- 1Institute for Physical Activity and Nutrition, School of Exercise and Nutrition Science, Deakin University, Geelong, VIC, Australia
- 2Sydney Nursing School, Faculty of Medicine and Health, University of Sydney and Sydney Local Health District, Sydney, NSW, Australia
- 3Deakin Health Economics, Institute for Health Transformation, School of Health and Social Development, Deakin University, Geelong, VIC, Australia
- 4La Trobe Analytics Lab, La Trobe University, Melbourne, VIC, Australia
- 5Institute for Health Transformation, School of Health and Social Development, Deakin University, Geelong, VIC, Australia
- 6Université de Paris, CRESS, INSERM, INRAE, Paris, France
- 7Biostatistics Unit, Deakin University, Geelong, VIC, Australia
Introduction: Promoting healthy eating and active play in early life is critical, however few interventions have been delivered or sustained at scale. The evaluation of interventions at scale is a crucial, yet under-researched aspect of modifying population-level health behaviours. INFANT is an evidence-based early childhood healthy lifestyle intervention that aims to improve parents’ knowledge and skills around promoting optimal energy balance-related behaviours that, in turn, influence children’s diet, activity and adiposity. It consists of: 1) Four group sessions delivered via first time parent groups across the first 12 months of life; 2) access to the My Baby Now app from birth to 18 months of age. This research aims to assess real-world implementation, effectiveness and cost-effectiveness of INFANT when delivered at scale across Victoria, Australia.
Methods and Analysis: A hybrid type II implementation-effectiveness trial applying a mixed methods design will be conducted. INFANT will be implemented in collaboration with practice and policy partners including maternal and child health services, population health and Aboriginal health, targeting all local government areas (n=79) in Victoria, Australia. Evaluation is based on criteria from the ‘Outcomes for Implementation Research’ and ‘RE-AIM’ frameworks. Implementation outcomes will be assessed using descriptive quantitative surveys and qualitative interviews with those involved in implementation, and include intervention reach, organisational acceptability, adoption, appropriateness, cost, feasibility, penetration and sustainability. Process measures include organizational readiness, fidelity, and adaptation. Effectiveness outcomes will be assessed using a sample of INFANT participants and a non-randomized comparison group receiving usual care (1,500 infants in each group), recruited within the same communities. Eligible participants will be first time primary caregivers of an infant aged 0-3 months, owning a personal mobile phone and able to communicate in English. Effectiveness outcomes include infant lifestyle behaviours and BMIz at 12 and 18 months of age.
Impact: This is the first known study to evaluate the scale up of an evidence based early childhood obesity prevention intervention under real world conditions. This study has the potential to provide generalisable implementation, effectiveness and cost-effectiveness evidence to inform the future scale up of public health interventions both in Australia and internationally.
Clinical Trial Registration: Australian and New Zealand Clinical Trial Registry https://www.anzctr.org.au/, identifier ACTRN12620000670976.
1 Introduction
The prevention of obesity in early life is a key priority identified by the World Health Organization (1). The dietary and activity (energy-balance) behaviours associated with overweight and obesity begin in and track from early childhood (2). In Australia, a minority of children meet dietary, physical activity or sedentary behaviour guidelines (3). Children experiencing rapid weight gain during the first two years of life are also nearly four times more likely to become overweight or obese later in life (4). Globally, an estimated 41 million children aged under five are affected by overweight or obesity (5), including nearly one quarter of Australian children aged 2-4 years (3). Addressing energy balance behaviours in early life is therefore critical (6).
The prevalence of overweight and obesity, and the energy balance behaviours that predict them, are culturally, geographically and socioeconomically patterned (7–9) such that the most disadvantaged are disproportionately affected (8). It is estimated that obesity and dietary risks are each responsible for approximately 15% of the gap in disease burden between Aboriginal and Torres Strait Islander peoples and non-Indigenous Australians (10). Preventive interventions must therefore reach families across social, cultural and geographic divides. Most population-based interventions to improve children’s health behaviours in early life occur via settings, such as child care. These do not reach most children in their first year of life, where the risks for later overweight and obesity begin. At this time, the main influences on children’s health behaviours are the family and home environment (11) with a key avenue for reaching parents through Maternal and Child Health (MCH) services.
In Australia, routine universal MCH services are provided in all Australian states and territories. In Victoria, Australia, this service includes 10 free ‘key age and stage’ consultations with a MCH nurse (nine occurring between birth and two years of age) with a strong focus on promoting optimal child development and growth (12). More than 95% of Victorian parents attend these consultations between birth and four months, with over 80% still attending at 12 months (12). Routine preventive care services, such as those provided through MCH services, therefore offer a circumscribed opportunity to support parents in the establishment of healthy behaviours during infancy and early childhood.
The Infant feeding activity and Nutrition Trial (INFANT) (13) tested the efficacy of a group-based intervention delivered via established first time parents’ groups by universal MCH services. The intervention aimed to improve parents’ knowledge and skills around promoting healthy eating and active play, and in turn, healthy growth from the start of life (14). The intervention was underpinned by the principles of anticipatory guidance and social support and was first delivered in the trial by trained dietitians. Six 1.5-2hour face-to-face group sessions with parents and infants were delivered at 3 monthly intervals when the infant was approximately 3, 6, 9, 12, 15 and 18 months of age. The randomized controlled trial (RCT) showed INFANT achieved high acceptance, with 70% of participants attending ≥ four of six sessions over 15 months and 85% reporting high usefulness (13, 15). While the RCT did not impact standardised body mass index (BMIz) or physical activity (13), it did demonstrate positive child outcomes at 18 months for television viewing and dietary quality (16), with additional benefits for children of younger and less educated mothers (17). Additional positive outcomes were observed for mothers’ own dietary patterns and feeding practices (15, 18). Importantly, despite no further intervention beyond 18 months of age, the impact of INFANT remained evident up to 5 years of age for several targeted child health behaviors but not adiposity (19, 20). It is important to note that as with most research trials, participants were predominantly well educated (54% had a university education or higher) and English speaking (94% spoke English as the main language at home). No data was collected on participation by Aboriginal and Torres Strait Islander families but it is likely they were under represented in the trial.
While evidence is accumulating in support of efficacious early life childhood obesity prevention interventions such as INFANT (21), few studies have examined the effectiveness of these interventions when implemented at scale, in a real-world context. Further, implementation evidence including information on reach (particularly amongst hard to reach groups), feasibility, acceptability, scalability, effectiveness and cost-effectiveness as well as sustainability are also lacking but vital to decision makers (22). This was reflected in a recent research prioritization exercise with child obesity prevention researchers, policy makers and practitioners who identified ‘implementation science’ and ‘how to integrate child obesity prevention into existing service structures’ as key priorities (23).
Interventions that can be integrated into existing delivery systems are more likely to be sustainable than resource intensive interventions (1). INFANT represents a low intensity intervention with the potential for wide scale integration into service delivery structures. In Victoria, Australia, INFANT is now promoted as a flagship state-wide intervention delivering on two priority areas within the Victorian Public Health and Well Being Plan (2019-23) (24) and is endorsed as an evidenced based program nationally by the Australian Institute of Family Studies. This provides an opportunity to evaluate the usefulness of implementation strategies and overall intervention effectiveness when delivering INFANT at scale in a real-world context.
2 Methods and Analysis
2.1 Study Aims and Objectives
This research aims to assess real-world implementation, effectiveness and cost-effectiveness of INFANT when delivered at scale across Victoria, Australia. It has two main objectives:
1) To assess reach, and system/organizational level barriers and enablers to the adoption, implementation and sustainability of INFANT when delivered at scale.
2) To assess effectiveness and cost-effectiveness of INFANT when delivered at scale.
2.2 INFANT Enhancements for Scale Up
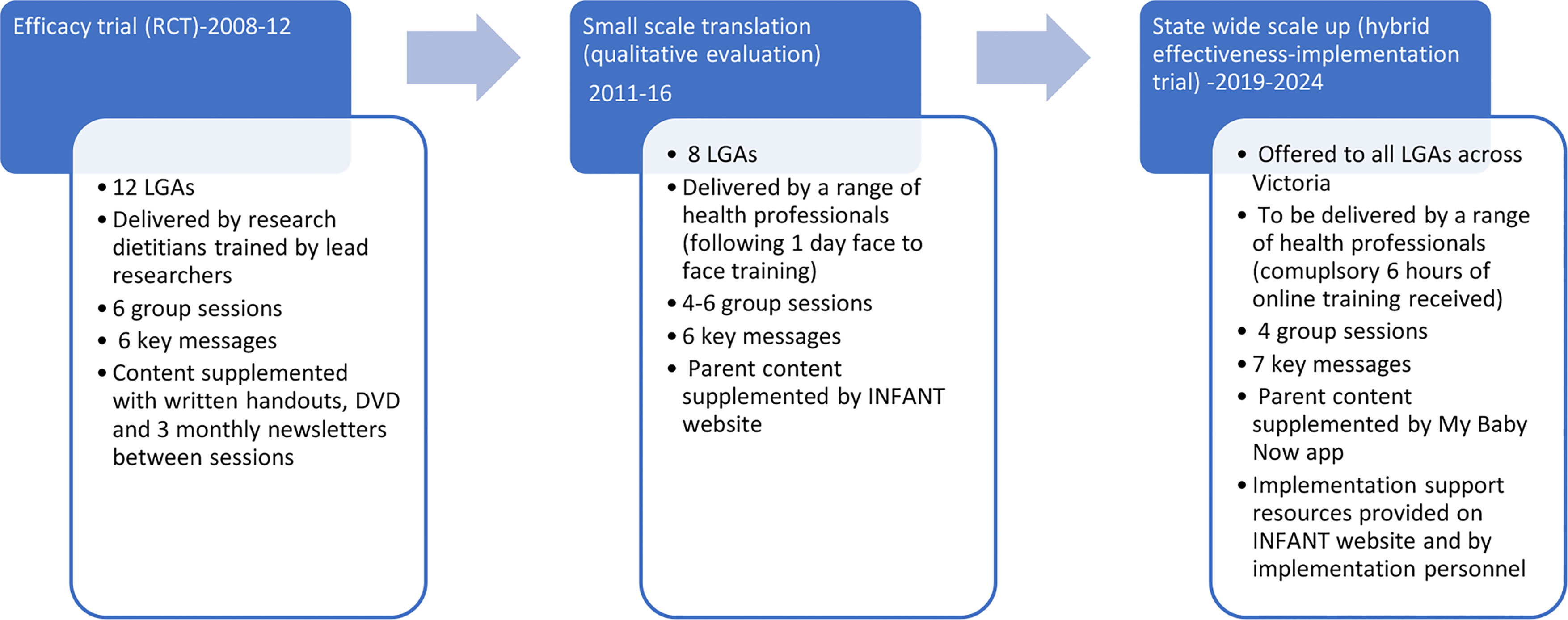
Figure 1 provides an overview of the evolution of INFANT from efficacy testing to state wide scale up. Small scale translation of INFANT across eight Victorian LGAs between 2011-2016 (25) identified a number of barriers to real-world implementation and scale up. These included a high organisational administrative burden coordinating and scheduling group sessions; challenges faced by facilitators in attending face-to-face training; limited staff time and confidence for evaluation (15); parents’ earlier return to work (between 9-12 months as opposed to 15-18 months in the original trial); and parental preference for online supplementary information (15, 26). These findings have informed a number of enhancements to INFANT to improve scalability. These include the provision of online facilitator training, accessible to MCH nurses, dietitians and other health professionals; a reduction in INFANT group sessions from six to four (3, 6, 9, 12 months) to accommodate parents’ earlier return to work; and the development of the My Baby Now app (27, 28). The app provides evidence-based information and support from birth to 18 months of age including two to three age-appropriate push notifications per week to reinforce INFANT key messages (Box 1; Supplementary File 1). The information from the trial (originally provided via handouts and a DVD) has been incorporated into the app, including information from sessions five and six offered at 15 and 18 months in the original trial. Additional information on milk feeding, including a new key message ‘feeding is a learning curve’, was also added to the app and program resources. An overview of the INFANT group sessions and key features of the My Baby Now app are provided in Supplementary Files 2, 3.
To reflect the move from trial phase to scale up, the acronym INFANT was changed from ‘Infant feeding activity and nutrition trial’ to INfant Feeding, Active play and NuTrition. Health services will be encouraged to offer the app from birth aligned with routine services and through INFANT group sessions starting at 3 months of age. Promotion of the app will be via flyers and posters in health services, and SMS and referral during MCH key age and stage consultations. Enrolment of parents to the group sessions will be via existing systems for group programs locally. In the original RCT, this involved inviting first time parent groups to continue to meet for INFANT sessions. In the small-scale translation study, areas continued to use this approach, with some making the last session of the first-time parent group (usually when infants were around three months old) the first INFANT session. This opt-in model of enrolment was identified as promoting intervention uptake with parents (26).
Box 1. INFANT key messages.

2.3 Study Design
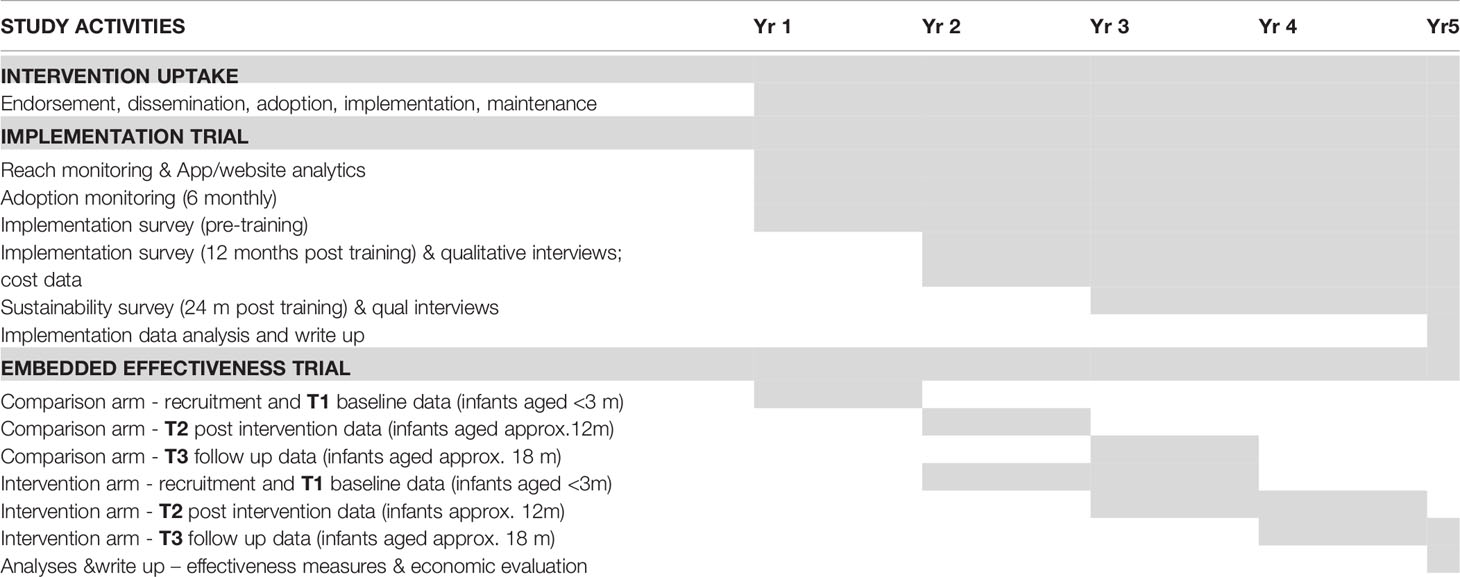
This study will use a hybrid type II implementation-effectiveness trial design as both implementation and effectiveness outcomes will be studied concurrently (29). Mixed methods will be used to collect data at a system (statewide stakeholders), organisational (health services) and individual (facilitator, parent, infant) level. This study will be conducted over a five year period to allow sufficient time to monitor implementation outcomes with organisations at 12 and 24 months, and for follow up of effectiveness outcomes with children at 18 months of age (Table 1).
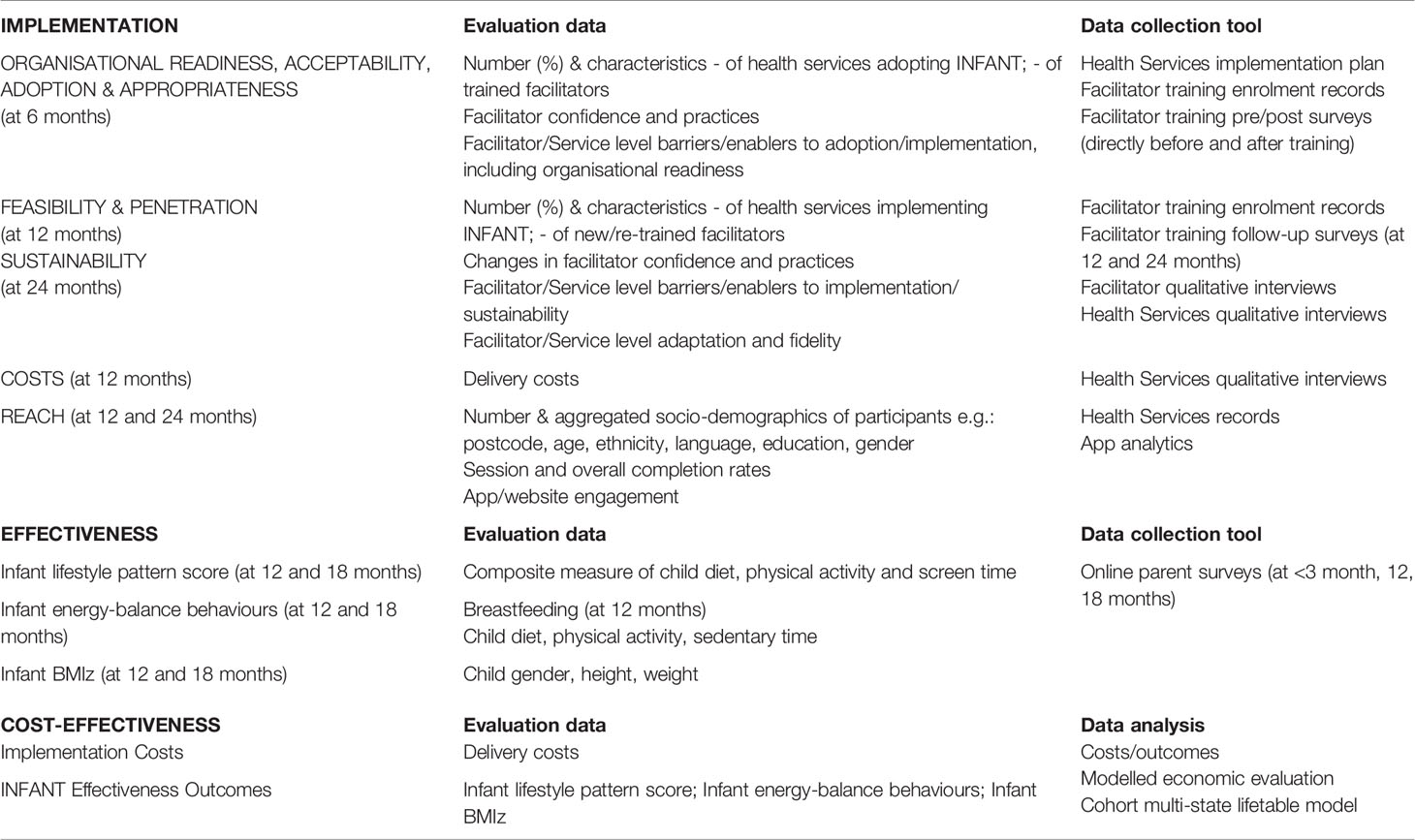
Two evaluation frameworks underpin the hybrid trial, ‘Outcomes for Implementation Research’ (30) (implementation trial) and ‘RE-AIM’ (31) (effectiveness trial), as outlined in Table 2.
2.4 Implementation Trial
2.4.1 Trial Design
INFANT will be implemented in collaboration with eight practice and policy partners including key agencies involved in MCH service delivery, population health and Aboriginal health. Partners will integrate the promotion and endorsement of INFANT to all Victorian LGAs (n=79), leveraging routine communication channels (e.g., statewide newsletters) and via presentations from the research implementation team at existing meetings of service providers. Health services seeking to adopt INFANT will express interest via the INFANT website (infantprogram.org) and be guided through the sign-up process by the research implementation team. Cultural and linguistic adaptations to meet the needs of multi-cultural and Aboriginal and Torres Strait Islander families within Victoria will be explored with relevant peak representative organizations as a sub-project, to be reported elsewhere.
2.4.2 Organisational Eligibility Criteria
To be eligible for adoption, health services will be required to nominate at least one staff member to complete the online INFANT implementation training. Organisational staff eligible to facilitate INFANT include MCH nurses, dietitians, community health workers or nominated personnel. INFANT implementation training comprises eight hours of free online training, providing guidance on all aspects of INFANT implementation and facilitation, with a focus on how to deliver group sessions and integration within existing delivery systems to maximise adoption and sustainability. The training includes case studies of successful implementation, an online discussion forum to promote peer networking (moderated by research staff), and interaction with the research implementation team to develop an implementation plan (a blueprint for local implementation). Following implementation training, health services will be eligible to have their trained staff deliver INFANT with yearly renewal contingent on facilitators completing a short online annual refresher course. Trained facilitators will be provided with online access to all resources including a facilitator and implementation manual as well as parent handouts (downloaded as pdfs) and the My Baby Now app.
2.4.3 Implementation Strategies
Implementation strategies have been selected from the Expert Recommendations for Implementing Change (ERIC) compilation (32) to address barriers identified from the RCT and related small scale implementation studies (15, 26, 28, 33). Key strategies to support local implementation include assessment of organisational readiness; development of an implementation blueprint; implementation support through site visits, resource sharing, and collaborative learning; support with accessing new funding; revising professional roles; implementation audit and feedback; and accessible, incentivised training. Supplementary File 4 presents the 16 INFANT implementation strategies, categorised according to the six key implementation processes (34): planning, educate, finance, restructure, quality management, and policy context. Implementation strategies are described using Proctor et al.’s (35) recommended ‘specifiying and reporting’ guidelines.
2.4.4 Implementation Outcomes
Implementation outcomes, as detailed below, will be explored to understand (i) the role of, and barriers and enablers to, the use of implementation strategies and perceived impacts on implementation and/or effectiveness, and (ii) the rationale for uptake and modification, and impacts on fidelity and/or effectiveness (30).
2.4.4.1 Organisational Readiness, Acceptability, Adoption, Appropriateness, and Feasibility
Staff who enroll in INFANT implementation training will be invited to complete a quantitative online survey at baseline (pre-training), immediately post-training and at 12 and 24-months post training to assess changes in confidence and practices, to assess barriers and facilitators to implementation, and use of core INFANT messages to inform health professional practice. Facilitators will also be invited to complete a short online survey immediately post-training to provide feedback on the training course. Organisational readiness questions will be embedded in the baseline, 12 and 24-months post training surveys to identify organisational level barriers to adoption, implementation and maintenance. Organisational readiness will be measured using the 12-item ORIC Tool (36) which explores the domains of change valence (motivation), change commitment, change efficacy and change capacity. ORIC scores will be aggregated at organisational (health service) level to inform the development of the implementation plan, tailored to the needs of the organisation. The research implementation team will provide support to organisations to address identified readiness barriers when developing their implementation plans, and during 12- and 24-month qualitative interviews informed by the Consolidated Framework for Implementation Research (CFIR) (37) and the Program Sustainabilty Assessment Tool (PSAT) (38). Number of organisational staff trained and delivering INFANT will be monitored by the research implementation team through six-monthly telephone support. Organisations who have expressed interest or have trained facilitators but do not proceed to implementation will also be invited to participate in qualitative interviews to explore barriers and facilitators to adoption.
2.4.4.2 Fidelity and Adaptation
It is anticipated that contextual modifications will occur, therefore fidelity and adaptation data will be explored using FRAME (39) (Framework for Reporting Adaptations and Modifications-Enhanced) to understand the rationale for modification and impacts on fidelity and/or effectiveness. Questions will be embedded into the 12-months post-training online survey to assess changes to planned INFANT implementation, and the types and context of adaptations made. Qualitative interviews will be conducted with a purposeful sample of these staff (with high/low fidelity, high/low barriers) to explore key factors influencing implementation, and adaptations made to implementation strategies. This will enable the fit of the intervention and implementation strategies to be documented and reviewed (40).
2.4.4.3 Penetration and Sustainability
Factors affecting sustained implementation will be assessed using a quantitative online survey of staff who completed INFANT implementation training 24-months post-training. Qualitative interviews will be conducted with a purposeful sample of organizational staff (continued/discontinued implementation) to explore key factors influencing sustained implementation. Qualitative interviews with key state level partners will also be conducted to explore the extent to which dissemination and promotion was embedded into existing systems, and barriers/facilitators to achieving integration into routine practice.
2.4.4.4 Costs
Costs of scaling up will be examined to assess intervention affordability and sustainability. A mixed-methods approach will evaluate the costs associated with implementation, using qualitative data collected from quarterly semi-structured interviews with key informants involved in implementation and unit costs and quantities by resource category (e.g., time, equipment).
2.4.4.5 Reach
Intervention enrolment, participant characteristics, attendance and completion rates will be monitored over the duration of the project using the MCH nurse record system. Use of the app and website will be measured via analytics using an Engagement Index Score, a novel methodology previously developed by members of the research team (41).
2.4.5 Data Analysis
Descriptive statistics will be reported for quantitative survey items related to organizational readiness, acceptability, appropriateness, feasibility, adaptation and penetration. Qualitative interview data will be transcribed verbatim and coded deductively [informed by CFIR (37), PSAT (38) and FRAME (39)] and inductively to capture issues within and outside of these frameworks. This has been successfully used by the team in previous implementation research (25, 42). Findings will be compared with the choice of INFANT implementation strategies to determine future refinements. Cost data analysis will use a healthcare funder perspective and detailed pathway analysis will specify scale-up and implementation activities in order to measure costs of associated resource use (2023 reference year, costs measured in Australian dollars (AUD)). Total and incremental cost, cost per local government area, cost per participant child and cost per eligible child will be reported.
2.5 Effectiveness Trial
2.5.1 Trial Design
An embedded non-randomised controlled trial will assess the effectiveness and cost-effectiveness of INFANT when delivered at scale. The trial will involve comparing primary and secondary effectiveness outcomes between a group of INFANT participants (intervention arm) and a non-randomised comparison group recruited within the same communities prior to the implementation of INFANT (usual care arm). This approach enables comparison of outcomes between parents and infants exposed and not exposed to the intervention living in the same geographical areas and therefore sharing socio-demographic characteristics and having access to the same health services. The risk of bias will be reduced by replicating strategies for recruitment, follow-up and assessment in both trial arms and adjusting for unbalanced baseline characteristics. This trial design was deemed appropriate for assessing the effect of INFANT when implemented at scale in real-world conditions, enabling natural dissemination and uptake to be captured. A cluster randomized design with communities allocated to intervention or usual care was deemed not feasible because the prospect of being randomised to the control arm is likely to reduce participation. Within communities, the prospect of parent groups being randomised to a control arm is unethical (given the known efficacy of the intervention) and contamination between groups would be unavoidable. A stepped-wedge cluster randomised study design (43) was also considered but ruled out as randomisation of LGAs to an implementation time period may interfere with natural uptake under real world conditions.
2.5.2 Eligibility Criteria
Parents will be eligible if they are a first-time parent/primary caregiver of an infant aged 0-3 months, owning an internet enabled personal mobile phone and able to communicate in English. Infants born before 37 weeks gestation will be excluded.
2.5.3 Study Procedures
Families will be recruited over a 6-month period for the comparison arm and over a two-year period for the intervention group (Table 1). The comparison (usual care) group will be recruited prior to the implementation of INFANT by health services who have expressed interest prior to undergoing INFANT training. If sample size is not achieved from these LGAs, recruitment will be extended to other LGAs ensuring a mix of metro, regional, rural LGAs and various levels of socioeconomic disadvantage. There will be a three-month gap between recruitment of the last comparison participant and the commencement of recruitment for INFANT implementation participants. This gap ensures comparison participant infants will be too old to enroll in INFANT sessions and thus reduce potential for contamination.
Eligible parents will be invited to participate via SMS (with up to 2 reminders) sent by participating organisations. The SMS will contain a link to an online screening and consent process with consenting parents invited to complete online surveys when infants are aged ≤ 3 months (T1), 12 months (T2) and 18 months (T3) (Table 1). Up to three reminders will be generated for non-responders to follow up survey invitations. Strategies will be employed to promote retention (e.g. personalised newsletters, reimbursement for time spent completing surveys) which have been used successfully in the original INFANT trial, which achieved 85% retention over two years across both study arms (13).
2.5.4 Primary and Secondary Outcomes
The primary outcome for the effectiveness trial will be child lifestyle pattern score ‘Discretionary food and screen time’ at 18 months. This is a composite measure of child diet, physical activity and screen time derived from a previous analysis (44) of the INFANT RCT (13) and INFANT Extend RCT (33) using principal component analysis. This has been selected as the primary outcome based on previous research (44) confirming the early clustering of these behaviours into unhealthy versus healthy lifestyle patterns in children as young as 18 months of age. Further, these lifestyle patterns track from early childhood into later childhood and adulthood, and are associated with overweight and obesity (45).
Secondary outcomes of the effectiveness trial will include (i) proportion of mothers with any breastfeeding at 12 months; (ii) energy-balance behaviours including child diet (fruit, vegetables, non-core drinks and snacks), child time physically active and sedentary, scores on the ‘Fruit, vegetables and outdoor time’ lifestyle pattern (12 & 18 months), and scores on the ‘Discretionary food and screen time’ lifestyle pattern (12 months) (44); (iii) infant BMIz at 12 months and 18 months, and (iv) child classification as overweight or obese according to WHO definitions (46).
2.5.5 Sample Size
The trial aims to recruit 1,500 primary carer/infant dyads in each arm (total 3,000). Assuming a conservative attrition rate of 33%, it is expected that complete data on 2,000 primary carer/infant dyads (1,000/arm) will be collected. The statistical software PASS version 14.0.9 (NCSS, LLC) was used for all power calculations, assuming that 190 first-time parent groups will run in each arm with an average number of 5.4 children with complete data per group and a coefficient of variability in group size of 0.32, as observed in the INFANT RCT (13) (α=0.05; two-sided tests). A target sample size of 1,000 infants per arm achieves 80% power to detect a mean difference between arms of at least 0.17 points in the lifestyle score ‘Discretionary food and screen time’ at 18 months [primary outcome; standard deviation within clusters (SD) =1.21, intracluster correlation coefficient (ICC) = 0.048]. In our former INFANT trial (44) the difference in “Discretionary food and screen time” pattern score was 0.29 (95% CI: 0.08, 0.49).
The target sample size (1,000 infants per arm) also achieves 80% power to detect the following differences in secondary outcomes:
1) 6.2% increase in the proportion of mothers with any breastfeeding at 12 months assuming the proportion in the control group will be 27% (ICC =0.05);
2) a mean difference (at 18m) between study arms of a) 0.24 serve/day in fruit [SD=1.7, ICC=0.071]; b) 0.25 serve/day in vegetables [SD=2.0, ICC=0.000]; c) 0.06 serve/day in non-core drinks [SD=0.44, ICC=0.034]; d) 0.06 serve/day in non-core sweet [SD=0.39, ICC=0.064]; e) 0.05 serve/day in non-core savoury [SD=0.33, ICC=0.047]; f) 6.7 min/day of screen use [SD=44, ICC=0.11]; and g) 18.4 min/day of physical activity [SD=143, ICC=0.018]. While individual energy-balance behaviour changes appear small, modest increments in energy consumption (126-189kJ/day (30-45 calories)) are estimated to promote population weight gain over time. Collectively these changes have capacity to influence weight gain trajectories at a population level (47, 48);
3) a mean difference between arms of at least 0.13 change in BMIz score by 12 months [SD=1.0, ICC= 0.021]. Assuming a mean BMIz of 0.41, a 0.13 reduction in BMIz to 0.28 equates to a 1.3% reduction in prevalence of overweight and obesity, considered meaningful at a population level.
2.5.6 Data Collection and Analysis
Infant diets will be measured by a validated Food Frequency questionnaire (FFQ) with average percentage categorical agreement against three days of 24 hour recall of 62-64% (49). Infant physical activity will be measured using a single parent-reported question with good test-retest reliability (ICC 0.62) (50). Infant screen time will be measured using a single parent-reported question with excellent reliability (ICC 0.84) (51). Change in BMIz measured at 12 and 18 months will be derived from child height and weight which is routinely collected by MCH nurses and will be made available in de-identified form to the research team. These data are typically available for 80% and 71% of infants at 12 and 18 months respectively (52). Lifestyle pattern scores will be calculated as linear combinations of the standardized dietary (fruit, vegetables, water, discretionary sweet foods, discretionary savory foods), outdoor and television viewing items, using the principal component analysis factor loadings previously published from the combined INFANT and INFANT Extend datasets (44).
Covariates will include self-reported parent socio-demographic characteristics (including marital status, education level, age, sex, Indigenous status, language spoken at home, country of birth, years lived in Australia, health care card holder) parent weight and height, pre-pregnancy weight, self-rated coping, sleep and health, infant sex, birth weight and temperament. Baseline characteristics will be compared between arms using linear mixed models (LMM) or generalized estimating equations (GEE) with link and distribution selected based on the type of variable. The effect of the intervention on the primary outcome (‘Discretionary food and screen time’ lifestyle pattern) will be estimated using a LMM including arm, time (12 and 18 months), interaction arm-time and any baseline variable showing clear imbalance between arms (covariates) as fixed effects. The same LMM model will be used for numerical secondary outcomes. All mixed models will include MCH group as a random effect to account for clustering. The effect of the intervention on proportion of mothers with any breastfeeding at 12 months will be estimated using a GEE model with logit link and binary distribution including arm, time (12 and 18 months), interaction arm-time and baseline confounders. If appropriate, multiple imputation will be used to handle missing data.
2.5.7 Cost Effectiveness
A cost-effectiveness analysis will determine if the implementation of INFANT represents ‘value for money’ when measured incrementally against usual practice. A ‘trial-based evaluation’ (i.e., costs/outcomes exactly as per the trial) will be conducted using primary and secondary outcomes. A ‘modelled economic evaluation’ will also be conducted, using the difference in BMIz between the intervention and control arms, and extending the target population, time horizon and decision context. A cohort multi-state lifetable model (53) will be used to translate changes in infants’ BMIz to long-term health outcomes (healthy adjusted life years). Simulation-modelling using the @RISK software package will calculate 95% uncertainty intervals around epidemiological inputs and cost estimates.
2.6 Governance
Practice and policy partners engaged in this proposal are key to the implementation, and importantly the sustained delivery of INFANT within Victorian local government areas. An Implementation Advisory Group consisting of Department of Health representatives (including MCH services), Municipal Association of Victoria representatives (responsible for delivery of MCH services), representatives of local health services who have successfully implemented INFANT for a number of years and the research team meet bi monthly. This group has input into all aspects of this proposal including recruitment and promotion approaches, development and delivery of INFANT implementation training, data collection and study measures. A larger Partnership Advisory committee has also been formed (consisting of all 8 practice and policy partners) meeting annually. This group provides strategic input and advice regarding the alignment of INFANT with organizational and policy directions important for the sustainability of this initiative into the future.
2.7 Data Management
All data will be collected and stored electronically in re-identifiable form on Deakin University’s online Research Data Store system. De-identified data will be made available to partners listed in the Partnership Grant and Multi-Institutional Agreement via a username and password. Online survey forms include error messages for response outside of plausible values. The de-identified data set may be made available on request to those with a methodologically sound proposal and pending ethics approval.
3 Discussion
The evaluation of effective interventions delivered at scale is a crucial, yet under-researched aspect of changing population-level health behaviours and essential to reducing the evidence to practice and policy gap. Examining implementation and effectiveness outcomes concurrently using a hybrid implementation and effectiveness trial design however comes with a number of challenges. Recruitment to the effectiveness trial is contingent on sufficient uptake of INFANT by organisations which in turn is shaped by a wide range of contextual factors outside of the control of the research team. Hence implementation outcomes determine the feasibility of conducting an embedded effectiveness trial within the timeframes of the study. Designing such a trial is also methodologically challenging as traditional trial designs such as RCT, cluster RCTs and stepped-wedge designs, which require organisations to be randomized to control arms, can interfere with the natural uptake of the intervention under ‘real world conditions’. The use of a non-randomized design in this effectiveness trial is therefore not without its limitations but will be minimized by recruiting parents exposed and not exposed to the intervention living in the same communities, replicating strategies for recruitment, follow-up and assessment in both trial arms, and adjusting for unbalanced baseline characteristics.
Successful, sustained implementation is likely due to a combination of factors, including the effectiveness of the intervention and the use of appropriate implementation strategies. An improved understanding of the interrelationship of these factors will therefore enable explicit and transparent decisions regarding intervention adoption, implementation and sustainability. To our knowledge, no obesity prevention interventions in early childhood have been implemented and evaluated at scale under real world conditions. The findings from this study will directly inform future delivery of INFANT in Victoria. It will also provide vital implementation, effectiveness and cost-effectiveness evidence to inform the adoption and sustained implementation of early childhood obesity prevention interventions both in Australia and internationally.
4 Ethics And Dissemination
This study has been approved by the Deakin University Human Ethics Research Committee (approval no: 2019-403), and is registered on the Australian New Zealand Clinical Trials Registry (ACTRN12620000670976). Written informed consent will be obtained from all participating implementation staff and parents. In recognition of the importance of research with Aboriginal communities being led by Aboriginal people, Aboriginal families will be the focus of a separate sub-project conducted in partnership with the Victorian Aboriginal Community Controlled Health Organisation and led by Aboriginal researchers. The protocol for this will be published elsewhere and subject to the NHMRC Guidelines for Ethical conduct in research with Aboriginal and Torres Strait Islander Peoples and communities [https://www.nhmrc.gov.au/about-us/resources/ethical-conduct-research-aboriginal-and-torres-strait-islander-peoples-and-communities]. Any future protocol modifications will be submitted for ethics approval and discussed and agreed with the project advisory committee including all chief investigators and partners. While it is unlikely that this health promotion research presents any potential risks associated with participation, if any participant expresses individual concerns or issues related to this study that requires medical attention, the health service will follow their standard procedures. Organisational staff will notify the lead investigator to determine the course of action. Research staff and organisations involved in delivering INFANT will be asked to report any unintended consequences to the research team.
Research findings will be disseminated in peer reviewed journals and at scientific and professional conferences. We will also aim to communicate the study findings at key practice and policy forums and using plain language summaries for participating parents, practice and policy stakeholders distributed via email and the program website infantprogram.org.
Data Availability Statement
The de-identified data set may be made available on request to those with a methodologically sound proposal and pending ethics approval.
Ethics Statement
The studies involving human participants were reviewed and approved by the Deakin University Human Ethics Research Committee (approval no: 2019-403), and is registered on the Australian New Zealand Clinical Trials Registry (ACTRN12620000670976). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors contributed to study design which was led by KC, RL with the implementation trial component led by PL, the economic evaluation plan led by VB and MM and the sample size calculation and statistical analysis plan led by LO. RL, PL, and KC developed the first draft of the manuscript. All authors reviewed and commented on the draft manuscript. RL and PL co-wrote the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research is funded by The National Health and Medical Research Council (Grant number: GNT1161223), the Victorian Health Promotion Foundation, and the Victorian Department of Health. KH is supported by an Australian Research Council Future Fellowship (FT130100637). VB is supported by an Alfred Deakin Postdoctoral Research Fellowship. Beyond the peer review process, the funding bodies do not have a role in study design; data management, analysis, nor interpretation; writing of the report; or final authority over the decision to submit study findings for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge all the practice and policy partners involved in this research including Victorian Health Promotion Foundation (VicHealth), Municipal Association of Victoria, Victorian Department of Health, Victorian Aboriginal Community Controlled Health Organisation, Raising Children’s Network, City of Whittlesea, Sunraysia Community Health Services, Western Alliance Academic Health Science Centre.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.717468/full#supplementary-material
References
1. WHO. Report of the Commission on Ending Childhood Obesity. Geneva: World Health Organisation (2016).
2. Moore TG, Arefadib N, Deery A, West S. The FirstThousand Days: An Evidence Paper. Parkville, Victoria: Centre for Community Child Health, Murdoch Children’s Research Institute (2017).
3. ABS. National Health Survey First Results, 2017-18. Canberra: Australian Bureau of Statistics (2015).
4. Zheng M, Lamb KE, Grimes C, Laws R, Bolton K, Ong KK, et al. Rapid Weight Gain During Infancy and Subsequent Adiposity: A Systematic Review and Meta-Analysis of Evidence. Obes Rev (2018) 19(3):321–32. doi: 10.1111/obr.12632
5. UNICEF. Levels and Trends in Child Malnutrition: Key Findings of the 2015 Edition. New York: UNICEF; World Health Organisation; World Bank (2015).
6. Wake M, Lycett K. Let’s Call It as It Is: On Results, Reach, and Resolution in Population-Based Obesity Trials. Pediatrics (2014) 134(3):e846–7. doi: 10.1542/peds.2014-1940
8. Chung A, Backholer K, Wong E, Palermo C, Keating C, Peeters A. Trends in Child and Adolescent Obesity Prevalence in Economically Advanced Countries According to Socioeconomic Position: A Systematic Review. Obes Rev an Off J Int Assoc Study Obes (2016) 17(3):276–95. doi: 10.1111/obr.12360
9. Cameron AJ, Spence AC, Laws R, Hesketh KD, Lioret S, Campbell KJ. A Review of the Relationship Between Socioeconomic Position and the Early-Life Predictors of Obesity. Curr Obes Rep (2015) 4(3):350–62. doi: 10.1007/s13679-015-0168-5
10. AIHW. Australian Burden of Disease Study: Impact and Causes of Illness and Death in Aboriginal and Torres Strait Islander People 2011. Australian Burden of Disease Study Series No. 6. Cat. No. BOD 7. Canberaa: AIHW (2016).
11. Baxter J, Hand K. Access to Early Childhood Education in Australia: Research Report No. 24. Melbourne: Australian Institute of Family Studies (2013).
12. DET. Maternal & Child Health Services Annual Report 2016-2017 Statewide. Melbourne: Victorian State Government Education and Training (2017).
13. Campbell K, Lioret S, McNaughton SA, Crawford D, Salmon J, Ball K, et al. A Parent-Focused Intervention to Reduce Infant Obesity Risk Behaviors: A Randomized Trial. Paediatrics (2013) 131(4):652–60. doi: 10.1542/peds.2012-2576
14. Campbell K, Hesketh K, Crawford D, Salmon J, Ball K, McCallum Z. The Infant Feeding Activity and Nutrition Trial (INFANT) an Early Intervention to Prevent Childhood Obesity: Cluster-Randomised Controlled Trial. BMC Public Health (2008) 8:103. doi: 10.1186/1471-2458-8-103
15. Lunn PL, Roberts S, Spence A, Hesketh KD, Campbell KJ. Mothers’ Perceptions of Melbourne InFANT Program: Informing Future Practice. Health Promot Int (2016) 31(3):614–22. doi: 10.1093/heapro/dav004
16. Lioret S, McNaughton SA, Spence AC, Crawford D, Campbell KJ. Tracking of Dietary Intakes in Early Childhood: The Melbourne InFANT Program. Eur J Clin Nutr (2013) 67(3):275–81. doi: 10.1038/ejcn.2012.218
17. Cameron AJ, Ball K, Hesketh KD, McNaughton SA, Salmon J, Crawford DA, et al. Variation in Outcomes of the Melbourne Infant, Feeding, Activity and Nutrition Trial (InFANT) Program According to Maternal Education and Age. Prev Med (2014) 58(1):58–63. doi: 10.1016/j.ypmed.2013.10.021
18. Lioret S, McNaughton SA, Crawford D, Spence AC, Hesketh K, Campbell KJ. Parents’ Dietary Patterns Are Significantly Correlated: Findings From the Melbourne Infant Feeding Activity and Nutrition Trial Program. Br J Nutr (2012) 108(3):518–26. doi: 10.1017/S0007114511005757
19. Delisle Nyström C, Cameron AJ, Campbell KJ, Hesketh KD. Variation in Outcomes of the Melbourne Infant, Feeding, Activity and Nutrition Trial (INFANT) According to Maternal Education and Age 2 and 3·5 Years Post-Intervention. Public Health Nutr (2021) 24(6):1460–8. doi: 10.1017/S1368980021000045
20. Hesketh KD, Salmon J, McNaughton SA, Crawford D, Abbott G, Cameron AJ, et al. Long-Term Outcomes (2 and 3.5 Years Post-Intervention) of the INFANT Early Childhood Intervention to Improve Health Behaviors and Reduce Obesity: Cluster Randomised Controlled Trial Follow-Up. Int J Behav Nutr Phys Activity (2020) 17(1):95. doi: 10.1186/s12966-020-00994-9
21. WHO. Consideration of the Evidence on Childhood Obesity for the Commission on Ending Childhood Obesity: Report of the Ad Hoc Working Group on Science and Evidence for Ending Childhood Obesity. Geneva: World Health Organisation (2016).
22. Milat AJ, King L, Bauman AE, Redman S. The Concept of Scalability: Increasing the Scale and Potential Adoption of Health Promotion Interventions Into Policy and Practice. Health Promot Int (2013) 28(3):285-98.
23. Hennessy M, Byrne M, Laws R, Mc Sharry J, O’Malley G, Heary C. Childhood Obesity Prevention: Priority Areas for Future Research and Barriers and Facilitators to Knowledge Translation, Coproduced Using the Nominal Group Technique. Translat Behavioral Med (2018) 9(4):759-67.
24. Victorian_Government. Victorian Public Health and Wellbeing Plan 2019–2023. In: Prevention_and_Population_Health_Branch. Melbourne: Victoria (2019).
25. Laws R, Hesketh KD, Ball K, Cooper C, Vrljic K, Campbell KJ. Translating an Early Childhood Obesity Prevention Program for Local Community Implementation: A Case Study of the Melbourne InFANT Program. BMC Public Health (2016) 16:748. doi: 10.1186/s12889-016-3361-x
26. Love P, Laws R, Litterbach E, Campbell KJ. Factors Influencing Parental Engagement in an Early Childhood Obesity Prevention Program Implemented at Scale: The Infant Program. Nutrients (2018) 10(4):509. doi: 10.3390/nu10040509
27. Litterbach EK, Russell CG, Taki S, Denney-Wilson E, Campbell KJ, Laws RA. Factors Influencing Engagement and Behavioral Determinants of Infant Feeding in an Mhealth Program: Qualitative Evaluation of the Growing Healthy Program. JMIR Mhealth Uhealth (2017) 5(12):e196. doi: 10.2196/mhealth.8515
28. Laws RA, Denney-Wilson EA, Taki S, Russell CG, Zheng M, Litterbach EK, et al. Key Lessons and Impact of the Growing Healthy Mhealth Program on Milk Feeding, Timing of Introduction of Solids, and Infant Growth: Quasi-Experimental Study. JMIR Mhealth Uhealth (2018) 6(4):e78. doi: 10.2196/mhealth.9040
29. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-Implementation Hybrid Designs: Combining Elements of Clinical Effectiveness and Implementation Research to Enhance Public Health Impact. Med Care (2012) 50(3):217–26. doi: 10.1097/MLR.0b013e3182408812
30. Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for Implementation Research: Conceptual Distinctions, Measurement Challenges, and Research Agenda. Adm Policy Ment Health (2011) 38(2):65–76. doi: 10.1007/s10488-010-0319-7
31. Glasgow RE, Harden SM, Gaglio B, Rabin B, Smith ML, Porter GC, et al. RE-AIM Planning and Evaluation Framework: Adapting to New Science and Practice With a 20-Year Review. Front Public Health (2019) 7:64. doi: 10.3389/fpubh.2019.00064
32. Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A Refined Compilation of Implementation Strategies: Results From the Expert Recommendations for Implementing Change (ERIC) Project. Implementation Sci IS (2015) 10:21. doi: 10.1186/s13012-015-0209-1
33. Campbell KJ, Hesketh KD, McNaughton SA, Ball K, McCallum Z, Lynch J, et al. The Extended Infant Feeding, Activity and Nutrition Trial (InFANT Extend) Program: A Cluster-Randomized Controlled Trial of an Early Intervention to Prevent Childhood Obesity. BMC Public Health (2016) 16:166. doi: 10.1186/s12889-016-2836-0
34. Powell BJ, McMillen JC, Proctor EK, Carpenter CR, Griffey RT, Bunger AC, et al. A Compilation of Strategies for Implementing Clinical Innovations in Health and Mental Health. Med Care Res Rev (2012) 69(2):123–57. doi: 10.1177/1077558711430690
35. Proctor EK, Powell BJ, McMillen JC. Implementation Strategies: Recommendations for Specifying and Reporting. Implementation Sci IS (2013) 8:139. doi: 10.1186/1748-5908-8-139
36. Sanders KA, Wolcott MD, McLaughlin JE, D’Ostroph A, Shea CM, Pinelli NR. Organizational Readiness for Change: Preceptor Perceptions Regarding Early Immersion of Student Pharmacists in Health-System Practice. Res Soc Adm Pharm (2017) 13(5):1028–35. doi: 10.1016/j.sapharm.2017.03.004
37. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering Implementation of Health Services Research Findings Into Practice: A Consolidated Framework for Advancing Implementation Science. Implementation Sci IS (2009) 4:50. doi: 10.1186/1748-5908-4-50
38. Luke DA, Calhoun A, Robichaux CB, Elliott MB, Moreland-Russel S. The Program Sustainability Assessment Tool: A New Instrument for Public Health Programs. Preventing Chronic Dis (2014) 23(11):130184. doi: 10.5888/pcd11.130184
39. Wiltsey Stirman S, Baumann AA, Miller CJ. The FRAME: An Expanded Framework for Reporting Adaptations and Modifications to Evidence-Based Interventions. Implementation Sci IS (2019) 14(1):58.
40. Glasgow RE, Harden SM, Gaglio B, Rabin B, Smith ML, Porter GC, et al. RE-AIM Planning and Evaluation Framework: Adapting to New Science and Practice With a 20-Year Review. Front Public Health (2019) 7(64). doi: 10.3389/fpubh.2019.00064
41. Taki S, Lymer S, Russell C, Campbell K, Laws R, Ong KL, et al. Assessing User Engagement of a mHeath Intervention: Development and Implementation of the Growing Healthy App Engagement Index. J Med Internet Res (2017) 5(6):e89.
42. Vidgen HA, Love PV, Wutzke SE, Daniels LA, Rissel CE, Innes-Hughes C, et al. A Description of Health Care System Factors in the Implementation of Universal Weight Management Services for Children With Overweight or Obesity: Case Studies From Queensland and New South Wales, Australia. Implementation Sci IS (2018) 13(1):109. doi: 10.1186/s13012-018-0801-2
43. Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The Stepped Wedge Cluster Randomised Trial: Rationale, Design, Analysis, and Reporting. BMJ Br Med J (2015) 350:h391. doi: 10.1136/bmj.h391
44. Lioret S, Campbell KJ, McNaughton SA, Cameron AJ, Salmon J, Abbott G, et al. Lifestyle Patterns Begin in Early Childhood, Persist and Are Socioeconomically Patterned, Confirming the Importance of Early Life Interventions. Nutrients (2020) 12(3):724. doi: 10.3390/nu12030724
45. Leech R, McNaughton S, Timperio A. Clustering of Diet, Physical Activity and Sedentary Behaviour Among Australian Children: Cross-Sectional and Longitudinal Associations With Overweight and Obesity. Int J Obes (2015) 39(7):1079–85. doi: 10.1038/ijo.2015.66
46. WHO. Child Growth Standards. World Health Organisation (2006). Available at: https://www.who.int/tools/child-growth-standards.
47. Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the Effect of Energy Imbalance on Bodyweight. Lancet (2011) 378(9793):826–37. doi: 10.1016/S0140-6736(11)60812-X
48. Wang YC, Orleans CT, Gortmaker SL. Reaching the Healthy People Goals for Reducing Childhood Obesity: Closing the Energy Gap. Am J Prev Med (2012) 42(5):437–44. doi: 10.1016/j.amepre.2012.01.018
49. Zheng M, Campbell KJ, Scanlan E, McNaughton SA. Development and Evaluation of a Food Frequency Questionnaire for Use Among Young Children. PloS One (2020) 15(3):e0230669. doi: 10.1371/journal.pone.0230669
50. Hesketh KD, Crawford DA, Abbott G, Campbell KJ, Salmon J. Prevalence and Stability of Active Play, Restricted Movement and Television Viewing in Infants. Early Child Dev Care (2015) 185(6):883–94. doi: 10.1080/03004430.2014.963066
51. Campbell KJ, Lioret S, McNaughton SA, Crawford DA, Salmon J, Ball K, et al. A Parent-Focused Intervention to Reduce Infant Obesity Risk Behaviors: A Randomized Trial. Pediatrics (2013) 131(4):652–60. doi: 10.1542/peds.2012-2576
52. Victoria_Government. Maternal and Child Health Services Annual Report: Statewide. Melourne Victoria: Department of Education and Training (2015-2016).
Keywords: child obesity prevention, infants (birth to 2 years), nutrition, physical activity, sedentary behavior, implementation science, obesity, scaling up
Citation: Laws R, Love P, Hesketh KD, Koorts H, Denney-Wilson E, Moodie M, Brown V, Ong K-L, Browne J, Marshall S, Lioret S, Orellana L and Campbell KJ (2021) Protocol for an Effectiveness-Implementation Hybrid Trial to Evaluate Scale up of an Evidence-Based Intervention Addressing Lifestyle Behaviours From the Start of Life: INFANT. Front. Endocrinol. 12:717468. doi: 10.3389/fendo.2021.717468
Received: 31 May 2021; Accepted: 21 September 2021;
Published: 08 November 2021.
Edited by:
Sally Radovick, The State University of New Jersey, United StatesReviewed by:
Vandana Jain, All India Institute of Medical Sciences, IndiaRona Macniven, University of New South Wales, Australia
Copyright © 2021 Laws, Love, Hesketh, Koorts, Denney-Wilson, Moodie, Brown, Ong, Browne, Marshall, Lioret, Orellana and Campbell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel Laws, ci5sYXdzQGRlYWtpbi5lZHUuYXU=
†These authors have contributed equally to this work and share first authorship
 Rachel Laws
Rachel Laws Penelope Love
Penelope Love Kylie D. Hesketh1
Kylie D. Hesketh1 Marj Moodie
Marj Moodie Vicki Brown
Vicki Brown Kok-Leong Ong
Kok-Leong Ong