- 1Department of Head Neck and Thyroid Surgery, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, Henan, China
- 2Department of Head and Neck Surgery, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China
- 3Office for Cancer Control and Research, Henan Cancer Hospital, The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, China
- 4Department of Radiation Oncology, The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, Henan, China
Objective: To analyze the incidence and risk factors for lateral lymph node metastases (LNMs) in T1a papillary thyroid carcinoma (PTC) with a focus on tumor location and size.
Materials and Methods: The incidence of lateral LNM in 345 cases of T1a PTC was retrospectively analyzed. Univariate and multivariate analyses were performed to assess the relationships between lateral LNM and clinicopathological characteristics.
Results: The incidence of skip metastasis to lateral LNM in T1a PTC located in the upper lobe was 12.1% (8/66). Logistic regression analysis indicated tumor size >5 mm (OR = 5.04, 95% CI = 1.79 to 14.18, P = 0.002), upper lobe location (OR = 7.68, 95% CI = 3.05–19.34, P < 0.001) and the number of central neck LNM (<2: OR = 24.79, 95% CI = 8.23–74.60, P < 0.001; ≥2: OR = 4.99, 95% CI = 1.95–12.73, P < 0.001) were independently associated with lateral LNM. Comparing the lateral and central LNM stratification based on tumor location revealed that both the incidences of lateral (33.3%) and central (30.3%) LNM of T1a PTC located in the upper lobe were higher than those of T1a PTC located in the middle and lower lobes. Of T1a PTC located in the upper lobe, the incidence of lateral LNM was 33.3% (22/66), which was higher than that [30.3% (20/66)] of central LNM. This finding is reversed in all T1a PTC cases and T1a PTC cases with tumor located in the middle and lower lobes.
Conclusion: A particularly high likelihood of lateral LNM was observed in T1a PTC patients with tumor located in the upper lobe of the thyroid gland, especially the tumor >5 mm in size, which could be considered a risk factor for lateral LNM in the clinical management of T1a PTC.
Introduction
The incidence of papillary thyroid carcinoma (PTC), especially occurrences smaller than 10 mm, continues to increase, arousing worldwide concern (1–5). A papillary thyroid carcinoma measuring ≤10 mm in its greatest dimension is defined as papillary thyroid microcarcinoma (PTMC) according to the World Health Organization classification system for thyroid tumors (6). The risk factors for the aggressiveness of PTMC are extrathyroidal extension (ETE) and clinically positive cervical lymph nodes (3). For PTMC without risk factors for aggressiveness, active surveillance can be considered an alternative option due to its indolent nature in biology (3, 7–10). For PTMC with risk factors for aggressiveness, surgery should be implemented in a limited time frame.
However, unlike ETE, it is typically not easy to assess the lymph node metastases (LNM) of PTMC on US or CT in clinical practice, especially in some hospitals without high-resolution US and thin-layer CT in developing countries. At present, there is a great difference in the study of the incidence of lateral LNM of PTMC. Zhao (11) found that lateral LNM was observed in 163 (75.8%) of 215 PTMC patients. However, Kim (12) found that approximately 4.4% of patients with PTMC presented with lateral LNM at the time of diagnosis. The effect of ETE on lateral LNM and prognosis has been well established (3, 13–17). Nevertheless, there are few studies on lateral LNM in PTMC without ETE. In this study, we focused on analyzing the lateral LNM of PTMC without ETE, that is, T1a PTC.
Patients and Methods
Ethics Statement
The Hospital Institutional Research Committee approved our study, and all participants provided written informed consent. All methods were performed in accordance with the relevant guidelines and regulations. All procedures in studies involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Patient Selection
From January 2017 to May 2017, the medical records of patients with surgically treated primary PTC were retrospectively reviewed. Enrolled patients met the following criteria: clinicopathological characteristics, including the location, size, multifocality, extrathyroidal extension of the tumor; lateral and central neck LNM; and BRAFV600E mutation status. Data were recorded in detail. PTMC was confirmed by pathologic analysis; that is, PTC measuring ≤10 mm in its greatest dimension (the largest disease if it is a multifocal PTC). Then, 367 PTMC patients were selected.
According to the American Joint Committee on Cancer (AJCC) TNM Staging For Thyroid-Medullary Carcinoma (8th ed., 2017), PTC with only minimal extrathyroidal extension invading strap muscles did not belong to T3b stage, so 16 cases with gross extrathyroidal extension invading strap muscles (T3b) and 6 cases with gross extrathyroidal extension into the nearby tissues of the neck, including the larynx, trachea, esophagus, or recurrent laryngeal nerve (T4a), were excluded. Therefore, a total of 345 T1a PTC patients were enrolled for analysis.
Treatment Principles
Based on 2016 NCCN guidelines for thyroid cancer (3), of 345 T1a PTC patients in this study, 81 patients received total thyroidectomy and 264 patients received lobectomy. Ipsilateral central neck lymph node dissection (LND) was routinely performed on PTC patients treated for the first time. In the lateral compartment, a formal modified radical LND including levels II, III, IV, and Vb was performed for pathologically positive lateral neck LNM (Table 1).
Pathologically positive lateral neck LNM was proven by preoperative biopsy or intraoperative frozen pathology with clinically positive lateral neck LNM. Clinical positive lateral neck LNM was suggested if the following features were noted on high-resolution ultrasound (US): absence of an echogenic hilum, round shape, microcalcification (Figure 1A), peripheral blood flow on color Doppler images, or cystic changes (18, 19). Clinical positive lateral LNM was suggested if the following features were noted on computed tomography (CT): area with clear evidence of non-fat, microcalcification (Figure 1B), low-density, or liquid components (Figures 1C, D); largest diameter >15 mm at level II and >10 mm at other levels; and the ratio of the longest to smallest diameter ≤ 2.
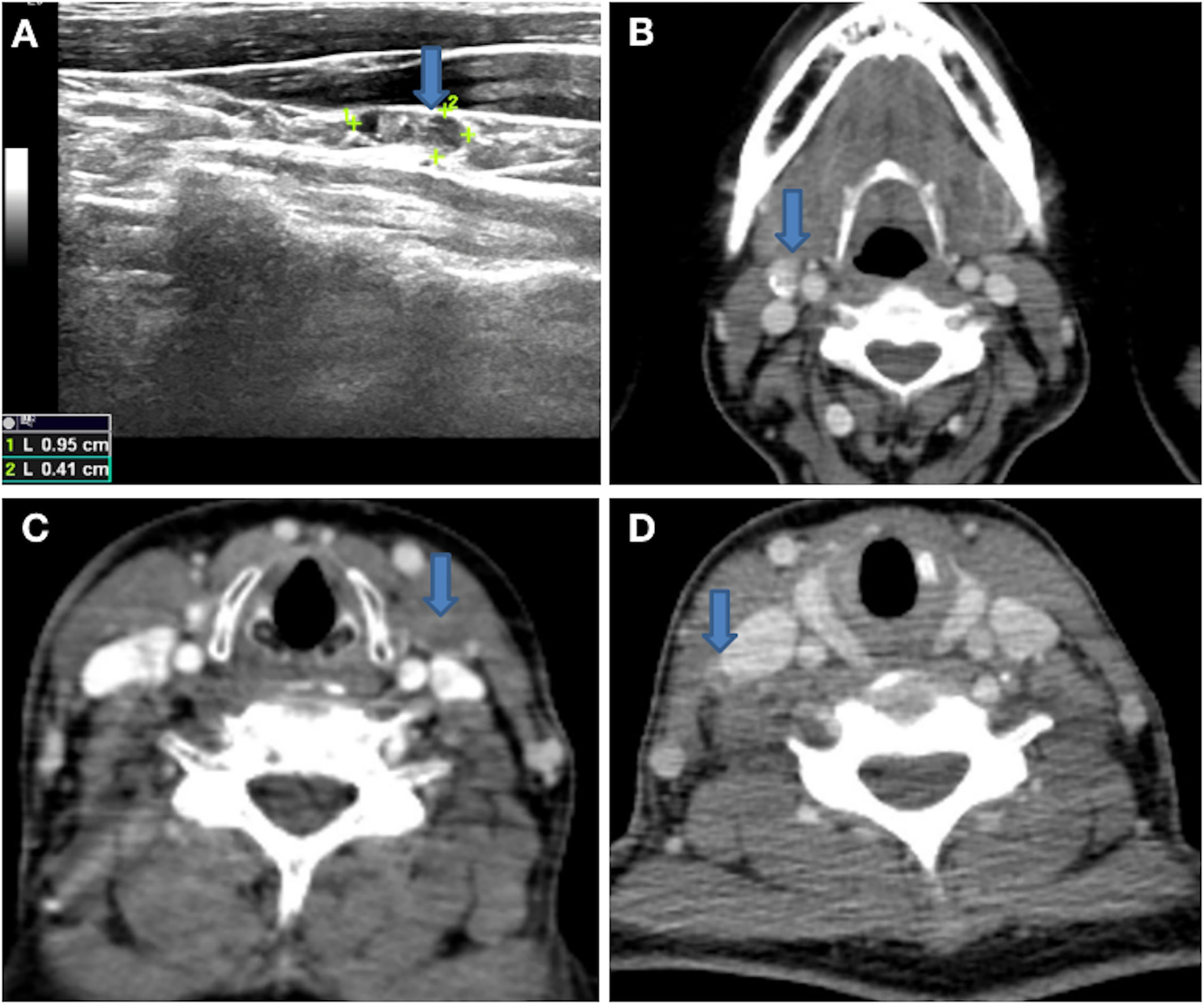
Figure 1 (A) Lateral neck lymph node with microcalcification on high-resolution US. (B) Lateral neck lymph node with microcalcification on 1-mm thin layer contrast-enhanced CT. (C, D) Lateral neck lymph nodes with low-density or liquid components on 1-mm thin layer contrast-enhanced CT.
Statistical Analysis
We used descriptive statistics to summarize patient demographics and the general characteristics of thyroid tumors. Data are presented as frequencies (percentages) for categorical variables with means (standard deviations) or (range) as appropriate for continuous variables.
The following factors were examined as possible factors associated with lateral LNM in T1a PTC: sex, age (<55 vs ≥55 years old), Hashimoto’s thyroiditis, location, size, multifocality of the tumor, number of central LNM and BRAFV600E mutation status. Tumor size was treated as a continuous measurement (in mm) and as a binary categorical variable with two categories (≤5 vs >5 mm). Based on US and CT images, the tumor location was categorized as upper lobe, middle lobe, or lower lobe. The thyroid was subjectively divided into thirds to differentiate the upper, middle, and lower lobes. Thyroid nodules occupying both the middle and upper thyroid lobes were included in the upper thyroid nodule category. Thyroid nodules occupying both the middle and lower thyroid lobes were included in the lower thyroid nodule category, as described in an early study (20). In addition, isthmus tumors were included in the middle lobe in this study, and the largest lesion was assessed if the tumor was multifocal. The number of central LNMs was treated as a continuous measurement and as a binary categorical variable with three categories (0 vs ≤2 vs >2).
Univariate analysis was performed using Pearson’s chi-square test; statistically significant results obtained from univariate analysis were submitted to multivariate logistic regression. The association of lateral LNM with each factor was expressed as odds ratios (ORs) and 95% confidence intervals (CIs). All statistical analyses were performed with SPSS 20.0 and P <0.05 was considered to be significant.
Results
Clinical and Pathologic Characteristics of Patients With T1a PTC
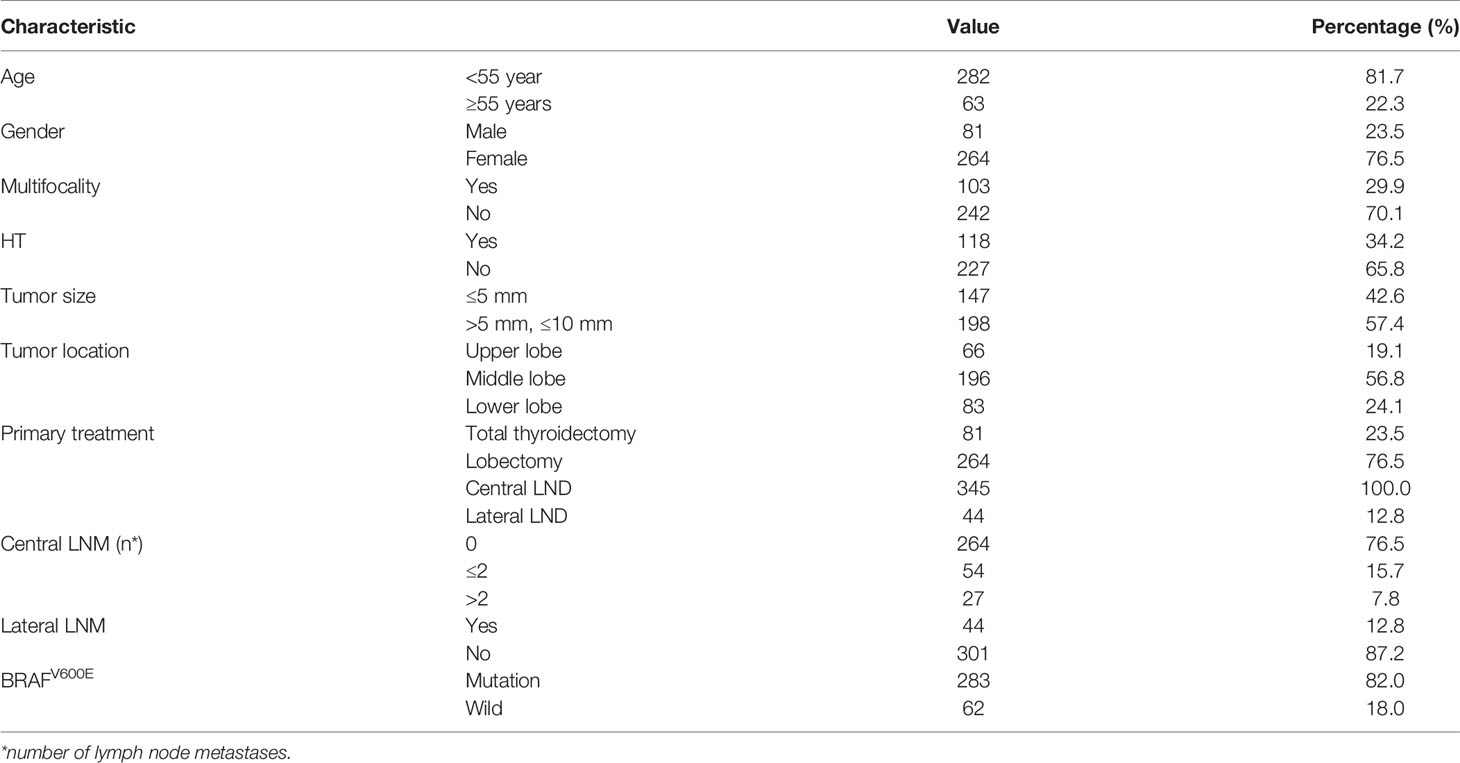
Ultimately, 345 T1a PTC patients (81 males and 264 females) were enrolled with a mean age of 47.0 (range: 20–74) years. Eighty-one (23.5%) patients underwent total thyroidectomy for bilateral tumors or lateral LNM, 81 (23.5%) patients had LNM, 44 (12.8%) patients had lateral neck LNM, 52 (15.1%) patients had bilateral thyroid lobe tumors, and 283 (82.0%) patients had BRAFV600E mutations (Table 1).
Of all T1a PTCs, the incidence of skip metastases to lateral LNM with central lymph node negativity was 4.3% (15/345), whereas that of T1a PTC with tumors located in the upper lobe with skip metastases was 12.1% (8/66).
Univariate Analysis of Factors Related to Lateral LNM in 345 Cases of T1a PTC
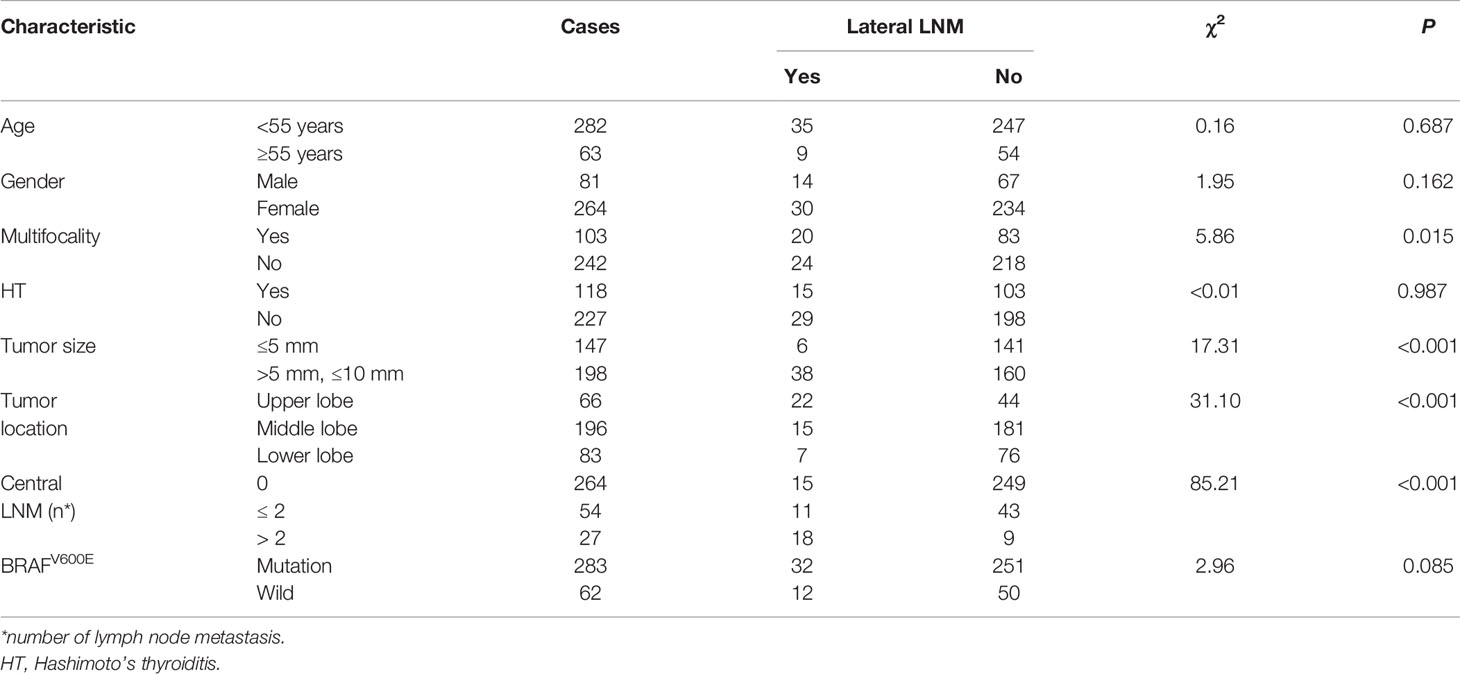
To explore the factors of lateral LNM of T1a PTC, the correlation between lateral LNM of T1a PTC and possible related clinicopathologic factors was further analyzed. The univariate analysis (Table 2) showed a statistically significant association between lateral LNM of T1a PTC and tumor size (≤5 mm vs >5 mm, P < 0.001), multifocality (P = 0.015), tumor location (upper, middle, and lower parts of thyroid lobe, P < 0.001) and the number of central LNM (>2, P < 0.001). No difference was observed between lateral LNM of T1a PTC and age (P = 0.156), sex (P = 0.162), Hashimoto’s thyroiditis (P = 0.987) or BRAFV600E (P = 0.085).
Multivariate Analysis of Factors Related to Lateral LNM in 345 Cases of T1a PTC
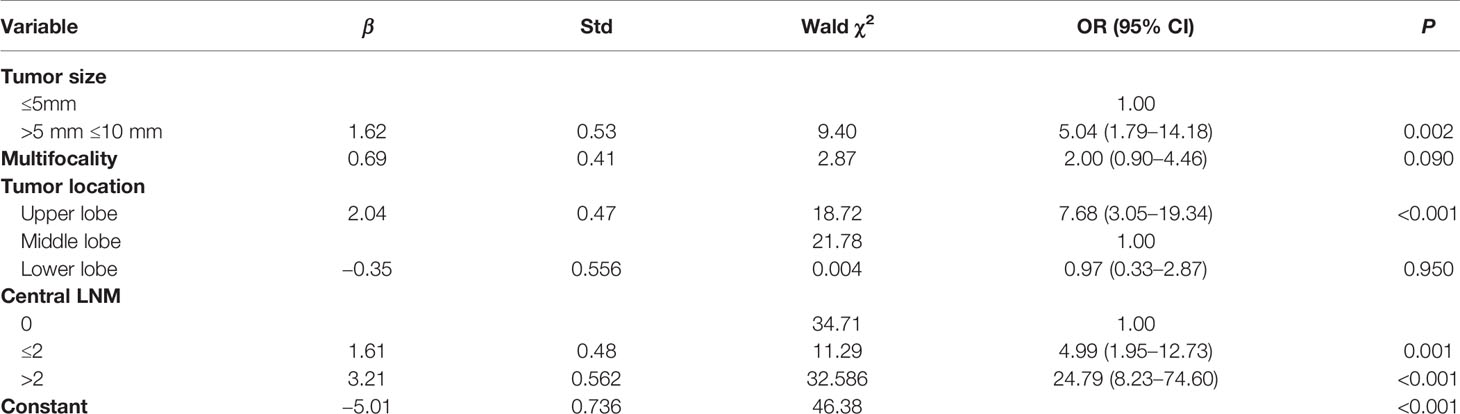
The results of the logistic regression analysis are shown in Table 3 and revealed three significant predictors for lateral neck LNM in T1a PTC: tumor size >5 mm (OR = 5.04, 95% CI = 1.79 to 14.18, P = 0.002), upper lobe location (OR = 7.68, 95% CI = 3.05–19.34, P < 0.001) and the number of central neck LNM (<2: OR = 24.79, 95% CI = 8.23–74.60, P < 0.001; ≥2: OR = 4.99, 95% CI =1.95–12.73, P < 0.001). However, the risk factor multifocality was not a significant predictor for lateral neck LNM in T1a PTC (OR = 2.00, 95% CI = 0.90–4.46, P < 0.001).
Incidence of Lateral LNM in T1a PTC Patients Stratified by Tumor Location And Size
Of 345 T1a PTC patients, 44 (12.8%) patients had lateral neck LNM. Stratification by location revealed that of 66 T1a PTC patients had tumors located in the upper part, 22 (33.3%) patients had lateral neck LNM, which was greater than 7.7% (15/196) in the middle part and 8.4% (7/83) in the lower part (Figure 2A).
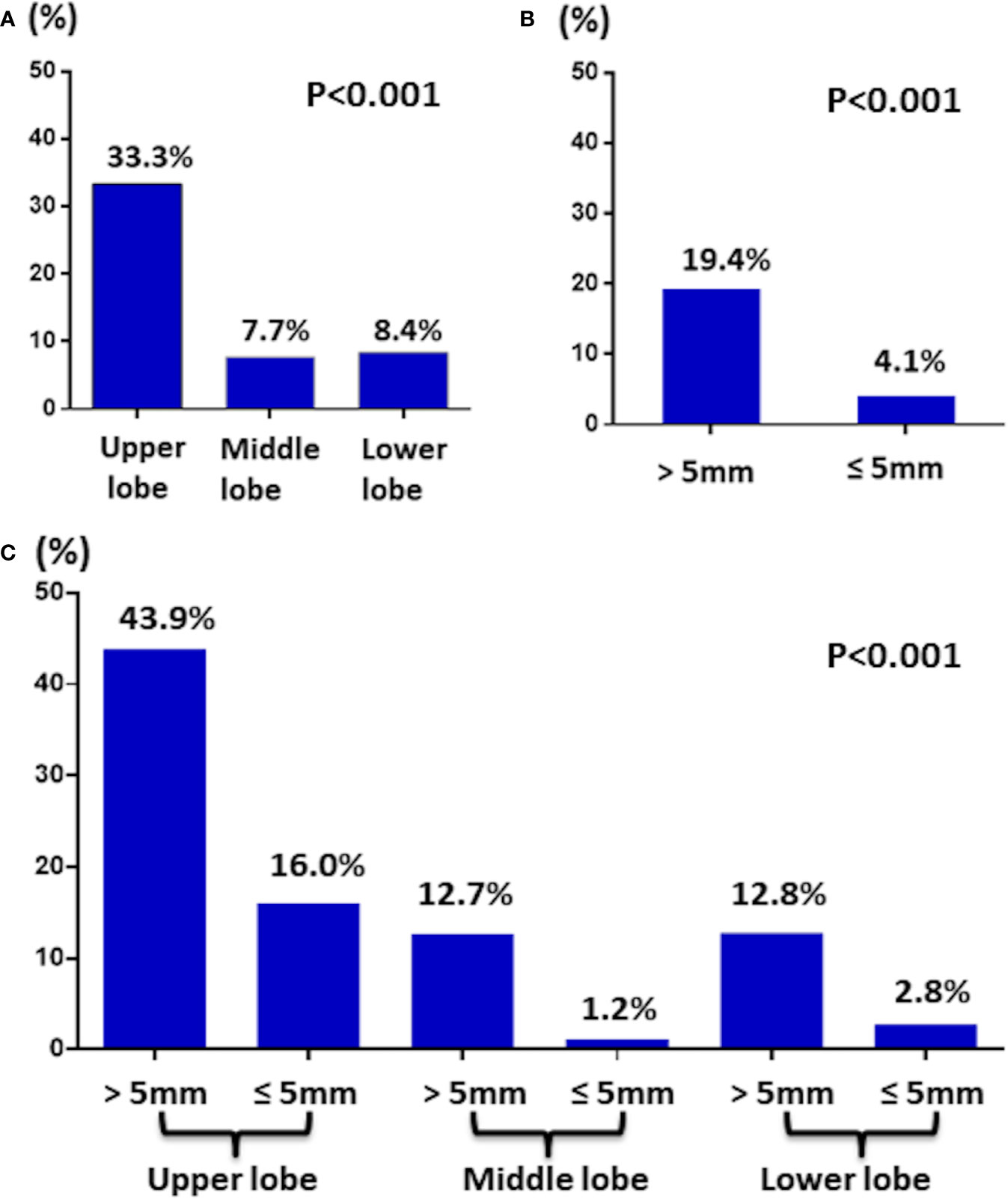
Figure 2 (A) The incidence of lateral LNM in T1a PTC by tumor location. (B) The incidence of lateral LNM in T1a PTC by tumor size. (C) The incidence of lateral LNM in T1a PTC by tumor location and size.
Then, stratification by size revealed that of 198 T1a PTC patients with tumors >5 mm, 38 (19.4%) patients had lateral LNM, which was greater than the 4.1% (6/147) noted for patients with tumors ≤5 mm (Figure 2B). Interestingly, of six T1a PTC patients with tumors ≤5 mm and lateral LNM, four tumors of the patients were located in the upper part.
Finally, stratification by location together with size revealed that of 41 T1a PTC patients with tumor >5 mm and located in the upper part, 18 (43.9%) patients had lateral LNM (Figure 2C).
Comparison of Lateral LNM and Central LNM Stratification by Tumor Location
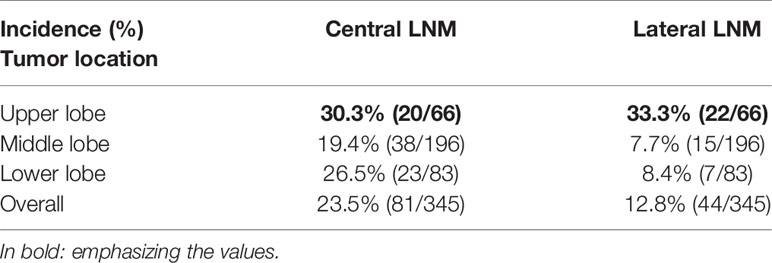
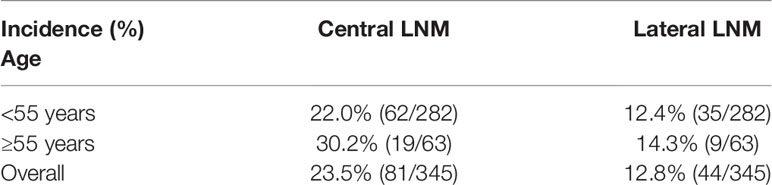
As shown in Table 4, 23.5% (81/345) of all T1a PTCs patients had central LNM, and 12.8% (44/345) of patients had lateral LNM. We compared lateral and central LNM stratified by tumor location and found that both the incidence of lateral (33.3%) and central (30.3%) LNM of T1a PTC with tumor located in upper lobe were higher than that of T1a PTC with tumor located in middle and lower lobes; of T1a PTC with tumor located on upper lobe, the incidence of lateral LNM was 33.3% (22/66), which is slightly higher than that noted for central LNM [30.3% (20/66)]. This finding is reversed in all T1a PTC and T1a PTC with tumors located in the middle and lower lobes.
Comparison of Lateral LNM and Central LNM Stratification by Age
As shown in Table 5, we compared the incidence of lateral and central LNMs of T1a PTC stratified by age and found that the incidences of central LNM of T1a PTC with age <55 and ≥55 years were 22.0% (62/282) and 30.2% (19/63) respectively (P = 0.166); the incidences of lateral LNM of T1a PTC with age <55 and ≥55 years were 12.4% (35/282) and 14.3% (9/63) respectively (P = 0.687). The incidence of central LNM of T1a PTC with age ≥55 year was slightly higher than that of T1a PTC with age <55; however, there was no statistical difference.
Discussion
The most important finding in the current study was that the rate of lateral LNM of T1a PTC with tumor located in the upper lobe was 33.3%, which is distinctly higher than that of T1a PTC with tumor located in the middle lobe (7.7%) and lower lobes (8.4%). Additionally, the rate of lateral LNM of T1a PTC with tumor >5 mm was 19.4%, which was also higher than that of T1a PTC with tumor ≤5 mm (4.1%). Finally, when considering location together with size, we found that the incidence reached 43.9% in T1a PTC patients with tumor >5 mm and located in the upper lobe.
Cervical LNM in PTC is common and is detected in up to 80% of patients as a result of radical neck dissection and postoperative histological examination (21). Kim (22) found that the overall frequencies of central and lateral LNMs in PTMC were 39.6% and 3.0%, respectively. A meta-analysis of 5,342 PTC cases showed that (23) the frequency of occult lateral neck lymph node metastases was 47.8%. A majority of studies have shown that cervical LNM, especially lateral LNM, carries prognostic significance (21, 24–26). When excluding invasive PTMC (T3–4) in this study, the overall frequencies of central and lateral LNMs in T1a PTC were 23.5% (81/345) and 12.8% (44/345), respectively.
Of T1a PTCs in this study, the incidence of skip metastases was 4.3% (15/345), whereas that of T1a PTC in the upper lobe was 12.1% (8/66). Zhao (27) reviewed 721 PTCs and found that the rate of skip metastases was 7.4%, and PTMC was an independent risk factor for skip metastases.
In this study, we found that lateral LNM of T1a PTC was associated with tumor size (≤5 mm vs >5 mm, P < 0.001), multifocality (P = 0.015), tumor location (upper, middle and lower lobes, P < 0.001), and central LNM. Further logistic regression analysis indicated that tumor size >5 mm (OR = 5.04, 95% CI = 1.79 to 14.18, P = 0.002), upper lobe location (OR = 7.68, 95% CI = 3.05–19.34, P < 0.001) and the number of central neck LNM (<2: OR = 24.79, 95% CI = 8.23–74.60, P < 0.001; ≥2: OR = 4.99, 95% CI = 1.95–12.73, P < 0.001) were significant predictors for lateral LNM in T1a PTC.
Similar to our results, Liu (28) reviewed 966 PTCs and found that only central LNM was independent risk factors for lateral LNM, but he also indicated that tumor located in the upper 1/3 of the lobe had the highest lateral LNM incidence (60%). Back (29) found that superior lesions, male sex, age under 45 years, and central lymph node metastasis were significant predictors of high-risk lateral LNM. Zhang (30) found that male sex, upper location of the tumor and tumor diameter ≥0.7 cm were predictive factors of lateral LNM in solitary PTMC without gross ETE. Kim (31) found that male sex, tumor size >0.5 cm, multiplicity, ETE, and central LNM were independent predictors of a high prevalence of lateral LNM in PTMC. In another study, multivariate analysis indicated that the significant risk factors for lateral LNM included ETE, multifocality, and central node metastases but not BRAF mutation (12).
Intriguingly, one of the major findings of the current study is the relatively high prevalence (33.3%) of lateral LNM of T1a PTC with tumors located in the upper lobe. Some studies (29, 30, 32–36) also indicated that the superior lesion or upper location of the tumor was an independent predictor for lateral LNM of PTMC with or without gross ETE. However, it is not entirely clear why the tumor location (upper lobe) is independently associated with lateral LNM in T1a PTC because the effects of ETE and tumor size were already excluded. This finding is probably due to the unique anatomical structure of the upper lobe, which has an abundant blood supply and lymphatic drainage. It is also likely that lymphatic vessels exist between the upper lobe of the thyroid and lateral neck by which tumor cells can metastasize directly to the lateral neck (37). In our observation, the proposed mechanisms may also include persistent physical pressure from adjacent thyroid cartilage. Recently, two small studies proposed integrating nodule location within the thyroid gland into risk stratification systems (20, 37, 38). In the two prospective clinical trials of active surveillance for low-risk PTMC from Japanese centers, a small number of PTMCs under active surveillance showed progression of tumor enlargement or new LNM appearance (39, 40). It is interesting to analyze whether the appearance of newly appeared LNM was associated with the tumor located in the upper lobe.
Our study has some limitations. First, there is inherent bias in all retrospective studies. Second, our sample size was relatively small, which potentially reduced the statistical power of this study. Third, lateral lymph node dissection is performed only when the suspected lateral lymph node is pathologically proven; thus, some micrometastases may be missed. Some merits of this study should also be noted. First, preoperative and lateral lymph nodes were accurately evaluated through high-resolution US and 1-mm thin layer contrast-enhanced CT (Figure 1), which was performed by a multidisciplinary team (MDT) composed of a surgeon, sonographer, radiologist, and pathologist specializing in thyroid carcinoma. Second, the characteristics of lateral LNM of T1a PTC were described in detail, especially the tumor located in the upper lobe.
In conclusion, the major findings of the current study are the relatively high incidence (33.3%) of lateral LNM of T1a PTC with tumor located in the upper lobe. Furthermore, when considering tumor size together with location, the incidence reached 43.9% in T1a PTC patients with tumor >5mm and located in the upper lobe.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All the authors made the contribution in study design, manuscript writing, studies selecting, data analysis, study quality evaluating, and manuscript revising. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Chen AY, Jemal A, Ward EM. Increasing Incidence of Differentiated Thyroid Cancer in the United States, 1988-2005. Cancer (2009) 115(16):3801–7. doi: 10.1002/cncr.24416
2. Polyzos SA, Anastasilakis AD. Clinical Complications Following Thyroid Fine-Needle Biopsy: A Systematic Review. Clin Endocrinol (2009) 71(2):157–65. doi: 10.1111/j.1365-2265.2009.03522.x
3. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Off J Am Thyroid Assoc (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
4. Ahn HS, Kim HJ, Welch HG. Korea’s Thyroid-Cancer “Epidemic”–Screening and Overdiagnosis. New Engl J Med (2014) 371(19):1765–7. doi: 10.1056/NEJMp1409841
5. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA (2017) 317(13):1338–48. doi: 10.1001/jama.2017.2719
6. Lloyd R, De Lellis R, Heitz P. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Endocrine Organs. Lyon, France: IARC Press International Agency for Research on Cancer (2000).
7. Ito Y, Oda H, Miyauchi A. Insights and Clinical Questions About the Active Surveillance of Low-Risk Papillary Thyroid Microcarcinomas [Review]. Endocr J (2016) 63(4):323–8. doi: 10.1507/endocrj.EJ15-0637
8. Brito JP, Ito Y, Miyauchi A, Tuttle RM. A Clinical Framework to Facilitate Risk Stratification When Considering an Active Surveillance Alternative to Immediate Biopsy and Surgery in Papillary Microcarcinoma. Thyroid Off J Am Thyroid Assoc (2016) 26(1):144–9. doi: 10.1089/thy.2015.0178
9. Fukuoka O, Sugitani I, Ebina A, Toda K, Kawabata K, Yamada K. Natural History of Asymptomatic Papillary Thyroid Microcarcinoma: Time-Dependent Changes in Calcification and Vascularity During Active Surveillance. World J Surg (2016) 40(3):529–37. doi: 10.1007/s00268-015-3349-1
10. Takami H, Ito Y, Noguchi H. Okamoto T 2012 Treatment of Thyroid Tumor: Japanese Clinical Guidelines. Tokyo: Springer (2012). p. 1–310.
11. Zhao W, Chen S, Hou X, Liao Q, Chen G, Zhao Y. Predictive Factors of Lateral Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Pathol Oncol Res POR (2019) 25(3):1245–51. doi: 10.1007/s12253-018-0511-8
12. Kim K, Zheng X, Kim JK, Lee CR, Kang SW, Lee J, et al. The Contributing Factors for Lateral Neck Lymph Node Metastasis in Papillary Thyroid Microcarcinoma (PTMC). Endocrine (2020) 69(1):149–56. doi: 10.1007/s12020-020-02251-2
13. Nixon IJ, Ganly I, Patel S, Palmer FL, Whitcher MM, Tuttle RM, et al. The Impact of Microscopic Extrathyroid Extension on Outcome in Patients With Clinical T1 and T2 Well-Differentiated Thyroid Cancer. Surgery (2011) 150(6):1242–9. doi: 10.1016/j.surg.2011.09.007
14. Radowsky JS, Howard RS, Burch HB, Stojadinovic A. Impact of Degree of Extrathyroidal Extension of Disease on Papillary Thyroid Cancer Outcome. Thyroid Off J Am Thyroid Assoc (2014) 24(2):241–4. doi: 10.1089/thy.2012.0567
15. Jain NK, Mostoufi-Moab S, Hawkes CP, Nelson ND, Surrey LF, Jones ZS, et al. Extrathyroidal Extension Is an Important Predictor of Regional Lymph Node Metastasis in Pediatric Differentiated Thyroid Cancer. Thyroid Off J Am Thyroid Assoc (2020) 30(7):1037–43. doi: 10.1089/thy.2019.0229
16. Park JS, Chang JW, Liu L, Jung SN, Koo BS. Clinical Implications of Microscopic Extrathyroidal Extension in Patients With Papillary Thyroid Carcinoma. Oral Oncol (2017) 72:183–7. doi: 10.1016/j.oraloncology.2017.02.008
17. Kim JW, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, et al. Extent of Extrathyroidal Extension as a Significant Predictor of Nodal Metastasis and Extranodal Extension in Patients With Papillary Thyroid Carcinoma. Ann Surg Oncol (2017) 24(2):460–8. doi: 10.1245/s10434-016-5594-4
18. Frasoldati A, Toschi E, Zini M, Flora M, Caroggio A, Dotti C, et al. Role of Thyroglobulin Measurement in Fine-Needle Aspiration Biopsies of Cervical Lymph Nodes in Patients With Differentiated Thyroid Cancer. Thyroid Off J Am Thyroid Assoc (1999) 9(2):105–11. doi: 10.1089/thy.1999.9.105
19. Al-Hilli Z, Strajina V, McKenzie TJ, Thompson GB, Farley DR, Regina Castro M, et al. Thyroglobulin Measurement in Fine-Needle Aspiration Improves the Diagnosis of Cervical Lymph Node Metastases in Papillary Thyroid Carcinoma. Ann Surg Oncol (2017) 24(3):739–44. doi: 10.1245/s10434-016-5625-1
20. Jasim S, Baranski TJ, Teefey SA, Middleton WD. Investigating the Effect of Thyroid Nodule Location on the Risk of Thyroid Cancer. Thyroid Off J Am Thyroid Assoc (2020) 30(3):401–7. doi: 10.1089/thy.2019.0478
21. Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The Prognostic Significance of Nodal Metastases From Papillary Thyroid Carcinoma can be Stratified Based on the Size and Number of Metastatic Lymph Nodes, as Well as the Presence of Extranodal Extension. Thyroid Off J Am Thyroid Assoc (2012) 22(11):1144–52. doi: 10.1089/thy.2012.0043
22. Kim YS. Patterns and Predictive Factors of Lateral Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Otolaryngol Head Neck Surg Off J Am Acad Otolaryngol Head Neck Surg (2012) 147(1):15–9. doi: 10.1177/0194599812439277
23. Zhan S, Luo D, Ge W, Zhang B, Wang T. Clinicopathological Predictors of Occult Lateral Neck Lymph Node Metastasis in Papillary Thyroid Cancer: A Meta-Analysis. Head Neck (2019) 41(7):2441–9. doi: 10.1002/hed.25762
24. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Torotti A. Ajcc Cancer Staging Manual Seventh Edition. New York: Springer-Verlag (2010).
25. Nixon IJ, Wang LY, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The Impact of Nodal Status on Outcome in Older Patients With Papillary Thyroid Cancer. Surgery (2014) 156(1):137–46. doi: 10.1016/j.surg.2014.03.027
26. Tran Cao HS, Johnston LE, Chang DC, Bouvet M. A Critical Analysis of the American Joint Committee on Cancer (AJCC) Staging System for Differentiated Thyroid Carcinoma in Young Patients on the Basis of the Surveillance, Epidemiology, and End Results (SEER) Registry. Surgery (2012) 152(2):145–51. doi: 10.1016/j.surg.2012.02.015
27. Zhao H, Huang T, Li H. Risk Factors for Skip Metastasis and Lateral Lymph Node Metastasis of Papillary Thyroid Cancer. Surgery (2019) 166(1):55–60. doi: 10.1016/j.surg.2019.01.025
28. Liu C, Xiao C, Chen J, Li X, Feng Z, Gao Q, et al. Risk Factor Analysis for Predicting Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Study of 966 Patients. BMC Cancer (2019) 19(1):622. doi: 10.1186/s12885-019-5835-6
29. Back K, Kim JS, Kim JH, Choe JH. Superior Located Papillary Thyroid Microcarcinoma Is a Risk Factor for Lateral Lymph Node Metastasis. Ann Surg Oncol (2019) 26(12):3992–4001. doi: 10.1245/s10434-019-07587-2
30. Zhang X, Zhang L, Xue S, Wang P, Chen G. Predictive Factors of Lateral Lymph Node Metastasis in Solitary Papillary Thyroid Microcarcinoma Without Gross Extrathyroidal Extension. Asian J Surg (2019) 42(4):563–70. doi: 10.1016/j.asjsur.2018.07.003
31. Kim SK, Park I, Woo JW, Lee JH, Choe JH, Kim JH, et al. Predictive Factors for Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Ann Surg Oncol (2016) 23(9):2866–73. doi: 10.1245/s10434-016-5225-0
32. Jeon MJ, Chung MS, Kwon H, Kim M, Park S, Baek JH, et al. Features of Papillary Thyroid Microcarcinoma Associated With Lateral Cervical Lymph Node Metastasis. Clin Endocrinol (2017) 86(6):845–51. doi: 10.1111/cen.13322
33. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Papillary Microcarcinoma of the Thyroid: How Should It Be Treated? World J Surg (2004) 28(11):1115–21. doi: 10.1007/s00268-004-7644-5
34. Yip L, Nikiforova MN, Yoo JY, McCoy KL, Stang MT, Armstrong MJ, et al. Tumor Genotype Determines Phenotype and Disease-Related Outcomes in Thyroid Cancer: A Study of 1510 Patients. Ann Surg (2015) 262(3):519–25. doi: 10.1097/SLA.0000000000001420
35. Kim SY, Kim BW, Pyo JY, Hong SW, Chang HS, Park CS. Macrometastasis in Papillary Thyroid Cancer Patients Is Associated With Higher Recurrence in Lateral Neck Nodes. World J Surg (2018) 42(1):123–9. doi: 10.1007/s00268-017-4158-5
36. Teixeira G, Teixeira T, Gubert F, Chikota H, Tufano R. The Incidence of Central Neck Micrometastatic Disease in Patients With Papillary Thyroid Cancer Staged Preoperatively and Intraoperatively as N0. Surgery (2011) 150(6):1161–7. doi: 10.1016/j.surg.2011.09.019
37. Zhang F, Oluwo O, Castillo FB, Gangula P, Castillo M, Farag F, et al. THYROID NODULE LOCATION ON ULTRASONOGRAPHY AS A PREDICTOR OF MALIGNANCY. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol (2019) 25(2):131–7. doi: 10.4158/EP-2018-0361
38. Ramundo V, Lamartina L, Falcone R, Ciotti L, Lomonaco C, Biffoni M, et al. Is Thyroid Nodule Location Associated With Malignancy Risk? Ultrason (Seoul Korea) (2019) 38(3):231–5. doi: 10.14366/usg.18050
39. Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, et al. An Observation Trial Without Surgical Treatment in Patients With Papillary Microcarcinoma of the Thyroid. Thyroid Off J Am Thyroid Assoc (2003) 13(4):381–7. doi: 10.1089/105072503321669875
Keywords: papillary thyroid carcinoma, lymph node metastases, upper lobe, tumor location, tumor size
Citation: Zhang X, Chen W, Fang Q, Fan J, Feng L, Guo L, Liu S, Ge H and Du W (2021) Lateral Lymph Node Metastases in T1a Papillary Thyroid Carcinoma: Stratification by Tumor Location and Size. Front. Endocrinol. 12:716082. doi: 10.3389/fendo.2021.716082
Received: 28 May 2021; Accepted: 25 June 2021;
Published: 15 July 2021.
Edited by:
Paolo Miccoli, University of Pisa, ItalyReviewed by:
Brian Lang, The University of Hong Kong, Hong Kong, SAR ChinaErivelto Martinho Volpi, Centro de referencia no ensino do diagnóstico por imagem (CETRUS), Brazil
Stefano Spiezia, Local Health Authority Naples 1 Center, Italy
Copyright © 2021 Zhang, Chen, Fang, Fan, Feng, Guo, Liu, Ge and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Du, ZHV3ZWl0akAxMjYuY29t; Hong Ge, Z2Vob25nNjE2QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Xiaojun Zhang
Xiaojun Zhang Wenkuan Chen2†
Wenkuan Chen2† Qigen Fang
Qigen Fang Lu Feng
Lu Feng