
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 03 November 2021
Sec. Gut Endocrinology
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.774519
This article is part of the Research TopicThe Impact of Nutrient-Gut-Brain-Signaling on Health and Disease: from Systems Biology to Mechanisms.View all 5 articles
Background: Diabetes is a risk factor for colorectal neoplasms. The association between the level of glycosylated hemoglobin (HbA1c) and the risk of colorectal adenomas (CRAs) in non-diabetic adults needs to be investigated.
Methods: A cross-sectional study was performed on non-diabetic adults with normal HbA1c level who underwent colonoscopy between January 2010 and December 2016 during health check-ups in our hospital in China. The association between HbA1c level and CRAs was assessed by multiple logistic regression models stratified by age group (<40, ≥40 and <50, and ≥50 years old). The age group-specified thresholds for HbA1c on elevated risk of CRAs were estimated using the piecewise logistic regression.
Results: Among the 2,764 subjects, 445 (16.1%) had CRA. The prevalence of CRA varied across the three age groups. A higher HbA1c level was found to be significantly associated with increased CRA risk in the 40–50 years group (odds ratio [OR]=1.70, 95% confidence interval [CI] 1.04–2.78, p=0.035) after adjusting for other related factors, while this association was borderline significant among the 50 years and older group (OR=1.57, 95% CI 0.97–2.54, p=0.067). Based on the piecewise logistic regression analysis results, the thresholds for HbA1c on elevated risk of CRA were 5.44% for the 40–50 years group and 4.81% for the 50 years and older group, respectively.
Conclusions: Higher levels of HbA1c, even within the normal range, were associated with elevated CRA risk among non-diabetic adults. The threshold effects of HbA1c on the risk of CRA varied across different age groups, and early screening colonoscopy might be needed for individuals in their 40s and with HbA1c levels ≥5.44%.
Colorectal cancer (CRC) is one of the most common cancers, with the third highest incidence and the second highest mortality in the world (1). The incidence and mortality of CRC have shown an increasing trend in China over the past decade (2). Understanding the risk factors for CRC can provide guidance for developing strategies targeted toward colorectal screening and its prevention. Several risk factors have been proposed, including age, smoking, alcohol consumption, obesity, high-calorie/fat diets, lack of physical exercise, and family history of CRC (3).
Colorectal adenoma (CRA), which is considered a precursor of most CRCs (4), allows for screening and prevention of CRC by colonoscopy examination and polypectomy, respectively (5). Hyperglycemia plays an important role in the pathogenesis of CRC and CRA (6, 7). A meta-analysis of 17 observational studies showed that type 2 diabetes mellitus is associated with a higher risk of developing CRA (8). Glycosylated hemoglobin (HbA1c) reflects the average blood glucose concentration over the preceding 2–3 months and is a sensitive and reliable marker of abnormal glucose metabolism. Chronic hyperglycemia may result in hyperinsulinemia, and HbA1c may indirectly reflect hyperinsulinemia. Many studies have reported that hyperglycemia and insulin resistance (IR) are associated with an increased risk of CRC (7, 9, 10). However, in contrast to CRC, there are limited data on the relationship between the levels of HbA1c and CRA, and the conclusions are not entirely consistent (6, 11–16). Only a few studies have reported that HbA1c levels are associated with CRAs, but the association between normal HbA1c levels and the risk of CRAs in non-diabetic adults has not been reported (11–14).
The American College of Gastroenterology and Asia Pacific Consensus CRC screening guidelines strongly recommend 50 years as the starting age for screening CRC, while the former conditionally recommends lowering the starting age to 45 years (17, 18). In view of the increasing trend in young-onset CRC worldwide, with newly diagnosed CRC cases increasing to 15% (19, 20), and the rapidly increasing incidence of CRC in patients as early as 40 years of age in China (21), we need to focus on average-risk individuals between 40 and 50 years of age. However, due to the availability of medical resources and national screening programs, CRC screening is less likely to be conducted in average-risk individuals, even in individuals between the ages of 40 and 50 years (22). So far, there is no consensus that persons aged 40–50 years should be screened.
Therefore, the current study aimed to explore the relationship between HbA1c level and the risk of CRAs among different age groups in non-diabetic Chinese adults with normal levels of HbA1c, and focused on identifying high-risk individuals of CRAs in young-onset adults, especially those aged 40–50 years.
We conducted a cross-sectional study on a consecutive series of asymptomatic adults who underwent colonoscopy examinations and had a serum HbA1c level determined during routine health check-ups at the Health Management Center, The Second Affiliated Hospital, School of Medicine, Zhejiang University, China, between January 2010 and December 2016. Among the 3895 participants, 615 were excluded for the following reasons: 1) medical history of first-degree relatives with CRC; 2) previous history of inflammatory bowel disease or colorectal neoplasm; 3) medical history of diabetes or HbA1c ≥6.5%; and 4) incomplete laboratory data available. In addition, 606 participants with colorectal polyps were excluded for the following reasons: 1) pathologic diagnosis of a non-adenomatous polyp (i.e., hyperplastic polyps, inflammatory polyps, juvenile polyps, or serrated polyps); 2) pathologic diagnosis of cancer; and 3) without biopsy. After excluding these participants, 445 patients with adenomatous polyps and 2319 polyp-free controls were enrolled in this study (Figure 1). The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and was approved by the Institutional Research Ethics Committee of the hospital (No.20210667), which waived the requirement for informed consent.
Medical history of hypertension, diabetes, CRC, colorectal polyps, colorectal surgery family history of CRC, and personal history of smoking and alcohol consumption were obtained by trained physicians. Height and weight were measured using an ultrasonic body scale (SK-CK, Shenzhen, China) with subjects standing barefoot while wearing light clothing. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Blood pressure (BP) was measured using an automatic sphygmomanometer (Omron HBP-9020, Shanghai, China) following a standard protocol after 10 min of rest.
Fasting blood samples were obtained from each participant in the morning after overnight fasting. Total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting plasma glucose (FPG), and serum uric acid (sUA) levels were analyzed using an automatic chemistry analyzer (Olympus AU4500, Tokyo, Japan) according to the manufacturer’s instructions. White blood cell (WBC) counts were measured using an automatic hematology analyzer (Sysmex XN-9000, Kobe, Japan). Fasting insulin levels were measured using an autoanalyzer (Roche E170, Mannheim, Germany). HbA1c levels were determined using an automatic analyzer (Arkray HA8160, Kyoto, Japan) using standard methods. Insulin resistance (IR) was estimated using the homeostatic model assessment of IR (HOMA-IR): fasting insulin (μU/mL) × FPG (mmol/L)/22.5 (23).
Before colonoscopy examination, participants were prescribed a liquid diet a day before and received 2–3 L of polyethylene glycol solution (HE SHUANG, Shenzhen, China) for bowel preparation. After the bowels were adequately prepared, all colonoscopies were performed by experienced endoscopists with more than ten years of experience. Participants were examined by video colonoscopy (Olympus CF-H290, CF-H260AI, or CF-Q260AI, Tokyo, Japan), after which they underwent total colonoscopy. When colorectal polyps were identified, the endoscopists decided whether biopsy is required as determined by the endoscopists. Each pathological diagnosis was performed by two histopathologists who were unaware of the laboratory test results or medical histories of the subjects.
Continuous variables are expressed as median and interquartile range, while categorical variables are presented as frequencies with percentages (%). Demographic, CRA status, history of smoking and drinking, anthropometric measurements, and laboratory test results were stratified by age group (<40, ≥40 and <50, and ≥50 years old) and were compared using the Kruskal-Wallis rank test for continuous variables and χ2 test for categorical variables. Univariable logistic regression analysis was performed to examine the unadjusted association between each factor and the presence of CRA for the entire cohort, as well as for each age subgroup. Multiple logistic regression on the effects of related factors on CRA status was applied based on stepwise model selection results to reduce collinearity and overfitting. To account for the rarity of CRA cases (6.52%) in the group aged less than 40 years, the Firth penalized maximum-likelihood method was applied on the logistic regression model for the purpose of bias reduction. The locally weighted scatterplot smoothing (LOWESS) curves were constructed by age groups to visually present the association between HbA1c and the probability of CRA, as well as to explore the potential trend changes of the association. To further investigate the threshold for the effects of HbA1c on the probability of CRA, piecewise logistic regression was performed to identify the breakpoint in each age group. All statistical analyses were performed using Stata/SE 16 (StataCorp, College Station, TX, USA). The Firth penalized maximum-likelihood method and piecewise logistic regression were performed using the user-written Stata programs firthlogit and loghockey, respectively. All statistical significance was set at P<0.05.
Among the 2,764 subjects in the selected cohort, 445 (16.1%) had CRA. Male subjects accounted for 65.85% of the entire cohort, and the mean age was 45.96 ± 0.19 and 46.56 ± 0.27 years old for males and females, respectively. Demographic and clinical characteristics were compared across the three age groups and are presented in Table 1. The prevalence of CRA was significantly different across the three age groups, with the group aged 50 years and older having the highest prevalence (23.97%), followed by the 40–50 years group (14.81%) and the group aged younger than 40 years (6.52%). Characteristics including age, sex, systolic BP (SBP), diastolic DP (DBP), BMI, TC, TG, LDL-C, HDL-C, WBC, FPG, fasting insulin, HbA1c, HOMA-IR, smoking, and medical history of hypertension, varied across the three age groups (P<0.05).
Univariable logistic regression analysis was performed to determine the association between clinical factors and CRA status (Table 2). For the entire group, all the factors examined were significantly associated with CRA. When stratified by age group, the factors associated with CRA differed across different age groups. For subjects younger than 40 years old, only age, sex, DBP, smoking, and medical history of hypertension were found to be significantly associated with CRA. For the 40–50 years group, age, SBP, DBP, BMI, TC, TG, LDL, WBC, sUA, fasting insulin, HbA1c, and HOMA-IR were all positively associated with increasing CRA risk, while higher HDL levels were associated with lower CRA risk. Within this group, being male, smoking, and drinking were found to be associated with CRA. The results for the 50 years old and over group were similar to those of the 40–50 years group, except that TG, LDL, and drinking were not found to be significantly associated with the risk of CRA.
Multiple logistic regression models were constructed for the entire cohort and for each age subgroup based on the stepwise model selection results (Table 3). Adjusting for other related factors, increasing HbA1c level was found to be associated with increased CRA risk when all subjects in the cohort were included in the analysis (odds ratio [OR]=1.56, 95% confidence interval [CI] 1.13–2.16, p=0.007). For the youngest group (age<40 years), no association was observed between HbA1c and CRA in both univariable and multiple analyses. For subjects aged 40–50 years, the adjusted analysis results indicated that every 1% increase in HbA1c was associated with a 1.70-fold higher risk of CRA (95% CI 1.04–2.78, p=0.035). In the oldest group, after adjusting for the effects of other influencing factors, HbA1c was borderline significantly associated with CRA (OR=1.57, 95% CI 0.97–2.54, p=0.067).
The LOWESS smoothing curves exhibited the relationship between HbA1c and logit of the probability of CRA for each age subgroup, which indicated a non-linear relationship, as well as changes in relationship patterns (Figure 2). Although HbA1c was found to be not associated with CRA in both unadjusted and adjusted analyses for the youngest group, the CRA risk increased with the increase in HbA1c for the 40–50 years group and the oldest group, and this trend became more evident after a certain breakpoint. Based on the piecewise logistic regression analysis, the breakpoints suggesting thresholds for the effects of HbA1c on the risk of CRA were identified for the 40–50 years group and the oldest group (Table 4). When the HbA1c levels were below 5.44% for the 40–50 years group and 4.81% for the oldest group, HbA1c had no significant effect on the risk of CRA; however, above these thresholds, every one unit increase in HbA1c was associated with a 3.17-fold (95% CI 1.40–7.12, p=0.005) and 1.71-fold higher risk (95% CI 1.05–2.78, p=0.030) of CRA in the 40–50 years old group and the oldest group, respectively.
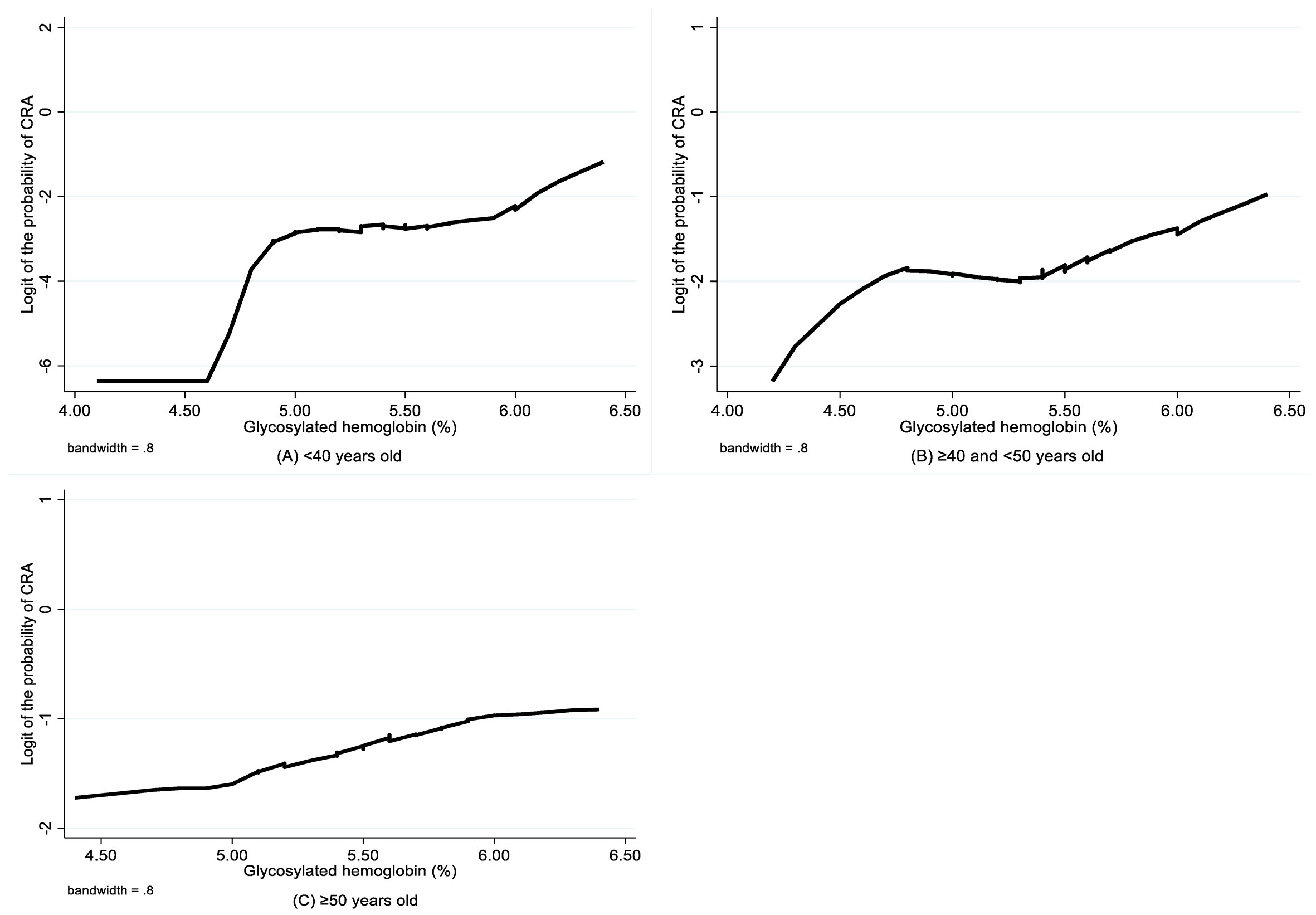
Figure 2 LOWESS smoothing curve on association between glycosylated hemoglobin and CRA by age group. CRA, colorectal adenoma.
In this study, we found that higher levels of HbA1c were independently associated with an elevated risk of CRAs in 40–50-year-old subjects after adjusting for related factors, whereas among subjects aged 50 years or older, HbA1c levels were only borderline significantly associated with increased risk of CRA. The association between HbA1c and CRAs was not detected in subjects younger than 40 years. Furthermore, we found that HbA1c has a threshold effect on the elevated risk of CRAs, which varied across different age groups. To the best of our knowledge, this study is the first to focus on normal HbA1c levels in non-diabetic adults and stratification by age when exploring the association between HbA1c and CRAs.
Our results support the hypothesis that perturbations in insulin levels and glucose control partially contribute to the etiology of colorectal neoplasms (9, 24). In this study, the results indicated that fasting insulin levels were almost the same in the 40–50 years group and the youngest group, but the levels in the 40–50 years group was considerably higher than those in the oldest group (P<0.001). Similarly, HOMA-IR was the highest in the 40–50 years group, followed by the youngest group and the oldest group successively (P<0.001). Hyperinsulinemia caused by IR is more apparent in 40–50-year-old subjects than in older subjects, since hyperinsulinemia is compromised by pancreatic β-cell dysfunction, which increases with age (25). However, IR was not apparent in the youngest group when compared to subjects aged 40–50 years, which is in agreement with the physiopathology of the earlier stage of hyperglycemia in the youngest group. Meanwhile, in this youngest group, islet function is almost in a compensative normal or high-level state, but the duration of IR has just begun for the younger subjects, whereas the islet function further decreases and may even have insulin deficiency in older subjects. Furthermore, the incidence of CRAs increases with age (26). Therefore, in this study, we speculate that the association between the level of HbA1c and CRAs varied across different age groups.
HbA1c is a reliable marker of hyperglycemia and may be a suitable marker of chronic hyperinsulinemia, as high glucose levels correspond to high insulin levels. Potential mechanisms for the association between hyperglycemia, hyperinsulinemia, and CRA risk have been reported. Hyperglycemia may induce colorectal neoplasia via chronic inflammation, oxidative stress, or adipokines (27). Hyperinsulinemia can stimulate the insulin-like growth factor (IGF) system, which plays an important role in cancer development and progression due to increased cell proliferation and anti-apoptotic effects through IGF-I receptor (IGF-IR) over-expression, over-activation, and endocrine/paracrine/autocrine production of its ligands, IGF-I, and IGF-II (28–32). Recently, a prospective cohort study including 397,380 participants provided a strong evidence for the positive association between circulating IGF-I levels and CRC risk (33). A meta-analysis of 19 epidemiological studies revealed that high levels of IGF-I and IGF-II significantly increased the risk of CRC (34).
Our results showed a significant association between HbA1c level and the risk of CRAs among non-diabetic adults with normal HbA1c levels, and this association varied among the different age groups. We also detected a threshold effect of HbA1c on the risk of CRA. When the HbA1c level exceeded 5.44% for subjects in their 40s or 4.81% for subjects aged 50 years and older, the risk of CRA significantly increased. The HbA1c threshold was not present among the group younger than 40 years old; however, since we only examined the effect of HbA1c within the normal range, we cannot exclude the possibility that there might also be a threshold effect in the younger group, but this is outside the scope of the study. Although our result was consistent with multiple recent studies, these studies did not focus on normal HbA1c levels in non-diabetic adults and were not stratified by age group. Two cross-sectional studies with subjects aged >40 years found that HbA1c >6.0% for non-diabetic adults and that HbA1c ≥6.5% was independently associated with CRA (13, 14). A cross-sectional study of 819 asymptomatic men aged 40–59 years found that higher levels of HbA1c were associated with a higher risk of CRA in those aged 40–50 years (11). A large-scale longitudinal study with 5,289 asymptomatic subjects aged >30 years revealed that HbA1c levels were significantly associated with the occurrence of CRA detected during surveillance colonoscopy (12). In contrast, some studies are inconsistent with the findings of this study. Two retrospective studies with diabetic subjects found no significant association between HbA1c level and CRA or high-risk CRA (15, 16). Meanwhile, a cross-sectional study with 19,361 asymptomatic subjects found that increasing levels of HbA1c were significantly associated with the prevalence of CRA, but there was no evidence of an association between HbA1c and CRA after adjusting for related risk factors (6). Discrepancies among these studies may result from different study designs, the composition of the study population, and the degree of control for potential confounders.
We also showed that there was a significant relationship between FPG and CRA in the whole population, but this relationship was not significant within each age group. Further analysis showed that there was no significant relationship between FPG and CRA (OR=1.13, 95% CI 0.94–1.37, P=0.194) after controlling for age, which implies that the influence of FPG on CRA was mainly attributed to the age effect. However, there was still a significant association between HbA1c and CRA (OR=1.79, 95% CI 1.30–2.50, P<0.001) in the whole population even after controlling for age, indicating that HbA1c still influenced the risk of CRA regardless of the influence of age. In the multiple analysis, after adjusting for other related factors, this association remained significant for subjects 40–50 years of age and was borderline significant for subjects in the oldest group. Therefore, we inferred that HbA1c may be a more effective predictor or risk marker for CRA than FPG. Consistent with our findings, Hsu et al. reported that HbA1c, compared to FPG, is more strongly and independently associated with colorectal neoplasia, even in non-diabetic participants (35). Our findings add to the evidence that HbA1c is superior to FPG in assessing the risk of complications associated with chronic hyperglycemia.
This study, however, is subject to limitations. First, we did not have data on postprandial blood glucose and postprandial insulin, so we cannot completely evaluate the blood glucose spectrum; otherwise, we can better evaluate the association between the level of HbA1c and CRA. Second, due to the nature of the cross-sectional design, the causal relationship between HbA1c level and the outcome of CRAs could not be established. A prospective study is needed to further examine the role of HbA1c in the pathological mechanisms of CRAs. Finally, the generalization of our results should be interpreted with caution. Since the subjects in the study were visitors of a health management center, they might be more concerned about their health than the general population.
In conclusion, higher HbA1c levels, even within the normal range, were associated with elevated CRA risk among non-diabetic adults, and this association varied among the different age groups. Furthermore, we found that there is an age group-specific threshold for HbA1c on elevated risk of CRA, and subjects aged 40–50 years with HbA1c level ≥5.44% were high-risk individuals for CRA. Therefore, we suggest that combining HbA1c level and age stratification might be necessary for non-diabetic adults when developing a plan for CRC, and early screening colonoscopy might be needed for subjects in their 40s when their HbA1c level is ≥5.44%. Currently, the ages between 40 to 50 years have no score in the revised risk-stratified scoring system of the Asia Pacific Consensus on CRC (17), and future insights will lead to an improved risk-stratified scoring system by adopting a more individualized approach to CRC screening.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Research Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University (No. 20210667). The ethics committee waived the requirement of written informed consent for participation.
XY: conception, design, interpretation, and drafting of the manuscript. CC: data analysis, interpretation, and drafting of the manuscript. XS: critical review. YG: critical review. YT: critical review. YZ: critical review. ZS: conception, design, and critical review. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the efforts of the health screening group at Health Management Center, the Second Affiliated Hospital, School of Medicine, Zhejiang University, China.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Xi L, Zhu J, Zhang H, Muktiali M, Xu C, Wu A. Epidemiological Trends in Gastrointestinal Cancers in China: An Ecological Study. Dig Dis Sci (2019) 64:532–43. doi: 10.1007/s10620-018-5335-6
4. Aran V, Victorino AP, Thuler LC, Ferreira CG. Colorectal Cancer: Epidemiology, Disease Mechanisms and Interventions to Reduce Onset and Mortality. Clin Colorectal Cancer (2016) 15:195–203. doi: 10.1016/j.clcc.2016.02.008
5. Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of Colorectal Cancer by Colonoscopic Polypectomy. The National Polyp Study Workgroup. N Engl J Med (1993) 329:1977–81. doi: 10.1056/NEJM199312303292701
6. Rampal S, Yang MH, Sung J, Son HJ, Choi YH, Lee JH, et al. Association Between Markers of Glucose Metabolism and Risk of Colorectal Adenoma. Gastroenterology (2014) 147:78–87.e73. doi: 10.1053/j.gastro.2014.03.006
7. Cheng HC, Chang TK, Su WC, Tsai HL, Wang JY. Narrative Review of the Influence of Diabetes Mellitus and Hyperglycemia on Colorectal Cancer Risk and Oncological Outcomes. Transl Oncol (2021) 14:101089. doi: 10.1016/j.tranon.2021.101089
8. Yu F, Guo Y, Wang H, Feng J, Jin Z, Chen Q. Type 2 Diabetes Mellitus and Risk of Colorectal Adenoma: A Meta-Analysis of Observational Studies. BMC Cancer (2016) 16:642. doi: 10.1186/s12885-016-2685-3
9. Xu J, Ye Y, Wu H, Duerksen-Hughes P, Zhang H, Li P, et al. Association Between Markers of Glucose Metabolism and Risk of Colorectal Cancer. BMJ Open (2016) 6:e011430. doi: 10.1136/bmjopen-2016-011430
10. Xu CX, Zhu HH, Zhu YM. Diabetes and Cancer: Associations, Mechanisms, and Implications for Medical Practice. World J Diabetes (2014) 5:372–80. doi: 10.4239/wjd.v5.i3.372
11. Kim BJ, Kim YH, Sinn DH, Kang KJ, Kim JY, Chang DK, et al. Clinical Usefulness of Glycosylated Hemoglobin as a Predictor of Adenomatous Polyps in the Colorectum of Middle-Aged Males. Cancer Causes Control (2010) 21:939–44. doi: 10.1007/s10552-010-9543-4
12. Kim NH, Suh JY, Park JH, Park DI, Cho YK, Sohn CI, et al. Parameters of Glucose and Lipid Metabolism Affect the Occurrence of Colorectal Adenomas Detected by Surveillance Colonoscopies. Yonsei Med J (2017) 58:347–54. doi: 10.3349/ymj.2017.58.2.347
13. Fliss-Isakov N, Zelber-Sagi S, Webb M, Halpern Z, Shibolet O, Kariv R. Distinct Metabolic Profiles Are Associated With Colorectal Adenomas and Serrated Polyps. Obes (Silver Spring) (2017) 25(Suppl 2):S72–80. doi: 10.1002/oby.22001
14. Hu KC, Wu MS, Chu CH, Wang HY, Lin SC, Liu SC, et al. Synergistic Effect of Hyperglycemia and Helicobacterpylori Infection Status on Colorectal Adenoma Risk. J Clin Endocrinol Metab (2017) 102:2744–50. doi: 10.1210/jc.2017-00257
15. Eddi R, Karki A, Shah A, DeBari VA, DePasquale JR. Association of Type 2 Diabetes and Colon Adenomas. J Gastrointest Cancer (2012) 43:87–92. doi: 10.1007/s12029-011-9316-7
16. Budzynska K, Passerman D, White-Perkins D, Rees DA, Xu J, Lamerato L, et al. Diabetes Mellitus and Hyperglycemia Control on the Risk of Colorectal Adenomatous Polyps: A Retrospective Cohort Study. BMC Fam Pract (2018) 19:145. doi: 10.1186/s12875-018-0835-1
17. Sung JJ, Ng SC, Chan FK, Chiu HM, Kim HS, Matsuda T, et al. An Updated Asia Pacific Consensus Recommendations on Colorectal Cancer Screening. Gut (2015) 64:121–32. doi: 10.1136/gutjnl-2013-306503
18. Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol (2021) 116:458–79. doi: 10.14309/ajg.0000000000001122
19. Lui RN, Tsoi KKF, Ho JMW, Lo CM, Chan FCH, Kyaw MH, et al. Global Increasing Incidence of Young-Onset Colorectal Cancer Across 5 Continents: A Joinpoint Regression Analysis of 1,922,167 Cases. Cancer Epidemiol Biomarkers Prev (2019) 28:1275–82. doi: 10.1158/1055-9965.EPI-18-1111
20. Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J, et al. The Increasing Incidence of Young-Onset Colorectal Cancer: A Call to Action. Mayo Clin Proc (2014) 89:216–24. doi: 10.1016/j.mayocp.2013.09.006
21. Du LB, Li HZ, Wang YQ, Zhu C, Zheng RS, Zhang SW, et al. Report of Colorectal Cancer Incidence and Mortality in China, 2013. Zhonghua Zhong Liu Za Zhi (2017) 39:701–6. doi: 10.3760/cma.j.issn.0253-3766.2017.09.012
22. Leung DY, Chow KM, Lo SW, So WK, Chan CW. Contributing Factors to Colorectal Cancer Screening Among Chinese People: A Review of Quantitative Studies. Int J Environ Res Public Health (2016) 13(5):506. doi: 10.3390/ijerph13050506
23. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function From Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia (1985) 28:412–9. doi: 10.1007/BF00280883
24. Yoon YS, Keum N, Zhang X, Cho E, Giovannucci EL. Hyperinsulinemia, Insulin Resistance and Colorectal Adenomas: A Meta-Analysis. Metabolism (2015) 64:1324–33. doi: 10.1016/j.metabol.2015.06.013
25. Vilchis-Flores LH, Barajas-Medina GA, Villa-Martínez AK, Salazar López SS, Luna-Patiño GA, Quiroz-Hernández ME, et al. Pancreatic β-Cell Dysfunction in Normoglycemic Patients and Risk Factors. Acta Diabetol (2019) 56:1305–14. doi: 10.1007/s00592-019-01411-9
26. Pendergrass CJ, Edelstein DL, Hylind LM, Phillips BT, Iacobuzio-Donahue C, Romans K, et al. Occurrence of Colorectal Adenomas in Younger Adults: An Epidemiologic Necropsy Study. Clin Gastroenterol Hepatol (2008) 6:1011–5. doi: 10.1016/j.cgh.2008.03.022
27. Wojciechowska J, Krajewski W, Bolanowski M, Kręcicki T, Zatoński T. Diabetes and Cancer: A Review of Current Knowledge. Exp Clin Endocrinol Diabetes (2016) 124:263–75. doi: 10.1055/s-0042-100910
28. Pollak MN, Schernhammer ES, Hankinson SE. Insulin-Like Growth Factors and Neoplasia. Nat Rev Cancer (2004) 4:505–18. doi: 10.1038/nrc1387
29. LeRoith D, Roberts CT Jr. The Insulin-Like Growth Factor System and Cancer. Cancer Lett (2003) 195:127–37. doi: 10.1016/S0304-3835(03)00159-9
30. Valentinis B, Baserga R. IGF-I Receptor Signalling in Transformation and Differentiation. Mol Pathol (2001) 54:133–7. doi: 10.1136/mp.54.3.133
31. Yu H, Rohan T. Role of the Insulin-Like Growth Factor Family in Cancer Development and Progression. J Natl Cancer Inst (2000) 92:1472–89. doi: 10.1093/jnci/92.18.1472
32. Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The Effects of Insulin-Like Growth Factors on Tumorigenesis and Neoplastic Growth. Endocr Rev (2000) 21:215–44. doi: 10.1210/edrv.21.3.0399
33. Murphy N, Carreras-Torres R, Song M, Chan AT, Martin RM, Papadimitriou N, et al. Circulating Levels of Insulin-Like Growth Factor 1 and Insulin-Like Growth Factor Binding Protein 3 Associate With Risk of Colorectal Cancer Based on Serologic and Mendelian Randomization Analyses. Gastroenterology (2020) 158:1300–12.e1320. doi: 10.1053/j.gastro.2019.12.020
34. Chi F, Wu R, Zeng YC, Xing R, Liu Y. Circulation Insulin-Like Growth Factor Peptides and Colorectal Cancer Risk: An Updated Systematic Review and Meta-Analysis. Mol Biol Rep (2013) 40:3583–90. doi: 10.1007/s11033-012-2432-z
Keywords: glycosylated hemoglobin (HbA1c), adenomatous polyp, non-diabetic adults, age factor, risk factors
Citation: Yu X, Chen C, Song X, Guo Y, Tong Y, Zhao Y and Song Z (2021) Glycosylated Hemoglobin as an Age-Specific Predictor and Risk Marker of Colorectal Adenomas in Non-Diabetic Adults. Front. Endocrinol. 12:774519. doi: 10.3389/fendo.2021.774519
Received: 12 September 2021; Accepted: 14 October 2021;
Published: 03 November 2021.
Edited by:
Stephen A. Whelan, Cedars Sinai Medical Center, United StatesReviewed by:
Andrei I. Tarasov, Ulster University, United KingdomCopyright © 2021 Yu, Chen, Song, Guo, Tong, Zhao and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenya Song, c29uZ3poZW55YUB6anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.