
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Endocrinol. , 15 December 2021
Sec. Cancer Endocrinology
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.793718
This article is part of the Research Topic Rare Endocrine Tumors View all 27 articles
 Yongchao Yu
Yongchao Yu Yue Wang
Yue Wang Qingcheng Wu
Qingcheng Wu Xuzi Zhao
Xuzi Zhao Deshun Liu
Deshun Liu Yongfu Zhao
Yongfu Zhao Yuguo Li
Yuguo Li Guangzhi Wang
Guangzhi Wang Jingchao Xu
Jingchao Xu Junzhu Chen
Junzhu Chen Ning Zhang*
Ning Zhang* Xiaofeng Tian*
Xiaofeng Tian*Background: Parathyroid carcinoma (PC) is a rare malignancy, the incidence of which is less than 1/1 million per year. Sarcomatoid parathyroid carcinoma (SaPC) is an extremely peculiar subtype; only three cases have been reported internationally. It consists of both malignant epithelial components and sarcomatoid components (mesenchymal origin) simultaneously. This “confusing” cancer exhibits higher invasiveness, and traditional surgery does not appear to achieve the expectation, which differs significantly from that of general PC.
Objective: To characterize the clinicopathologic features of SaPC and explore similarities and differences between SaPC and general PC.
Materials and Methods: We collected clinical data of SaPC cases from our center and literature. The SaPC case in our center was presented. To better understand the characteristics of SaPC, we also reviewed clinical information in general PC cases from our center and literature within the last 5 years, and a systematic review was performed for further comparison.
Results: A 60-year-old woman was admitted for a neck mass and hoarseness. After the surgery, she was confirmed as SaPC and ultimately developed local recurrence at 3 months. Together with the reported cases from literature, four cases of SaPC (three cases from literature) and 203 cases of general PC (200 cases from literature) were reviewed. Both tumors showed obvious abnormalities in parathormone (PTH) level and gland size. Compared to general PC, SaPC has a later age of onset (60.50 ± 7.42 vs. 51.50 ± 8.29), relatively low levels of PTH (110.28 ± 59.32 vs. 1,156.07 ± 858.18), and a larger tumor size (6.00 ± 1.63 vs. 3.14 ± 0.70). For SaPC, all four cases were initially misdiagnosed as thyroid tumors (4/4). Spindle cell areas or transitional zones were common pathological features in SaPC cases (3/4).
Conclusion: SaPC is a very rare pathologic subtype of PC and appears to be much more easily misdiagnosed as a thyroid tumor. Spindle cell areas or transitional zones are highly possible to be pathological features in its sarcomatoid components. Despite many similarities, there are some differences between SaPC and general PC—SaPC does not show the obvious endocrine feature but stronger aggressiveness. Surgical treatment of SaPC does relieve life-threatening symptoms and improve quality of life even with recurrence in the short term.
Parathyroid carcinoma (PC) is a rare malignant neoplasm. The incidence is less than 1 in 1 million per year (1). At present, general PC is believed to be a slowly progressive disease characterized by specific clinical features (2), and radical surgery is believed to be the only effective curative treatment for this disease (3–5). Sarcomatoid carcinoma is an exceedingly rare tumor subtype. It refers to a bipolar malignancy with both carcinoma and sarcomatoid components. In PC, this tumor subtype was initially named parathyroid carcinosarcoma or parathyroid carcinoma with sarcomatoid differentiation (6–8). We utilized the term “sarcomatoid carcinoma” referring to nomenclature of other tumors with similar features. Sarcomatoid parathyroid carcinoma (SaPC) was first reported in 2002 by Nacamuli et al. (6) and characterized by strong invasiveness and life-threatening recurrence shortly after the operation, which is quite different from general PC. For sarcomatoid carcinoma, there are several hypotheses about its formation, and epithelial–mesenchymal transformation (EMT) appears to play a major role (9, 10).
Here, we introduce similarities and differences between SaPC and general PC based on our experience with cases in our center and the literature. In addition, we provide more insights into the diagnosis and surgical strategies of SaPC.
From January 2016 to September 2021, four patients with PC were enrolled in this study, including one patient with SaPC (one female) and three patients with general PC (three females). They underwent surgery in our department and were followed up. We obtained clinicopathologic and prognostic information of the patients after approval by the Ethics Committee of the Second Hospital of Dalian Medical University.
The systematic review was conducted under the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (11). Literature that contained general PC cases was searched using terms “parathyroid carcinoma” OR “parathyroid cancer” in PubMed (MeSH) and Cochrane Library (Title Abstract Keyword) from 2016 to present. General PC cases were defined as an age of onset >18 years old, having elevated preoperative parathormone (PTH) level (>65 pg/ml), and finally diagnosed with PC (based on postoperative histopathology). Literature related to SaPC was obtained by manual searches. Only publications in English were considered. The date of the last retrieval was September 18, 2021, and literature was reviewed independently by two authors. For all enrolled cases, we reviewed age, gender, preoperative total serum calcium level, preoperative PTH, and tumor size (the greatest diameter) for further analysis. Prognostic information is also reviewed in SaPC cases. SPSS software 23.0 is used for statistical analyses, and GraphPad Prism 8.0 software is used for data plotting. Data are expressed as mean ± SD. Two-tailed independent samples t-test is used for evaluating statistical differences; p < 0.05 is considered significant (*), and p < 0.01 is considered extremely significant (**).
A 60-year-old woman was admitted with a continuously enlarged neck mass for 1 year and hoarseness for 1 week. In addition, she presented with dyspnea for 5 months. The patient had no family history of parathyroid diseases or hyperparathyroidism-jaw tumor syndrome. Physical examination showed a firm left neck mass of approximately 6.0 cm * 5.0 cm. Laboratory findings revealed elevated serum PTH (188.1 pg/ml, reference range: 15–65 pg/ml) and hypercalcemia (total serum calcium: 3.29 mmol/L, reference range: 2.1–2.6 mmol/L). Indicators related to thyroid function were within normal limits. Laryngoscopy showed left vocal cord paralysis. Ultrasonography showed that the left thyroid lobe was enlarged significantly, a hypoechoic lesion nearly occupied the whole lobe, and comparable signs were presented on the neck CT (Figure 1A). Tc-99m sestamibi scintigraphy demonstrated two-phase nuclide accumulation on the left thyroid (Figure 1B). Chest CT showed multiple micro pulmonary nodules (Figure 1C).
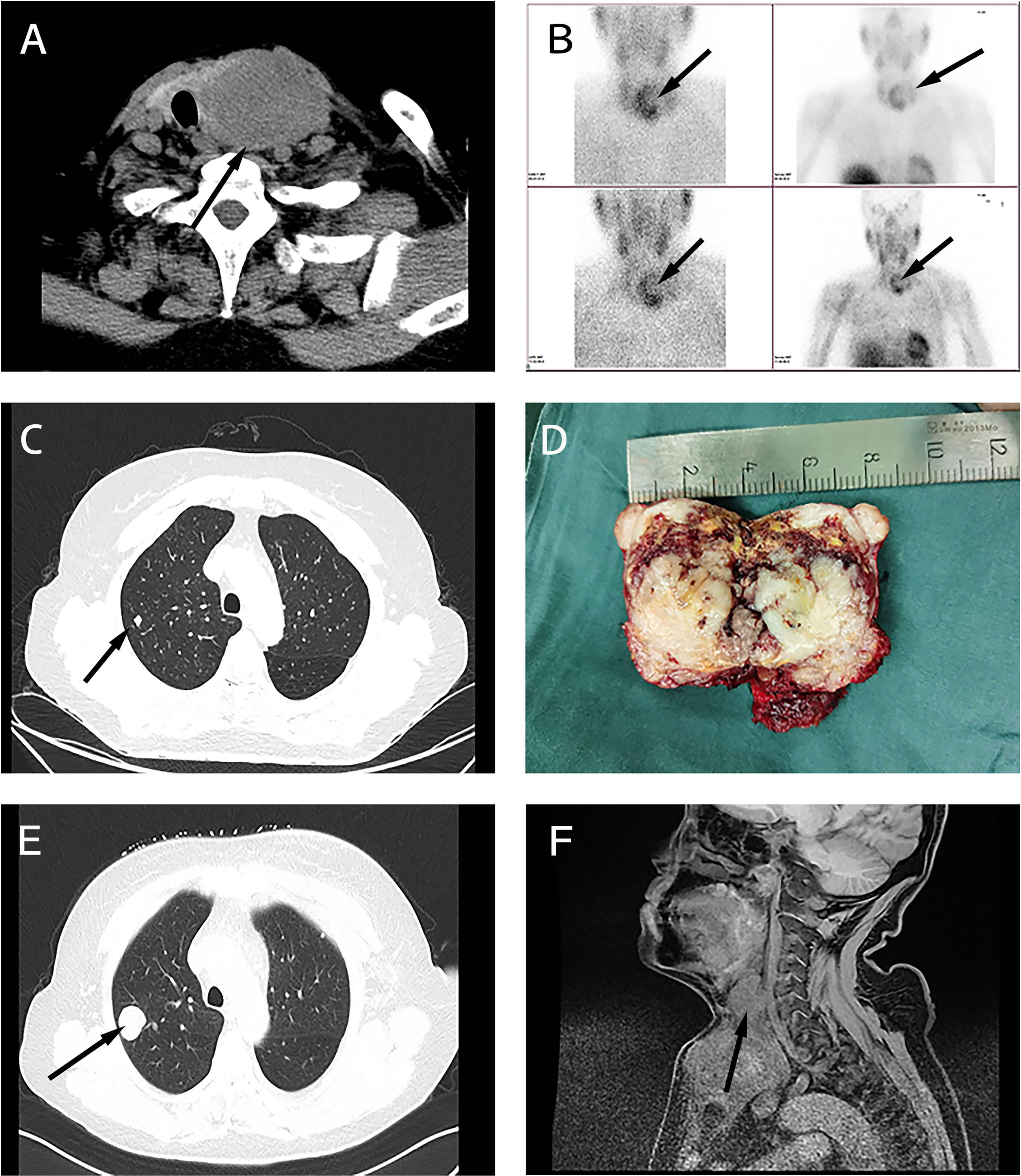
Figure 1 Clinical characteristics of the sarcomatoid parathyroid carcinoma (SaPC) case. (A) Neck CT showed the giant tumor. (B) Tc-99m sestamibi scintigraphy suggests abnormal nuclide uptake in the left thyroid. (C) Chest CT presented micro pulmonary nodule before surgery. (D) The profile of the resected tumor. (E) Chest CT shows pulmonary metastasis at the closely located position in preoperative chest CT (C). (F) Enhanced MRI showed extensive local organ and tissue invasion.
During the surgical exploration, we found that the tumor invaded the anterior cervical muscle group and left recurrent laryngeal nerve. Only the superior parathyroid was found in the left neck. En bloc resection (including part of the invaded recurrent laryngeal nerve and muscle tissue and entire thyroid) and left central lymph node dissection were performed to completely remove the affected tissue. The tumor profile showed that the thyroid was markedly infiltrated, and the normal gland was almost invisible (Figure 1D). Postoperative histopathological findings revealed that SaPC widely invaded the ipsilateral thyroid, and 1/6 of the lymph nodes showed metastasis. Immunohistochemical staining was further performed to confirm the diagnosis (Figure 2); results were presented below: (1) Carcinomatous components: Some PC cells show negative nuclear staining of parafibromin (Figure 2A); Cytokeratin (AE1/AE3) (+); Chromogranin A (+); E-Cadherin (+); PTH (+); Calcitonin (–); Thyroglobulin (-); Desmin (-); KI-67 index 10%; (2) Spindle cell components: Desmin (+; Figure 2B); Cytokeratin (AE1/AE3) (-); Chromogranin A (-); E-Cadherin (-); Calcitonin (-); KI-67 index 30%. In addition, the existence of transition zones (Figure 2C) and positive N-cad staining in both carcinomatous and sarcomatoid components (Figure 2D) was found during pathological examination.
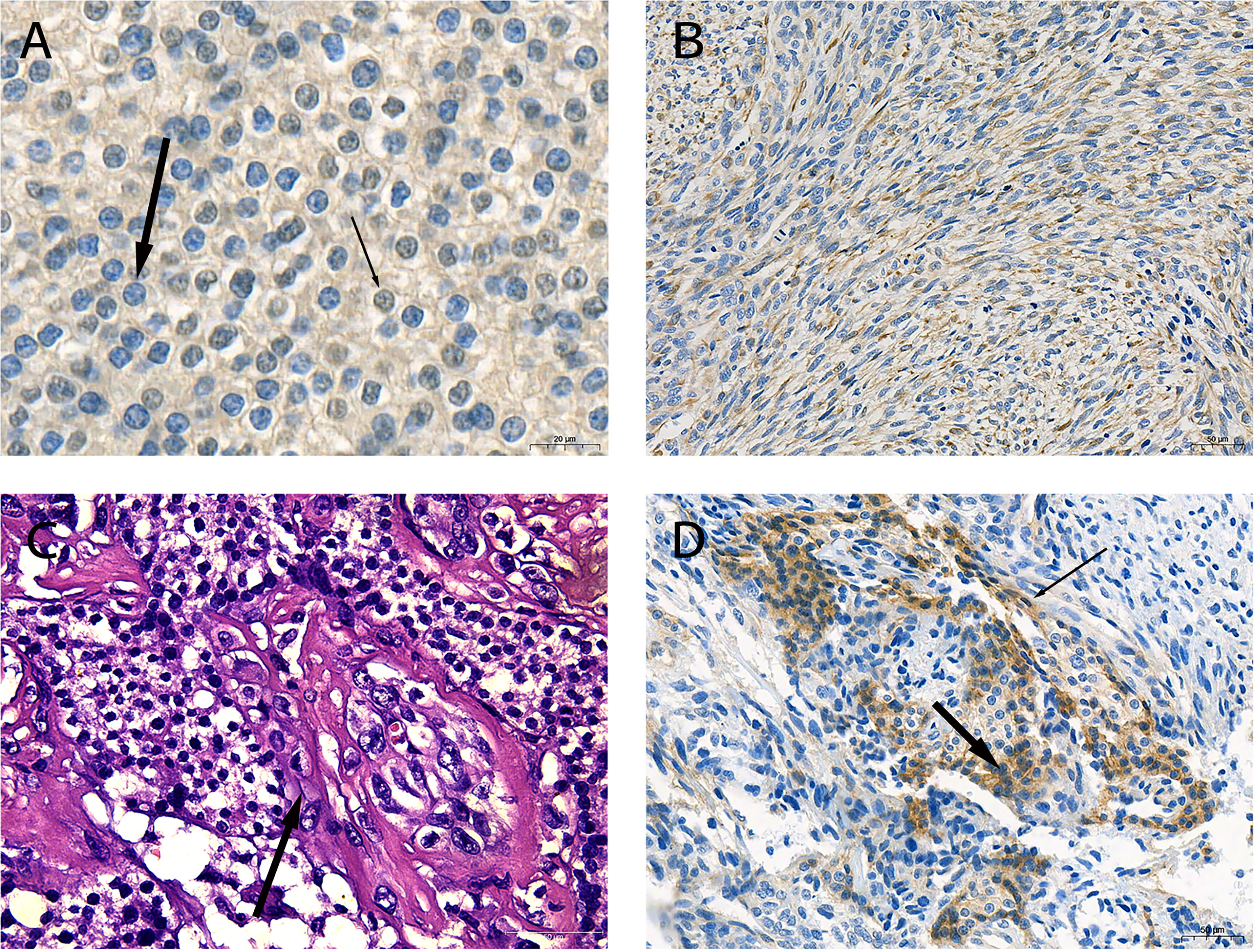
Figure 2 Pathological characteristics of the sarcomatoid parathyroid carcinoma (SaPC) case. (A) Negative staining of parafibromin in the nucleus (thick arrow) and contrast-positive staining (thin arrow) of parathyroid carcinoma (PC) cells (×630). (B) Spindle cells shuttled through carcinomatous component regions showed positive desmin staining (×200). (C) The morphology of cell changes between two component regions (arrow) (H&E, ×400). (D) N-cad was positive in carcinomatous (thick arrow) and spindle cell components (thin arrow) (×200).
The patient recovered soon postoperatively and remained hoarse. She did not experience choking when drinking water, and dyspnea significantly improved. Three months later, the patient complained of progressively aggravating dyspnea and a gradually growing neck mass. Serum calcium and PTH levels were without abnormal elevation during this time (Figure S1). Clinical examinations suggested regional relapse and multiple pulmonary metastases (Figure 1E). In contrast to the chest CT before, it seemed that pulmonary metastasis had occurred before the first surgery. Enhanced MRI showed extensive local organ and tissue invasion by the recurred tumor (Figure 1F). At last, the patient gave up the medical treatments.
Together with the reported cases, four cases of SaPC (male:female = 1:1, three cases from literature) and 203 cases of general PC (male:female = 0.85:1, 200 cases from literature) were included in this study. The process of the assessment of literature related to general PC was shown in the PRISMA flowchart (Figure 3). The details of all four SaPC cases are present in Table 1. A total of 197 PTH results, 169 total serum calcium results and 189 tumor size results of general PC cases are reviewed, which are presented in the online supplementary material [Table S1 (12–47)]. Overall, both SaPC and general PC cases showed abnormal PTH levels and gland size. Compared with general PC, SaPC presented a later age of onset (60.50 ± 7.42 vs. 51.50 ± 8.29, p = 0.032*), lower levels of PTH (110.28 ± 59.32 vs. 1,156.07 ± 858.18, p = 0.016*), and larger tumor size (6.00 ± 1.63 vs. 3.14 ± 0.70, p = 0.039*). Besides, SaPC also shows a lower serum calcium level (2.70 ± 0.42 vs. 3.41 ± 0.91), but it is not significant (p = 0.120) due to small samples. For SaPC cases (Table 1), all four cases were initially suspected as thyroid tumors (4/4), three of four patients showed hoarseness (3/4), and two of four patients showed a neck mass (2/4). Spindle cell areas or transitional zones are common pathological features in SaPC cases (3/4).
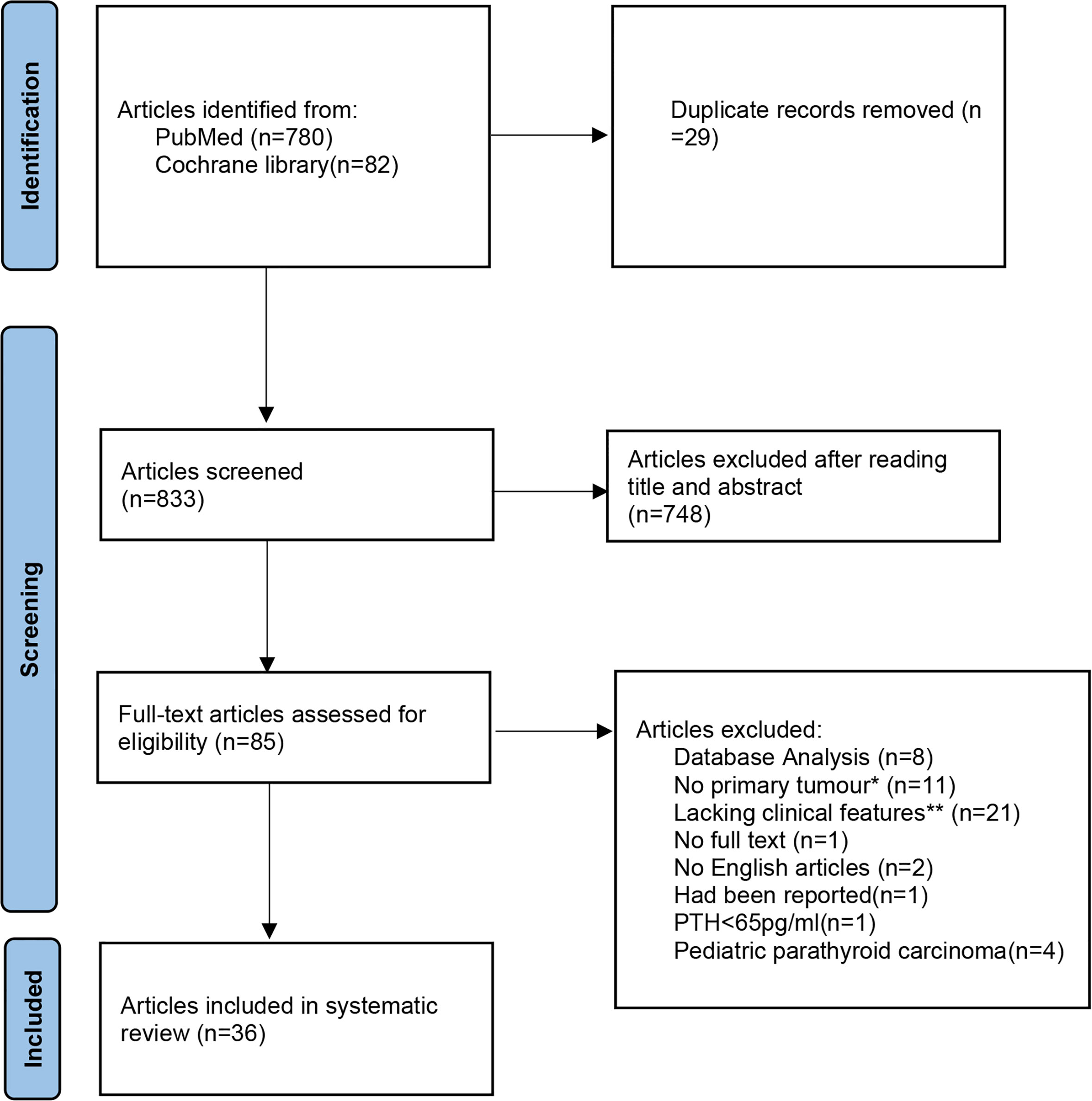
Figure 3 The detailed literature search process presented in a Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flowchart. *No primary tumours: secondary to other parathyroid diseases or not identified as parathyroid cancer in the initial surgery. **Lacking clinical features: Articles which lacking more than two study elements in the systematic review (age, gender, preoperative PTH, preoperative total serum calcium or tumour size.
PC is a rare malignancy in the endocrine system (48). The prevalence of PC in the USA is approximately 30 cases per year (49). For most patients, uncontrolled hypercalcemia is the main cause of death (50). SaPC is a rare and special subtype of PC, and only three cases have been reported internationally. Tumor invasion and metastasis seem to be associated with a patient’s death in SaPC.
Collectively, it is difficult to do the differentiation diagnosis of PC before surgery—how to distinguish malignant from benign, or how to distinguish PC from thyroid tumors, especially in non-functional PC (51) and SaPC. In recent years, the medical circle has made a breakthrough in the management of thyroid cancer, especially with the publication of the 2015 American Thyroid Association (ATA) guidelines (52) in the year 2016. According to the 2015 ATA guidelines, all patients suspected of thyroid cancer need assessment of parathyroid function. Thus, more atypical PC can be found, just like our case. Simultaneously, more cases and clinical experience of PC have been accumulated in recent years. Recognition of parathyroid carcinoma has been enlarged and deepened than any before. Well-recognized clinical manifestations of PC include extremely elevated serum calcium levels and serum PTH levels and a prominent neck mass (53). The significantly increased level of serum alkaline phosphatase has a predictive value for PC (54). Owing to the unclear cytologic atypia between benign and malignant parathyroid tumors, fine-needle aspiration (FNA) results do not diagnose the disease preoperatively instead of increasing the risk of needle tract metastasis (40). Therefore, unlike the thyroid cancer (52), FNA is not applicable when parathyroid diseases are suspected. Imaging examination is routinely used to localize the abnormal parathyroid gland. Ultrasonography and CT are common preoperative examinations before surgery. Methoxy Isobutyl Isonitrile (MIBI) scan is effective in differentiating parathyroid and thyroid tumors when high PTH levels are found in patients. It can also distinguish PC from benign parathyroid diseases by differences in the retention level of 99mTc-MIBI (55). In our study, MIBI scan showed that a large range of abnormal uptake occurred on the left thyroid (Figure 1B); this might be a useful adjunct to reveal evidence of invasion when PC is suspected. Definitive diagnosis of PC is restricted to histological findings, which contain observable vascular or peripheral nerve invasion, penetration of the capsule, and infiltration of surrounding tissues (56). Currently, it is believed that parafibromin (encoded by CDC73/HRPT2) is a significant immunohistochemical marker, and there is evidence that its negative expression in parathyroid cells indicates PC (57). Similarly, invasion and negative staining of parafibromin (Figure 2A) can be seen in SaPC cases. Recent studies showed that germline CDC73 mutation is closely related to HPT-JT syndrome and other variant phenotypes of sporadic PC (58, 59). There might be a connection between germline CDC73 mutation and SaPC. Unfortunately, we did not conserve the blood sample for the gene test. And the patient was lost to follow-up from December 2020. This is the limitation of this work, and more studies are needed to pursue this connection.
Interestingly, there are some differences in manifestations between SaPC and general PC. Firstly, the endocrine feature of SaPC is less distinct—although the PTH level is higher than normal, it is about one-tenth of general PC, which may be related to functional carcinomatous regions replaced by non-functional sarcomatoid regions at different levels. Moreover, all four cases of SaPC were suspected as thyroid tumors before surgery, which might be associated with the anatomical location and tumor invasiveness, but more cases are needed for validation. Notable signs, such as hoarseness and a larger tumor size, are common in this subtype, which might be an embodiment of the fast disease progression. Pathologically, the diagnosis of SaPC also includes the presence of mesenchymal cell areas and corresponding immunohistochemical markers. Specific spindle cell regions or transitional zones can also be observed in reported cases and our case of SaPC (Table 1; Figures 2B, C), which might be potential histomorphological characteristics of this subtype. In addition, the positive staining of N-cad in both components (Figure 2D) and the appearance of transitional zones seem to support the hypothesis of EMT. Combined with our case (Figure 2B), sarcomatoid regions showed a positive expression of different mesenchymal tissue markers (desmin or vimentin), indicating that the direction of sarcomatoid differentiation in PC may be different between individuals.
Presently, surgery is the overriding therapeutic modality for PC patients (12, 20, 60). Margin status in the initial surgery is crucial to the prognosis (61), and early salvage surgery can still achieve reasonably good therapeutic efficacy (19). En bloc resection with ipsilateral thyroid lobectomy and ipsilateral central lymph node dissection seems to be an appropriate surgical approach (62). However, a study suggested that resection of the ipsilateral thyroid cannot prolong survival (63). Thus, in-depth studies on the extent of resection are needed.
Nevertheless, the effect and extent of surgery in SaPC require further study. In our case (No. 1, Table 1), we performed the radical surgical procedure, and dyspnea improved temporarily, but tumor recurrence occurred 3 months later. Combined with reported cases, SaPC showed early recurrence after radical surgery. Surgical treatments in SaPC indeed achieve improvements in clinical symptoms, but recurrence and death occurred about 6 months later. In general, surgery for SaPC does relieve tumor compression, correct hypercalcemia, and improve quality of life even with recurrence in the short term.
According to the literature, radiotherapy and chemotherapy after surgery for general PC are ineffective (64, 65). The result shows no difference in SaPC with these treatments—the first two patients received postoperative chemotherapy but relapsed in months. In terms of sarcomatoid carcinoma in other organs such as kidney (66) and lung (67), they show the similar clinicopathological characteristics as SaPC, and targeted therapy has been confirmed to be potentially beneficial. This may be the future direction of treatment for SaPC.
Due to the rarity of the disease, there are currently no well-accepted staging criteria for PC. Patients who receive radical resection often have a favorable outcome, with a 5-year survival of up to 82.3% and 66% in 10 years (68). In our center, all four patients with typical PC received radical surgery, and none experienced recurrence (follow-up period was between 15 and 66 months; data not shown). However, SaPC was highly aggressive, with postoperative recurrence and early systemic metastases in all four patients (Table 1), which led to the patient’s death in a short time—two of the four patients with SaPC died within 7 months. We noticed that a longer period of the systematic review might benefit the research, which is a limitation of this work. We will continue to concern relative research henceforth.
SaPC is a highly unusual subtype of PC, and patients eventually succumb to direct tumor invasion and metastasis, while general PC has a good prognosis after radical surgery. Patients with SaPC seem to be easily misdiagnosed as thyroid tumors. Spindle cell areas or transitional zones are likely to be pathological features of SaPC. Despite many similarities, there are also some differences between SaPC and general PC—SaPC does not present a significant endocrinological feature but presents more aggressiveness. Surgery in SaPC cases indeed improves severe symptoms and quality of life even in patients experiencing a relapse in the short term, and other effective treatments are needed to explore.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Hospital of Dalian Medical University. The patients/participants provided their written informed consent to participate in this study.
YY designed the study. YY, YW, QW, and XZ reviewed the literature and collected data. DL, GW, JX, and JC analyzed data and assessed the accuracy of results. YY wrote the first paper draft of the article. YL and YZ provided assistance during the writing process. XT and NZ reviewed and edited the article. All authors contributed to the article and approved the submitted version.
This study was supported by the Scientific Research Fund of Liaoning Provincial Education Department, China (grant no. LZ2019057).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.793718/full#supplementary-material
1. Lee PK, Jarosek SL, Virnig BA, Evasovich M, Tuttle TM. Trends in the Incidence and Treatment of Parathyroid Cancer in the United States. Cancer (2007) 109(9):1736–41. doi: 10.1002/cncr.22599
2. Quaglino F, Manfrino L, Cestino L, Giusti M, Mazza E, Piovesan A, et al. Parathyroid Carcinoma: An Up-To-Date Retrospective Multicentric Analysis. Int J Endocrinol (2020) 2020:7048185. doi: 10.1155/2020/7048185
3. Wei CH, Harari A. Parathyroid Carcinoma: Update and Guidelines for Management. Curr Treat Options Oncol (2012) 13(1):11–23. doi: 10.1007/s11864-011-0171-3
4. Shane E. Clinical Review 122: Parathyroid Carcinoma. J Clin Endocrinol Metab (2001) 86(2):485–93. doi: 10.1210/jcem.86.2.7207
5. Rubello D, Casara D, Dwamena BA, Shapiro B. Parathyroid Carcinoma. A Concise Review. Minerva Endocrinol (2001) 26(2):59–64.
6. Nacamuli R, Rumore GJ, Clark G. Parathyroid Carcinosarcoma: A Previously Unreported Entity. Am Surg (2002) 68(10):900–3.
7. Taggart JL, Summerlin DJ, Moore MG. Parathyroid Carcinosarcoma: A Rare Form of Parathyroid Carcinoma With Normal Parathyroid Hormone Levels. Int J Surg Pathol (2013) 21(4):394–8. doi: 10.1177/1066896913480830
8. Hu L, Xie X. Parathyroid Carcinoma With Sarcomatoid Differentiation: A Case Report and Literature Review. Diagn Pathol (2020) 15(1):142. doi: 10.1186/s13000-020-01060-5
9. Pang A, Carbini M, Moreira AL, Maki RG. Carcinosarcomas and Related Cancers: Tumors Caught in the Act of Epithelial-Mesenchymal Transition. J Clin Oncol (2018) 36(2):210–6. doi: 10.1200/jco.2017.74.9523
10. McCluggage WG. Malignant Biphasic Uterine Tumours: Carcinosarcomas or Metaplastic Carcinomas? J Clin Pathol (2002) 55(5):321–5. doi: 10.1136/jcp.55.5.321
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Bmj (2021) 372:n71. doi: 10.1136/bmj.n71
12. Ferraro V, Sgaramella LI, Di Meo G, Prete FP, Logoluso F, Minerva F, et al. Current Concepts in Parathyroid Carcinoma: A Single Centre Experience. BMC Endocr Disord (2019) 19(Suppl 1):46. doi: 10.1186/s12902-019-0368-1
13. Campennì A, Giovinazzo S, Pignata SA, Di Mauro F, Santoro D, Curtò L, et al. Association of Parathyroid Carcinoma and Thyroid Disorders: A Clinical Review. Endocrine (2017) 56(1):19–26. doi: 10.1007/s12020-016-1147-7
14. Takenobu M, Moritani S, Kawamoto K, Yoshioka K, Kitano H. Parathyroid Carcinoma Coexisting With Multiple Parathyroid Adenomas: A Case Report. Endocr J (2020) 67(9):963–7. doi: 10.1507/endocrj.EJ20-0139
15. Xin Y, Zhao T, Wei B, Gu H, Jin M, Shen H, et al. Intrapericardial Parathyroid Carcinoma: A Case Report. Endocrine (2020) 69(2):456–60. doi: 10.1007/s12020-020-02283-8
16. Vodopivec DM, Thomas DD, Palermo NE, Steenkamp DW, Lee SL. Looking for the Outsider. N Engl J Med (2020) 383(23):2275–81. doi: 10.1056/NEJMcps2004935
17. Medas F, Erdas E, Loi G, Podda F, Pisano G, Nicolosi A, et al. Controversies in the Management of Parathyroid Carcinoma: A Case Series and Review of the Literature. Int J Surg (2016) 28 Suppl 1:S94–8. doi: 10.1016/j.ijsu.2015.12.040
18. Tkachenko R, Zakhartseva L, Golovko A, Kuryk O, Lazarenko G. Parathyroid Carcinoma: A Case Report. Exp Oncol (2019) 41(1):72–5. doi: 10.32471/exp-oncology.2312-8852.vol-41-no-1.12775
19. Xue S, Chen H, Lv C, Shen X, Ding J, Liu J, et al. Preoperative Diagnosis and Prognosis in 40 Parathyroid Carcinoma Patients. Clin Endocrinol (Oxf) (2016) 85(1):29–36. doi: 10.1111/cen.13055
20. Ryhänen EM, Leijon H, Metso S, Eloranta E, Korsoff P, Ahtiainen P, et al. A Nationwide Study on Parathyroid Carcinoma. Acta Oncol (2017) 56(7):991–1003. doi: 10.1080/0284186x.2017.1306103
21. Libánský P, Adámek S, Broulík P, Fialová M, Kubinyi J, Lischke R, et al. Parathyroid Carcinoma in Patients That Have Undergone Surgery for Primary Hyperparathyroidism. In Vivo (2017) 31(5):925–30. doi: 10.21873/invivo.11148
22. Gao Y, Yu C, Xiang F, Xie M, Fang L. Acute Pancreatitis as an Initial Manifestation of Parathyroid Carcinoma: A Case Report and Literature Review. Med (Baltimore) (2017) 96(44):e8420. doi: 10.1097/md.0000000000008420
23. Shruti S, Siraj F. Parathyroid Carcinoma: An Unusual Presentation of a Rare Neoplasm. Ger Med Sci (2017) 15:Doc21. doi: 10.3205/000262
24. Dikmen K, Bostanci H, Gobut H, Yildiz A, Ertunc O, Celik A, et al. Nonfunctional Double Parathyroid Carcinoma With Incidental Thyroid Micropapillary Carcinoma: A Rare Case. Pan Afr Med J (2017) 27:241. doi: 10.11604/pamj.2017.27.241.11503
25. Liu R, Xia Y, Chen C, Ye T, Huang X, Ma L, et al. Ultrasound Combined With Biochemical Parameters Can Predict Parathyroid Carcinoma in Patients With Primary Hyperparathyroidism. Endocrine (2019) 66(3):673–81. doi: 10.1007/s12020-019-02069-7
26. Shah R, Gosavi V, Mahajan A, Sonawane S, Hira P, Kurki V, et al. Preoperative Prediction of Parathyroid Carcinoma in an Asian Indian Cohort. Head Neck (2021) 43(7):2069–80. doi: 10.1002/hed.26677
27. Triggiani V, Castellana M, Basile P, Renzulli G, Giagulli VA. Parathyroid Carcinoma Causing Mild Hyperparathyroidism in Neurofibromatosis Type 1: A Case Report and Systematic Review. Endocr Metab Immune Disord Drug Targets (2019) 19(3):382–8. doi: 10.2174/1871530318666180910123316
28. Sadacharan D, Mahadevan S, Ferdinant J, Rakeshchandru K. Hypercalcaemic Encephalopathy Due to Metastatic Parathyroid Carcinoma. BMJ Case Rep (2017) 2017. doi: 10.1136/bcr-2017-219664
29. Rizwan A, Jamal A, Uzzaman M, Fatima S. Case Report: Lady With Bone Pains for 5 Years-Parathyroid Carcinoma. BMC Res Notes (2018) 11(1):617. doi: 10.1186/s13104-018-3711-0
30. Takumi K, Fukukura Y, Hakamada H, Nagano H, Kumagae Y, Arima H, et al. CT Features of Parathyroid Carcinomas: Comparison With Benign Parathyroid Lesions. Jpn J Radiol (2019) 37(5):380–9. doi: 10.1007/s11604-019-00825-3
31. Baek CO, Kim KH, Song SK. Synchronous Parathyroid Carcinoma and Papillary Thyroid Carcinoma in a Patient With Long-Standing Schizophrenia. Korean J Intern Med (2017) 32(6):1104–7. doi: 10.3904/kjim.2015.072
32. Agarwal S, Kumar T, Sharma MC, Damle NA, Gandhi AK. Parathyroid Carcinoma With Contralateral Subcutaneous and Breast Recurrences: A Rare Presentation. Head Neck (2016) 38(5):E115–8. doi: 10.1002/hed.24317
33. Kapur A, Singh N, Mete O, Hegele RA, Fantus IG. A Young Male With Parafibromin-Deficient Parathyroid Carcinoma Due to a Rare Germline HRPT2/CDC73 Mutation. Endocr Pathol (2018) 29(4):374–9. doi: 10.1007/s12022-018-9552-5
34. Shahid A, Iftikhar F. Pathological Bone Fractures in a Patient With Parathyroid Carcinoma - A Case Report. J Pak Med Assoc (2017) 67(12):1956–8.
35. Singh P, Vadi SK, Saikia UN, Sood A, Dahiya D, Arya AK, et al. Minimally Invasive Parathyroid Carcinoma-A Missing Entity Between Parathyroid Adenoma and Carcinoma: Scintigraphic and Histological Features. Clin Endocrinol (Oxf) (2019) 91(6):842–50. doi: 10.1111/cen.14088
36. Morand GB, Helmchen BM, Steinert HC, Schmid C, Broglie MA. 18f-Choline-PET in Parathyroid Carcinoma. Oral Oncol (2018) 86:314–5. doi: 10.1016/j.oraloncology.2018.09.009
37. Mahajan G, Sacerdote A. Previously Unreported Deletion of CDC73 Involving Exons 1-13 was Detected in a Patient With Recurrent Parathyroid Carcinoma. BMJ Case Rep (2018) 11(1):e225784. doi: 10.1136/bcr-2018-225784
38. Chen Z, Fu J, Shao Q, Zhou B, Wang F. 99mtc-MIBI Single Photon Emission Computed Tomography/Computed Tomography for the Incidental Detection of Rare Parathyroid Carcinoma. Med (Baltimore) (2018) 97(40):e12578. doi: 10.1097/md.0000000000012578
39. Dagang DJ, Gutierrez JB, Sandoval MA, Lantion-Ang FL. Multiple Brown Tumours From Parathyroid Carcinoma. BMJ Case Rep (2016) 2016. doi: 10.1136/bcr-2016-215961
40. Song A, Yang Y, Liu S, Nie M, Jiang Y, Li M, et al. Prevalence of Parathyroid Carcinoma and Atypical Parathyroid Neoplasms in 153 Patients With Multiple Endocrine Neoplasia Type 1: Case Series and Literature Review. Front Endocrinol (Lausanne) (2020) 11:557050. doi: 10.3389/fendo.2020.557050
41. Tarbunova M, Trimaldi J, Saremian J. A Case of Parathyroid Carcinoma Accompanied by a Brown Tumor. Diagn Cytopathol (2016) 44(8):685–7. doi: 10.1002/dc.23511
42. Balakrishnan M, George SA, Rajab SH, Francis IM, Kapila K. Cytological Challenges in the Diagnosis of Intrathyroidal Parathyroid Carcinoma: A Case Report and Review of Literature. Diagn Cytopathol (2018) 46(1):47–52. doi: 10.1002/dc.23847
43. Kawagishi S, Funaki S, Ose N, Kimura K, Mukai K, Otsuki M, et al. Debulking Surgery for Functional Pleural Dissemination of Parathyroid Carcinoma-Case Report. J Cardiothorac Surg (2021) 16(1):86. doi: 10.1186/s13019-021-01477-z
44. Russo M, Borzì G, Ilenia M, Frasca F, Malandrino P, Gullo D. Challenges in the Treatment of Parathyroid Carcinoma: A Case Report. Hormones (Athens) (2019) 18(3):325–8. doi: 10.1007/s42000-019-00104-w
45. do Vale RH, Queiroz MA, Coutinho AM, Buchpiguel CA, de Menezes MR. 18f-FDG PET/CT Osteometabolic Activity in Metastatic Parathyroid Carcinoma. Clin Nucl Med (2016) 41(9):724–5. doi: 10.1097/rlu.0000000000001278
46. Omi Y, Horiuchi K, Haniu K, Tokura M, Nagai E, Isozaki O, et al. Parathyroid Carcinoma Occurred in Two Glands in Multiple Endocrine Neoplasia 1: A Report on a Rare Case. Endocr J (2018) 65(2):245–52. doi: 10.1507/endocrj.EJ17-0409
47. Çalapkulu M, Sencar ME, Unsal IO, Sakiz D, Duger H, Özbek M, et al. Tumor Volume Can Be Used as a Parameter Indicating the Severity of Disease in Parathyroid Cancer. Endocr Pract (2021) 27(7):706–9. doi: 10.1016/j.eprac.2021.01.006
48. Cetani F, Pardi E, Marcocci C. Parathyroid Carcinoma. Front Horm Res (2019) 51:63–76. doi: 10.1159/000491039
49. Dudney WC, Bodenner D, Stack BC Jr. Parathyroid Carcinoma. Otolaryngol Clin North Am (2010) 43(2):441–53, xi. doi: 10.1016/j.otc.2010.01.011
50. Salcuni AS, Cetani F, Guarnieri V, Nicastro V, Romagnoli E, de Martino D, et al. Parathyroid Carcinoma. Best Pract Res Clin Endocrinol Metab (2018) 32(6):877–89. doi: 10.1016/j.beem.2018.11.002
51. Wang L, Han D, Chen W, Zhang S, Wang Z, Li K, et al. Non-Functional Parathyroid Carcinoma: A Case Report and Review of the Literature. Cancer Biol Ther (2015) 16(11):1569–76. doi: 10.1080/15384047.2015.1070989
52. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
53. Givi B, Shah JP. Parathyroid Carcinoma. Clin Oncol (R Coll Radiol) (2010) 22(6):498–507. doi: 10.1016/j.clon.2010.04.007
54. Bae JH, Choi HJ, Lee Y, Moon MK, Park YJ, Shin CS, et al. Preoperative Predictive Factors for Parathyroid Carcinoma in Patients With Primary Hyperparathyroidism. J Korean Med Sci (2012) 27(8):890–5. doi: 10.3346/jkms.2012.27.8.890
55. Zhang M, Sun L, Rui W, Guo R, He H, Miao Y, et al. Semi-Quantitative Analysis of (99m)Tc-Sestamibi Retention Level for Preoperative Differential Diagnosis of Parathyroid Carcinoma. Quant Imaging Med Surg (2019) 9(8):1394–401. doi: 10.21037/qims.2019.07.02
56. Schulte KM, Talat N. Diagnosis and Management of Parathyroid Cancer. Nat Rev Endocrinol (2012) 8(10):612–22. doi: 10.1038/nrendo.2012.102
57. Erickson LA, Mete O. Immunohistochemistry in Diagnostic Parathyroid Pathology. Endocr Pathol (2018) 29(2):113–29. doi: 10.1007/s12022-018-9527-6
58. Shattuck TM, Välimäki S, Obara T, Gaz RD, Clark OH, Shoback D, et al. Somatic and Germ-Line Mutations of the HRPT2 Gene in Sporadic Parathyroid Carcinoma. N Engl J Med (2003) 349(18):1722–9. doi: 10.1056/NEJMoa031237
59. Chiofalo MG, Sparaneo A, Chetta M, Franco R, Baorda F, Cinque L, et al. A Novel CDC73 Gene Mutation in an Italian Family With Hyperparathyroidism-Jaw Tumour (HPT-JT) Syndrome. Cell Oncol (Dordr) (2014) 37(4):281–8. doi: 10.1007/s13402-014-0187-3
60. Rodrigo JP, Hernandez-Prera JC, Randolph GW, Zafereo ME, Hartl DM, Silver CE, et al. Parathyroid Cancer: An Update. Cancer Treat Rev (2020) 86:102012. doi: 10.1016/j.ctrv.2020.102012
61. McClenaghan F, Qureshi YA. Parathyroid Cancer. Gland Surg (2015) 4(4):329–38. doi: 10.3978/j.issn.2227-684X.2015.05.09
62. Schulte KM, Talat N, Galata G, Gilbert J, Miell J, Hofbauer LC, et al. Oncologic Resection Achieving R0 Margins Improves Disease-Free Survival in Parathyroid Cancer. Ann Surg Oncol (2014) 21(6):1891–7. doi: 10.1245/s10434-014-3530-z
63. Zhou L, Huang Y, Zeng W, Chen S, Zhou W, Wang M, et al. Surgical Disparities of Parathyroid Carcinoma: Long-Term Outcomes and Deep Excavation Based on a Large Database. J Oncol (2021) 2021:8898926. doi: 10.1155/2021/8898926
64. Limberg J, Stefanova D, Ullmann TM, Thiesmeyer JW, Bains S, Beninato T, et al. The Use and Benefit of Adjuvant Radiotherapy in Parathyroid Carcinoma: A National Cancer Database Analysis. Ann Surg Oncol (2021) 28(1):502–11. doi: 10.1245/s10434-020-08825-8
65. Sharretts JM, Kebebew E, Simonds WF. Parathyroid Cancer. Semin Oncol (2010) 37(6):580–90. doi: 10.1053/j.seminoncol.2010.10.013
66. Pichler R, Compérat E, Klatte T, Pichler M, Loidl W, Lusuardi L, et al. Renal Cell Carcinoma With Sarcomatoid Features: Finally New Therapeutic Hope? Cancers (Basel) (2019) 11(3):422. doi: 10.3390/cancers11030422
67. Li X, Wu D, Liu H, Chen J. Pulmonary Sarcomatoid Carcinoma: Progress, Treatment and Expectations. Ther Adv Med Oncol (2020) 12:1758835920950207. doi: 10.1177/1758835920950207
Keywords: sarcomatoid, PTH, parathyroid carcinoma, diagnosis, surgery
Citation: Yu Y, Wang Y, Wu Q, Zhao X, Liu D, Zhao Y, Li Y, Wang G, Xu J, Chen J, Zhang N and Tian X (2021) Case Report and Systematic Review: Sarcomatoid Parathyroid Carcinoma—A Rare, Highly Malignant Subtype. Front. Endocrinol. 12:793718. doi: 10.3389/fendo.2021.793718
Received: 12 October 2021; Accepted: 24 November 2021;
Published: 15 December 2021.
Edited by:
Enzo Lalli, UMR7275 Institut de Pharmacologie Moléculaire et Cellulaire (IPMC), FranceReviewed by:
Jessica Costa-Guda, University of Connecticut, United StatesCopyright © 2021 Yu, Wang, Wu, Zhao, Liu, Zhao, Li, Wang, Xu, Chen, Zhang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofeng Tian, dHhmZGxAZG11LmVkdS5jbg==; Ning Zhang, ZHJkb3Jpc2NoYW5nQHllYWgubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.