
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 14 June 2021
Sec. Pediatric Endocrinology
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.687834
 Ari Song1†
Ari Song1† Su Jin Kim2†
Su Jin Kim2† Min-Sun Kim3
Min-Sun Kim3 Jiyeon Kim3
Jiyeon Kim3 Insung Kim3
Insung Kim3 Ga Young Bae3
Ga Young Bae3 Eunseop Seo3
Eunseop Seo3 Young Seok Cho4
Young Seok Cho4 Joon Young Choi4
Joon Young Choi4 Sung Yoon Cho3*‡
Sung Yoon Cho3*‡ Dong-Kyu Jin3‡
Dong-Kyu Jin3‡Background/purpose: Graves’ disease (GD) is the most common cause of thyrotoxicosis in children and adolescents. There is some debate regarding the optimal treatment and predicting factors of remission or relapse in children and adolescents with GD. In this study, we report a retrospective study of 195 children and adolescents with GD treated at a single tertiary institution in Korea.
Methods: This study included children and adolescents with GD diagnosed before 19 years of age from January of 2000 to October of 2020. The diagnosis of GD was based on clinical features, high thyroxine (FT4), suppressed thyroid-stimulating hormone, and a positive titer of thyrotropin receptor antibodies. Remission was defined as maintenance of euthyroid status for more than six months after discontinuing antithyroid drug (ATD).
Results: A total of 195 patients with GD were included in this study. The mean age at diagnosis was 12.9 ± 3.2 years, and 162 patients (83.1%) were female. Among all 195 patients, five underwent thyroidectomy and three underwent radioactive iodine therapy. The mean duration of follow-up and ATD treatment were 5.9 ± 3.8 years and 4.7 ± 3.4 years, respectively. The cumulative remission rates were 3.3%, 19.6%, 34.1%, 43.5%, and 50.6% within 1, 3, 5, 7, and 10 years of starting ATD, respectively. FT4 level at diagnosis (P = 0.001) was predicting factors for remission [HR, 0.717 (95% CI, 0.591 – 0.870), P = 0.001]. Methimazole (MMI)-related adverse events (AEs) occurred in 11.3% of patients, the most common of which were rash and hematologic abnormalities. Of a total of 26 AEs, 19 (73.1%) occurred within the first month of taking MMI.
Conclusions: In this study, the cumulative remission rate increased according to the ATD treatment duration. Long-term MMI treatment is a useful treatment option before definite treatment in children and adolescents with GD.
Graves’ disease (GD) is an autoimmune disorder characterized by hyperthyroidism, diffuse goiter, and/or ophthalmopathy that is caused by the activation of the thyroid-stimulating hormone (TSH) receptor by thyrotropin receptor antibodies (TRAb). GD is the most common cause of thyrotoxicosis in children and adolescents and contributes to 10-15% of all pediatric thyroid disease (1). The incidence of GD in children and adolescents varies by country, ranging from 1 to 14 per 100,000 person-years. It is relatively rare in children compared to adults, as less than 5% of all GD patients are children (2). However, the incidence of GD in children and adolescents is currently estimated to be rising (3–5).
Treatment options of GD in children and adolescents include antithyroid drug (ATD), radioactive iodine (RAI) therapy, and thyroidectomy, which are the same as in adults. There is no consensus regarding the optimal treatment of GD in children and adolescents, and the subject remains a matter of debate (6–8). Definite treatments such as thyroidectomy and RAI therapy may cause permanent hypothyroidism, requiring life-long thyroid hormone replacement, and RAI therapy has the potential for oncogenic damage. For these reasons, the majority of clinicians choose ATD as the first-line treatment option (8–10).
Long-term ATD treatment may lead to the development of drug-related adverse effects (AEs), a high rate of relapse after drug discontinuation, and compliance problems. In children and adolescents, previous studies have focused on predicting factors of remission or relapse after ATD discontinuation (11–14). However, no congruous predicting factors have been determined yet. The treatment policy of GD in children and adolescents varies between countries and depends on local traditions and resources, patient age, the preferences of the patients and their parents, and disease severity. This is a retrospective study of 195 children and adolescents with GD treated at a single tertiary institution in Korea.
We reviewed the medical records of 195 patients diagnosed with GD before the age of 19 at Samsung Medical Center from January of 2000 to October of 2020. The diagnosis of GD was based on the presence of typical clinical features (tachycardia, tremor, exophthalmos, goiter, etc.), high levels of free thyroxine (FT4), suppressed levels of TSH, and a positive titer of TRAb. Exclusion criteria were as follows: (1) patients who had been followed up for less than six months, (2) patients treated with ATD for more than six months at another hospital, and (3) patients who had other thyroid diseases (e.g., chronic lymphocytic thyroiditis, papillary carcinoma, etc.). Clinical data such as age, sex, height, weight, body mass index (BMI), GD symptoms and signs, drug-related AEs, a personal history of non-thyroid autoimmune diseases, and family history of thyroid autoimmune diseases in first-degree relatives were collected retrospectively.
Goiter was evaluated as present or absent at physical examination. The absence of goiter was defined as Grade 0, and the presence of goiter was defined as Grade 1 or 2 according to the WHO goiter classification. Graves’ ophthalmopathy (GO) was evaluated as present or absent. Presence of GO was defined as abnormal ocular signs and symptoms (e.g., eyelid retraction, eyelid lag, exophthalmos) by an endocrinologist and an ophthalmologist if needed. Laboratory data included FT4, total triiodothyronine (T3), TSH, anti-thyroglobulin antibody (ATA), anti-microsomal antibody (AMA), TRAb, complete blood count (CBC), creatine kinase (CK), and liver function test (LFT). Two different TRAb assays were used over the study period. During the first part of the study (before March of 2011), we used a TRAK-Assay (Brahms, Berlin, Germany; reference range, < 10% negative, 10–15% borderline, > 15% positive), which is a first-generation assay and a conventional radioreceptor assay using porcine thyrocytes membrane. Thereafter (after March of 2011), we used a TRAK human RIA (Brahms; reference range, < 1.0 IU/L negative, 1.0–1.5 IU/L borderline, > 1.5 IU/L positive), which is a second-generation assay and a coated tube radioimmunoassay using recombinant human TSH receptor. Thyroid function test (TFT) was measured by radioimmunoassay using an RIA kit (Immunotech Inc., Praha, Czech Republic) or chemiluminescence immunoassay using ADVIA CENTAUR XP (Seimens Healthcare Diagnostics, Massachusetts, USA). For accurate statistical analysis, the TFT values were standardized considering the reference ranges of each analysis kit (supplementary data). Laboratory data, including TFT, CBC, and LFT, were regularly evaluated at least every 3 months during the follow-up period. This study was approved by the Institutional Review Board of Samsung Medical Center (SMC 2021-01-051).
All patients were initially treated with an ATD, including methimazole (MMI) or propylthiouracil (PTU), although MMI was primarily used in this study. The initial doses of MMI were 0.5 to 1.0 mg/kg/day, (maximum 30 mg/day), which were gradually reduced in a titration regimen and discontinued when euthyroidism was maintained at the lowest dose (2.5 mg/day) for 6-12 months. In this study, two pediatric endocrinologists made a decision according to the treatment policy of the center. Remission was defined as maintenance of euthyroid status for more than six months after discontinuation of ATD. Relapse was defined as elevated serum FT4 with suppressed TSH during the follow-up period after ATD withdrawal.
Continuous variables are presented as mean ± standard deviation (SD). Categorical variables are expressed as rates and proportions. Kaplan–Meier survival analysis was used to estimate the time to remission. A comparison of categorical variables was performed using the Chi-square test or the Fisher exact test (in case of expected frequencies < 5). A comparison of continuous variables was performed using the Mann-Whitney U test (nonparametric test). The Cox regression model was used to identify the significant predictors of remission. Total T3 was not included in the Cox regression model because of collinearity with free T4. The results are expressed as hazard ratios (HR) with 95% confidence intervals (CI). A P-value < 0.05 indicates statistical significance. Statistical analyses were performed using SPSS Statistics software, version 23 (IBM Corp., Armonk, NY, USA) and SAS version 9.4 (SAS Institute, Cary, NC, USA).
Among the 195 patients, there were 162 (83.1%) females and 33 (16.9%) males. Their mean age at diagnosis was 12.9 ± 3.2 years (Figure 1). The mean duration of follow-up and ATD treatment were 5.9 ± 3.8 years and 4.7 ± 3.4 years, respectively. Among the 195 patients, 152 (77.9%) had a goiter and 64 (32.8%) had ophthalmopathy. Five patients (2.6%) had non-thyroid autoimmune diseases such as insulin-dependent diabetes mellitus or myasthenia gravis and one (0.5%) presented with thyrotoxic periodic paralysis (TPP). Among all subjects, 68 (34.9%) had a familial history of thyroid autoimmune diseases in first-degree relatives (Table 1).
Figure 2 demonstrates the clinical course of all patients. Among the 195 patients, eight (4.1%) underwent definite treatment: five thyroidectomies and three RAI therapies. Definite treatment was performed due to poorly controlled hyperthyroidism despite ATD treatment or was based on the patient’s preferences. Of the 187 patients treated with ATD, 50 (26.7%) have continued ATD treatment without remission, 104 (55.6%) discontinued ATD, and 33 (16.9%) were lost to follow-up. Of the 104 patients who discontinued ATD, 41 (39.4%) achieved remission while 63 (60.6%) relapsed. Of the 63 patients who experienced their first relapse, 15 (23.8%) continued ATD, 34 (54.0%) discontinued ATD, and 14 (22.2%) were lost to follow-up. Of the 34 patients who discontinued ATD, 19 patients experienced a second relapse and two experienced a third relapse.
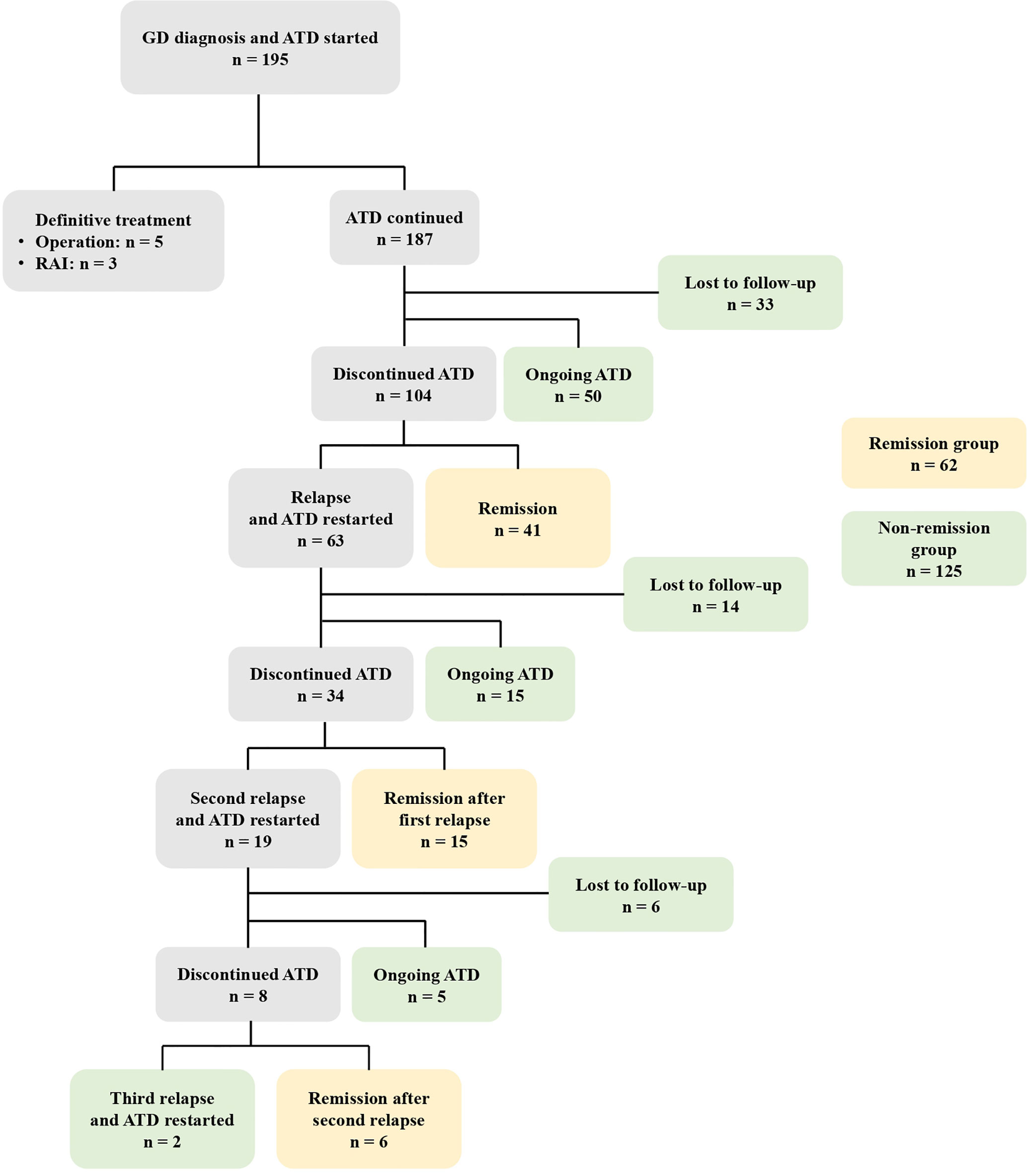
Figure 2 The clinical course of 195 patients with GD initially treated with ATD. GD, Graves’ disease; ATD, Antithyroid drugs; RAI, Radioactive iodine.
Among the 187 patients treated with ATD, 41 achieved remission without relapse, 15 achieved remission after the first relapse, and six achieved remission after the second relapse. These 62 patients were defined as the remission group. The non-remission group (n = 125) included the 53 patients who were lost to follow-up, the 50 patients who continued ATD treatment without remission, the 15 patients who continued ATD after their first relapse, the five patients who continued ATD after their second relapse, and the two patients who continued ATD after their third relapse.
There were no significant differences in clinical and biochemical characteristics between the patients who were lost to follow-up and all patients (Supplementary Table 1). In the remission group, the time to achieve remission was 43.2 ± 26.4 months, and the time to relapse was 9.7 ± 13.4 months. In the 187 patients treated with ATD, Kaplan-Meier survival analyses predicted that the cumulative remission rates were 3.3%, 19.6%, 34.1%, 43.5%, and 50.6% within 1 year, 3 years, 5 years, 7 years, and 10 years, respectively (Figure 3).
The total T3 level at diagnosis was higher in the non-remission group than in the remission group (502.6 ± 218.8 vs. 408.2 ± 192.7 pg/mL, P = 0.009). FT4 level at diagnosis was higher in the non-remission group than in the remission group (5.0 ± 1.9 vs. 3.9 ± 1.5 ng/dL, P = 0.002). Other variables, including sex, past medical history of autoimmune disease, family history of thyroid disease, ophthalmopathy, goiter, age at diagnosis, BMI score at diagnosis, ATA at diagnosis, and AMA at diagnosis, were not significantly different between the two groups (Table 2).
In the Cox regression model, the FT4 level at diagnosis [HR, 0.717 (95% CI, 0.591–0.870), P = 0.001] was identified as a predictive factor for GD remission in children and adolescents in this study (Table 3).
Most patients in our study were initially treated with MMI, although 13 patients started PTU at another hospital and then changed to MMI at our center. Among the 195 patients who had taken MMI at least once, 22 (11.3%) experienced a total of 26 AEs (Figure 4). Rash was the most common AE reported (n = 11), followed by hematologic abnormalities (n = 5), elevated liver enzymes (n = 4), arthralgia (n = 3), myalgia with elevated CK (n = 2), and alopecia (n = 1). Among the 26 AEs, 19 occurred within one month of taking MMI and 22 occurred within the first three months. Five were late AEs that occurred after more than 3 months of taking MMI. These were rash, alopecia, abnormal LFTs, and two events of neutropenia, which occurred at 30 months, 3 years, 5 years, 11 months, and 6 years after taking MMI, respectively.
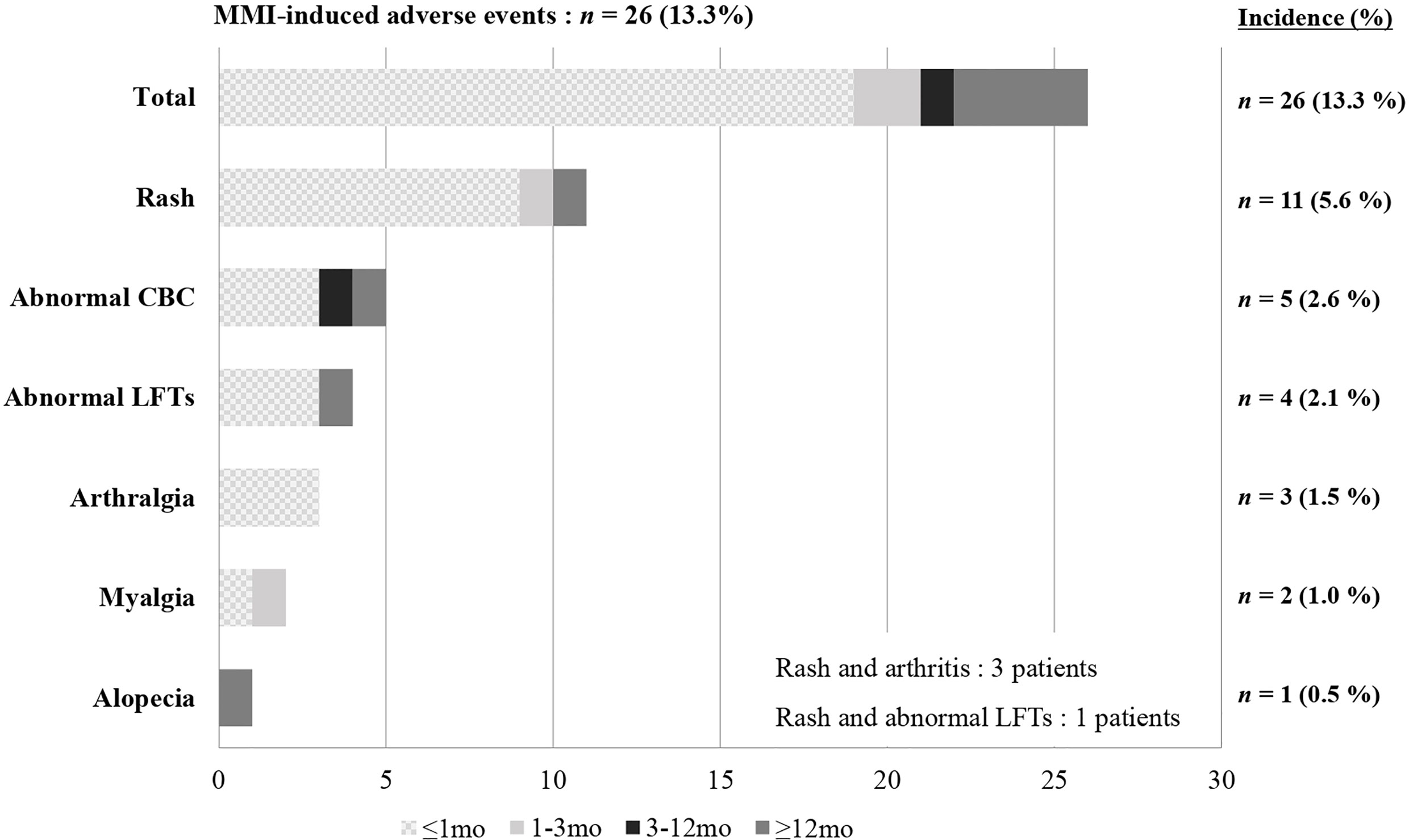
Figure 4 Incidence and time to onset of MMI-induced adverse events. MMI, Methimazole; CBC, Complete blood count; LFTs, Liver function tests.
Rash occurred in most of the patients (9 of 11) within one month, and three patients with rash also reported arthralgia. The rash and arthralgia were suitably controlled by antihistamine drugs and non-steroidal anti-inflammatory drugs and spontaneously disappeared. However, one patient switched to PTU because both symptoms persisted despite medical therapy. Of the five patients with hematologic abnormalities, one patient showed severe agranulocytosis (absolute neutrophil count (ANC) to 50/μL) with fever and abdominal pain on the fifth day of taking MMI. They recovered immediately after stopping MMI. Also, four patients had a slightly decreased WBC count and neutropenia (ANC > 500/μL). LFT abnormalities and elevated CK were mild and recovered spontaneously.
This is a retrospective study of long-term ATD treatment in children and adolescents with GD at a single tertiary institution in Korea.
Generally, the remission rates for GD in children and adolescents are 21-49% (12, 15–17). These rates may vary according to the ATD regimen or definition of the term remission. The remission rate tends to increase as the duration of ATD administration increases, reaching up to 50% in several studies of more than 10 years. However, it does not appear to increase beyond that, and some research indicates that these rates plateau (15, 16, 18). In this study, the cumulative remission rate was 3.3% at 1 year after starting ATD treatment, 19.6% at 3 years, 34.1% at 5 years, 43.5% at 7 years, and 50.6% at 10 years. In the Kaplan-Meier survival curve, remission rates hardly increased after 10 years. This may be due to the small number of patients who were followed for more than 10 years.
Several previous studies on predicting factors associated with remission or relapse in children and adolescents with GD of a similar size or larger than this study are summarized in Table 4. The literature reports different predictors for GD remission or relapse in children and adolescents, such as high TRAb levels, high FT4 levels, goiter, age or BMI-SDS at time of diagnosis, and ethnicity. Kaguelidou et al. reported that high serum TRAb levels at diagnosis were associated with a higher risk of relapse (HR = 1.21 by 10 U, P = 0.03) in a prospective, multi-center cohort study of 154 children with GD (19). Gasaldi et al. reported that lower median TRAb levels at diagnosis (less than 2.5 times the upper limit of the reference range) might be associated with positive outcomes (P = 0.031) in a retrospective, multi-center study of 115 children with GD (12). In our study, the TRAb measurement method was changed during the study period, therefore, the analysis of each measurement method was performed separately. However, we could not find consistent significant differences in TRAb levels within each group (Supplementary Table 2).
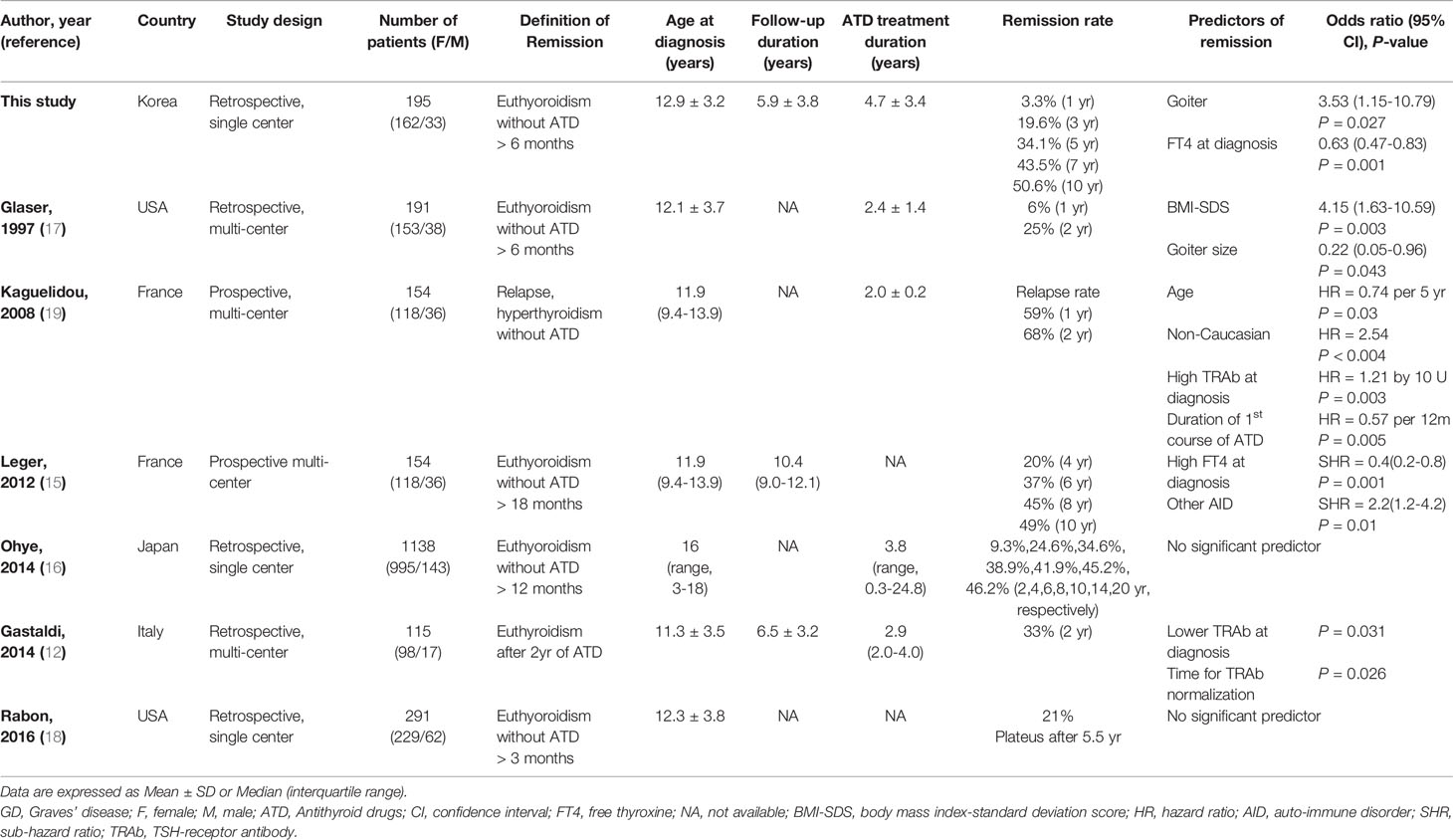
Table 4 Literature review of predicting factors associated with GD remission or relapse in children and adolescents.
We found that a low FT4 level at diagnosis was a predictor of remission, which was consistent with some previous research. Leger et al. reported that less severe parameters at the time of diagnosis (patients with FT4 <35 pmol/liter) and the presence of other autoimmune diseases had an independent positive effect on remission rate (15).
However, Ohye reported that no significant predictors of remission were found in a large, single-center cohort study involving 1,138 children with GD that has been conducted to date (16). Another single-center study of 291 children with GD by Rabon et al. found no significant factors predicting remission (18).
There is a debate about the appropriate treatment policy for GD in children and adolescents (6, 7, 20, 21). In a Cochrane review involving 3,388 adults with GD in 26 randomized trials published in 2010, the optimal duration of ATD treatment was 12-18 months (22). However, recently published studies suggest long-term medical therapy with the lowest dose of ATD is effective and safe in both adults and children (21, 23–27). A recent review article in children with GD suggested that ATD treatment maintains normal homeostasis of the hypothalamus-pituitary-thyroid axis and that long-term ATD treatment with the lowest dose of MMI/carbimazole (CMZ) should be offered to increase the possibility of remission (21). Azizi et al. reported that long-term MMI treatment for a mean of 9.1 years is a safer and more effective treatment option than short-term MMI treatment in a randomized, parallel-group trial of 66 adolescent patients with GD (24). In an ongoing study, 59 patients underwent very long-term ATD treatment with low-dose MMI for an average of 14.2 years, and most had euthyroidism without significant MMI-related AEs (25). There are few studies with a mean duration of ATD treatment of four years or more in children and adolescents with GD (15, 24, 28, 29). In our study, patients received relatively long-term MMI treatment for a mean of 4.7 ± 3.4 years and the remission rate increased according to the duration of MMI treatment in the Kaplan-Meier graph.
In long-term ATD treatment, the appropriate dosage, administration duration, and AEs of drugs are important issues. The American Thyroid Association and the American Association of Clinical Endocrinologists recommend that MMI be used to treat GD in children because PTU sometimes causes severe AEs, such as liver failure and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (30). In our study, MMI was mainly used for ATD treatment, except in cases of intolerable MMI-related AEs. The American Thyroid Association guidelines published in 2016 recommend that the initial MMI dose be 0.2-0.5 mg/kg, with a range of 0.1-1.0 mg/kg/day (30). However, treatment strategies vary between researchers and countries. In Japan, most studies used an initial dose of 1mg/kg/day (16, 31). Kaguelidou et al. used 0.5-0.7mg/kg/day (19), and Rabon et al. used 0.2-0.8 mg/kg/day (18). Several studies have reported that the AEs of MMI occur more frequently with higher doses than lower doses, and most occur early in treatment (29, 32, 33). The AEs of MMI include minor allergic reactions such as rash and serious AEs such as agranulocytosis, vasculitis, and hepatic damage. In adults with GD, reported AEs of MMI were cutaneous allergic reaction (13-24%), increased liver enzymes (2.7-3.8%), and agranulocytosis (0.3-0.7%) (33–36). In children and adolescents with GD, the prevalence of MMI-related AEs varied (6-35%), but fatal AEs were rare, and most occurred within the first three months (16, 19, 20, 37). Accurate prevalence data for agranulocytosis in children and adolescents taking MMI have not yet been reported (30).
In our study, MMI was initially dosed at 0.5-1.0 mg/kg/day, depending on the patient’s FT4 level at diagnosis, and was maintained at the lowest dose of 2.5 mg/day after euthyroidism. MMI-related AEs occurred in 11.3% of all patients, the most common of which were rash and hematologic abnormalities, followed by increased liver enzymes and arthralgia. The rash and arthralgia were suitably controlled by medical therapy, and the increased liver enzymes were within five times the normal range, with no associated jaundice. Most AEs disappeared spontaneously. Serious agranulocytosis occurred on the fifth day of MMI treatment in one patient (0.5% of all patients) who recovered immediately after drug discontinuation. The majority of AEs (19 cases, 73.0%) occurred within the first month, and only four cases (15.4%) occurred after one year of treatment. Therefore, careful monitoring to detect AEs is imperative, especially early in MMI treatment. It is essential to use the lowest MMI dose to increase the chance of remission and minimize side effects.
This study has several limitations. One is that 28.3% of patients were lost to follow-up over the 20-year study period. Some of these patients likely became adults and were transferred from pediatric endocrinologists to endocrinologists. However, those lost to follow-up showed similar baseline clinical and biochemical characteristics compared with those who remained in the study (Supplementary Table 1). Another limitation is the insufficient number of patients to identify predicting factors of remission. TRAb was not included in the statistical results due to a change in the measurement method during the study. Nevertheless, the main strength of this study is that the safety of long-term MMI treatment was assessed with a mean treatment duration of 4.7 ± 3.4 years and was conducted with the same regimen for 20 years at a single institution.
In this study, the cumulative remission rate increased according to the ATD treatment duration. The drug-related AEs usually occur early in treatment, and the majority of AEs were tolerable. Fatal side effects were rare even with long-term treatment. Therefore, long-term MMI treatment with careful monitoring of drug-related AEs is a useful treatment option before definite treatment in children and adolescents with GD. Compliance with long-term medical therapy is also important for these subjects to achieve remission. Further research is needed to determine the safety and optimal duration of long-term ATD treatment in children and adolescents with GD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Institutional Review Board of Samsung Medical Center (SMC 2021-01-051). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
AS oversaw data collection, interpretation, management, and statistical analysis for this study. SK contributed to data analysis and interpretation, management, and drafting of the article. M-SK and JK developed the structure and arguments for the paper and were responsible for clinical data collection. IK, GB, and ES analyzed and interpreted the data. YC and JC measured, analyzed, and interpreted the data. SC contributed to the research design, data analysis and interpretation, the critical review of the manuscript and approval of the submitted paper. D-KJ also approved the submitted paper. SC and D-KJ contributed equally to this work. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We wish to thank all of the individuals who are living with endocrine disorders and their families and all of the clinical and research laboratory staff.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.687834/full#supplementary-material
1. Braverman LE, M.D ed. Werner and Ingbar’s the Thyroid. In: A Fundamental and Clinical Text. Philadelphia: Lippincott Williams & Wilkins.
2. Metso S, Jaatinen P, Salmi J. Graves’ Disease. N Engl J Med (2008) 359(13):1408–9; author reply 1409. doi: 10.1056/NEJMc081426
3. Leger J, Oliver I, Rodrigue D, Lambert AS, Coutant R. Graves’ Disease in Children. Ann Endocrinol (Paris) (2018) 79(6):647–55. doi: 10.1016/j.ando.2018.08.001
4. Havgaard Kjaer R, Smedegard Andersen M, Hansen D. Increasing Incidence of Juvenile Thyrotoxicosis in Denmark: A Nationwide Study, 1998-2012. Horm Res Paediatr (2015) 84(2):102–7. doi: 10.1159/000430985
5. Wong GW, Cheng PS. Increasing Incidence of Childhood Graves’ Disease in Hong Kong: A Follow-Up Study. Clin Endocrinol (Oxf) (2001) 54(4):547–50. doi: 10.1046/j.1365-2265.2001.01252.x
6. Okawa ER, Grant FD, Smith JR. Pediatric Graves’ Disease: Decisions Regarding Therapy. Curr Opin Pediatr (2015) 27(4):442–7. doi: 10.1097/MOP.0000000000000241
7. Rivkees SA. Controversies in the Management of Graves’ Disease in Children. J Endocrinol Invest (2016) 39(11):1247–57. doi: 10.1007/s40618-016-0477-x
8. Brito JP, Schilz S, Singh Ospina N, Rodriguez-Gutierrez R, Maraka S, Sangaralingham LR, et al. Antithyroid Drugs-The Most Common Treatment for Graves’ Disease in the United States: A Nationwide Population-Based Study. Thyroid (2016) 26(8):1144–5. doi: 10.1089/thy.2016.0222
9. Escobar-Jimenez F, Fernandez-Soto ML, Luna-Lopez V, Quesada-Charneco M, Glinoer D. Trends in Diagnostic and Therapeutic Criteria in Graves’ Disease in the Last 10 Years. Postgrad Med J (2000) 76(896):340–4. doi: 10.1136/pmj.76.896.340
10. Moon JH, Yi KH. The Diagnosis and Management of Hyperthyroidism in Korea: Consensus Report of the Korean Thyroid Association. Endocrinol Metab (Seoul) (2013) 28(4):275–9. doi: 10.3803/EnM.2013.28.4.275
11. Glaser NS, Styne DM, Organization of Pediatric Endocrinologists of Northern California Collaborative Graves’ Disease Study, G. Predicting the Likelihood of Remission in Children With Graves’ Disease: A Prospective, Multicenter Study. Pediatrics (2008) 121(3):e481–8. doi: 10.1542/peds.2007-1535
12. Gastaldi R, Poggi E, Mussa A, Weber G, Vigone MC, Salerno M, et al. Graves Disease in Children: Thyroid-Stimulating Hormone Receptor Antibodies as Remission Markers. J Pediatr (2014) 164(5):1189–94.e1181. doi: 10.1016/j.jpeds.2013.12.047
13. Hwang SM, Kim MS, Lee DY. Predictive Factors for Early Response to Methimazole in Children and Adolescents With Graves Disease: A Single-Institute Study Between 1993 and 2013. Ann Pediatr Endocrinol Metab (2016) 21(2):70–4. doi: 10.6065/apem.2016.21.2.70
14. Wang SY, Wang CT, Tien KJ, Chang CC, Liu TH. Thyroid-Stimulating Hormone Receptor Antibodies During Follow-Up as Remission Markers in Childhood-Onset Graves’ Disease Treated With Antithyroid Drugs. Kaohsiung J Med Sci (2020) 36(4):281–6. doi: 10.1002/kjm2.12167
15. Leger J, Gelwane G, Kaguelidou F, Benmerad M, Alberti C, French Childhood Graves’ Disease Study, G. Positive Impact of Long-Term Antithyroid Drug Treatment on the Outcome of Children With Graves’ Disease: National Long-Term Cohort Study. J Clin Endocrinol Metab (2012) 97(1):110–9. doi: 10.1210/jc.2011-1944
16. Ohye H, Minagawa A, Noh JY, Mukasa K, Kunii Y, Watanabe N, et al. Antithyroid Drug Treatment for Graves’ Disease in Children: A Long-Term Retrospective Study at a Single Institution. Thyroid (2014) 24(2):200–7. doi: 10.1089/thy.2012.0612
17. Glaser NS, Styne DM. Predictors of Early Remission of Hyperthyroidism in Children. J Clin Endocrinol Metab (1997) 82(6):1719–26. doi: 10.1210/jcem.82.6.3986
18. Rabon S, Burton AM, White PC. Graves’ Disease in Children: Long-Term Outcomes of Medical Therapy. Clin Endocrinol (Oxf) (2016) 85(4):632–5. doi: 10.1111/cen.13099
19. Kaguelidou F, Alberti C, Castanet M, Guitteny MA, Czernichow P, Leger J, et al. Predictors of Autoimmune Hyperthyroidism Relapse in Children After Discontinuation of Antithyroid Drug Treatment. J Clin Endocrinol Metab (2008) 93(10):3817–26. doi: 10.1210/jc.2008-0842
20. Rivkees SA. Pediatric Graves’ Disease: Controversies in Management. Horm Res Paediatr (2010) 74(5):305–11. doi: 10.1159/000320028
21. Leger J, Carel JC. Management of ENDOCRINE Disease: Arguments for the Prolonged Use of Antithyroid Drugs in Children With Graves’ Disease. Eur J Endocrinol (2017) 177(2):R59–67. doi: 10.1530/EJE-16-0938
22. Abraham P, Avenell A, McGeoch SC, Clark LF, Bevan JS. Antithyroid Drug Regimen for Treating Graves’ Hyperthyroidism. Cochrane Database Syst Rev (2010) 2010(1):CD003420. doi: 10.1002/14651858.CD003420.pub4
23. Azizi F, Malboosbaf R. Safety of Long-Term Antithyroid Drug Treatment? A Systematic Review. J Endocrinol Invest (2019) 42(11):1273–83. doi: 10.1007/s40618-019-01054-1
24. Azizi F, Takyar M, Madreseh E, Amouzegar A. Long-Term Methimazole Therapy in Juvenile Graves’ Disease: A Randomized Trial. Pediatrics (2019) 143(5):e20183034. doi: 10.1542/peds.2018-3034
25. Azizi F, Abdi H, Amouzegar A. Control of Graves’ Hyperthyroidism With Very Long-Term Methimazole Treatment: A Clinical Trial. BMC Endocr Disord (2021) 21(1):16. doi: 10.1186/s12902-020-00670-w
26. Villagelin D, Romaldini JH, Santos RB, Milkos AB, Ward LS. Outcomes in Relapsed Graves’ Disease Patients Following Radioiodine or Prolonged Low Dose of Methimazole Treatment. Thyroid (2015) 25(12):1282–90. doi: 10.1089/thy.2015.0195
27. Azizi F, Malboosbaf R. Long-Term Antithyroid Drug Treatment: A Systematic Review and Meta-Analysis. Thyroid (2017) 27(10):1223–31. doi: 10.1089/thy.2016.0652
28. Sato H, Minagawa M, Sasaki N, Sugihara S, Kazukawa I, Minamitani K, et al. Comparison of Methimazole and Propylthiouracil in the Management of Children and Adolescents With Graves’ Disease: Efficacy and Adverse Reactions During Initial Treatment and Long-Term Outcome. J Pediatr Endocrinol Metab (2011) 24(5-6):257–63. doi: 10.1515/jpem.2011.194
29. Yasuda K, Miyoshi Y, Tachibana M, Namba N, Miki K, Nakata Y, et al. Relationship Between Dose of Antithyroid Drugs and Adverse Events in Pediatric Patients With Graves’ Disease. Clin Pediatr Endocrinol (2017) 26(1):1–7. doi: 10.1297/cpe.26.1
30. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid (2016) 26(10):1343–421. doi: 10.1089/thy.2016.0229
31. Sato H, Harada S, Yokoya S, Tanaka T, Asayama K, Mori M, et al. Treatment for Childhood-Onset Graves’ Disease in Japan: Results of a Nationwide Questionnaire Survey of Pediatric Endocrinologists and Thyroidologists. Thyroid (2007) 17(1):67–72. doi: 10.1089/thy.2006.0193
32. Kahaly GJ, Bartalena L, Hegedus L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism. Eur Thyroid J (2018) 7(4):167–86. doi: 10.1159/000490384
33. Nakamura H, Miyauchi A, Miyawaki N, Imagawa J. Analysis of 754 Cases of Antithyroid Drug-Induced Agranulocytosis Over 30 Years in Japan. J Clin Endocrinol Metab (2013) 98(12):4776–83. doi: 10.1210/jc.2013-2569
34. Rho JG, Park KJ, Lee HS, Hwang JS. Thyrotoxic Hypokalemic Periodic Paralysis Due to Grave’s Disease in 2 Adolescents. Ann Pediatr Endocrinol Metab (2019) 24(2):133–6. doi: 10.6065/apem.2019.24.2.133
35. Otsuka F, Noh JY, Chino T, Shimizu T, Mukasa K, Ito K, et al. Hepatotoxicity and Cutaneous Reactions After Antithyroid Drug Administration. Clin Endocrinol (Oxf) (2012) 77(2):310–5. doi: 10.1111/j.1365-2265.2012.04365.x
36. Sundaresh V, Brito JP, Wang Z, Prokop LJ, Stan MN, Murad MH, et al. Comparative Effectiveness of Therapies for Graves’ Hyperthyroidism: A Systematic Review and Network Meta-Analysis. J Clin Endocrinol Metab (2013) 98(9):3671–7. doi: 10.1210/jc.2013-1954
Keywords: Graves’ disease, hyperthyroidism, antithyroid drugs, remission, children
Citation: Song A, Kim SJ, Kim M-S, Kim J, Kim I, Bae GY, Seo E, Cho YS, Choi JY, Cho SY and Jin D-K (2021) Long-Term Antithyroid Drug Treatment of Graves’ Disease in Children and Adolescents: A 20-Year Single-Center Experience. Front. Endocrinol. 12:687834. doi: 10.3389/fendo.2021.687834
Received: 30 March 2021; Accepted: 31 May 2021;
Published: 14 June 2021.
Edited by:
Ronald Cohen, University of Chicago, United StatesReviewed by:
Onyebuchi Okosieme, Cwm Taf University Health Board, United KingdomCopyright © 2021 Song, Kim, Kim, Kim, Kim, Bae, Seo, Cho, Choi, Cho and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sung Yoon Cho, bmFkcmkxMjE3QG5hdmVyLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.