- Department of Endocrine, Xiangya Hospital of Central South University, Changsha, China
Background: Hypoglycemia is an important event that could be related to increased mortality in patients with diabetes. The risk of hypoglycemia is not clearly illustrated to increase when Sodiumglucose co-transporter 2 (SGLT-2) inhibitors are used concomitantly with sulfonylureas. The present study will assess the risk of hypoglycemia associated with the concomitant use of SGLT-2 inhibitors and sulfonylureas compared with placebo and sulfonylureas.
Method: We searched Medline, EMBASE, Cochrane Central Register of Controlled Trials, and Clinicaltrial.gov and identified the randomized trials comparing SGLT-2 inhibitors with placebo for type 2 diabetes treated with sulfonylureas. The risk of bias in each trial was assessed using the Cochrane tool. The risk ratio of hypoglycemia was measured using the Mantel Haenszel method. We also performed subgroup analysis to examine the dosage effects. The number needed to harm (NNH) was measured according to the duration of intervention.
Results: A total of 12 studies, including 3761 participants, were enrolled in our systematic review and meta-analysis. The risk ratio of hypoglycemia was 1.67 (95% CI 1.42 to 1.97). The NNH was 13 (95% CI 9 to 21) for a treatment duration of 24 weeks or less, 11 (8 to 18) for 25 to 48 weeks, and 7 (5 to 10) for more than 48 weeks. Subgroup analysis showed that no difference was found between higher and lower doses of SGLT-2 inhibitors. The risk ratio related to lower dose SGLT-2 inhibitors was 1.56 (95% CI 1.30 to 1.88), and the risk ratio related to higher dose SGLT-2 inhibitors was 1.70 (95% CI 1.42 to 2.04).
Conclusions: The risk of hypoglycemia was significantly increased in subjects treated with SGLT-2 inhibitors compared with placebo. Addition of SGLT-2 inhibitors to sulfonylureas would lead to one more case of hypoglycemia in every 13 patients with a treatment duration less than 24 weeks. This suggests that a decrease in sulfonylureas dose may be an important recommendation when adding SGLT-2 inhibitors to sulfonylureas.
Introduction
Hypoglycemia is a common adverse effect of antihyperglycemic agents. There is a strong relationship between hypoglycemia and increased risk of cardiovascular disease, and mortality. Although glycemic control reduced the cardiovascular events in diabetic patients, intensive glycemic control neither resulted in reduction of cardiovascular events nor decreased all-cause mortality in type 2 diabetic patients (1). Moreover, data from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial demonstrated that intensive treatment increased all-cause mortality and cardiovascular mortality compared with standard treatment, which possibly related to the negative effect of hypoglycemia (2).
The hospital admissions rate for hypoglycemia have declined slowly since 2007, while hypoglycemia is still the main complication of old diabetic patients (>60 years) with longer disease duration (3, 4). The immediate effects of hypoglycemia can affect the everyday activities because of impairment of balance, coordination, vision and level of consciousness, and cause vehicle accidents, falls and injury (5, 6). In addition, hypoglycemia induces consequences well beyond its immediate effects. Hypoglycemia is able to frustrate the goal of glycemic control, which limits full archiving of the benefit from improvement in glycemic control (5, 7). It also impairs the awareness of hypoglycemia because of deficient counter regulatory hormonal responses to hypoglycemia (8), which would greatly increase the risk of severe hypoglycemia (8, 9).
SGLT-2 inhibitors are a new class of oral antihyperglycemic drugs. SGLT-2 inhibitors improve hyperglycemia by inhibiting renal glucose reabsorption (10) and decrease cardiovascular events and kidney damage in patients with diabetes (11).
A variety of randomized clinical trials have studied SGLT-2 inhibitors both as monotherapy and in patients treated with other glucose lowering drugs, DPP-4 inhibitors, metformin, sulfonylureas, and thiazolidinediones (12). The risk of hypoglycemia with SGLT-2 inhibitors is similar to that of placebo or other oral agents (metformin, DPP-4 inhibitors, thiazolidinediones, and α-Glucosidase inhibitors) when used as monotherapy (12–16). When used in patients with metformin, all above oral agents associate with the similar HbA1c content, while SGLT-2 inhibitors have the lowest hypoglycemia risk (16). Previous studies reported that SGLT-2 in conjunction with sulfonylureas increased incidence of hypoglycemia significantly (14, 17). This could be related to the higher risk of hypoglycemia among subjects treated with sulfonylureas, which is further increased when patients are treated by a second antidiabetic agent. The summaries of the product characteristics of SGLT-2 inhibitors suggested that the hypoglycemia risk is common due to association with sulfonylureas (18–20). However, some clinical trials reported that SGLT-2 plus sulfonylureas did not increase incidence of hypoglycemia (21, 22). Therefore, this risk is not clearly illustrated yet. We assessed the risk of hypoglycemia associated with the use of SGLT-2 inhibitors and sulfonylureas in patients with type 2 diabetes.
Methods
Systematic Review Protocol and Registration
The systematic review and meta-analysis is reported in accordance with the preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). All research was conducted according to protocol registered in the PROSPERO database (CRD42018096476).
Search Strategy and Study Selection
We did a search in the PUBMED, EMBASE, and the Cochrane Central Register of Controlled Trials on 10 Apr 2021 using keywords related to SGLT-2 inhibitors and randomized controlled trials (supplement 1). There were no language restrictions. We also screened the reference list of identified studies and performed forward citation searches to consider studies not identified by our online search. In addition, we checked Clinicaltrials.gov (last search April 2021) to identify unpublished but eligible trials.
We identify the eligible trials for this meta-analysis were those that: They were randomized clinical trials in adults with type 2 diabetes; studied the effect of adding one SGLT2 inhibitor to sulfonylureas, with or without other antidiabetic drugs; studied one SGLT2 inhibitor used at daily doses approved in clinical practice-luseogliflozin (Takeda Pharmaceutical), dapagliflozin (Astra-Zeneca), ipragliflozin (Astellas Pharma Inc), canagliflozin (Janssen Research & Development, LLC), empagliflozin (Boehringer Ingelheim), ertugliflozin (Merck Sharp & Dohme Corp), and included no less than 50 patients treated with SGLT2 inhibitors. Studies concerning extension phases of randomized controlled trials were excluded.
Two investigators (MJ and ML) reviewed the titles and abstracts of potentially relevant trials and determined final eligibility through full-text assessment. Disagreements were resolved through discussion with another investigator.
Data Extraction and Risk of Bias
The following information was collected from each study: number of subjects, sex, age, country, setting, study duration, and change in glycated hemoglobin A1C (HbA1c) level; intervention details (SGLT-2 inhibitors and sulfonylureas names, doses, and number of treated patients); and the number of patients with any hypoglycemia. Disagreements were resolved through discussion with another investigator.
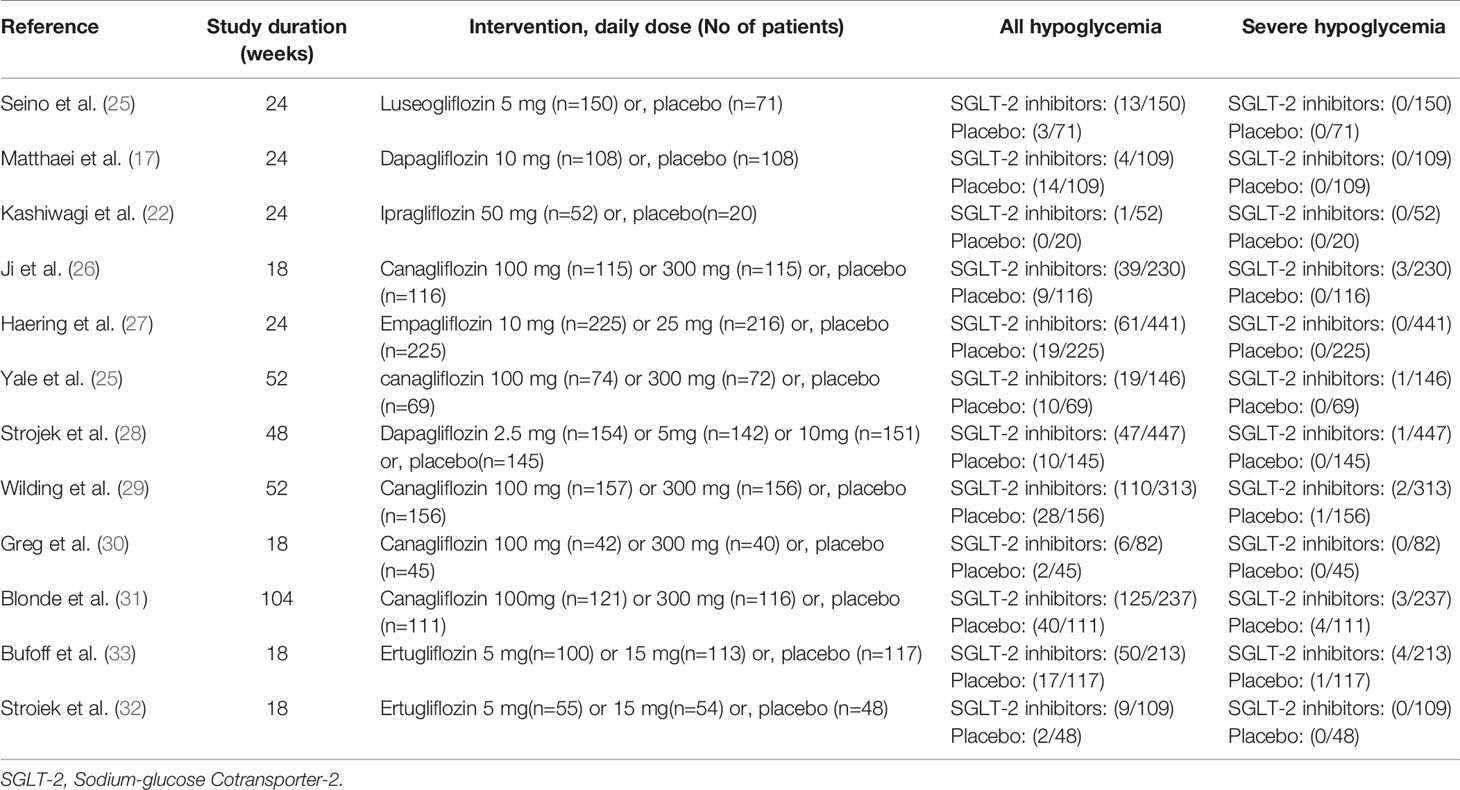
Three categories of hypoglycemia were captured: total hypoglycemia (irrespective of definition, severity, blood glucose value or documentation), severe hypoglycemia (a hypoglycemic event that required the assistance of third party), hypoglycaemia (plasma glucose levels ≤ 3.9mmol/L).
The risk of bias of each study was assessed by using the Cochrane Collaboration risk of bias tool in randomized trials. The quality of the studies was determined by examination of the original study protocol. We graded random sequence generation; allocation concealment; blinding of participants and personnel, blinding of outcome assessment; incomplete outcome data; selective outcome reporting; and other sources of biases. Two investigators independently graded the seven parts as low, unclear or high risk of bias. The quality of evidence resulting from a meta-analysis would be rated from very low to high using the GRADE framework.
Data Analysis
We compared the risk of hypoglycemia in patients treated with SGLT-2 inhibitors plus sulfonylureas with that in patients treated with placebo plus sulfonylureas.
We calculated the risk ratio for hypoglycemia along with the 95% confidence interval. The fixed effect model (Mantel Haenszel method) was used to calculate the pooled risk ratio.
Statistical heterogeneity was assessed with the I2 statistic, considering values below 50% indicative of low heterogeneity (23). The subgroup analysis was undertaken based on the dosage of SGLT-2 inhibitors (eTable 1 in the supplement).
Publication bias was tested by inspection of funnel plots and Egger’s tests (P<0.05 considered to be significant). The number needed to harm (NNH) (ie, number of patients who receive treatment that would be associated with 1 hypoglycemia) was measured according to the Cochrane recommendations. On the assumption that the risk of hypoglycemia is associated with the duration of treatment, we calculated the assumed control risk for different follow-up scenarios: 24 weeks or less, 25 to 48 weeks, and more than 48 weeks.
We also conducted a secondary analysis according to different doses of SGLT-2 inhibitors. We classified the SGLT-2 inhibitors doses into a lower dose group and higher dose group (eTable 1 in the supplement) according to previous studies (24) (low daily doses are mostly recommended in patients with renal impairment).
Results
Study Selection
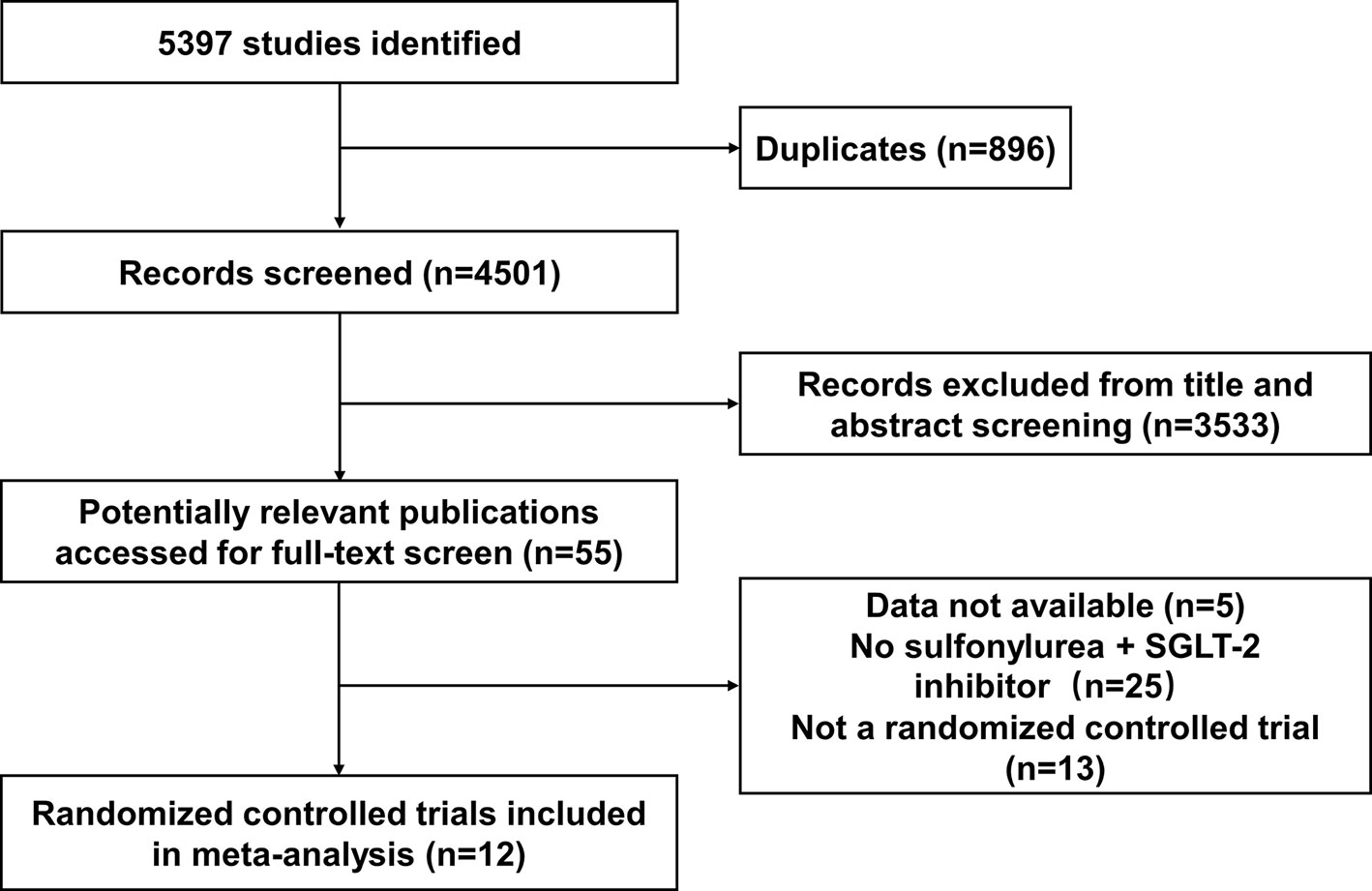
Twelve studies were identified as being eligible for this meta-analysis and systemic review (Figure 1). The total number of participants from the 12 studies was 3761, 2529 of whom received SGLT-2 inhibitors plus sulfonylureas and 1232 placebo plus sulfonylureas. One study included only subjects aged 55 years or more. In eight of the 12 studies (17, 21, 22, 25–33) the planned follow-up was 24 weeks or less; one study followed patients for a median time of 48 weeks; three studies followed patients for a median time of over 48 weeks. It was impossible to get specific NNHs for the follow-up period range. Therefore, we calculated the assumed control risk of hypoglycemia in patients treated with sulfonylureas from another meta-analysis (34, 35). The assumed control risk was 11.6% for follow-up of 24 weeks or less (seven studies), 13.3% from 25 to 48 weeks (nine studies), and 22.8% for over 48 weeks (11 studies).
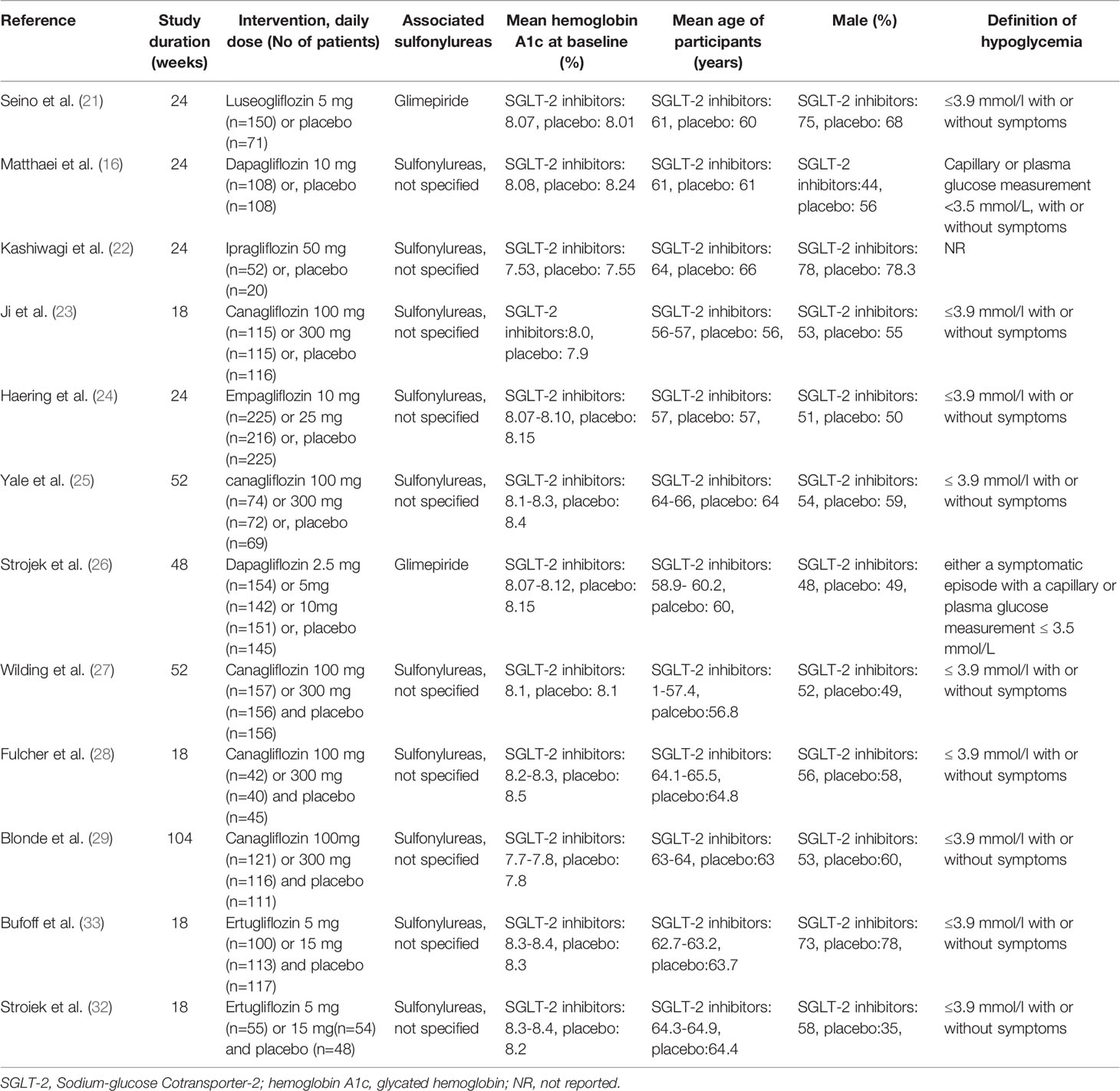
The characteristics of studies included in the present study are summarized in Table 1. The type of associated sulfonylureas varied across the trials. Five studies enrolled patients only receiving sulfonylureas, while the remaining trials recruited receiving sulfonylureas and metformin. Baseline patient characteristics (mean glycated hemoglobin A1C, mean age, and sex) were well balanced among the patients in each of the groups. In addition, the severe and all hypoglycemic events are summarized in Table 2. The severe hypoglycemic events were reported in six studies.
One trial studied luseogliflozin 2.5 mg/day in a total of 150 patients (25). Dapagliflozin 2.5 mg/day(n=154) was studied in one trial (28) and dapagliflozin 5 mg/day (n=142) in one trial (28) and dapagliflozin 10 mg/day (n=170) in two trials (17, 28). Ipragliflozin was studied at 50 mg/day (n=52) in one trial (22). Canagliflozin was studied at 100 mg/day (n=509) to 300 mg/day (n=499) in 5 trials (21, 26, 29–31). Empagliflozin (10 mg/day (n=225) and 25 mg/day (n=216)) was studied in one trial (27). Ertugliflozin (5 mg/day (n=155) and 10 mg/day (n=167)) was studied in two trials. Overall, 1232 patients receiving placebo plus sulfonylureas were identified in the 12 included trials (Table 1). One of the 12 trials failed to clearly report the definition of hypoglycemia (Table 1) (22, 25).
Risk of Bias Assessment
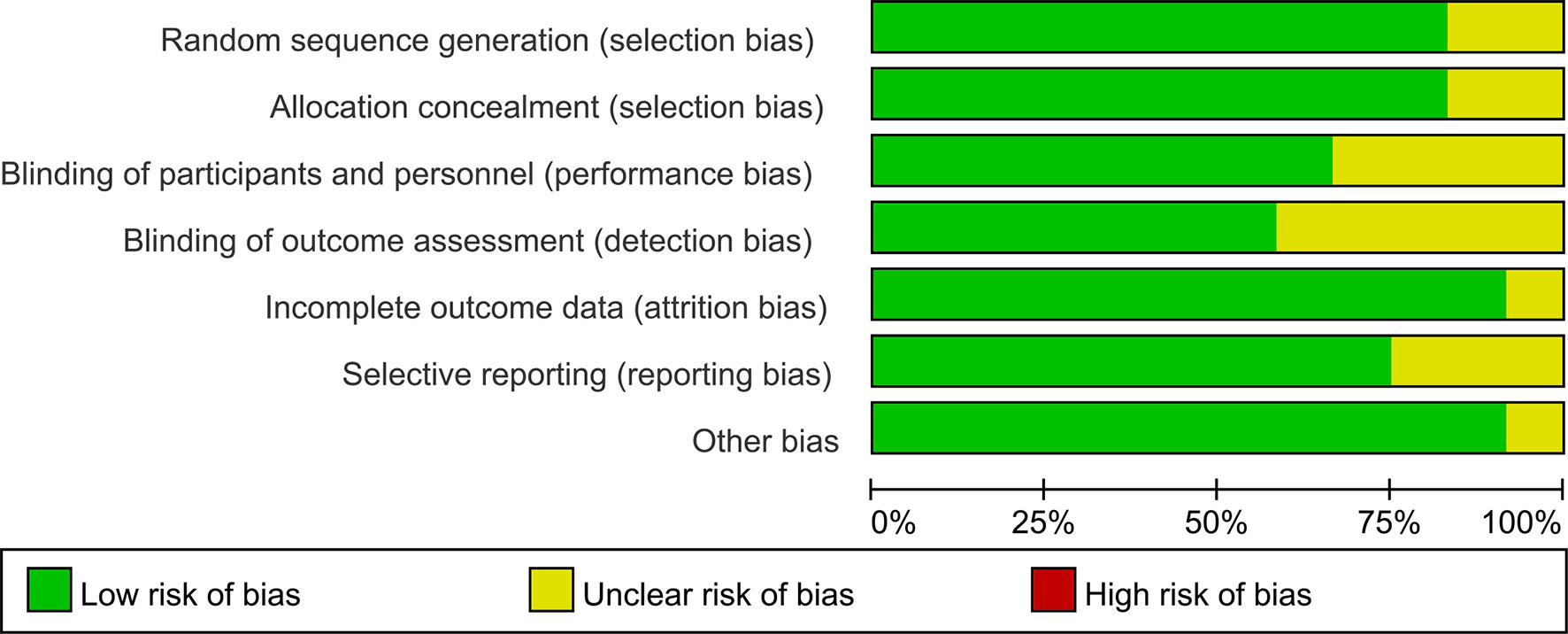
Assessment of risk of bias is summarized in Figure 2. Method of random sequence generation was reported and found to be adequate in 12 studies. Ten trials provided sufficient information concerning the proceedings of allocation concealment. Eight studies blinded study participants and personnel to the dietary interventions. Seven studies reported blinding of outcome assessors. One study had an unclear risk of attrition bias as the result of incomplete reporting of outcome data, as the dropout rate placebo group is higher. Selective reporting was found in ten trials.
Meta-Analysis
The risk ratio of hypoglycemia for SGLT-2 inhibitors plus sulfonylureas versus placebo plus sulfonylureas was 1.67 (1.42 to 1.97), with no evidence of heterogeneity across the trials (P=0.78, I2 = 0%; Figure 3).
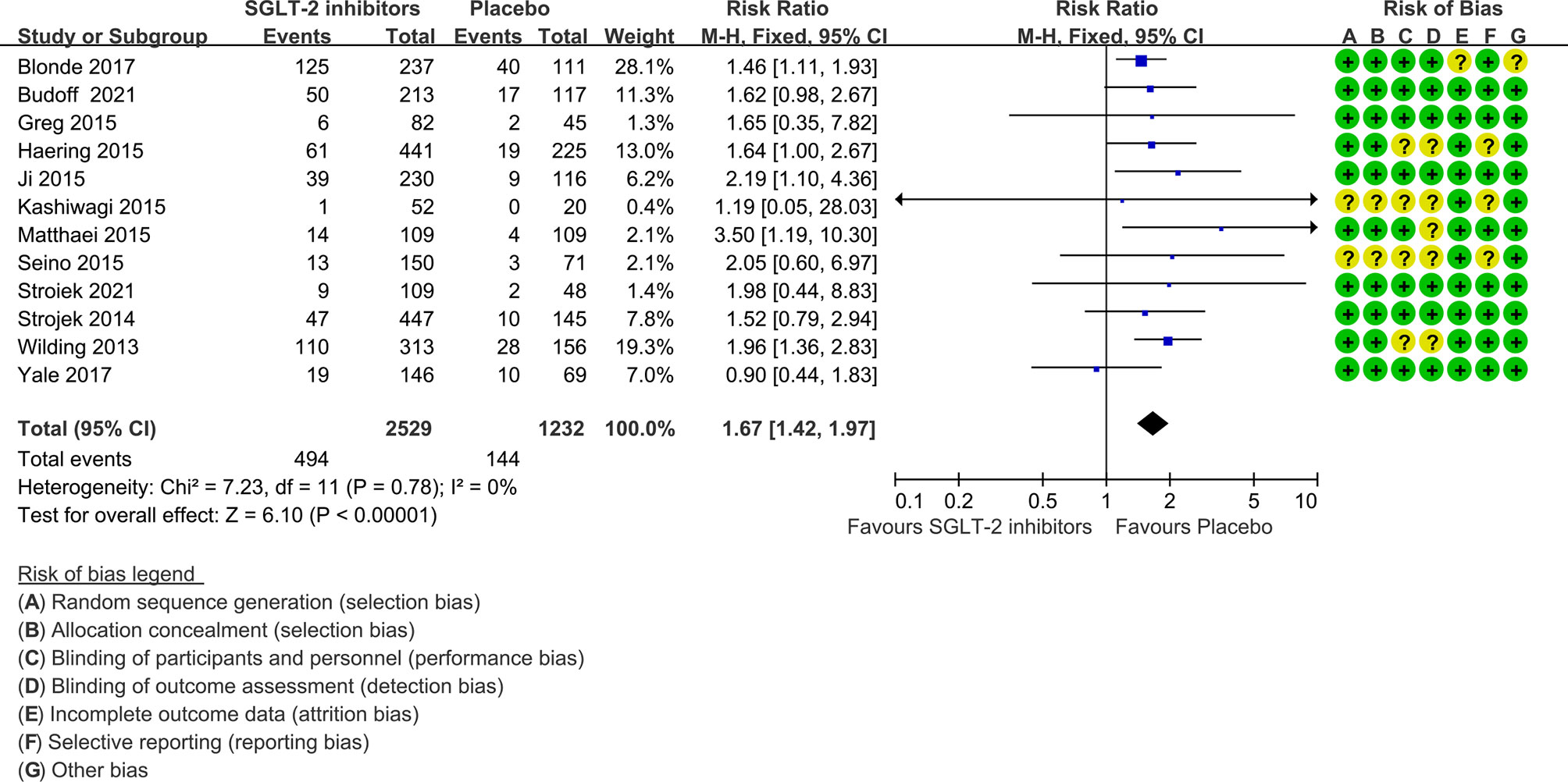
Figure 3 Risk of hypoglycemia in all included trials. Forest plot showing risk of hypoglycemia in patients treated with SGLT-2 inhibitors plus sulfonylureas compared with placebo plus sulfonylureas.
The NNH was 13 (95% confidence interval 9 to 21) for a follow-up period of 24 weeks or less, 11 (8 to 18) for 25 to 48 weeks, and 7 (5 to 10) for more than 48 weeks.
In nine of the 12 studies definitions of hypoglycemia (≤ 3.9 mmol/l with or without symptoms) are the same, while the remaining three studies have different or do not report a definition of hypoglycemia. The risk ratio was 1.64(1.38 to 1.95) when trials with different or not report a definition of hypoglycemia were excluded (Figure 4). Severe hypoglycemic events were reported in six of the 12 studies (Table 2). In addition, the risk of severe hypoglycemia did not have significant difference between the two groups (Figure 5).
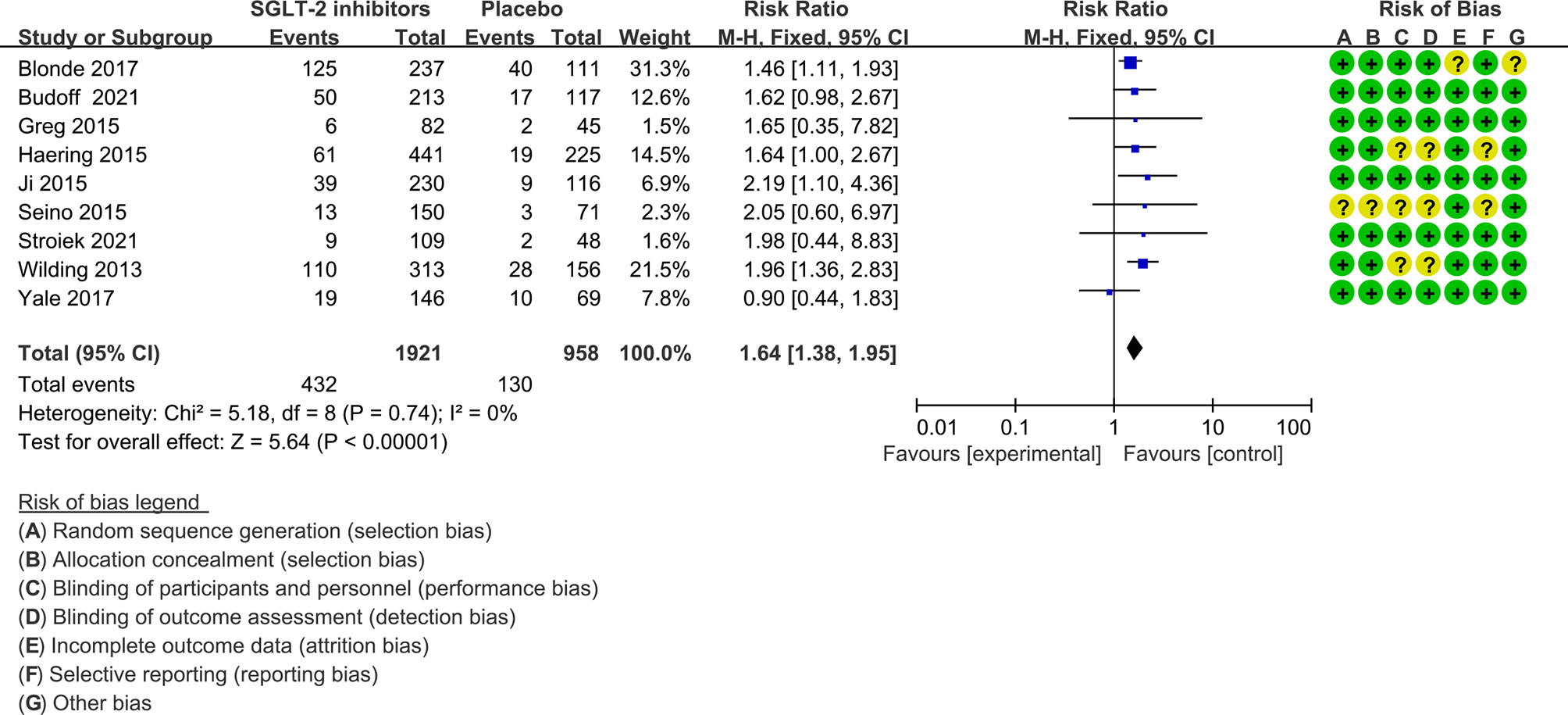
Figure 4 Risk of hypoglycemia in trials with the same definition of hyperglycemia. Forest plot showing risk of hypoglycemia in patients treated with SGLT-2 inhibitors plus sulfonylureas compared with placebo plus sulfonylureas (Trials with different or not report a definition of hypoglycemia were excluded).
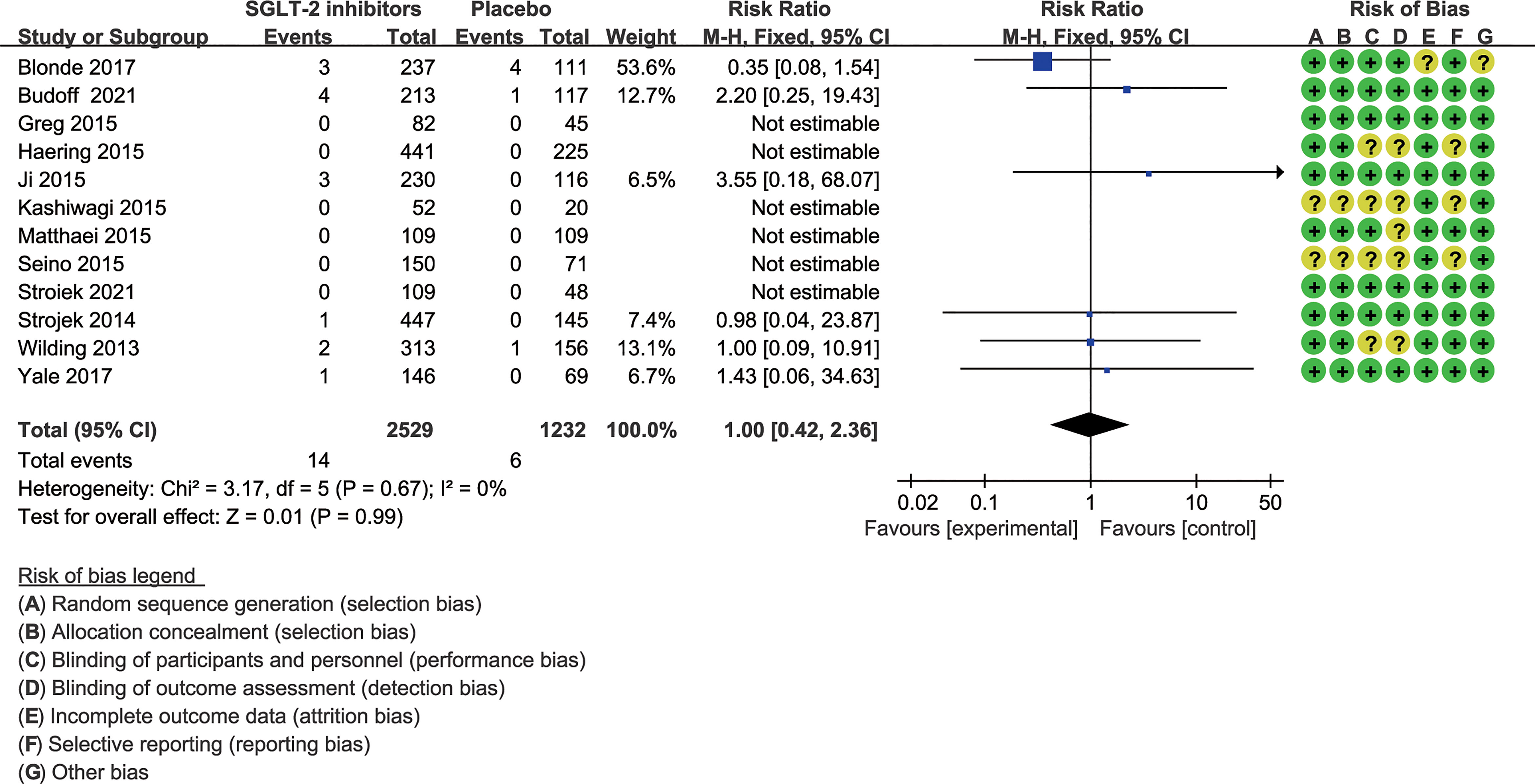
Figure 5 Risk of severe hypoglycemia. Forest plot showing risk of severe hypoglycemia in patients treated with SGLT-2 inhibitors plus sulfonylureas compared with placebo plus sulfonylureas.
In subgroup analysis, there was no difference between lower and higher dose SGLT-2 inhibitors for risk of hypoglycemia (P=0.51, I2 = 0%; Figure 6). The risk for SGLT-2 inhibitors remained statistically significantly increased at lower dose (1.56, 1.30 to 1.88) and higher dose (1.70, 1.43 to 1.86).
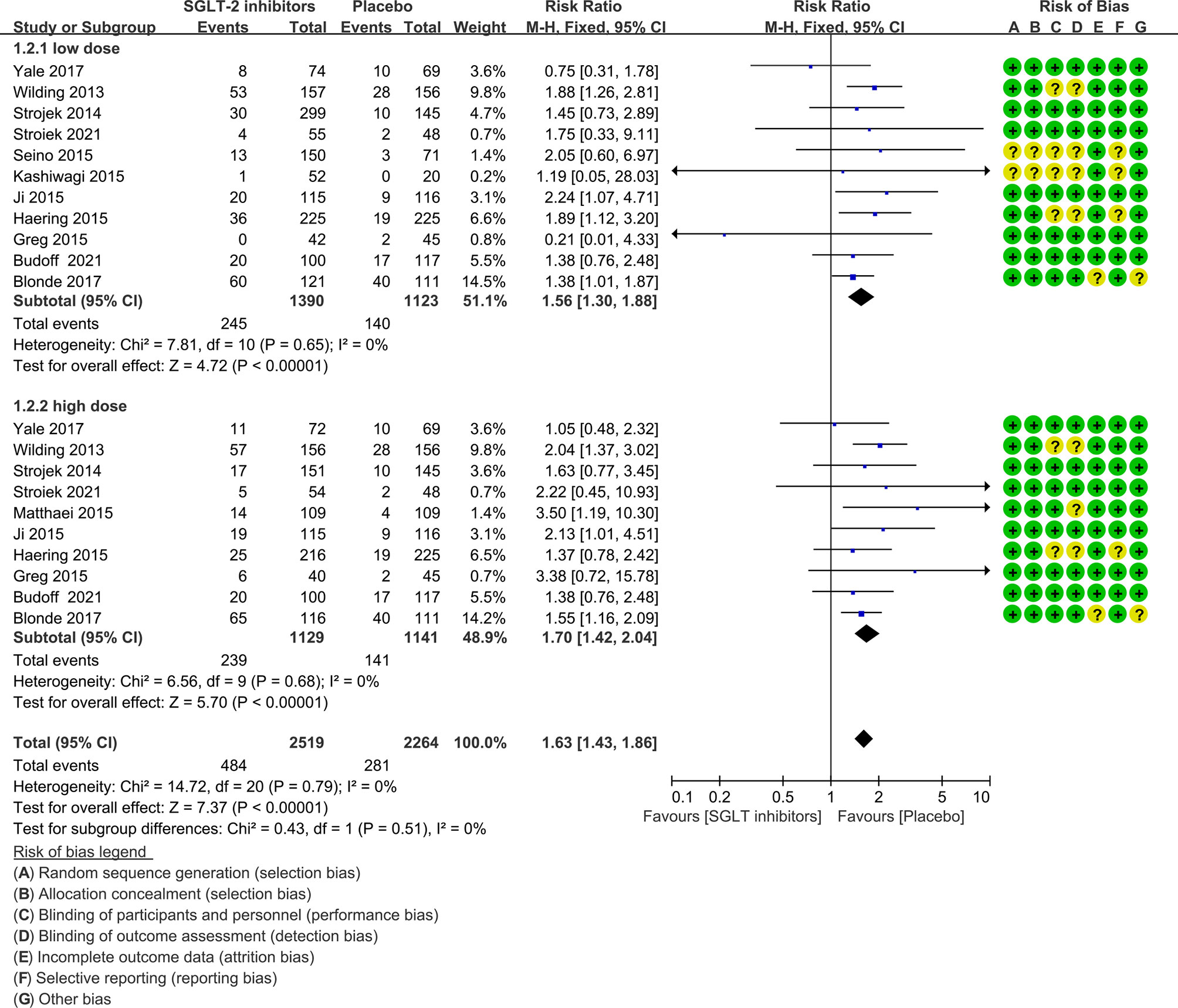
Figure 6 Risk of hypoglycemia in subgroups. Forest plot showing risk of hypoglycemia in patients treated with higher or lower SGLT-2 inhibitors plus sulfonylureas compared with placebo plus sulfonylureas.
The funnel plots for all studies did not show any asymmetry (Figure 7), and the Egger’s test did not suggest any publication bias (t=-0.62, p=0.547). The strength of evidence of this meta-analysis was graded as high according to GRADE (eTable 2 in the supplement).
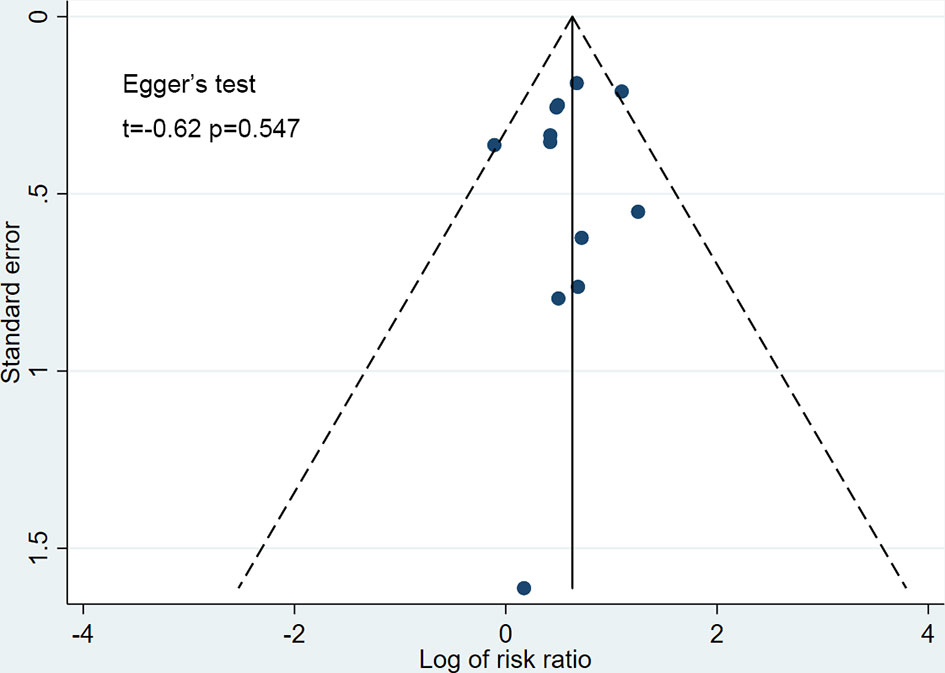
Figure 7 Funnel plot for publication bias. Scatter plot reporting risk ratio of the studies testing SGLT-2 inhibitors plus sulfonylureas compared with placebo plus sulfonylureas (horizontal axis) against their standard error (vertical axis).
Discussion
This systemic review and meta-analysis shows that over a 50% increase in the risk of hypoglycemia when an SGLT-2 inhibitor was added to sulfonylureas to treat diabetic patients, and this combination therapy lead to one more case of hypoglycemia for every 13 patients for treatment duration less than first six months of treatment. The present study is based on a large number of patients, which enables us to arrive plausible conclusions. Other strengths included the high quality of each study, which was assessed according to the Cochrane Collaboration tool for risk of bias assessment. In addition, funnel plots for the different outcomes did not suggest any publication bias. In conclusion, the quality of evidence of the present study was rated as high according to GRADE.
A number of limitations do exist in the present meta-analysis. First, because we cannot get the data for the risk of hypoglycemia in patients receiving sulfonylureas, five studies could not be included (36–40). However, given the size of the present meta-analysis and the high number of hypoglycemia cases, including results from these studies may not be able to change the results. There was no heterogeneity across trials further, which supports this hypothesis. Second, the definition of hypoglycemia varied among the included trials, and it was not reported in one. However, an analysis excluded these trials did not substantially change the results of the meta-analysis (Figure 4). Third, the results might be more strongly affected by trials with lager participants, although our sensitivity analysis found that removing any trial did not substantially change the results of the meta-analysis. Fourth, the incidence of hypoglycemia is different among studies; however, this did not affect the estimation of the pooled risk nor on the NNH, which was calculated according to an assumed control risk of hypoglycemia from a meta-analysis with 27 clinical trials included (34). Fifth, the effects of different sulfonylureas and different levels of renal function on the study results cannot be determined because of a lack of sufficient data.
SGLT-2 inhibitors increase the glucose excretion to lower hyperglycemia, which is a unique insulin-independent mode of action (41) with a limited risk of hyperglycemia. Because of the beneficial effects on cardio-renal outcomes, the guidelines propose a new paradigm in diabetes management with a preferential place for SGLT-2 inhibitors. However, insulin secretion is already stimulated independently of glycemia in patients treated with sulfonylureas, and the addition of a glucose execration effect may result in an increase in the incidence of hypoglycemia. Given the extensive use of sulfonylureas and preferential place of SGLT-2 inhibitors for the treatment of type 2 diabetes, the risk associated with the combination therapy would result in a substantial number of cases of induced hypoglycemia.
Addition of an SGLT-2 inhibitor to sulfonylureas increase the risk of hypoglycemia, which is described in the product characteristics for SGLT-2 inhibitors. From our study, we knew that the low doses of SGLT-2 inhibitors also increased hypoglycemia risk statistically significant. Therefore, we may recommend using full dose SGLT-2 inhibitors but a reduced sulfonylurea dose in patients taking such combinations. In consistent with our findings, a recent study suggested that maintaining sulfonylurea treatment at the lowest dose could ensure better glycemic control without increasing hypoglycemia in patients treated with such combinations (42). However, owing to the small number of cases and other limitations, further studies are required to confirm the conclusions.
Hyperglycemia increases the risk of hospital admissions and cardiovascular mortality. In addition, it is able to activate the renin-angiotensin-aldosterone and sympathetic systems, which leads to higher risk of cardiovascular events. The risk of severe hypoglycemia does not increase significantly in the present study. However, mild or moderate hypoglycemia is able to decrease the usual adrenergic response to hyperglycemia. It may induce hypoglycemia unawareness and compromise behavioral responses, which can result in severe hypoglycemia. It is inadvisable to achieve optimal glycemic control at the expense of hypoglycemic events. Overall, the clinicians should pay attention to the information of any degree of hypoglycemia.
Conclusion
In conclusion, the addition of SGLT-2 inhibitors to sulfonylureas was associated with an increased risk of hypoglycemia in type 2 diabetic patients. In addition, the combination therapy would lead to one more case of hypoglycemia in every 13 treated patients in the first 24 weeks of treatment. This negative effect may induce a remarkedly increase the cases of hypoglycemia worldwide. These outcomes strongly suggest that dose reduction of sulfonylureas is important when adding an SGLT-2 inhibitor to sulfonylurea treatment, and it is important to determine the efficacy of this measure in minimizing the risk of hypoglycemia.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
ML and MJ had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. MJ and ML conceived the research idea. All authors performed the meta-analysis and the draft manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grant 2017SK1020 from Department of Science and Technology of Hunan province.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.713192/full#supplementary-material
References
1. Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, et al. Intensive Glycaemic Control for Patients With Type 2 Diabetes: Systematic Review With Meta-Analysis and Trial Sequential Analysis of Randomised Clinical Trials. BMJ (2011) 343:d6898. doi: 10.1136/bmj.d6898
2. Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, et al. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N Engl J Med (2008) 358(24):2545–59. doi: 10.1056/NEJMoa0802743
3. Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of Complications and Mortality in Older Patients With Diabetes Mellitus: The Diabetes and Aging Study. JAMA Intern Med (2014) 174(2):251–8. doi: 10.1001/jamainternmed.2013.12956
4. Lipska KJ, Ross JS, Wang Y, Inzucchi SE, Minges K, Karter AJ, et al. National Trends in US Hospital Admissions for Hyperglycemia and Hypoglycemia Among Medicare Beneficiaries, 1999 to 2011. JAMA Intern Med (2014) 174(7):1116–24. doi: 10.1001/jamainternmed.2014.1824
5. Frier BM. Hypoglycaemia in Diabetes Mellitus: Epidemiology and Clinical Implications. Nat Rev Endocrinol (2014) 10(12):711–22. doi: 10.1038/nrendo.2014.170
6. Graveling AJ, Frier BM. Hypoglycaemia: An Overview. Prim Care Diabetes (2009) 3(3):131–9. doi: 10.1016/j.pcd.2009.08.007
7. International Hypoglycaemia Study Group. Minimizing Hypoglycemia in Diabetes. Diabetes Care (2015) 38(8):1583–91. doi: 10.2337/dc15-0279
8. Graveling AJ, Frier BM. Impaired Awareness of Hypoglycaemia: A Review. Diabetes Metab (2010) 36(Suppl 3):S64–74. doi: 10.1016/S1262-3636(10)70470-5
9. Cryer PE. Mechanisms of Hypoglycemia-Associated Autonomic Failure in Diabetes. N Engl J Med (2013) 369(4):362–72. doi: 10.1056/NEJMra1215228
10. Vallon V. The Mechanisms and Therapeutic Potential of SGLT2 Inhibitors in Diabetes Mellitus. Annu Rev Med (2015) 66:255–70. doi: 10.1146/annurev-med-051013-110046
11. McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney D, Dagogo-Jack S, et al. Association of SGLT2 Inhibitors With Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes: A Meta-Analysis. JAMA Cardiol (2021) 6(2):148–58. doi: 10.1001/jamacardio.2020.4511
12. Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al. Sodium-Glucose Cotransporter 2 Inhibitors for Type 2 Diabetes: A Systematic Review and Meta-Analysis. Ann Intern Med (2013) 159(4):262–74. doi: 10.7326/0003-4819-159-4-201308200-00007
13. Yang XP, Lai D, Zhong XY, Shen HP, Huang YL. Efficacy and Safety of Canagliflozin in Subjects With Type 2 Diabetes: Systematic Review and Meta-Analysis. Eur J Clin Pharmacol (2014) 70(10):1149–58. doi: 10.1007/s00228-014-1730-x
14. Monami M, Nardini C, Mannucci E. Efficacy and Safety of Sodium Glucose Co-Transport-2 Inhibitors in Type 2 Diabetes: A Meta-Analysis of Randomized Clinical Trials. Diabetes Obes Metab (2014) 16(5):457–66. doi: 10.1111/dom.12244
15. de Wit HM, Te Groen M, Rovers MM, Tack CJ. The Placebo Response of Injectable GLP-1 Receptor Agonists vs. Oral DPP-4 Inhibitors and SGLT-2 Inhibitors: A Systematic Review and Meta-Analysis. Br J Clin Pharmacol (2016) 82(1):301–14. doi: 10.1111/bcp.12925
16. Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, et al. Comparison of Clinical Outcomes and Adverse Events Associated With Glucose-Lowering Drugs in Patients With Type 2 Diabetes: A Meta-Analysis. JAMA (2016) 316(3):313–24. doi: 10.1001/jama.2016.9400
17. Matthaei S, Bowering K, Rohwedder K, Grohl A, Parikh S. Dapagliflozin Improves Glycemic Control and Reduces Body Weight as Add-on Therapy to Metformin Plus Sulfonylurea: A 24-Week Randomized, Double-Blind Clinical Trial. Diabetes Care (2015) 38(3):365–72. doi: 10.2337/dc14-0666
18. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002649/WC500156456.pdf.
19. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002322/WC500136026.pdf.
20. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002677/WC500168592.pdf.
21. Yale JF, Xie J, Sherman SE, Garceau C. Canagliflozin in Conjunction With Sulfonylurea Maintains Glycemic Control and Weight Loss Over 52 Weeks: A Randomized, Controlled Trial in Patients With Type 2 Diabetes Mellitus. Clin Ther (2017) 39(11):2230–42.e2. doi: 10.1016/j.clinthera.2017.10.003
22. Kashiwagi A, Takahashi H, Ishikawa H, Yoshida S, Kazuta K, Utsuno A, et al. A Randomized, Double-Blind, Placebo-Controlled Study on Long-Term Efficacy and Safety of Ipragliflozin Treatment in Patients With Type 2 Diabetes Mellitus and Renal Impairment: Results of the Long-Term ASP1941 Safety Evaluation in Patients With Type 2 Diabetes With Renal Impairment (LANTERN) Study. Diabetes Obes Metab (2015) 17(2):152–60. doi: 10.1111/dom.12403
23. Higgins J GSP, Wiley I CC. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: Wiley-Blackwell. (2008).
24. Wang W, Zhang L, Pei X, Pan Q, Guo L. Evaluation of the Safety of Sodium-Glucose Co-Transporter-2 Inhibitors for Treating Patients With Type 1 Diabetes. Diabetes Obes Metab (2020) 22(10):1767–76. doi: 10.1111/dom.14092
25. Seino Y, Inagaki N, Haneda M, Kaku K, Sasaki T, Fukatsu A, et al. Efficacy and Safety of Luseogliflozin Added to Various Oral Antidiabetic Drugs in Japanese Patients With Type 2 Diabetes Mellitus. J Diabetes Investig (2015) 6(4):443–53. doi: 10.1111/jdi.12316
26. Ji L, Han P, Liu Y, Yang G, Dieu Van NK, Vijapurkar U, et al. Canagliflozin in Asian Patients With Type 2 Diabetes on Metformin Alone or Metformin in Combination With Sulphonylurea. Diabetes Obes Metab (2015) 17(1):23–31. doi: 10.1111/dom.12385
27. Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, et al. Empagliflozin as Add-on to Metformin Plus Sulfonylurea in Patients With Type 2 Diabetes: A 24-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Care (2013) 36(11):3396–404. doi: 10.2337/dc12-2673
28. Strojek K, Yoon KH, Hruba V, Sugg J, Langkilde AM, Parikh S. Dapagliflozin Added to Glimepiride in Patients With Type 2 Diabetes Mellitus Sustains Glycemic Control and Weight Loss Over 48 Weeks: A Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Trial. Diabetes Ther (2014) 5(1):267–83. doi: 10.1007/s13300-014-0072-0
29. Wilding JP, Charpentier G, Hollander P, González-Gálvez G, Mathieu C, Vercruysse F, et al. Efficacy and Safety of Canagliflozin in Patients With Type 2 Diabetes Mellitus Inadequately Controlled With Metformin and Sulphonylurea: A Randomised Trial. Int J Clin Pract (2013) 67(12):1267–82. doi: 10.1111/ijcp.12322
30. Fulcher G, Matthews DR, Perkovic V, de Zeeuw D, Mahaffey KW, Weiss R, et al. Efficacy and Safety of Canagliflozin Used in Conjunction With Sulfonylurea in Patients With Type 2 Diabetes Mellitus: A Randomized, Controlled Trial. Diabetes Ther (2015) 6(3):289–302. doi: 10.1007/s13300-015-0117-z
31. Blonde L, Stenlöf K, Fung A, Xie J, Canovatchel W, Meininger G. Effects of Canagliflozin on Body Weight and Body Composition in Patients With Type 2 Diabetes Over 104 Weeks. Postgrad Med (2016) 128(4):371–80. doi: 10.1080/00325481.2016.1169894
32. Strojek K, Pandey AS, Dell V, Sisson M, Wang S, Huyck S, et al. Efficacy and Safety of Ertugliflozin in Patients With Diabetes Mellitus Inadequately Controlled by Sulfonylurea Monotherapy: A Substudy of VERTIS Cv. Diabetes Ther (2021) 12(4):1175–92. doi: 10.1007/s13300-021-01018-w
33. Budoff MJ, Davis T, Palmer AG, Frederich R, Lawrence DE, Liu J, et al. Efficacy and Safety of Ertugliflozin in Patients With Type 2 Diabetes Inadequately Controlled by Metformin and Sulfonylurea: A Sub-Study of VERTIS Cv. Diabetes Ther (2021) 12(5):1279–97. doi: 10.1007/s13300-021-01033-x
34. Hemmingsen B, Schroll JB, Lund SS, Wetterslev J, Gluud C, Vaag A, et al. Sulphonylurea Monotherapy for Patients With Type 2 Diabetes Mellitus. Cochrane Database Syst Rev (2013) CD009008. doi: 10.1002/14651858.CD009008.pub2
35. Salvo F, Moore N, Arnaud M, Robinson P, Raschi E, De Ponti F, et al. Addition of Dipeptidyl Peptidase-4 Inhibitors to Sulphonylureas and Risk of Hypoglycaemia: Systematic Review and Meta-Analysis. BMJ (2016) 353:i2231. doi: 10.1136/bmj.i2231
36. Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, et al. Efficacy and Safety of Empagliflozin Added to Existing Antidiabetes Treatment in Patients With Type 2 Diabetes and Chronic Kidney Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol (2014) 2(5):369–84. doi: 10.1016/S2213-8587(13)70208-0
37. Cefalu WT, Leiter LA, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin’s Effects on Glycemia and Cardiovascular Risk Factors in High-Risk Patients With Type 2 Diabetes: A 24-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study With a 28-Week Extension. Diabetes Care (2015) 38(7):1218–27. doi: 10.2337/dc14-0315
38. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med (2015) 373(22):2117–28. doi: 10.1056/NEJMoa1504720
39. Abstracts of the 48th EASD (European Association for the Study of Diabetes) Annual Meeting of the European Association for the Study of Diabetes. October 1-5, 2012. Berlin, Germany. Diabetologia. (2012) 55(Suppl 1):S7–537. doi: 10.1007/s00125-012-2688-9
40. Yale JF, Bakris G, Cariou B, Nieto J, David-Neto E, Yue D, et al. Efficacy and Safety of Canagliflozin Over 52 Weeks in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease. Diabetes Obes Metab (2014) 16(10):1016–27. doi: 10.1111/dom.12348
41. van Baar M, van Ruiten CC, Muskiet M, van Bloemendaal L, IJzerman RG, van Raalte DH. SGLT2 Inhibitors in Combination Therapy: From Mechanisms to Clinical Considerations in Type 2 Diabetes Management. Diabetes Care (2018) 41(8):1543–56. doi: 10.2337/dc18-0588
42. Takahashi K, Cho KY, Nakamura A, Miya A, Miyoshi A, Yamamoto C, et al. Should Sulfonylurea Be Discontinued or Maintained at the Lowest Dose When Starting Ipragliflozin? A Multicenter Observational Study in Japanese Patients With Type 2 Diabetes. J Diabetes Investig (2018). doi: 10.1111/jdi.12913
Keywords: SGLT-2 (sodium glucose co transporter 2) inhibitors, sulfonylureas, type 2 diabetes, meta – analysis, systematic review
Citation: Jiang M, Liu Q, Jiang T, Nizigiyimana P and Lei M (2021) Adding Sodium–Glucose Co-Transporter 2 Inhibitors to Sulfonylureas and Risk of Hypoglycemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 12:713192. doi: 10.3389/fendo.2021.713192
Received: 22 May 2021; Accepted: 04 October 2021;
Published: 21 October 2021.
Edited by:
Stefan Zoltán Lutz, Bad Sebastiansweiler, GermanyReviewed by:
Peter Fasching, Vienna Health Association, AustriaMuath Alanbaei, Kuwait University, Kuwait
Baptist Gallwitz, Tübingen University Hospital, Germany
Copyright © 2021 Jiang, Liu, Jiang, Nizigiyimana and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minxiang Lei, bGVpbWlueGlhbmdAZ21haWwuY29t
 Meng Jiang
Meng Jiang Qiaoshu Liu
Qiaoshu Liu Minxiang Lei
Minxiang Lei