Natural products are vital sources of lead compounds and are important in drug discovery. Several natural products are widely used in obesity treatment (10). Various natural products (11–14), including flavonoids, alkaloids, and terpenoids control obesity by stimulating lipolysis, inhibiting adipogenesis and lipogenesis, and promoting energy expenditure. However, the activities and mechanisms of natural products in modulating lipolytic activity have not yet been systematically summarized. In the present review, from a lipolysis perspective, we describe the biosynthesis and metabolism of TG in adipose tissue and review the regulatory mechanisms of lipolysis. Furthermore, we summarize a wide diversity of plant secondary metabolites that have demonstrated anti-obesity effects via the promotion of lipolysis. We also focus on the progress of research on inhibitors of lipolysis with different mechanisms of action in adipose tissue dysfunction. This review provides insight into the precise biochemical and molecular mechanisms by which plant secondary metabolites inhibit the onset and/or progression of obesity and, by extension, its related co-morbidities. In addition, it highlights the potential of lipolysis as a therapeutic target for obesity and its complications.
2 Triglyceride biosynthesis and metabolism
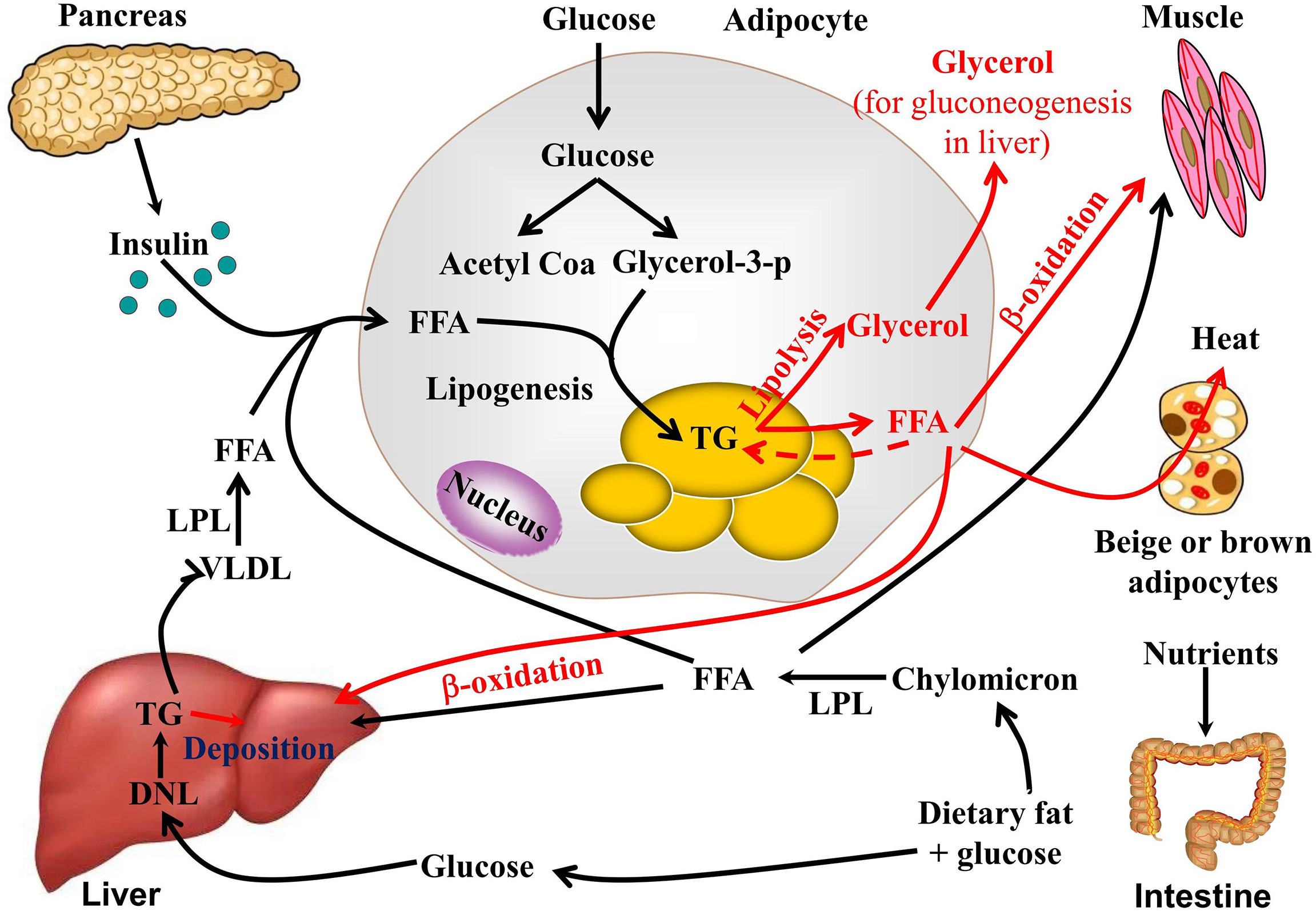
Adipose tissue, the liver, and skeletal muscle are the mains organs responsible for the regulation of lipid metabolism. TG biosynthesis and decomposition (lipolysis) in WAT equilibrate lipid metabolism. After feeding, glucose and lipids from food are absorbed in the intestine in the form of chylomicrons and enter the bloodstream. The chylomicrons are then hydrolyzed into FFAs by lipoprotein lipase and absorbed and utilized by adipocytes and liver and muscle tissue. Insulin is secreted by β-cells in the pancreas and promotes FFA and glucose uptake, while insulin inhibits lipolysis via lipase inhibition. Adipocytes absorb excess FFA and glucose and produce TG as an energy storage form (15). During this process, adipogenesis and lipogenesis increase, while lipolysis, thermogenesis, and browning decrease. De novo lipogenesis involves TG biosynthesis and occurs in the adipocytes and liver. To maintain normal blood glucose levels, the liver converts excess glucose into glycogen and stores it in liver cells, or hepatocytes, which can also synthesize TG through the de novo TG synthesis pathway. TG subsequently is transported from the liver to adipose tissue by very low-density lipid (VLDL) (16). An important contributor to hepatic fat accumulation is the insufficient hepatic export of TG in the form of VLDL particles. TG synthesis and metabolism are illustrated in Figure 1.
During fasting and starvation, TG is decomposed into glycerol and FFA (17). Adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and monoacylglycerol lipase (MAGL) hydrolyze TG to FFAs and glycerol. The glycerol is used to make glucose in gluconeogenesis. FFAs are then released into circulation, where they are utilized by the peripheral tissues and/or re-esterified into TG in the adipocytes. Skeletal muscle and the liver are the most important organs involved in FFA metabolism via β-oxidation and subsequent ATP generation. Mitochondrial-rich beige adipose tissue or BAT are the major sites responsible for non-shivering thermogenesis in mammals. Cold exposure, β-adrenergic receptor (β-AR) agonists, peroxisome proliferator-activated receptor-γ, and exercise can induce the browning of WAT. FFA produced by lipolysis is also absorbed and utilized by beige adipocytes or BAT through UCP-1-dependent shiver-independent thermogenesis.
3 Lipolysis and its mechanisms
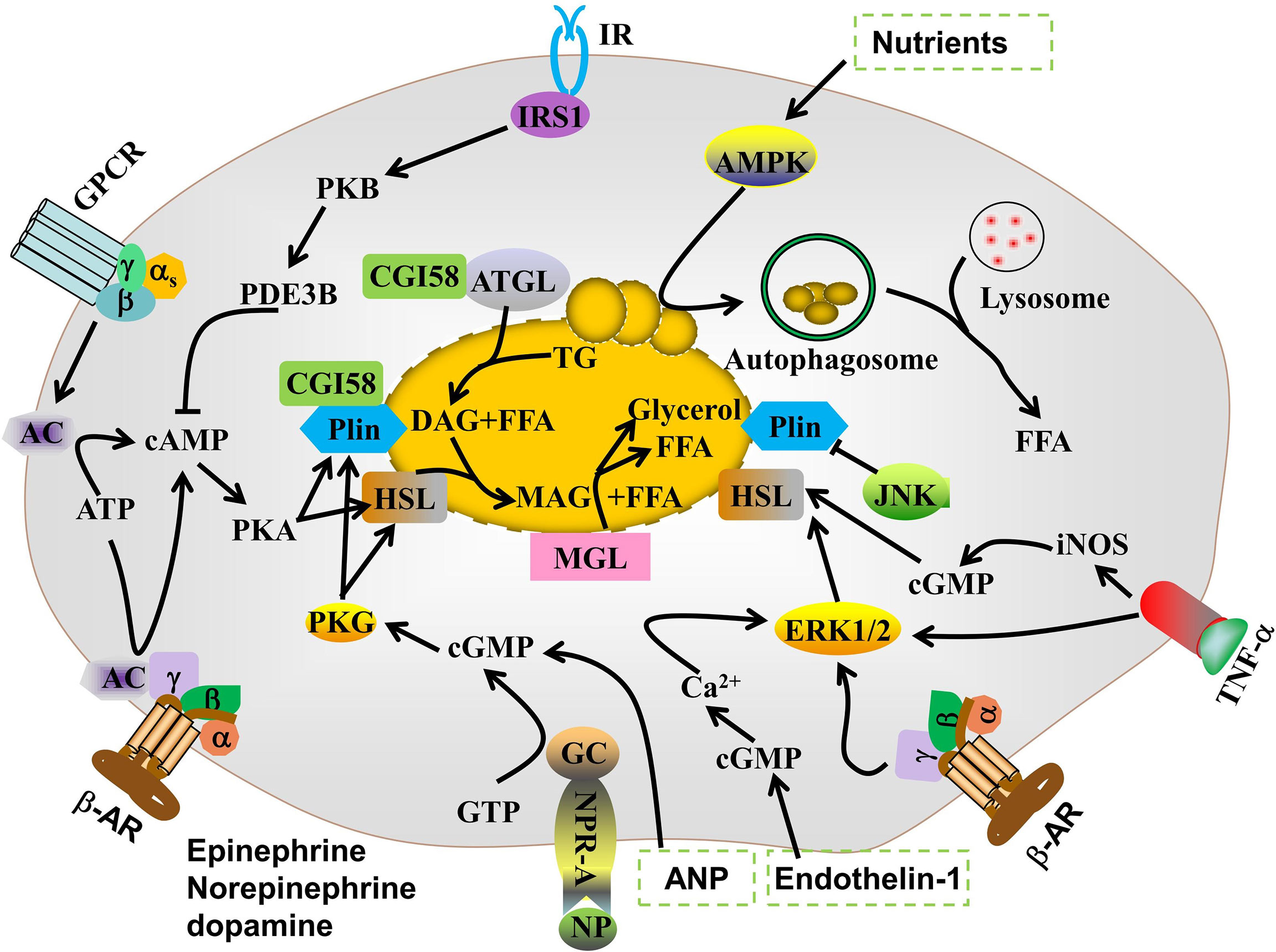
Lipolysis is a finely regulated process mediated by the consecutive actions of ATGL, HSL, and MAGL. ATGL or HSL first hydrolyzes TG to diglycerides and FFA. HSL then hydrolyzes diglycerides to monoglycerides and FFA. MAGL then hydrolyzes monoglycerides to glycerol and FFA (18). Lipid droplet autophagy or lipophagy is a complementary cellular lipid breakdown pathway (19). Sex, age, physical activity, fat deposit location, and genetic variation regulate basal lipolytic activity in adipocytes (20). The proinflammatory cytokines TNF-α (21), IL-6 (22), and IL-1β (23) as well as lipopolysaccharide (LPS) (24) and hypoxia (25) may induce TG lipolysis. Lipid droplet-associated proteins (LDAPs) (26), cyclic guanosine monophosphate dependent-protein kinase G (cGMP-PKG) (8), mitogen-activated protein kinase (MAPK) (27), and adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) (28) are also implicated in TG lipolysis.
3.1 LDAPs
Lipid droplets (LD) are dynamic lipid storage organelles surrounded by single layers of polar and amphipathic phospholipids and structural proteins. They are now considered major fat storage, lipid secretion, and lipolysis regulators (29). The perilipins, including perilipin1, perilipin2, and perilipin5, as well as the cell death-inducing DNA fragmentation factor alpha (DFFA)-like effector (CIDE) family proteins, including Cidea, Cideb, and Cidec/Fsp27, have emerged as key lipolysis regulators (30, 31). Perilipin1 is a scaffold for organized protein-protein interactions on LD surfaces. It binds CGI-58 and suppresses HSL translocation to LD under basal conditions. During stimulatory conditions, however, phosphorylation causes perilipin to dissociate from CGI-58. The free CGI-58 then binds phosphorylated ATGL and co-activates TG hydrolysis (32). Perilipin phosphorylation recruits HSL from the cytosols to the surfaces of the LDs, and diglycerides are then hydrolyzed (26). FSP27-deficient cells exhibite increased basal lipolysis and reduced lipid storage capacity (33). The mechanisms by which perilipin1 regulates lipolysis are generally understood. However, the roles and mechanism of perilipin2, perilipin5, and the CIDE family in lipolysis remain to be elucidated.
3.2 cAMP-PKA pathway
In vivo, dynamic lipolysis processes are mainly regulated by hormones, such as catecholamines, ghrelin, adiponectin, and insulin. Under conditions of fasting, cold stress, and other compound treatment, norepinephrine is released from sympathetic nerve terminals. β-AR agonists, such as epinephrine, norepinephrine, and dopamine, upregulate cyclic AMP (cAMP) by linking various AR subtypes to the G-protein receptor complex that controls adenylate cyclase in the cell membrane. Thereafter, protein kinase A (PKA) is activated by cAMP (34). PKA phosphorylates both HSLs at Ser563, Ser659, and Ser660, thereby activating them and promoting their translocation from the cytoplasm to the surfaces of LDs (35).
cAMP degradation is mediated by phosphodiesterase (PDE). Insulin inhibits lipolysis mainly by activating the phosphoinositide 3-kinase/protein kinase B/PDE 3B (PDE3B) pathway, which leads to p-HSL and p-perilipin dephosphorylation (36). In addition, ligands of Gi protein-coupled receptors, such as succinic acid, nicotinic acid, beta-hydroxybutyric acid, and neuropeptide Y, inhibit the formation of cAMP by binding to their receptors, thereby exerting an anti-lipolytic effect.
3.3 cGMP-PKG
Cyclic guanosine 3’5’-monophosphate (cGMP) is an important intracellular secondary messenger of hormone-induced lipolysis. Atrial and b-type natriuretic peptides are nitric oxide (NO) donors that stimulate lipolysis in adipocytes via the cGMP/PKG pathway (8). PKG phosphorylates proteins associated with lipolysis, including HSL and perilipin, thereby promoting TG breakdown (37). The cGMP is also involved in TNF-α (iNOS/NO/GC/cGMP-dependent pathway)- and endothelin-1 (GC/cGMP/Ca2+/ERK/CaMKIII signaling pathway)-induced lipolysis in adipocytes (38–40). Few studies have reported on the involvement of cGMP/PKG in lipolysis regulation. Moreover, the roles of cGMP/PKG in lipolytic enzymes regulation and LDAPs remain to be clarified.
3.4 Mitogen-activated protein kinase
The mitogen-activated protein kinase (MAPK) family, which including extracellular signal-regulated kinases (ERKs), jun aminoterminal kinase (JNK), and p38 mitogen-activated protein kinases (p38) plays vital roles in adipogenesis and lipolysis. (β−AR) stimulation by catecholamine activates ERK1/2, which is sufficient to induce lipolysis by direct HSL phosphorylation at Ser600. JNK regulates lipolysis. JNK1/2 deficiency accelerates basal lipolysis in mouse adipocytes (41). The MEK1/2-ERK1/2 pathway controls TNF-α-stimulated lipolysis in human adipocytes (42).
3.5 AMPK pathway
AMPK is a Ser/Thr protein and an important regulatory sensor of cellular energy metabolism. Activated AMPK inhibits sterol regulatory element binding protein-1, CCAAT/enhancer binding protein alpha, peroxisome proliferator activated receptor gamma, and acetyl-CoA carboxylase (ACC). Hence, AMPK suppresses adipocyte differentiation (43). AMPK also phosphorylates ATGL Ser406, which promotes TG decomposition (44). However, the roles of AMPK in regulating TG lipolysis in adipocytes are controversial. AMPK may phosphorylate HSL at Ser565 to inhibit phosphorylation at HSL Ser660 and Ser563. In this manner, it reduces HSL activity and suppresses lipolysis (45). AMPK is implicated in chaperone-mediated autophagy which selectively degrades perilipins and initiates lipolysis (46). Therefore, proteins and signaling pathways that modulate AMPK expression and activity, such as SIRT (47) and SIRT3 (48), mobilize TG in adipocytes.
Protein kinase C (49), Ca2+ (50), inositol hexakisphosphate kinase-1 (51), transient receptor potential vanilloid channels (38, 52), and endoplasmic reticulum (ER) stress (53) regulate lipolysis in adipocytes either alone or by interacting with the aforementioned signaling pathways (Figure 2).
4 Natural products involved in lipolysis
The structural diversity of natural products determines their wide range of pharmacological activity. Natural products may be used to treat obesity and its associated metabolic diseases. Traditional and complementary medicines including various herbs and extracts have been widely used to prevent and treat metabolic disorders (54, 55). Flavonoids (56), alkaloids (57), terpenoids (58), and polyphenols (59) stimulate lipolysis in adipocytes, thereby causing weight loss and improving metabolic status. Their modes of action involve the PKA-HSL, PKC, AMPK, MAPK, and other signaling pathways.
4.1 Natural products promote lipolysis
4.1.1 Flavonoids
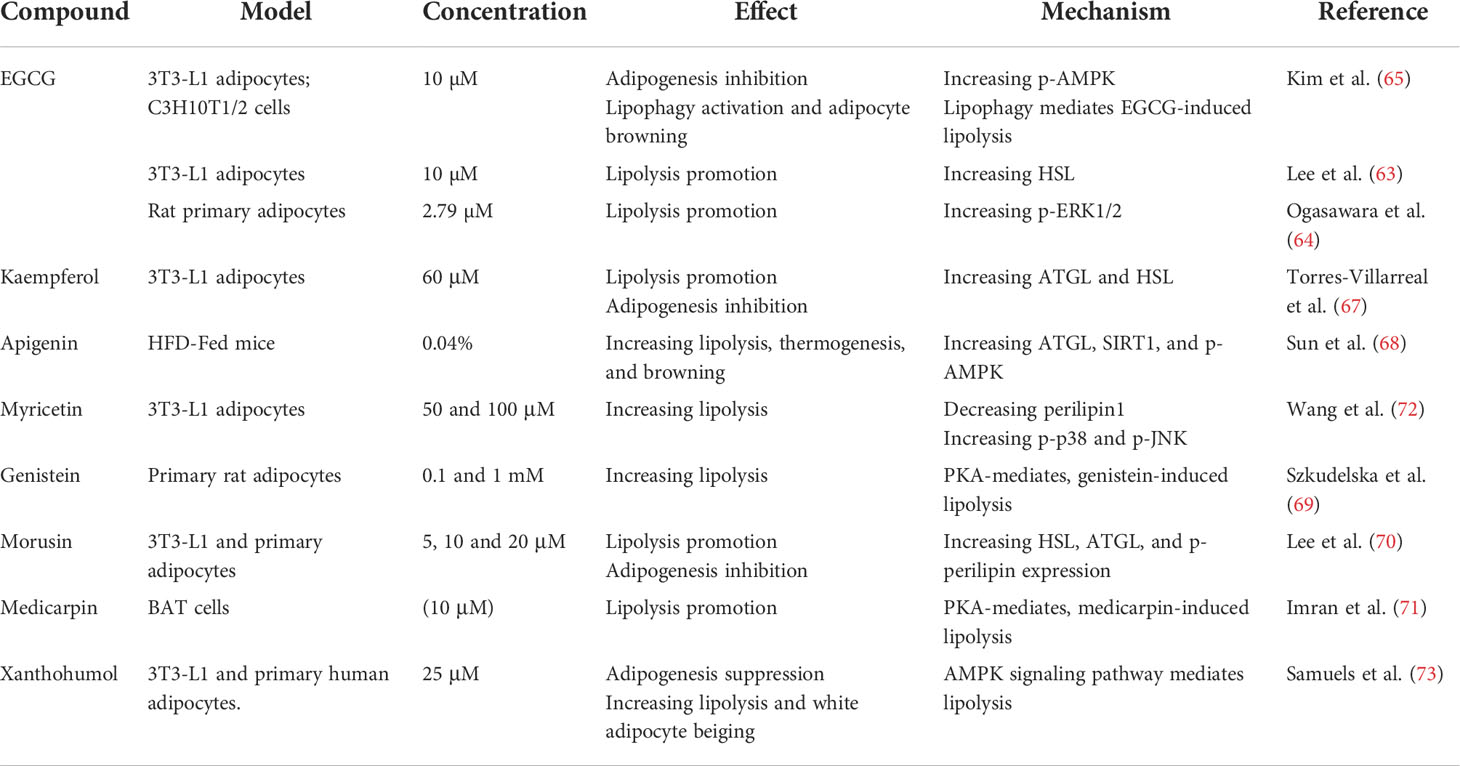
Flavonoids comprise a large family of natural substances sharing a molecular structure characterized by at least one phenolic ring. Flavonoids are reputed for their health benefits. Epigallocatechin-3-gallate (EGCG) is a polyphenolic catechin in green tea that improves the lipid prolife and reduces body weight (60). EGCG inhibits adipogenesis and adipocyte differentiation, reduces energy intake, and increases energy expenditure and lipolysis (61, 62). EGCG-stimulated lipolysis is mediated by activating HSL (63), ERK1/2 (64), and p-AMPK (65). Lipophagy is also associated with EGCG-induced lipolysis. Rab7 knockdown attenuates EGCG-dependent lipid reduction (65). However, a clinical trial demonstrated no effect of EGCG on obesity reduction, lipolysis, or white adipocyte browning in humans (66).
Kaempferol (67), apigenin (68), genistein (69), morusin (70), medicarpin (71), and myricetin (72) commonly occur in fruits, vegetables, and tea. These flavonoids have anti-obesity and pro-lipolysis efficacy. Elevated lipolysis upregulates thermogenic genes and increases mitochondrial biogenesis by supplying FFAs for mitochondrial β-oxidation. Apigenin activates lipolysis via the ATGL/FOXO1/SIRT1 pathway and increases FFA consumption by upregulating fatty acid oxidation (FAO) (AMPK/ACC), thermogenesis, and browning (UCP-1, PGC-1α) (68). Lipolysis is also associated with activated BAT or beiging which is regarded as an alternative strategy against diet-induced obesity. Xanthohumol (73), apigenin (68), and EGCG (65) inhibit adipogenesis, stimulate adipocyte lipolysis, and may act as browning or beiging agents because they upregulate the thermogenic protein UCP1 (Table 1).
4.1.2 Alkaloids
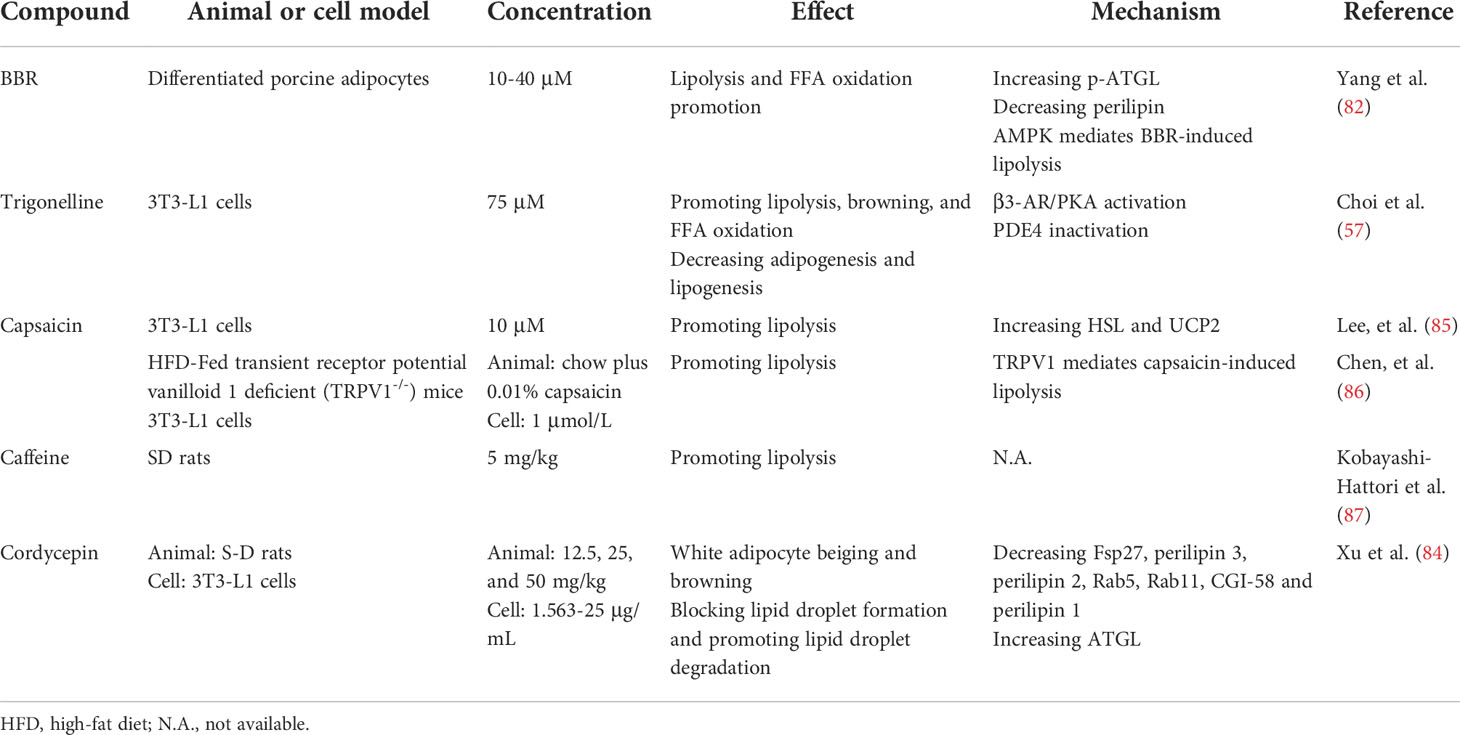
Consumption of coffee, ephedrine, or capsaicin increases lipolytic responses, raise metabolic rates, and increase energy expenditure and weight loss (74, 75). Caffeine is the main alkaloid in tea, coffee, and cacao. It decreases body fat, improves glucose tolerance and insulin sensitivity (76), and increases lipolysis by raising cAMP levels and upregulating lipolytic enzymes (77). Ephedrine is an α- and β-adrenergic receptor agonist with efficacy as a bronchodilator. It also activates the β-adrenergic receptors, contributing to lipolysis (78). Capsaicin analogs significantly increase cAMP levels and PKA activity in BAT (79). While ephedrine, caffeine, capsaicin, and synephrine strongly induce lipolysis, they are also associated with various cardiovascular and gastrointestinal side effects when they are administered for weight loss (80). Therefore, novel lipolytic compounds with minimal adverse reactions merit further investigation.
Berberine (BBR) is an isoquinoline alkaloid derived from the Chinese herb Coptis chinensis. It has anti-obesity, anti-diabetic, and anti-hyperlipidemic efficacy. BBR stimulates basal lipolysis in 3T3−L1 adipocytes by upregulating ATGL via the AMPK pathway (81, 82). However, Zhou et al. found that BBR attenuates isoprenaline-stimulated lipolysis in 3T3−L1 adipocytes by reducing phosphodiesterase-3B and -4 inhibition, thereby decreasing cAMP production and inhibiting HSL activation (83). Trigonelline (N-methylnicotinic acid) is a pyridine derivative that increases brown and beige fat-specific markers as well as mitochondrial biogenesis in 3T3-L1 adipocytes (57). Trigonelline as well as cordycepin from Cordyceps militaris promotes white adipocytes beiging and browning and increases lipolysis by various mechanisms (57, 84) (Table 2).
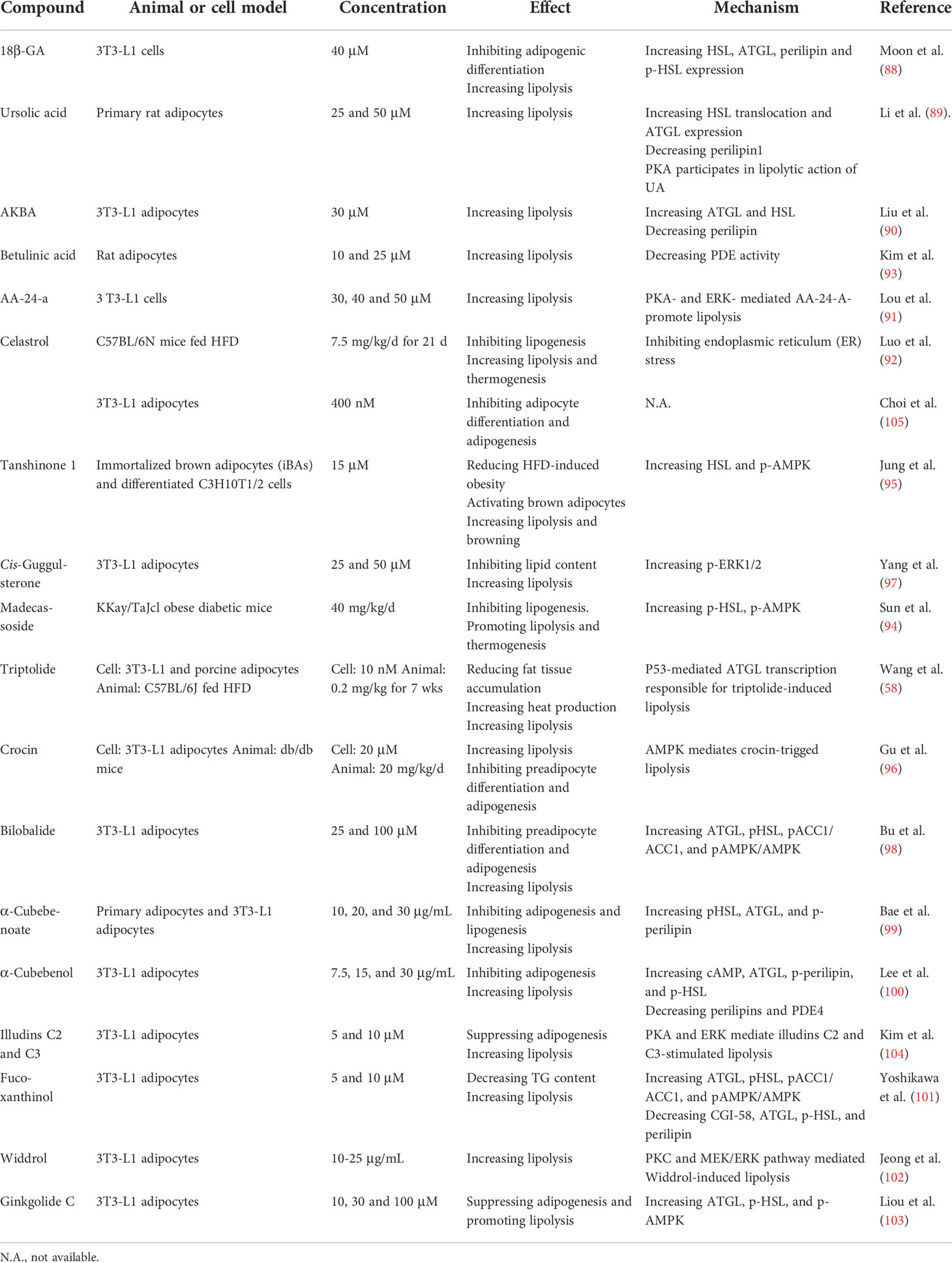
4.1.3 Terpenoids
Terpenoids comprise five-carbon isoprene units and have diverse effects on obesity and its associated metabolic diseases. Triterpenoids include 18β-glycyrrhetinic acid (18β-GA) (88), ursolic acid (89), acetyl-keto-β-boswellic acid (AKBA) (90), alisol A 24-acetate (AA-24-a) (91), celastrol (92), and betulinic acid (93). All of these reduce neutral lipids in the cytosol and increase FFA release. Madecassoside (94), tanshinone 1 (95), triptolide (58), crocin (96), guggulsterone (97), bilobalide (98), α-cubebenoate (99, 100), betulinic acid (93), fucoxanthinol (101), widdrol (102), ginkgolide C (103), and illudins C2 and C3 (104) could all potentially treat obesity either by inhibiting adipocyte differentiation and lipogenesis or by increasing lipolysis. The LDAP (88–90), PKA (89, 90), AMPK (96, 98), and PKC-MEK-ERK (102) pathways are involved in the lipolytic mechanisms induced by these compounds (Table 3).
Celastrol and triptolide are the main bioactive constituents in the root of Tripterygium wilfordii. The administration of celastrol and triptolide reduces body and fat weight, suppresses lipogenesis (58, 92), increases heat production in BAT, and enhances lipolysis (58). Celastrol rapidly lowers body weight by covalently inhibiting GRP78 chaperone activity and disconnecting ER stress signal transduction (92). Elevated lipolysis induced by triptolide is mediated by p53 which directly binds and promotes the transcription of the ATGL promoter (58). Although triptolide and celastrol have good anti-obesity efficacy, their potential toxicity must be established.
4.1.4 Other compounds
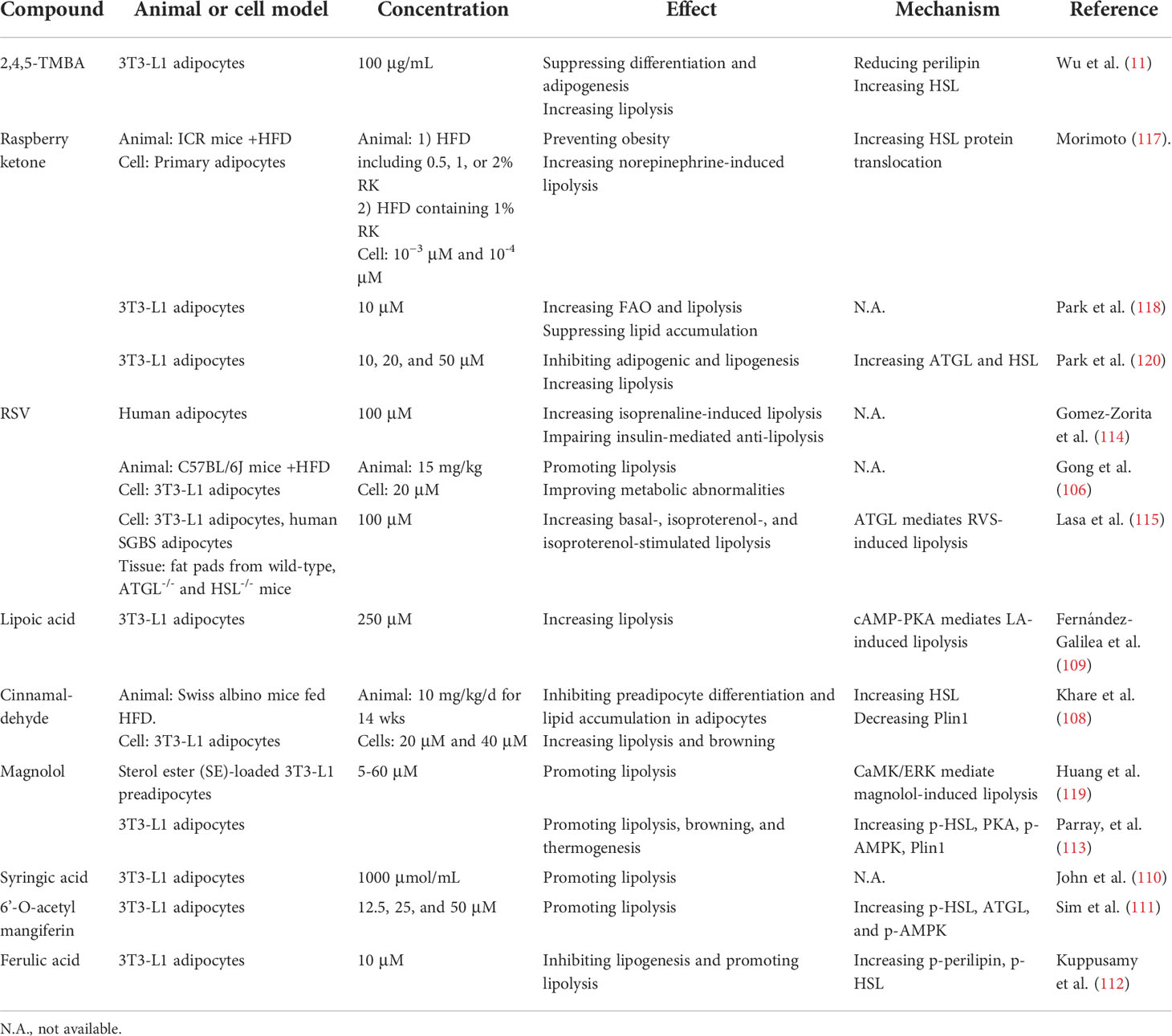
Resveratrol (RSV) (106), 2,4,5-trimethoxybenzaldehyde (2,4,5-TMBA) (11), raspberry ketone (RK) (107), cinnamaldehyde (108), lipoic acid (109), syringic acid (110), 6’-O-acetyl mangiferin (111), ferulic acid (112), and magnolol (113) have all demonstrated potential prophylactic and therapeutic efficacy against obesity. RSV directly affectes isoprenaline-stimulated lipolysis in vitro in fac cells from overweight humans (114). It also increases FFA and glycerol content in high-fat diet (HFD)-fed mice or 3T3-L1 adipocytes (106). Arrate et al. showed ATGL-mediated, RSV-induced lipolysis in vivo (115). However, a randomized, double-blind, crossover study revealed that RSV improved adipose tissue lipolysis and decreased plasma FFA and glycerol levels (116). This apparent contradiction in the anti-obesity effects of RSV in rodents and humans necessitates the re-evaluation of RSV as a putative anti-obesity drug.
RK has a structure resembling those of capsaicin and synephrine and can prevent HFD-induced obesity (117). 3T3-L1 adipocytes treated with 10 µM RK presented with elevated FAO and inhibition of lipid accumulation (118). Magnolol is the main bioactive compound in Magnolia officinalis. Its lipolytic effect is mediated by the calcium/calmodulin-dependent protein kinase (CaMK)/ERK1/2 signaling pathway and not by PKA (119). Magnolol may cause browning in white adipocytes and augment thermogenesis (113) (Table 4). Further research in the form of animal models is required to validate the lipolytic potential and clinical value of the foregoing compounds.
The lipolytic effects of the compounds above have already been established in in vivo or in vitro experiments. For compounds with pro-lipolytic activity tested only in vivo, preclinical pharmacodynamics and safety evaluations are required. In pharmacodynamics experiments, primary outcome measures, such as change in body weight, food intake, resting metabolic rate, blood lipids, and biochemistry, need to be tested. In addition to general and specific toxicities of drugs, the safety evaluation should pay special attention to liver and kidney toxicity caused by long-term use of lipolysis agonists, as well as pancreatic damage, insulin resistance, and cardiovascular events that may be caused by elevated FFA.
4.2 Natural products that inhibit lipolysis
Adipose tissue dysfunction increases circulating FFA levels. Elevated FFAs are often observed in patients with IR and T2DM (9). Impaired lipogenic capacity driven by insulin signaling and re-esterification of FFA with adipocytes results in impaired buffering capacity for FFA and high concentrations of circulating FFA (26). Long-term over-activation of lipolysis may promote lipid ‘overflow’ into the muscle, liver, endothelium, heart, and β-cells, thereby causing muscular/hepatic IR, CVD, and impaired insulin secretion (121). For example, adipocyte-derived FFA is involved in regulating hepatic energy metabolism (122). FFA impairs the insulin signaling pathway by forming diacylglycerol and ceramides and increases gluconeogenesis via the hepatic acetyl-CoA pathway in liver during diseased states (26, 123), which leads to TG accumulation in the liver. In patients with adipose tissue dysfunction, then, the inhibition of lipolysis may ameliorate IR- and obesity-associated metabolic diseases. Thiazolidinedione antidiabetic drugs improve insulin sensitivity and reduce circulating FFA levels by attenuating lipolysis and FFA release (124).
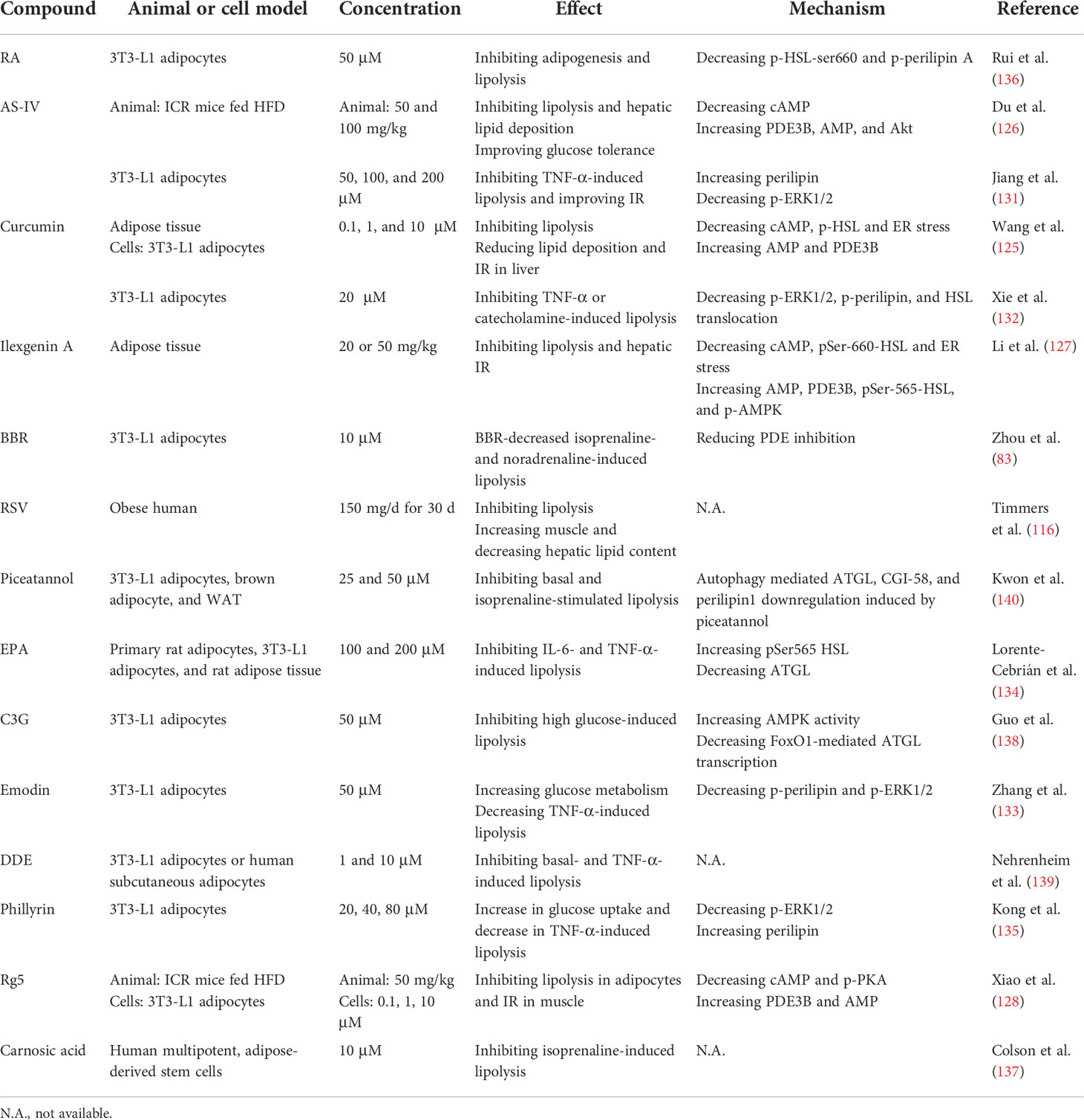
Curcumin (125), astragaloside IV (AS-IV) (126), and ilexgenin A (127) attenuate lipolysis by modulating the cAMP/PKA/HSL pathway. The inhibition of lipolysis in adipose tissue may improve hepatic insulin sensitivity (125, 126). Ginsenoside Rg5 (Rg5) suppresses lipolysis and inhibited IR in muscle (128). The foregoing findings suggest that a decrease in adipose tissue lipolysis mediated by natural bioactive components is a potentially efficacious therapy for hepatic IR and related disorders.
TNF-α is a proinflammatory cytokine expressed in adipose tissue that might link obesity and IR (129) and increases plasma FFA levels in obesity and T2DM (130). AS-IV (131), curcumin (132), emodin (133), eicosapentaenoic acid (EPA) (134), and phillyrin (135) attenuates TNF-α-induced lipolysis by suppressing p-ERK1/2 and reversing perilipin or p-perilipin downregulation. Rosmarinic acid (RA) (136, 137), RSV (116), BBR (83), cyanidin-3-O-β-glucoside (C3G) (138), dihydrodehydrodiisoeugenol (DDE) (139), carnosic acid (137), and piceatannol (140) may also inhibit lipolysis. The effects and mechanisms of these compounds are summarized in Table 5.
5 Conclusions and perspectives
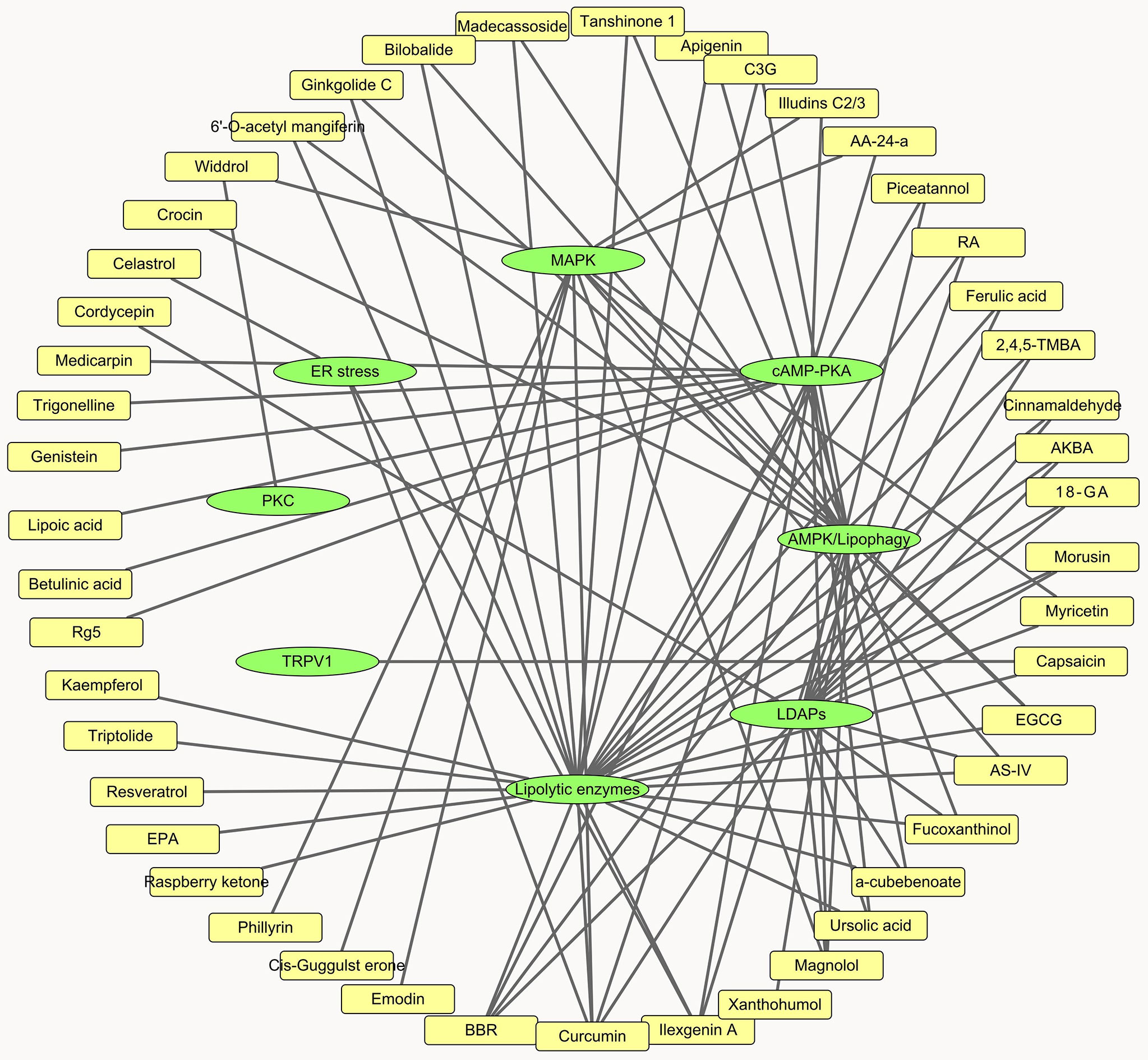
In the present review, we summarized the effect and modes of action of a wide range of natural products on lipolysis. Overall, these compounds individually or synergistically affect lipolytic enzymes, LDAPs, ER stress, and the cAMP-PKA, MAPK, AMPK, and PKC signaling pathways (Figure 3). The lipolytic effects of certain compounds have already been established. Nevertheless, their influences and mechanisms in fat synthesis and metabolism, their toxicity, and their effects on whole-body phenotypes, appetite, energy expenditure, and thermogenesis remain to be determined. About half the compounds evaluated herein affect lipolytic enzyme expression. However, in vitro enzyme activity assay and compound-enzyme interaction data were lacking for them. These experiments may help identify novel lipolysis inhibitors and agonists.
Our understanding of adipocyte lipolysis has progressed from basic knowledge of its associated enzymatic processes to elucidtation of the dynamic and complex regulatory mechanisms involved. Lipolysis interacts with other related processes, including thermogenesis, adipocyte browning, and lipogenesis. Clarification of the mechanisms of lipolysis and the changes it causes in whole-body energy metabolism has positive clinical value and socioeconomic benefits in that it may help develop modalities to prevent and treat obesity and its associated metabolic disorders. Lipolysis regulates TG metabolism and weight loss. Certain compounds with lipolytic activity, such as celastrol (141), apigenin (142), cordycepin (84), and BBR (143), have demonstrated anti-obesity efficacy. Theoretically, activating lipolysis may be a rational therapeutic approach for obesity. Thus far, however, no anti-obesity drugs targeting lipolytic enzymes or its related targets have been marketed.
The pathologies of obesity and its related metabolic conditions are highly complex. Simply targeting lipolysis can achieve weight loss. From the perspective of energy metabolism, however, weight loss is the result of multiple factors, including dietary restrictions and increases in lipolysis and energy utilization. It remains to be established whether lipolysis triggered by lipolytic agonists may damage certain cells, tissues, and organs or cause complications. The ideal anti-obesity drug should safely suppress appetite, increase lipolysis, and activate energy expenditure. Finally, the physiological functions of adipocytes should be rationally exploited, and their roles during metabolic disease should be identified. For patients with adipose dysfunction, the dynamic regulation of lipolysis and the amelioration of adipocyte dysfunction could improve obesity-associated metabolic conditions. For example, AS-IV and curcumin inhibit adipose lipolysis and thus prevent hepatic IR, which demonstrates their potential as treatments for metabolic-associated fatty liver disease through the regulation of lipolysis in adipose tissue during diseased states.
Author contributions
X-DY and Y-YY wrote the manuscript. X-CG and S-YJ collected and checked the data. Y-YY contributions to design of the work and revised the work. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation (No. 81603171) and the Natural Science Foundation of Hunan Province (2022JJ30860 and 2022JJ30862).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO. Obesity and overweight (2016). Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
2. Mili N, Paschou SA, Goulis DG, Dimopoulos MA, Lambrinoudaki I, Psaltopoulou T. Obesity, metabolic syndrome, and cancer: Pathophysiological and therapeutic associations. Endocrine (2021) 74:478–97. doi: 10.1007/s12020-021-02884-x
3. Drolet R, Richard C, Sniderman AD, Mailloux J, Fortier M, Huot C, et al. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int J Obes (Lond) (2008) 32:283–91. doi: 10.1038/sj.ijo.0803708
4. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell (2012) 150:366–76. doi: 10.1016/j.cell.2012.05.016
5. Giralt M, Villarroya F. White, brown, beige/brite: Different adipose cells for different functions? Endocrinology (2013) 154:2992–3000. doi: 10.1210/en.2013-1403
6. Tanaka K, Fukuda D, Sata M. Roles of epicardial adipose tissue in the pathogenesis of coronary atherosclerosis - an update on recent findings. Circ J (2020) 85:2–8. doi: 10.1253/circj.CJ-20-0935
7. Obert J, Pearlman M, Obert L, Chapin S. Popular weight loss strategies: A review of four weight loss techniques. Curr Gastroenterol Rep (2017) 19:61. doi: 10.1007/s11894-017-0603-8
8. Ceddia RP, Collins S. A compendium of G-protein-coupled receptors and cyclic nucleotide regulation of adipose tissue metabolism and energy expenditure. Clin Sci (Lond) (2020) 134:473–512. doi: 10.1042/cs20190579
9. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol (2008) 9:367–77. doi: 10.1038/nrm2391
10. Negi H, Gupta M, Walia R, Khataibeh M, Sarwat M. Medicinal plants and natural products: More effective and safer pharmacological treatment for the management of obesity. Curr Drug Metab (2021) 22:918–30. doi: 10.2174/1389200222666210729114456
11. Wu MR, Hou MH, Lin YL, Kuo CF. 2,4,5-TMBA, a natural inhibitor of cyclooxygenase-2, suppresses adipogenesis and promotes lipolysis in 3T3-L1 adipocytes. J Agric Food Chem (2012) 60:7262–9. doi: 10.1021/jf302285k
12. Martín M, Ramos SA-O. Dietary flavonoids and insulin signaling in diabetes and obesity. Cells (2021) 10:1474. doi: 10.3390/cells10061474
13. Islam MA-O, Ali ES, Mubarak MA-O. Anti-obesity effect of plant diterpenes and their derivatives: A review. Phytother Res (2020) 34:1216–25. doi: 10.1002/ptr.6602
14. Li R, Lan YA-O, Chen CA-OX, Cao Y, Huang QA-O, Ho CA-O, et al. Anti-obesity effects of capsaicin and the underlying mechanisms: A review. Food Funct (2020) 11:7356–70. doi: 10.1039/d0fo01467b
15. Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose-tissue plasticity in health and disease. Cell (2022) 185:419–46. doi: 10.1016/j.cell.2021.12.016
16. Sanders FW, Griffin JL. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol Rev Camb Philos Soc (2016) 91:452–68. doi: 10.1111/brv.12178
17. Sethi JK, Vidal-Puig AJ. Thematic review series: Adipocyte biology. Adipose Tissue Funct Plasticity Orchestrate Nutr Adapt J Lipid Res (2007) 48:1253–62. doi: 10.1194/jlr.R700005-JLR200
18. Taschler U, Radner FP, Heier C, Schreiber R, Schweiger M, Schoiswohl G, et al. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J Biol Chem (2011) 286:17467–77. doi: 10.1074/jbc.M110.215434
19. Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol (2015) 17:759–70. doi: 10.1038/ncb3166
20. Fruhbeck G, Mendez-Gimenez L, Fernandez-Formoso JA, Fernandez S, Rodriguez A. Regulation of adipocyte lipolysis. Nutr Res Rev (2014) 27:63–93. doi: 10.1017/S095442241400002X
21. Jin D, Sun J, Huang J, He Y, Yu A, Yu X, et al. TNF-alpha reduces g0s2 expression and stimulates lipolysis through PPAR-gamma inhibition in 3T3-L1 adipocytes. Cytokine (2014) 69:196–205. doi: 10.1016/j.cyto.2014.06.005
22. Morisset AS, Huot C, Legare D, Tchernof A. Circulating IL-6 concentrations and abdominal adipocyte isoproterenol-stimulated lipolysis in women. Obes (Silver Spring) (2008) 16:1487–92. doi: 10.1038/oby.2008.242
23. Feingold KR, Doerrler W, Dinarello CA, Fiers W, Grunfeld C. Stimulation of lipolysis in cultured fat cells by tumor necrosis factor, interleukin-1, and the interferons is blocked by inhibition of prostaglandin synthesis. Endocrinology (1992) 130:10–6. doi: 10.1210/endo.130.1.1370149
24. Hoch M, Eberle AN, Peterli R, Peters T, Seboek D, Keller U, et al. LPS induces interleukin-6 and interleukin-8 but not tumor necrosis factor-alpha in human adipocytes. Cytokine (2008) 41:29–37. doi: 10.1016/j.cyto.2007.10.008
25. Musutova M, Weiszenstein M, Koc M, Polak J. Intermittent hypoxia stimulates lipolysis, but inhibits differentiation and De novo lipogenesis in 3T3-L1 cells. Metab Syndr Relat Disord (2020) 18:146–53. doi: 10.1089/met.2019.0112
26. Yang A, Mottillo EP. Adipocyte lipolysis: from molecular mechanisms of regulation to disease and therapeutics. Biochem J (2020) 477:985–1008. doi: 10.1042/BCJ20190468
27. Mottillo EP, Shen XJ, Granneman JG. beta3-adrenergic receptor induction of adipocyte inflammation requires lipolytic activation of stress kinases p38 and JNK. Biochim Biophys Acta (2010) 1801:1048–55. doi: 10.1016/j.bbalip.2010.04.012
28. Boone-Villa DA-O, Ventura-Sobrevilla JA-O, Aguilera-Méndez AA-O, Jiménez-Villarreal JA-O. The effect of adenosine monophosphate-activated protein kinase on lipolysis in adipose tissue: An historical and comprehensive review. Arch Physiol Biochem (2022) 128:7–23. doi: 10.1080/13813455.2019.1661495
29. Klug YA, Deme JC, Corey RA, Renne MF, Stansfeld PJ, Lea SM, et al. Mechanism of lipid droplet formation by the yeast Sei1/Ldb16 seipin complex. Nat Commun (2021) 12:5892. doi: 10.1038/s41467-021-26162-6
30. Chen FJ, Yin Y, Chua BT, Li P. CIDE family proteins control lipid homeostasis and the development of metabolic diseases. Traffic (2020) 21:94–105. doi: 10.1111/tra.12717
31. Sztalryd C, Brasaemle DL. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim Biophys Acta Mol Cell Biol Lipids (2017) 1862:1221–32. doi: 10.1016/j.bbalip.2017.07.009
32. Yu L, Li Y, Grisé A, Wang H. CGI-58: Versatile regulator of intracellular lipid droplet homeostasis. Adv Exp Med Biol (2020) 1276:197–222. doi: 10.1007/978-981-15-6082-8_13
33. Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, et al. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest (2008) 118:2808–21. doi: 10.1172/JCI34090
34. Corbin JD, Reimann EM, Walsh DA, Krebs EG. Activation of adipose tissue lipase by skeletal muscle cyclic adenosine 3’,5’- monophosphate-stimulated protein kinase. J Biol Chem (1970) 245:4849–51. doi: 10.1016/S0021-9258(18)62871-6
35. Langin D. Control of fatty acid and glycerol release in adipose tissue lipolysis. C R Biol (2006) 329:598–607. doi: 10.1016/j.crvi.2005.10.008
36. Ahmadian M, Wang Y, Sul HS. Lipolysis in adipocytes. Int J Biochem Cell Biol (2010) 42:555–9. doi: 10.1016/j.biocel.2009.12.009
37. Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab (2008) 19:130–7. doi: 10.1016/j.tem.2007.11.006
38. Lien CC, Yin WH, Yang DM, Chen LK, Chen CW, Liu SY, et al. Endothelin-1 induces lipolysis through activation of the GC/cGMP/Ca(2+)/ERK/CaMKIII pathway in 3T3-L1 adipocytes. Biochim Biophys Acta Mol Cell Biol Lipids (2022) 1867:159071. doi: 10.1016/j.bbalip.2021.159071
39. Chin CH, Tsai Fc Fau - Chen S-P, Chen Sp Fau - Wang K-C, Wang Kc Fau - Chang C-C, Chang Cc Fau - Pai M-H, Pai Mh Fau - Fong T-H, et al. YC-1, a potent antithrombotic agent, induces lipolysis through the PKA pathway in rat visceral fat cells. Eur J Pharmacol (2012) 689:1–7. doi: 10.1016/j.ejphar.2012.05.013
40. Lien CC, Au Lc Fau - Tsai Y-L, Tsai Yl Fau - Ho L-T, Ho Lt Fau - Juan C-C, Juan CC. Short-term regulation of tumor necrosis factor-alpha-induced lipolysis in 3T3-L1 adipocytes is mediated through the inducible nitric oxide synthase/nitric oxide-dependent pathway. Endocrinology (2009) 150:4892–900. doi: 10.1210/en.2009-0403
41. Rozo AV, Vijayvargia R, Weiss HR, Ruan H. Silencing Jnk1 and Jnk2 accelerates basal lipolysis and promotes fatty acid re-esterification in mouse adipocytes. Diabetologia (2008) 51:1493–504. doi: 10.1007/s00125-008-1036-6
42. Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes (2002) 51:2929–35. doi: 10.2337/diabetes.51.10.2929
43. Wang Q, Liu S, Zhai A, Zhang B, Tian G. AMPK-mediated regulation of lipid metabolism by phosphorylation. Biol Pharm Bull (2018) 41:985–93. doi: 10.1248/bpb.b17-00724
44. Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab (2011) 13:739–48. doi: 10.1016/j.cmet.2011.05.002
45. Anthony NM, Gaidhu MP, Ceddia RB. Regulation of visceral and subcutaneous adipocyte lipolysis by acute AICAR-induced AMPK activation. Obesity (2009) 17:1312–7. doi: 10.1038/oby.2008.645
46. Kaushik S, Cuervo AM. AMPK-dependent phosphorylation of lipid droplet protein PLIN2 triggers its degradation by CMA. Autophagy (2016) 12:432–8. doi: 10.1080/15548627.2015.1124226
47. Chakrabarti P, English T Fau - Karki S, Karki S Fau - Qiang L, Qiang L Fau - Tao R, Tao R Fau - Kim J, Kim J Fau - Luo Z, et al. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J Lipid Res (2011) 52:1693–701. doi: 10.1194/jlr.M014647
48. Zhang T, Liu J, Tong Q, Lin L. SIRT3 acts as a positive autophagy regulator to promote lipid mobilization in adipocytes via activating AMPK. Int J Mol Sci (2020) 21:372. doi: 10.3390/ijms21020372
49. Gorin E, Tai LR, Honeyman TW, Goodman HM. Evidence for a role of protein kinase c in the stimulation of lipolysis by growth hormone and isoproterenol. Endocrinology (1990) 126:2973–82. doi: 10.1210/endo-126-6-2973
50. Turovsky EA-O, Varlamova EA-O, Turovskaya MV. Activation of Cx43 hemichannels induces the generation of Ca(2+) oscillations in white adipocytes and stimulates lipolysis. Int J Mol Sci (2021) 22:8095. doi: 10.3390/ijms22158095
51. Ghoshal S, Tyagi R, Zhu Q, Chakraborty A. Inositol hexakisphosphate kinase-1 interacts with perilipin1 to modulate lipolysis. Int J Biochem Cell Biol (2016) 78:149–55. doi: 10.1016/j.biocel.2016.06.018
52. Sánchez JC, Valencia-Vásquez A, García AM. Role of TRPV4 channel in human white adipocytes metabolic activity. Endocrinol Metab (Seoul) (2021) 36:997–1006. doi: 10.3803/EnM.2021.1167
53. Bogdanovic E, Kraus N, Patsouris D, Patsouris D, Diao L, Diao L, et al. Endoplasmic reticulum stress in adipose tissue augments lipolysis. J Cell Mol Med (2015) 19:82–91. doi: 10.1111/jcmm.12384
54. Li J, Bai L, Wei F, Zhao J, Wang D, Xiao Y, et al. Therapeutic mechanisms of herbal medicines against insulin resistance: A review. Front Pharmacol (2019) 10:661. doi: 10.3389/fphar.2019.00661
55. Bahmani M, Eftekhari Z, Saki K, Fazeli-Moghadam E, Jelodari M, Rafieian-Kopaei M. Obesity phytotherapy: Review of native herbs used in traditional medicine for obesity. J Evidence-Based Complement Altern Med (2016) 21:228–34. doi: 10.1177/2156587215599105
56. Carrasco-Pozo C, Cires MJ, Gotteland M. Quercetin and epigallocatechin gallate in the prevention and treatment of obesity: From molecular to clinical studies. J Med Food (2019) 22:753–70. doi: 10.1089/jmf.2018.0193
57. Choi M, Mukherjee S, Yun JW. Trigonelline induces browning in 3T3-L1 white adipocytes. Phytother Res (2021) 35:1113–24. doi: 10.1002/ptr.6892
58. Wang X, Xu M, Peng Y, Naren Q, Xu Y, Wang X, et al. Triptolide enhances lipolysis of adipocytes by enhancing ATGL transcription via upregulation of p53. Phytother Res (2020) 34:3298–310. doi: 10.1002/ptr.6779
59. Pan H, Gao Y, Tu Y. Mechanisms of body weight reduction by black tea polyphenols. Molecules (2016) 21:1659. doi: 10.3390/molecules21121659
60. Li F, Gao C, Yan P, Zhang M, Wang Y, Hu Y, et al. EGCG reduces obesity and white adipose tissue gain partly through AMPK activation in mice. Front Pharmacol (2018) 9:1366. doi: 10.3389/fphar.2018.01366
61. Lee MS, Kim CT, Kim Y. Green tea (-)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. Ann Nutr Metab (2009) 54:151–7. doi: 10.1159/000214834
62. Moon HS, Chung CS, Lee HG, Kim TG, Choi YJ, Cho CS. Inhibitory effect of (-)-epigallocatechin-3-gallate on lipid accumulation of 3T3-L1 cells. Obes (Silver Spring) (2007) 15:2571–82. doi: 10.1038/oby.2007.309
63. Lee MS, Kim CT, Kim IH, Kim Y. Inhibitory effects of green tea catechin on the lipid accumulation in 3T3-L1 adipocytes. Phytother Res (2009) 23:1088–91. doi: 10.1002/ptr.2737
64. Ogasawara J, Kitadate K, Nishioka H, Fujii H, Sakurai T, Kizaki T, et al. Comparison of the effect of oligonol, a new lychee fruit-derived low molecular form of polyphenol, and epigallocatechin-3-gallate on lipolysis in rat primary adipocytes. Phytother Res (2011) 25:467–71. doi: 10.1002/ptr.3296
65. Kim SN, Kwon HJ, Akindehin S, Jeong HW, Lee YH. Effects of epigallocatechin-3-Gallate on autophagic lipolysis in adipocytes. Nutrients (2017) 9:680. doi: 10.3390/nu9070680
66. Chatree S, Sitticharoon C, Maikaew P, Pongwattanapakin K, Keadkraichaiwat I, Churintaraphan M, et al. Epigallocatechin gallate decreases plasma triglyceride, blood pressure, and serum kisspeptin in obese human subjects. Exp Biol Med (Maywood) (2021) 246:163–76. doi: 10.1177/1535370220962708
67. Torres-Villarreal D, Camacho A, Castro H, Ortiz-Lopez R, de la Garza AL. Anti-obesity effects of kaempferol by inhibiting adipogenesis and increasing lipolysis in 3T3-L1 cells. J Physiol Biochem (2019) 75:83–8. doi: 10.1007/s13105-018-0659-4
68. Sun YS, Qu W. Dietary apigenin promotes lipid catabolism, thermogenesis, and browning in adipose tissues of HFD-fed mice. Food Chem Toxicol (2019) 133:110780. doi: 10.1016/j.fct.2019.110780
69. Szkudelska K, Nogowski L, Szkudelski T. Genistein affects lipogenesis and lipolysis in isolated rat adipocytes. J Steroid Biochem Mol Biol (2000) 75:265–71. doi: 10.1016/s0960-0760(00)00172-2
70. Lee MR, Kim JE, Choi JY, Park JJ, Kim HR, Song BR, et al. Morusin functions as a lipogenesis inhibitor as well as a lipolysis stimulator in differentiated 3T3-L1 and primary adipocytes. Molecules (2018) 23: 2004. doi: 10.3390/molecules23082004
71. Imran KM, Yoon D, Lee TJ, Kim YS. Medicarpin induces lipolysis via activation of protein kinase a in brown adipocytes. BMB Rep (2018) 51:249–54. doi: 10.5483/bmbrep.2018.51.5.228
72. Wang Q, Wang ST, Yang X, You PP, Zhang W. Myricetin suppresses differentiation of 3 T3-L1 preadipocytes and enhances lipolysis in adipocytes. Nutr Res (2015) 35:317–27. doi: 10.1016/j.nutres.2014.12.009
73. Samuels JS, Shashidharamurthy R, Rayalam S. Novel anti-obesity effects of beer hops compound xanthohumol: Role of AMPK signaling pathway. Nutr Metab (Lond) (2018) 15:42. doi: 10.1186/s12986-018-0277-8
74. Mougios V, Ring S, Petridou A, Nikolaidis MG. Duration of coffee- and exercise-induced changes in the fatty acid profile of human serum. J Appl Physiol (1985) (2003) 94:476–84. doi: 10.1152/japplphysiol.00624.2002
75. Diepvens K, Westerterp KR, Westerterp-Plantenga MS. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am J Physiol Regul Integr Comp Physiol (2007) 292:R77–85. doi: 10.1152/ajpregu.00832.2005
76. Panchal SK, Wong WY, Kauter K, Ward LC, Brown L. Caffeine attenuates metabolic syndrome in diet-induced obese rats. Nutrition (2012) 28:1055–62. doi: 10.1016/j.nut.2012.02.013
77. Han LK, Takaku T, Li J, Kimura Y, Okuda H. Anti-obesity action of oolong tea. Int J Obes Relat Metab Disord (1999) 23:98–105. doi: 10.1038/sj.ijo.0800766
78. De Matteis R, Arch JR, Petroni ML, Ferrari D, Cinti S, Stock MJ. Immunohistochemical identification of the beta(3)-adrenoceptor in intact human adipocytes and ventricular myocardium: Effect of obesity and treatment with ephedrine and caffeine. Int J Obes Relat Metab Disord (2002) 26:1442–50. doi: 10.1038/sj.ijo.0802148
79. Ohyama K, Nogusa Y, Suzuki K, Shinoda K, Kajimura S, Bannai M. A combination of exercise and capsinoid supplementation additively suppresses diet-induced obesity by increasing energy expenditure in mice. Am J Physiol Endocrinol Metab (2015) 308:E315–23. doi: 10.1152/ajpendo.00354.2014
80. Perova IB, Eller KI, Musatov AV, Tymolskaya EV. Synephrine in dietary supplements and specialized foodstuffs: Biological activity, safety and methods of analysis]. Vopr Pitan (2021) 90:101–13. doi: 10.33029/0042-8833-2021-90-6-101-113
81. Jiang D, Wang D, Zhuang X, Wang Z, Ni Y, Chen S, et al. Berberine increases adipose triglyceride lipase in 3T3-L1 adipocytes through the AMPK pathway. Lipids Health Dis (2016) 15:214. doi: 10.1186/s12944-016-0383-4
82. Yang Y, Lu R, Gao F, Zhang J, Liu F. Berberine induces lipolysis in porcine adipocytes by activating the AMPactivated protein kinase pathway. Mol Med Rep (2020) 21:2603–14. doi: 10.3892/mmr.2020.11070
83. Zhou L, Wang X, Yang Y, Wu L, Li F, Zhang R, et al. Berberine attenuates cAMP-induced lipolysis via reducing the inhibition of phosphodiesterase in 3T3-L1 adipocytes. Biochim Biophys Acta (2011) 1812:527–35. doi: 10.1016/j.bbadis.2010.10.001
84. Xu H, Wu B, Wang X, Ma F, Li Y, An Y, et al. Cordycepin regulates body weight by inhibiting lipid droplet formation, promoting lipolysis and recruiting beige adipocytes. J Pharm Pharmacol (2019) 71:1429–39. doi: 10.1111/jphp.13127
85. Lee MS, Kim CT, Kim IH, Kim Y. Effects of capsaicin on lipid catabolism in 3T3-L1 adipocytes. Phytother Res (2011) 25:935–9. doi: 10.1002/ptr.3339
86. Chen J, Li L, Li Y, Liang X, Sun Q, Yu H, et al. Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx. Cardiovasc Diabetol (2015) 14:22. doi: 10.1186/s12933-015-0183-6
87. Kobayashi-Hattori K, Mogi A, Matsumoto Y, Takita T. Effect of caffeine on the body fat and lipid metabolism of rats fed on a high-fat diet. Biosci Biotechnol Biochem (2005) 69:2219–23. doi: 10.1271/bbb.69.2219
88. Moon MH, Jeong JK, Lee YJ, Seol JW, Ahn DC, Kim IS, et al. 18beta-glycyrrhetinic acid inhibits adipogenic differentiation and stimulates lipolysis. Biochem Biophys Res Commun (2012) 420:805–10. doi: 10.1016/j.bbrc.2012.03.078
89. Li Y, Kang Z, Li S, Kong T, Liu X, Sun C. Ursolic acid stimulates lipolysis in primary-cultured rat adipocytes. Mol Nutr Food Res (2010) 54:1609–17. doi: 10.1002/mnfr.200900564
90. Liu JJ, Toy WC, Liu S, Cheng A, Lim BK, Subramaniam T, et al. Acetyl-keto-beta-boswellic acid induces lipolysis in mature adipocytes. Biochem Biophys Res Commun (2013) 431:192–6. doi: 10.1016/j.bbrc.2012.12.136
91. Lou HX, Fu WC, Chen JX, Li TT, Jiang YY, Liu CH, et al. Alisol a 24-acetate stimulates lipolysis in 3 T3-L1 adipocytes. BMC Complement Med Ther (2021) 21:128. doi: 10.1186/s12906-021-03296-0
92. Luo D, Fan N, Zhang X, Ngo FY, Zhao J, Zhao W, et al. Covalent inhibition of endoplasmic reticulum chaperone GRP78 disconnects the transduction of ER stress signals to inflammation and lipid accumulation in diet-induced obese mice. Elife (2022) 11:e72182. doi: 10.7554/eLife.72182
93. Kim J, Lee YS, Kim CS, Kim JS. Betulinic acid has an inhibitory effect on pancreatic lipase and induces adipocyte lipolysis. Phytother Res (2012) 26:1103–6. doi: 10.1002/ptr.3672
94. Sun B, Hayashi M, Kudo M, Wu L, Qin L, Gao M, et al. Madecassoside inhibits body weight gain via modulating SIRT1-AMPK signaling pathway and activating genes related to thermogenesis. Front Endocrinol (Lausanne) (2021) 12:627950. doi: 10.3389/fendo.2021.627950
95. Jung DY, Suh N, Jung MH. Tanshinone 1 prevents high fat diet-induced obesity through activation of brown adipocytes and induction of browning in white adipocytes. Life Sci (2022) 298:120488. doi: 10.1016/j.lfs.2022.120488
96. Gu M, Luo L, Fang K. Crocin inhibits obesity via AMPK-dependent inhibition of adipocyte differentiation and promotion of lipolysis. Biosci Trends (2018) 12:587–94. doi: 10.5582/bst.2018.01240
97. Yang JY, Della-Fera MA, Baile CA. Guggulsterone inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 cells. Obes (Silver Spring) (2008) 16:16–22. doi: 10.1038/oby.2007.24
98. Bu S, Yuan CY, Xue Q, Chen Y, Cao F. Bilobalide suppresses adipogenesis in 3T3-L1 adipocytes via the AMPK signaling pathway. Molecules (2019) 24:3503. doi: 10.3390/molecules24193503
99. Bae SJ, Kim JE, Choi YJ, Lee SJ, Gong JE, Choi YW, et al. Novel function of alpha-cubebenoate derived from schisandra chinensis as lipogenesis inhibitor, lipolysis stimulator and inflammasome suppressor. Molecules (2020) 25:4495. doi: 10.3390/molecules25214995
100. Lee SJ, Kim JE, Choi YJ, Gong JE, Jin YJ, Lee DW, et al. Anti-obesity effect of α-cubebenol isolated from schisandra chinensis in 3T3-L1 adipocytes. Biomolecules (2021) 11:1650. doi: 10.3390/biom11111650
101. Yoshikawa M, Hosokawa M, Miyashita K, Fujita T, Nishino H, Hashimoto T. Fucoxanthinol attenuates oxidative stress-induced atrophy and loss in myotubes and reduces the triacylglycerol content in mature adipocytes. Mol Biol Rep (2020) 47:2703–11. doi: 10.1007/s11033-020-05369-8
102. Jeong HY, Yun HJ, Kim BW, Lee EW, Kwon HJ. Widdrol-induced lipolysis is mediated by PKC and MEK/ERK in 3T3-L1 adipocytes. Mol Cell Biochem (2015) 410:247–54. doi: 10.1007/s11010-015-2558-0
103. Liou CJ, Lai XY, Chen YL, Wang CL, Wei CH, Huang WC. Ginkgolide c suppresses adipogenesis in 3T3-L1 adipocytes via the AMPK signaling pathway. Evid Based Complement Alternat Med (2015) 2015:298635. doi: 10.1155/2015/298635
104. Kim SO, Sakchaisri K, Asami Y, Ryoo IJ, Choo SJ, Yoo ID, et al. Illudins C2 and C3 stimulate lipolysis in 3T3-L1 adipocytes and suppress adipogenesis in 3T3-L1 preadipocytes. J Nat Prod (2014) 77:744–50. doi: 10.1021/np400520a
105. Choi SK, Park S, Jang S, Cho HH, Lee S, You S, et al. Cascade regulation of PPARgamma(2) and C/EBPalpha signaling pathways by celastrol impairs adipocyte differentiation and stimulates lipolysis in 3T3-L1 adipocytes. Metabolism (2016) 65:646–54. doi: 10.1016/j.metabol.2016.01.009
106. Gong L, Guo S, Zou Z. Resveratrol ameliorates metabolic disorders and insulin resistance in high-fat diet-fed mice. Life Sci (2020) 242:117212. doi: 10.1016/j.lfs.2019.117212
107. Mehanna ET, Barakat BM, ElSayed MH, Tawfik MK. An optimized dose of raspberry ketones controls hyperlipidemia and insulin resistance in male obese rats: Effect on adipose tissue expression of adipocytokines and aquaporin 7. Eur J Pharmacol (2018) 832:81–9. doi: 10.1016/j.ejphar.2018.05.028
108. Khare P, Jagtap S, Jain Y, Baboota RK, Mangal P, Boparai RK, et al. Cinnamaldehyde supplementation prevents fasting-induced hyperphagia, lipid accumulation, and inflammation in high-fat diet-fed mice. Biofactors (2016) 42:201–11. doi: 10.1002/biof.1265
109. Fernandez-Galilea M, Perez-Matute P, Prieto-Hontoria PL, Martinez JA, Moreno-Aliaga MJ. Effects of lipoic acid on lipolysis in 3T3-L1 adipocytes. J Lipid Res (2012) 53:2296–306. doi: 10.1194/jlr.M027086
110. John CM, Arockiasamy S. Syringic acid (4-hydroxy-3,5-dimethoxybenzoic acid) inhibits adipogenesis and promotes lipolysis in 3T3-L1 adipocytes. Nat Prod Res (2020) 34:3432–36. doi: 10.1080/14786419.2019.1573820
111. Sim MO, Lee HJ, Jeong DE, Jang JH, Jung HK, Cho HW. 6’-o-acetyl mangiferin from iris rossii baker inhibits lipid accumulation partly via AMPK activation in adipogenesis. Chem Biol Interact (2019) 311:108755. doi: 10.1016/j.cbi.2019.108755
112. Kuppusamy P, Ilavenil S, Hwang IH, Kim D, Choi KA-O. Ferulic acid stimulates adipocyte-specific secretory proteins to regulate adipose homeostasis in 3T3-L1 adipocytes. Molecules (2021) 26:1984. doi: 10.3390/molecules26071984
113. Parray HA, Lone J, Park JP, Choi JW, Yun JW. Magnolol promotes thermogenesis and attenuates oxidative stress in 3T3-L1 adipocytes. Nutrition (2018) 50:82–90. doi: 10.1016/j.nut.2018.01.017
114. Gomez-Zorita S, Treguer K, Mercader J, Carpene C. Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. J Physiol Biochem (2013) 69:585–93. doi: 10.1007/s13105-012-0229-0
115. Lasa A, Schweiger M, Kotzbeck P, Churruca I, Simon E, Zechner R, et al. Resveratrol regulates lipolysis via adipose triglyceride lipase. J Nutr Biochem (2012) 23:379–84. doi: 10.1016/j.jnutbio.2010.12.014
116. Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab (2011) 14:612–22. doi: 10.1016/j.cmet.2011.10.002
117. Morimoto C, Satoh Y, Hara M, Inoue S, Tsujita T, Okuda H. Anti-obese action of raspberry ketone. Life Sci (2005) 77:194–204. doi: 10.1016/j.lfs.2004.12.029
118. Park KS. Raspberry ketone increases both lipolysis and fatty acid oxidation in 3T3-L1 adipocytes. Planta Med (2010) 76:1654–8. doi: 10.1055/s-0030-1249860
119. Huang SH, Shen WJ, Yeo HL, Wang SM. Signaling pathway of magnolol-stimulated lipolysis in sterol ester-loaded 3T3-L1 preadipocyes. J Cell Biochem (2004) 91:1021–9. doi: 10.1002/jcb.10788
120. Park KS. Raspberry ketone, a naturally occurring phenolic compound, inhibits adipogenic and lipogenic gene expression in 3T3-L1 adipocytes. Pharm Biol (2015) 53:870–5. doi: 10.3109/13880209.2014.946059
121. Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. BioMed Pharmacother (2021) 137:111315. doi: 10.1016/j.biopha.2021.111315
122. Duwaerts CC, Maher JJ. Macronutrients and the adipose-liver axis in obesity and fatty liver. Cell Mol Gastroenterol Hepatol (2019) 7:749–61. doi: 10.1016/j.jcmgh.2019.02.001
123. Roden M, Stingl H, Chandramouli V, Schumann WC, Hofer A, Landau BR, et al. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes (2000) 49:701–7. doi: 10.2337/diabetes.49.5.701
124. He J, Xu C, Kuang J, Liu Q, Jiang H, Mo L, et al. Thiazolidinediones attenuate lipolysis and ameliorate dexamethasone-induced insulin resistance. Metabolism (2015) 64:826–36. doi: 10.1016/j.metabol.2015.02.005
125. Wang L, Zhang B, Huang F, Liu B, Xie Y. Curcumin inhibits lipolysis via suppression of ER stress in adipose tissue and prevents hepatic insulin resistance. J Lipid Res (2016) 57:1243–55. doi: 10.1194/jlr.M067397
126. Du Q, Zhang S, Li A, Mohammad IS, Liu B, Li Y. Astragaloside IV inhibits adipose lipolysis and reduces hepatic glucose production via akt dependent PDE3B expression in HFD-fed mice. Front Physiol (2018) 9:15. doi: 10.3389/fphys.2018.00015
127. Li LZ, Zhang T, Yang L, Zhang L, Wang L, Liu B, et al. Inhibition of lipolysis by ilexgenin a via AMPK activation contributes to the prevention of hepatic insulin resistance. Eur J Pharmacol (2017) 813:84–93. doi: 10.1016/j.ejphar.2017.07.038
128. Xiao N, Yang LL, Yang YL, Liu LW, Li J, Liu B, et al. Ginsenoside Rg5 inhibits succinate-associated lipolysis in adipose tissue and prevents muscle insulin resistance. Front Pharmacol (2017) 8:43. doi: 10.3389/fphar.2017.00043
129. Stagakis I, Bertsias G, Karvounaris S, Kavousanaki M, Virla D, Raptopoulou A, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther (2012) 14:R141. doi: 10.1186/ar3874
130. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science (1993) 259:87–91. doi: 10.1126/science.7678183
131. Jiang B, Yang Y, Jin H, Shang W, Zhou L, Qian L, et al. Astragaloside IV attenuates lipolysis and improves insulin resistance induced by TNFalpha in 3T3-L1 adipocytes. Phytother Res (2008) 22:1434–9. doi: 10.1002/ptr.2434
132. Xie XY, Kong PR, WU JF, Li Y, Li YX. Curcumin attenuates lipolysis stimulated by tumor necrosis factor-α or isoproterenol in 3T3-L1 adipocytes. Phytomedicine (2012) 20:3–8. doi: 10.1016/j.phymed.2012.09.003
133. Zhang X, Zhang R, Lv P, Yang J, Deng Y, Xu J, et al. Emodin up-regulates glucose metabolism, decreases lipolysis, and attenuates inflammation. Vitro J Diabetes (2015) 7:360–8. doi: 10.1111/1753-0407.12190
134. Lorente-Cebrián S, Bustos M, Marti A, Fernández-Galilea M, Martinez JA, Moreno-Aliaga JM. Eicosapentaenoic acid inhibits tumour necrosis factor-α-induced lipolysis in murine cultured adipocytes. J Nutr Biochem (2012) 23:218–27. doi: 10.1016/j.jnutbio.2010.11.018
135. Kong P, Zhang L, Guo Y, Lu Y, Lin D. Phillyrin, a natural lignan, attenuates tumor necrosis factor α-mediated insulin resistance and lipolytic acceleration in 3T3-L1 adipocytes. Planta Med (2014) 80:880–6. doi: 10.1055/s-0034-1368614
136. Rui Y, Tong L, Cheng J, Wang G, Qin L, Wan Z. Rosmarinic acid suppresses adipogenesis, lipolysis in 3T3-L1 adipocytes, lipopolysaccharide-stimulated tumor necrosis factor-alpha secretion in macrophages, and inflammatory mediators in 3T3-L1 adipocytes. Food Nutr Res (2017) 61:1330096. doi: 10.1080/16546628.2017.1330096
137. Colson C, Batrow PL, Gautier N, Rochet N, Ailhaud G, Peiretti F, et al. The rosmarinus bioactive compound carnosic acid is a novel PPAR antagonist that inhibits the browning of white adipocytes. Cells (2020) 9:2433. doi: 10.3390/cells9112433
138. Guo H, Guo J, Jiang X, Li Z, Ling W. Cyanidin-3-O-beta-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food Chem Toxicol (2012) 50:3040–7. doi: 10.1016/j.fct.2012.06.015
139. Nehrenheim K, Meyer I, Brenden H, Vielhaber G, Krutmann J, Grether-Beck S. Dihydrodehydrodiisoeugenol enhances adipocyte differentiation and decreases lipolysis in murine and human cells. Exp Dermatol (2013) 22:638–43. doi: 10.1111/exd.12218
140. Kwon JY, Kershaw J, Chen CY, Komanetsky SM, Zhu Y, Guo X, et al. Piceatannol antagonizes lipolysis by promoting autophagy-lysosome-dependent degradation of lipolytic protein clusters in adipocytes. J Nutr Biochem (2022) 105:108998. doi: 10.1016/j.jnutbio.2022.108998
141. Wang B, Yang X, Zhao M, Su Z, Hu Z, Zhang C, et al. Celastrol prevents high-fat diet-induced obesity by promoting white adipose tissue browning. Clin Transl Med (2021) 11:e641. doi: 10.1002/ctm2.641
142. Su T, Huang C, Yang C, Jiang T, Su J, Chen M, et al. Apigenin inhibits STAT3/CD36 signaling axis and reduces visceral obesity. Pharmacol Res (2020) 152:104586. doi: 10.1016/j.phrs.2019.104586
Keywords: adipose tissue, insulin resistance, lipolysis, natural product, obesity
Citation: Yang X-D, Ge X-C, Jiang S-Y and Yang Y-Y (2022) Potential lipolytic regulators derived from natural products as effective approaches to treat obesity. Front. Endocrinol. 13:1000739. doi: 10.3389/fendo.2022.1000739
Received: 22 July 2022; Accepted: 23 August 2022;
Published: 13 September 2022.
Edited by:
Bruno Melo Carvalho, Universidade de Pernambuco, BrazilReviewed by:
Monica Colitti, University of Udine, ItalyAlisson Oliveira, Federal University of Pernambuco, Brazil
Copyright © 2022 Yang, Ge, Jiang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Yu Yang, eW9uZ3l1eWFuZ0Bjc3UuZWR1LmNu
 Xi-Ding Yang
Xi-Ding Yang Xing-Cheng Ge3
Xing-Cheng Ge3