- 1Department of Maternal and Child Health, School of Public Health, Peking University, Beijing, China
- 2Environmental and Spatial Epidemiology Research Center, National Human Genetic Resources Center, Beijing, China
- 3Human Genetic Resources Center, National Research Institute for Family Planning, Beijing, China
- 4Obstetrical Department, Tongzhou Maternal and Child Health Hospital of Beijing, Beijing, China
- 5Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, China
- 6Institute of Reproductive and Child Health, Peking University/Key Laboratory of Reproductive Health, National Health and Family Planning Commission of the People’s Republic of China, Beijing, China
- 7Department of Laboratorial Science and Technology, School of Public Health, Peking University, Beijing, China
Objective: The extensive use of rare earth elements (REEs) in many technologies was found to have effects on human health, but the association between early pregnancy exposure to REEs and gestational diabetes mellitus (GDM) is still unknown.
Methods: This nested case-control study involved 200 pregnant women with GDM and 200 healthy pregnant women from the Peking University Birth Cohort in Tongzhou. We examined the serum concentrations of 14 REEs during early pregnancy and analyzed their associations with the risk of GDM.
Results: When the elements were considered individually in the logistic regression model, no significant associations were found between REEs and GDM, after adjusting for confounding variables (P > 0.05). In weighted quantile sum (WQS) regression, each quartile decrease in the mixture index for REEs resulted in a 1.67-fold (95% CI: 1.12-2.49) increased risk of GDM. Neodymium (Nd), Praseodymium (Pr), and Lanthanum (La) were the most important contributors in the mixture.
Conclusion: The study findings indicated that early pregnancy exposure to lower levels of REE mixture was associated with an increased risk of GDM, and Nd, Pr, and La exhibited the strongest effects in the mixture.
Introduction
Gestational diabetes mellitus (GDM), defined as any degree of glucose intolerance with onset or first recognition during pregnancy (1), affects a considerable number of pregnant women worldwide (2). GDM is associated with many short- and long-term adverse health outcomes for mothers and their offspring. Mothers with GDM are more likely to develop preeclampsia during pregnancy and type 2 diabetes mellitus (T2DM) 5-10 years after delivery (3, 4). The offspring of mothers with GDM have a higher incidence of macrosomia and a higher risk of diabetes and other cardiometabolic diseases later in life (5–7). Both genetic susceptibility and lifestyle risk factors have been shown to play a role in the etiology of GDM, yet the impact of environmental exposure remains unclear (8–10). Recently, the effects of environmental metal exposure on GDM risk have attracted increasing attention worldwide. Different metals exhibited protective (11–13) or deleterious (14–17) effects on the development of GDM.
Rare earth elements (REEs) have gained worldwide attention due to the rapid development of industrial, agricultural and medical technologies in the last decades. China has the world’s richest reserves of REEs, sufficient to meet the global demand (18, 19). REEs are beneficial elements for plants and have been used in fertilizers in Chinese agriculture for about 40 years (20). REEs are also used in medicine. For example, rare earth-doped nanoparticles have the potential to target and treat glioblastoma (21). With the exploitation and application of REEs in multiple fields, there is an increasing possibility for humans to absorb REEs from the workplace, environment and food (22, 23). The health effects of REEs are controversial (24). While some studies found that REEs impair human health (25–33), other studies showed that higher than background concentrations of REEs in human tissues did not have significant health effects (34, 35).
Oxidative stress plays a key role in the development and progression of diabetes. The vulnerability to oxidative stress during pregnancy suggests that oxidative stress may also be an important factor in triggering GDM (35, 36). It is known that low doses of REEs have antioxidant effects, while high concentrations of REEs contribute to oxidative stress (37). This unique redox property makes rare earth oxalates show great potential for the treatment of oxidative stress-induced diseases, such as diabetes, in several animal studies (38–41). In 2011, Pourkhalili N et al. first reported the beneficial effect of the combination of cerium oxide nanoparticles/sodium selenite in diabetes treatment (42). Treatment of rats with this combination resulted in a significant reduction in blood glucose. Hence, a small intake of REEs might reduce the risk of developing GDM. However, to our knowledge, we did not find epidemiological evidence of an association between REEs exposure and GDM.
Therefore, this study aimed to investigate whether early pregnancy exposure to REEs (single metal elements or a metal mixture) is associated with the risk of GDM.
Methods
Study Design
This was a nested case-control study based on the Peking University Birth Cohort in Tongzhou (PKUBC-T). The primary aim of the prospective cohort was to investigate the short- and long-term health effects of pre-pregnancy and prenatal exposures on mothers and their children. This cohort has been registered in ClinicalTrials.gov (NCT 03814395, see the website for details). Baseline recruitment was conducted between June 2018 and February 2019, and a total of 5,426 pregnant women who visited the outpatient clinic for the first prenatal examination at Tongzhou maternal and child health hospital and met the following inclusion criteria were recruited: 1) age between 18 to 45 years old, 2) <14 gestational weeks, 3) resided in Tongzhou during the past half year and have no plan to move out after delivery, 4) plan to have antenatal care and delivery in Tongzhou Maternal and Child Hospital. The study was approved by the institutional review boards at Peking University (IRB00001052-18003), and all participants gave written informed consent at the enrollment.
After excluding participants who were diagnosed with diabetes before pregnancy, had a family history of diabetes, polycystic ovary syndrome, thyroid disease, hypertension, heart disease, autoimmune diseases, infectious diseases, chronic liver or kidney diseases, cigarette smoking, and alcohol consumption, a total of 200 women with GDM were selected as cases. We randomly selected 200 controls from participants without GDM. Cases and controls were matched to age ( ± 2 years old) and gestation week of taking oral glucose tolerance test (OGTT), but not to other factors.
Data Collection
The trained nurses administrated interview questionnaires face to face to collect information including maternal characteristics and lifestyle habits at the first prenatal visit (7-13 gestational weeks), such as demographic information, pre-pregnancy weight, pregnancy history, smoking status, alcohol intake, and family history of diabetes. Dietary intake for early pregnant women was assessed using 24-hour dietary recall for two inconsecutive days within 7 days and daily intake of calories was calculated. Physical activity was evaluated using the last 7-day, short form of the International Physical Activity Questionnaire and quantified using metabolic equivalents of task (MET-min week-1) (43). The heights and weights of pregnant women were measured by trained nurses. Pre-pregnancy body mass index (BMI) was calculated as pre-pregnancy weight in kilograms divided by height squared in meters.
Diagnosis of GDM
We obtained the information on GDM diagnosed by the obstetricians in the hospital from the medical records. The OGTT was implemented between 24 and 28 gestational weeks. Venous blood samples were collected at 0, 1, and 2 hours after a 75g glucose load. According to current guidelines of the American Diabetes Association (ADA) (44), women who met one or more of the following criteria were diagnosed with GDM: fasting plasma glucose ≥ 5.1mmol/L, 1 h ≥ 10.0 mmol/L, and 2 h ≥ 8.5 mmol/L.
Metal Analysis
Blood samples were obtained from all participants at the first prenatal visit (<14 gestational weeks) in the first trimester. Samples were processed within 24 hours of collection and stored at −80°C. Fasting plasma glucose (FPG) and total vitamin D concentrations were measured using standard detection methods.
Each serum sample was transported to the laboratory on dry ice and stored at −80°C until assay. After thawing on a 4°C low-temperature console, 0.1 mL of serum sample was transferred to a 2 mL centrifuge tube. Then 0.1 mL of indium (In) internal standard and 1.8 mL of 1% nitric acid (ultrapure grade) were added, and the sample was shaken sufficiently. We measured the concentrations of elements using inductively coupled plasma mass spectrometry (ICP-MS, ELAN DRC II, PerkinElmer Sciex, USA). A total of 14 REEs were included in this study (see Table 2): lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb) and lutetium (Lu). The limits of detection (LODs, μg/L) were 0.012 (La), 0.008 (Ce), 0.002 (Pr), 0.060 (Nd), 0.008 (Sm), 0.020 (Eu), 0.040 (Gd), 0.008 (Tb), 0.020 (Dy), 0.040 (Ho), 0.240 (Er), 0.140 (Tm), 0.020 (Yb) and 0.002 (Lu). We defined the concentration below the LOD as LOD/√2 for statistical analysis.
For quality assurance, the quantitative analysis was conducted in the Central Laboratory of Biological Elements in the Peking University Health Science Center, and the protocol was qualified by the China Metrology Accreditation (CMA) system.
Statistical Analyses
We first performed descriptive statistical analyses of the sociodemographic, lifestyle, and clinical characteristics of the participants. Data were presented as mean ± standard deviation (SD) for normally distributed continuous variables and as median (interquartile range, IQR) for non-normally distributed continuous variables. Categorical variables were presented as frequency and percentage (N, %). The independent samples t-test and the Mann-Whitney U test were used to compare the differences between two groups for normally and non-normally distributed continuous variables, respectively. The Chi-square test was performed for the categorical variables. Because the concentrations of the elements were not normally distributed, the concentrations of all REEs were described by the median with upper and lower quartiles (P25-P75). Non-parametric analyses were used to examine the differences in REEs between the GDM group and the control group. We also conducted Spearman’s rank correlation analysis to examine correlations between different metals.
We then performed dose-response analyses. REEs concentrations were grouped based on the quartiles of their distribution among all participants (with the highest quartile as the reference). The unconditional logistic regression analysis was used to assess the association of serum REEs with the risk of GDM. The crude model only included each REEs (quartiles) without any adjustment, and the subsequent models were adjusted for pre-pregnancy BMI, education, parity, dietary energy, physical activity intensity, total vitamin D concentrations and fetal sex. K-Nearest Neighbors method was used to interpolate missing values of physical activity intensity and total vitamin D concentrations (11.8% and 5.0%, respectively). The linear trend was tested by using the median of each quartile as a continuous variable in the models.
The association of the mixture of REEs with GDM was estimated using weighted quantile sum (WQS) regression. WQS regression could combine highly correlated exposures into an index to assess the association between multiple exposures and outcomes. This method is based on the assumption that all components of the index are associated with the outcome in the same direction (all negative or all positive). After grouping different REE metals into ordinal variables (quartiles), the WQS approach was used to estimate a weighted linear index (WQS index), which identifies the significant variables in a set of multiple correlated metals and further estimates the association with GDM (45). The WQS index is a value between 0 and 1, summing to 1, determined by bootstrap sampling (n = 100). In our analysis, we set β1 as a non-constrained negative coefficient. The significance level was set at P < 0.05 (two-tailed test). All data analyses were conducted using R software (version 4.0.2).
Results
General Characteristics of Participants
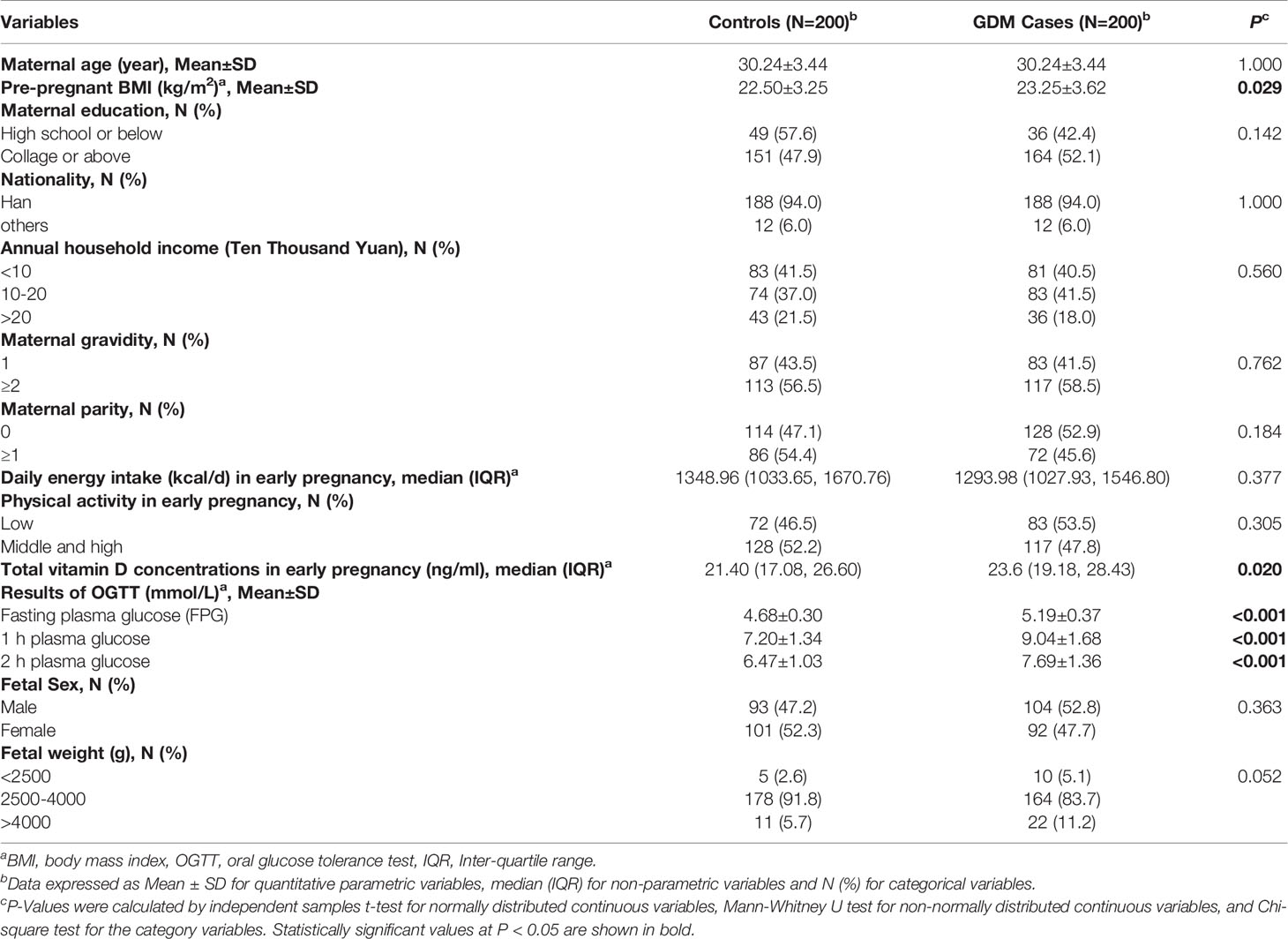
The distributions of the baseline characteristics of pregnant women with GDM and controls are shown in Table 1. The mean age of the 400 participants was 30.24 ± 3.43 years. Compared with controls, pregnant women with GDM had a higher pre-pregnancy BMI (23.25 ± 3.62 kg/m2 vs. 22.50 ± 3.25 kg/m2, P = 0.029). No significant differences were observed between the cases and controls in regard to the educational level, nationality, annual household income, gravidity, parity, daily energy intake, and physical activity during early pregnancy (P > 0.05). For the 75g OGTT, the mean glucose levels of fasting, 1 h, and 2 h among controls were 4.68 ± 0.30, 7.20 ± 1.34, and 6.47 ± 1.03 mmol/L, respectively, while the mean levels of fasting, 1 h, and 2 h among GDM cases were 5.19 ± 0.37, 9.04 ± 1.68, and 7.69 ± 1.36 mmol/L, respectively (P < 0.05). Compared with controls, pregnant women with GDM had a higher total vitamin D concentrations in early pregnancy (21.40 (17.08, 26.60) ng/ml vs. 23.6 (19.18, 28.43) ng/ml, P = 0.020). In neonates, no differences were found between the two groups in terms of fetal sex and birth weight (P > 0.05).
The Concentrations of REEs
We compared the concentrations of REEs between two groups (see Table 2). La, Ce, Pr, Nd, and Sm were detected in 100% of the samples. And the detection rates of Dy and Lu were 89.25% and 80%, respectively. The least frequently detected elements were Er (0%), Tm (0%), Ho (0.25%), Yb (24.0%), Gd (42.75%), Tb (59.25%), and Eu (72.75%). Because the detection rates of these seven elements were under 80%, we excluded these metals in subsequent analyses. The median concentrations (μg/L) of the REEs in all women were 0.178 for Nd, 0.137 for Ce, 0.123 for Sm, 0.120 for Er, 0.076 for La, 0.070 for Tm, 0.040 for Dy, 0.029 for Pr, 0.027 for Eu, 0.020 for Gd, 0.020 for Ho, 0.010 for Yb, 0.009 for Tb, and 0.004 for Lu, respectively. We compared the REE concentrations in our study with those reported in other regions (see Table S1). No statistically significant differences in the concentrations of REEs were observed between cases and controls in this study (P > 0.05). The correlation coefficients between most of the REEs were significantly positive (P < 0.05) (see Table S2).

Table 2 Concentrations (μg/L) of the rare earth elements (REEs) in maternal blood between cases and controls.
REEs Exposure and GDM
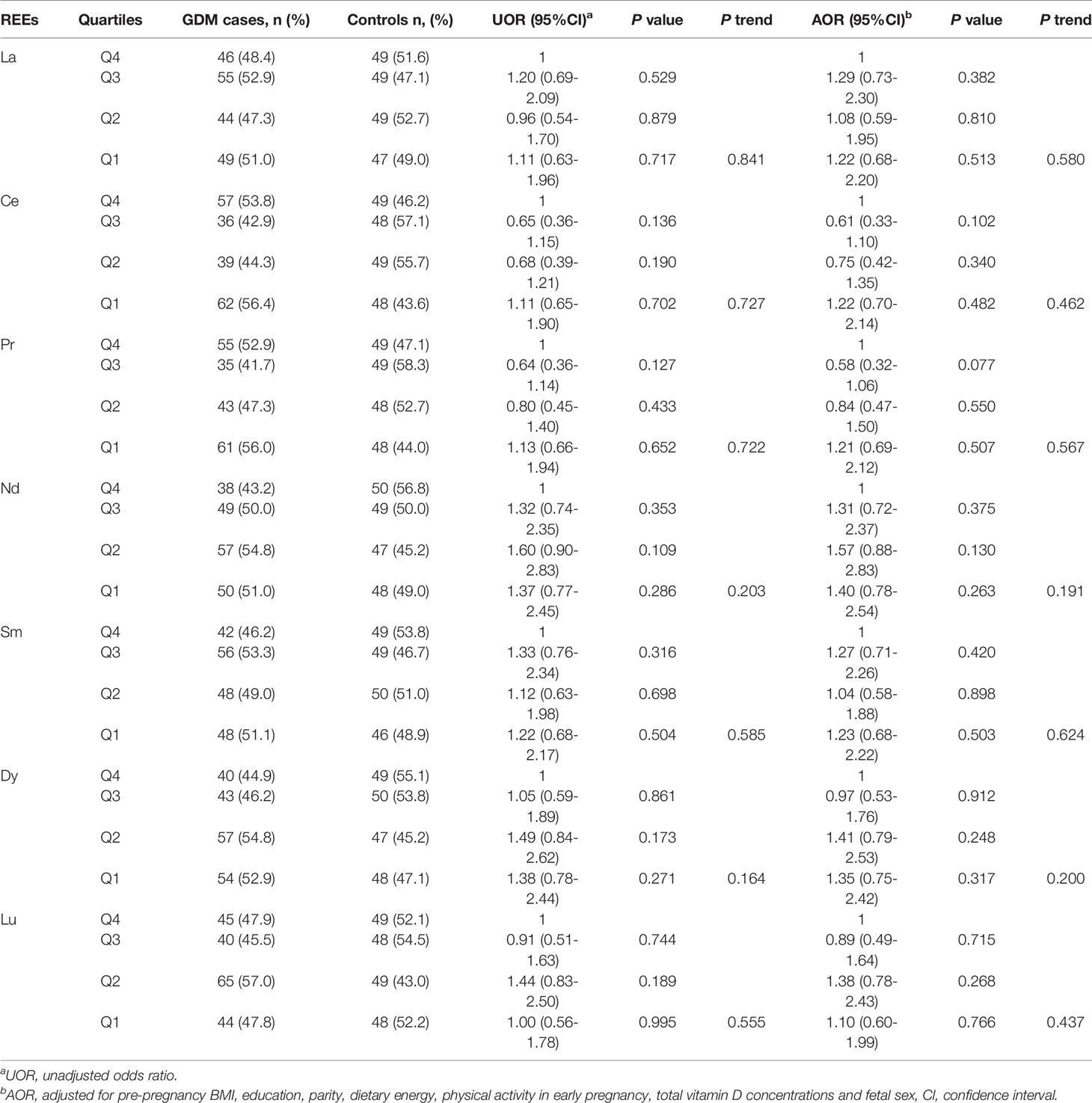
In order to explore the associations between REEs and GDM, we further classified the concentrations of the REEs based on quartiles (see Table 3). Compared with the 4th quartile, we didn’t find significant associations between REEs in other quartiles and GDM risk (P > 0.05). After adjusting for pre-pregnancy BMI, education, parity, dietary energy, physical activity in early pregnancy, total vitamin D concentrations and fetal sex, the associations remained not statistically significant (P > 0.05). Besides, no statistically significant trend was observed (P > 0.05).
WQS Regression
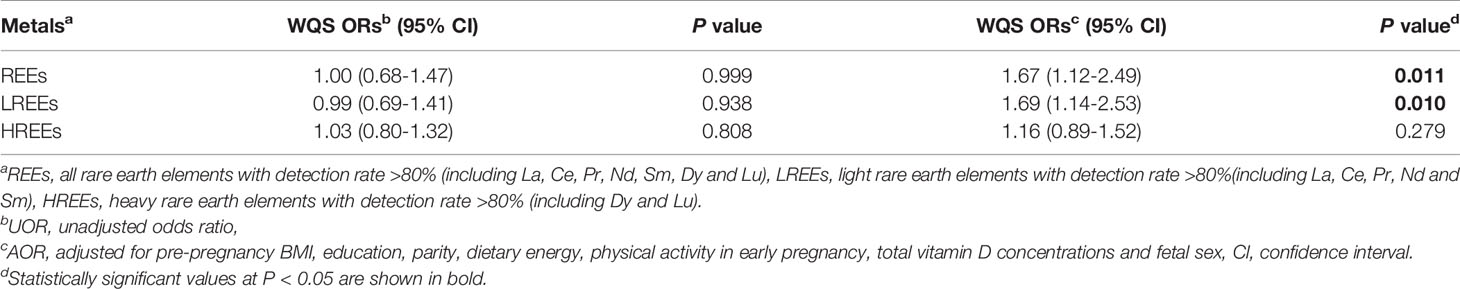
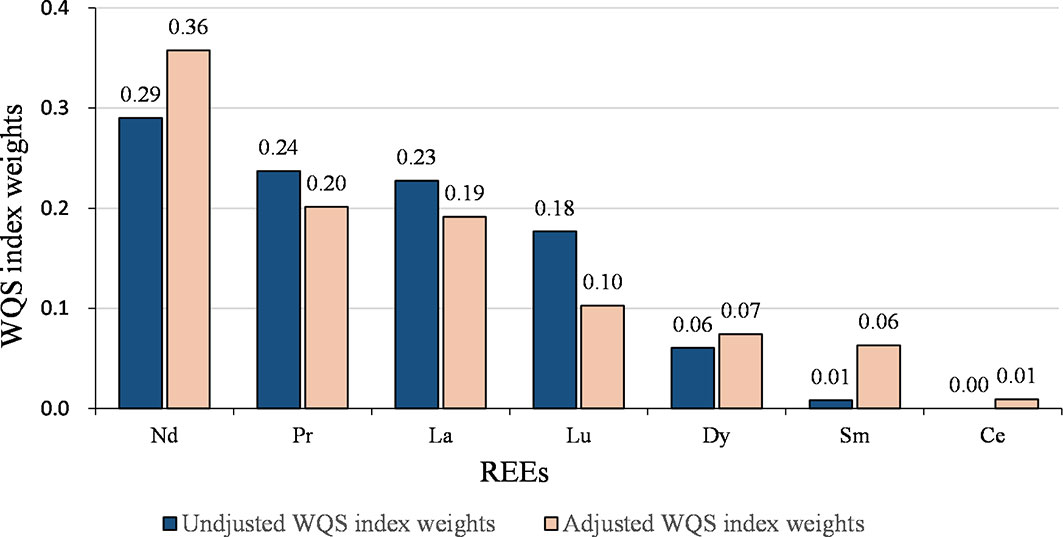
We calculated the WQS index for all REEs, where the contribution of each element reflected its relative effect on GDM. The detailed results are presented in Table 4. No statistically significant association was found in the crude model (P > 0.05). After adjusting for confounding variables, each quartile decrease in the WQS index was associated with a 1.67-fold (95% CI: 1.12-2.49, P = 0.011) higher risk of GDM. The assigned weights for each REE element are shown in Figure 1. Based on the weighted mean empirical weights for each REEs, the major contributors to the metal mixture index (WQS index) were Nd (35.8%), Pr (20.1%), and La (19.1%), followed by Lu (10.3%), Dy (7.4%), and Sm (6.3%), while Ce (0.9%) made almost no contribution.
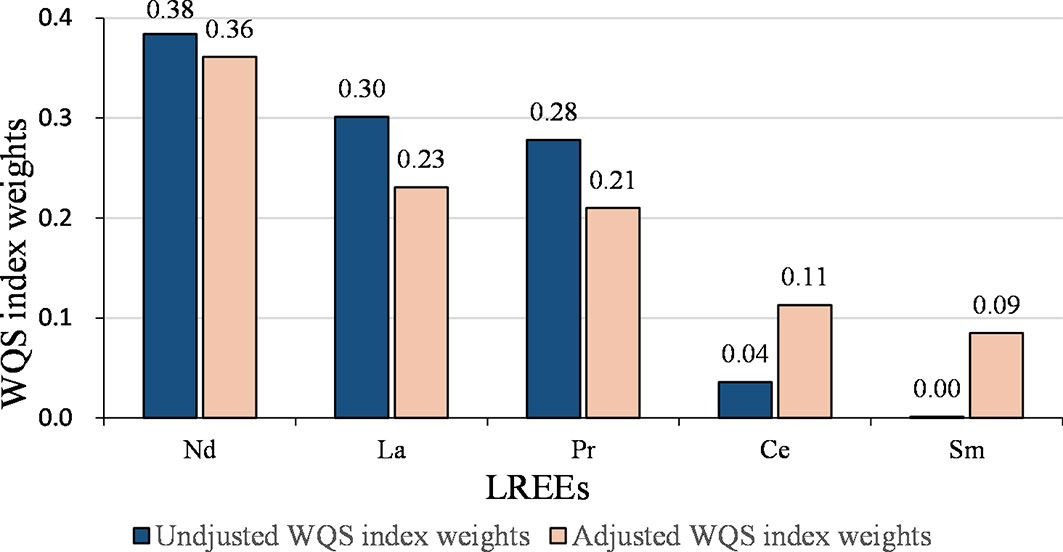
The joint effect of the LREEs on GDM was similar to the results of WQS analysis for all REEs (Table 4 and Figure 2). After adjusting for confounding variables, each quartile decrease in the WQS index for LREEs resulted in a 1.69-fold (95% CI: 1.14-2.53, P = 0.010) increase in GDM risk, with Nd (36.1%), La (23.1%), and Pr (21.0%) receiving the highest weights. However, we did not observe a significant association between WQS index for HREEs and GDM (figure not shown).
Discussion
In this study, we investigated the association between serum REE concentrations and GDM risk among pregnant women in Beijing, China. We didn’t observe a significant association between REEs and GDM by logistic regression analysis. However, the WQS model indicated that exposure to lower levels of REEs mixtures was associated with an increased risk of GDM, and three elements, Nd, Pr, and La, were important contributors to the REE mixture.
Blood concentrations of REEs in unexposed populations vary considerably by race, region, age, gender, and the different analytical methods used (28). As shown in Table S1, previous studies (28, 31, 46–49) showed that the range of the median values was 0.01-0.854 μg/L for La, 0.02-2.546 μg/L for Ce, 0.01-0.132 μg/L for Pr, 0.01-0.839 μg/L for Nd, 0.01-0.132 μg/L for Sm, 0.01-0.034 μg/L for Eu, 0.049-0.059 μg/L for Gd, 0.001-0.011 μg/L for Tb, 0.01-0.047 μg/L for Dy, 0.001-0.01 μg/L for Lu, 0.01-0.529 μg/L for Yb among the non-occupationally exposed population in China, Spain, Romania, and Sub-Saharan Africa. The concentrations of all the serum REEs in our study fell in these ranges. The high detection rates in blood for most REEs in humans are remarkable, considering that to date there are very few references in the scientific literature dealing with the effects of chronic exposure to REEs on human health (24–28). Therefore, it remains unclear whether the exposure to REEs during pregnancy is related to GDM or not.
In the present study, we didn’t observe a significant association between individual REEs and GDM risk. When we classified the concentrations of the REEs according to quartiles, we found no statistically significant association. Considering the absence of clear toxicological criteria, some authors analyzed the combined effects of these elements, as they were often used in combination in the manufacture of multiple devices (50). The WQS model can not only examine the effects of the REE mixture but also identify those elements most strongly associated with GDM (45). In WQS analysis, lower levels of REE mixture were significantly associated with a higher risk of GDM, and Nd, Pr and La were likely to be the most important contributors. The joint effect of the LREEs on GDM was similar to the results of WQS analysis for all REEs. A possible explanation for the similar results is that the three most important contributors (Nd, Pr, and La) belong to LREEs. To the best of our knowledge, there is no published report in the literature on REEs in maternal serum and risk for GDM. A previous study conducted in Shanxi province of China reported that higher concentrations of REEs were associated with an increased risk of hypertension (24). Two studies conducted in Shanxi and Hebei provinces of China found that high concentrations of REEs were associated with increased risks of fetal neural tube defects (NTDs) (25, 26). Besides, Liu et al. found that increased maternal urinary REEs (Ce and Yb) were associated with decreased neonatal thyroid-stimulating hormone (TSH) levels (27). Another study suggested that REEs might play a role in the development of anemia (28). Liu et al. showed that prenatal exposure to REEs and REE mixtures were associated with an increased risk of premature rupture of membranes (29). Liu et al. found that in utero exposure to REE mixtures increased the risk of orofacial clefts (30). According to these studies, exposure to higher levels of REEs was a risk factor for some diseases, while in our study, exposure to higher levels of REEs might be a protective factor for GDM.
The possible underlying mechanisms of this inconsistency are as follows. REEs may act either as antioxidants or pro-oxidants, depending on the environment, the nature of the bonding in their compounds, and their concentration in the tissues (51). Results of previous studies might be explained by oxidative stress induced by exposure to REEs. However, some REEs have been reported to have antioxidant effects in clinical applications (52). For example, cerium oxide nanoparticles could exhibit superoxide dismutase (SOD) activity and act as a free radical scavenger (42). GDM is usually accompanied by increased levels of oxidative stress and inadequate antioxidant defense responses (36). Therefore, REEs may reduce oxidative stress associated with GDM. Moreover, individual REEs and REE mixtures have been shown to have hormetic effects on several health endpoints. In other words, REEs can increase or improve biological events (e.g. growth) at low concentrations and exhibit inhibitory or toxic effects at increasing doses/concentrations (35). Another possible explanation is related to the relatively low concentrations of REEs in our study. Low concentrations of REEs might stimulate pancreatic beta (β) cells to secrete insulin to regulate glucose metabolism and maintain blood glucose stability (53–55). Within a certain range of concentrations, exposure to lower concentrations of REEs might be a risk factor for GDM. Further studies are needed to validate the role of REEs in the pathophysiology of GDM.
To the best of our knowledge, this study is the first to evaluate the association between early pregnancy exposure to REEs and the risk of GDM. This is one of the strengths of our study. Second, comprehensive information regarding potential confounders was prospectively collected in the birth cohort and controlled in our statistical analyses. Third, the sample size of this prospective cohort study was relatively large, and we collected blood samples in early pregnancy to evaluate metal exposure, which meets the criterion of temporality for causal inference. Finally, the information in our questionnaire was collected using standardized interview procedures by trained local health workers at the hospital. However, there were some limitations in our study. First, causality cannot be made based on the findings of the current study because of the observational nature of the study design. Second, everyone is exposed to multiple toxic substances in real life, and it is difficult to fully rule out the effects of residual confounders. Thirdly, the data of dietary intake were retrospectively collected and had recall bias, causing underestimation of energy intake in this study. However, we think that the underestimation of energy intake may not affect the main results, because it was a covariate in our study. More validation studies are needed in the future.
Conclusion
In conclusion, we observed that early pregnancy exposure to lower levels of REE mixtures increased the risk of GDM. Nd, Pr, and La showed the greatest contribution to the metal mixture index related to GDM. Our findings suggested that REEs are more likely to play a role through oxidative stress or other pathways. Animal studies are needed to uncover the possible mechanisms underlying the association between the mixture of REEs and GDM risk. The results of this study warrant further epidemiological investigation in other populations.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional review boards at Peking University (IRB00001052-18003). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization, XX, YW, LY, XM, and H-JW. Methodology, XX, YW, SL, ZL, and HB. Software, XX and XY. Validation, XY and H-JW. Formal Analysis, XX, YW, and YJ. Data Curation, NH, XY, JL, CJ, LL, and SZ. Writing - Original Draft Preparation, XX and YW. Writing–Review and Editing, LY, HW, and BW. Supervision, LY, XM, and H-JW. Project Administration, XX. Funding Acquisition, H-JW. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Key Research and Development Program (No. 2016YFC1000300 and No. 2016YFC1000307) and National Natural Science Foundation of China (81973053 and 81703240).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely thank the staff in the Maternal and Child Health Care Hospital of Tongzhou District for data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.774142/full#supplementary-material
References
1. American Diabetes Association. Classification and Diagnosis of Diabetes : Standards of Medical Care in Diabetes—2018. Diabetes Care (2018) 41:S13–27. doi: 10.2337/dc18-S002
2. Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr Diabetes Rep (2016) 16:1–11. doi: 10.1007/s11892-015-0699-x
3. Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. High Prevalence of Type 2 Diabetes and Pre-Diabetes in Adult Offspring of Women With Gestational Diabetes Mellitus or Type 1 Diabetes: The Role of Intrauterine Hyperglycemia. Diabetes Care (2008) 31:340–6. doi: 10.2337/dc07-1596
4. Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The Hyperglycemia and Adverse Pregnancy Outcome Study: Associations of GDM and Obesity With Pregnancy Outcomes. Diabetes Care (2012) 35:780–6. doi: 10.2337/dc11-1790
5. Yang G, Dye TD, Li D. Effects of Pre-Gestational Diabetes Mellitus and Gestational Diabetes Mellitus on Macrosomia and Birth Defects in Upstate New York. Diabetes Res Clin Pract (2019) 155:107811. doi: 10.1016/j.diabres.2019.107811
6. Tam WH, Ma R, Ozaki R, Li AM, Chan M, Yuen LY, et al. In Utero Exposure to Maternal Hyperglycemia Increases Childhood Cardiometabolic Risk in Offspring. Diabetes Care (2017) 40:679–86. doi: 10.2337/dc16-2397
7. Cho NH, Jang HC. Better Understanding and New Insight of Genetic Risk Loci for Gestational Diabetes Mellitus. J Diabetes Investig (2012) 3:424–6. doi: 10.1111/j.2040-1124.2012.00228.x
8. Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational Diabetes in the United States: Temporal Changes in Prevalence Rates Between 1979 and 2010. BJOG: Int J Obstet Gynaecol (2017) 124:804–13. doi: 10.1111/1471-0528.14236
9. Carroll X, Liang X, Zhang W, Zhang W, Liu G, Turner N, et al. Socioeconomic, Environmental and Lifestyle Factors Associated With Gestational Diabetes Mellitus: A Matched Case-Control Study in Beijing, China. Sci Rep (2018) 8:8103. doi: 10.1038/s41598-018-26412-6
10. Franzago M, Fraticelli F, Stuppia L, Vitacolonna E. Nutrigenetics, Epigenetics and Gestational Diabetes: Consequences in Mother and Child. Epigenetics (2019) 14:215–35. doi: 10.1080/15592294.2019.1582277
11. Wilson RL, Bianco-Miotto T, Leemaqz SY, Grzeskowiak LE, Dekker GA, Roberts CT. Early Pregnancy Maternal Trace Mineral Status and the Association With Adverse Pregnancy Outcome in a Cohort of Australian Women. J Trace Elements Med Biol (2018) 46:103–9. doi: 10.1016/j.jtemb.2017.11.016
12. Arbuckle TE, Liang CL, Morisset A, Fisher M, Weiler H, Cirtiu CM, et al. Maternal and Fetal Exposure to Cadmium, Lead, Manganese and Mercury: The MIREC Study. Chemosphere (2016) 163:270–82. doi: 10.1016/j.chemosphere.2016.08.023
13. Kong F, Ma L, Chen S, Li G, Zhou J. Serum Selenium Level and Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutr J (2016) 15:94. doi: 10.1186/s12937-016-0211-8
14. Zhang Q, Li X, Liu X, Dong M, Xiao J, Wang J, et al. Association Between Maternal Antimony Exposure and Risk of Gestational Diabetes Mellitus: A Birth Cohort Study. Chemosphere (2020) 246:125732. doi: 10.1016/j.chemosphere.2019.125732
15. Wang X, Gao D, Zhang G, Zhang X, Li Q, Gao Q, et al. Exposure to Multiple Metals in Early Pregnancy and Gestational Diabetes Mellitus: A Prospective Cohort Study. Environ Int (2020) 135:105370. doi: 10.1016/j.envint.2019.105370
16. Wang Y, Zhang P, Chen X, Wu W, Feng Y, Yang H, et al. Multiple Metal Concentrations and Gestational Diabetes Mellitus in Taiyuan, China. Chemosphere (2019) 237:124412. doi: 10.1016/j.chemosphere.2019.124412
17. Xia X, Liang C, Sheng J, Yan S, Huang K, Li Z, et al. Association Between Serum Arsenic Levels and Gestational Diabetes Mellitus: A Population-Based Birth Cohort Study. Environ Pollut (2018) 235:850–6. doi: 10.1016/j.envpol.2018.01.016
18. Zhou B, Li Z, Chen C. Global Potential of Rare Earth Resources and Rare Earth Demand From Clean Technologies. Minerals (2017) 7:203. doi: 10.3390/min7110203
19. Alonso E, Sherman AM, Wallington TJ, Everson MP, Field FR, Roth R, et al. Correction to Evaluating Rare Earth Element Availability: A Case With Revolutionary Demand From Clean Technologies. Environ Ence Technol (2012) 46:4684–4. doi: 10.1021/es3011354
20. Thomas PJ, Carpenter D, Boutin C, Allison JE. Rare Earth Elements (REEs): Effects on Germination and Growth of Selected Crop and Native Plant Species. Chemosphere (2014) 96:57–66. doi: 10.1016/j.chemosphere.2013.07.020
21. Lu VM, Mcdonald KL, Townley HE. Realizing the Therapeutic Potential of Rare Earth Elements in Designing Nanoparticles to Target and Treat Glioblastoma. Nanomedicine (2017) 12:2389–401. doi: 10.2217/nnm-2017-0193
22. Gwenzi W, Mangori L, Danha C, Chaukura N, Dunjana N, Sanganyado E. Sources, Behaviour, and Environmental and Human Health Risks of High-Technology Rare Earth Elements as Emerging Contaminants. Sci Total Environ (2018) 636:299–313. doi: 10.1016/j.scitotenv.2018.04.235
23. Wang L, He J, Xia A, Cheng M, Yang Q, Du C, et al. Toxic Effects of Environmental Rare Earth Elements on Delayed Outward Potassium Channels and Their Mechanisms From a Microscopic Perspective. Chemosphere (2017) 181:690–8. doi: 10.1016/j.chemosphere.2017.04.141
24. Pagano G, Guida M, Tommasi F, Oral R. Health Effects and Toxicity Mechanisms of Rare Earth Elements—Knowledge Gaps and Research Prospects. Ecotoxicol Environ Saf (2015) 115:40–8. doi: 10.1016/j.ecoenv.2015.01.030
25. Wang B, Yan L, Huo W, Lu Q, Cheng Z, Zhang J, et al. Rare Earth Elements and Hypertension Risk Among Housewives: A Pilot Study in Shanxi Province, China. Environ Pollut (2017) 220:837–42. doi: 10.1016/j.envpol.2016.10.066
26. Huo W, Zhu Y, Li Z, Pang Y, Wang B, Li Z. A Pilot Study on the Association Between Rare Earth Elements in Maternal Hair and the Risk of Neural Tube Defects in North China. Environ Pollut (2017) 226:89–93. doi: 10.1016/j.envpol.2017.03.046
27. Wei J, Wang C, Yin S, Pi X, Jin L, Li Z, et al. Concentrations of Rare Earth Elements in Maternal Serum During Pregnancy and Risk for Fetal Neural Tube Defects. Environ Int (2020) 137:105542. doi: 10.1016/j.envint.2020.105542
28. Liu Y, Wu M, Zhang L, Bi J, Song L, Wang L, et al. Prenatal Exposure of Rare Earth Elements Cerium and Ytterbium and Neonatal Thyroid Stimulating Hormone Levels: Findings From a Birth Cohort Study. Environ Int (2019) 133:105222. doi: 10.1016/j.envint.2019.105222
29. Henríquez-Hernández LA, Boada LD, Carranza C, Pérez-Arellano JL, González-Antuña A, Camacho M, et al. Blood Levels of Toxic Metals and Rare Earth Elements Commonly Found in E-Waste may Exert Subtle Effects on Hemoglobin Concentration in Sub-Saharan Immigrants. Environ Int (2017) 109:20–8. doi: 10.1016/j.envint.2017.08.023
30. Liu Y, Wu M, Song L, Bi J, Wang L, Chen K, et al. Association Between Prenatal Rare Earth Elements Exposure and Premature Rupture of Membranes: Results From a Birth Cohort Study. Environ Res (2021) 193:110534. doi: 10.1016/j.envres.2020.110534
31. Liu L, Wang L, Ni W, Pan Y, Chen Y, Xie Q, et al. Rare Earth Elements in Umbilical Cord and Risk for Orofacial Clefts. Ecotoxicol Environ Saf (2021) 207:111284. doi: 10.1016/j.ecoenv.2020.111284
32. Guo C, Wei Y, Yan L, Li Z, Qian Y, Liu H, et al. Rare Earth Elements Exposure and the Alteration of the Hormones in the Hypothalamic-Pituitary-Thyroid (HPT) Axis of the Residents in an E-Waste Site: A Cross-Sectional Study. Chemosphere (2020) 252:126488. doi: 10.1016/j.chemosphere.2020.126488
33. Liu Y, Wu M, Liu B, Song L, Bi J, Wang L, et al. Association of Prenatal Exposure to Rare Earth Elements With Newborn Mitochondrial DNA Content: Results From a Birth Cohort Study. Environ Int (2020) 143:105863. doi: 10.1016/j.envint.2020.105863
34. Li X, Chen Z, Chen Z, Zhang Y. A Human Health Risk Assessment of Rare Earth Elements in Soil and Vegetables From a Mining Area in Fujian Province, Southeast China. Chemosphere (2013) 93:1240–6. doi: 10.1016/j.chemosphere.2013.06.085
35. Zhuang M, Zhao J, Li S, Liu D, Wang K, Xiao P, et al. Concentrations and Health Risk Assessment of Rare Earth Elements in Vegetables From Mining Area in Shandong, China. Chemosphere (2017) 168:578–82. doi: 10.1016/j.chemosphere.2016.11.023
36. Chen X, Scholl TO. Oxidative Stress: Changes in Pregnancy and With Gestational Diabetes Mellitus. Curr Diabetes Rep (2005) 5:282–8. doi: 10.1007/s11892-005-0024-1
37. Pagano G, Thomas PJ, Di Nunzio A, Trifuoggi M. Human Exposures to Rare Earth Elements: Present Knowledge and Research Prospects. Environ Res (2019) 171:493–500. doi: 10.1016/j.envres.2019.02.004
38. Hasanvand D, Amiri I, Soleimani Asl S, Saidijam M, Shabab N, Artimani T. Effects of CeO2nanoparticles on theHO-1,NQO1, Andgclcexpression in the Testes of Diabetic Rats. Can J Physiol Pharmacol (2018) 96:963–9. doi: 10.1139/cjpp-2017-0784
39. Lopez-Pascual A, Urrutia-Sarratea A, Lorente-Cebrián S, Martinez JA, González-Muniesa P. Cerium Oxide Nanoparticles Regulate Insulin Sensitivity and Oxidative Markers in 3T3-L1 Adipocytes and C2C12 Myotubes. Oxid Med Cell Longevity (2019) 2019:1–10. doi: 10.1155/2019/2695289
40. Khaksar MR, Rahimifard M, Baeeri M, Maqbool F, Navaei-Nigjeh M, Hassani S, et al. Protective Effects of Cerium Oxide and Yttrium Oxide Nanoparticles on Reduction of Oxidative Stress Induced by Sub-Acute Exposure to Diazinon in the Rat Pancreas. J Trace Elements Med Biol (2017) 41:79–90. doi: 10.1016/j.jtemb.2017.02.013
41. Vafaei-Pour Z, Shokrzadeh M, Jahani M, Shaki F. Embryo-Protective Effects of Cerium Oxide Nanoparticles Against Gestational Diabetes in Mice. Iran J Pharm Res (2018) 17:964–75. doi: 10.22037/IJPR.2018.2253
42. Pourkhalili N, Hosseini A, Nili-Ahmadabadi A, Hassani S, Pakzad M, Baeeri M, et al. Biochemical and Cellular Evidence of the Benefit of a Combination of Cerium Oxide Nanoparticles and Selenium to Diabetic Rats. World J Diabetes (2011) 2:204–10. doi: 10.4239/wjd.v2.i11.204
43. Bassett DR. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med Sci Sports Exercise (2003) 35:1396. doi: 10.1249/01.MSS.0000078923.96621.1D
44. American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care (2011) 34:S62–9. doi: 10.2337/dc11-s062
45. Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat (2015) 20:100–20. doi: 10.1007/s13253-014-0180-3
46. Badea M, Luzardo OP, González-Antuña A, Zumbado M, Rogozea L, Floroian L, et al. Body Burden of Toxic Metals and Rare Earth Elements in Non-Smokers, Cigarette Smokers and Electronic Cigarette Users. Environ Res (2018) 166:269–75. doi: 10.1016/j.envres.2018.06.007
47. Bai Y, Long C, Hu G, Zhou D, Gao X, Chen Z, et al. Association of Blood Chromium and Rare Earth Elements With the Risk of DNA Damage in Chromate Exposed Population. Environ Toxicol Pharmacol (2019) 72:103237. doi: 10.1016/j.etap.2019.103237
48. Cabrera-Rodríguez R, Luzardo OP, González-Antuña A, Boada LD, Almeida-González M, Camacho M, et al. Occurrence of 44 Elements in Human Cord Blood and Their Association With Growth Indicators in Newborns. Environ Int (2018) 116:43–51. doi: 10.1016/j.envint.2018.03.048
49. Bao TM, Tian Y, Wang LX, Wu T, Lu LN, Ma HY, et al. An Investigation of Lanthanum and Other Metals Levels in Blood Urine and Hair Among Residents in the Rare Earth Mining Area of a City in China. Chin J Ind Hyg Occup Dis (2018) 2:99–101.
50. Tansel B. From Electronic Consumer Products to E-Wastes: Global Outlook, Waste Quantities, Recycling Challenges. Environ Int (2017) 98:35–45. doi: 10.1016/j.envint.2016.10.002
51. Kostova I, Saso L, Valcheva-Traykova M. Involvement of Lanthanides in the Free Radicals Homeostasis. Curr Top Med Chem (2014) 14:2508–19. doi: 10.2174/1568026614666141203123620
52. Wong LL, McGinnis JF. Nanoceria as Bona Fide Catalytic Antioxidants in Medicine: What We Know and What We Want to Know. Adv Exp Med Biol (2014) 801:821–8. doi: 10.1007/978-1-4614-3209-8_103
53. Yan YE, Xiu-Juan LI, Zeng ZZ. Synthesis,anti-Diabete and Antioxidative Action of Dimethylbiguamide Rare Earth Complexes. Chin J Appl Chem (2005) 22:1060–4.
54. Wen J, Shen C, Ying S, Wu Y, Hong W, Hong T, et al. Gadolinium Chloride Down-Regulated Glucose Level of Type I Diabetic Mice. J Chin Rare Earth Soc (2008), 108–11.
Keywords: rare earth elements (REEs), gestational diabetes mellitus (GDM), serum, early pregnancy, weighted quantile sum (WQS)
Citation: Xu X, Wang Y, Han N, Yang X, Ji Y, Liu J, Jin C, Lin L, Zhou S, Luo S, Bao H, Liu Z, Wang B, Yan L, Wang H-J and Ma X (2021) Early Pregnancy Exposure to Rare Earth Elements and Risk of Gestational Diabetes Mellitus: A Nested Case-Control Study. Front. Endocrinol. 12:774142. doi: 10.3389/fendo.2021.774142
Received: 11 September 2021; Accepted: 03 December 2021;
Published: 20 December 2021.
Edited by:
Sarah Taki, Sydney Local Health District, AustraliaReviewed by:
Lorenzo Iughetti, University of Modena and Reggio Emilia, ItalyDimitrios T. Papadimitriou, National and Kapodistrian University of Athens, Greece
Copyright © 2021 Xu, Wang, Han, Yang, Ji, Liu, Jin, Lin, Zhou, Luo, Bao, Liu, Wang, Yan, Wang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-Jun Wang, d2hqdW5AcGt1LmVkdS5jbg==; Lailai Yan, eWxsQGJqbXUuZWR1LmNu; Xu Ma, TkZQQ0NfbWFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xiangrong Xu
Xiangrong Xu Yuanyuan Wang2,3†
Yuanyuan Wang2,3† Yuelong Ji
Yuelong Ji Zheng Liu
Zheng Liu Xu Ma
Xu Ma