- 1Medical Department, ART Fertility Clinics, Abu Dhabi, United Arab Emirates
- 2Medical Department, Advanced Reproductive Technologies (ART) Fertility Clinics, Dubai, United Arab Emirates
- 3Biostatistics Department, Consofi Pharma, Dubai, United Arab Emirates
- 4Departamento de Biomedicina y Biotecnología, Universidad de Alcalá, Madrid, Spain
- 5Medical Department, Women’s University Hospital Tuebingen, Tuebingen, Germany
Background: Anti-Müllerian hormone (AMH) and antral follicle count (AFC) age-specific reference values form the basis of infertility treatments, yet they were based upon studies performed primarily on Caucasian populations. However, they may vary across different age-matched ethnic populations. This study aimed to describe age-specific serum AMH and AFC for women native to the Arabian Peninsula.
Methods: A retrospective large-scale study was performed including 2,495 women, aged 19 to 50 years, native to the Arabian Peninsula. AMH and AFC were measured as part of their fertility assessment at tertiary-care fertility centres. Age-specific values and nomograms were calculated.
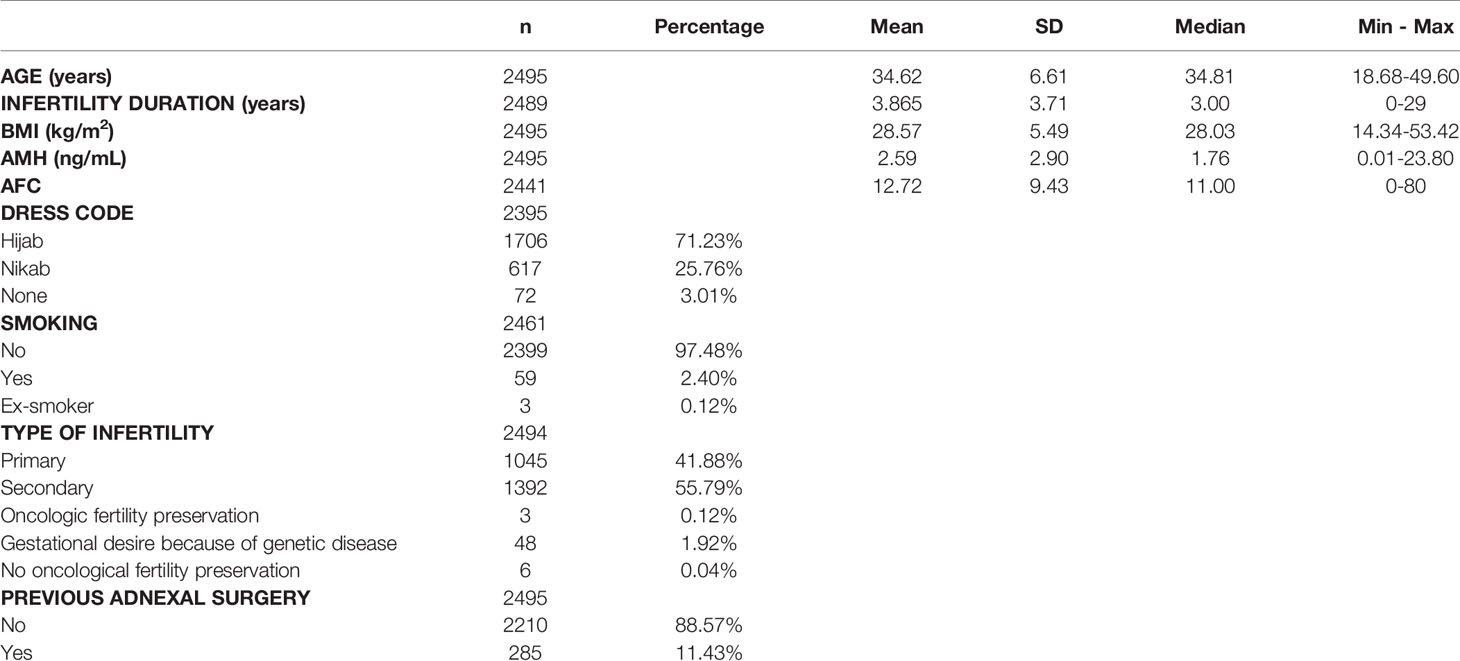
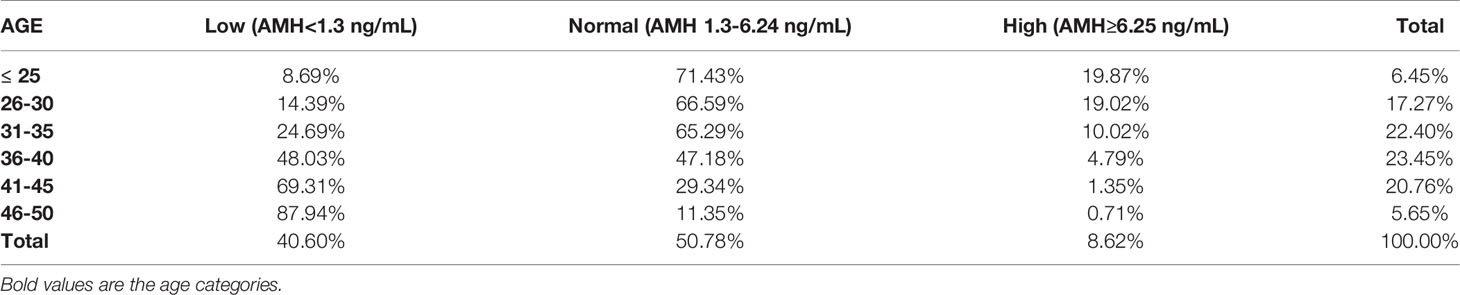
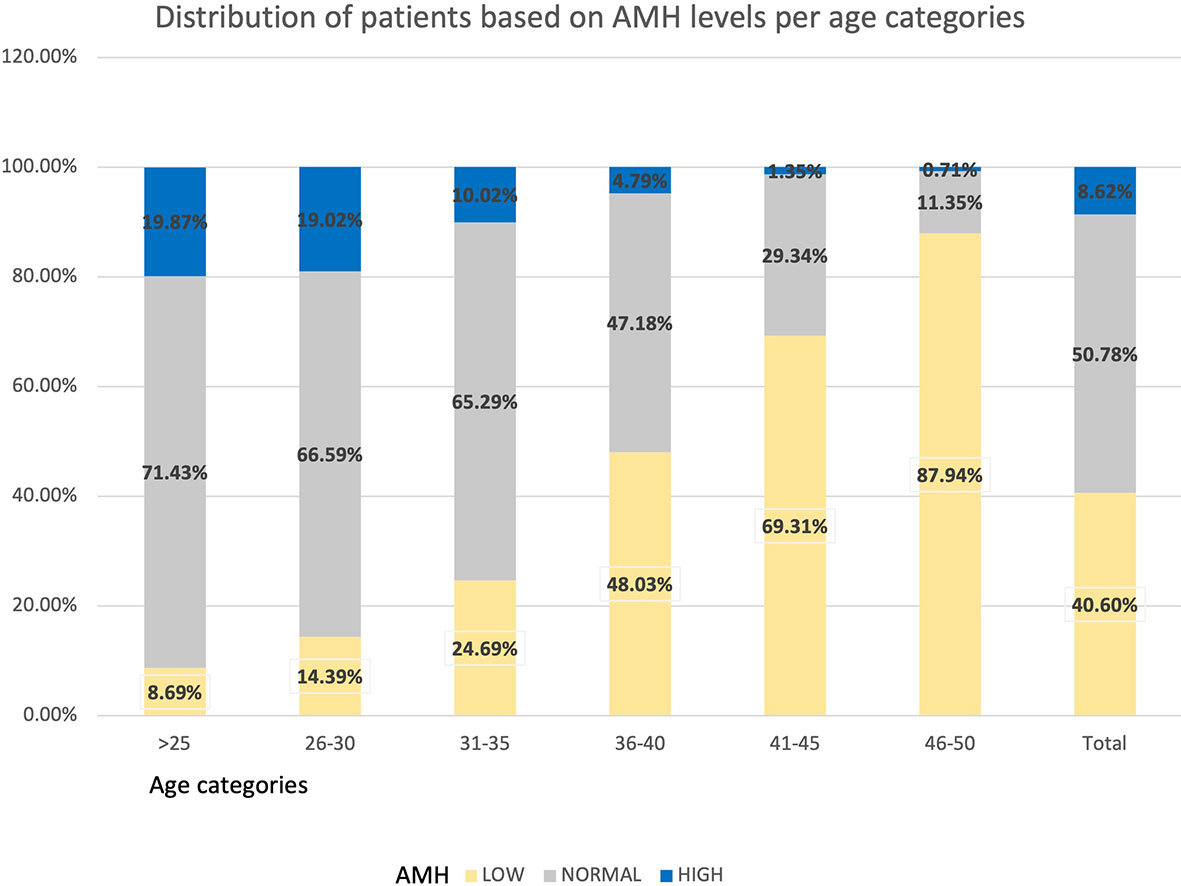
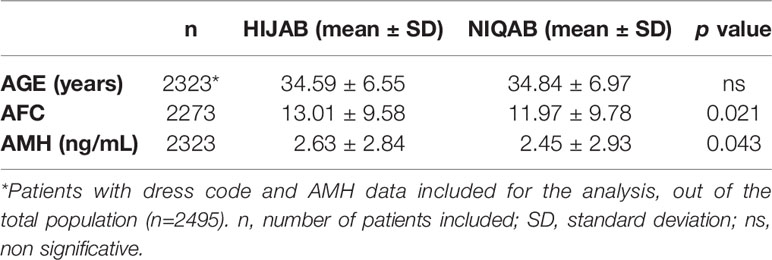
Results: 2,495 women were evaluated. Mean, standard deviation and median values were calculated for AMH and AFC by 1-year and 5-years intervals. Median age was 34.81 years, median AMH was 1.76ng/ml and median AFC was 11. From the total group, 40.60% presented with AMH levels below 1.3ng/mL. For women <45 years old, the decrease in AFC was between -0.6/-0.8 per year. Up to 36 years old, the decrease of AMH was 0.1ng/ml. However, from 36 to 40 years old, an accelerated decline of 0.23ng/ml yearly was noted. In keeping with local customs, 71.23% of women wore the hijab and 25.76% the niqab. AMH and AFC were significantly lower for niqab group compared with hijab group (p=0.02 and p=0.04, respectively).
Conclusion: This is to-date the largest data set on age-specific AMH and AFC values in women from the Arabian Peninsula aiming to increase clinical awareness of the ovarian reserve in this population.
Introduction
In recent years, Anti-Müllerian hormone (AMH) and antral follicle count (AFC) have become widely used markers for baseline assessment of ovarian reserve, especially preceding assisted reproductive techniques (ART) (1). AMH is used to individualize the treatment regime, not only due to its ability to predict the ovarian response to gonadotropins and the risk of cycle cancellation (2–4), but also as an indicator for oocyte and embryo quality (5, 6), euploidy rate (5, 7) and miscarriage rate (8) and oocyte survival rates following vitrification (9).
Ovarian reserve declines over the reproductive lifespan and has a relevant impact on present and future fertility. This is why AMH and AFC have an important influence on treatment decisions. However, these markers can be influenced by different factors. The so far biggest database on age-specific AMH values, which includes more than 17,000 patients, was published by Seifer et al. in 2011 (10). They report age-specific mean and median AMH values in serum samples from patients, aged 24 to 50 years, obtained from U.S. fertility centers in 37 different states and measured by the first-generation immunoassay DSL. AMH ranges over the life span of a women were published by Kelsey et al. (11) by combining data from 20 studies (3260 data points). Likewise, most studies have evaluated the ovarian reserve parameters in Caucasian populations and only few publications have considered other ethnicities (12–14). However, it has been shown that AMH and AFC varies across different age-matched ethnic populations (14–16). Studies comparing patients from Middle East/North Africa (MENA) region with Caucasian patients described a reduced ovarian reserve and lower number of retrieved oocytes for the MENA population (17, 18). These findings point to significant race-dependent differences in the ovarian reserve and in ovarian ageing (19, 20).
Due to the lack of large-scale age-specific ovarian reserve parameters for women from the Arabian Peninsula and with the aim to close this gap in knowledge, this study describes age-specific AMH and AFC values from women native to the Arabian Peninsula region and evaluates them in the context of the existing sociocultural and religious habits from the Middle East. These values can be used as reference by infertility centers treating patients of similar ethnicity, racial and sociocultural characteristics.
Methods
Study Design and Participants
This is a large-scale retrospective, observational study for which data from the clinical documentation program had been extracted and analyzed after the ethical approval had been obtained. Included were data from women, native to the Arabian Peninsula, who had their serum AMH and AFC (sum of small antral follicles in both ovaries) measured as part of their fertility assessment at ART Fertility Clinics Abu Dhabi (UAE), Dubai (UAE) and Muscat (Oman), from May 2015 to November 2019. The Arabian peninsula is composed of the countries Yemen, Oman, Qatar, Bahrain, Kuwait, Saudi Arabia and the United Arab Emirates (21).
Serum AMH concentrations were measured by Elecsys® AMH automated assay (for Cobas 601 platform, Roche®) for all patients. Imprecision expected from the assay was <5%, as described by the manufacturer; intra-assay and inter-assay co-efficient of variation for Elecsys® AMH automated assay has been reported as 0.5 – 1.4% and 0.7 – 1.9%, respectively (22). To assess AFC, women underwent transvaginal 2D-sonography (Voluson E8, GE Healthcare, United States) during the menstrual cycle (23). The patients were asked to empty their bladders and were placed in the lithotomy position. The ultrasound-scans were performed by reproductive medicine specialists and a systematic ultrasound technique for AFC measurement was used in order to avoid any bias through different techniques: identification of the ovaries and evaluation of the dimensions in two planes; measurement of the largest follicle in two dimensions; the probe was then positioned along the long axis of the ovary and count the follicles from outer ovarian margin to the opposite margin for both ovaries. The number of follicles in each ovary was combined to obtain the AFC. The number of antral follicles of 2 to 10mm in diameter were counted (24).
A strict dress code is imposed on the women from the Arabian Peninsula due to sociocultural and religious habits in the Middle East region. “Hijab” describes a type of modest clothing with a veil covering the head, neck and chest and “Niqab” is a veil that covers the face while leaving the eyes uncovered (25).
Each patient’s data was recorded only once in the study. Patients, who had been on any hormonal treatment within three months before AMH and AFC measurement, were excluded. Ethical approval was obtained from the Research Ethics Committee (REFA040) of ART Fertility clinic, Abu Dhabi, UAE.
Statistical Analysis
Patient characteristics are described using mean ± SD, median, minimum, and maximum values for continuous variables, frequencies and percentages for categorical variables. The normality of data distribution was tested through Kolmogorov-Smirnov normality test. Mean ± SD and median values were calculated for AMH and AFC for women ages 19–50 at 1-year intervals (31 age groups in total) and by five age categories (≤25, 26-30, 31-35, 36-40, 41-45, 46-50). Five empirical centiles, including the 5th, 25th, 50th, 75th and 95th were calculated, and nomogram tables were constructed. Graphics based on the percentile age for each variable were drawn using Proc Quantreg. AMH was categorized in 3 groups to evaluate the frequency of low (<1.3 ng/ml), normal (1.3 - 6.24 ng/ml) or high (≥6.25 ng/ml) levels of AMH. The low AMH cut-off level was set at <1.3 ng/mL following the Bologna Criteria (26, 27). The high AMH cut-off level was set at ≥ 6.25 ng/mL, as proposed by Calzada et al. (28) in order to define a cut-off level for PCOS (polycystic ovarian syndrome) patients. Association between dress code and AMH and age were tested using General Linear Model procedure. R-squared were calculated using regression model to measure the strength of the relationship between AMH, AFC, age and BMI. A multiple regression analysis for both AMH and AFC as dependent variables was performed, including the variables studied (age, BMI and dress code) as predictor variables.
As the lower limit of detection for Elecsys assay was 0.01ng/mL, values lower than the detection limit were considered equal to zero. All analyses and the graph plotting were performed using SAS studio.
Results
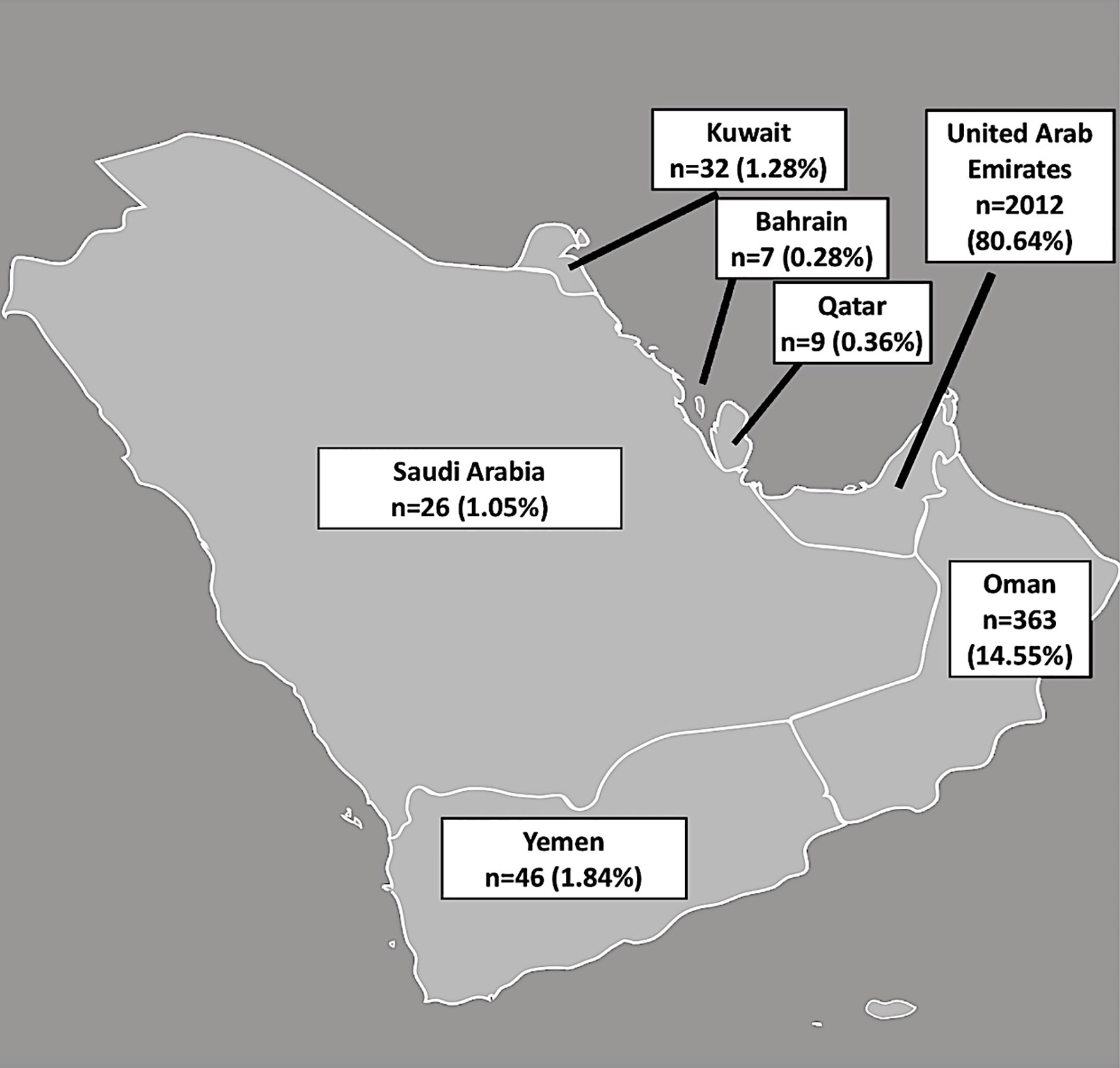
A total of 2,495 patients, native to the Arabian Peninsula with data on AMH, dress code and BMI were included for analysis. AFC values were available for 2,441 patients. The number and percentage of women included from each country is shown in Figure 1.
Patient characteristics were as follows (in mean ± SD): age 34.62 ± 6.61 years, BMI 28.57 ± 5.49 kg/m2. The smoking rate among the patients was 2.40%. Most frequent dress code was hijab (71.23%), followed by niqab (25.76%). Mean AMH was 2.59 ± 2.9 ng/mL, whereas median AMH was 1.76 ng/mL. The percentage of women with previous adnexal surgery in the total group analyzed was 11.43%, with a similar distribution between the hijab and niqab groups (12.31% and 14.47%, respectively; p=0.704). Patient’ characteristics are displayed in Table 1. Single-year specific median, mean and SD for AMH and AFC are summarized in Table 2 and graphical comparisons of AMH and AFC in 1-year intervals are shown in Figures 2A, B respectively; Table 3 and Figures 3A, B include median, mean, SD values, and average decrease per age category. The decrease of AMH was highest in the age group 36-40 (AMH median: -1.15 ng/mL between age 36 and 40), showing a yearly decrease of -0.23 ng/mL.
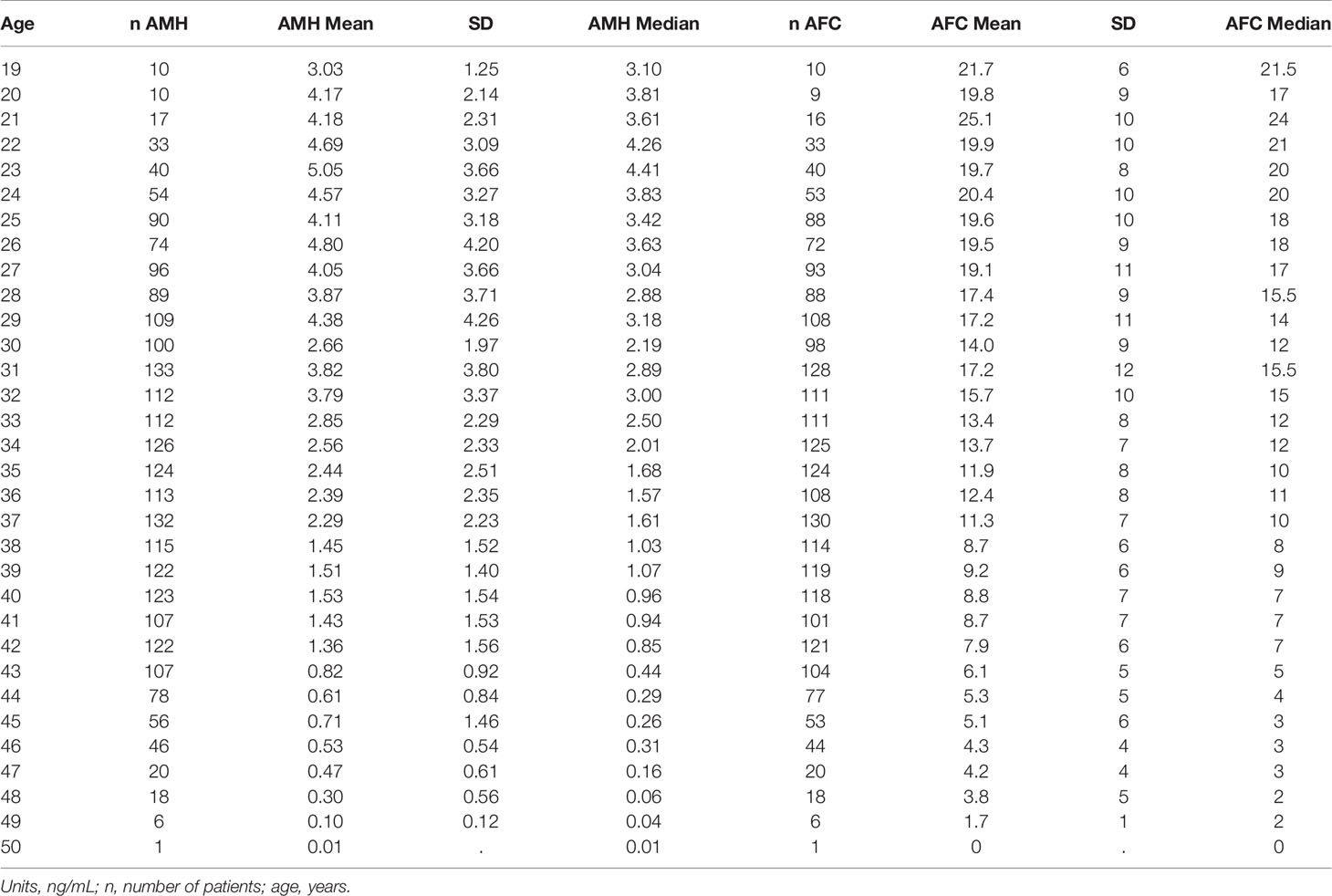
Table 2 Single-age specific AMH mean with standard deviation (SD) and median values obtained by Elecsys AMH immunoassay, and single-age specific AFC mean with standard deviation (SD) and median.
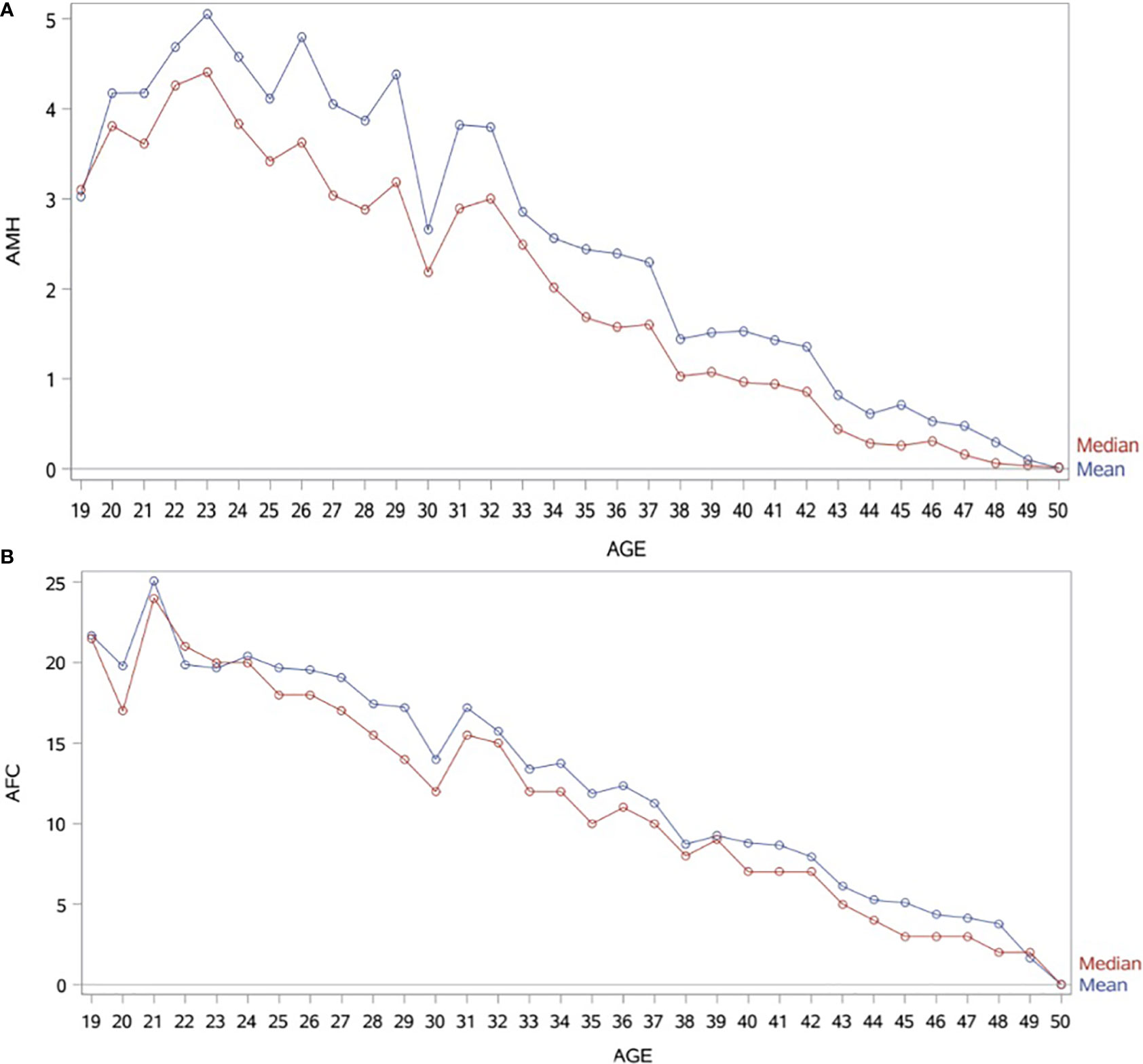
Figure 2 (A) Graphical comparisons of AMH mean and median in 1-year intervals. AMH (ng/mL). (B) Graphical comparisons of AFC mean and median in 1-year intervals.
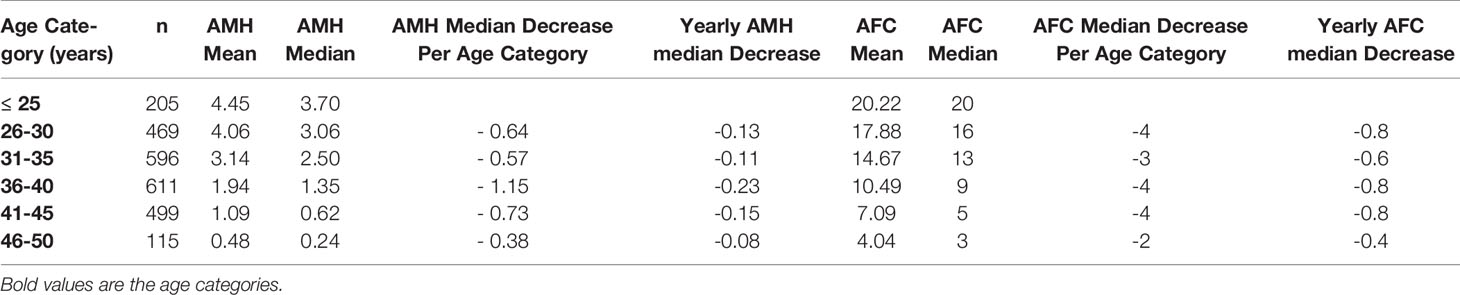
Table 3 Median, mean, SD values, and average decrease per age category for serum AMH levels (ng/mL) and AFC.
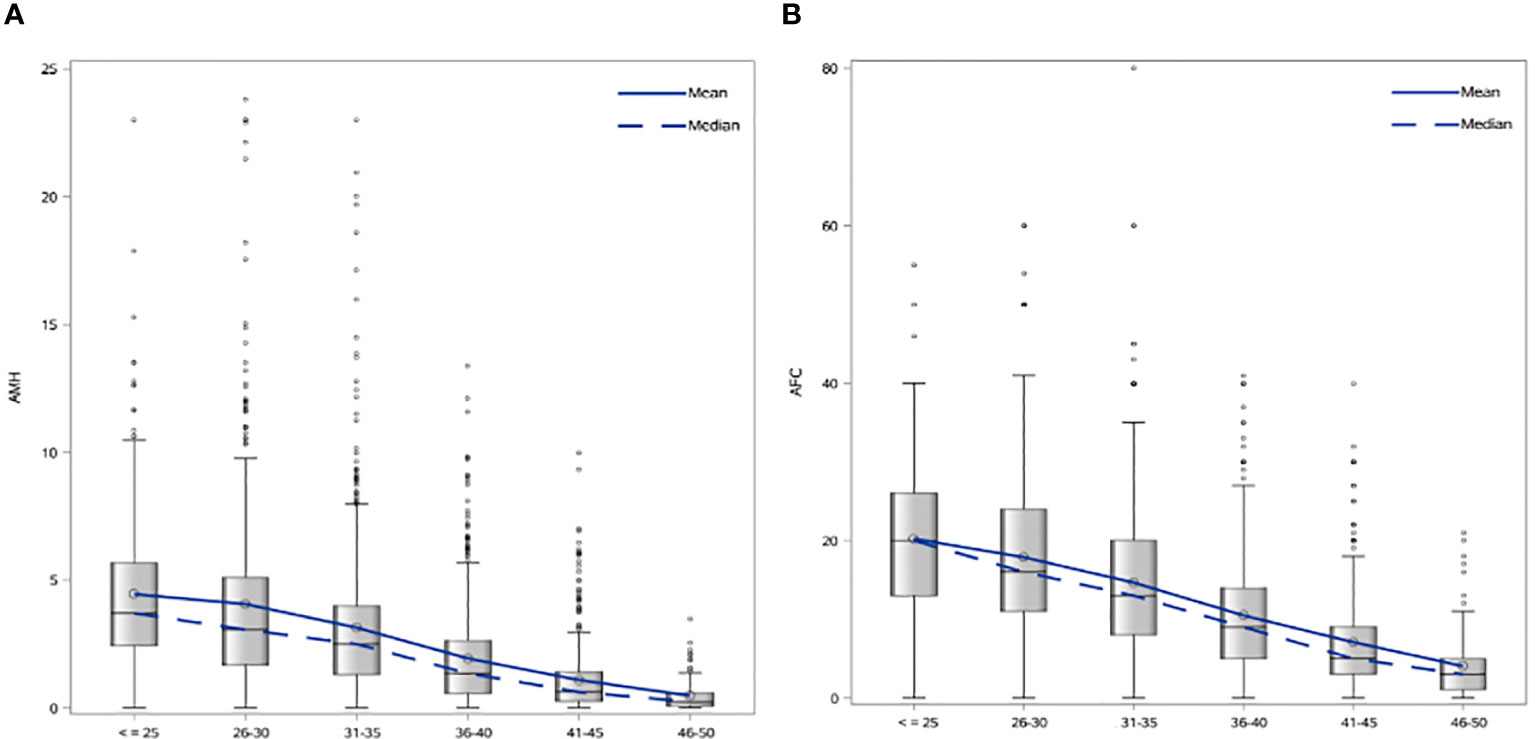
Figure 3 (A) Age-specific AMH mean and median values in 5-year intervals. (B) Age-specific AFC mean and median values in 5-year intervals.
AMH and AFC values of 5th, 25th, 50th, 75th and 95th centiles are shown as Supplementary Data (Supplementary Tables 1, 2 and Supplementary Figures 1, 2). Correlation (R-square) for AMH and age was 18.8% and 25.9% for AFC and age (Supplementary Figure 3). AMH and AFC showed a R-square of 57.6%. Very low R-square was found for BMI and AMH (R-square=0.08%).
The distribution of women based on their levels of AMH is shown in Table 4 and Figure 4. In the total population, 40.60% of women presented AMH serum levels <1.3 ng/mL, 50.78% between 1.3 - 6.24 ng/mL, and 8.62% had AMH ≥6.25 ng/mL.
Data on dress code was available for 2,323 women. There was no difference in age between the two groups of dress code (Hijab versus Niqab), but AMH serum levels and AFC were significantly lower for patients wearing the niqab (p=0.043 and p=0.021, respectively). The results are shown in Table 5.
A multiple regression analysis for both AMH and AFC as dependent variables was performed, including age, BMI and dress code as predictor variables. Age and BMI showed a significant correlation to AFC (p< 0.0001 for both predictor variables) and to AMH (p<0.0001 and p=0.004, respectively). Regarding the dress code, a significant negative correlation was observed for the niqab dress code to AFC (p=0.025), however, not significant for AMH (p=0.22) (Table 6).
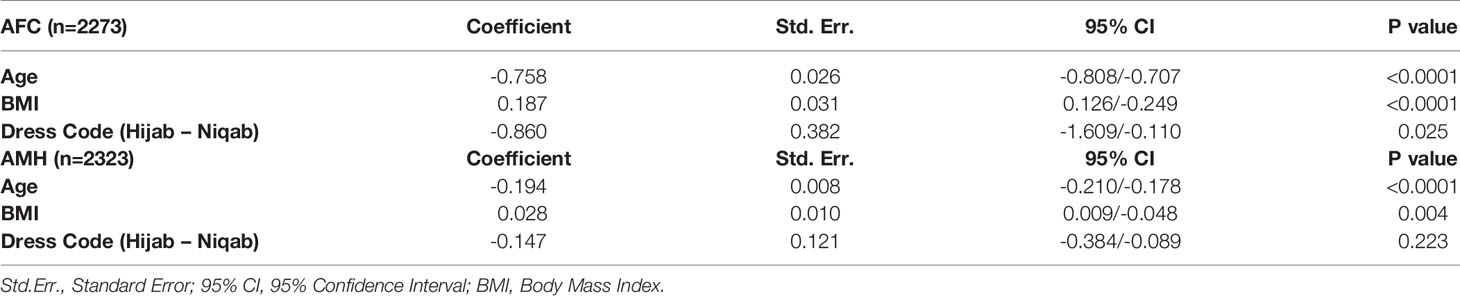
Table 6 Multiple regression analysis for AFC and AMH as dependent variables, including age, BMI and dress code as predictor variables.
Discussion
Whereas it is well known that age is the most significant factor to impact the ovarian reserve, the influence of other factors is often less considered and/or neglected, especially in populations less included in research. To the best of our knowledge, this is the first large-scale retrospective data set reporting age, BMI and dress-code in relation to AMH and AFC values specific to women native to the Arabian Peninsula, with all AMH measurements performed with 3rd-generation automated assay (Elecsys, Roche®) in a single laboratory. In this specific study population, the peak AMH level (median AMH of 4.41 ng/ml) was exhibited at an age of 23 years, a finding which is in line with the data from Kelsey et al. (11), who also demonstrated an AMH peak at the age of 24.5 years and described lower AMH levels in the population below 24 years of age. A large percentage of women (40.60%) from the herein presented study population were affected by a low ovarian reserve, expressed as AMH serum levels <1.3ng/mL (26). While an age-dependent physiological decrease for both ovarian reserve markers was expected at any given age, it is worth noting that AMH presented an accelerate decrease for the age category of 36-40 years old. This significant decrease over those five years is in keeping with the already well recognized decrease in ovarian reserve and fertility for women above 35 years old (29). The multiple regression analysis showed that age and BMI were strong predictor variables for the ovarian reserve markers. The dress code was a significant predictor variable for AFC, however, no significant value was reached for AMH.
There is growing evidence that ovarian reserve is, besides age, influenced by several factors including the sociocultural environment, habits and ethnicity. Previous publications have demonstrated, that Asian, African American, Hispanic, Indian American have lower live birth rates (LBR) and higher miscarriage rates after ART when compared to a reference population of white/Caucasian women (30–37). Despite the fact that the Arabian culture is a pro-family society and families commonly have a higher number of children compared to couples in Western societies (38, 39), there is a high prevalence of infertility in the MENA region (40) and very limited knowledge about ovarian reserve parameters and ART outcomes in the Arab population.
Women native to the MENA (Middle East North Africa) region, which is a region comprising 17 countries with multiple ethnicities (The Middle East Population 2021 (Demographics, Maps, Graphs), no date), are only included into few studies (17, 18, 35, 37). Feichtinger et al. (17) found a significantly lower oocyte yield in MENA patients compared to their European counterparts, despite being of younger age and having a higher prevalence of PCOS. Salem et al. (37) described poorer IVF outcomes with decreased live birth rates per blastocyst transfer and increased miscarriage rates in MENA women compared to Caucasian women. In the study of Jayaprakasan et al. (35), the Middle Eastern women presented the lowest LBR (21.4%), yet only 14 women were included for the analysis. Similar data are described by Tabbalat et al. (18), who compared the IVF outcomes for infertile couples from the Arabian Peninsula to Caucasian couples from the U.S. The Arabian Peninsula women showed statistically higher FSH levels, lower AMH levels and lower AFC, as well as lower number of mature oocytes. These results suggest that Arabian Peninsula ethnicity is associated with lower ovarian reserve and ovarian response, and these findings are corroborated by the AMH and AFC values from the herein presented data when compared to the ovarian reserve parameters described in other ethnicities. Loy et al. (12) presented percentiles and nomograms for AMH and AFC for 1,009 Chinese sub-fertile women, using Gen II ELISA assay for AMH. Considering the possible variations due to the different assays used [serum AMH concentrations seem to be 20% lower when Elecsys is compared with conventional AMH Gen II assays (41)], AMH values for Chinese ethnicity seem clearly higher than for the Arab population. Compared to Japanese population, the median AMH values presented in this study are 38.4% lower using Access assay (14). This percentage is far from the 10% expected due to the different assays used (42). Likewise, the percentage of women with low AMH values (<1.3 ng/mL) in the herein presented study is 40.60%, a high rate when compared to 13.2% described for a group of 423 infertile women from Pakistan (13) and apparently, these disparities are more distinct in the younger groups (43).
The reduced ovarian reserve identified in the Middle Eastern ethnical group highlights the influence of ethnicity, sociocultural, religious, environmental and genetic factors on the ovarian reserve and consequently on fertility (20). The high rates of consanguineous marriages, which amount up to 80% in certain regions in the Middle East, have been related to a reduced ovarian reserve (20, 44). Environmental factors have an impact as well, e.g. vitamin D deficiency, which has one of the world’s highest prevalence rates in the Middle East (45). Lately, the positive impact of 1α,25-dihydroxyvitamin D3, which is the biologically active form, on the ovarian reserve has been demonstrated in the ovarian tissue of rhesus macaques (46, 47) as well as in patients, who received a single, high dosage of vitamin D at the beginning of their cycle (48). It is important to acknowledge that the dress-code of women correlates with the extent of vitamin D deficiency (49, 50) and most women of the Arabian Peninsula are following a concealing dress-code with coverage of large parts of the skin due to sociocultural/religious habits. Arefi et al. (19) previously showed a negative influence of the dress-code on the ovarian reserve parameters. The data of the present study support the previous findings as, indeed, women using the most covering dress code (Niqab) have significantly lower values for AMH and AFC compared to women with a less concealing dress-code. Furthermore, dress code was a significant predictive variable for AFC in the regression analysis. No significant result was observed for the association with AMH, hence, this discrepancy could be explained by the lower number of included AMH results and a non-normal distribution for the AMH values. Body weight and BMI are factors which exhibit a negative impact on the ovarian reserve parameters and it is well known that the prevalence of obesity (BMI>30 Kg/m2) in adults aged 18 and above in the Arabian Peninsula are above 40% (51). In line with these data, the women included in the herein study presented a median BMI of 28.03 Kg/m2 and the regression analysis showed BMI as a strong predictive variable for both ovarian reserve parameters (AFC: p< 0.0001; AMH: p=0.004).
Although the retrospective design might be considered as a limitation, the main strengths of the present study are firstly, the large number of included women with similar geographical, sociocultural/religious and ethnical similarities; and secondly, the use of a 3rd-generation automated assay (Elecsys, Roche®) for the AMH analysis, performed in a single clinical reference laboratory, leading to an improved accuracy and homogeneity of the results. Further on, this study provides median reference values in addition to mean values for more adequate information regarding AMH and AFC values, as data are non-normally distributed.
This study presents age and dress code specific AMH and AFC in women native to the Arabian Peninsula, facilitating an increased understanding of ovarian reserve in this population and the rates of low, normal and high ovarian reserve. Reproductive specialists should be aware of the influence of ethnicity on the ovarian reserve and to adopt region specific counseling strategies and personalize treatment plans accordingly.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The study involving human participants were reviewed and approved by the Research Ethics Committee (REFA040) of ART Fertility clinic, Abu Dhabi, UAE. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LM, HF, and BL contributed to the study conception and design, data acquisition, analysis, interpretation of data, and drafting of the bulk of the article. LB contributed to the analysis and interpretation of data, conception and design of the statistical analysis. RV, CC, AA, IE, and ND contributed to the data acquisition and updated bibliography. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Mr. Vivek KM for data handling and graph design.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.735116/full#supplementary-material
Supplementary Table 1 | AMH (ng/mL) by centile and age.
Supplementary Table 2 | AFC by centile and age.
Supplementary Figure 1 | Centiles of Anti-Mullerian hormone (ng/mL) by age.
Supplementary Figure 2 | Centiles of AFC by age.
Supplementary Figure 3 | Relations between AMH, AFC and age for the total population (n=2495). Left, relation between AMH values and age (R-square = 0,188). Right, relation between total AFC and age (R-square = 0,259).
References
1. Usta T, Oral E. Is the Measurement of Anti-Müllerian Hormone Essential? Curr Opin Obstet Gynecol (2012) 24(3):151–7. doi: 10.1097/GCO.0b013e3283527dcf
2. La Marca A, Sunkara SK. Individualization of Controlled Ovarian Stimulation in IVF Using Ovarian Reserve Markers: From Theory to Practice. Hum Reprod Update (2014) 20(1):124–40. doi: 10.1093/humupd/dmt037
3. Kavoussi SK, Odenwald KC, Boehnlein LM, Summers-Colquitt RB, Pool TB, Swain JE, et al. Antimüllerian Hormone as a Predictor of Good-Quality Supernumerary Blastocyst Cryopreservation Among Women With Levels <1 Ng/mL Versus 1-4 Ng/Ml. Fertil Steril (2015) 104(3):633–6. doi: 10.1016/j.fertnstert.2015.06.007
4. da Silva JB, Panaino TR, Tamm MA, Lira P, Arêas PCF, Mancebo ACA, et al. Prediction of Metaphase II Oocytes According to Different Serum Anti-Müllerian Hormone (AMH) Levels in Antagonist ICSI Cycles. JBRA Assist Reprod (2016) 20(4):222–6. doi: 10.5935/1518-0557.20160043
5. La Marca A, Minasi MG, Sighinolfi G, Greco P, Argento C, Grisendi V, et al. Female Age, Serum Antimüllerian Hormone Level, and Number of Oocytes Affect the Rate and Number of Euploid Blastocysts in In Vitro Fertilization/Intracytoplasmic Sperm Injection Cycles. Fertil Steril (2017) 108(5):777–83.e2. doi: 10.1016/j.fertnstert.2017.08.029
6. Melado Vidales L, Fernández-Nistal A, Martínez Fernández V, Verdú Merino V, Bruna Catalán I, Bajo Arenas JM. Anti-Müllerian Hormone Levels to Predict Oocyte Maturity and Embryo Quality During Controlled Ovarian Hyperstimulation. Minerva Ginecol (2017) 69(3):225–32. doi: 10.23736/S0026-4784.16.03958-7
7. Arnanz A. ‘Anti-Müllerian Hormone as a Quantitative and Qualitative Marker of Euploid Blastocysts’. Vienna: ESHRE Congress (2019).
8. Tarasconi B, Tadros T, Ayoubi J-M, Belloc S, de Ziegler D, Fanchin R. Serum Antimüllerian Hormone Levels Are Independently Related to Miscarriage Rates After In Vitro Fertilization-Embryo Transfer. Fertil Steril (2017) 108(3):518–24. doi: 10.1016/j.fertnstert.2017.07.001
9. Melado L, Arnanz A, Bayram A, Elkhatib I, De Munck N, Navarro AT, et al. Anti-Müllerian Hormone Is an Independent Marker for Oocyte Survival After Vitrification. Reprod BioMed Online (2020) 41(1):119–27. doi: 10.1016/j.rbmo.2020.03.014
10. Seifer DB, Baker VL, Leader B. Age-Specific Serum Anti-Müllerian Hormone Values for 17,120 Women Presenting to Fertility Centers Within the United States. Fertil Steril (2011) 95(2):747–50. doi: 10.1016/j.fertnstert.2010.10.011
11. Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WHB. A Validated Model of Serum Anti-Müllerian Hormone From Conception to Menopause. PloS One (2011) 6(7):e22024. doi: 10.1371/journal.pone.0022024
12. Loy SL, Cheung YB, Fortier MV, Ong CL, Tan HH, Nadarajah S, et al. Age-Related Nomograms for Antral Follicle Count and Anti-Mullerian Hormone for Subfertile Chinese Women in Singapore. PloS One (2017) 12(12):e0189830. doi: 10.1371/journal.pone.0189830
13. Khan HL, Bhatti S, Suhail S, Gul R, Awais A, Hamayun H, et al. Antral Follicle Count (AFC) and Serum Anti-Müllerian Hormone (AMH) Are the Predictors of Natural Fecundability Have Similar Trends Irrespective of Fertility Status and Menstrual Characteristics Among Fertile and Infertile Women Below the Age of 40 Years. Reprod Biol Endocrinol (2019) 17(1):20. doi: 10.1186/s12958-019-0464-0
14. Segawa T, Omi K, Watanabe Y, Sone Y, Handa M, Kuroda M, et al. Age-Specific Values of Access Anti-Müllerian Hormone Immunoassay Carried Out on Japanese Patients With Infertility: A Retrospective Large-Scale Study. BMC Womens Health (2019) 19(1):57. doi: 10.1186/s12905-019-0752-z
15. Seifer DB, Golub ET, Lambert-Messerlian G, Benning L, Anastos K, Watts DH, et al. Variations in Serum Müllerian Inhibiting Substance Between White, Black, and Hispanic Women. Fertil Steril (2009) 92(5):1674–8. doi: 10.1016/j.fertnstert.2008.08.110
16. Iglesias C, Banker M, Mahajan N, Herrero L, Meseguer M, Garcia-Velasco JA. Ethnicity as a Determinant of Ovarian Reserve: Differences in Ovarian Aging Between Spanish and Indian Women. Fertil Steril (2014) 102(1):244–9. doi: 10.1016/j.fertnstert.2014.03.050
17. Feichtinger M, Göbl C, Weghofer A, Feichtinger W. Reproductive Outcome in European and Middle Eastern/North African Patients. Reprod BioMed Online (2016) 33(6):684–9. doi: 10.1016/j.rbmo.2016.09.003
18. Tabbalat AM, Pereira N, Klauck D, Melhem C, Elias RT, Rosenwaks Z. Arabian Peninsula Ethnicity Is Associated With Lower Ovarian Reserve and Ovarian Response in Women Undergoing Fresh ICSI Cycles. J Assist Reprod Genet (2018) 35(2):331–7. doi: 10.1007/s10815-017-1071-7
19. Arefi S, Khalili G, Iranmanesh H, Farifteh F, Hosseini A, Fatemi HM, et al. Is the Ovarian Reserve Influenced by Vitamin D Deficiency and the Dress Code in an Infertile Iranian Population? J Ovarian Res (2018) 11(1):62. doi: 10.1186/s13048-018-0435-7
20. Lawrenz B, Coughlan C, Melado L, Fatemi HM. Ethnical and Sociocultural Differences Causing Infertility Are Poorly Understood-Insights From the Arabian Perspective. J Assist Reprod Genet (2019) 36(4):661–5. doi: 10.1007/s10815-019-01411-2
21. Cohen SB. Geopolitics of the World System. Lanham: Rowman & Littlefield Publishers (2003). p. 337.
22. Li HWR, Wong BPC, Ip WK, Yeung WSB, Ho PC, Ng EHY. Comparative Evaluation of Three New Commercial Immunoassays for Anti-Müllerian Hormone Measurement. Hum Reprod Oxf Engl (2016) 31(12):2796–802. doi: 10.1093/humrep/dew248
23. Deb S, Campbell BK, Clewes JS, Pincott-Allen C, Raine-Fenning NJ. Intracycle Variation in Number of Antral Follicles Stratified by Size and in Endocrine Markers of Ovarian Reserve in Women With Normal Ovulatory Menstrual Cycles: Intracycle Variation in Markers of Ovarian Reserve. Ultrasound Obstet Gynecol (2013) 41(2):216–22. doi: 10.1002/uog.11226
24. Broekmans FJM, de Ziegler D, Howles CM, Gougeon A, Trew G, Olivennes F. The Antral Follicle Count: Practical Recommendations for Better Standardization. Fertil Steril (2010) 94(3):1044–51. doi: 10.1016/j.fertnstert.2009.04.040
25. Hijab. In: Wikipedia [Internet]. 2021 [Cited 2021 Feb 1]. Available at: https://en.wikipedia.org/w/index.php?title=Hijab&oldid=1003061422.
26. Ferraretti AP, Gianaroli L. The Bologna Criteria for the Definition of Poor Ovarian Responders: Is There a Need for Revision? Hum Reprod (2014) 29(9):1842–5. doi: 10.1093/humrep/deu139
27. Ferraretti AP, La Marca A, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE Consensus on the Definition of ‘Poor Response’ to Ovarian Stimulation for In Vitro Fertilization: The Bologna Criteria. Hum Reprod Oxf Engl (2011) 26(7):1616–24. doi: 10.1093/humrep/der092
28. Calzada M, López N, Noguera JA, Mendiola J, Hernández AI, Corbalán S, et al. AMH in Combination With SHBG for the Diagnosis of Polycystic Ovary Syndrome. J Obstet Gynaecol (2019) 39(8):1130–6. doi: 10.1080/01443615.2019.1587604
29. ESHRE Capri Workshop Group. Fertility and Ageing. Hum Reprod Update (2005) 11(3):261–76. doi: 10.1093/humupd/dmi006
30. Sharara FI, McClamrock HD. Differences in In Vitro Fertilization (IVF) Outcome Between White and Black Women in an Inner-City, University-Based IVF Program. Fertil Steril (2000) 73(6):1170–3. doi: 10.1016/S0015-0282(00)00524-0
31. Purcell K, Schembri M, Frazier LM, Rall MJ, Shen S, Croughan M, et al. Asian Ethnicity Is Associated With Reduced Pregnancy Outcomes After Assisted Reproductive Technology. Fertil Steril (2007) 87(2):297–302. doi: 10.1016/j.fertnstert.2006.06.031
32. Shahine LK, Lamb JD, Lathi RB, Milki AA, Langen E, Westphal LM. Poor Prognosis With In Vitro Fertilization in Indian Women Compared to Caucasian Women Despite Similar Embryo Quality. PloS One (2009) 4(10):e7599. doi: 10.1371/journal.pone.0007599
33. Butts SF, Seifer DB. Racial and Ethnic Differences in Reproductive Potential Across the Life Cycle. Fertil Steril (2010) 93(3):681–90. doi: 10.1016/j.fertnstert.2009.10.047
34. Seifer DB, Zackula R, Grainger DA, Society for Assisted Reproductive Technology Writing Group Report. Trends of Racial Disparities in Assisted Reproductive Technology Outcomes in Black Women Compared With White Women: Society for Assisted Reproductive Technology 1999 and 2000 vs. 2004-2006. Fertil Steril (2010) 93(2):626–35. doi: 10.1016/j.fertnstert.2009.02.084
35. Jayaprakasan K, Pandian D, Hopkisson J, Campbell B, Maalouf W. Effect of Ethnicity on Live Birth Rates After In Vitro Fertilisation or Intracytoplasmic Sperm Injection Treatment. BJOG Int J Obstet Gynaecol (2014) 121(3):300–7. doi: 10.1111/1471-0528.12504
36. Quinn M, Fujimoto V. Racial and Ethnic Disparities in Assisted Reproductive Technology Access and Outcomes. Fertil Steril (2016) 105(5):1119–23. doi: 10.1016/j.fertnstert.2016.03.007
37. Salem WH, Abdullah A, Abuzeid O, Bendikson K, Sharara FI, Abuzeid M. Decreased Live Births Among Women of Middle Eastern/North African Ethnicity Compared to Caucasian Women. J Assist Reprod Genet (2017) 34(5):581–6. doi: 10.1007/s10815-017-0904-8
38. Hamadeh RR, Al-Roomi K, Masuadi E. Determinants of Family Size in a Gulf Arab State: A Comparison Between Two Areas. J R Soc Promot Health (2008) 128(5):226–32. doi: 10.1177/1466424008092795
39. Green KE, Smith DE. Change and Continuity: Childbirth and Parenting Across Three Generations of Women in the United Arab Emirates. Child Care Health Dev (2007) 33(3):266–74. doi: 10.1111/j.1365-2214.2006.00667.x
40. Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PloS Med (2012) 9(12):e1001356. Low N, editor. doi: 10.1371/journal.pmed.1001356
41. Tadros T, Tarasconi B, Nassar J, Benhaim J-L, Taieb J, Fanchin R. New Automated Antimüllerian Hormone Assays Are More Reliable Than the Manual Assay in Patients With Reduced Antral Follicle Count. Fertil Steril (2016) 106(7):1800–6. doi: 10.1016/j.fertnstert.2016.08.045
42. Iliodromiti S, Salje B, Dewailly D, Fairburn C, Fanchin R, Fleming R, et al. Non-Equivalence of Anti-Müllerian Hormone Automated Assays—Clinical Implications for Use as a Companion Diagnostic for Individualised Gonadotrophin Dosing. Hum Reprod (2017) 32(8):1710–5. doi: 10.1093/humrep/dex219
43. Bleil ME, Gregorich SE, Adler NE, Sternfeld B, Rosen MP, Cedars MI. Race/ethnic Disparities in Reproductive Age: An Examination of Ovarian Reserve Estimates Across Four Race/Ethnic Groups of Healthy, Regularly Cycling Women. Fertil Steril (2014) 101(1):199–207. doi: 10.1016/j.fertnstert.2013.09.015
44. Seher T, Thiering E, al Azemi M, Heinrich J, Schmidt-Weber CB, Kivlahan C, et al. Is Parental Consanguinity Associated With Reduced Ovarian Reserve? Reprod BioMed Online (2015) 31(3):427–33. doi: 10.1016/j.rbmo.2015.06.003
45. Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, et al. Current Vitamin D Status in European and Middle East Countries and Strategies to Prevent Vitamin D Deficiency: A Position Statement of the European Calcified Tissue Society. Eur J Endocrinol (2019) 180(4):P23–54. doi: 10.1530/EJE-18-0736
46. Xu J, Lawson MS, Xu F, Du Y, Tkachenko OY, Bishop CV, et al. Vitamin D3 Regulates Follicular Development and Intrafollicular Vitamin D Biosynthesis and Signaling in the Primate Ovary. Front Physiol (2018) 9:1600. doi: 10.3389/fphys.2018.01600
47. Xu J, Hennebold JD, Seifer DB. Direct Vitamin D3 Actions on Rhesus Macaque Follicles in Three-Dimensional Culture: Assessment of Follicle Survival, Growth, Steroid, and Antimüllerian Hormone Production. Fertil Steril (2016) 106(7):1815–20.e1. doi: 10.1016/j.fertnstert.2016.08.037
48. Dennis NA, Houghton LA, Pankhurst MW, Harper MJ, McLennan IS. Acute Supplementation With High Dose Vitamin D3 Increases Serum Anti-Müllerian Hormone in Young Women. Nutrients (2017) 9(7):E719. doi: 10.3390/nu9070719
49. Hatun S, Islam Ö, Cizmecioglu F, Kara B, Babaoglu K, Berk F, et al. Subclinical Vitamin D Deficiency Is Increased in Adolescent Girls Who Wear Concealing Clothing. J Nutr (2005) 135(2):218–22. doi: 10.1093/jn/135.2.218
50. Gannagé-Yared M, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D in a Sunny Country: Relation to Lifestyle and Bone Markers. J Bone Miner Res (2000) 15(9):1856–62. doi: 10.1359/jbmr.2000.15.9.1856
51. World Health Organization (WHO). Prevalence of Obesity in Adults Aged 18 and Above. World Health Organization (WHO) (2017). Available at: https://www.who.int.
Keywords: Anti-Müllerian hormone, antral follicle count, Arab Peninsula, ovarian reserve, ethnic
Citation: Melado L, Vitorino R, Coughlan C, Bixio LD, Arnanz A, Elkhatib I, De Munck N, Fatemi HM and Lawrenz B (2021) Ethnic and Sociocultural Differences in Ovarian Reserve: Age-Specific Anti-Müllerian Hormone Values and Antral Follicle Count for Women of the Arabian Peninsula. Front. Endocrinol. 12:735116. doi: 10.3389/fendo.2021.735116
Received: 02 July 2021; Accepted: 20 September 2021;
Published: 21 October 2021.
Edited by:
Yingying Qin, Shandong University, ChinaReviewed by:
Biljana Popovic Todorovic, University Hospital Brussels, BelgiumLeif Johan Bungum, Trianglen Fertility Clinic, Denmark
Copyright © 2021 Melado, Vitorino, Coughlan, Bixio, Arnanz, Elkhatib, De Munck, Fatemi and Lawrenz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Melado, TGF1cmEubWVsYWRvQGFydGZlcnRpbGl0eWNsaW5pY3MuY29t
 Laura Melado
Laura Melado Raquel Vitorino
Raquel Vitorino Carol Coughlan2
Carol Coughlan2 Leyla Depret Bixio
Leyla Depret Bixio Ana Arnanz
Ana Arnanz Ibrahim Elkhatib
Ibrahim Elkhatib Neelke De Munck
Neelke De Munck Human M. Fatemi
Human M. Fatemi Barbara Lawrenz
Barbara Lawrenz