
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 17 February 2022
Sec. Obesity
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.740703
This article is part of the Research Topic Mechanisms involved in the Development of Obesity with PCOS View all 7 articles
 Manjunath Ramanjaneya1*
Manjunath Ramanjaneya1* Ibrahem Abdalhakam1
Ibrahem Abdalhakam1 Ilham Bettahi1
Ilham Bettahi1 Milin Bensila1
Milin Bensila1 Jayakumar Jerobin1
Jayakumar Jerobin1 Myint Myint Aye2
Myint Myint Aye2 Meis Alkasem1
Meis Alkasem1 Thozhukat Sathyapalan2
Thozhukat Sathyapalan2 Stephen Lawrence Atkin3
Stephen Lawrence Atkin3 Abdul-Badi Abou-Samra1
Abdul-Badi Abou-Samra1Background: The complement system is pivotal in host defense mechanisms, protecting against pathogenic infection by regulating inflammation and cell immunity. Complement-related protein activation occurs through three distinct pathways: classical, alternative, and lectin-dependent pathways, which are regulated by cascades of multiple proteins. Complement activation is recognized in polycystic ovary syndrome (PCOS) to be associated with obesity and insulin sensitivity. Exercise reduces insulin resistance and may help reduce obesity, and therefore, this study was undertaken to determine the effect of exercise on the activation of complement-related proteins in PCOS and control women.
Subjects and Measurements: In this study, 10 controls and 11 PCOS subjects who were age- and weight-matched underwent an 8-week supervised exercise program at 60% maximal oxygen consumption. Weight was unchanged though insulin sensitivity was increased in PCOS subjects and controls. Fasting baseline and post-exercise samples were collected and 14 complement-related proteins belonging to classical, alternative, and lectin-dependent pathways were measured.
Results: Baseline levels of complement C4b and complement C3b/iC3b were higher in PCOS (P < 0.05) compared with controls. Exercise reduced complement C1q (P < 0.05), C3 (P < 0.001), C4 (P < 0.01), factor B (P < 0.01), factor H (P < 0.01), and properdin (P < 0.05) in controls, but not in PCOS women.
Conclusion: Exercise induced complement changes in controls that were not seen in PCOS subjects, suggesting that these pathways remain dysregulated even in the presence of improved insulin sensitivity and not improved by moderate aerobic exercise.
Clinical Trial Registration: ISRCTN registry, ISRCTN42448814.
Polycystic ovarian syndrome (PCOS) is one of the most common endocrine illnesses affecting 5%–7% of women of reproductive age (1). Insulin resistance, obesity, and dyslipidemia, leading to increased cardiovascular risk and type 2 diabetes (T2D), are common features seen in PCOS women (1–3). Seventy-five percent of women diagnosed with PCOS exhibit obesity, with the majority showing increased central adiposity (4, 5). This PCOS phenotype is associated with hyperandrogenism and insulin resistance, resulting in a higher frequency of impaired glucose tolerance (IGT), T2D, and metabolic syndrome in women with PCOS (4–6). Despite the high prevalence of PCOS, its underlying pathophysiology and etiology still remain unknown, and its management in clinical practice is disjointed between gynecologists, endocrinologists, and general practitioners.
The complement system controls inflammation and comprises three activation pathways: classical, alternative, and lectin, with 50 proteins accounting for around 15% of the globulin fraction (7, 8). Antigen–antibody immune complexes, acute-phase proteins, and apoptotic and necrotic cells like C-reactive protein all activate the classical pathway. The lectin pathway identifies patterns of carbohydrate ligands on the surface of microorganisms by using mannose-binding lectins and ficolin. The alternative pathway is constitutively active in the typical host at low levels in preparation for rapid activation upon stimulus (8). In both the lectin and classical pathways, convertases require the cleavage of C2 and C4 (9) that necessitates the use of factors B and D (9). Both the classical and alternative activation pathways require complement C3. The immune system is impaired by a C3 deficit, making the body more susceptible to infection (10). Traditionally, C1s splits the C4 component in two halves, C4a and C4b. C4b connects to the cell membrane after binding to C2, divided in two subunits, C2a and C2b. Attributable to the serine protease activity of the C2a portion, a heterodimer known as classical C3 convertase is generated by merging the two C4b and C2a elements. As one of the steps of the healthy process, C3 convertase is needed to separate C3 protein further into C3a and C3b elements, which also exists in the lectin activation pathway (11, 12). Factor H and properdin are important in the regulation of alternative pathway activation: factor H acts as an inhibitor, dissociating C3 convertase; elevated levels of both indicate alternative pathway dysregulation, whereas properdin acts as a stabilizing agent of C3 convertase, resulting in prolonged complement activation (9). Only a few studies have examined complement-related protein levels in women with PCOS and reported higher factor D (13) and C3a levels (14, 15) and higher (15, 16) or no difference (17, 18) in C3 levels compared with matched controls. However, basal complement C3 levels are increased in insulin resistance with increased factor H levels associated with obesity in PCOS (9).
Physical activity is a natural stimulus that affects defensive and immune systems in both primary humoral and cellular immunity, influencing complement system activation (19). This interaction is complicated since routine moderate-intensity exercise will activate the immune system, while repetitive high-intensity activity (with inadequate recovery) may inhibit the immune system (20–23). Furthermore, strenuous exercise exertion may trigger oxidative stress with the release of heat shock proteins, catecholamines, cortisol, and insulin-like growth factor 1 (IGF-1) (20, 24), all of which may lead to immune stimulation or suppression based on other co-factors. Given that there are no prior reported studies on the effects of exercise on complement-related protein expression, this study was undertaken in weight-matched and age-matched women with and without PCOS before and after aerobic exercise of moderate duration.
The diagnosis of PCOS was established according to the Rotterdam criteria (13). Ten controls and 11 PCOS subjects were recruited as described previously (25). All the study participants attended fasting for baseline measurements. Following this, the study participants were enrolled in a supervised exercise program that consisted of 1-h supervised exercise three times per week for 8 weeks in the Department of Sports, Health and Exercise Science, University of Hull.
Where possible, each session was 1 h in duration depending on their ability to complete the sessions with no complications. The program used either a Woodway ELG55 motorized treadmill (Woodway, Weil am Rhein, Germany) or an HP Cosmos Pulsar Treadmill (H/P/Cosmos) with the same protocol. Participants performed all sessions on a motorized treadmill working at or as closely as possible to 60% VO2max. VO2/kg was measured (using an Oxycon Pro Metabolic System Jaegger, Hoechberg, Germany) after the warmup, which lasted for 5 min at 4.5 km h−1 and for a period of 10 min in order to confirm the appropriate exercise intensity. The intensity of exercise was then adjusted by altering the speed of the treadmill if this value was not within ±2.5% of the target oxygen uptake. Following this 10-min gas collection, the facemask was withdrawn with the speed of the treadmill remaining as it was. A further gas collection was made at 40 min to confirm the desired intensity for a 5-min period. If this intensity was out of range, then the treadmill speed was once again altered if required. Heart rate (HR) and rate of perceived exertion (RPE) (26) were monitored every 15 min throughout the session. If participants felt that they could not continue with the exercise for reasons such as injury or fatigue, they were able to stop at any time if necessary. Likewise, if it meant reducing the intensity for a period of time in order for them to recover, then this was permitted; otherwise, the intensity remained at the level predetermined. Each session ended with a 5-min cool down at 4.5 km h−1, and participants would then be free to leave once HR returned to within 120% of basal levels. During each exercise session, heart rate and inspired/expired gas fractions were continuously monitored and heart rate equivalent to 60% of baseline VO2max was achieved during each session of exercise training (27). Insulin sensitivity was measured using the gold standard euglycemic clamp technique as described previously (28, 29). Within a week following the completion of exercise protocol, all participants were invited to provide blood samples and underwent insulin sensitivity test.
The study was approved by the Yorkshire and the Humber Research Ethics Committee (reference number 10/H1313/44) and The Medical Research Center at Hamad Medical Corporation (reference number RP #17180/17). All study participants gave their written informed consent prior to participation in the study.
Plasma levels of human complement-related proteins were measured using MILLIPLEX MAP Kit Human Complement Magnetic Bead Panels 1 and 2 (HCMP1MAG-19K and HCMP2MAG-19K, Merck Millipore, USA) according to the instructions of the kit manufacturer as described previously (30). Protein levels in the plasma samples were quantified using 5PL logistic regression algorithms that are built into the Bio-Plex Manager 6 software, which was used for the quantification of all plasma samples with reference to the standards provided by the kit manufacturer using overnight incubation protocol. The samples were run on a Bio-Plex 200 (Bio-Rad, Hertfordshire, UK) instrument. The plasma samples were diluted 200 times for the measurement of complement panel-1 proteins (C2, C4b, C5, C5a, C9, factor D, mannose-binding lectin, and factor I) and 40,000 times for the measurements of complement panel 2 (C1q, C3, C3b/iC3b, C4, factor B, factor H, and properdin)-related proteins to attain the levels of the proteins within the reference range of the standard curve. The working range and assay precision for different complement-related proteins were reported previously (30).
Plasma levels of other variables were measured as reported previously (27). Serum insulin was measured using a competitive chemiluminescent immunoassay (Euro/DPC, Llanberis, UK). Plasma glucose was measured by a Synchon LX 20 analyzer (Bechman-Coulter, High Wycombe, UK). Triglycerides (TG) and total cholesterol measurements were done using Synchon LX 20 analyzer (Beckman-Coulter). The free androgen index (FAI) was calculated by dividing the total testosterone by sex hormone-binding globulin (SHBG) and then multiplying by 100. Serum testosterone (nmol/L) was assessed by high-performance liquid chromatography linked to tandem mass spectrometry (Waters Corporation, Manchester, UK); SHBG (nmol/L) levels were measured by immunometric assay with fluorescence detection on the DPC Immulite 2000 analyzer (Euro/DPC, Llanberis UK). FSH (iU/L) was measured by an Architect analyzer (Abbott Laboratories, Maidenhead, UK); TCH (mmol/L), TG (mmol/L), and HDL (mmol/L) were measured using Synchron LX 20 analyzer (Beckman Coulter); and LDL (mmol/L) was calculated using the Friedewald equation. Plasma glucose was measured using Synchron LX 20 analyzer (Beckman-Coulter). NEFA was measured using an enzymatic colorimetric method (Wako NEFA-H2) on a Konelab20 auto analyzer with a coefficient of variation of 1.4%. All the above measurements were done according to the recommended protocol of the manufacturer and as mentioned previously (25).
All the data are expressed as mean ± standard deviation (SD). Baseline differences between the control and PCOS and their expression levels after exercise were determined by unpaired Student’s t-test. The effects of exercise within and between the control and PCOS groups were determined by general linear model repeated measure ANOVA. This statistical method of analysis compares the exercise intervention effects within groups and the interaction of group and time and also compares between control and PCOS subjects. Spearman bivariate correlation analysis was performed to study the associations between clinical and biochemical measurements and the complement-related proteins. The statistical package SPSS 22.0 software was used for the data analysis and a P-value <0.05 (two-tailed) was considered statistically significant.
The baseline measurement of both control and PCOS subjects was previously reported (31). The samples used in this study are a subset from a previous study (31) in which weight did not differ between baseline and 8 weeks exercise for both PCOS and control subjects, though insulin sensitivity increased in both groups. In this study, no significant differences in complement C1q, C2, C5, C5a, C3, C4, factor B, factor H, factor D, mannose-binding lectin, properdin, and complement factor I at baseline were shown between PCOS and controls. Only complement C3b/iC3b (P = 0.048) and C4b (P = 0.036) were found to be significantly higher in PCOS subjects compared with controls (Table 1). Data comparing the differences between controls and PCOS groups following exercise showed significant differences for C1q (P = 0.005), C3 (P = 0.036), C4 (P = 0.01), factor B (P = 0.004), factor H (P = 0.005), properdin (P = 0.005), and C4b (P = 0.009) (Table 1).
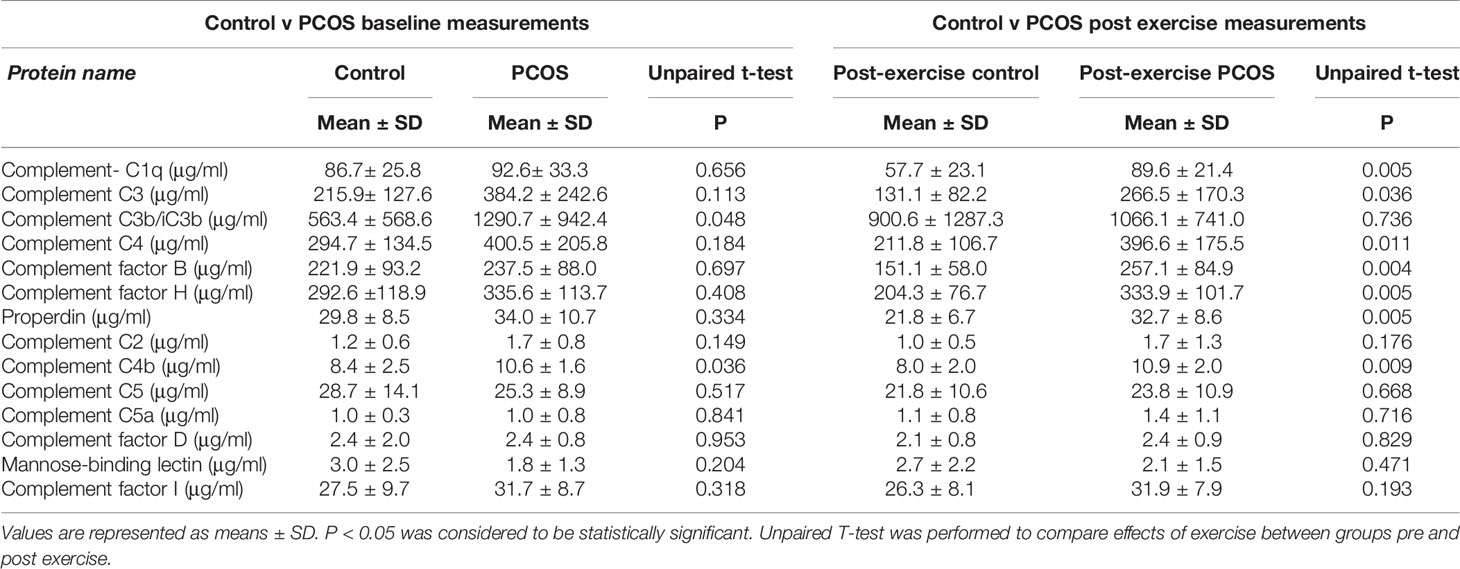
Table 1 Comparison of baseline and exercise induced changes in complement related proteins in control and PCOS subjects.
Following exercise, there were no significant changes in weight and BMI. However, there was a reduction in waist size both within groups (P = 0.031) and between groups (P = 0.025). Hip size within groups did not differ (P = 0.251) but did differ between groups (P = 0.045). A trend to an increase in M-value was seen within groups (P = 0.057) but differed between groups (P = 0.016). An increase in VO2max was seen both within groups (P = 0.001) and between groups (P < 0.0001, Table 2).
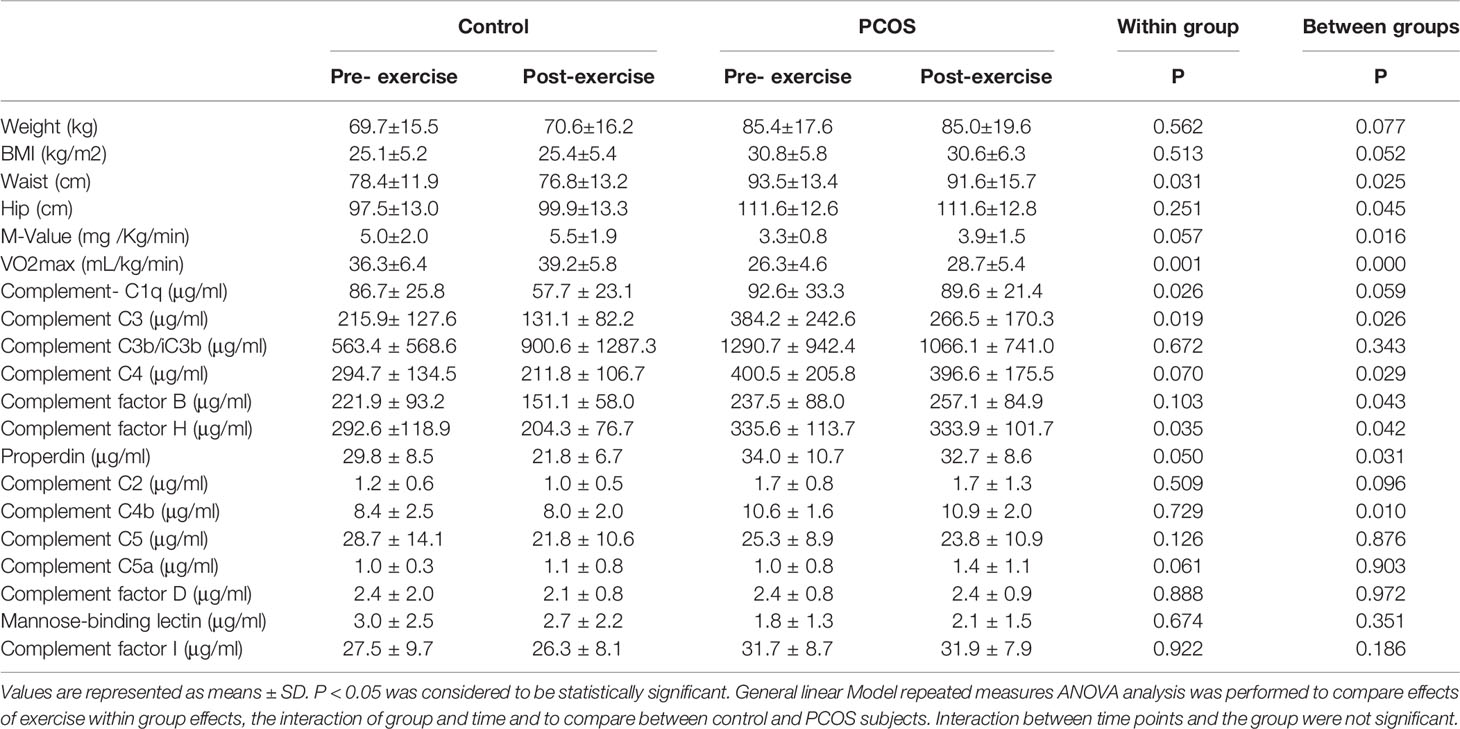
Table 2 Exercise induced changes in physiological variables and complement related proteins within and between control and PCOS subjects.
Exercise effects were analyzed by general linear model repeated measures to compare the effects of exercise within groups and between control and PCOS group effects. Our data showed that exercise significantly reduced the levels of complement C1q (P = 0.026) within groups, but between groups, there was no change. C3 (P = 0.019) differed both within groups and between groups (P = 0.026). C4 and complement factor B did not show any changes within groups; however, the control and PCOS group comparison was significantly altered for C4 (P = 0.029) and complement factor B (P = 0.043), following exercise. Complement factor H was significantly reduced both within groups (P = 0.035) and between groups (P = 0.042). Properdin differed significantly between control and PCOS (P = 0.031); similarly, complement C4b was also significantly reduced (P = 0.042) between group analysis of control and PCOS subjects following exercise. We did not observe any differences for complement C2, C3b/iC3b, C5, C5a, factor D, mannose-binding lectin, and complement factor I both within and between groups following exercise (Table 2). The interactions between time points and the groups were found to be similar (data not shown).
The association of complement-related proteins with baseline clinical, hormonal, and biochemical parameters was examined using the Spearman correlation coefficient (Tables 3A, B). In the control group, complement C3 levels correlated positively with waist circumference (P = 0.047), WHR (P = 0.042), and TG (P = 0.049). C4 (P = 0.015) and factor H (P = 0.026) also showed a positive correlation with waist circumference. Properdin showed positive correlations with waist circumference (P = 0.026) and ALT (P = 0.027). Similarly, complement C4b showed positive correlations with weight (P = 0.033) and BMI (P = 0.042) in controls (Table 3A). In the PCOS group, C3 showed a positive correlation with SBP (P = 0.042), and C4 showed a positive correlation with SBP (P = 0.011) and a negative correlation with TC (P = 0.003). Factor B did not show any significant correlations with measured clinical and biochemical variables. Factor H showed a positive correlation with SBP (P = 0.007) and a negative association with TC (P = 0.024). Properdin showed a positive correlation with SBP (P = 0.042) and C4b showed a negative correlation with ALT (P = 0.029, Table 3B).
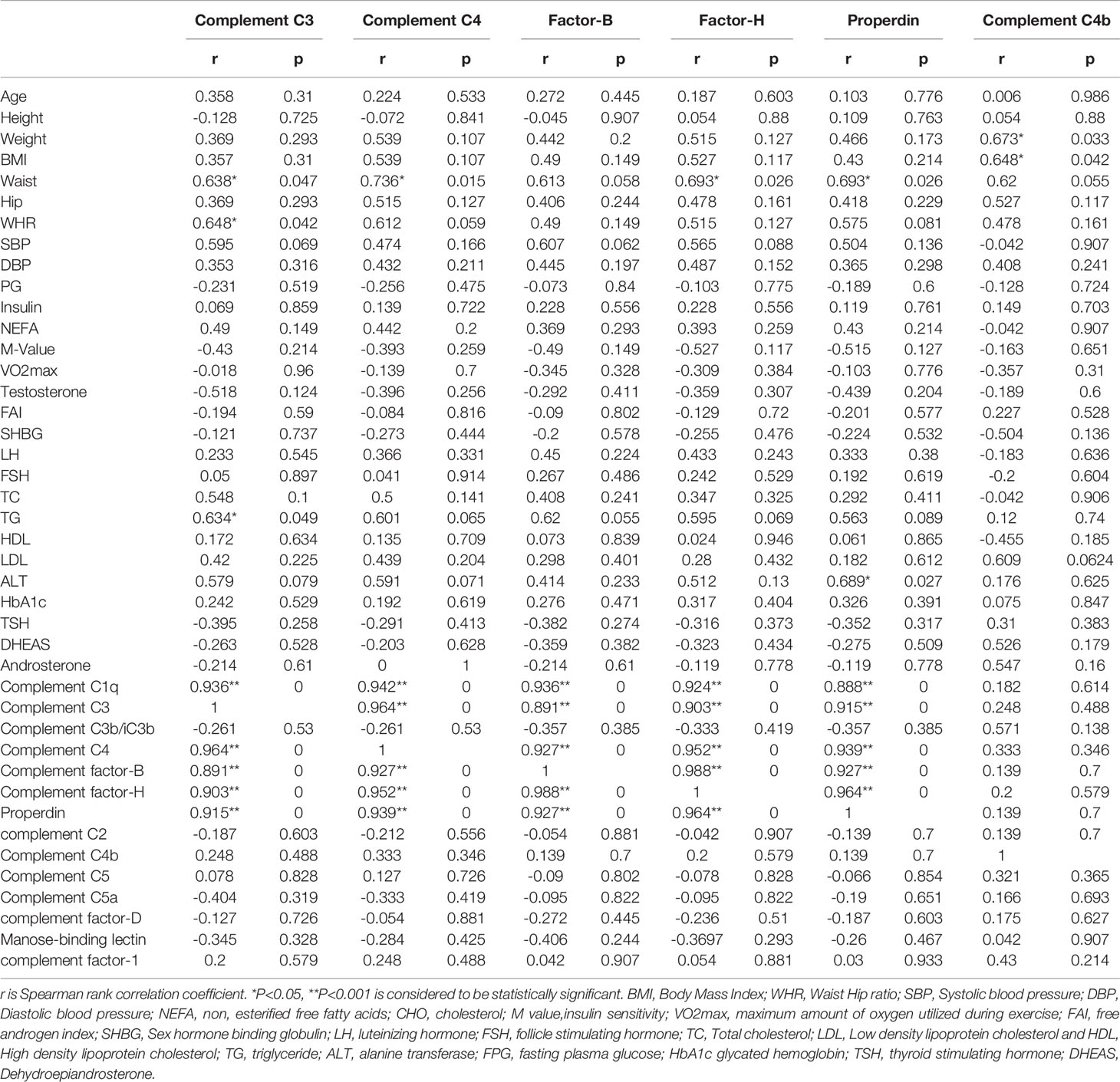
Table 3A Spearman rank correlation analysis of complement related proteins with anthropometric and hormonal variables before exercise controls.
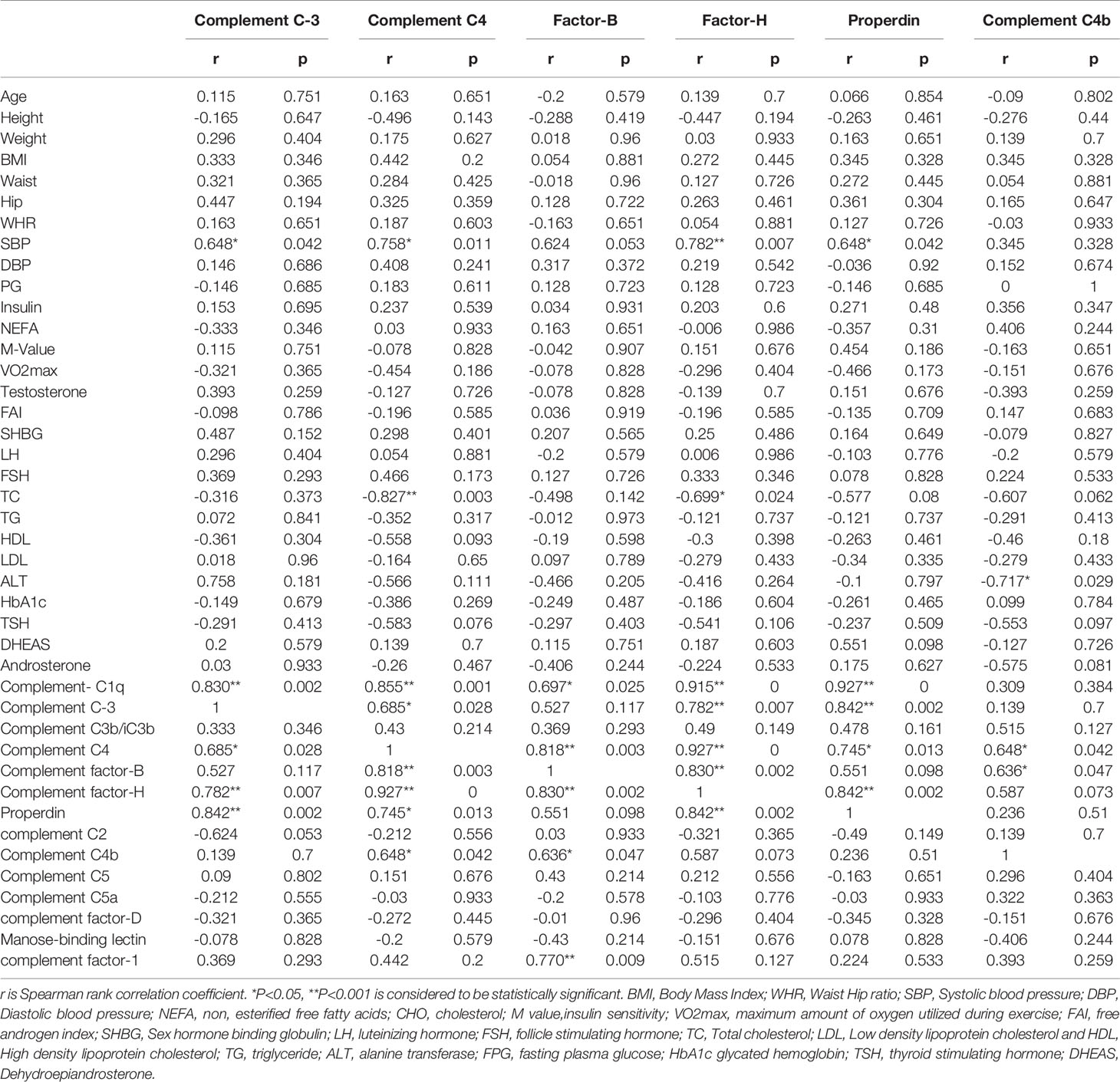
Table 3B Spearman rank correlation analysis of complement related proteins with anthropometric and hormonal variables before exercise in PCOS subjects.
In control subjects, complement C3 was positively associated with complement C1q (P = 0.001), C4 (P < 0.0001), factor B (P < 0.0001), factor H (P < 0.0001), and properdin (P = 0.0001). Complement C4 was positively associated with C1q (P < 0.0001), C3 (P < 0.0001), factor B (P < 0.0001), factor H (P < 0.0001), and properdin (P < 0.0001). Factor B was positively associated with C1q (P < 0.0001), C3 (P < 0.0001), C4 (P < 0.0001), factor H (P < 0.0001), and properdin (P < 0.0001). Similarly, factor H was positively associated with C1q (P < 0.0001), C3 (P < 0.0001), C4 (P < 0.0001), factor B (P < 0.0001), and properdin (P < 0.0001). Properdin was associated positively with C1q (P < 0.0001), C3 (P = 0.001), C4 (P < 0.0001), factor B (P < 0.0001), and factor H (P < 0.0001). Complement C4b did not show any significant associations with clinical or biochemical parameters measured in control subjects (Table 3A).
In PCOS subjects, complement C3 was positively associated with complement C1q (P = 0.002), C4 (P < 0.028), factor H (P < 0.007), and properdin (P = 0.002). Complement C4 was positively associated with C1q (P = 0.001), C3 (P = 0.028), factor B (P = 0.003), factor H (P < 0.0001), properdin (P = 0.013), and C4b (P = 0.042). Factor B was positively associated with C1q (P = 0.025), C4 (P = 0.003), factor H (P = <0.002), C4b (P = 0.047), and complement factor 1 (P = 0.009). Similarly, factor H was positively associated with C1q (P < 0.0001), C3 (P = 0.007), C4 (P < 0.0001), factor B (P = 0.002), and properdin (P = 0.002). Properdin was associated positively with C1q (P < 0.0001), C3 (P = 0.002), C4 (P = 0.013), and factor H (P = 0.002). Complement C4b showed a positive association with C4 (P = 0.042) and factor B (P = 0.047) in PCOS subjects (Table 3B).
In the control group, complement C3 (P = 0.034), C4 (P = 0.010), factor B (P = 0.030), factor H (P = 0.012), and properdin (P = 0.012) showed positive association with waist circumference (Table 3C). In PCOS subjects, complement C3 negatively correlated with VO2max (P = 0.026). C4 negatively correlated with VO2max (P = 0.001) and TC (P = 0.030). Factor B (P = 0.039) and factor H (P = 0.007) also showed a negative correlation with VO2max. Complement properdin showed a negative correlation with VO2max (P = 0.009) and a positive correlation with FSH (P = 0.035) in the PCOS group (Table 3D).
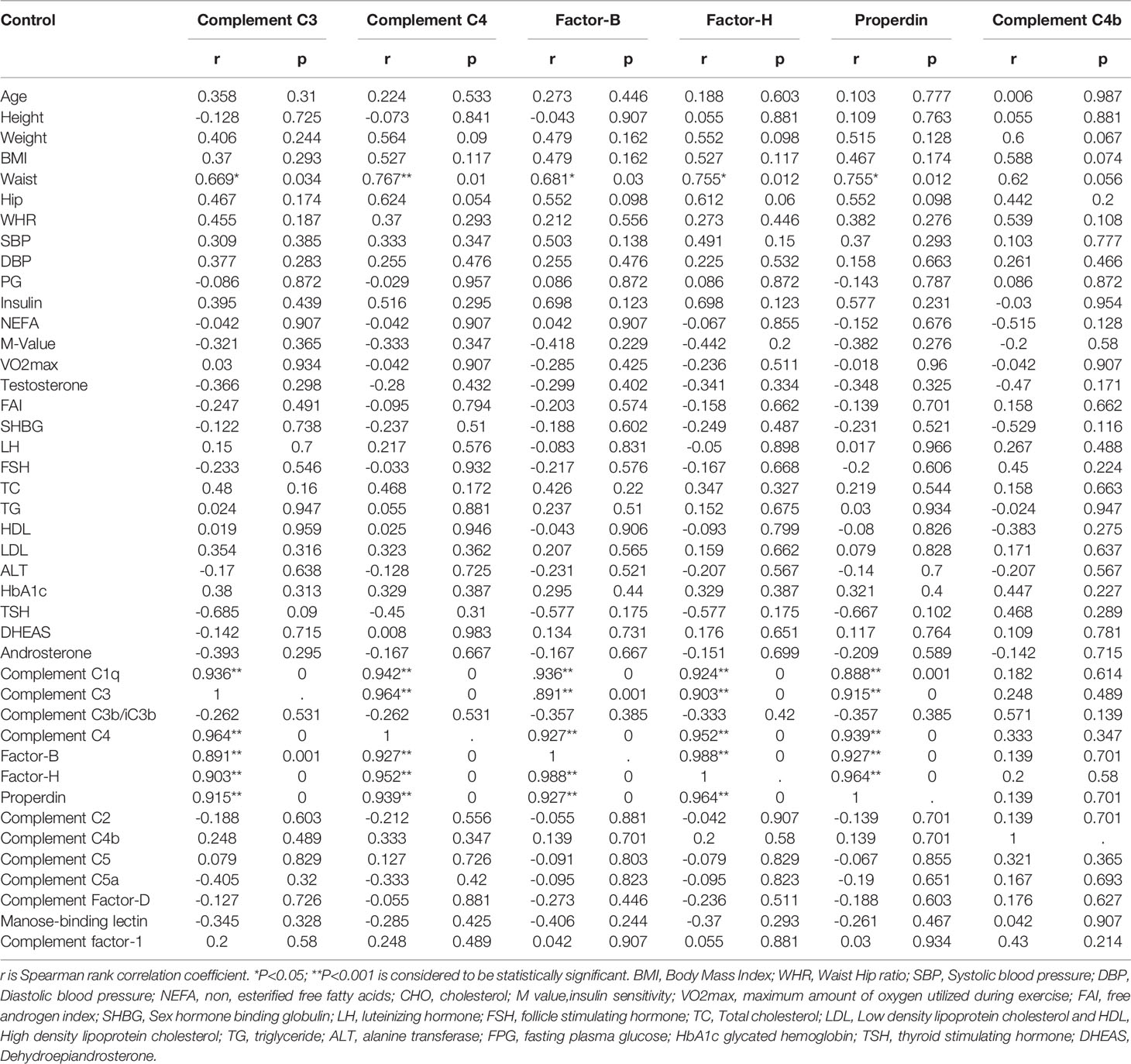
Table 3C Spearman rank correlation analysis of complement related proteins with anthropometric and hormonal variables after exercise controls.
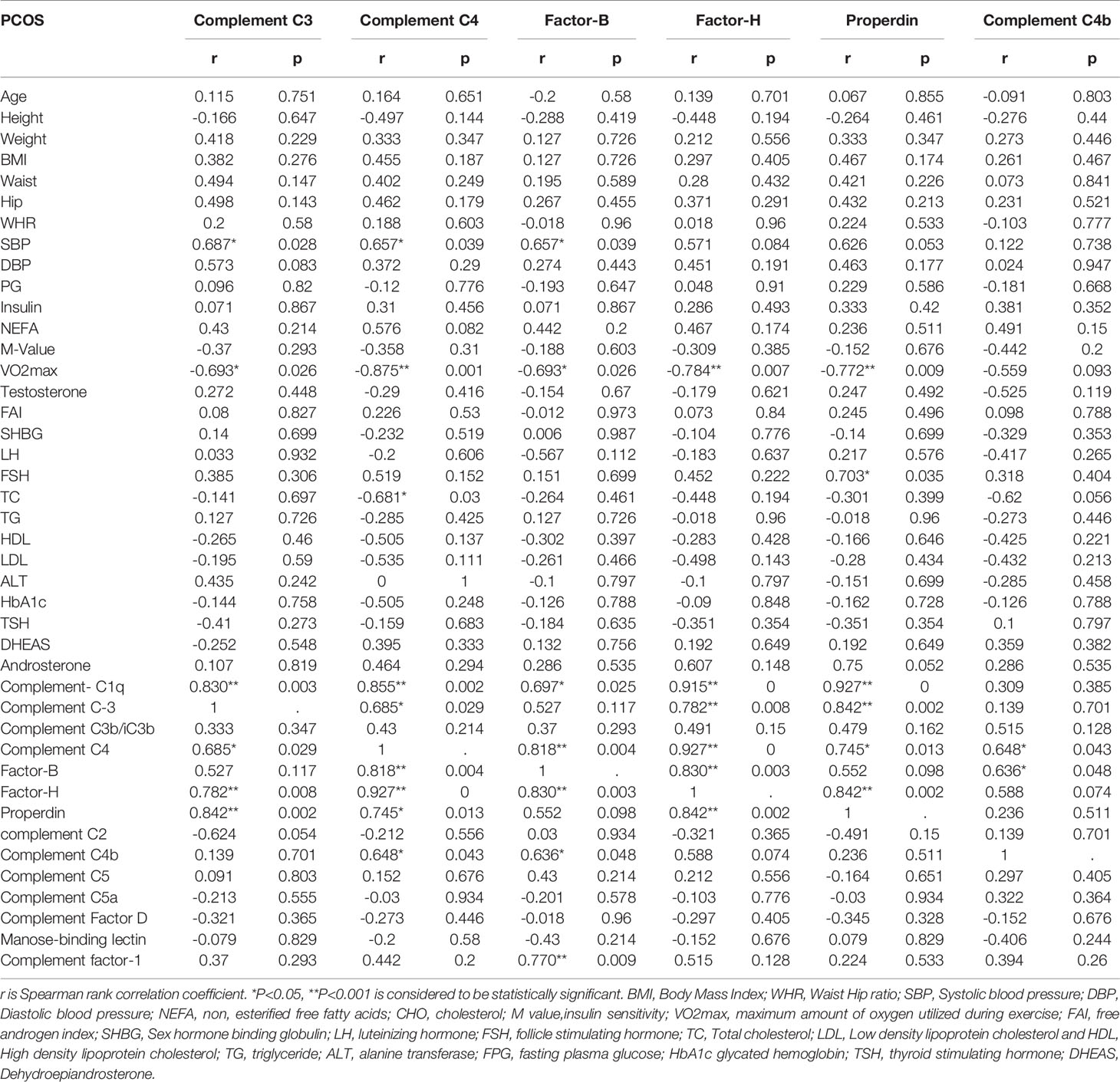
Table 3D Spearman rank correlation analysis of complement related proteins with anthropometric and hormonal variables after exercise PCOS subjects.
In control subjects, complement C3 was positively associated with complement C1q (P < 0.0001), C4 (P < 0.0001), factor B (P = 0.001), factor H (P < 0.0001), and properdin (P < 0.0001). Complement C4 was positively associated with C1q (P < 0.0001), C3 (P < 0.0001), factor B (P < 0.0001), factor H (P < 0.0001), and properdin (P < 0.0001). Factor B was positively associated with C1q (P < 0.0001), C3 (P = 0.001), C4 (P < 0.0001), factor H (P < 0.0001), and properdin (P < 0.0001). Similarly, factor H was positively associated with C1q (P < 0.0001), C3 (P < 0.0001), C4 (P < 0.0001), factor B (P < 0.0001), and properdin (P < 0.0001). Properdin was associated positively with C1q (P = 0.001), C3 (P < 0.0001), C4 (P < 0.0001), factor B (P < 0.0001), and factor H (P < 0.0001). Complement C4b did not show any association with clinical or biochemical variables in the control group of subjects (Table 3C). In PCOS subjects, C3 was positively associated with complement C1q (P = 0.003), C4 (P = 0.029), factor H (P = 0.008), and properdin (P = 0.002). Complement C4 was positively associated with C1q (P = 0.002), C3 (P = 0.0293), factor B (P = 0.004), factor H (P < 0.0001), properdin (P = 0.013), and C4b (P = 0.043). Factor B was positively associated with C1q (P = 0.025), C4 (P = 0.004), factor H (P = 0.003), C4b (P = 0.048), and complement factor 1 (P = 0.009). Similarly, factor H was positively associated with C1q (P < 0.0001), C3 (P = 0.008), C4 (P < 0.0001), factor B (P = 0.003), and properdin (P = 0.002). Properdin was associated positively with C1q (P < 0.0001), C3 (P = 0002), C4 (P = 0.013), and factor H (P = 0.002). Complement C4b was associated positively with C4 (P = 0.043) and factor B (P = 0.048, Table 3D).
The complement system plays important roles in immunity and inflammation (8). Obesity and PCOS are characterized by dysregulation of several components of the complement system (9). Aerobic exercises were shown to induce the activation of the alternative pathway of the complement system (19). Our study shows that moderate aerobic exercise improves insulin sensitivity and cardiometabolic fitness in both the control and the PCOS subjects; however, it predominantly reduced the components of the complement system in the control subjects, but not in the subjects with PCOS.
At baseline, we found significantly elevated complement C4b in PCOS subjects compared with controls. However, no significant differences were observed between controls and PCOS subjects for complement factor I, C1q, C2, C3, C3b/iC3b, C4, C5, C5a, complement factor D, mannose-binding lectin, factor B, factor H, and properdin. Our findings are consistent with some (17, 18) but not all (14, 16) previously reported studies, where no differences in C3 level in women with PCOS compared with controls were found. These differences may be due to the relationship of the complement proteins to insulin resistance and obesity (9) as PCOS women were weight- and age-matched to the controls with no difference in the baseline insulin resistance, and therefore, those complement changes due to these parameters would have been taken into account.
In this study, moderate aerobic exercise showed a significant reduction of complement-related protein family members both within-group (C1q, C3, and factor H) and between-group (C3, C4, factor B, factor H, properdin, and C4b) analyses. However, these changes appear to be predominantly restricted to the control group of subjects. In the PCOS group, exercise did not change the levels of the secreted complement components C1q, C3, and factor H, and therefore, it appears that complement pathways were not activated in PCOS subjects following moderate exercise. This may be due to dysregulation of the complement system in women with PCOS (Figure 1B). In comparison, members of complement proteins C1q, C3, and factor H were significantly downregulated following exercise in controls (Figure 1A). The downregulation of these complement proteins following exercise may make individuals more susceptible to infections, particularly C3 (10). The significant decreases in C3 protein in the control group were similar to those reported in previous studies (32–34). The effect of the complement system on the immune system has a wide range of biological consequences, implying a wide range of relationships. It is thought that high-intensity physical exertion impairs the immune system of an organism (20, 35, 36) and that their long-term effects might lead to immunosuppression (20, 21). Regular, moderate-intensity physical exercise, on the other hand, promotes development and improves immunity (20, 35).
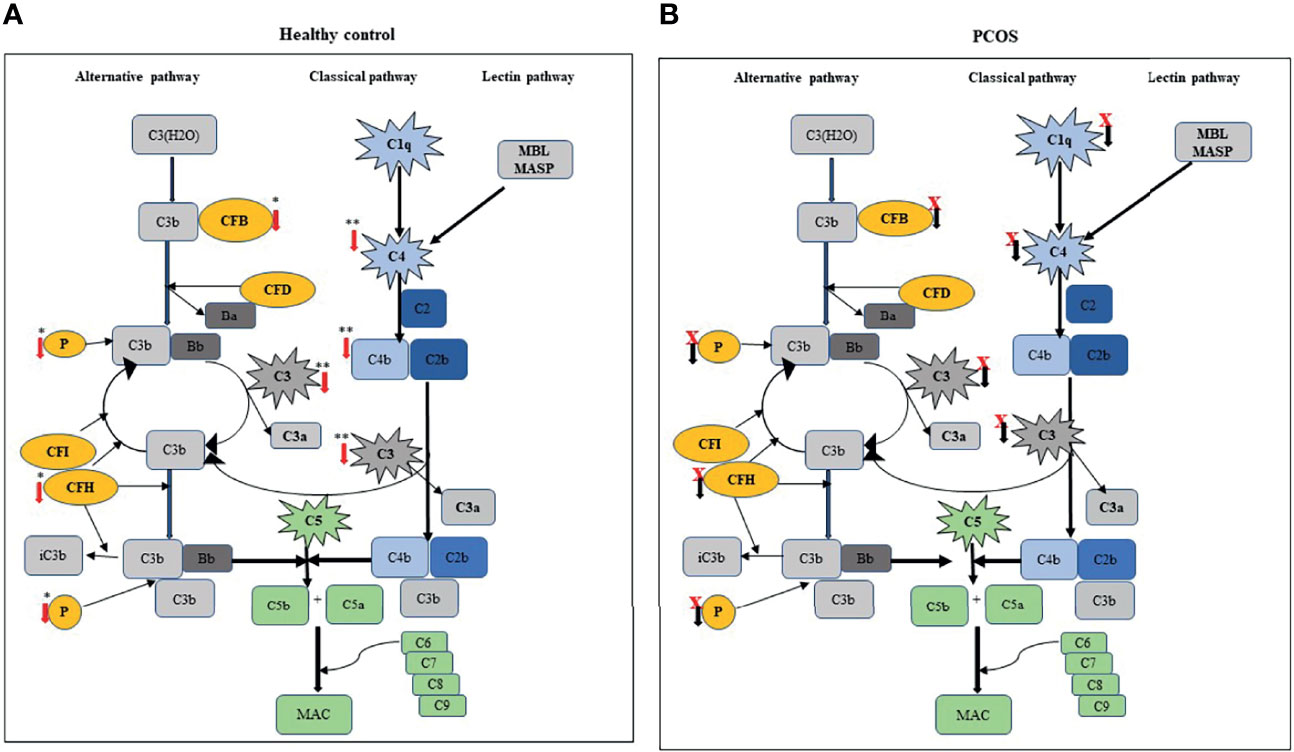
Figure 1 Comparison of exercise-induced changes in complement-related proteins between control and PCOS subjects: (A) schematic represents the level of secreted complement components, such as C1q, C3, C3b/iC3b, C4, and FB; FH, properdin (P), and C4b were significantly decreased after exercise in a healthy subject. (B) In PCOS subjects, circulating component-related proteins were not significantly altered following exercise. Red (↓) arrow indicates the complement-related proteins that are reduced following exercise. In the PCOS group, black (↓) arrow with a red cross indicates no significant difference following exercise.
In our study, complement C3 and C4, as well as factor B, factor H, properdin, and C4b levels, were significantly different between groups after exercise. Assessment of the relationships between these complement-related proteins with anthropometric and hormonal characteristics of the subjects were in accord with the relationship of complement proteins with weight and BMI (9); we found a positive correlation for waist circumference in the control group of subjects. VO2max is a factor that determines cardiometabolic status, and here VO2max negatively correlated with complement C3 and C4b, as well as factor B, factor H, and properdin levels, suggesting that lower VO2max (i.e., more unfit) was associated with higher complement levels at baseline. Similarly, another cardiometabolic-related factor SBP showed significant positive correlations following exercise, with complement factors C3, C4, and factor B in the PCOS group suggesting the link between complement factors and cardiovascular risk, which is usually higher in PCOS subjects. As expected, most of the family members of complement-related proteins regulate each other; therefore, significant positive correlations of complement-related proteins and its family members were seen both at baseline and following exercise.
The limitations of this study include the small number of participants from one single ethnicity (Caucasian) and did not account for metabolic differences and phenotypic differences that usually exists in PCOS subjects. Therefore, the findings may not be generalized to the wider population or to different ethnicities.
In conclusion, our data showed that exercise reduced several components of the complement system—C1q, C3, C4, factor B, factor H, and properdin—in control subjects, but not in PCOS women, although they were age- and weight-matched. The data suggest that the component system pathways remain dysregulated after moderate aerobic exercise in PCOS compared with control subjects, although insulin sensitivity after exercise was improved in both groups.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Yorkshire and the Humber Research Ethics Committee (reference number 10/H1313/44) and The Medical Research Center at Hamad Medical Corporation (reference number RP #17180/17). All study participants gave their written informed consent prior to participation in the study. The patients/participants provided their written informed consent to participate in this study.
MR, IB, JJ, and MB performed the measurements and contributed to the manuscript. IA and MR wrote the manuscript. SA, TS, and MM recruited the subjects and were involved in sample collection and data analysis. MA researched the data and contributed to the manuscript. MR, A-BA-S, and SA conceptualized the study, designed the experiments, supervised the progress, analyzed the data, and approved the final version of the article. All the authors reviewed and revised the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past collaboration with several of the authors (MR, IB, SA, A-BA-S).
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Qatar Metabolic Institute, Medical Research Center, Hamad Medical Corporation, Doha, Qatar for supporting the study with funding, and the Medical Research Center, Hamad Medical Corporation for the article processing fees support.
1. Ehrmann DA. Polycystic Ovary Syndrome. N Engl J Med (2005) 352(12):1223–36. doi: 10.1056/NEJMra041536
2. Jayasena CN, Franks S. The Management of Patients With Polycystic Ovary Syndrome. Nat Rev Endocrinol (2014) 10(10):624. doi: 10.1038/nrendo.2014.102
3. Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, et al. Women With Polycystic Ovary Syndrome Have Intrinsic Insulin Resistance on Euglycaemic–Hyperinsulaemic Clamp. Hum Reprod (2013) 28(3):777–84. doi: 10.1093/humrep/des463
4. Kyrou I, Weickert MO, Randeva HS. Diagnosis and Management of Polycystic Ovary Syndrome (PCOS). In: Ajjan R, Orme S, editors. Endocrinology and Diabetes. London: Springer (2015) 99–113. doi: 10.1007/978-1-4471-2789-5_13
5. Kyrou I, Randeva HS, Tsigos C, Kaltsas G, Weickert MO. Clinical Problems Caused by Obesity. Endotext (2018).
6. Randeva HS, Tan BK, Weickert MO, Lois K, Nestler JE, Sattar N, et al. Cardiometabolic Aspects of the Polycystic Ovary Syndrome. Endocrine Rev (2012) 33(5):812–41. doi: 10.1210/er.2012-1003
7. Carroll MC. A Protective Role for Innate Immunity in Autoimmune Disease. Clin Immunol (2000) 95(1):S30–8. doi: 10.1006/clim.1999.4813
9. Lewis RD, Narayanaswamy AK, Farewell D, Rees DA. Complement Activation in Polycystic Ovary Syndrome Occurs in the Postprandial and Fasted State and Is Influenced by Obesity and Insulin Sensitivity. Clin Endocrinol (2021) 94(1):74–84. doi: 10.1111/cen.14322
10. Matsuyama W, Nakagawa M, Takashima H, Muranaga F, Sano Y, Osame M. Molecular Analysis of Hereditary Deficiency of the Third Component of Complement (C3) in Two Sisters. Intern Med (2001) 40(12):1254–8. doi: 10.2169/internalmedicine.40.1254
11. Sarma JV, Ward PA. The Complement System. Cell Tissue Res (2011) 343(1):227–35. doi: 10.1007/s00441-010-1034-0
12. Prohászka Z, Kirschfink M, Frazer-Abel A. Complement Analysis in the Era of Targeted Therapeutics. Mol Immunol (2018) 102:84–8. doi: 10.1016/j.molimm.2018.06.001
13. Gursoy Calan O, Calan M, Yesil Senses P, Unal Kocabas G, Ozden E, Sari KR, et al. Increased Adipsin Is Associated With Carotid Intima Media Thickness and Metabolic Disturbances in Polycystic Ovary Syndrome. Clin Endocrinol (2016) 85(6):910–7. doi: 10.1111/cen.13157
14. Wu Y, Zhang J, Wen Y, Wang H, Zhang M, Cianflone K. Increased Acylation-Stimulating Protein, C-Reactive Protein, and Lipid Levels in Young Women With Polycystic Ovary Syndrome. Fertil Steril (2009) 91(1):213–9. doi: 10.1016/j.fertnstert.2007.11.031
15. Oktenli C, Ozgurtas T, Dede M, Sanisoglu YS, Yenen MC, Yesilova Z, et al. Metformin Decreases Circulating Acylation-Stimulating Protein Levels in Polycystic Ovary Syndrome. Gynecological Endocrinol (2007) 23(12):710–5. doi: 10.1080/09513590701666571
16. Yang S, Li Q, Song Y, Tian B, Cheng Q, Qing H, et al. Serum Complement C3 has a Stronger Association With Insulin Resistance Than High-Sensitivity C-Reactive Protein in Women With Polycystic Ovary Syndrome. Fertil Steril (2011) 95(5):1749–53. doi: 10.1016/j.fertnstert.2011.01.136
17. Dehdashtihaghighat S, Mehdizadehkashi A, Arbabi A, Pishgahroudsari M, Chaichian S. Assessment of C-Reactive Protein and C3 as Inflammatory Markers of Insulin Resistance in Women With Polycystic Ovary Syndrome: A Case-Control Study. J Reprod Infertil (2013) 14(4):197.
18. Snyder ML, Shields KJ, Korytkowski MT, Sutton-Tyrrell K, Talbott EO. Complement Protein C3 and Coronary Artery Calcium in Middle-Aged Women With Polycystic Ovary Syndrome and Controls. Gynecological Endocrinol (2014) 30(7):511–5. doi: 10.3109/09513590.2014.895985
19. Kostrzewa-Nowak D, Kubaszewska J, Nowakowska A, Nowak R. Effect of Aerobic and Anaerobic Exercise on the Complement System of Proteins in Healthy Young Males. J Clin Med (2020) 9(8):2357. doi: 10.3390/jcm9082357
20. Njernqn D. Exercise Immunology: Practical Applications. J Sports Med (1997) 18(1):91–100. doi: 10.1055/s-2007-972705
21. Peake JM, Neubauer O, Della Gatta PA, Nosaka K. Muscle Damage and Inflammation During Recovery From Exercise. J Appl Physiol (2017) 122(3):559–70. doi: 10.1152/japplphysiol.00971.2016
22. Peake JM, Neubauer O, Walsh NP, Simpson RJ. Recovery of the Immune System After Exercise. J Appl Physiol (2017) 122(5):1077–87. doi: 10.1152/japplphysiol.00622.2016
23. Pedersen BK, Hoffman-Goetz L. Exercise and the Immune System: Regulation, Integration, and Adaptation. Physiol Rev (2000). doi: 10.1152/physrev.2000.80.3.1055
24. Liu X, Zeng Z, Zhao L, Xiao W, Chen P. Changes in Inflammatory and Oxidative Stress Factors and the Protein Synthesis Pathway in Injured Skeletal Muscle After Contusion. Exp Ther Med (2018) 15(2):2196–202. doi: 10.3892/etm.2017.5625
25. Aye MM, Kilpatrick ES, Aburima A, Wraith KS, Magwenzi S, Spurgeon B, et al. Acute Hypertriglyceridemia Induces Platelet Hyperactivity That Is Not Attenuated by Insulin in Polycystic Ovary Syndrome. J Am Heart Assoc (2014) 3(1):e000706. doi: 10.1161/JAHA.113.000706
26. Foster C, Florhaug JA, Franklin J, Gottschall L, Hrovatin LA, Parker S, et al. A New Approach to Monitoring Exercise Training. J Strength Conditioning Res (2001) 15(1):109–15.
27. Kirk RJ, Madden LA, Peart DJ, Aye MM, Atkin SL, Vince RV. Circulating Endothelial Microparticles Reduce in Concentration Following an Exercise Programme in Women With Polycystic Ovary Syndrome. Front Endocrinol (2019) 10:200. doi: 10.3389/fendo.2019.00200
28. Ramanjaneya M, Bensila M, Bettahi I, Jerobin J, Samra TA, Aye MM, et al. Dynamic Changes in Circulating Endocrine FGF19 Subfamily and Fetuin-A in Response to Intralipid and Insulin Infusions in Healthy and PCOS Women. Front Endocrinol (2020) 11:568500. doi: 10.3389/fendo.2020.568500
29. Halama A, Aye MM, Dargham SR, Kulinski M, Suhre K, Atkin SL. Metabolomics of Dynamic Changes in Insulin Resistance Before and After Exercise in PCOS. Front Endocrinol (2019) 10:116. doi: 10.3389/fendo.2019.00116
30. Ramanjaneya M, Butler AE, Alkasem M, Bashir M, Jerobin J, Godwin A, et al. Association of Complement-Related Proteins in Subjects With and Without Second Trimester Gestational Diabetes. Front Endocrinol (2021) 12:641361. doi: 10.3389/fendo.2021.641361
31. Ramanjaneya M, Jerobin J, Bettahi I, Bensila M, Aye M, Siveen KS, et al. Lipids and Insulin Regulate Mitochondrial-Derived Peptide (MOTS-C) in PCOS and Healthy Subjects. Clin Endocrinol (Oxf) (2019) 91: (2):278–87. doi: 10.1111/cen.14007
32. Karacabey K, Saygin O, Ozmerdivenli R, Zorba E, Godekmerdan A, Bulut V. The Effects of Exercise on the Immune System and Stress Hormones in Sportswomen. Neuroendocrinol Lett (2005) 26(4):361–6.
33. Mashiko T, Umeda T, Nakaji S, Sugawara K. Position Related Analysis of the Appearance of and Relationship Between Post-Match Physical and Mental Fatigue in University Rugby Football Players. Br J Sports Med (2004) 38(5):617–21. doi: 10.1136/bjsm.2003.007690
34. Karacabey K, Peker I, Saygın Ö, Cıloglu F, Ozmerdivenli R, Bulut V. Effects of Acute Aerobic and Anaerobic Exercise on Humoral Immune Factors in Elite Athletes. Biotechnol Biotechnol Equip (2005) 19(1):175–80. doi: 10.1080/13102818.2005.10817177
35. Simpson RJ, Kunz H, Agha N, Graff R. Exercise and the Regulation of Immune Functions. Prog Mol Biol Trans Sci (2015) 135:355–80. doi: 10.1016/bs.pmbts.2015.08.001
Keywords: PCOS, T2D, complement-related proteins, M value, VO2max, exercise
Citation: Ramanjaneya M, Abdalhakam I, Bettahi I, Bensila M, Jerobin J, Aye MM, Alkasem M, Sathyapalan T, Atkin SL and Abou-Samra A-B (2022) Effect of Moderate Aerobic Exercise on Complement Activation Pathways in Polycystic Ovary Syndrome Women. Front. Endocrinol. 12:740703. doi: 10.3389/fendo.2021.740703
Received: 21 July 2021; Accepted: 23 December 2021;
Published: 17 February 2022.
Edited by:
Amelia Palermo, University of California, Los Angeles, United StatesReviewed by:
Harpal Singh Randeva, University Hospitals Coventry and Warwickshire NHS Trust, United KingdomCopyright © 2022 Ramanjaneya, Abdalhakam, Bettahi, Bensila, Jerobin, Aye, Alkasem, Sathyapalan, Atkin and Abou-Samra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manjunath Ramanjaneya, TXJhbWFuamFuZXlhQGhhbWFkLnFh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.