- 1Department of Endocrinology and Metabolism, The Affiliated Hospital of Southwest Medical University, Luzhou, China
- 2Cardiovascular and Metabolic Diseases Key Laboratory of Luzhou, The Affiliated Hospital of Southwest Medical University, Luzhou, China
- 3Sichuan Clinical Research Center for Nephropathy, The Affiliated Hospital of Southwest Medical University, Luzhou, China
The relationship between dietary inflammatory index (DII) scores and the risk of diabetes mellitus (DM) is unclear; therefore, a systematic review and meta-analysis of the current published literature was conducted. Relevant studies published online (PubMed, Embase, and Web of Science) until February 1, 2021 were identified for review. The initial search yielded 13 reports, and after perusing their titles, abstracts, and full texts, 5 studies were deemed appropriate for inclusion in the systematic review and meta-analysis. Individuals with higher DII scores (representing a more proinflammatory diet) had a higher risk of DM (pooled odds ratio 1.32, 95% confidence interval 1.01–1.72, I2 58.6%, p < 0.05). Although the current meta-analysis indicated a trend toward a positive association between DII and DM, further evidence—especially from larger prospective studies in different countries—is needed to clarify this association.
Introduction
Diabetes mellitus (DM) has become a global epidemic that affects millions of people worldwide. Dietary intervention is obviously effective in the treatment of DM and its associated complications. Multiple factors may contribute to the development of DM, and among them, chronic inflammation is widely considered an underlying pathophysiological mechanism involved in the development of DM (1). Studies suggest that multiple factors that affect inflammation may be associated with DM, such as sex, age, smoking, alcohol, physical activity, the application of some medications, and diet (2). The premise that diet can increase or reduce inflammation has been extensively investigated (3–5), but the degree to which modulating diet can have desirable effects in DM is unclear. It is therefore important to clarify relationships between diet, inflammation, and DM.
The dietary inflammatory index (DII) is a useful tool created by Cavicchia et al. (6) in 2009 and Shivappa et al. (7) in 2014 that has been developed for use in different populations. It can be used to estimate the overall inflammatory potential of an individual’s diet by utilizing a 24-h dietary record interview or food frequency questionnaires (FFQs) (7). The DII is based on 1943 eligible articles that reported the effects of 45 dietary parameters on serum levels of six biomarkers; the proinflammatory biomarkers interleukin (IL)-1β, IL-6, tumor necrosis factor alpha, and C-reactive protein, and the anti-inflammatory biomarkers IL-4 and IL-10 (7). Positive DII scores indicate a proinflammatory diet that significantly increases serum proinflammatory biomarker levels, whereas negative DII scores indicate the contrary. Therefore, DII scores can constitute a quantitative measure for the assessment of the relationships between diet and health outcomes. They can be used to identify the associations between diet and parameters ranging from serum concentrations of inflammatory factors to various types of chronic disease indicators (8) such as cardiovascular diseases (9), metabolic syndrome (10), obesity (11), cancer (12), and DM (13).
The relationship between the DII score and DM is controversial. Some studies suggest a positive relationship between the DII score and DM (13–15), whereas other studies indicate no positive association (16, 17) or a negative association (18). To date, only one narrative review by Phillips et al. has assessed the relationships between DII and DM (19), but no systematic review and meta-analysis has been reported. A systematic review and meta-analyses using published study data may be of great value with respect to clarifying the associations between DII and DM and may provide a theoretical basis for dietary management in DM patients.
Materials and Methods
The current systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (20).
Search Strategy
Relevant studies published until February 1, 2021 pertaining to DII and DM were searched for in the online databases PubMed, Embase, and Web of Science. In the current study, articles were identified by utilizing the following keywords: “DII” OR “dietary inflammatory index” OR “diet” OR “dietary” in combination with “diabetes mellitus” OR “diabetes.” The literature search had no limitations on country or race. Additionally, all reference lists in the articles identified were manually checked in an effort to locate any missed relevant papers. The full texts of all the articles located were read to determine whether they met the inclusion criteria. All the above processes were conducted independently by two authors. Any discrepancies were resolved by reaching a consensus via discussion.
Inclusion and Exclusion Criteria
Publications were considered eligible if they met the following criteria: (i) studies conducted using adult subjects; (ii) studies that reported odds ratios (ORs), hazard ratios (HRs), or risk ratios (RRs) with 95% confidence intervals (CIs) for the highest to the lowest DII scores; (iii) studies that reported definitions of outcomes; and (iv) studies with either a cohort, case-control, or cross-sectional design. Studies were excluded if they were (i) studies on other diseases or dietary patterns; (ii) studies without complete data; (iii) dietary pattern (Mediterranean diet); (iv) review papers; or (v) so-called “gray” literature (dissertations, evaluations, working papers, book chapters, conference abstracts, and interviews).
Data Extraction
Data were extracted from the reports and summarized by one reviewer, and then another reviewer independently checked the accuracy of that information. Data extracted from original articles included (i) study characteristics (the first author’s name, year of publication, country, study design), (ii) participant information (sex, age), (iii) follow-up duration, (iv) sample size, (v) number of cases, (vi) DII measure and dietary assessment tool, (vii) risk estimates with CIs, and (viii) adjustments. In cases of missing data, the first author was contacted in an effort to obtain the missing information.
Methodological Quality Assessment
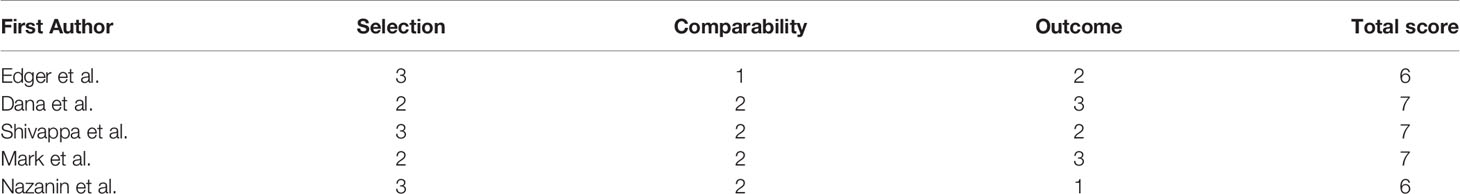
The quality of the studies included was assessed based on the Newcastle-Ottawa scale, which is a useful tool for estimating risks of bias in nonrandomized studies. The scale has three main grouping items: selection, comparability, and outcome (cohort study) or exposure (case-control study). The maximum total score is 9, and the respective maximum scores for selection, comparability, and outcome or exposure are 4, 2, and 3. A score of >5 points is considered to indicate high study quality. This step was also conducted by two authors independently, and any discrepancy was resolved by reaching a consensus via discussion.
Statistical Analysis
HRs, RRs, or ORs extracted from the reports were pooled with random effect models, and then summary ORs were calculated by comparing the relationships between the two extreme categories of DII scores and DM outcomes, or using continuous DII measures when feasible. The I2 index was used to evaluate the presence and severity of between-study heterogeneity. I2 values ≤ 25%, 25%–50%, 50%–75%, and >75% respectively represented no, small, moderate, and high heterogeneity. Given the existence of moderate heterogeneity (I2 > 50%), subgroup analyses were conducted based on the following stratification: body mass index (BMI), age, dietary assessment tool, location, BMI adjustment, physical activity adjustment, and energy adjustment. A sensitivity analysis was performed to examine the robustness of the results by repeating the meta-analysis an additional five times, each time with one of the five studies excluded. Egger’s regression test was conducted to assess the publication bias for each outcome. All of the above statistical analyses were conducted using the STATA version 15.1 software.
Results
Literature Research
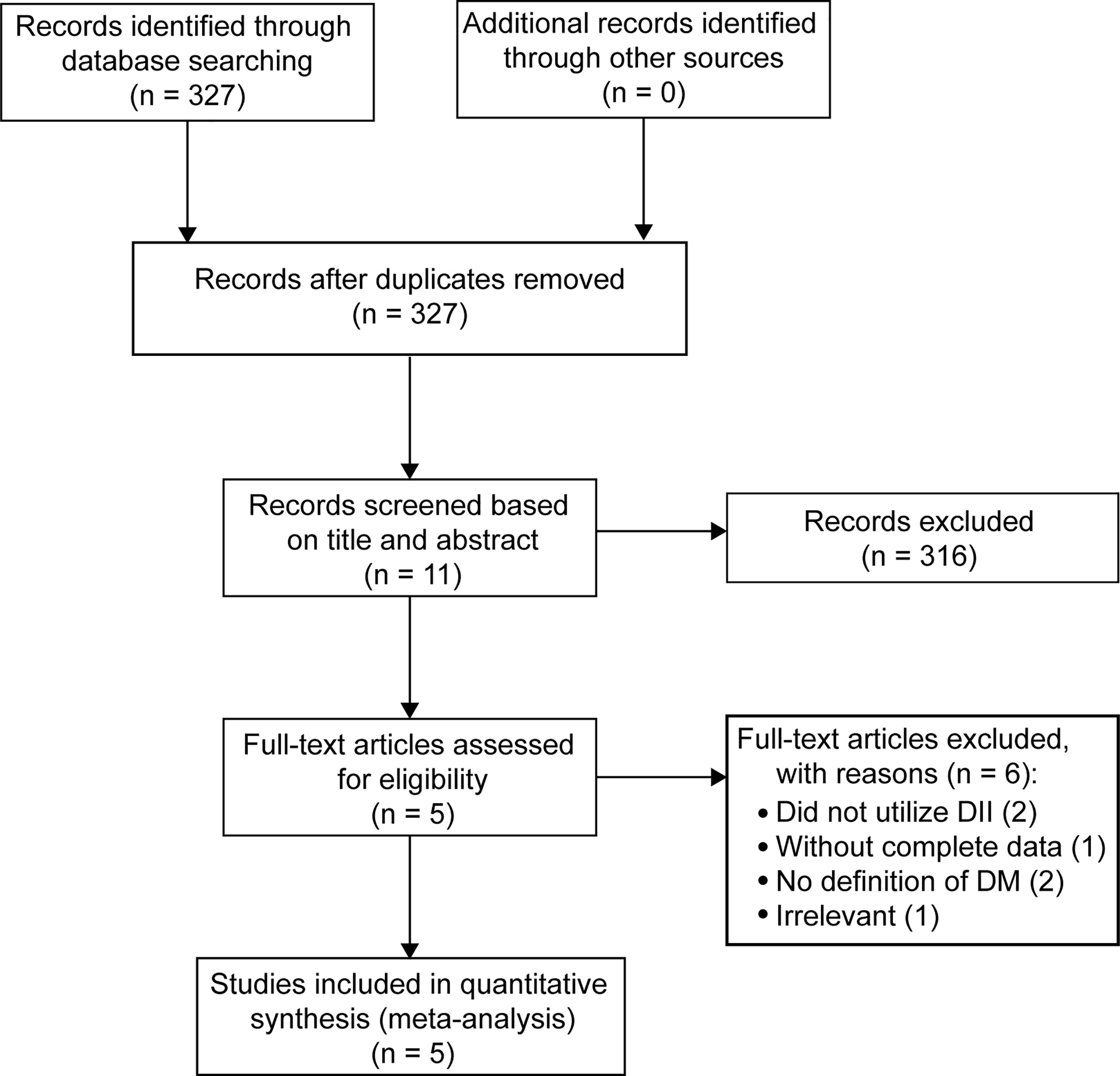
A total of 327 records were initially identified via database searching. After screening titles and abstracts, 316 were initially eliminated based on the inclusion and exclusion criteria, or because they were duplicates. After further assessment by reading the full texts, a further six articles were excluded because they did not utilize the DII (one study), had incomplete data (one study), did not include an accurate definition of DM (two studies), or were irrelevant (one study). Five studies ultimately met the inclusion criteria and were merged to evaluate associations between the two extreme categories of the DII score and risk of DM (13–17). The flow of the above-described procedures is shown in Figure 1.
Study Characteristics
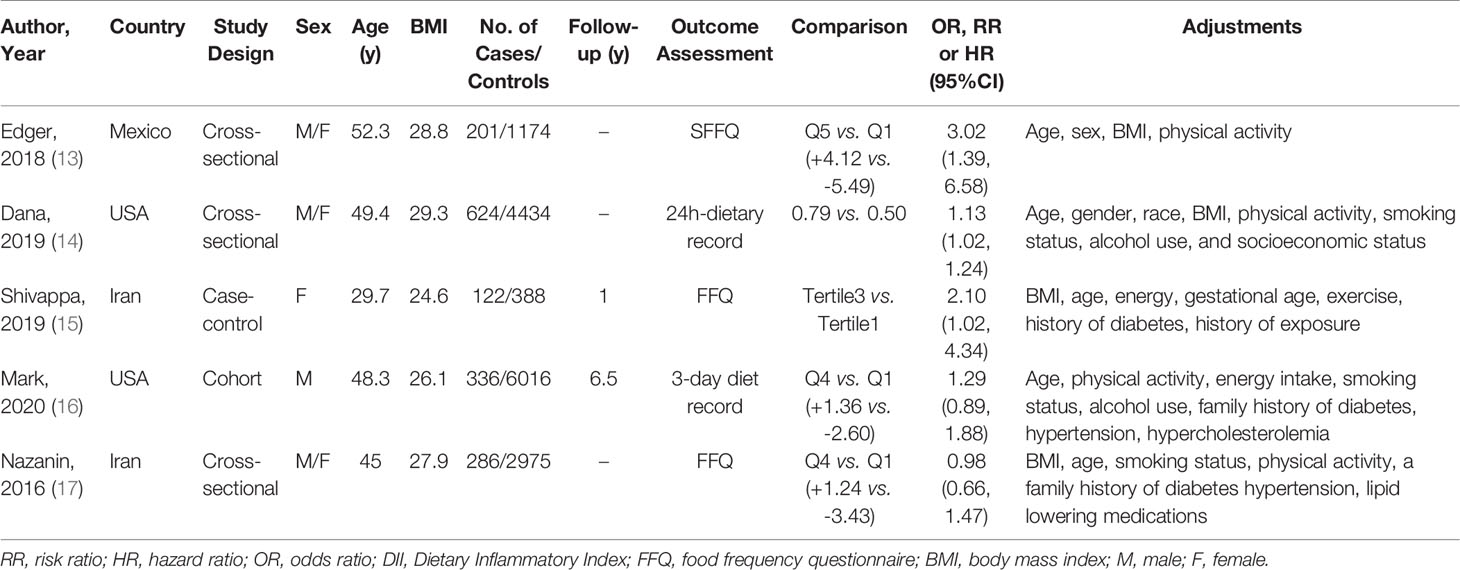
Five studies were included in the meta-analysis, involving a total of 14,987 participants and 1,366 cases from various countries, including the USA (two studies), Iran (two studies), and Mexico (one study). All five studies were published in the years from 2016 to 2020 inclusively (the DII was first introduced in 2009) (6). With regard to study design, three studies had a cross-sectional design, one was a prospective cohort study, and one had a case-control design. The sample sizes ranged from 388 to 6,016 participants, with mean ages ranging from 31.0 to 52.3 years. Follow-up duration was reported in the prospective cohort study and the case-control study, but not in any of the three cross-sectional studies. All the studies utilized either FFQs (three studies) or dietary records (two studies) as a dietary assessment tool to calculate DII scores. DII scores were used to assign study participants into a highest group (most proinflammatory diet) and a lowest group (most anti-inflammatory diet) to estimate the relationship between the DII score and the risk of DM. Adjusted ORs/RRs/HRs were reported in all five studies, and they were controlled for different types of confounding factors such as age, sex, and energy intake. Not all studies categorized DII measures into highest and lowest groups, and the study conducted by Dana et al. only reported continuous data for overall measures, not for stratified measures. In three of the five studies, there was a positive association between the most proinflammatory diet versus the most anti-inflammatory diet and the incidence of DM. Notably, however, in the remaining two studies, there was no such statistically significant association. Detailed information about the five studies in the analyses is presented in Table 1. According to the Newcastle-Ottawa scale, the methodological quality of all five studies was high (scores ≥ 5 points) (Table 2). Their findings on the association between the DII score and DM were inconsistent.
Higher DII Score and Increased Risk of DM
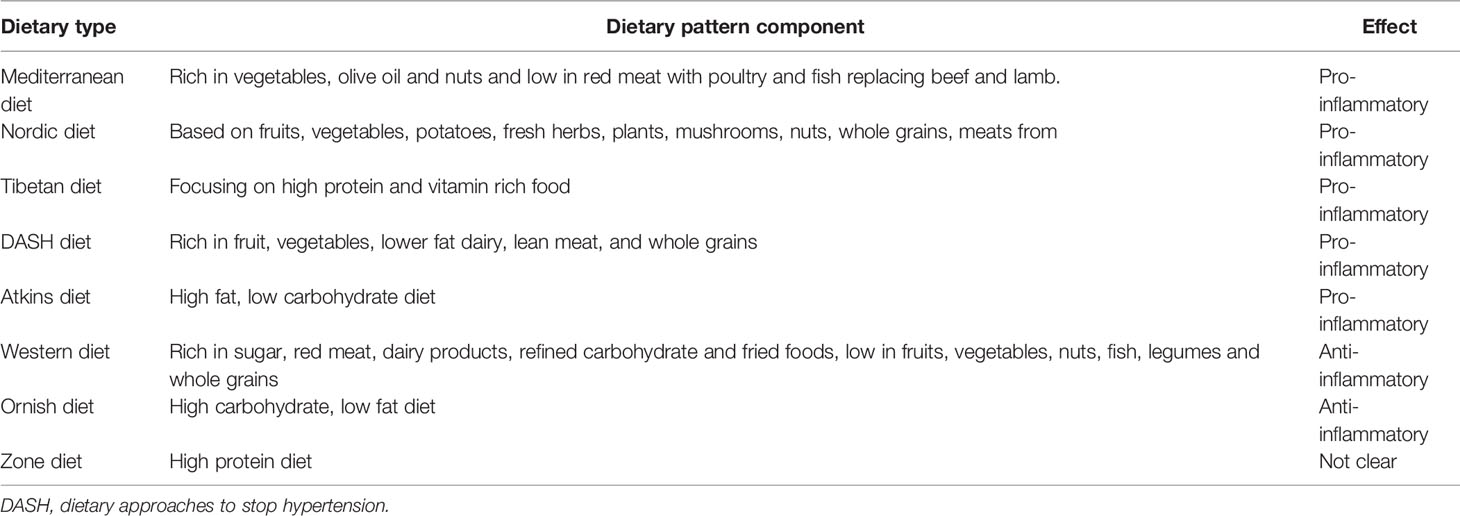
In the present meta-analysis, there was a significant association between the DII score and the incidence of DM (pooled OR 1.32, 95% CI 1.01–1.72) with moderate heterogeneity (I2 58.6%, p < 0.05) (Figure 2). Further subgroup analyses were conducted to identify the main possible source of the heterogeneity observed between the results of the five studies.
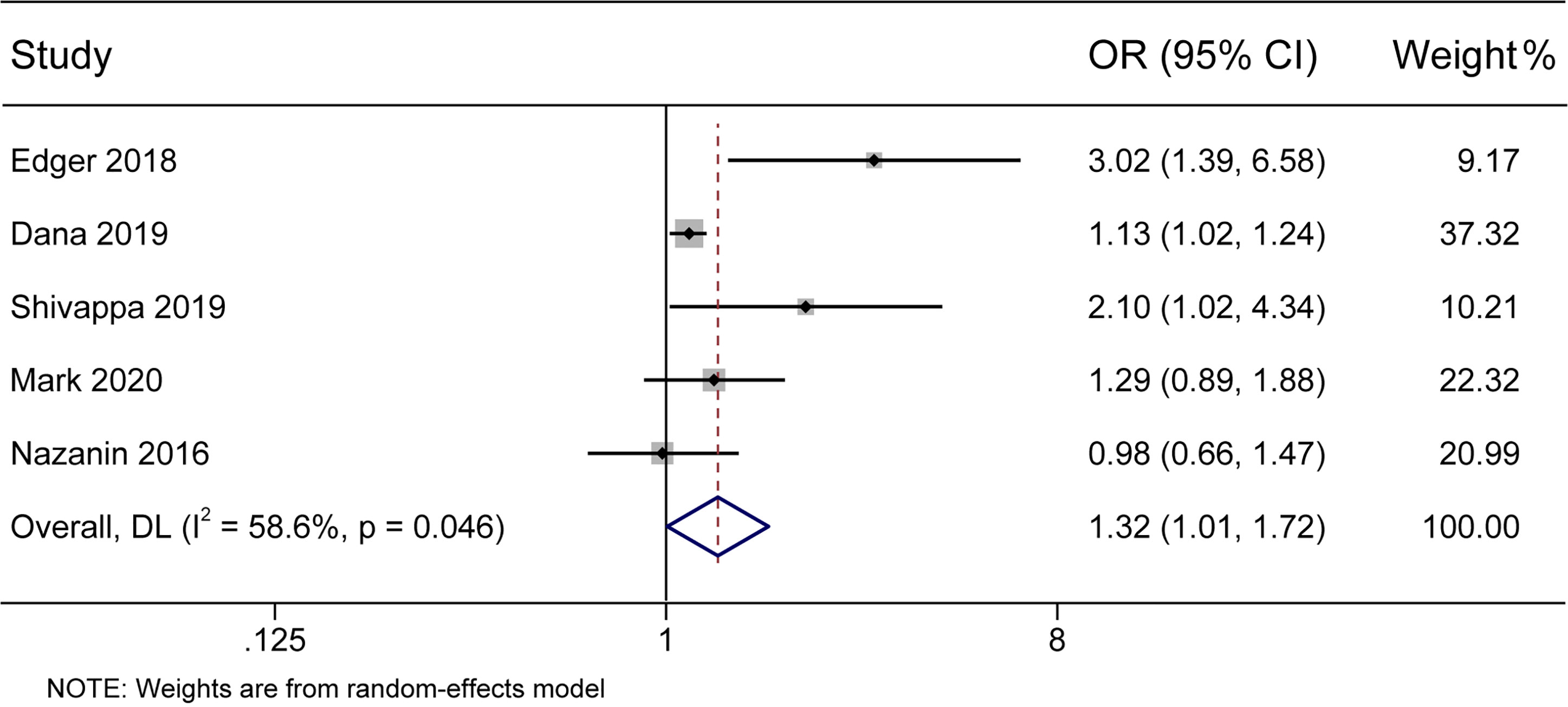
Figure 2 Forest plot showing the association between the DII and the risk of diabetes mellitus. Higher DII scores were associated with an increased incidence of diabetes mellitus.
BMI
A higher DII score predicted a greater risk of DM, especially in individuals with a BMI ≥ 28 (pooled OR 1.71, 95% CI 0.66–4.42, I2 83.5%, p = 0.014) (Table 3). Because obesity is associated with a variety of metabolic disorder risk factors including high blood pressure, high cholesterol, and insulin resistance (IR), obese individuals are more susceptible to the effects of proinflammatory diets (21).
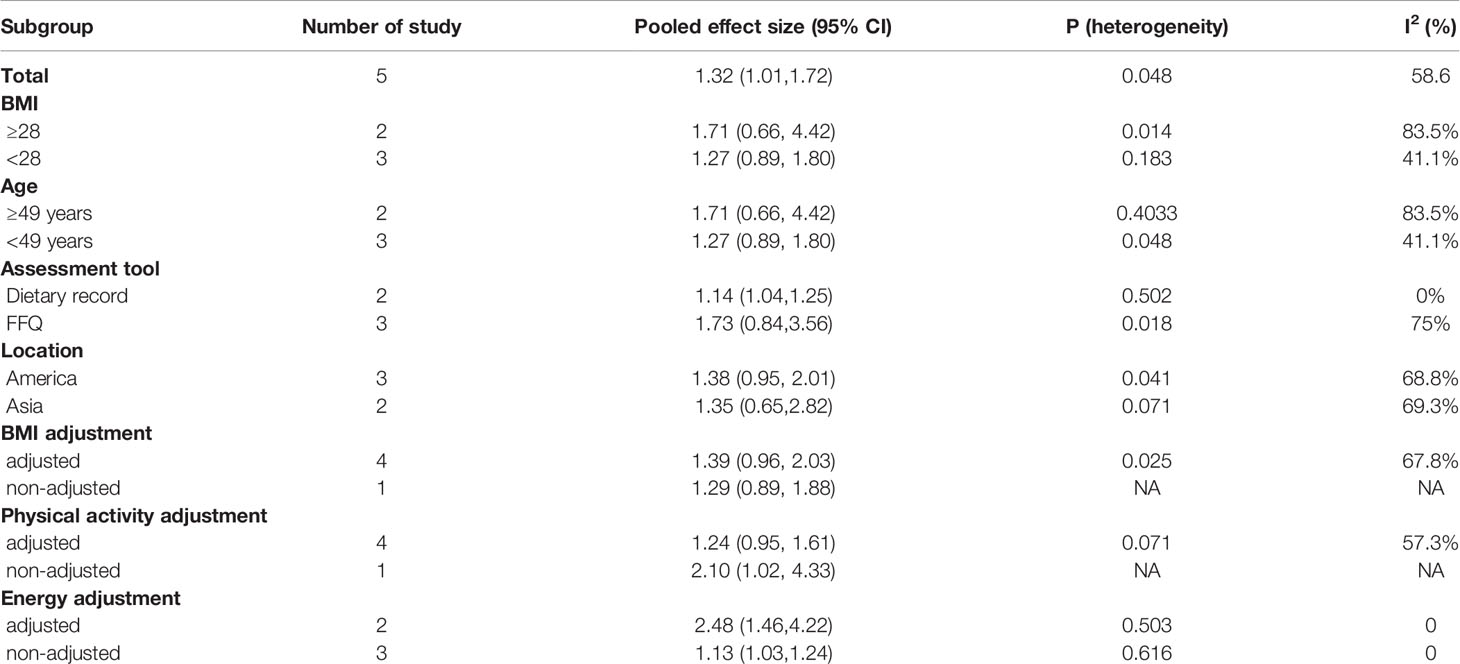
Table 3 Subgroup analyses of the association between the DII score and diabetes mellitus. Risk estimates refer to the highest vs. lowest DII categories.
Dietary Assessment Tool
Stratification via a dietary assessment tool and age partly reduced the heterogeneity between studies, and the association differed significantly between studies that used FFQs (pooled OR 1.73, 95% CI 0.84–3.56, I2 75%, p = 0.018) and those that used dietary records (pooled OR 1.14, 95% CI 1.04–1.25, I2 0, p = 0.502).
Energy Intake Adjustment
Among the variables investigated, adjustment for energy intake (pooled OR 2.48, 95% CI 1.46–4.22, I2 = 0, p = 0.503) was critical for achieving significant results as compared to studies that did not utilize such adjustment (pooled OR 1.13, 95% CI 1.03–1.24, I2 0, p = 0.616). Because higher energy intake reflects greater food intake that could be variably distributed among proinflammatory and anti-inflammatory-associated foods, paying no attention to total energy intake may lead to bias.
Sensitivity Analysis
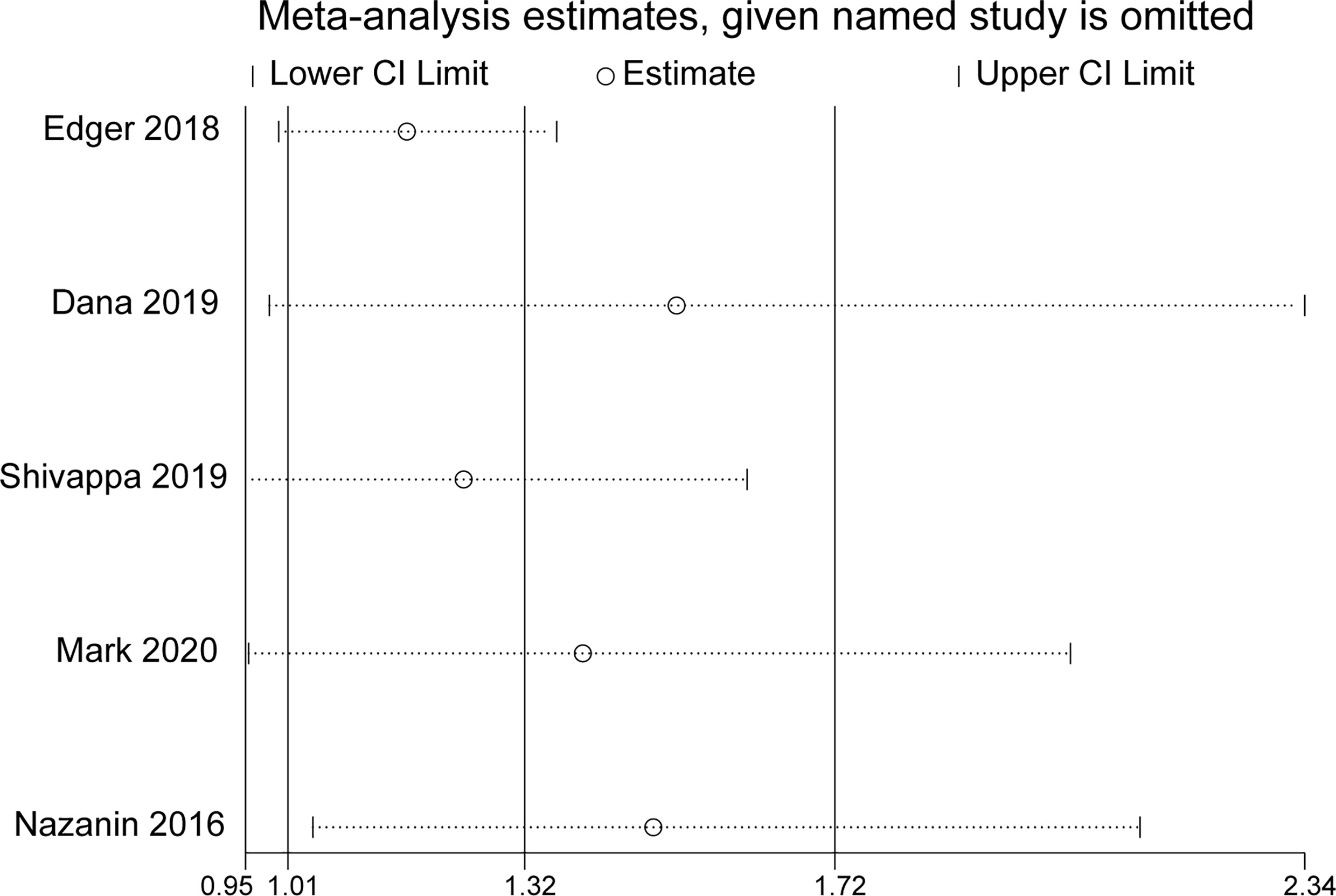
In sensitivity analysis, the overall effect estimates were not considerably altered by removing any one of the five studies (Figure 3).
Publication Bias
The results of Egger’s test (t = 1.70, p = 0.187) indicated that there was no obvious publication bias in the five studies.
Discussion
In the present meta-analysis, individuals with a more proinflammatory diet were at a 32% higher risk of DM. Similarly, in a review by Catherine et al., it was concluded that the DII can be used as an effective tool to evaluate the overall inflammatory nature of diet, and that it helps to identify relationships between diet, inflammation, and DM. However, that review only reported qualified synthesis, whereas the quantitative rate of the relationship remains unclear (19). On the whole, previous evidence about potential associations between DII scores and DM has been limited largely because of a lack of studies, small sample sizes, and differences in participants’ characteristics including age, sex, and race. The present systematic review and meta-analysis incorporated results from five published studies and, thus, identified stronger associations.
Diet is widely believed to be a main contributor to chronic inflammation (5). A Western diet is generally rich in proinflammatory foods such as sugar, red meat, dairy products, refined carbohydrates, and fried foods, and this may increase the serum levels of inflammatory markers (22) and the risk of DM in both males (23) and females (24). In contrast, a Mediterranean diet is generally rich in vegetables, olive oil, and nuts, and low in red meat, with poultry and fish replacing beef and lamb. Several epidemiological studies have assessed the protective effects of a Mediterranean diet with respect to several diseases associated with chronic low-grade inflammation such as cancer, diabetes, obesity, metabolic syndrome, and atherosclerosis (25). Therefore, we investigated and summarized the current evidence on associations between several normal dietary patterns and inflammation, as derived from epidemiological studies (26–31) (Table 4).
The correlation between diet and DM risk can be partly explained by chronic inflammation that may lead to IR and β cell dysfunction (32). The medium function of inflammation may involve multiple mechanisms, as described below. Activation of c-Jun N-terminal kinase by the excessive production of inflammatory factors induces phosphorylation of insulin receptor substrate-1 (IRS-1) at Ser307 leading to its inactivation, thus suppressing insulin receptor signaling (33, 34). Inflammatory factors also reduce insulin-dependent glucose transport and peripheral glucose utilization respectively by inducing the suppressor of cytokine signaling-3 and inhibiting lipoprotein lipase activity (35). Oxidative stress damage induced by inflammation may cause β cell apoptosis and deficiency of insulin secretion. In addition, sustained activation of mTORC1/S6K1 under conditions of nutrient overload (glucose, amino acids—especially branched-chain amino acids such as leucine) via AMP-activated protein kinase generates a feedback loop that suppresses IRS-1 mRNA and protein levels and IRS-1 activity. mTORC1/S6K1 plays a dual role in β cells, regulating β cell size and inhibiting insulin/insulin-like growth factor 1 signal transduction, which may lead to increased apoptosis (36, 37).
The original DII was created as a useful tool for categorizing the diets of individuals on a continuum from maximally anti-inflammatory to maximally proinflammatory, and it can be used in epidemiologic studies to estimate the potential burden of DM related to diet. All dietary indices prior to the DII such as the Healthy Eating Index, Alternative Healthy Eating Index, Dietary Approaches to Stop Hypertension, and Mediterranean Dietary Index were not specifically designed to evaluate the overall inflammatory potential of diet (38–42). In contrast, the DII was developed to reflect all evidence from a wide range of populations as well as laboratory animal and cell culture experiments.
The results of subgroup analyses indicated that individuals with a higher BMI are more susceptible to the effects of inflammatory stimuli. The strong associations between obesity, inflammation, and DM can be partly explained by the recruitment of macrophages into adipose tissue. Excessive inflammatory factors produced by macrophages and adipocytes may lead to the activation of c-Jun N-terminal kinase and subsequently inhibit phosphorylation of IRS-1 serine residues that induce IR (34). Studies that used FFQs identified stronger associations than those that used dietary records. This may be because DII scores can be affected by dietary patterns and dietary assessment tools. Although completing an FFQ requires more time than completing a dietary record, it is more accurate, enabling investigators to estimate relatively long-range dietary intake, focus on specific foods or nutrients consumed by a given population, and understand relationships between diet and health outcomes (43, 44).
The current meta-analysis had several limitations. Only five studies were eligible for the analysis of DM risk. Because the DII was first created in 2009, to date, few studies have used it, particularly studies investigating associations between DII scores and DM. Different DII score categorical cutoff points (such as quintile and quartile) rendered comparisons less straightforward; thus, we could only obtain a quantitative rate of the association between the DII and DM but not a specific range. As a part of individual investigations and the basis of DII scores, the availability of FFQ data is offset by well-documented limitations such as recall bias, limitations associated with assessing specific cultural food items, and lack of validation in different research settings. Lastly, substantial heterogeneity was observed in the current meta-analysis. One possible explanation for this is the differences in energy intake adjustment used in the studies included. Another may relate to the different demographic characteristics and designs of the studies. Despite the above-described limitations, the DII calculation method used was the same in each study, thus increasing comparability. The present meta-analysis also provides greater statistical power and a more robust pooled estimate of associations by pooling results from all of the included studies.
Conclusion
Diets resulting in high DII scores are associated with an increased risk of DM, particularly in obese individuals. However, the results of the current meta-analysis were based on a small number of studies, and further high-quality prospective studies with larger sample sizes conducted in different populations and countries are required to elucidate this association.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
YX and C-LG designed this study. Q-QT and X-YD extracted and confirmed the data. Q-QT performed the statistical analysis. Q-QT was responsible for manuscript preparation. YX and C-LG commented on the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of China (NO.81900764, NO.81970676), Sichuan Science and Technology Program (NO.2019YFS0537, NO.2020YFS0456), and Luzhou-Southwest Medical University cooperation project (NO.2020LZXNYDJ32).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express our thanks to the Natural Science Foundation of China (NO.81900764, NO.81970676), Sichuan Science and Technology Program (NO.2019YFS0537, NO.2020YFS0456), and Luzhou-Southwest Medical University cooperation project (NO.2020LZXNYDJ32) for financial support.
Abbreviation
DII, dietary inflammatory index; DM, diabetes mellitus; BMI, body mass index; IR, insulin resistance; NOS, Newcastle-Ottawa scale; FFQ, food frequency questionnaire; WPD, western pattern diet; IRS-1, insulin receptor substrate-1; SOCS-3, suppressor of cytokine signaling-3; LPL, lipoprotein lipase; JNK, c-Jun N-terminal kinase; WOSCOPS, West of Scotland Coronary Prevention Study; HEI, Healthy Eating Index; AHEI, Alternative Healthy Eating Index; DASH, Dietary Approaches to Stop Hypertension; MDI, Mediterranean Dietary Index; LPL, low-density lipoprotein.
References
1. Namazi N, Larijani B, Azadbakht L. Dietary Inflammatory Index and its Association With the Risk of Cardiovascular Diseases, Metabolic Syndrome, and Mortality: A Systematic Review and Meta-Analysis. Horm Metab Res (2018) 50(5):345–58. doi: 10.1055/a-0596-8204
2. Libby P. Inflammatory Mechanisms: The Molecular Basis of Inflammation and Disease. Nutr Rev (2007) 65(12 Pt 2):S140–6. doi: 10.1301/nr.2007.dec.S140-S146
3. Galland L. Diet and Inflammation. Nutr Clin Pract (2010) 25(6):634–40. doi: 10.1177/0884533610385703
4. Emerson SR, Kurti SP, Harms CA, Haub MD, Melgarejo T, Logan C, et al. Magnitude and Timing of the Postprandial Inflammatory Response to a High-Fat Meal in Healthy Adults: A Systematic Review. Adv Nutr (2017) 8(2):213–25. doi: 10.3945/an.116.014431
5. Giugliano D, Ceriello A, Esposito K. The Effects of Diet on Inflammation: Emphasis on the Metabolic Syndrome. J Am Coll Cardiol (2006) 48(4):677–85. doi: 10.1016/j.jacc.2006.03.052
6. Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, et al. A New Dietary Inflammatory Index Predicts Interval Changes in Serum High-Sensitivity C-Reactive Protein. J Nutr (2009) 139(12):2365–72. doi: 10.3945/jn.109.114025
7. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and Developing a Literature-Derived, Population-Based Dietary Inflammatory Index. Public Health Nutr (2014) 17(8):1689–96. doi: 10.1017/S1368980013002115
8. Hébert JR, Shivappa N, Wirth MD, Hussey JR, Hurley TG. Perspective: The Dietary Inflammatory Index (DII)-Lessons Learned, Improvements Made, and Future Directions. Adv Nutr (2019) 10(2):185–95. doi: 10.1093/advances/nmy071
9. Neufcourt L, Assmann KE, Fezeu LK, Touvier M, Graffouillère L, Shivappa N, et al. Prospective Association Between the Dietary Inflammatory Index and Cardiovascular Diseases in the SUpplémentation En VItamines Et Minéraux AntioXydants (SU.VI.MAX) Cohort. J Am Heart Assoc (2016) 5(3):e002735. doi: 10.1161/JAHA.115.002735
10. Naja F, Shivappa N, Nasreddine L, Kharroubi S, Itani L, Hwalla N, et al. Role of Inflammation in the Association Between the Western Dietary Pattern and Metabolic Syndrome Among Lebanese Adults. Int J Food Sci Nutr (2017) 68(8):997–1004. doi: 10.1080/09637486.2017.1312297
11. San KMM, Fahmida U, Wijaksono F, Lin H, Zaw KK, Htet MK. Chronic Low Grade Inflammation Measured by Dietary Inflammatory Index and its Association With Obesity Among School Teachers in Yangon, Myanmar. Asia Pac J Clin Nutr (2018) 27(1):92–8. doi: 10.6133/apjcn.042017.06
12. Vidal AC, Oyekunle T, Howard LE, Shivappa N, De Hoedt A, Figueiredo JC, et al. Dietary Inflammatory Index (DII) and Risk of Prostate Cancer in a Case-Control Study Among Black and White US Veteran Men. Prostate Cancer Prostatic Dis (2019) 22(4):580–7. doi: 10.1038/s41391-019-0143-4
13. Denova-Gutiérrez E, Muñoz-Aguirre P, Shivappa N, Hébert JR, Tolentino-Mayo L, Batis C, et al. Dietary Inflammatory Index and Type 2 Diabetes Mellitus in Adults: The Diabetes Mellitus Survey of Mexico City. Nutrients (2018) 10(4):385. doi: 10.3390/nu10040385
14. King DE, Xiang J. The Dietary Inflammatory Index Is Associated With Diabetes Severity. J Am Board Fam Med (2019) 32(6):801–6. doi: 10.3122/jabfm.2019.06.190092
15. Shivappa N, Hébert JR, Akhoundan M, Mirmiran P, Rashidkhani B. Association Between Inflammatory Potential of Diet and Odds of Gestational Diabetes Mellitus Among Iranian Women. J Matern Fetal Neona (2019) 32(21):3552–8. doi: 10.1080/14767058.2018.1466275
16. Guinter MA, Merchant AT, Tabung FK, Wirth MD, Shivappa N, Hurley TG, et al. Adiposity Does Not Modify the Effect of the Dietary Inflammatory Potential on Type 2 Diabetes Incidence Among a Prospective Cohort of Men. J Nutr Intermed Metab (2019) 16:100095. doi: 10.1016/j.jnim.2019.100095
17. Moslehi N, Ehsani B, Mirmiran P, Shivappa N, Tohidi M, Hébert J, et al. Inflammatory Properties of Diet and Glucose-Insulin Homeostasis in a Cohort of Iranian Adults. Nutrients (2016) 8(11):735. doi: 10.3390/nu8110735
18. Laouali N, Mancini FR, Hajji-Louati M, El Fatouhi D, Balkau B, Boutron-Ruault M-C, et al. Dietary Inflammatory Index and Type 2 Diabetes Risk in a Prospective Cohort of 70,991 Women Followed for 20 Years: The Mediating Role of BMI. Diabetologia (2019) 62(12):2222–32. doi: 10.1007/s00125-019-04972-0
19. Phillips CM, Chen L-W, Heude B, Bernard JY, Harvey NC, Duijts L, et al. Dietary Inflammatory Index and Non-Communicable Disease Risk: A Narrative Review. Nutrients (2019) 11(8):1873. doi: 10.3390/nu11081873
20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
21. Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Dannenberg AJ, et al. Metabolic Dysfunction, Obesity, and Survival Among Patients With Early-Stage Colorectal Cancer. J Clin Oncol (2016) 34(30):3664–71. doi: 10.1200/JCO.2016.67.4473
22. King DE, Egan BM, Geesey ME. Relation of Dietary Fat and Fiber to Elevation of C-Reactive Protein. Am J Cardiol (2003) 92(11):1335–9. doi: 10.1016/j.amjcard.2003.08.020
23. Drake I, Sonestedt E, Ericson U, Wallström P. Orho-Melander M. A Western Dietary Pattern is Prospectively Associated With Cardio-Metabolic Traits and Incidence of the Metabolic Syndrome. Br J Nutr (2018) 119(10):1168–76. doi: 10.1017/S000711451800079X
24. Drewnowski A. The Real Contribution of Added Sugars and Fats to Obesity. Epidemiol Rev (2007) 29:160–71. doi: 10.1093/epirev/mxm011
25. Casas R, Sacanella E, Estruch R. The Immune Protective Effect of the Mediterranean Diet Against Chronic Low-Grade Inflammatory Diseases. Endocr Metab Immune Disord Drug Targets (2014) 14(4):245–54. doi: 10.2174/1871530314666140922153350
26. Poulsen SK, Due A, Jordy AB, Kiens B, Stark KD, Stender S, et al. Health Effect of the New Nordic Diet in Adults With Increased Waist Circumference: A 6-Mo Randomized Controlled Trial. Am J Clin Nutr (2014) 99(1):35–45. doi: 10.3945/ajcn.113.069393
27. von Haehling S, Stellos K, Qusar N, Gawaz M, Bigalke B. Weight Reduction in Patients With Coronary Artery Disease: Comparison of Traditional Tibetan Medicine and Western Diet. Int J Cardiol (2013) 168(2):1509–15. doi: 10.1016/j.ijcard.2013.07.034
28. Roussell MA, Hill AM, Gaugler TL, West SG, Heuvel JPV, Alaupovic P, et al. Beef in an Optimal Lean Diet Study: Effects on Lipids, Lipoproteins, and Apolipoproteins. Am J Clin Nutr (2012) 95(1):9–16. doi: 10.3945/ajcn.111.016261
29. Ruth MR, Port AM, Shah M, Bourland AC, Istfan NW, Nelson KP, et al. Consuming a Hypocaloric High Fat Low Carbohydrate Diet for 12 Weeks Lowers C-Reactive Protein, and Raises Serum Adiponectin and High Density Lipoprotein-Cholesterol in Obese Subjects. Metabolism (2013) 62(12):1779–87. doi: 10.1016/j.metabol.2013.07.006
30. Azadbakht L, Izadi V, Surkan PJ, Esmaillzadeh A. Effect of a High Protein Weight Loss Diet on Weight, High-Sensitivity C-Reactive Protein, and Cardiovascular Risk Among Overweight and Obese Women: A Parallel Clinical Trial. Int J Endocrinol (2013) 2013:971724. doi: 10.1155/2013/971724
31. Christ A, Lauterbach M, Latz E. Western Diet and the Immune System: An Inflammatory Connection. Immunity (2019) 51(5):794–811. doi: 10.1016/j.immuni.2019.09.020
32. Li Z-Z, Liu J-B, Li L, Jiao L, Chen L. Intensive Therapy for Diabetes Through Influence on Innate Immune System. Med Hypotheses (2009) 72(6):675–6. doi: 10.1016/j.mehy.2009.01.028
33. Kohn LD, Wallace B, Schwartz F, McCall K. Is Type 2 Diabetes an Autoimmune-Inflammatory Disorder of the Innate Immune System? Endocrinology (2005) 146(10):4189–91. doi: 10.1210/en.2005-0920
34. Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, et al. A Central Role for JNK in Obesity and Insulin Resistance. Nature (2002) 420(6913):333–6. doi: 10.1038/nature01137
35. Karlsen AE, Rønn SG, Lindberg K, Johannesen J, Galsgaard ED, Pociot F, et al. Suppressor of Cytokine Signaling 3 (SOCS-3) Protects Beta -Cells Against Interleukin-1beta - and Interferon-Gamma -Mediated Toxicity. Proc Natl Acad Sci USA (2001) 98(21):12191–6. doi: 10.1073/pnas.211445998
36. Leibowitz G, Cerasi E, Ketzinel-Gilad M. The Role of mTOR in the Adaptation and Failure of Beta-Cells in Type 2 Diabetes. Diabetes Obes Metab (2008) 10(Suppl 4):157–69. doi: 10.1111/j.1463-1326.2008.00952.x
37. Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, et al. Hypoinsulinaemia, Glucose Intolerance and Diminished Beta-Cell Size in S6K1-Deficient Mice. Nature (2000) 408(6815):994–7. doi: 10.1038/35050135
38. Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: Design and Applications. J Am Diet Assoc (1995) 95(10):1103–8. doi: 10.1016/S0002-8223(95)00300-2
39. McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, et al. Diet Quality and Major Chronic Disease Risk in Men and Women: Moving Toward Improved Dietary Guidance. Am J Clin Nutr (2002) 76(6):1261–71. doi: 10.1093/ajcn/76.6.1261
40. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-Style Diet and Risk of Coronary Heart Disease and Stroke in Women. Arch Intern Med (2008) 168(7):713–20. doi: 10.1001/archinte.168.7.713
41. Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary Patterns: A Mediterranean Diet Score and its Relation to Clinical and Biological Markers of Cardiovascular Disease Risk. Nutr Metab Cardiovasc Dis (2006) 16(8):559–68. doi: 10.1016/j.numecd.2005.08.006
42. Tabung FK, Wang W, Fung TT, Hu FB, Smith-Warner SA, Chavarro JE, et al. Development and Validation of Empirical Indices to Assess the Insulinaemic Potential of Diet and Lifestyle. Br J Nutr (2016) 116(10):1787–98. doi: 10.1017/S0007114516003755
43. El Kinany K, Garcia-Larsen V, Khalis M, Deoula MMS, Benslimane A, Ibrahim A, et al. Adaptation and Validation of a Food Frequency Questionnaire (FFQ) to Assess Dietary Intake in Moroccan Adults. Nutr J (2018) 17(1):61. doi: 10.1186/s12937-018-0368-4
44. Koole JL, Bours MJL, Breedveld-Peters JJL, van Roekel EH, van Dongen MCJM, Eussen SJPM, et al. Evaluating the Validity of a Food Frequency Questionnaire in Comparison With a 7-Day Dietary Record for Measuring Dietary Intake in a Population of Survivors of Colorectal Cancer. J Acad Nutr Diet (2020) 120(2):245–57. doi: 10.1016/j.jand.2019.09.008
Keywords: body mass index, diabetes mellitus, diet, dietary inflammatory index, inflammation
Citation: Tan Q-Q, Du X-Y, Gao C-L and Xu Y (2021) Higher Dietary Inflammatory Index Scores Increase the Risk of Diabetes Mellitus: A Meta-Analysis and Systematic Review. Front. Endocrinol. 12:693144. doi: 10.3389/fendo.2021.693144
Received: 09 April 2021; Accepted: 20 July 2021;
Published: 11 August 2021.
Edited by:
Pradeep Kumar, University of the Witwatersrand, South AfricaReviewed by:
Uma Shanker Navik, Central University of Punjab, IndiaDonatella Stilli, University of Parma, Italy
Arti Singh, Indo-Soviet Friendship College of Pharmacy, India
Copyright © 2021 Tan, Du, Gao and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Xu, eHl3eWxsQGFsaXl1bi5jb20=; Chen-Lin Gao, Z2FvY2hlbmxpbjAwQDEyNi5jb20=
 Qing-Qing Tan
Qing-Qing Tan Xin-Yi Du1,2,3
Xin-Yi Du1,2,3 Chen-Lin Gao
Chen-Lin Gao Yong Xu
Yong Xu